The Influence of Orthodontic Treatment on Periodontal Health between Challenge and Synergy: A Narrative Review
Abstract
1. Introduction
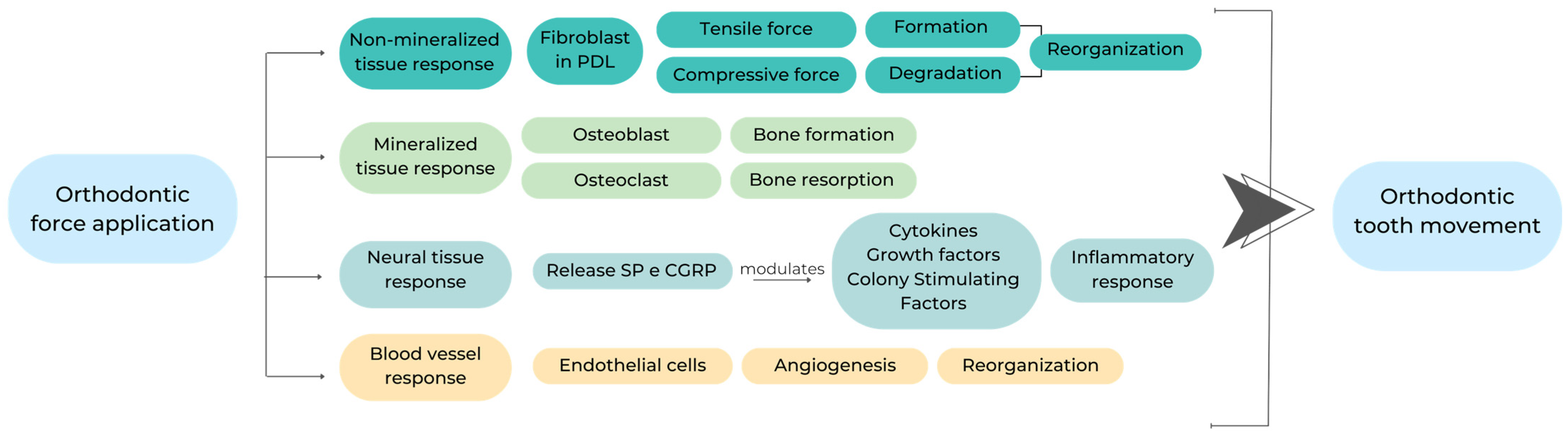
2. Mechanisms of Action
3. Periodontal and Bone Biomarkers Related to Orthodontic Force Application
4. Orthodontic Fixed Appliance
4.1. Self-Ligating Brackets (SLBs) and Conventional Brackets (CBs)
4.2. Changes in Oral Microbiota
4.3. Changes in Periodontal Health
5. Orthodontic Removable Appliance and Periodontal Health
6. Discussion and Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Anezi, S.A. The effect of orthodontic bands or tubes upon periodontal status during the initial phase of orthodontic treatment. Saudi Dent. J. 2015, 27, 120–124. [Google Scholar] [CrossRef]
- Alfuriji, S.; Alhazmi, N.; Alhamlan, N.; Al-Ehaideb, A.; Alruwaithi, M.; Alkatheeri, N.; Geevarghese, A. The effect of orthodontic therapy on periodontal health: A review of the literature. Int. J. Dent. 2014, 2014, 585048. [Google Scholar] [CrossRef]
- Bollen, A.M.; Cunha-Cruz, J.; Bakko, D.W.; Huang, G.J.; Hujoel, P.P. The effects of orthodontic therapy on periodontal health: A systematic review of controlled evidence. J. Am. Dent. Assoc. 2008, 139, 413–422. [Google Scholar] [CrossRef]
- Ren, Y.; Jongsma, M.A.; Mei, L.; van der Mei, H.C.; Busscher, H.J. Orthodontic treatment with fixed appliances and biofilm formation--a potential public health threat? Clin. Oral Investig. 2014, 18, 1711–1718. [Google Scholar] [CrossRef]
- Rossini, G.; Parrini, S.; Castroflorio, T.; Deregibus, A.; Debernardi, C.L. Periodontal health during clear aligners treatment: A systematic review. Eur. J. Orthod. 2015, 37, 539–543. [Google Scholar] [CrossRef]
- Talic, N.F. Adverse effects of orthodontic treatment: A clinical perspective. Saudi Dent. J. 2011, 23, 55–59. [Google Scholar] [CrossRef]
- Sandić, M.Z.; Popović, B.; Carkić, J.; Nikolić, N.; Glisić, B. Changes in subgingival microflora after placement and removal of fixed orthodontic appliances. Srp. Arh. Celok. Lek. 2014, 142, 301–305. [Google Scholar] [CrossRef]
- Dannan, A. An update on periodontic-orthodontic interrelationships. J. Indian Soc. Periodontol. 2010, 14, 66–71. [Google Scholar] [CrossRef]
- Gorbunkova, A.; Pagni, G.; Brizhak, A.; Farronato, G.; Rasperini, G. Impact of Orthodontic Treatment on Periodontal Tissues: A Narrative Review of Multidisciplinary Literature. Int. J. Dent. 2016, 2016, 4723589. [Google Scholar] [CrossRef]
- Feller, L.; Khammissa, R.A.; Schechter, I.; Thomadakis, G.; Fourie, J.; Lemmer, J. Biological Events in Periodontal Ligament and Alveolar Bone Associated with Application of Orthodontic Forces. Sci. World J. 2015, 2015, 876509. [Google Scholar] [CrossRef]
- Othman, S.A.; Mansor, N.; Saub, R. Randomized controlled clinical trial of oral health-related quality of life in patients wearing conventional and self-ligating brackets. Korean J. Orthod. 2014, 44, 168–176. [Google Scholar] [CrossRef]
- Boas Nogueira, A.V.; Chaves de Souza, J.A.; Kim, Y.J.; Damião de Sousa-Neto, M.; Chan Cirelli, C.; Cirelli, J.A. Orthodontic force increases interleukin-1β and tumor necrosis factor-α expression and alveolar bone loss in periodontitis. J. Periodontol. 2013, 84, 1319–1326. [Google Scholar] [CrossRef]
- Antoun, J.S.; Mei, L.; Gibbs, K.; Farella, M. Effect of orthodontic treatment on the periodontal tissues. Periodontol. 2000 2017, 74, 140–157. [Google Scholar] [CrossRef]
- Pender, N. Aspects of oral health in orthodontic patients. Br. J. Orthod. 1986, 13, 95–103. [Google Scholar] [CrossRef]
- Davies, T.M.; Shaw, W.C.; Worthington, H.V.; Addy, M.; Dummer, P.; Kingdon, A. The effect of orthodontic treatment on plaque and gingivitis. Am. J. Orthod. Dentofac. Orthop. 1991, 99, 155–161. [Google Scholar] [CrossRef]
- Camerlingo, C.; d‘Apuzzo, F.; Grassia, V.; Perillo, L.; Lepore, M. Micro-Raman spectroscopy for monitoring changes in periodontal ligaments and gingival crevicular fluid. Sensors 2014, 14, 22552–22563. [Google Scholar] [CrossRef]
