Late Complications in Long-Term Childhood Cancer Survivors: What the Oral Health Professional Needs to Know
Abstract
1. Introduction
2. Methods
3. Late oral and Craniofacial Complications
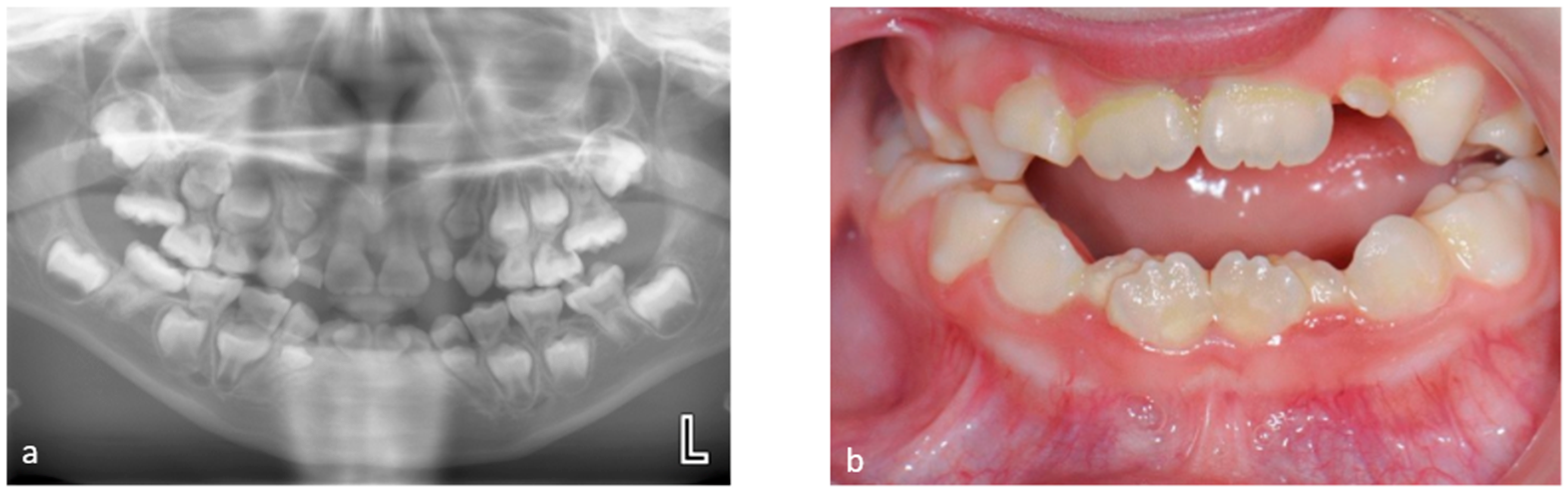
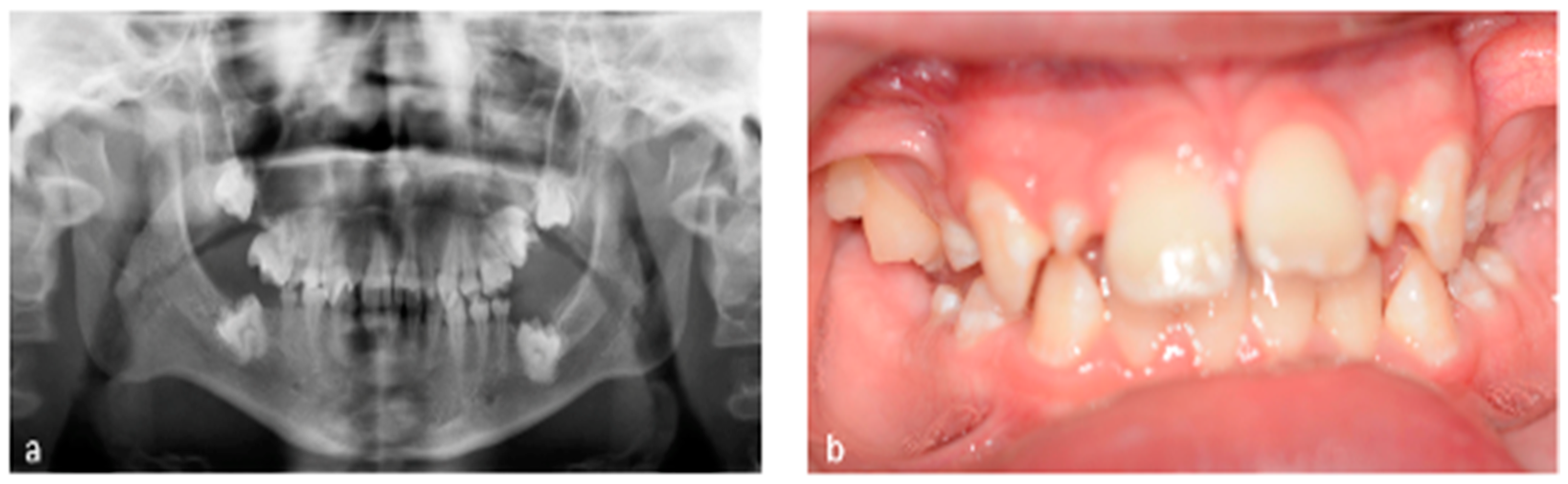
3.1. Tooth Development Disturbances
3.2. Disturbed Craniofacial Growth
3.3. Craniomandibular Dysfunction
3.4. Salivary Gland Dysfunction
3.5. Dental Caries and Periodontal Diseases
3.6. Oral Graft-versus-Host-Disease
3.7. Osteoradionecrosis and Medication-Related Osteonecrosis of the Jaw
3.8. Subsequent Primary Malignancies
3.9. Oral Health Related Quality of Life
4. Late Non-Oral Sequelae That May Affect Oral Health and Provision of Dental Care
4.1. Endocrine Dysfunction
4.2. Diabetes Mellitus
4.3. Thyroid Dysfunction
4.4. Adrenal Insufficiency
4.5. Gonadal Dysfunction
4.6. Cardiovascular Disease
4.7. Increased Infection Risk
4.8. Mental Health Considerations
5. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, J.; Wang, J.; Li, S. Advances in the treatment of solid tumors in children and adolescents. Cancer Innov. 2023, 2, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Pieters, R.; Mullighan, C.G.; Hunger, S.P. Advancing Diagnostics and Therapy to Reach Universal Cure in Childhood ALL. J. Clin. Oncol. 2023, 41, 5579–5591. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review (CSR) 1975–2017. 2020. Available online: https://seer.cancer.gov/csr/1975_2017/ (accessed on 12 September 2023).
