Post-Orthodontic Relapse Prevention through Administration of a Novel Synthetic Carbonated Hydroxyapatite–Chitosan Hydrogel Derived from Blood Cockle Shell (Anadara granosa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Synthesis of CHA-CS from Blood Cockle Shells
2.3. Production of CHA-CS Hydrogel
2.4. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.5. Evaluation of the CHA-CS Hydrogel
- a.
- pH test
- b.
- Dispersion test
- c.
- Contact angle test
- d.
- Organoleptic test
2.6. Animal and Experimental Procedures
2.7. Determination of Tooth Displacement (Distance of Relapse)
2.8. Histological Preparation and Analysis
2.9. Data Analysis
3. Results
3.1. Synthesis of CHA and CS of Blood Cockle Shells
3.2. CHA and CS Fourier Transform Infra-Red (FTIR) Test of Blood Shells
3.3. Evaluation of Hydrogel Preparations
3.3.1. pH Test
3.3.2. Dispersion Test
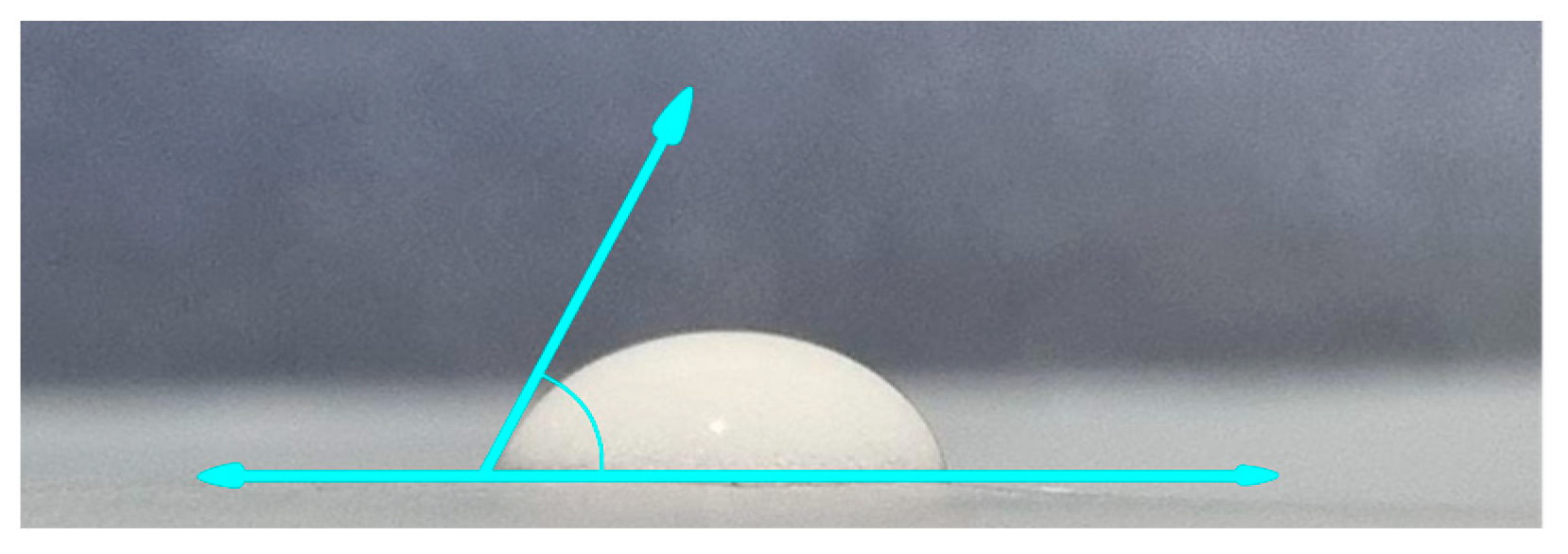
3.3.3. Contact Angle Test
3.3.4. Organoleptic Test
3.4. Relapse Distance and Histological Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Indriasari, V.; Suparwitri, S.; Christnawati, C.; Alhasyimi, A.A. Different effects of soybean isoflavone genistein on transforming growth factor levels during orthodontic tooth movement among young and old rabbits. F1000Research 2019, 8, 2074. [Google Scholar] [CrossRef]
- Alam, M.K.; Kanwal, B.; Abutayyem, H.; Alswairki, H.J.; Alfawzan, A.A.; Shqaidef, A.; Almakrami, L.H.; Alaqidi, S.F.S.; Alaskar, A.A.; Almutairi, I.A.; et al. Complications arising due to orthodontic treatment—A systematic review and meta-analysis. Appl. Sci. 2023, 13, 4035. [Google Scholar] [CrossRef]
- Kalina, E.; Grzebyta, A.; Zadurska, M. Bone remodeling during orthodontic movement of lower incisors—Narrative review. Int. J. Environ. Res. Public Health 2022, 19, 15002. [Google Scholar] [CrossRef] [PubMed]
- Rosyida, N.F.; Ana, I.D.; Alhasyimi, A.A. The use of polymers to enchance post-orthodontic tooth stability. Polymers 2023, 15, 103. [Google Scholar] [CrossRef] [PubMed]
- Alhasyimi, A.A.; Pudyani, P.P.; Asmara, W.; Ana, I.D. Enhancement of post-orthodontic tooth stability by carbonated hydroxyapatite-incorporated advanced platelet-rich fibrin in rabbits. Orthod. Craniofacial Res. 2018, 21, 112–118. [Google Scholar] [CrossRef]
- Kaan, M.; Madléna, M. Retenció és recidiva az ortodonciában. Irodalmi áttekintés (Retention and relapse. Review of the literature). Fogorv. Sz. 2011, 104, 139–146. [Google Scholar]
- Al Yami, E.A.; Kuijpers-Jagtman, A.M.; van’t Hof, M.A. Stability of orthodontic treatment outcome: Follow-up until 10 years postretention. Am. J. Orthod. Dentofac. Orthop. 1999, 115, 300–304. [Google Scholar] [CrossRef]