- Reitan, K.; Righ, P. Biomechanical principles and reactions. In Orthodontics, Current Principles and Techniques, 2nd ed.; Graber, T.H., Vanarsdall, T.M., Eds.; CV Mosby: St Louis, MO, USA, 1994; pp. 96–192. [Google Scholar]
- Krishnan, V.; Davidovitch, Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 469.e1-32. [Google Scholar] [CrossRef]
- Guo, L.; Feng, Y.; Guo, H.G.; Liu, B.W.; Zhang, Y. Consequences of orthodontic treatment in malocclusion patients: Clinical and microbial effects in adults and children. BMC Oral Health 2016, 16, 112. [Google Scholar] [CrossRef]
- Katta, M.; Cumpătă, C.N.; Ţuculină, M.J.; Lazăr, A.C.; Manolea, H.O.; Mocanu, H.; Mărăşescu, F.I.; Petrescu, S.M.S.; Dascălu, I.T. Clinical, histopathological and immunohistochemical changes of the superficial marginal periodontium caused by orthodontic treatment with fixed metallic orthodontic appliances. Rom. J. Morphol. Embryol. 2022, 63, 431–438. [Google Scholar] [CrossRef]
- Perinetti, G.; Di Leonardo, B.; Di Lenarda, R.; Contardo, L. Repeatability of gingival crevicular fluid collection and quantification, as determined through its alkaline phosphatase activity: Implications for diagnostic use. J. Periodontal Res. 2013, 48, 98–104. [Google Scholar] [CrossRef]
- Reichert, C.; Hagner, M.; Jepsen, S.; Jäger, A. Interfaces between orthodontic and periodontal treatment: Their current status. J. Orofac. Orthop. 2011, 72, 165–186. [Google Scholar] [CrossRef]
- Amid, R.; Kadkhodazadeh, M.; Moscowchi, A.; Tavakol Davani, S.; Soleimani, M.; Dehghani Soltani, A.; Al-Shuhayeb, M. Effect of gingival biotype on orthodontic treatment-inducedperiodontal complications: A systematic review. J. Adv. Periodontol. Implant. Dent. 2020, 12, 3–10. [Google Scholar] [CrossRef]
- Steegmans, P.A.J.; Meursinge Reynders, R.A. Fixed orthodontic retainers and periodontal health. Evid. Based Dent. 2020, 21, 146–149. [Google Scholar] [CrossRef]
- Kaku, M.; Motokawa, M.; Tohma, Y.; Tsuka, N.; Koseki, H.; Sunagawa, H.; Arturo Marquez Hernandes, R.; Ohtani, J.; Fujita, T.; Kawata, T.; et al. VEGF and M-CSF levels in periodontal tissue during tooth movement. Biomed. Res. 2008, 29, 181–187. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, H. Comparative study on the effect of fixed appliances and removable aligners. Stomatology 2014, 34, 784–786. [Google Scholar]
- Eroglu, A.K.; Baka, Z.M.; Arslan, U. Comparative evaluation of salivary microbial levels and periodontal status of patients wearing fixed and removable orthodontic retainers. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 186–192. [Google Scholar] [CrossRef]
- Jurela, A.; Repic, D.; Pejda, S.; Juric, H.; Vidakovic, R.; Matic, I.; Bosnjak, A. The effect of two different bracket types on the salivary levels of S mutans and S sobrinus in the early phase of orthodontic treatment. Angle Orthod. 2013, 83, 140–145. [Google Scholar] [CrossRef]
- Behm, C.; Nemec, M.; Blufstein, A.; Schubert, M.; Rausch-Fan, X.; Andrukhov, O.; Jonke, E. Interleukin-1β Induced Matrix Metalloproteinase Expression in Human Periodontal Ligament-Derived Mesenchymal Stromal Cells under In Vitro Simulated Static Orthodontic Forces. Int. J. Mol. Sci. 2021, 22, 1027. [Google Scholar] [CrossRef]
- Behm, C.; Nemec, M.; Weissinger, F.; Rausch, M.A.; Andrukhov, O.; Jonke, E. MMPs and TIMPs Expression Levels in the Periodontal Ligament during Orthodontic Tooth Movement: A Systematic Review of In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2021, 22, 6967. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, Y.; Liu, X.; He, J.; Zheng, B.; Liu, Y. The Experimental Study of Periodontal Ligament Stem Cells Derived Exosomes with Hydrogel Accelerating Bone Regeneration on Alveolar Bone Defect. Pharmaceutics 2022, 14, 2189. [Google Scholar] [CrossRef]
- Kook, S.H.; Jang, Y.S.; Lee, J.C. Involvement of JNK-AP-1 and ERK-NF-κB signaling in tension-stimulated expression of type I collagen and MMP-1 in human periodontal ligament fibroblasts. J. Appl. Physiol. 2011, 111, 1575–1583. [Google Scholar] [CrossRef]