- American Cancer Society Journal, CA: A Cancer Journal for Clinicians. American Cancer Society. Cancer Facts & Figures 2022. American Cancer Society. Atlanta, GA, USA, 2021, Childhood Cancer Inequalities in the WHO European Region. Copenhagen: WHO Regional Office for Europe. 2022. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html (accessed on 8 August 2023).
- Landier, W.; Armenian, S.; Bhatia, S. Late effects of childhood cancer and its treatment. Pediatr. Clin. N. Am. 2015, 62, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Ness, K.K.; Armstrong, G.T.; Bhakta, N.; Yeh, J.M.; Bhatia, S.; Landier, W.; Constine, L.S.; Hudson, M.M.; Nathan, P.C. Current and coming challenges in the management of the survivorship population. Semin. Oncol. 2020, 47, 23–39. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Geenen, M.M.; Cardous-Ubbink, M.C.; Kremer, L.C.; van den Bos, C.; van der Pal, H.J.; Heinen, R.C.; Jaspers, M.W.; Koning, C.C.; Oldenburger, F.; Langeveld, N.E.; et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 2007, 297, 2705–2715. [Google Scholar] [CrossRef]
- Calaminus, G.; Baust, K.; Berger, C.; Byrne, J.; Binder, H.; Casagranda, L.; Grabow, D.; Grootenhuis, M.; Kaatsch, P.; Kaiser, M.; et al. Health-Related Quality of Life in European Childhood Cancer Survivors: Protocol for a Study Within PanCareLIFE. JMIR Res. Protoc. 2021, 10, e21851. [Google Scholar] [CrossRef]
- Clemens, E.; van der Kooi, A.L.F.; Broer, L.; van Dulmen-den Broeder, E.; Visscher, H.; Kremer, L.; Tissing, W.; Loonen, J.; Ronckers, C.M.; Pluijm, S.M.F.; et al. The influence of genetic variation on late toxicities in childhood cancer survivors: A review. Crit. Rev. Oncol. Hematol. 2018, 126, 154–167. [Google Scholar] [CrossRef]
- Dixon, S.B.; Liu, Q.; Chow, E.J.; Oeffinger, K.C.; Nathan, P.C.; Howell, R.M.; Leisenring, W.M.; Ehrhardt, M.J.; Ness, K.K.; Krull, K.R.; et al. Specific causes of excess late mortality and association with modifiable risk factors among survivors of childhood cancer: A report from the Childhood Cancer Survivor Study cohort. Lancet 2023, 401, 1447–1457. [Google Scholar] [CrossRef]
- Effinger, K.E.; Migliorati, C.A.; Hudson, M.M.; McMullen, K.P.; Kaste, S.C.; Ruble, K.; Guilcher, G.M.; Shah, A.J.; Castellino, S.M. Oral and dental late effects in survivors of childhood cancer: A Children’s Oncology Group report. Support. Care Cancer 2014, 22, 2009–2019. [Google Scholar] [CrossRef]
- Stolze, J.; Raber-Durlacher, J.E.; Loonen, J.J.; Teepen, J.C.; Ronckers, C.M.; Tissing, W.J.E.; de Vries, A.C.H.; Neggers, S.; Dulmen-den Broeder, E.; Heuvel-Eibrink, M.M.; et al. Self-reported outcomes on oral health and oral health-related quality of life in long-term childhood cancer survivors—A DCCSS-LATER 2 Study. Support. Care Cancer 2023, 31, 344. [Google Scholar] [CrossRef]
- Socie, G.; Curtis, R.E.; Deeg, H.J.; Sobocinski, K.A.; Filipovich, A.H.; Travis, L.B.; Sullivan, K.M.; Rowlings, P.A.; Kingma, D.W.; Banks, P.M.; et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J. Clin. Oncol. 2000, 18, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Sunguc, C.; Hawkins, M.M.; Winter, D.L.; Dudley, I.M.; Heymer, E.J.; Teepen, J.C.; Allodji, R.S.; Belle, F.N.; Bagnasco, F.; Byrne, J.; et al. Risk of subsequent primary oral cancer in a cohort of 69,460 5-year survivors of childhood and adolescent cancer in Europe: The PanCareSurFup study. Br. J. Cancer 2023, 128, 80–90. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). 2023. Available online: https://www.cancer.gov (accessed on 12 September 2023).
