Propolis Controlled Delivery Systems for Oral Therapeutics in Dental Medicine: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Research Question (PICO)
- P: the drug delivery systems DDSs
- I: application and efficacy of propolis in drug delivery systems, in dental medicine
- C: comparison between DDSs without propolis extracts and/or DDSs with other substances
- O: effectiveness of propolis-based DDSs for dental medicine
- S: clinical, in vitro and in vivo studies.
2.3. Eligibility Criteria and Selection Process
2.4. Information Sources and Search Strategy
2.5. Data Collection Process
2.6. Data Items
2.7. Study Risk of Bias Assessment and Synthesis of Results
3. Results
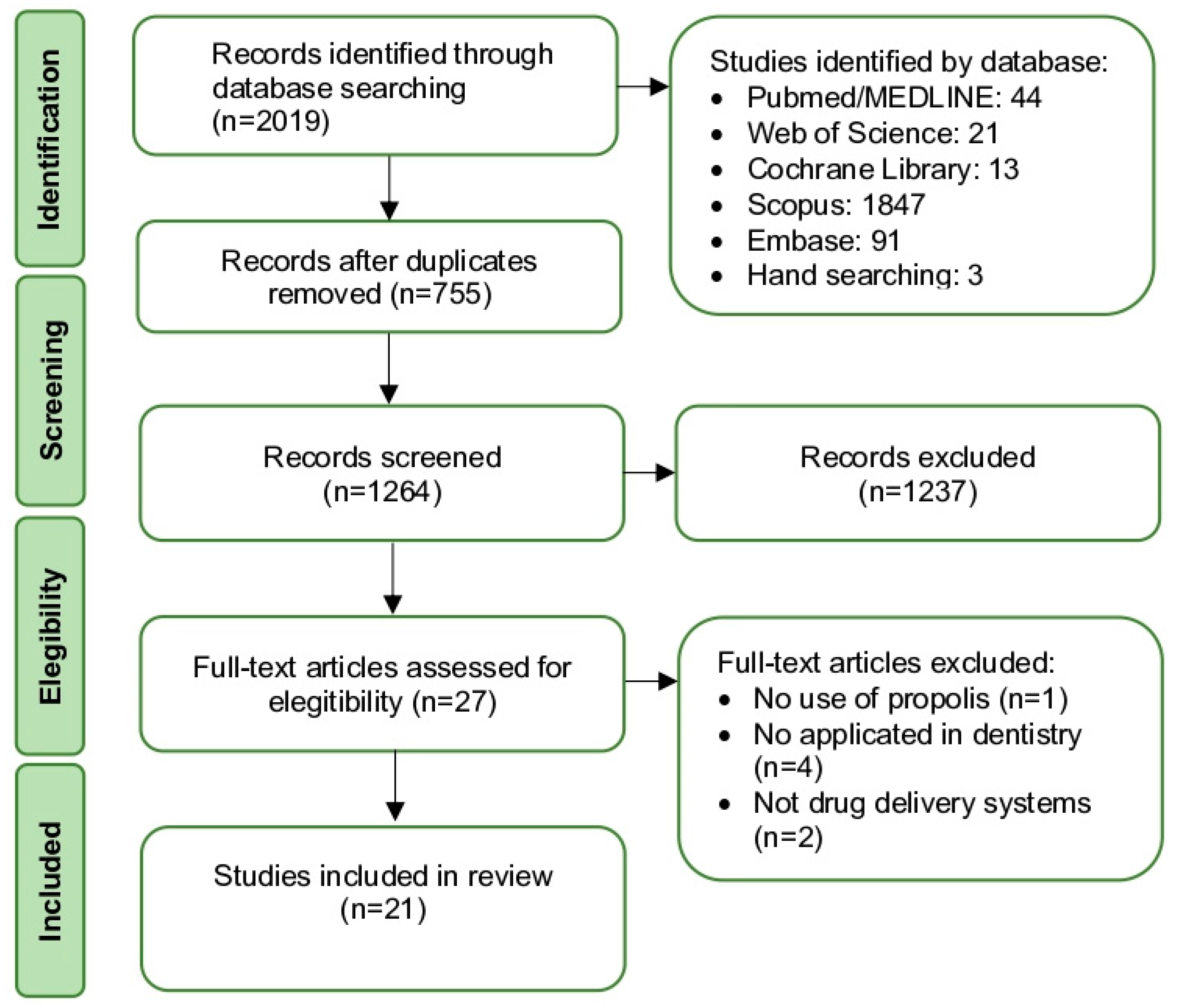
3.1. Study Selection
3.2. Study Characteristics
3.3. Results of Individual Studies and Results of Syntheses
3.4. Risk of Bias in Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Prim. 2017, 3, 17030. [Google Scholar] [CrossRef]
- Ahmed, G.M.; Abouauf, E.A.; AbuBakr, N.; Dörfer, C.E.; El-Sayed, K.F. Tissue engineering approaches for enamel, dentin, and pulp regeneration: An update. Stem. Cells Int. 2020, 2020, 5734539. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Hiorth, M. Advanced drug delivery systems for local treatment of the oral cavity. Ther Deliv. 2015, 6, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Current status and future prospects of drug delivery systems. Drug Deliv Syst. 2014, 1141, 1–56. [Google Scholar] [CrossRef]
- Costa, J.V.; Portugal, J.; Neves, C.B.; Bettencourt, A.F. Should local drug delivery systems be used in dentistry? Drug Deliv. Transl. Res. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Devi, V.K.; Jain, N.; Valli, K.S. Importance of novel drug delivery systems in herbal medicines. Pharmacogn. Rev. 2010, 4, 27. [Google Scholar] [PubMed]
- Sarangi, M.K.; Padhi, S. Novel herbal drug delivery system: An overview. Arch. Med. Health Sci. 2018, 6, 171. [Google Scholar] [CrossRef]
- Simu, M.R.; Pall, E.; Radu, T.; Miclaus, M.; Culic, B.; Mesaros, A.S.; Muntean, A.; Filip, G.A. Development of a novel biomaterial with an important osteoinductive capacity for hard tissue engineering. Tissue Cell 2018, 52, 101–107. [Google Scholar] [CrossRef]