- Nakao, Y.; Fukuda, T.; Zhang, Q.; Sanui, T.; Shinjo, T.; Kou, X.; Chen, C.; Liu, D.; Watanabe, Y.; Hayashi, C.; et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021, 122, 306–324. [Google Scholar] [CrossRef]
- Trindade, F.; Oppenheim, F.G.; Helmerhorst, E.J.; Amado, F.; Gomes, P.S.; Vitorino, R. Uncovering the molecular networks in periodontitis. Proteom. Clin. Appl. 2014, 8, 748–761. [Google Scholar] [CrossRef]
- Ganther, S.; Fenno, C.J.; Kapila, Y.L. Stimulation of Human Periodontal Ligament Fibroblasts Using Purified Dentilisin Extracted from Treponema denticola. Bio Protoc. 2022, 12, 4571. [Google Scholar] [CrossRef]
- Dudic, A.; Kiliaridis, S.; Mombelli, A.; Giannopoulou, C. Composition changes in gingival crevicular fluid during orthodontic tooth movement: Comparisons between tension and compression sides. Eur. J. Oral Sci. 2006, 114, 416–422. [Google Scholar] [CrossRef]
- Kim, T.; Handa, A.; Iida, J.; Yoshida, S. RANKL expression in rat periodontal ligament subjected to a continuous orthodontic force. Arch. Oral Biol. 2007, 52, 244–250. [Google Scholar] [CrossRef]
- Zuppardo, M.L.; Santamaria, M.; Ferreira, C.L.; Longo, M.; Cirelli, J.A.; Santamaria, M.P.; Jardini, M.A.N. Effect of two corticotomy protocols on periodontal tissue and orthodontic movement. J. Appl. Oral Sci. 2020, 28, 20190766. [Google Scholar] [CrossRef]
- Brooks, P.J.; Nilforoushan, D.; Manolson, M.F.; Simmons, C.A.; Gong, S.G. Molecular markers of early orthodontic tooth movement. Angle Orthod. 2009, 79, 1108–1113. [Google Scholar] [CrossRef]
- Castroflorio, T.; Gamerro, E.F.; Caviglia, G.P.; Deregibus, A. Biochemical markers of bone metabolism during early orthodontic tooth movement with aligners. Angle Orthod. 2017, 87, 74–81. [Google Scholar] [CrossRef]
- Küchler, E.C.; Schröder, A.; Corso, P.; Scariot, R.; Spanier, G.; Proff, P.; Kirschneck, C. Genetic polymorphisms influence gene expression of human periodontal ligament fibroblasts in the early phases of orthodontic tooth movement. Odontology 2020, 108, 493–502. [Google Scholar] [CrossRef]
- Miyagawa, A.; Chiba, M.; Hayashi, H.; Igarashi, K. Compressive force induces VEGF production in periodontal tissues. J. Dent. Res. 2009, 88, 752–756. [Google Scholar] [CrossRef]
- Kaku, M.; Kohno, S.; Kawata, T.; Fujita, I.; Tokimasa, C.; Tsutsui, K.; Tanne, K. Effects of vascular endothelial growth factor on osteoclast induction during tooth movement in mice. J. Dent. Res. 2001, 80, 1880–1883. [Google Scholar] [CrossRef]
- Caviedes-Bucheli, J.; Lopez-Moncayo, L.F.; Muñoz-Alvear, H.D.; Gomez-Sosa, J.F.; Diaz-Barrera, L.E.; Curtidor, H.; Munoz, H.R. Expression of substance P, calcitonin gene-related peptide and vascular endothelial growth factor in human dental pulp under different clinical stimuli. BMC Oral Health 2021, 21, 152. [Google Scholar] [CrossRef]
- Farahani, M.; Safavi, S.M.; Dianat, O.; Khoramian Tusi, S.; Younessian, F. Acid and Alkaline Phosphatase Levels in GCF during Orthodontic Tooth Movement. J. Dent. 2015, 16, 237–245. [Google Scholar]
- Kumar, A.A.; Saravanan, K.; Kohila, K.; Kumar, S.S. Biomarkers in orthodontic tooth movement. J. Pharm. Bioallied Sci. 2015, 7 (Suppl. S2), S325–S330. [Google Scholar] [CrossRef]
- Kakali, L.; Giantikidis, I.; Sifakakis, I.; Kalimeri, E.; Karamani, I.; Mavrogonatou, E.; Kloukos, D. Fluctuation of bone turnover markers’ levels in samples of gingival crevicular fluid after orthodontic stimulus: A systematic review. Syst. Rev. 2022, 11, 3. [Google Scholar] [CrossRef]
- Jeyraj, Y.; Katta, A.K.; Vannala, V.; Lokanathan, D.; Reddy, S.N.; Rajasigamani, K. Estimation of alkaline phosphatase in the gingival crevicular fluid during orthodontic tooth movement in premolar extraction cases to predict therapeutic progression. J. Nat. Sci. Biol. Med. 2015, 6, 343–346. [Google Scholar]