- Franzen, T.J.; Monjo, M.; Rubert, M.; Vandevska-Radunovic, V. Expression of bone markers, and micro-CT analysis of alveolar bone during orthodontic relapse. Orthod. Craniofacial Res. 2014, 17, 249–258. [Google Scholar] [CrossRef]
- Martin, T.J. Bone biology and anabolic therapies for bone: Current status and future prospects. J. Bone Metab. 2014, 21, 8–20. [Google Scholar] [CrossRef]
- Alhasyimi, A.A.; Suparwitri, S.; Christnawati, C. Effect of carbonate apatite hydrogel-advanced platelet-rich fibrin injection on osteoblastogenesis during orthodontic relapse in rabbits. Eur. J. Dent. 2021, 15, 412–419. [Google Scholar] [CrossRef]
- Lin, Y.; Fu, M.L.; Harb, I.; Ma, L.X.; Tran, S.D. Functional biomaterials for local control of orthodontic tooth movement. J. Funct. Biomater. 2023, 14, 294. [Google Scholar] [CrossRef] [PubMed]
- Montero Jiménez, O.G.; Dib Kanán, A.; Dipp Velázquez, F.A.; Aristizábal Pérez, J.F.; Moyaho Bernal, M.d.l.Á.; Salas Orozco, M.F.; Casillas Santana, M.A. Use of hydrogels to regulate orthodontic tooth movement in animal models: A systematic review. Appl. Sci. 2022, 12, 6683. [Google Scholar] [CrossRef]
- Asefi, S.; Seifi, M.; Fard, G.H.; Lotfi, A. Innovative evaluation of local injective gel of curcumin on the orthodontic tooth movement in rats. Dent. Res. J. 2018, 15, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Prado, H.J.; Matulewicz, M.C. Cationization of polysaccharides: A path to greener derivatives with many industrial applications. Eur. Polym. J. 2014, 52, 53–75. [Google Scholar] [CrossRef]
- Chalermwat, K.; Szuster, B.W.; Flaherty, M. Shellfish aquaculture in Thailand. Aquac. Econ. Manag. 2003, 7, 249–261. [Google Scholar] [CrossRef]
- Xu, Z.; Valeo, C.; Chu, A.; Zhao, Y. The Efficacy of Whole Oyster Shells for Removing Copper, Zinc, Chromium, and Cadmium Heavy Metal Ions from Stormwater. Sustainability 2021, 13, 4184. [Google Scholar] [CrossRef]
- Panpho, P.; Vittayakorn, N.; Sumang, R. Synthesis, scrutiny, and applications of bio-adsorbents from cockle shell waste for the adsorption of Pb and Cd in aqueous solution. Crystals 2023, 13, 552. [Google Scholar] [CrossRef]
- Muhammad Mailafiya, M.; Abubakar, K.; Danmaigoro, A.; Musa Chiroma, S.; Bin Abdul Rahim, E.; Aris Mohd Moklas, M.; Abu Bakar Zakaria, Z. Cockle shell-derived calcium carbonate (aragonite) nanoparticles: A dynamite to nanomedicine. Appl. Sci. 2019, 9, 2897. [Google Scholar] [CrossRef]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and fluorapatite in conservative dentistry and oral implantology-a review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef]
