Clinical Outcomes After Dental Surgery with Two Antiseptic Protocols
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Selection
- Scheduled dental surgery, tooth extraction with a flap elevation, single implant placement, and odontoma removal;
- Absence of periodontitis;
- Absence of allergy to chlorhexidine and/or cetylpyridinium chloride;
- Good general health, in particular no disease that could potentially delay wound healing;
- No intake of any medical drug that could influence the outcome of the study, such as immunosuppressors (azathioprine, everolimus, mycophenolic acid).
2.2. Subject Allocation
2.3. Treatment Protocol
2.4. Clinical Measurements
2.5. Statistical Analysis
3. Results
Limitation of the Study
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toma, A.I.; Fuller, J.M.; Willett, N.J.; Goudy, S.L. Oral wound healing models and emerging regenerative therapies. Transl. Res. 2021, 236, 17–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albanese, M.; Zangani, A.; Manfrin, F.; Bertossi, D.; De Manzoni, R.; Tomizioli, N.; Faccioni, P.; Pardo, A. Influence of Surgical Technique on Post-Operative Complications in the Extraction of the Lower Third Molar: A Retrospective Study. Dent. J. 2023, 11, 238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waasdorp, M.; Krom, B.P.; Bikker, F.J.; van Zuijlen, P.P.M.; Niessen, F.B.; Gibbs, S. The Bigger Picture: Why Oral Mucosa Heals Better Than Skin. Biomolecules 2021, 11, 1165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kiencało, A.; Jamka-Kasprzyk, M.; Panaś, M.; Wyszyńska-Pawelec, G. Analysis of complications after the removal of 339 third molars. Dent. Med. Probl. 2021, 58, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Silva, C.O.; Misawa, M.; Sukekava, F. Alveolar socket healing: What can we learn? Periodontol. 2000 2015, 68, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Rossi-Fedele, G.; Doğramacı, E.J. Post-operative instructions following minor oral surgery—The quality and level of evidence: A cross-sectional study. Br. Dent. J. 2020, 228, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, A.P. Minimizing post-operative edema and ecchymoses by the use of an oral enzyme preparation (bromelain). A controlled study of 53 rhinoplasty cases. Eye Ear Nose Throat Mon. 1962, 41, 813–817. [Google Scholar] [PubMed]
- Shibl, M.; Ali, K.; Burns, L. Effectiveness of pre-operative oral corticosteroids in reducing pain, trismus and oedema following lower third molar extractions: A systematic review. Br. Dent. J. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.L.; Rodrigues, M.T.; Ferreira Júnior, O.; Garlet, G.P.; de Carvalho, P.S. Clinical concepts of dry socket. J. Oral Maxillofac. Surg. 2010, 68, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.C.; Spera, J.F. Management of acute postoperative pain after oral surgery. Dent. Clin. N. Am. 2012, 56, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Brar, P.; Jakubowski, J.; Kaltman, S.; Lopez, E. The use of corticosteroids and nonsteroidal antiinflammatory medication for the management of pain and inflammation after third molar surgery: A review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Egierska, D.; Perszke, M.; Mazur, M.; Duś-Ilnicka, I. Platelet-rich plasma and platelet-rich fibrin in oral surgery: A narrative review. Dent. Med. Probl. 2023, 60, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Stolarz, A.; Mikulewicz, M.; Laskowska, J.; Karolewicz, B.; Owczarek, A. The Importance of Chitosan Coatings in Dentistry. Mar. Drugs 2023, 21, 613. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Agostino, S.; Dolci, M. Antibiotic therapy in oral surgery: A cross sectional survey among Italian dentists. J. Biol. Regul. Homeost. Agents 2020, 34, 1549–1552. [Google Scholar] [CrossRef] [PubMed]
- Laraki, M.; Chbicheb, S.; El Wady, W. Les alvéolites: Revue de littérature [Alveolitis: Review of the literature]. Odontostomatol. Trop. 2012, 35, 19–25. (In French) [Google Scholar] [PubMed]
- Norman, G.; Dumville, J.C.; Mohapatra, D.P.; Owens, G.L.; Crosbie, E.J. Antibiotics and antiseptics for surgical wounds healing by secondary intention. Cochrane Database Syst. Rev. 2016, 3, CD011712. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thangavelu, A.; Kaspar, S.S.; Kathirvelu, R.P.; Srinivasan, B.; Srinivasan, S.; Sundram, R. Chlorhexidine: An Elixir for Periodontics. J. Pharm. Bioallied Sci. 2020, 12 (Suppl. 1), S57–S59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, W.; Wang, R.E.; Finger, M.; Lang, N.P. Evaluation of the antigingivitis effect of a chlorhexidine mouthwash with or without an antidiscoloration system compared to placebo during experimental gingivitis. J. Investig. Clin. Dent. 2014, 5, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Poppolo Deus, F.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pitten, F.A.; Kramer, A. Efficacy of cetylpyridinium chloride used as oropharyngeal antiseptic. Arzneimittelforschung 2001, 51, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Navabi, N.; Afshari, Z.; Kamyabi, H.; Mohammadi, M. Side effects and short effects of using three common mouthwashes on oral health and quality of life: A quasi-experimental study. Int. J. Dent. Hyg. 2023, 22, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, G.C.; Plous, S. Research Randomizer (Version 4.0) [Computer Software]. 2013. Available online: http://www.randomizer.org/ (accessed on 3 April 2024).