- American Academy of Pediatric Dentistry. Dental Management of Pediatric Patients Receiving Immunosuppressive Therapy and or Head and Neck Radiation; Best Practices: Immunosuppressive and/or Radiation; American Academy of Pediatric Dentistry: Chicago, IL, USA, 2023. [Google Scholar]
- Stolze, J.; Vlaanderen, K.C.E.; Holtbach, F.; Teepen, J.C.; Kremer, L.C.M.; Loonen, J.J.; van Dulmen-den Broeder, E.; Heuvel-Eibrink, M.; Pal, H.; Versluys, B.; et al. Long-Term Effects of Childhood Cancer Treatment on Dentition and Oral Health: A Dentist Survey Study from the DCCSS LATER 2 Study. Cancers 2021, 13, 5264. [Google Scholar] [CrossRef] [PubMed]
- Bei, M. Molecular genetics of tooth development. Curr. Opin. Genet. Dev. 2009, 19, 504–510. [Google Scholar] [CrossRef]
- Cubukcu, C.E.; Sevinir, B.; Ercan, I. Disturbed dental development of permanent teeth in children with solid tumors and lymphomas. Pediatr. Blood Cancer 2012, 58, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Al-Ansari, S.; Jalali, R.; Bronckers, T.; Raber-Durlacher, J.; Logan, R.; de Lange, J.; Rozema, F. The effect of a single injection of irinotecan on the development of enamel in the Wistar rats. J. Cell. Mol. Med. 2018, 22, 1501–1506. [Google Scholar] [CrossRef]
- Carrillo, C.M.; Correa, F.N.; Lopes, N.N.; Fava, M.; Odone Filho, V. Dental anomalies in children submitted to antineoplastic therapy. Clinics 2014, 69, 433–437. [Google Scholar] [CrossRef]
- Wilberg, P.; Kanellopoulos, A.; Ruud, E.; Hjermstad, M.J.; Fossa, S.D.; Herlofson, B.B. Dental abnormalities after chemotherapy in long-term survivors of childhood acute lymphoblastic leukemia 7-40 years after diagnosis. Support. Care Cancer 2016, 24, 1497–1506. [Google Scholar] [CrossRef]
- Goho, C. Chemoradiation therapy: Effect on dental development. Pediatr. Dent. 1993, 15, 6–12. [Google Scholar]
- Lopes, N.N.; Petrilli, A.S.; Caran, E.M.; Franca, C.M.; Chilvarquer, I.; Lederman, H. Dental abnormalities in children submitted to antineoplastic therapy. J. Dent. Child. 2006, 73, 140–145. [Google Scholar]
- Maciel, J.C.; de Castro, C.G., Jr.; Brunetto, A.L.; Di Leone, L.P.; da Silveira, H.E. Oral health and dental anomalies in patients treated for leukemia in childhood and adolescence. Pediatr. Blood Cancer 2009, 53, 361–365. [Google Scholar] [CrossRef]
- Busenhart, D.M.; Erb, J.; Rigakos, G.; Eliades, T.; Papageorgiou, S.N. Adverse effects of chemotherapy on the teeth and surrounding tissues of children with cancer: A systematic review with meta-analysis. Oral Oncol. 2018, 83, 64–72. [Google Scholar] [CrossRef]
- Oguz, A.; Cetiner, S.; Karadeniz, C.; Alpaslan, G.; Alpaslan, C.; Pinarli, G. Long-term effects of chemotherapy on orodental structures in children with non-Hodgkin’s lymphoma. Eur. J. Oral Sci. 2004, 112, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Holtta, P.; Hovi, L.; Saarinen-Pihkala, U.M.; Peltola, J.; Alaluusua, S. Disturbed root development of permanent teeth after pediatric stem cell transplantation. Dental root development after SCT. Cancer 2005, 103, 1484–1493. [Google Scholar] [CrossRef]
- Proc, P.; Szczepanska, J.; Herud, A.; Zubowska, M.; Fendler, W.; Mlynarski, W. Dental caries among childhood cancer survivors. Medicine 2019, 98, e14279. [Google Scholar] [CrossRef]
- Dahllof, G.; Rozell, B.; Forsberg, C.M.; Borgstrom, B. Histologic changes in dental morphology induced by high dose chemotherapy and total body irradiation. Oral Surg. Oral Med. Oral Pathol. 1994, 77, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Holtta, P.; Alaluusua, S.; Saarinen-Pihkala, U.M.; Wolf, J.; Nystrom, M.; Hovi, L. Long-term adverse effects on dentition in children with poor-risk neuroblastoma treated with high-dose chemotherapy and autologous stem cell transplantation with or without total body irradiation. Bone Marrow Transpl. 2002, 29, 121–127. [Google Scholar] [CrossRef]
- Holtta, P.; Alaluusua, S.; Saarinen-Pihkala, U.M.; Peltola, J.; Hovi, L. Agenesis and microdontia of permanent teeth as late adverse effects after stem cell transplantation in young children. Cancer 2005, 103, 181–190. [Google Scholar] [CrossRef]
- van der Pas-van Voskuilen, I.G.; Veerkamp, J.S.; Raber-Durlacher, J.E.; Bresters, D.; van Wijk, A.J.; Barasch, A.; McNeal, S.; Gortzak, R.A. Long-term adverse effects of hematopoietic stem cell transplantation on dental development in children. Support. Care Cancer 2009, 17, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Rakhshan, V. Congenitally missing teeth (hypodontia): A review of the literature concerning the etiology, prevalence, risk factors, patterns and treatment. Dent. Res. J. 2015, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Avsar, A.; Elli, M.; Darka, O.; Pinarli, G. Long-term effects of chemotherapy on caries formation, dental development, and salivary factors in childhood cancer survivors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 104, 781–789. [Google Scholar] [CrossRef]
- Wuollet, E.; Laisi, S.; Salmela, E.; Ess, A.; Alaluusua, S. Molar-incisor hypomineralization and the association with childhood illnesses and antibiotics in a group of Finnish children. Acta Odontol. Scand. 2016, 74, 416–422. [Google Scholar] [CrossRef]
- Nasman, M.; Forsberg, C.M.; Dahllof, G. Long-term dental development in children after treatment for malignant disease. Eur. J. Orthod. 1997, 19, 151–159. [Google Scholar] [CrossRef]