- Alhadlaq, A.M. Biomarkers of Orthodontic Tooth Movement in Gingival Crevicular Fluid: A Systematic Review. J. Contemp. Dent. Pract. 2015, 16, 578–587. [Google Scholar] [CrossRef]
- Kapoor, P.; Kharbanda, O.P.; Monga, N.; Miglani, R.; Kapila, S. Effect of orthodontic forces on cytokine and receptor levels in gingival crevicular fluid: A systematic review. Prog. Orthod. 2014, 9, 65. [Google Scholar] [CrossRef]
- Attin, R.; Thon, C.; Schlagenhauf, U.; Werner, C.; Wiegand, A.; Hannig, C.; Attin, T. Recolonization of mutans streptococci on teeth with orthodontic appliances after antimicrobial therapy. Eur. J. Orthod. 2005, 27, 489–493. [Google Scholar] [CrossRef]
- Ristic, M.; Vlahovic Svabic, M.; Sasic, M.; Zelic, O. Clinical and microbiological effects of fixed orthodontic appliances on periodontal tissues in adolescents. Orthod. Craniofac. Res. 2007, 10, 187–195. [Google Scholar] [CrossRef]
- Pandis, N.; Vlachopoulos, K.; Polychronopoulou, A.; Madianos, P.; Eliades, T. Periodontal condition of the mandibular anterior dentition in patients with conventional and self-ligating brackets. Orthod. Craniofac. Res. 2008, 11, 211–215. [Google Scholar] [CrossRef]
- Yáñez-Vico, R.M.; Iglesias-Linares, A.; Cadenas de Llano-Pérula, M.; Solano-Reina, A.; Solano-Reina, E. Management of occlusal canting with miniscrews. Angle Orthod. 2014, 84, 737–747. [Google Scholar] [CrossRef]
- Mummolo, S.; Marchetti, E.; Giuca, M.R.; Gallusi, G.; Tecco, S.; Gatto, R.; Marzo, G. In-office bacteria test for a microbial monitoring during the conventional and self-ligating orthodontic treatment. Head Face Med. 2013, 9, 7. [Google Scholar] [CrossRef]
- Türkkahraman, H.; Sayın, O.; Bozkurt, Y.; Yetkin, Z.; Kaya, S.; Onal, S. Archwire ligation techniques, microbial colonization, and periodontal status in orthodontically treated patients. Angle Orthod. 2005, 75, 231–236. [Google Scholar]
- Pellegrini, P.; Sauerwein, R.; Finlayson, T.; McLeod, J.; Covell, D.A., Jr.; Maier, T.; Machida, C.A. Plaque retention by self-ligating vs elastomeric orthodontic brackets: Quantitative comparison of oral bacteria and detection with adenosine triphosphate-driven bioluminescence. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 426.e-9; discussion 426-7. [Google Scholar] [CrossRef]
- van Gastel, J.; Quirynen, M.; Teughels, W.; Coucke, W.; Carels, C. Influence of bracket design on microbial and periodontal parameters in vivo. J. Clin. Periodontol. 2007, 34, 423–431. [Google Scholar] [CrossRef]
- Li, Y.R.; Yuan, J.J.; Li, J.Q.; Bai, Y.; Yan, Y.Z.; Liu, L.Y.; Zhang, C.X. Comparison of the effects of two kinds of aesthetic appliances on periodontal health of adult orthodontic patients. Henan Med. Res. 2015, 26, 1757–1759. [Google Scholar]
- Sallum, E.J.; Nouer, D.F.; Klein, M.I.; Gonçalves, R.B.; Machion, L.; Sallum, A.W.; Sallum, E.A. Clinical and microbiologic changes after removal of orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 363–366. [Google Scholar] [CrossRef]
- Paolantonio, M.; Festa, F.; di Placido, G.; D’Attilio, M.; Catamo, G.; Piccolomini, R. Site-specific subgingival colonization by Actinobacillus actinomycetemcomitans in orthodontic patients. Am. J. Orthod. Dentofac. Orthop. 1999, 115, 423–428. [Google Scholar] [CrossRef]
- Contaldo, M.; Lucchese, A.; Lajolo, C.; Rupe, C.; Di Stasio, D.; Romano, A.; Petruzzi, M.; Serpico, R. The oral microbiota changes in orthodontic patients and effects on oral health: An overview. J. Clin. Med. 2021, 10, 780. [Google Scholar] [CrossRef]
- Lucchese, A.; Bondemark, L.; Marcolina, M.; Manuelli, M. Changes in oral microbiota due to orthodontic appliances: A systematic review. J. Oral Microbiol. 2018, 10, 1476645. [Google Scholar] [CrossRef]
- Nalçacı, R.; Özat, Y.; Çokakoğlu, S.; Türkkahraman, H.; Önal, S.; Kaya, S. Effect of bracket type on halitosis, periodontal status, and microbial colonization. Angle Orthod. 2014, 84, 479–485. [Google Scholar] [CrossRef]