- Wilson, W.R.; Gewitz, M.; Lockhart, P.B.; Bolger, A.F.; DeSimone, D.C.; Kazi, D.S.; Couper, D.J.; Beaton, A.; Kilmartin, C.; Miro, J.M.; et al. Prevention of Viridans Group Streptococcal Infective Endocarditis: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e963–e978, Erratum in Circulation 2021, 144, e192; Erratum in Circulation 2022, 145, e868. [Google Scholar] [CrossRef] [PubMed]
- Marini, L.; Rojas, M.A.; Sahrmann, P.; Aghazada, R.; Pilloni, A. Early Wound Healing Score: A system to evaluate the early healing of periodontal soft tissue wounds. J. Periodontal Implant Sci. 2018, 48, 274–283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar] [PubMed]
- Downie, W.W.; Leatham, P.A.; Rhind, V.M.; Wright, V.; Branco, J.A.; Anderson, J.A. Studies with pain rating scales. Ann. Rheum. Dis. 1978, 37, 378–381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lorusso, F.; Tartaglia, G.; Inchingolo, F.; Scarano, A. Early Response and Clinical Efficacy of a Mouthwash Containing Chlorhexidine, Anti Discoloration System, Polyvinylpyrrolidone/Vinyl Acetate and Sodium DNA in Periodontitis Model: A Triple-Blind Randomized Controlled Clinical Trial. Dent. J. 2022, 10, 101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lorusso, F.; Tartaglia, G.; Inchingolo, F.; Scarano, A. Peri-Implant Mucositis Treatment with a Chlorexidine Gel with A.D.S. 0.5%, PVP-VA and Sodium DNA vs. a Placebo Gel: A Randomized Controlled Pilot Clinical Trial. Front. Biosci. 2022, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Gent, J.F.; Frank, M.E.; Hettinger, T.P. Taste confusions following chlorhexidine treatment. Chem. Senses 2002, 27, 73–80. [Google Scholar] [CrossRef] [PubMed]
