In Vitro Evaluation of the Effectiveness and pH Variation of Dental Bleaching Gels and Their Effect on Enamel Surface Roughness
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection and Preparation
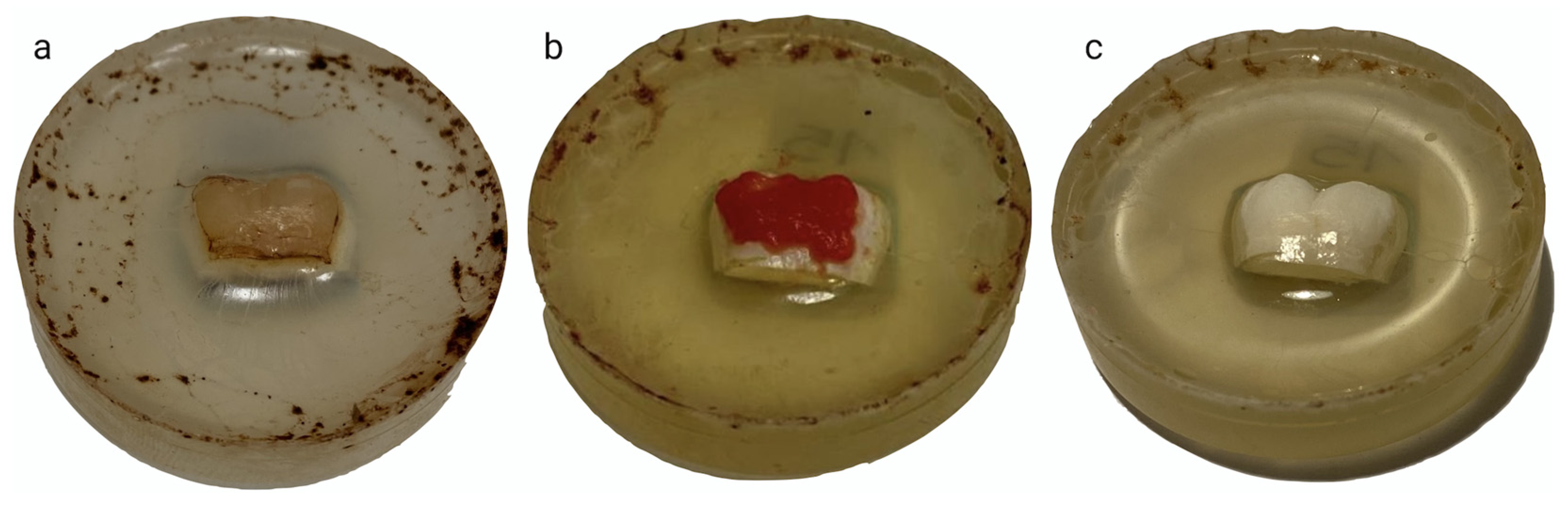
2.2. Staining Procedure
2.3. Bleaching Procedure
2.4. Color Change Evaluation
2.5. pH Analysis
2.6. Surface Roughness Evaluation
2.7. Statistical Analysis
3. Results
3.1. Color Change Evaluation
3.2. pH Analysis
3.3. Surface Roughness Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Checchi, V.; Forabosco, E.; Dall’Olio, F.; Kaleci, S.; Giannetti, L.; Generali, L. Assessment of colour modifications in two different composite resins induced by the influence of chlorhexidine mouthwashes and gels, with and without anti-staining properties: An in vitro study. Int. J. Dent. Hyg. 2024, 22, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Checchi, V.; Forabosco, E.; Della Casa, G.; Kaleci, S.; Giannetti, L.; Generali, L.; Bellini, P. Color Stability Assessment of Single- and Multi-Shade Composites Following Immersion in Staining Food Substances. Dent. J. 2024, 12, 285. [Google Scholar] [CrossRef]
- De Geus, J.; Wambier, L.; Kossatz, S.; Loguercio, A.; Reis, A. At-home vs In-office Bleaching: A Systematic Review and Meta-analysis. Oper. Dent. 2016, 41, 341–356. [Google Scholar] [CrossRef]
- Maghaireh, G.A.; AIzraikat, H.; Taha, N.A. Satisfaction with Dental Appearance and Attitude toward improving Dental Esthetics among Patients attending a Dental Teaching Center. J. Contemp. Dent. Pract. 2016, 17, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Altınışık, H.; Akgül, S.; Nezir, M.; Özcan, S.; Özyurt, E. The Effect of In-Office Bleaching with Different Concentrations of Hydrogen Peroxide on Enamel Color, Roughness, and Color Stability. Materials 2023, 16, 1389. [Google Scholar] [CrossRef]
- Omar, F.; Ab-Ghani, Z.; Rahman, N.A.; Halim, M.S. Nonprescription Bleaching versus Home Bleaching with Professional Prescriptions: Which One is Safer? A Comprehensive Review of Color Changes and Their Side Effects on Human Enamel. Eur. J. Dent. 2019, 13, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Greenwall, L. (Ed.) Bleaching Techniques in Restorative Dentistry, 1st ed.; CRC Press: London, UK, 2001; ISBN 978-0-203-41743-0. [Google Scholar]
- De Mendonça, R.; Baliza, J.; Burey, A.; Cavalcante, L.; Loguercio, A.; Calazans, F.; Barceleiro, M. In vitro analysis of the pH stability of dental bleaching gels during in-office procedures. J. Clin. Exp. Dent. 2021, 13, e22–e29. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.A.; Strazzi-Sahyon, H.B.; Suzuki, T.Y.U.; Briso, A.L.F.; Dos Santos, P.H. Effect of dental bleaching on the microhardness and surface roughness of sealed composite resins. Restor. Dent. Endod. 2020, 45, e12. [Google Scholar] [CrossRef]