- Németh, O. Dental and Craniofacial Effects on Childhood Cancer Survivors; IntechOpen: London, UK, 2017. [Google Scholar]
- Denys, D.; Kaste, S.C.; Kun, L.E.; Chaudhary, M.A.; Bowman, L.C.; Robbins, K.T. The effects of radiation on craniofacial skeletal growth: A quantitative study. Int. J. Pediatr. Otorhinolaryngol. 1998, 45, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, N.; Toth, B.B.; Hoar, R.E.; Ried, H.L.; Sullivan, M.P.; McNeese, M.D. Dental and maxillofacial abnormalities in long-term survivors of childhood cancer: Effects of treatment with chemotherapy and radiation to the head and neck. Pediatrics 1984, 73, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Gevorgyan, A.; La Scala, G.C.; Neligan, P.C.; Pang, C.Y.; Forrest, C.R. Radiation-induced craniofacial bone growth disturbances. J. Craniofac. Surg. 2007, 18, 1001–1007. [Google Scholar] [CrossRef]
- Bhat, M. The human face: Genes, embryological development and dysmorphology. Int. J. Dev. Biol. 2020, 64, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.; Stock, H. Effects of radiation on bone. Curr. Osteoporos. Rep. 2013, 11, 299–304. [Google Scholar] [CrossRef]
- Dahllof, G. Craniofacial growth in children treated for malignant diseases. Acta Odontol. Scand. 1998, 56, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, O.; Hermann, P.; Kivovics, P.; Garami, M. Long-term effects of chemotherapy on dental status of children cancer survivors. Pediatr. Hematol. Oncol. 2013, 30, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Vesterbacka, M.; Ringden, O.; Remberger, M.; Huggare, J.; Dahllof, G. Disturbances in dental development and craniofacial growth in children treated with hematopoietic stem cell transplantation. Orthod. Craniofac. Res. 2012, 15, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Sonis, A.L.; Tarbell, N.; Valachovic, R.W.; Gelber, R.; Schwenn, M.; Sallan, S. Dentofacial development in long-term survivors of acute lymphoblastic leukemia. A comparison of three treatment modalities. Cancer 1990, 66, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Nordforsk. ALLTogether—A European Treatment Protocol for Children and Young Adults with Acute Lymphoblastic Leukaemia (ALL). 2023. Available online: https://www.nordforsk.org/projects/alltogether-european-treatment-protocol-children-and-young-adults-acute-lymphoblastic (accessed on 12 September 2023).
- Bianchi, A.; Crimi, S.; Cipriani, R.; De Ponte, F.S.; Cicciu, M.; Marchetti, C. Comprehensive Treatment of Facial Deformity Due to Radiotherapy in Rhabdomyosarcoma Patients: Distraction Osteogenesis and Free Flaps Surgical Technique. J. Craniofac. Surg. 2019, 30, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Allam, K.A.; Lim, A.A.; Elsherbiny, A.; Bradley, J.P.; Kawamoto, H.K. Radiation-induced craniofacial deformities: A new classification and management algorithm. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 1088–1095. [Google Scholar] [CrossRef]
- Gawade, P.L.; Hudson, M.M.; Kaste, S.C.; Neglia, J.P.; Wasilewski-Masker, K.; Constine, L.S.; Robison, L.L.; Ness, K.K. A systematic review of selected musculoskeletal late effects in survivors of childhood cancer. Curr. Pediatr. Rev. 2014, 10, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.L.; Sonis, S.T. Mechanisms of cellular fibrosis associated with cancer regimen-related toxicities. Front. Pharmacol. 2014, 5, 51. [Google Scholar] [CrossRef]
- Dahllof, G.; Krekmanova, L.; Kopp, S.; Borgstrom, B.; Forsberg, C.M.; Ringden, O. Craniomandibular dysfunction in children treated with total-body irradiation and bone marrow transplantation. Acta Odontol. Scand. 1994, 52, 99–105. [Google Scholar] [CrossRef]
- Kupeli, S.; Varan, A.; Ozyar, E.; Atahan, I.L.; Yalcin, B.; Kutluk, T.; Akyuz, C.; Buyukpamukcu, M. Treatment results of 84 patients with nasopharyngeal carcinoma in childhood. Pediatr. Blood Cancer 2006, 46, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Mawardi, H.; Hashmi, S.K.; Elad, S.; Aljurf, M.; Treister, N. Chronic graft-versus-host disease: Current management paradigm and future perspectives. Oral Dis. 2019, 25, 931–948. [Google Scholar] [CrossRef]
- Kaste, S.C.; Goodman, P.; Leisenring, W.; Stovall, M.; Hayashi, R.J.; Yeazel, M.; Beiraghi, S.; Hudson, M.M.; Sklar, C.A.; Robison, L.L.; et al. Impact of radiation and chemotherapy on risk of dental abnormalities: A report from the Childhood Cancer Survivor Study. Cancer 2009, 115, 5817–5827. [Google Scholar] [CrossRef] [PubMed]
- Stolze, J.; Teepen, J.C.; Raber-Durlacher, J.E.; Loonen, J.J.; Kok, J.L.; Tissing, W.J.E.; de Vries, A.C.H.; Neggers, S.; van Dulmen-den Broeder, E.; van den Heuvel-Eibrink, M.M.; et al. Prevalence and Risk Factors for Hyposalivation and Xerostomia in Childhood Cancer Survivors Following Different Treatment Modalities-A Dutch Childhood Cancer Survivor Study Late Effects 2 Clinical Study (DCCSS LATER 2). Cancers 2022, 14, 3379. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, O.; Kivovics, M.; Pinke, I.; Marton, K.; Kivovics, P.; Garami, M. Late effects of multiagent chemotherapy on salivary secretion in children cancer survivors. J. Am. Coll. Nutr. 2014, 33, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Al-Ansari, S.; Jalali, R.; Bronckers, A.; van Tellingen, O.; Raber-Durlacher, J.; Nadjmi, N.; Brook, A.H.; de Lange, J.; Rozema, F.R. Tooth Formation as Experimental Model to Study Chemotherapy on Tissue Development: Effect of a Specific Dose of Temozolomide/Veliparib. Genes 2022, 13, 1198. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.; Joshi, R.K.; Ekstrom, J.; Aframian, D.; Pedersen, A.M.; Proctor, G.; Narayana, N.; Villa, A.; Sia, Y.W.; Aliko, A.; et al. A Guide to Medications Inducing Salivary Gland Dysfunction, Xerostomia, and Subjective Sialorrhea: A Systematic Review Sponsored by the World Workshop on Oral Medicine VI. Drugs R D 2017, 17, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Dahllof, G.; Wondimu, B.; Barr-Agholme, M.; Garming-Legert, K.; Remberger, M.; Ringden, O. Xerostomia in children and adolescents after stem cell transplantation conditioned with total body irradiation or busulfan. Oral Oncol. 2011, 47, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Connell, C.L.; Abati, S. Diagnosis and management of xerostomia and hyposalivation. Ther. Clin. Risk Manag. 2015, 11, 45–51. [Google Scholar] [CrossRef]
- Mercadante, V.; Jensen, S.B.; Smith, D.K.; Bohlke, K.; Bauman, J.; Brennan, M.T.; Coppes, R.P.; Jessen, N.; Malhotra, N.K.; Murphy, B.; et al. Salivary Gland Hypofunction and/or Xerostomia Induced by Nonsurgical Cancer Therapies: ISOO/MASCC/ASCO Guideline. J. Clin. Oncol. 2021, 39, 2825–2843. [Google Scholar] [CrossRef]
- Pajari, U.; Ollila, P.; Lanning, M. Incidence of dental caries in children with acute lymphoblastic leukemia is related to the therapy used. ASDC J. Dent. Child. 1995, 62, 349–352. [Google Scholar]
- Wogelius, P.; Dahllof, G.; Gorst-Rasmussen, A.; Sorensen, H.T.; Rosthoj, S.; Poulsen, S. A population-based observational study of dental caries among survivors of childhood cancer. Pediatr. Blood Cancer 2008, 50, 1221–1226. [Google Scholar] [CrossRef]
- Seremidi, K.; Kavvadia, K.; Kattamis, A.; Polychronopoulou, A. Dental caries and dental developmental defects as adverse effects of antineoplastic treatment in childhood cancer survivors. Eur. Arch. Paediatr. Dent. 2023, 24, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Patni, T.; Lee, C.T.; Li, Y.; Kaste, S.; Zhu, L.; Sun, R.; Hudson, M.M.; Ness, K.K.; Neumann, A.; Robison, L.L. Factors for poor oral health in long-term childhood cancer survivors. BMC Oral Health 2023, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.H.; Napenas, J.J.; Hodgson, B.D.; Stokman, M.A.; Mathers-Stauffer, V.; Elting, L.S.; Spijkervet, F.K.; Brennan, M.T.; Dental Disease Section, Oral Care Study Group, Multi-national Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). A systematic review of dental disease in patients undergoing cancer therapy. Support. Care Cancer 2010, 18, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Robertson, M.; Emerton, S.; Phillips, N.; Stevenson-Moore, P. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck 2001, 23, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Schuurhuis, J.M.; Stokman, M.A.; Witjes, M.J.H.; Reintsema, H.; Langendijk, J.A.; Vissink, A.; Spijkervet, F.K.L. Patients with advanced periodontal disease before intensity-modulated radiation therapy are prone to develop bone healing problems: A 2-year prospective follow-up study. Support. Care Cancer 2018, 26, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.S.; Mendes, E.M.; Borges, J.S.; Osuna, L.G.; Rabelo, G.D.; Soares, P.B. Periodontal therapy for patients before and after radiotherapy: A review of the literature and topics of interest for clinicians. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, e524–e530. [Google Scholar] [CrossRef] [PubMed]
- Horner, A.J.; Nativio, D.G. Unique Factors Affecting the Management and Prevention of Caries in the Childhood Cancer Survivor. J. Pediatr. Health Care 2019, 33, 53–57. [Google Scholar] [CrossRef]
- Majorana, A.; Schubert, M.M.; Porta, F.; Ugazio, A.G.; Sapelli, P.L. Oral complications of pediatric hematopoietic cell transplantation: Diagnosis and management. Support. Care Cancer 2000, 8, 353–365. [Google Scholar] [CrossRef]
- Treister, N.S.; Woo, S.B.; O’Holleran, E.W.; Lehmann, L.E.; Parsons, S.K.; Guinan, E.C. Oral chronic graft-versus-host disease in pediatric patients after hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2005, 11, 721–731. [Google Scholar] [CrossRef]
- Dahllof, G.; Hingorani, S.R.; Sanders, J.E. Late effects following hematopoietic cell transplantation for children. Biol. Blood Marrow Transplant. 2008, 14 (Suppl. S1), 88–93. [Google Scholar] [CrossRef][Green Version]
- Cantile, T.; Coppola, N.; Canfora, F.; Adamo, D.; Ruoppo, E.; Mignogna, M.D.; Leuci, S. Oral Cancer in HSCT Pediatric Patients Arising on GVHD: A Comprehensive Review. Cancers 2022, 14, 5775. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, P.A.; Kitko, C.L.; Elad, S.; Flowers, M.E.; Gea-Banacloche, J.C.; Halter, J.P.; Hoodin, F.; Johnston, L.; Lawitschka, A.; McDonald, G.B.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol. Blood Marrow Transplant. 2015, 21, 1167–1187. [Google Scholar] [CrossRef] [PubMed]
- Haverman, T.M.; Raber-Durlacher, J.E.; Raghoebar, I.I.; Rademacher, W.M.H.; Rozema, F.R.; Hazenberg, M.D.; Epstein, J.B.; Treister, N.S. Oral chronic graft-versus-host disease: What the general dental practitioner needs to know. J. Am. Dent. Assoc. 2020, 151, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Cancer.gov. Oral Complications of Chemotherapy and Head/Neck Radiation (PDQ®)–Health Professional Version. 2022. Available online: https://www.cancer.gov/about-cancer/treatment/side-effects/mouth-throat/oral-complications-hp-pdq#_207 (accessed on 4 January 2023).