- Ishikawa, K.; Hayashi, K. Carbonate apatite artificial bone. Sci. Technol. Adv. Mater. 2021, 22, 683–694. [Google Scholar] [CrossRef]
- Chen, C.; Chang, N.; Wu, Y.; Fu, E.; Shen, E.; Feng, C.; Wen, Z. Bone formation using Cross-Linked chitosan scaffolds in rat calvarial defects. Implant. Dent. 2018, 27, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Shetty, C.; Shetty, A.; Shetty, S.; Kaur, G.; Hegde, M.N.; Nidhi, L. Applications of chitosan in dentistry. Indian J. Public Health Res. Dev. 2020, 11, 99–105. [Google Scholar]
- Elkattan, A.E.; Gheith, M.; Fayed, M.S.; El Yazeed, M.A.; Farrag, A.H.; Khalil, W.K.B. Effects of different parameters of diode laser on acceleration of orthodontic tooth movement and its effect on relapse: An experimental animal study. Maced. J. Med. Sci. 2019, 7, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Abifarin, J.K.; Obada, D.O.; Dauda, E.T.; Dodoo-Arhin, D. Experimental data on the characterization of hydroxyapatite synthesized from biowates. Data Brief 2019, 26, 104485. [Google Scholar] [CrossRef]
- Sayyidah, N.A.; Darjati; Suryono, H. The Examination of the quality of chitosan from bamboo shell waste with variations of NaOH concentration in the deacetylation process. ICoEH 2021, 1, 71–79. [Google Scholar]
- Maslii, Y.; Ruban, O.; Kasparaviciene, G.; Kalveniene, Z.; Materiienko, A.; Ivanauskas, L.; Mazurkeviciute, A.; Kopustinskiene, D.M.; Bernatoniene, J. The Influence of pH values on the rheological, textural, and release properties of carbomer. Molecules 2020, 25, 5018. [Google Scholar] [CrossRef]
- Alghunaim, A.; Kirdponpattara, S.; Newby, B.Z. Techniques for determining contact angle and wettability of powders. Powder Technol. 2016, 287, 201–215. [Google Scholar] [CrossRef]
- Wang, Y.; Burgess, D.J. Influence of storage temperature and moisture on the performance of microsphere/hydrogel composites. Int. J. Pharm. 2013, 454, 310–315. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Tsuru, K.; Nagai, H.; Fujisawa, K.; Kudoh, T.; Ohe, G.; Ishikawa, K.; Miyamoto, Y. Fabrication and evaluation of carbonate apatite-coated calcium carbonate bone substitutes for bone tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, 2077–2087. [Google Scholar] [CrossRef]