- Araújo, L.S.N.D.; Dos Santos, P.H.; Anchieta, R.B.; Catelan, A.; Fraga Briso, A.L.; Fraga Zaze, A.C.S.; Sundfeld, R.H. Mineral loss and color change of enamel after bleaching and staining solutions combination. J. Biomed. Opt. 2013, 18, 108004. [Google Scholar] [CrossRef]
- De Carvalho, A.; De Souza, T.; Liporoni, P.; Pizi, E.; Matuda, L.; Catelan, A. Effect of bleaching agents on hardness, surface roughness and color parameters of dental enamel. J. Clin. Exp. Dent. 2020, 12, e670–e675. [Google Scholar] [CrossRef] [PubMed]
- Martini, E.C.; Parreiras, S.O.; Szesz, A.L.; Coppla, F.M.; Loguercio, A.D.; Reis, A. Bleaching-induced tooth sensitivity with application of a desensitizing gel before and after in-office bleaching: A triple-blind randomized clinical trial. Clin. Oral Investig. 2020, 24, 385–394. [Google Scholar] [CrossRef]
- Goyal, K.; Saha, S.G.; Bhardwaj, A.; Saha, M.K.; Bhapkar, K.; Paradkar, S. A comparative evaluation of the effect of three different concentrations of in-office bleaching agents on microhardness and surface roughness of enamel—An in vitro study. Dent. Res. J. 2021, 18, 49. [Google Scholar] [CrossRef]
- Lilaj, B.; Dauti, R.; Agis, H.; Schmid-Schwap, M.; Franz, A.; Kanz, F.; Moritz, A.; Schedle, A.; Cvikl, B. Comparison of Bleaching Products With Up to 6% and With More Than 6% Hydrogen Peroxide: Whitening Efficacy Using BI and WID and Side Effects—An in vitro Study. Front. Physiol. 2019, 10, 919. [Google Scholar] [CrossRef]
- Pimentel De Oliveira, R.; Baia, J.C.P.; Ribeiro, M.E.S., Jr.; e Souza, M.H.D.S.; Loretto, S.C. Influence of Time Intervals between Bleaching Procedures on Enamel Microhardness and Surface Roughness. Open Dent. J. 2018, 12, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Favoreto, M.W.; Cordeiro, D.C.F.; Centenaro, G.G.; Bosco, L.D.; Arana-Gordillo, L.A.; Reis, A.; Loguercio, A.D. Evaluating color change and hydrogen peroxide penetration in human and bovine teeth through in-office bleaching procedures. J. Esthet. Restor. Dent. 2024, 36, 1171–1178. [Google Scholar] [CrossRef]
- Acuña, E.D.; Parreiras, S.O.; Favoreto, M.W.; Cruz, G.P.; Gomes, A.; Borges, C.P.F.; Loguercio, A.D.; Reis, A. In-office bleaching with a commercial 40% hydrogen peroxide gel modified to have different pHs: Color change, surface morphology, and penetration of hydrogen peroxide into the pulp chamber. J. Esthet. Restor. Dent. 2022, 34, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, A.D.; Servat, F.; Stanislawczuk, R.; Mena-Serrano, A.; Rezende, M.; Prieto, M.V.; Cereño, V.; Rojas, M.F.; Ortega, K.; Fernandez, E.; et al. Effect of acidity of in-office bleaching gels on tooth sensitivity and whitening: A two-center double-blind randomized clinical trial. Clin. Oral Investig. 