- McCoy, L.C.; Wehler, C.J.; Rich, S.E.; Garcia, R.I.; Miller, D.R.; Jones, J.A. Adverse events associated with chlorhexidine use: Results from the Department of Veterans Affairs Dental Diabetes Study. J. Am. Dent. Assoc. 2008, 139, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.S.; Abdullah, D.; Liew, A.K.C.; Khazin, S.M. Chlorhexidine hypersensitivity. Br. Dent. J. 2021, 230, 273. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, M.; Trivedi, H.M.; Chen, D.; Gajendrareddy, P.; Chen, L. Recent Development of Active Ingredients in Mouthwashes and Toothpastes for Periodontal Diseases. Molecules 2021, 26, 2001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, X.; Li, Y.; Zhang, X.; Zhang, P.; Tian, Q.; Ma, C.; Shi, C. Combination of Cetylpyridinium Chloride and Chlorhexidine Acetate: A Promising Candidate for Rapid Killing of Gram-Positive/Gram-Negative Bacteria and Fungi. Curr. Microbiol. 2023, 80, 97. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuyyakanond, T.; Quesnel, L.B. The mechanism of action of chlorhexidine. FEMS Microbiol. Lett. 1992, 100, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Tanno, O.; Ota, Y.; Kitamura, N.; Katsube, T.; Inoue, S. Nicotinamide increases biosynthesis of ceramides as well as other stratum corneum lipids to improve the epidermal permeability barrier. Br. J. Dermatol. 2000, 143, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Wessels, Q.; Pretorius, E.; Smith, C.M.; Nel, H. The potential of a niacinamide dominated cosmeceutical formulation on fibroblast activity and wound healing in vitro. Int. Wound J. 2014, 11, 152–158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wertz, P.W. Roles