- Carillo, L.E.; Montiel-Bastida, N.M.; Sanchez-Perez, L.; Alanis-Tavira, J. Effect of orthodontic treatment on saliva, plaque and the levels of Streptococcus mutans and Lactobacillus. Med. Oral Patol. Oral Cir. Bucal. 2010, 15, 924–929. [Google Scholar] [CrossRef]
- Coronado-Castellote, L.; Jiménez-Soriano, Y. Clinical and microbiological diagnosis of oral candidiasis. J. Clin. Exp. Dent. 2013, 5, 279. [Google Scholar] [CrossRef]
- Hernández-Solís, S.E.; Rueda-Gordillo, F.; Flota-Alcocer, A.D.; Agullar-Ayala, F.J.; Rodríguez-Fernández Mdel, S.; Lama-González, E.M. Influence of orthodontic appliances on the occurrence of Candida spp. in the oral cavity. Rev. Chil. Infectol. 2016, 33, 293–297. [Google Scholar] [CrossRef]
- Perkowski, K.; Baltaza, W.; Conn, D.B.; Marczyńska-Stolarek, M.; Chomicz, L. Examination of oral biofilm microbiota in patients using fixed orthodontic appliances in order to prevent risk factors for health complications. Ann. Agric. Env. Med. 2019, 26, 231–235. [Google Scholar] [CrossRef]
- Grzegocka, K.; Krzyściak, P.; Hille-Padalis, A.; Loster, J.E.; Talaga-Ćwiertnia, K.; Loster, B.W. Candida prevalence and oral hygiene due to orthodontic therapy with conventional brackets. BMC Oral Health 2020, 20, 277. [Google Scholar] [CrossRef]
- Guo, R.; Lin, Y.; Zheng, Y.; Li, W. The microbial changes in subgingival plaques of orthodontic patients: A systematic review and meta-analysis of clinical trials. BMC Oral Health 2017, 17, 90. [Google Scholar] [CrossRef]
- Bergamo, A.Z.; de Oliveira, K.M.; Matsumoto, M.A.; Nascimento, C.D.; Romano, F.L.; da Silva, R.A.; da Silva, L.A.; Nelson-Filho, P. Orthodontic appliances did not increase risk of dental caries and periodontal disease under preventive protocol. Angle Orthod. 2019, 89, 25–32. [Google Scholar] [CrossRef]
- Santonocito, S.; Polizzi, A. Oral Microbiota Changes during Orthodontic Treatment. Front. Biosci. (Elite Ed.) 2022, 14, 19. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, J.B.; Wang, B.; Zhang, X.; Yin, Y.L.; Bai, H. Alterations of the oral microbiome in patients treated with the Invisalign system or with fixed appliances. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 633–640. [Google Scholar] [CrossRef]
- Cortellini, P.; Bissada, N.F. Mucogingival conditions in the natural dentition: Narrative review, case definitions, and diagnostic considerations. J. Periodontol. 2018, 89, 204–213. [Google Scholar] [CrossRef]
- Liu, H.; Sun, J.; Dong, Y.; Lu, H.; Zhou, H.; Hansen, B.F.; Song, X. Periodontal health and relative quantity of subgingival Porphyromonas gingivalis during orthodontic treatment. Angle Orthod. 2011, 81, 609–615. [Google Scholar] [CrossRef]
- Madariaga, A.C.P.; Bucci, R.; Rongo, R.; Simeon, V.; D’Antò, V.; Valletta, R. Impact of Fixed Orthodontic Appliance and Clear Aligners on Periodontal Health: A Prospective Clinical Study. Dent. J. 2020, 8, 4. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, L.; Cong, J. The periodontal status of removable appliances vs fixed appliances: A comparative meta-analysis. Medicine 2020, 99, 23165. [Google Scholar] [CrossRef]
- Li, W.; Huang, Y.T. Comparison of periodontal health of patients treated by the invisalign system with those treated by fixed orthodontic appliance. J. Pract. Stomatol. 2017, 33, 270–272. [Google Scholar]
- Sun, M.Y.; Huang, Q.B.; Wang, K.H. Impact of Invisalign and fixed appliance on the periodontal health of orthodontic patients. Stomatology 2018, 38, 149–153. [Google Scholar]
- Chhibber, A.; Agarwal, S.; Yadav, S.; Kuo, C.L.; Upadhyay, M. Which orthodontic appliance is best for oral hygiene? A randomized clinical trial. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 175–183. [Google Scholar] [CrossRef]
- Levrini, L.; Mangano, A.; Montanari, P.; Margherini, S.; Caprioglio, A.; Abbate, G.M. Periodontal health status in patients treated with the Invisalign® system and fixed orthodontic appliances: A 3 months clinical and microbiological evaluation. Eur. J. Dent. 2015, 9, 404–410. [Google Scholar] [CrossRef]