- Anitua, E.; Fuente, M.d.l.; Troya, M.; Zalduendo, M.; Alkhraisat, M.H. Autologous platelet rich plasma (PRGF) preserves genomic stability of gingival fibroblasts and alveolar osteoblasts after long-term cell culture. Dent. J. 2022, 10, 173. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kishida, T.; Sato, Y.; Nishioka, K.; Ejima, A.; Fujiwara, H.; Kubo, T.; Yamamoto, T.; Kanamura, N.; Mazda, O. Direct conversion of human fibroblasts into functional osteoblasts by defined factors. Proc. Natl. Acad. Sci. USA 2015, 112, 6152–6157. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Itoh, N.; Taniguchi, T.; Nakanishi, T.; Tanaka, K. Requirement of calcium and phosphate ions in expression of sodium-dependent vitamin C transporter 2 and osteopontin in MC3T3-E1 osteoblastic cells. Biochim. Biophys. Acta 2003, 1641, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kanatani, M.; Sugimoto, T.; Kano, J.; Kanzawa, M.; Chihara, K. Effect of high phosphate concentration on osteoclast differentiation as well as bone-resorbing activity. J. Cell Physiol. 2003, 196, 180–189. [Google Scholar] [CrossRef]
- Jindarojanakul, C.; Chanmanee, P.; Samruajbenjakun, B. Analysis of osteoclasts and root resorption in corticotomy-facilitated orthodontics with ibuprofen administration—An animal study. Dent. J. 2022, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- D’Apuzzo, F.; Cappabianca, S.; Ciavarella, D.; Monsurrò, A.; Silvestrini-Biavati, A.; Perillo, L. Biomarkers of periodontal tissue remodeling during orthodontic tooth movement in mice and men: Overview and clinical relevance. Sci. World J. 2013, 2013, 105873. [Google Scholar]
- Maarof, N.N.N.; Abdulmalek, E.; Fakurazi, S.; Rahman, M.B.A. Biodegradable carbonate apatite nanoparticle as a delivery system to promote afatinib delivery for non-small cell lung cancer treatment. Pharmaceutics 2022, 14, 1230. [Google Scholar] [CrossRef]
- Saito, T.; Tabata, Y. Preparation of gelatin hydrogels incorporating low-molecular-weight heparin for anti-fibrotic therapy. Acta Biomater. 2012, 8, 646–652. [Google Scholar] [CrossRef]
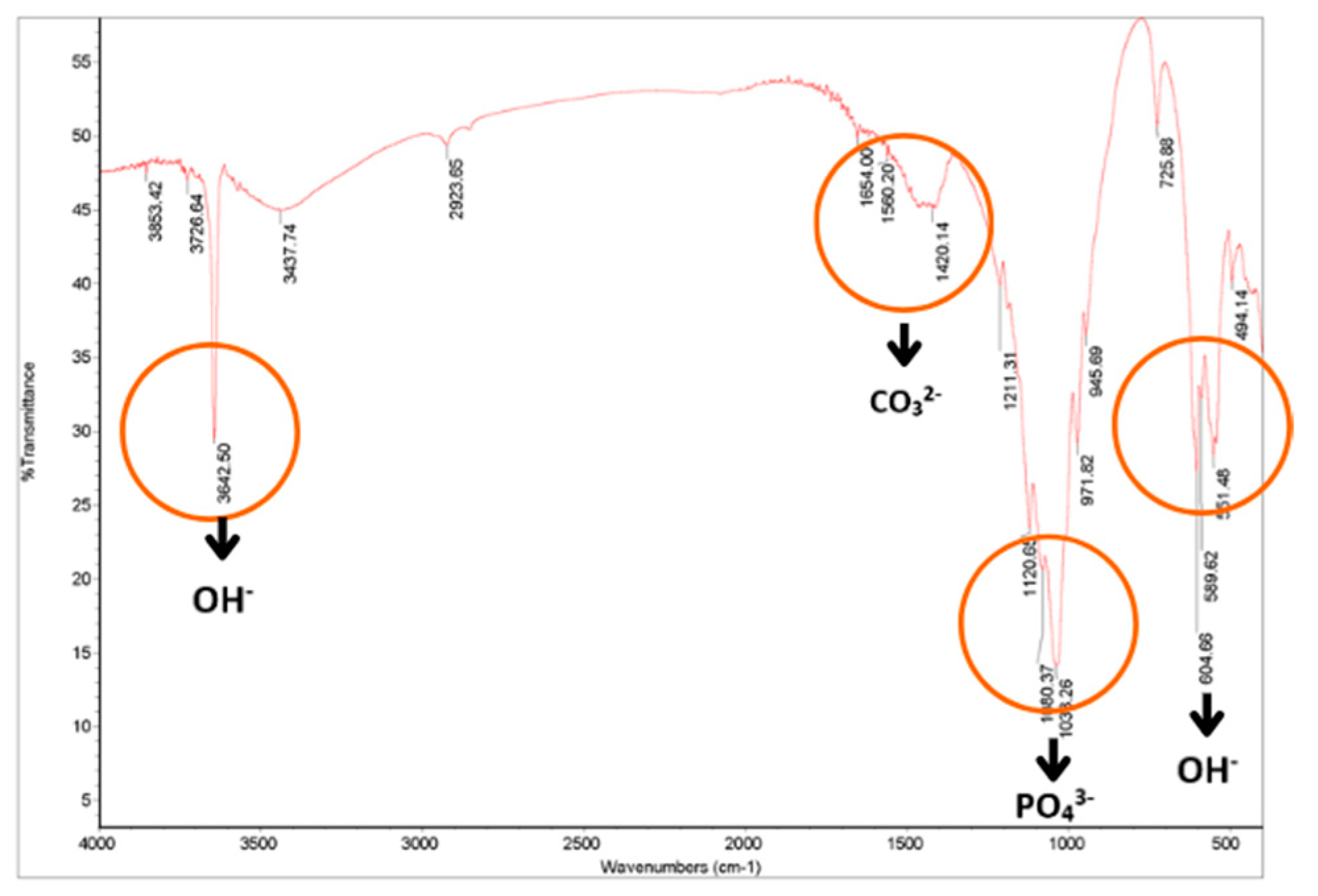



| Types of Bonding | Wavenumber (cm−1) of Hydroxyapatite Blood Shell | Wavenumber (cm−1) of Hydroxyapatite According to the Literature [24] |
|---|---|---|
| OH− | 3642.50 dan 604.66 | 3575 |
| OH hydration | 3437.74 | 3423 |
| CO32− | 1420.14 | 1470–1420 |
| PO43− | 1080.37 dan 1038.26 | 1085–1092 |
| Types of Bonding | Wavenumber (cm−1) of Chitosan Blood Cockle Shell | Chitosan Wavenumber (cm−1) of Hydroxyapatite According to the Literature [25] |
|---|---|---|
| N-H bond | 3404.63 | 3500–3100 |
| O-H bond | 3404.63 | 3400–3200 |
| C-H bond | 2925.36 | 3000–2850 |
| C=O bond | 1651.00 | 1654–1541 |
| C-N amine bond | 1420.29 | 1400 |
