Artificial Intelligence through Wireless Sensors Applied in Restorative Dentistry: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
- P: Studies involving the application of wireless sensors in restorative dentistry.
- I: Utilization of wireless sensors in restorative dentistry procedures.
- C: Comparative control experiments.
- O: Efficacy of wireless sensors.
2.3. Information Sources
2.4. Search Strategy
2.5. Data Collection
2.6. Evaluation of Bias Risk and Research Quality in Specific Studies
2.7. Summary Measurements
3. Results
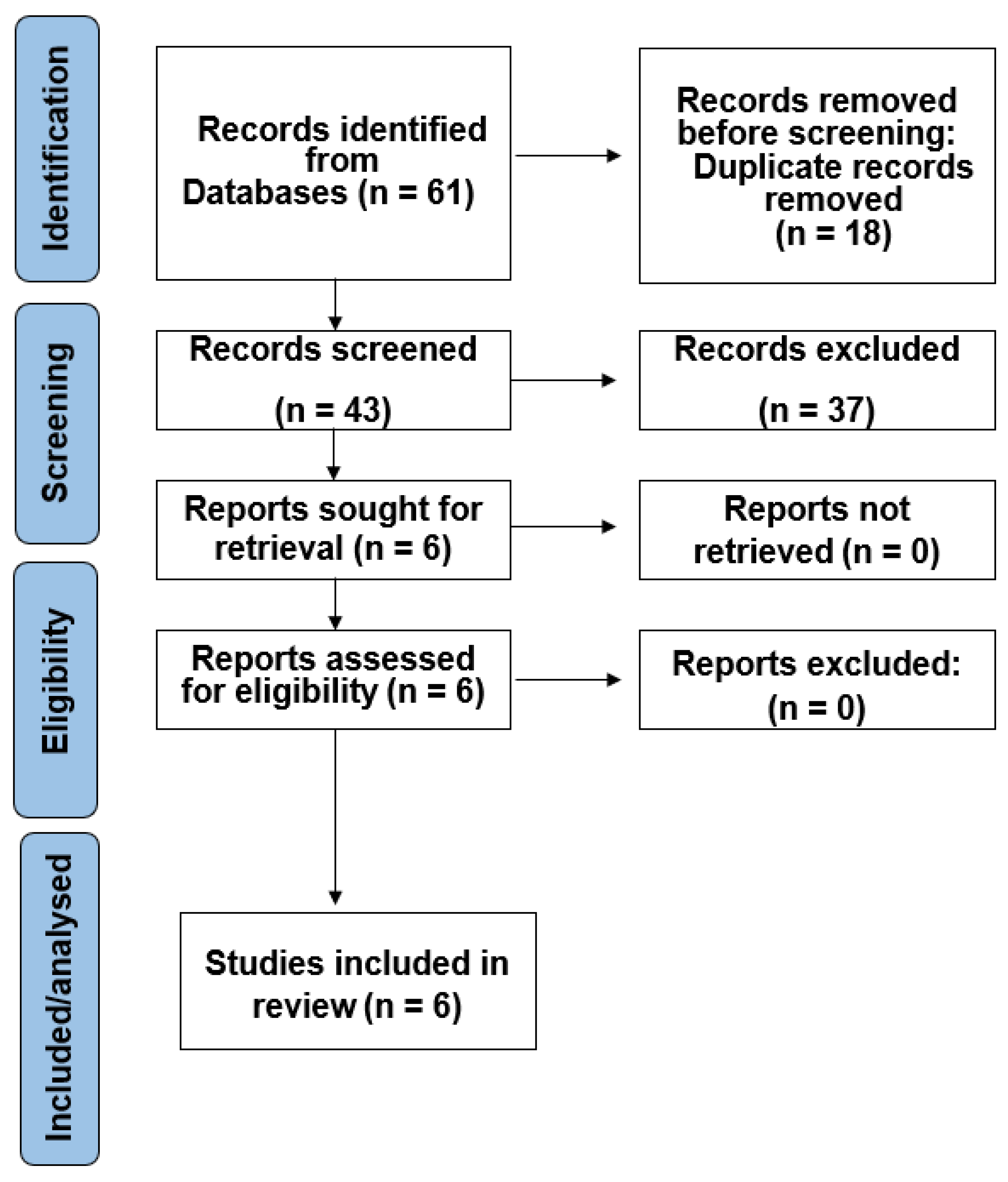
3.1. Study Selection
3.2. Features of the Studies
3.3. Main Outcomes
3.4. Results’ Synthesis
3.5. Bias Risk and Study Quality in Individual Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyers, I.; Hallett, K. Restorative Dentistry and Teeth for Life. Aust. Dent. J. 2019, 64, S3. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Fellows, C.; An, H. Digital Technologies for Restorative Dentistry. Dent. Clin. N. Am. 2022, 66, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, C.J.P.; Jiang, B.; Chen, J.; Song, J.; Liu, Z.; He, Z.; Wong, S.Y.; Fang, P.-H.; Ming, W.-K. Artificial Intelligence Versus Clinicians in Disease Diagnosis: Systematic Review. JMIR Med. Inform. 2019, 7, e10010. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Li, K. Application of Artificial Intelligence System Based on WIRELESS SENSOR Network in Enterprise Management. Comput. Intell. Neurosci. 2022, 2022, 2169521. [Google Scholar] [CrossRef] [PubMed]
- Masengo Wa Umba, S.; Abu-Mahfouz, A.M.; Ramotsoela, D. Artificial Intelligence-Driven Intrusion Detection in Software-Defined WIRELESS SENSOR Networks: Towards Secure IoT-Enabled Healthcare Systems. Int. J. Environ. Res. Public Health 2022, 19, 5367. [Google Scholar] [CrossRef]
- Lin, T.-E.; Chien, M.-C.; Chen, P.-F.; Yang, P.-W.; Chang, H.-E.; Wang, D.-H.; Lin, T.-Y.; Hsu, Y.-J. A Sensor-Integrated Face Mask Using Au@SnO2 Nanoparticle Modified Fibers and Augmented Reality Technology. ACS Omega 2022, 7, 42233–42241. [Google Scholar] [CrossRef]
- Tabata, M.; Ratanaporncharoen, C.; Ishihara, N.; Masu, K.; Sriyudthsak, M.; Kitasako, Y.; Ikeda, M.; Tagami, J.; Miyahara, Y. Surface analysis of dental caries using a wireless pH sensor and Raman spectroscopy for chairside diagnosis. Talanta 2021, 235, 122718. [Google Scholar] [CrossRef]
- Melo, D.P.; Pontual, A.D.A.; Haiter-Neto, F.; Alves, M.C.; Bóscolo, F.N.; Flores Campos, P.S. Effect of different exposure times on caries detection and pixel value in a wireless digital system. Indian J. Dent. Res. 2019, 30, 665–669. [Google Scholar] [CrossRef]
- Li, Y.J.; Lu, C.C. A Novel Scheme and Evaluations on a Long-Term and Continuous Biosensor Platform Integrated with a Dental Implant Fixture and Its Prosthetic Abutment. Sensors 2015, 15, 24961–24976. [Google Scholar] [CrossRef]
- Janusz, K.; Nelson, B.; Bartizek, R.D.; Walters, P.A.; Biesbrock, A.R. Impact of a novel power toothbrush with SmartGuide technology on brushing pressure and thoroughness. J. Contemp. Dent. Pract. 2008, 9, 1–8. [Google Scholar] [PubMed]