- Duarte, N.T.; Rech, B.O.; Martins, I.G.; Franco, J.B.; Ortega, K.L. Can children be affected by bisphosphonate-related osteonecrosis of the jaw? A systematic review. Int. J. Oral Maxillofac. Surg. 2020, 49, 183–191. [Google Scholar] [CrossRef]
- Rosales, H.D.; Garcia Guevara, H.; Requejo, S.; Jensen, M.D.; Acero, J.; Olate, S. Medication-Related Osteonecrosis of the Jaws (MRONJ) in Children and Young Patients-A Systematic Review. J. Clin. Med. 2023, 12, 1416. [Google Scholar] [CrossRef]
- Hernandez, M.; Phulpin, B.; Mansuy, L.; Droz, D. Use of new targeted cancer therapies in children: Effects on dental development and risk of jaw osteonecrosis: A review. J. Oral Pathol. Med. 2017, 46, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Tortora, C.; Paoletta, M.; Marrapodi, M.M.; Argenziano, M.; Di Paola, A.; Pota, E.; Di Pinto, D.; Di Martino, M.; Iolascon, G. Osteoporosis in Childhood Cancer Survivors: Physiopathology, Prevention, Therapy and Future Perspectives. Cancers 2022, 14, 4349. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws—2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef]
- Bassal, M.; Mertens, A.C.; Taylor, L.; Neglia, J.P.; Greffe, B.S.; Hammond, S.; Ronckers, C.M.; Friedman, D.L.; Stovall, M.; Yasui, Y.Y.; et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2006, 24, 476–483. [Google Scholar] [CrossRef]
- Rizzo, J.D.; Curtis, R.E.; Socie, G.; Sobocinski, K.A.; Gilbert, E.; Landgren, O.; Travis, L.B.; Travis, W.D.; Flowers, M.E.; Friedman, D.L.; et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood 2009, 113, 1175–1183. [Google Scholar] [CrossRef]
- Bhatti, P.; Veiga, L.H.; Ronckers, C.M.; Sigurdson, A.J.; Stovall, M.; Smith, S.A.; Weathers, R.; Leisenring, W.; Mertens, A.C.; Hammond, S.; et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: An update from the childhood cancer survivor study. Radiat. Res. 2010, 174, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Teepen, J.C.; van Leeuwen, F.E.; Tissing, W.J.; van Dulmen-den Broeder, E.; van den Heuvel-Eibrink, M.M.; van der Pal, H.J.; Loonen, J.J.; Bresters, D.; Versluys, B.; Neggers, S.; et al. Long-Term Risk of Subsequent Malignant Neoplasms After Treatment of Childhood Cancer in the DCOG LATER Study Cohort: Role of Chemotherapy. J. Clin. Oncol. 2017, 35, 2288–2298. [Google Scholar] [CrossRef] [PubMed]
- Lebbink, C.A.; Waguespack, S.G.; van Santen, H.M. Thyroid Dysfunction and Thyroid Cancer in Childhood Cancer Survivors: Prevalence, Surveillance and Management. Front. Horm. Res. 2021, 54, 140–153. [Google Scholar] [PubMed]
- Teepen, J.C.; Kok, J.L.; Kremer, L.C.; Tissing, W.J.E.; van den Heuvel-Eibrink, M.M.; Loonen, J.J.; Bresters, D.; van der Pal, H.J.; Versluys, B.; van Dulmen-den Broeder, E.; et al. Long-Term Risk of Skin Cancer Among Childhood Cancer Survivors: A DCOG-LATER Cohort Study. J. Natl. Cancer Inst. 2019, 111, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Yeazel, M.W.; Oeffinger, K.C.; Gurney, J.G.; Mertens, A.C.; Hudson, M.M.; Emmons, K.M.; Chen, H.; Robison, L.L. The cancer screening practices of adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2004, 100, 631–640. [Google Scholar] [CrossRef]
- Sischo, L.; Broder, H.L. Oral health-related quality of life: What, why, how, and future implications. J. Dent. Res. 2011, 90, 1264–1270. [Google Scholar] [CrossRef]
- van Erp, L.M.E.; Maurice-Stam, H.; Kremer, L.C.M.; Tissing, W.J.E.; van der Pal, H.J.H.; de Vries, A.C.H.; van den Heuvel-Eibrink, M.M.; Versluys, B.A.B.; Loonen, J.J.; Bresters, D.; et al. Health-related quality of life in Dutch adult survivors of childhood cancer: A nation-wide cohort study. Eur. J. Cancer 2021, 152, 204–214. [Google Scholar] [CrossRef]
- Wogelius, P.; Rosthoj, S.; Dahllof, G.; Poulsen, S. Oral health-related quality of life among survivors of childhood cancer. Int. J. Paediatr. Dent. 2011, 21, 465–467. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Kawashima, T.; Leisenring, W.; Stratton, K.; Stovall, M.; Hudson, M.M.; Sklar, C.A.; Robison, L.L.; Oeffinger, K.C. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J. Clin. Oncol. 2014, 32, 1218–1227. [Google Scholar] [CrossRef]
- Gebauer, J.; Higham, C.; Langer, T.; Denzer, C.; Brabant, G. Long-Term Endocrine and Metabolic Consequences of Cancer Treatment: A Systematic Review. Endocr. Rev. 2019, 40, 711–767. [Google Scholar] [CrossRef]
- Laznickova, P.; Bendickova, K.; Kepak, T.; Fric, J. Immunosenescence in Childhood Cancer Survivors and in Elderly: A Comparison and Implication for Risk Stratification. Front. Aging 2021, 2, 708788. [Google Scholar] [CrossRef] [PubMed]