| C-O bond | 1155.02 | 1200–1180 |
| Day | 0 | 1 | 3 |
| pH test results | 6 | 6 | 6 |
| Control | Smell | Color | Taste | Consistency | Deposits |
|---|---|---|---|---|---|
| Cold Temperature | Less concentrated | Cloudy white suspension | Sweet–sour | Solid gel | >30 min |
| Room Temperature | Concentrated | Clear white suspension | Sweet–sour | Liquid gel | <20 min |
| Parameters | CHA-CS Group | Control Group | p-Value |
|---|---|---|---|
| Relapse Distance | |||
| Day 0 | 1.13 ± 0.58 | 0.75 ± 0.05 | 0.105 |
| Day 5 | 0.85 ± 0.09 | 0.77 ± 0.31 | 0.507 |
| Day 7 | 0.75 ± 0.05 | 0.80 ± 0.30 | 0.790 |
| Osteoblasts | |||
| Day 1 | 24.80 ± 5.59 | 20.61 ± 1.82 | 0.179 |
| Day 5 | 21.34 ± 1.87 | 17.06 ± 3.41 | 0.076 |
| Day 7 | 26.68 ± 3.72 | 18.14 ± 2.71 | 0.004 * |
| Osteoclasts | |||
| Day 1 | 2.80 ± 0.27 | 2.81 ± 0.45 | 0.699 |
| Day 5 | 1.29 ± 0.45 | 2.31 ± 0.57 | 0.025 * |
| Day 7 | 1.32 ± 0.27 | 2.22 ± 0.84 | 0.095 |
| Fibroblasts | |||
| Day 1 | 13.63 ± 3.41 | 8.21 ± 3.11 | 0.032 * |
| Day 5 | 13.12 ± 2.53 | 7.20 ± 2.77 | 0.016 * |
| Day 7 | 12.81 ± 2.33 | 6.20 ± 1.79 | 0.008 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadi, A.F.N.; Aghniya, S.N.; Haidar, G.A.; Sihombing, W.S.M.; Sutedjo, A.; Alhasyimi, A.A. Post-Orthodontic Relapse Prevention through Administration of a Novel Synthetic Carbonated Hydroxyapatite–Chitosan Hydrogel Derived from Blood Cockle Shell (Anadara granosa L.). Dent. J. 2024, 12, 18. https://doi.org/10.3390/dj12010018
Hadi AFN, Aghniya SN, Haidar GA, Sihombing WSM, Sutedjo A, Alhasyimi AA. Post-Orthodontic Relapse Prevention through Administration of a Novel Synthetic Carbonated Hydroxyapatite–Chitosan Hydrogel Derived from Blood Cockle Shell (Anadara granosa L.). Dentistry Journal. 2024; 12(1):18. https://doi.org/10.3390/dj12010018
Chicago/Turabian StyleHadi, Aanisah Fauziyyah Nurul, Sabrina Noor Aghniya, Gayuh Abi Haidar, Windy Sepry Marcelina Sihombing, Angelina Sutedjo, and Ananto Ali Alhasyimi. 2024. "Post-Orthodontic Relapse Prevention through Administration of a Novel Synthetic Carbonated Hydroxyapatite–Chitosan Hydrogel Derived from Blood Cockle Shell (Anadara granosa L.)" Dentistry Journal 12, no. 1: 18. https://doi.org/10.3390/dj12010018
APA StyleHadi, A. F. N., Aghniya, S. N., Haidar, G. A., Sihombing, W. S. M., Sutedjo, A., & Alhasyimi, A. A. (2024). Post-Orthodontic Relapse Prevention through Administration of a Novel Synthetic Carbonated Hydroxyapatite–Chitosan Hydrogel Derived from Blood Cockle Shell (Anadara granosa L.). Dentistry Journal, 12(1), 18. https://doi.org/10.3390/dj12010018