- Abbate, G.M.; Caria, M.P.; Montanari, P.; Mannu, C.; Orrù, G.; Caprioglio, A.; Levrini, L. Periodontal health in teenagers treated with removable aligners and fixed orthodontic appliances. J. Orofac. Orthop. 2015, 76, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Azaripour, A.; Weusmann, J.; Mahmoodi, B.; Peppas, D.; Gerhold-Ay, A.; Van Noorden, C.J.F.; Willershausen, B. Braces versus Invisalign®: Gingival parameters and patients’ satisfaction during treatment: A cross-sectional study. BMC Oral Health 2015, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Miethke, R.R.; Vogt, S. A comparison of the periodontal health of patients during treatment with the Invisalign® system and with fixed orthodontic appliances. J. Orofac. Orthop. 2005, 66, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Issa, F.H.K.M.; Issa, Z.H.K.M.; Rabah, A.F.; Hu, L. Periodontal parameters in adult patients with clear aligners orthodontics treatment versus three other types of brackets: A cross-sectional study. J. Orthod. Sci. 2020, 9, 4. [Google Scholar]
- Robertson, L.; Kaur, H.; Fagundes, N.C.F.; Romanyk, D.; Major, P.; Flores Mir, C. Effectiveness of clear aligner therapy for orthodontic treatment: A systematic review. Orthod. Craniofac. Res. 2020, 23, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Shokeen, B.; Viloria, E.; Duong, E.; Rizvi, M.; Murillo, G.; Mullen, J.; Shi, B.; Dinis, M.; Li, H.; Tran, N.C.; et al. The impact of fixed orthodontic appliances and clear aligners on the oral microbiome and the association with clinical parameters: A longitudinal comparative study. Am. J. Orthod. Dentofac. Orthop. 2022, 161, 475–485. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Fukasawa, S. Is Inflammation a Friend or Foe for Orthodontic Treatment? Inflammation in Orthodontically Induced Inflammatory Root Resorption and Accelerating Tooth Movement. Int. J. Mol. Sci. 2021, 22, 2388. [Google Scholar] [CrossRef]
- Bayir, F.; Bolat Gumus, E. External apical root resorption after orthodontic treatment: Incidence, severity and risk factors. J. Dent. Res. Dent. Clin. Dent. Prospect. 2021, 15, 100–105. [Google Scholar] [CrossRef]
- Iglesias-Linares, A.; Hartsfield, J.K., Jr. Cellular and Molecular Pathways Leading to External Root Resorption. J. Dent. Res. 2017, 96, 145–152. [Google Scholar] [CrossRef]
- Sameshima, G.T.; Iglesias-Linares, A. Orthodontic root resorption. J. World Fed. Orthod. 2021, 10, 135–143. [Google Scholar] [CrossRef]
- Villaman-Santacruz, H.; Torres-Rosas, R.; Acevedo-Mascarúa, A.E.; Argueta-Figueroa, L. Root resorption factors associated with orthodontic treatment with fixed appliances: A systematic review and meta-analysis. Dent. Med. Probl. 2022, 59, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Lupi, J.E.; Handelman, C.S.; Sadowsky, C. Prevalence and severity of apical root resorption and alveolar bone loss in orthodontically treated adults. Am. J. Orthod. Dentofac. Orthop. 1996, 109, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Al-Kyssi, H.A.; Al-Mogahed, N.M.; Altawili, Z.M.; Dahan, F.N.; Almashraqi, A.A.; Aldhorae, K.; Alhammadi, M.S. Predictive factors associated with adjacent teeth root resorption of palatally impacted canines in Arabian population: A cone-beam computed tomography analysis. BMC Oral Health 2022, 22, 220. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yang, F.; Li, J.; Hua, F.; He, M.; Song, G. Treatment Outcomes of Regenerative Endodontic Procedures in Traumatized Immature Permanent Necrotic Teeth: A Retrospective Study. J. Endod. 2022, 48, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Ronsivalle, V.; Barbato, E.; Lagravère, M.; Flores-Mir, C.; Lo Giudice, A. External root resorption (ERR) and rapid maxillary expansion (RME) at post-retention stage: A comparison between tooth-borne and bone-borne RME. Prog. Orthod. 2022, 23, 45. [Google Scholar] [CrossRef] [PubMed]