- Haiter-Neto, F.; dos Anjos Pontual, A.; Frydenberg, M.; Wenzel, A. A comparison of older and newer versions of intraoral digital radiography systems: Diagnosing noncavitated proximal carious lesions. J. Am. Dent. Assoc. 2007, 138, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Revilla-León, M.; Gómez-Polo, M.; Vyas, S.; Barmak, A.B.; Özcan, M.; Att, W.; Krishnamurthy, V.R. Artificial intelligence applications in restorative dentistry: A systematic review. J. Prosthet. Dent. 2022, 128, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Corbella, S.; Srinivas, S.; Cabitza, F. Applications of deep learning in dentistry. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Sirriyeh, R.; Lawton, R.; Gardner, P.; Armitage, G. Reviewing studies with diverse designs: The development and evaluation of a new tool. J. Eval. Clin. Pract. 2012, 18, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.; Pollard, M.; Cleaton-Jones, P.; Preston, A. A comparison of three electrodes for the measurement of pH in small volumes. Caries Res. 1997, 31, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Galiano, V.; Basile, M.; Di Luca, F.; Gitto, S.; Messina, C.; Cagetti, M.G.; Del Fabbro, M.; Tartaglia, G.M.; Sconfienza, L.M. Artificial intelligence for radiographic imaging detection of caries lesions: A systematic review. BMC Oral Health 2024, 24, 274. [Google Scholar] [CrossRef]
- Marinho-Vieira, L.E.; Martins, L.A.C.; Freitas, D.Q.; Haiter-Neto, F.; Oliveira, M.L. Revisiting dynamic range and image enhancement ability of contemporary digital radiographic systems. Dentomaxillofac. Radiol. 2022, 51, 20210404. [Google Scholar] [CrossRef]
- Kitagawa, H.; Sheetz, J.P.; Farman, A.G. Comparison of complementary metal oxide semiconductor and charge-coupled device intraoral X-ray detectors using subjective image quality. Dentomaxillofac. Radiol. 2003, 32, 408–411. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Zhang, Q.; Li, M.; Zhao, Z.; Lin, B.; Peng, J.; Shen, H.; He, Q. Fenton-like system of UV/Glucose-oxidase@Kaolin coupled with organic green rust: UV-enhanced enzyme activity and the mechanism of UV synergistic degradation of photosensitive pollutants. Environ. Res. 2024, 247, 118257. [Google Scholar] [CrossRef] [PubMed]
- Habte-Asres, H.H.; Jiang, Y.; Rosenthal, M.; Wheeler, D.C. Burden of impaired awareness of hypoglycemia in people with diabetes undergoing hemodialysis. BMJ Open Diabetes Res. Care. 2024, 12, e003730. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Scholte, N.T.B.; Ebrahimkheil, K.; Brouwer, M.A.; Beukema, R.J.; Mafi-Rad, M.; Vernooy, K.; Yap, S.-C.; Ronner, E.; van Mieghem, N.; et al. Automated cardiac arrest detection using a photoplethysmography wristband: Algorithm development and validation in patients with induced circulatory arrest in the DETECT-1 study. Lancet Digit. Health 2024, 6, e201–e210. [Google Scholar] [CrossRef]
- Fontes, L.; Machado, P.; Vinkemeier, D.; Yahaya, S.; Bird, J.J.; Ihianle, I.K. Enhancing Stress Detection: A Comprehensive Approach through rPPG Analysis and Deep Learning Techniques. Sensors 2024, 24, 1096. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E. Bacterial species and factors influencing the contamination of the inner and outer layers of face masks used in dentistry. Dent. Med. Probl. 2023, 60, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Yaacob, M.; Worthington, H.V.; Deacon, A.S.; Deery, C.; Walmsley, A.D.; Robinson, P.G.; Glenny, A.-M. Powered versus manual toothbrushing for oral health. Cochrane Database Syst. Rev. 2014, 2014, CD002281. [Google Scholar] [CrossRef]
- Ardila, C.M.; Arrubla-Escobar, D.E.; Vivares-Builes, A.M. Efficacy of microchips and 3D sensors for orthodontic force measurement: A systematic review of in vitro studies. Orthod. Craniofac. Res. 2024. [Google Scholar] [CrossRef]
| Authors and Publication Year | Country | Study Design | Main Aim |
|---|---|---|---|
| Lin et al., 2022 [7] | Taiwan | In vitro | A wireless sensor-integrated face mask was created with Au@SnO2 nanoparticle-modified conductive structures, utilizing amplified reality machinery. |