of Lipids in the Permeability Barriers of Skin and Oral Mucosa. Int. J. Mol. Sci. 2021, 22, 5229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guerra, F.; Pasqualotto, D.; Rinaldo, F.; Mazur, M.; Corridore, D.; Nofroni, I.; Ottolenghi, L.; Nardi, G.M. Therapeutic efficacy of chlorhexidine-based mouthwashes and its adverse events: Performance-related evaluation of mouthwashes added with Anti-Discoloration System and cetylpyridinium chloride. Int. J. Dent. Hyg. 2019, 17, 229–236. [Google Scholar] [CrossRef] [PubMed]


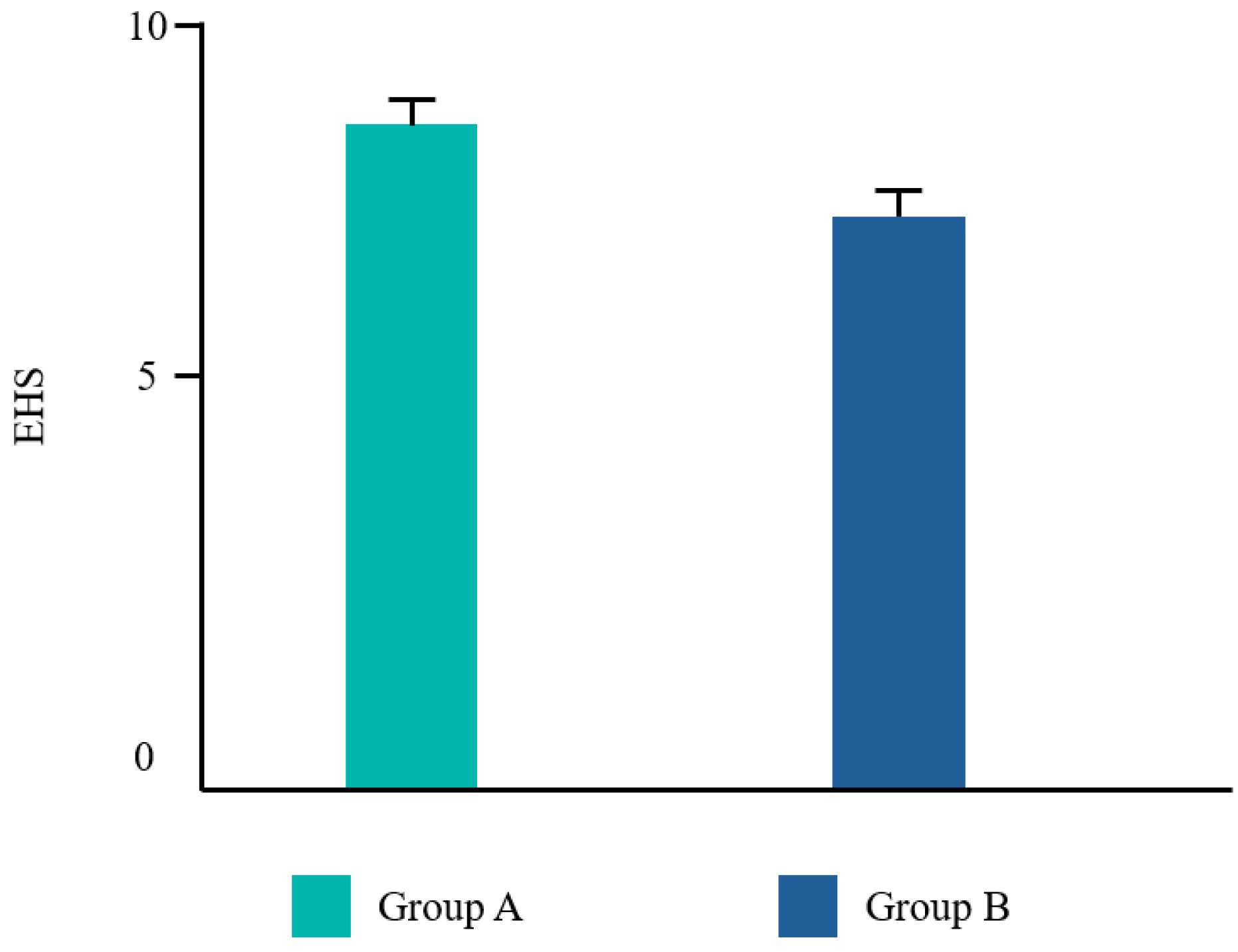
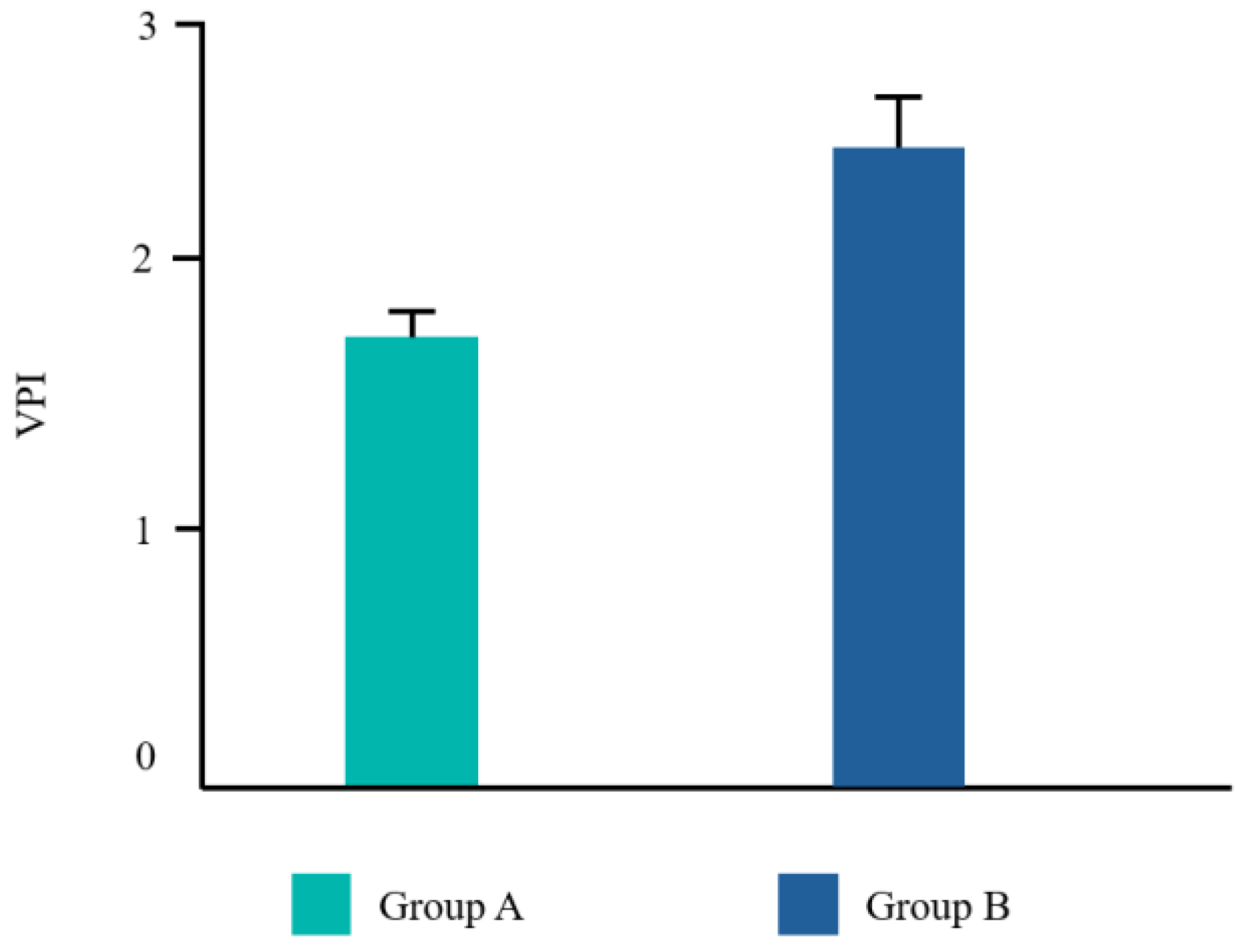


| EHS | VPI | NRS | Taste | |
|---|---|---|---|---|
| Group A | 8.5 ± 1.29 | 1.7 ± 1.27 | 0.9 ± 1.32 | 3.7 ± 1.04 |
| Group B | 6.8 ± 0.75 | 2.5 ± 0.68 | 1.7 ± 1.34 | 1.5 ± 0.07 |
| EHS | VPI | NRS | Taste | |
|---|---|---|---|---|
| p-value | 0.002 | 0.115 | 0.128 | 0.000 |
| Significance | Yes | No | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Agostino, S. Clinical Outcomes After Dental Surgery with Two Antiseptic Protocols. Dent. J. 2024, 12, 389. https://doi.org/10.3390/dj12120389
D’Agostino S. Clinical Outcomes After Dental Surgery with Two Antiseptic Protocols. Dentistry Journal. 2024; 12(12):389. https://doi.org/10.3390/dj12120389
Chicago/Turabian StyleD’Agostino, Silvia. 2024. "Clinical Outcomes After Dental Surgery with Two Antiseptic Protocols" Dentistry Journal 12, no. 12: 389. https://doi.org/10.3390/dj12120389
APA StyleD’Agostino, S. (2024). Clinical Outcomes After Dental Surgery with Two Antiseptic Protocols. Dentistry Journal, 12(12), 389. https://doi.org/10.3390/dj12120389