2017, 21, 2811–2818. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, Z.M.; Surmelioglu, D. Effects of different bleaching application durations on enamel in terms of tooth color, microhardness, and surface roughness. Color Res. Appl. 2022, 47, 204–212. [Google Scholar] [CrossRef]
- Wijetunga, C.L.; Otsuki, M.; Hiraishi, N.; Luong, M.N.; Tagami, J. Effect of pH of bleaching agent on tooth bleaching action in vitro. Dent. Mater. J. 2021, 40, 566–572. [Google Scholar] [CrossRef]
- Wijetunga, C.L.; Otsuki, M.; Abdou, A.; Luong, M.N.; Qi, F.; Tagami, J. The effect of in-office bleaching materials with different pH on the surface topography of bovine enamel. Dent. Mater. J. 2021, 40, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- CIE 15:2004; Colorimetry, 3rd ed. Central Bureau of the CIE: Vienna, Austria, 2004; ISBN 978-3-901906-33-6.
- Nogueira, J.; Lins-Filho, P.; Dias, M.; Silva, M.; Guimaraes, R. Does comsumption of staining drinks compromise the result of tooth whitening? J. Clin. Exp. Dent. 2019, 11, e1012–e1017. [Google Scholar] [CrossRef]
- Forabosco, E.; Consolo, U.; Mazzitelli, C.; Kaleci, S.; Generali, L.; Checchi, V. Effect of bleaching on the color match of single-shade resin composites. J. Oral Sci. 2023, 65, 232–236. [Google Scholar] [CrossRef]
- VITA Zahnfabrik. VITA Easyshade V—Instructions for Use; VITA Zahnfabrik: Bad Säckingen, Germany, 2023. [Google Scholar]
- Luo, M.R.; Cui, G.; Rigg, B. The development of the CIE 2000 colour-difference formula: CIEDE2000. Color Res. Appl. 2001, 26, 340–350. [Google Scholar] [CrossRef]
- CIE. Improvement to Industrial Colour-Difference Evaluation; International Commission on Illumination, CIE Technical Committee 1-47, Eds.; Technical Report; CIE Central Bureau: Vienna, Austria, 2001; ISBN 978-3-901906-08-4. [Google Scholar]
- ISO 11664-2:2007(E)/CIE S 014-2/E:2006; Colorimetry—Part 2: CIE Standard Illuminants for Colorimetry. CIE: Vienna, Austria, 2007.
- Sharma, G.; Wu, W.; Dalal, E.N. The CIEDE2000 color-difference formula: Implementation notes, supplementary test data, and mathematical observations. Color Res. Appl. 2005, 30, 21–30. [Google Scholar] [CrossRef]
- ISO 25178-2:2021; Geometrical product specifications (GPS)—Surface texture: Areal-Part 2: Terms, definitions and surface texture parameters. International Organization for Standardization ISO Central Secretariat: Geneva, Switzerland, 2021.