- Cupit-Link, M.C.; Kirkland, J.L.; Ness, K.K.; Armstrong, G.T.; Tchkonia, T.; LeBrasseur, N.K.; Armenian, S.H.; Ruddy, K.J.; Hashmi, S.K. Biology of premature ageing in survivors of cancer. ESMO Open 2017, 2, e000250. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, M.F.; Xie, L.; Rogers, P.C.; Pritchard, S.; Goddard, K.; McBride, M.L. Hospital-related morbidity among childhood cancer survivors in British Columbia, Canada: Report of the childhood, adolescent, young adult cancer survivors (CAYACS) program. Int. J. Cancer 2011, 128, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Oeffinger, K.C.; Hudson, M.M.; Landier, W. Survivorship: Childhood cancer survivors. Prim. Care 2009, 36, 743–780. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.; Chemaitilly, W. Endocrinopathies in survivors of childhood neoplasia. Front. Pediatr. 2014, 2, 101. [Google Scholar] [CrossRef]
- Chemaitilly, W.; Cohen, L.E.; Mostoufi-Moab, S.; Patterson, B.C.; Simmons, J.H.; Meacham, L.R.; van Santen, H.M.; Sklar, C.A. Endocrine Late Effects in Childhood Cancer Survivors. J. Clin. Oncol. 2018, 36, 2153–2159. [Google Scholar] [CrossRef]
- Casano-Sancho, P.; Izurieta-Pacheco, A.C. Endocrine Late Effects in Childhood Cancer Survivors. Cancers 2022, 14, 2630. [Google Scholar] [CrossRef]
- Friedman, D.N.; Tonorezos, E.S.; Cohen, P. Diabetes and Metabolic Syndrome in Survivors of Childhood Cancer. Horm. Res. Paediatr. 2019, 91, 118–127. [Google Scholar] [CrossRef]
- Wu, C.Z.; Yuan, Y.H.; Liu, H.H.; Li, S.S.; Zhang, B.W.; Chen, W.; An, Z.J.; Chen, S.Y.; Wu, Y.Z.; Han, B.; et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health 2020, 20, 204. [Google Scholar] [CrossRef]
- Simpson, T.C.; Clarkson, J.E.; Worthington, H.V.; MacDonald, L.; Weldon, J.C.; Needleman, I.; Iheozor-Ejiofor, Z.; Wild, S.H.; Qureshi, A.; Walker, A.; et al. Treatment of periodontitis for glycaemic control in people with diabetes mellitus. Cochrane Database Syst. Rev. 2022, 4, CD004714. [Google Scholar]
- Available online: https://www.ada.org/resources/research/science-and-research-institute/oral-health-topics/diabetes (accessed on 5 January 2023).
- Huber, M.A.; Terezhalmy, G.T. Risk stratification and dental management of the patient with thyroid dysfunction. Quintessence Int. 2008, 39, 139–150. [Google Scholar] [PubMed]
- Chemaitilly, W.; Li, Z.; Brinkman, T.M.; Delaney, A.; Huang, S.; Bjornard, K.L.; Lam, C.G.; Wilson, C.L.; Barnes, N.; Clark, K.L.; et al. Primary hypothyroidism in childhood cancer survivors: Prevalence, risk factors, and long-term consequences. Cancer 2022, 128, 606–614. [Google Scholar] [CrossRef]
- Young, E.R. The thyroid gland and the dental practitioner. J. Can. Dent. Assoc. 1989, 55, 903–907. [Google Scholar] [PubMed]
- Pinto, A.; Glick, M. Management of patients with thyroid disease: Oral health considerations. J. Am. Dent. Assoc. 2002, 133, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Chandna, S.; Bathla, M. Oral manifestations of thyroid disorders and its management. Indian J. Endocrinol. Metab. 2011, 15 (Suppl. S2), S113–S116. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S.R.; Allolio, B.; Arlt, W.; Barthel, A.; Don-Wauchope, A.; Hammer, G.D.; Husebye, E.S.; Merke, D.P.; Murad, M.H.; Stratakis, C.A.; et al. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 364–389. [Google Scholar] [CrossRef] [PubMed]
- van Santen, H.M.; van den Heuvel-Eibrink, M.M.; van de Wetering, M.D.; Wallace, W.H. Hypogonadism in Children with a Previous History of Cancer: Endocrine Management and Follow-Up. Horm. Res. Paediatr. 2019, 91, 93–103. [Google Scholar] [CrossRef]
- Claude, F.; Ubertini, G.; Szinnai, G. Endocrine Disorders in Children with Brain Tumors: At Diagnosis, after Surgery, Radiotherapy and Chemotherapy. Children 2022, 9, 1617. [Google Scholar] [CrossRef]
- Cornejo Ulloa, P.; Krom, B.P.; van der Veen, M.H. Sex Steroid Hormones as a Balancing Factor in Oral Host Microbiome Interactions. Front. Cell. Infect. Microbiol. 2021, 11, 714229. [Google Scholar] [CrossRef]
- Wactawski-Wende, J. Periodontal diseases and osteoporosis: Association and mechanisms. Ann. Periodontol. 2001, 6, 197–208. [Google Scholar] [CrossRef]
- Giro, G.; Chambrone, L.; Goldstein, A.; Rodrigues, J.A.; Zenobio, E.; Feres, M.; Figueiredo, L.C.; Cassoni, A.; Shibli, J.A. Impact of osteoporosis in dental implants: A systematic review. World J. Orthop. 2015, 6, 311–315. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Cochran, T.R.; Franco, V.I.; Miller, T.L. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat. Rev. Clin. Oncol. 2013, 10, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.; Jaiswal, R.; Sachdeva, S. Dental considerations in cardiovascular patients: A practical perspective. Indian Heart J. 2016, 68, 572–575. [Google Scholar] [CrossRef]