- Baghaei, N.N.; Zhai, G.; Lamani, E. Genetic and other factors contributing to external apical root resorption in orthodontic patients. Orthod. Craniofac. Res. 2023, 26 (Suppl. S1), 64–72. [Google Scholar] [CrossRef] [PubMed]
- Leite, V.; Conti, A.C.; Navarro, R.; Almeida, M.; Oltramari-Navarro, P.; Almeida, R. Comparison of root resorption between self-ligating and conventional preadjusted brackets using cone beam computed tomography. Angle Orthod. 2012, 82, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Çınarsoy Ciğerim, S.; Ozlek, E. Evaluation of the Effect of Different Bracket Systems on External Apical Root Resorption Using Cone-Beam Computed Tomography. Turk. J. Orthod. 2021, 34, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Harry, M.R.; Sims, M.R. Root resorption in bicuspid intrusion. A scanning electron microscope study. Angle Orthod. 1982, 52, 235–258. [Google Scholar]
- Owman-Moll, P.; Kurol, J.; Lundgren, D. The effects of a four-fold increased orthodontic force magnitude on tooth movement and root resorptions. An intra-individual study in adolescents. Eur. J. Orthod. 1996, 18, 287–294. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiao, L.; Yuan, X. Displacement and stress distribution of mandibular incisors after orthodontic treatment in the presence of alveolar bone loss under occlusal loads: A finite element analysis. Am. J. Orthod. Dentofac. Orthop. 2022, 161, e456–e465. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.T.; Huang, H.L.; Yang, S.G.; Su, K.C.; Fuh, L.J.; Hsu, J.T. Biomechanical analysis of occlusal modes on the periodontal ligament while orthodontic force applied. Clin. Oral Investig. 2021, 25, 5661–5670. [Google Scholar] [CrossRef] [PubMed]
- Sameshima, G.T.; Sinclair, P.M. Predicting and preventing root resorption: Part I. Diagnostic factors. Am. J. Orthod. Dentofac. Orthop. 2001, 119, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Alkan, Ö.; Kaya, Y.; Tunca, M.; Keskin, S. Changes in the gingival thickness and keratinized gingival width of maxillary and mandibular anterior teeth after orthodontic treatment. Angle Orthod. 2021, 91, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Di Febo, G.; Bedendo, A.; Romano, F.; Cairo, F.; Carnevale, G. Fixed prosthodontic treatment outcomes in the long-term management of patients with periodontal disease: A 20-year follow-up report. Int. J. Prosthodont. 2015, 28, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Liu, Y.; Si, Y.; Zhang, Q.; Wang, L.; Liu, J.; Wang, C.; Xiao, S. Prevalence of fimA genotypes of Porphyromonas gingivalis in adolescent orthodontic patients. PLoS ONE 2017, 12, e0188420. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, D.; Jang, I.; Cha, B.; Jost-Brinkmann, P.; Song, J. Microbiologic changes in subgingival plaque before and during the early period of orthodontic treatment. Angle Orthod. 2012, 82, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.S.; Park, H.J.; Hwang, H.R.; Kwon, A.; Lim, W.H.; Yi, W.J.; Han, D.H.; Kim, Y.H.; Baek, J.H. The effect of antioxidants on the production of pro-inflammatory cytokines and orthodontic tooth movement. Mol. Cells 2011, 32, 189–196. [Google Scholar] [CrossRef]
- Bletsa, A.; Berggreen, E.; Brudvik, P. Interleukin-1alpha and tumor necrosis factor-alpha expression during the early phases of orthodontic tooth movement in rats. Eur. J. Oral Sci. 2006, 114, 423–429. [Google Scholar] [CrossRef]
- Soares Bonato, R.C.; Abel Mapengo, M.A.; de Azevedo-Silva, L.J.; Janson, G.; de Carvalho Sales-Peres, S.H. Tooth movement, orofacial pain, and leptin, interleukin-1β, and tumor necrosis factor-α levels in obese adolescents. Angle Orthod. 2022, 92, 95–100. [Google Scholar] [CrossRef]