- Alkahtani, R.; Stone, S.; German, M.; Waterhouse, P. A review on dental whitening. J. Dent. 2020, 100, 103423. [Google Scholar] [CrossRef]
- Kawamoto, K.; Tsujimoto, Y. Effects of the Hydroxyl Radical and Hydrogen Peroxide on Tooth Bleaching. J. Endod. 2004, 30, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.Q. Tooth-bleaching procedures and their controversial effects: A literature review. Saudi Dent. J. 2014, 26, 33–46. [Google Scholar] [CrossRef]
- Forabosco, E.; Generali, L.; Mancuso, E.; Kaleci, S.; Consolo, U.; Checchi, V. Color match of single-shade restorations after professional dental bleaching: An in vitro study. J. Conserv. Dent. Endod. 2024, 27, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Morsy, N.; Holiel, A.A. Color difference for shade determination with visual and instrumental methods: A systematic review and meta-analysis. Syst. Rev. 2023, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Polo, C.; Gómez-Polo, M.; Celemin-Viñuela, A.; Martínez Vázquez De Parga, J.A. Differences between the human eye and the spectrophotometer in the shade matching of tooth colour. J. Dent. 2014, 42, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, D.T.; Wee, A.G. Perceptibility and acceptability of CIELAB color differences in computer-simulated teeth. J. Dent. 2007, 35, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Herrera, L.J.; Pulgar, R.; Santana, J.; Cardona, J.C.; Guillén, A.; Rojas, I.; Pérez, M.D.M. Prediction of color change after tooth bleaching using fuzzy logic for Vita Classical shades identification. Appl. Opt. 2010, 49, 422. [Google Scholar] [CrossRef]
- Kim, J.; Yu, B.; Lee, Y. Correlations between Color Differences Based on Three Color-Difference Formulas Using Dental Shade Guide Tabs. J. Prosthodont. 2009, 18, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Kose, C.; Calixto, A.; Bauer, J.; Reis, A.; Loguercio, A. Comparison of the Effects of In-office Bleaching Times on Whitening and Tooth Sensitivity: A Single Blind, Randomized Clinical Trial. Oper. Dent. 2016, 41, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Mena-Serrano, A.; Garcia, E.; Luque-Martinez, I.; Grande, R.; Loguercio, A.; Reis, A. A Single-Blind Randomized Trial About the Effect of Hydrogen Peroxide Concentration on Light-Activated Bleaching. Oper. Dent. 2016, 41, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Otsuki, M.; Hiraishi, N.; Hatayama, T.; Wijethunge, C.L.; Tagami, J. Effect of photo-thermal acceleration on in-office bleaching. Odontology 2021, 109, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Rezende, M.; Ferri, L.; Kossatz, S.; Loguercio, A.; Reis, A. Combined Bleaching Technique Using Low and High Hydrogen Peroxide In-Office Bleaching Gel. Oper. Dent. 2016, 41, 388–396. [Google Scholar] [CrossRef]
- Young, N.; Fairley, P.; Mohan, V.; Jumeaux, C. A study of hydrogen peroxide chemistry and photochemistry in tea stain solution with relevance to clinical tooth whitening. J. Dent. 2012, 40 (Suppl. S2), e11–e16. [Google Scholar] [CrossRef]
- Sun, L.; Liang, S.; Sa, Y.; Wang, Z.; Ma, X.; Jiang, T.; Wang, Y. Surface alteration of human tooth enamel subjected to acidic and neutral 30% hydrogen peroxide. J. Dent. 2011, 39, 686–692. [Google Scholar] [CrossRef]