| Tabata et al., 2021 [8] | Japan | In vitro | A wireless caries sensing tool, comparable in size to a dental explorer, was designed to compare two sensing modalities: Raman readings and pH readings, for the evaluation of tooth decay. |
| Melo et al., 2019 [9] | Brazil | In vitro | A wireless system was employed to assess the impact of dissimilar exposure durations on caries’ detection and pixel intensity values. |
| Li et al., 2015 [10] | Taiwan | In vitro | A novel “miniature intra-oral dental implant system”, which incorporates a “built-in biosensor device”, is being suggested. The implant fixture’s placement enables “continuous blood analysis and management via the maxillary bone marrow, facilitated by the dental implant platform”. |
| Janusz et al., 2008 [11] | USA | Randomized clinical trial | This report assessed the efficacy of a “power brush” equipped with a “wireless remote display” in enhancing brushing force and thoroughness. |
| Haiter-Neto et al., 2007 [12] | Brazil | In vitro | In this study, the accuracy of two intra-oral digital systems for the radiographic identification of proximal carious lesions was evaluated. |
| Authors | Sensor Characteristics and Operation | Main Results |
|---|---|---|
| Lin et al. [7] | The mask’s interior contained an electronic microprocessor with two sensors: a temperature sensor and a respiration sensor. These sensors monitored “body temperature and respiratory rate”, respectively. The microcontroller then combined the data and wirelessly communicated it to the smartwatch. | The “intelligent mask” not only enhances security by incorporating “conductive textiles and nanoparticles”, but it also integrates a variety of sensor systems for real-time monitoring without requiring the removal of the mask. |
| Tabata et al. [8] | The measurement system circuit included an analog–digital converter (ADC), a “wireless module, and a 3.3 V lithium-ion battery”. The pH conductor and reference electrode were directly connected to the ADC component. All components were housed “in a 3D-printed PLA polymer” casing. “The wireless sensor’s pH” reaction was evaluated with seven pH solutions (pH 3 to pH 9). “The wireless module’s software was written in Arduino, and real-time data collection was done using a LabVIEW-based application”. | “The pH of the dental caries surface was gauged with a wireless pH sensor”, revealing pH values of 6.9 and 5.7 in non-caries and caries areas, respectively. This wireless pH sensor could prove beneficial in comprehending dental caries conditions and aiding dentists in selectively removing caries while maintaining the non-caries structure. |
| Melo et al. [9] | “The data collected are transmitted through radiofrequency waves. When the wireless sensor’s digital receptor is exposed to radiation, the electric charge generated by the exposure of the silicon crystals is converted into radiofrequency waves. These waves are captured by the system’s base station antenna and converted into binary units, which are then transmitted to the computer via a fiber optic cable”. | “The wireless sensor was evaluated with histological confirmation, and it was determined that the optimal exposure time for caries diagnosis was 0.25 s (15 i), with an acceptable range of 0.06–0.20 s (4–12 i)”. |