- Lo Russo, L.; Zhurakivska, K.; Montaruli, G.; Salamini, A.; Gallo, C.; Troiano, G.; Ciavarella, D. Effects of crown movement on periodontal biotype: A digital analysis. Odontology 2018, 106, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zhang, Y.; Niu, W.; Liu, K.; Xue, L.; Zhou, K. Reactive oxygen species-mediated endoplasmic reticulum stress contributes to osteocyte death induced by orthodontic compressive force. Microsc. Res. Tech. 2023, 86, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Alijani, S.; Fotovat, F.; Rezaei Soufi, L.; Alafchi, B.; Mohammadkhani, M.H. Shear bond strength of orthodontic brackets to specimens fabricated from temporary restorative materials by 3D-printing, CAD/CAM technology, and the conventional technique after surface treatment by sandblasting and laser. Int. Orthod. 2023, 21, 100790. [Google Scholar] [CrossRef] [PubMed]
- Alabbadi, A.A.; Abdalla, E.M.; Hanafy, S.A.; Yousry, T.N. A comparative study of CAD/CAM fabricated polyether ether ketone and fiber-glass reinforcement composites versus metal lingual retainers under vertical load (an in vitro study). BMC Oral Health 2023, 23, 583. [Google Scholar] [CrossRef]
- Chaturvedi, T.P.; Singh, D.; Sharma, V.K.; Priyadarshani, P.; Turkiya, S. Effect of orthodontic retraction force on thick and thin gingival biotypes in different grades of gingival recession and alveolar bone quality: A finite element analysis. J. Orthod. Sci. 2023, 12, 22. [Google Scholar]

| Authors | Year of Publication | Parameters | Outcome Measures |
|---|---|---|---|
| Liu et al. [75] (clinical study) | 2011 | PLI, GI, PD | PLI, GI, and PD increased significantly in the first 3 months following appliance insertion but decreased significantly in the first 6 months after appliance removal. |
| Madariaga et al. [76] (prospective clinical study) | 2020 | PLI, GI, GBI, PD | According to the linear regression models, appliance type did not affect periodontal variables. Thus, fixed appliance and clear aligner orthodontic patients had similar gingival health statuses. |
| Wu Y. et al. [77] (meta-analysis) | 2020 | PLI, GI, PD | Removable appliances had significantly lower PLIs than fixed equipment. At 6 months, removable appliances reduced GI, but they did not do so at 3 months. At 3 months, mobile devices had lower PDs than fixed appliances. |
| Li W. et al. [78] (clinical study) | 2017 | PLI, GI, PD, GBI | After 3 months, Invisalign therapy lowered GBI and PD levels compared to fixed treatment (p < 0.05); however, there was no significant difference after 6 months (p > 0.05). |
| Eroglu et al. [27] (clinical study) | 2019 | PLI, GI, PD, GBI | The authors found no significant differences in the plaque index, gingival index, bleeding on probing, or probing depth (p > 0.05). |
| Sun et al. [79] (prospective clinical study) | 2018 | PLI, GI, PD, GBI | Before and three months after treatment, the two patient groups had similar periodontal indicators. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luchian, I.; Surlari, Z.; Goriuc, A.; Ioanid, N.; Zetu, I.; Butnaru, O.; Scutariu, M.-M.; Tatarciuc, M.; Budala, D.-G. The Influence of Orthodontic Treatment on Periodontal Health between Challenge and Synergy: A Narrative Review. Dent. J. 2024, 12, 112. https://doi.org/10.3390/dj12040112
Luchian I, Surlari Z, Goriuc A, Ioanid N, Zetu I, Butnaru O, Scutariu M-M, Tatarciuc M, Budala D-G. The Influence of Orthodontic Treatment on Periodontal Health between Challenge and Synergy: A Narrative Review. Dentistry Journal. 2024; 12(4):112. https://doi.org/10.3390/dj12040112
Chicago/Turabian StyleLuchian, Ionut, Zenovia Surlari, Ancuta Goriuc, Nicoleta Ioanid, Irina Zetu, Oana Butnaru, Monica-Mihaela Scutariu, Monica Tatarciuc, and Dana-Gabriela Budala. 2024. "The Influence of Orthodontic Treatment on Periodontal Health between Challenge and Synergy: A Narrative Review" Dentistry Journal 12, no. 4: 112. https://doi.org/10.3390/dj12040112
APA StyleLuchian, I., Surlari, Z., Goriuc, A., Ioanid, N., Zetu, I., Butnaru, O., Scutariu, M.-M., Tatarciuc, M., & Budala, D.-G. (2024). The Influence of Orthodontic Treatment on Periodontal Health between Challenge and Synergy: A Narrative Review. Dentistry Journal, 12(4), 112. https://doi.org/10.3390/dj12040112