- Saini, R.; Saini, S.; Sharma, S. Periodontal disease linked to cardiovascular disease. J. Cardiovasc. Dis. Res. 2010, 1, 161–162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef] [PubMed]
- Little, J.W.; Miller, C.S.; Rhodus, N.L. Little and Falace’s Dental Management of the Medically Compromised Patient, 9th ed.; Elsevier, Inc.: St. Louis, MO, USA, 2018; p. xxxviii. 675p. [Google Scholar]
- Weil, B.R.; Madenci, A.L.; Liu, Q.; Howell, R.M.; Gibson, T.M.; Yasui, Y.; Neglia, J.P.; Leisenring, W.M.; Smith, S.A.; Tonorezos, E.S.; et al. Late Infection-Related Mortality in Asplenic Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. J. Clin. Oncol. 2018, 36, 1571–1578. [Google Scholar] [CrossRef]
- Hussein, H.; Brown, R.S. Risk-benefit assessment for antibiotic prophylaxis in asplenic dental patients. Gen. Dent. 2016, 64, 62–65. [Google Scholar]
- Brinkman, T.M.; Li, C.; Vannatta, K.; Marchak, J.G.; Lai, J.S.; Prasad, P.K.; Kimberg, C.; Vuotto, S.; Di, C.; Srivastava, D.; et al. Behavioral, Social, and Emotional Symptom Comorbidities and Profiles in Adolescent Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. J. Clin. Oncol. 2016, 34, 3417–3425. [Google Scholar] [CrossRef]
- Wogelius, P.; Rosthoj, S.; Dahllof, G.; Poulsen, S. Dental anxiety among survivors of childhood cancer: A cross-sectional study. Int. J. Paediatr. Dent. 2009, 19, 121–126. [Google Scholar] [CrossRef]
- Castellino, S.M.; Ullrich, N.J.; Whelen, M.J.; Lange, B.J. Developing interventions for cancer-related cognitive dysfunction in childhood cancer survivors. J. Natl. Cancer Inst. 2014, 106, dju186. [Google Scholar] [CrossRef]
- Norsker, F.N.; Pedersen, C.; Armstrong, G.T.; Robison, L.L.; McBride, M.L.; Hawkins, M.; Kuehni, C.E.; de Vathaire, F.; Berbis, J.; Kremer, L.C.; et al. Late Effects in Childhood Cancer Survivors: Early Studies, Survivor Cohorts, and Significant Contributions to the Field of Late Effects. Pediatr. Clin. N. Am. 2020, 67, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.B.; Chow, E.J.; Hjorth, L.; Hudson, M.M.; Kremer, L.C.M.; Morton, L.M.; Nathan, P.C.; Ness, K.K.; Oeffinger, K.C.; Armstrong, G.T. The Future of Childhood Cancer Survivorship: Challenges and Opportunities for Continued Progress. Pediatr. Clin. N. Am. 2020, 67, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, C.; McLoone, J.K.; Wakefield, C.E.; Cohn, R.J. Dental hygiene in childhood cancer survivors: The importance of tertiary long term follow-up care. Pediatr. Blood Cancer 2015, 62, 921. [Google Scholar] [CrossRef] [PubMed]
- Haupt, R.; Essiaf, S.; Dellacasa, C.; Ronckers, C.M.; Caruso, S.; Sugden, E.; Zadravec Zaletel, L.; Muraca, M.; Morsellino, V.; Kienesberger, A.; et al. The ‘Survivorship Passport’ for childhood cancer survivors. Eur. J. Cancer 2018, 102, 69–81. [Google Scholar] [CrossRef]
- Hudson, M.M.; Bhatia, S.; Casillas, J.; Landier, W.; Section on Hematology/Oncology, Children’s Oncology Group, American Society of Pediatric Hematology/Oncology. Long-term Follow-up Care for Childhood, Adolescent, and Young Adult Cancer Survivors. Pediatrics 2021, 148, e2021053127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ansari, S.; Stolze, J.; Bresters, D.; Brook, A.H.; Laheij, A.M.G.A.; Brand, H.S.; Dahllöf, G.; Rozema, F.R.; Raber-Durlacher, J.E. Late Complications in Long-Term Childhood Cancer Survivors: What the Oral Health Professional Needs to Know. Dent. J. 2024, 12, 17. https://doi.org/10.3390/dj12010017
Al-Ansari S, Stolze J, Bresters D, Brook AH, Laheij AMGA, Brand HS, Dahllöf G, Rozema FR, Raber-Durlacher JE. Late Complications in Long-Term Childhood Cancer Survivors: What the Oral Health Professional Needs to Know. Dentistry Journal. 2024; 12(1):17. https://doi.org/10.3390/dj12010017
Chicago/Turabian StyleAl-Ansari, Sali, Juliette Stolze, Dorine Bresters, Alan Henry Brook, Alexa M. G. A. Laheij, Henk S. Brand, Göran Dahllöf, Frederik R. Rozema, and Judith E. Raber-Durlacher. 2024. "Late Complications in Long-Term Childhood Cancer Survivors: What the Oral Health Professional Needs to Know" Dentistry Journal 12, no. 1: 17. https://doi.org/10.3390/dj12010017
APA StyleAl-Ansari, S., Stolze, J., Bresters, D., Brook, A. H., Laheij, A. M. G. A., Brand, H. S., Dahllöf, G., Rozema, F. R., & Raber-Durlacher, J. E. (2024). Late Complications in Long-Term Childhood Cancer Survivors: What the Oral Health Professional Needs to Know. Dentistry Journal, 12(1), 17. https://doi.org/10.3390/dj12010017