- Reis, A.; Kossatz, S.; Martins, G.; Loguercio, A. Efficacy of and Effect on Tooth Sensitivity of In-office Bleaching Gel Concentrations: A Randomized Clinical Trial. Oper. Dent. 2013, 38, 386–393. [Google Scholar] [CrossRef]
- Trentino, A.C.; Soares, A.F.; Duarte, M.A.H.; Ishikiriama, S.K.; Mondelli, R.F.L. Evaluation of pH Levels and Surface Roughness After Bleaching and Abrasion Tests of Eight Commercial Products. Photomed. Laser Surg. 2015, 33, 372–377. [Google Scholar] [CrossRef]
- Polydorou, O.; Scheitza, S.; Spraul, M.; Vach, K.; Hellwig, E. The effect of long-term use of tooth bleaching products on the human enamel surface. Odontology 2018, 106, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Uthappa, R.; Suprith, M.; Bhandary, S.; Dash, S. A Comparative Study of Different Bleaching Agents on the Morphology of Human Enamel: An in vitro SEM Study. J. Contemp. Dent. Pract. 2012, 13, 756–759. [Google Scholar] [CrossRef]
- Al-Angari, S.S.; Eckert, G.J.; Sabrah, A.H.A. Color stability, Roughness, and Microhardness of Enamel and Composites Submitted to Staining/Bleaching Cycles. Saudi Dent. J. 2021, 33, 215–221. [Google Scholar] [CrossRef]
- He, B.; Ding, S.; Shi, Z. A comparison between profile and areal surface roughness parameters. Metrol. Meas. Syst. 2023, 28, 413–438. [Google Scholar] [CrossRef]
- Feagin, F.; Patel, P.R.; Koulourides, T.; Pigman, W. Study of the effect of calcium, phosphate, fluoride and hydrogen ion concentrations on the remineralization of partially demineralized human and bovine enamel surfaces. Arch. Oral Biol. 1971, 16, 535–548. [Google Scholar] [CrossRef]
- Bollenl, C.M.L.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Da Mata Galvão, A.; Campolina, M.G.; De Mendonça, L.C.; Soares, C.J.; Carvalho, C.N.; Da Silva, G.R. Does polishing of bleached enamel affect roughness and tooth color stability after exposure to coffee? J. Esthet. Restor. Dent. 2022, 34, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Lopes, I.A.D.; Monteiro, P.J.V.C.; Mendes, J.J.B.; Gonçalves, J.M.R.; Caldeira, F.J.F. The effect of different finishing and polishing techniques on surface roughness and gloss of two nanocomposites. Saudi Dent. J. 2018, 30, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Cadenaro, M.; Breschi, L.; Antoniolli, F.; Mazzoni, A.; Di Lenarda, R. Influence of whitening on the degree of conversion of dental adhesives on dentin. Eur. J. Oral Sci. 2006, 114, 257–262. [Google Scholar] [CrossRef]
- Grobler, S.R.; Majeed, A.; Moola, M.H. Effect of various tooth-whitening products on enamel microhardness. SADJ 2009, 64, 474–479. [Google Scholar]
- Majeed, A.; Grobler, S.R.; Moola, M.H.; Rossouw, R.J.; van Kotze, T.J.W. Effect of four different opalescence tooth-whitening products on enamel microhardness. SADJ 2008, 63, 282–286. [Google Scholar] [PubMed]