| Li et al. [10] | “The dental implant system comprises an implant fixture, a prosthetic abutment, a biosensor module, a Bluetooth 4.0 wireless module, and a DC button cell battery. The electrochemical biosensor features three electrodes: working, reference, and counter, which are positioned to penetrate the titanium implant fixture beneath the biosensor module. These electrodes are exposed to the blood pool within the maxillary bone marrow and engage in oxidation/reduction reactions with the biosensing enzyme coating”. | To validate the planned proposal, the restriction course of glucose oxidase (GOD) enzyme is successfully executed, and in vitro glucose level detections are performed, demonstrating “the sensitivity, linearity, and repeatability of the glucose biosensor system. Additionally, a preliminary canine animal model utilizing the new pathway” exhibits substantial agreement with the conventional technique of blood sugar detection via dermal pricks. |
| Janusz et al. [11] | “The wireless display and toothbrush” interconnect through an automatic chip embedded in the toothbrush handle. The display can be situated up to 10–15 feet from the patient, enabling them to conveniently “view the two-minute timer, brushing mode, quadrant timer, and pressure signal, which illuminates when a force of over 3 N is exerted”. | Following 30 days of at-home use, participants utilizing the power brush equipped with a wireless display experienced a reduction in pressure sensor activation time by 88.5% compared to the baseline, while those using the power brush alone saw a reduction of 53.4% (p = 0.034). Individuals using the power brush with the wireless display applied less force than those using the power brush alone. |
| Haiter-Neto et al. [12] | The data collected “are transmitted via radiofrequency waves”. When the digital receptor of the wireless sensor is “exposed to radiation, the electric charge generated by the silicon” crystals’ exposure is converted into radiofrequency waves. These waves are captured by the “system’s base station antenna and converted into binary units, which are then transmitted to the computer via a fiber optic cable”. | The wireless sensor digital systems exhibited significantly higher sensitivities compared to their precursors. The investigators noted no substantial differences in “specificity among the Digora FMX, Schick CDR, and CDR wireless systems, all of which had significantly higher specificity than the Digora Optime system (p < 0.02). The positive predictive value for the Digora Optime system was influenced by its high sensitivity and low specificity, resulting in a lower value compared to the two CDR systems (p < 0.02)”. |
| Study | Criteria Completely Satisfied | Percentage Score of Compliance |
|---|---|---|
| Lin et al., 2022 [7] | 15 | 94% |
| Tabata et al., 2021 [8] | 14 | 88% |
| Melo et al., 2019 [9] | 14 | 88% |
| Li et al., 2015 [10] | 14 | 88% |
| Janusz et al., 2008 [11] | 14 | 88% |
| Haiter-Neto et al. [12] | 15 | 94% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardila, C.M.; Vivares-Builes, A.M. Artificial Intelligence through Wireless Sensors Applied in Restorative Dentistry: A Systematic Review. Dent. J. 2024, 12, 120. https://doi.org/10.3390/dj12050120
Ardila CM, Vivares-Builes AM. Artificial Intelligence through Wireless Sensors Applied in Restorative Dentistry: A Systematic Review. Dentistry Journal. 2024; 12(5):120. https://doi.org/10.3390/dj12050120
Chicago/Turabian StyleArdila, Carlos M., and Annie Marcela Vivares-Builes. 2024. "Artificial Intelligence through Wireless Sensors Applied in Restorative Dentistry: A Systematic Review" Dentistry Journal 12, no. 5: 120. https://doi.org/10.3390/dj12050120
APA StyleArdila, C. M., & Vivares-Builes, A. M. (2024). Artificial Intelligence through Wireless Sensors Applied in Restorative Dentistry: A Systematic Review. Dentistry Journal, 12(5), 120. https://doi.org/10.3390/dj12050120