- Kwon, S.; Pallavi, F.; Shi, Y.; Oyoyo, U.; Mohraz, A.; Li, Y. Effect of Bleaching Gel Viscosity on Tooth Whitening Efficacy and Pulp Chamber Penetration: An In Vitro Study. Oper. Dent. 2018, 43, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Llena, C.; Martínez-Galdón, O.; Forner, L.; Gimeno-Mallench, L.; Rodríguez-Lozano, F.; Gambini, J. Hydrogen Peroxide Diffusion through Enamel and Dentin. Materials 2018, 11, 1694. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, G.; Valente, L.L.; Isolan, C.P.; Pinheiro, H.A.; Duarte, C.G.; Münchow, E.A. Bleaching and enamel surface interactions resulting from the use of highly-concentrated bleaching gels. Arch. Oral Biol. 2018, 87, 157–162. [Google Scholar] [CrossRef]
- Silva, B.G.; Gouveia, T.H.N.; Da Silva, M.D.A.P.; Ambrosano, G.M.B.; Aguiar, F.H.B.; Lima, D.A.N.L. Evaluation of home bleaching gel modified by different thickeners on the physical properties ofenamel: An in situ study. Eur. J. Dent. 2018, 12, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Otsuki, M.; Tagami, J. Effect of pH conditioners on tooth bleaching. Clin. Exp. Dent. Res. 2019, 5, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Koudriavtsev, T.; Herrera-Sancho, Ó. Effect of tooth-bleaching on the carbonate concentration in dental enamel by Raman spectroscopy. J. Clin. Exp. Dent. 2016, 9, e101–e106. [Google Scholar] [CrossRef] [PubMed]


| Treatment Groups | Color Change | |
|---|---|---|
| T0–T1 | T1–T2 | |
| ΔE00 ± SD | ΔE00 ± SD | |
| HP 40% (n = 14) | 22.91 ± 13.05 a | 25.32 ± 12.77 a |
| HP 35% (n = 14) | 24.94 ± 13.60 a | 27.64 ± 12.95 a |
| CP 16% (n = 14) | 20.87 ± 6.55 a | 14.78 ± 7.99 b |
| Treatment Groups | pH Before Bleaching (T1) | pH After Bleaching (T2) | ΔpH |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| HP 40% (n = 14) | 7.01 ± 0.00 | 6.60 ± 0.55 | −0.41 ± 0.55 a |
| HP 35% (n = 14) | 7.08 ± 0.00 | 7.34 ± 0.03 | 0.26 ± 0.03 b |
| CP 16% (n = 14) | 6.90 ± 0.00 | 6.93 ± 0.02 | 0.03 ± 0.02 a |
| Treatment Groups | Surface Roughness | ||
|---|---|---|---|
| Before Bleaching | After Bleaching | ||
| Sa ± SD [μm] | Sa ± SD [μm] | p | |
| HP 40% (n = 14) | 0.53 ± 0.10 a | 0.46 ± 0.08 a | 0.02 * |
| HP 35% (n = 14) | 0.58 ± 0.20 a | 0.48 ± 0.10 a | 0.067 |
| CP 16% (n = 14) | 0.47 ± 0.11 a | 0.44 ± 0.08 a | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veneri, F.; Cavani, F.; Bolelli, G.; Checchi, V.; Bizzi, A.; Setti, G.; Generali, L. In Vitro Evaluation of the Effectiveness and pH Variation of Dental Bleaching Gels and Their Effect on Enamel Surface Roughness. Dent. J. 2024, 12, 415. https://doi.org/10.3390/dj12120415
Veneri F, Cavani F, Bolelli G, Checchi V, Bizzi A, Setti G, Generali L. In Vitro Evaluation of the Effectiveness and pH Variation of Dental Bleaching Gels and Their Effect on Enamel Surface Roughness. Dentistry Journal. 2024; 12(12):415. https://doi.org/10.3390/dj12120415
Chicago/Turabian StyleVeneri, Federica, Francesco Cavani, Giovanni Bolelli, Vittorio Checchi, Alessia Bizzi, Giacomo Setti, and Luigi Generali. 2024. "In Vitro Evaluation of the Effectiveness and pH Variation of Dental Bleaching Gels and Their Effect on Enamel Surface Roughness" Dentistry Journal 12, no. 12: 415. https://doi.org/10.3390/dj12120415
APA StyleVeneri, F., Cavani, F., Bolelli, G., Checchi, V., Bizzi, A., Setti, G., & Generali, L. (2024). In Vitro Evaluation of the Effectiveness and pH Variation of Dental Bleaching Gels and Their Effect on Enamel Surface Roughness. Dentistry Journal, 12(12), 415. https://doi.org/10.3390/dj12120415