- Fabri, F.V.; Cupertino, R.R.; Hidalgo, M.M.; de Oliveira, R.M.M.W.; Bruschi, M.L. Preparation and characterization of bioadhesive systems containing propolis or sildenafil for dental pulp protection. Drug Dev. Ind. Pharm. 2011, 37, 1446–1454. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.Y.; Woo, S.M.; Jeong, H.N.; Jung, J.Y.; Kim, S.M.; Lim, H.S. Combination of mineral trioxide aggregate and propolis promotes odontoblastic differentiation of human dental pulp stem cells through ERK signaling pathway. Food Sci. Biotechnol. 2019, 28, 1801–1809. [Google Scholar] [CrossRef]
- Bonadies, I.; Cimino, F.; Guarino, V. In vitro degradation of zein nanofibres for propolis release in oral treatments. Mater Res. Express 2019, 6, 075407. [Google Scholar] [CrossRef]
- Abdelsalam, N.; Abu-Seida, A.M.; Fayyad, D.; Tawfik, H. Radiographic and histopathologic outcomes of immature dog teeth with apical periodontitis after revascularization using propolis. An in vivo study. Saudi Endod. J. 2020, 10, 199. [Google Scholar]
- Barboza, A.S.; Aitken-Saavedra, J.P.; Ferreira, M.L.; Aranha, A.M.F.; Lund, R.G. Are propolis extracts potential pharmacological agents in human oral health?-A scoping review and technology prospecting. J. Ethnopharmacol. 2021, 271, 113846. [Google Scholar] [CrossRef]
- Chua, E.G.; Parolia, A.A.; Ahlawat, P.P.; Pau, A.; Amalraj, F.D. Antifungal effectiveness of various intracanal medicaments against Candida albicans: An ex-vivo study. BMC Oral Health 2014, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Franca, J.R.; De Luca, M.P.; Ribeiro, T.G.; Castilho, R.O.; Moreira, A.N.; Santos, V.R.; Faraco, A.A. Propolis-based chitosan varnish: Drug delivery, controlled release and antimicrobial activity against oral pathogen bacteria. BMC Complement. Altern. Med. 2014, 14, 478. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Naseem, M.; Zafar, M.S.; Najeeb, S.; Zohaib, S. Propolis: A natural biomaterial for dental and oral healthcare. J. Dent. Res. Dent. Clin. Dent. Prospect. 2017, 11, 265. [Google Scholar]
- Jautová, J.; Zelenková, H.; Drotarová, K.; Nejdková, A.; Grünwaldová, B.; Hladiková, M. Lip creams with propolis special extract GH 2002 0.5% versus aciclovir 5.0% for herpes labialis (vesicular stage). Wien. Med. Wochenschr. 2019, 169, 193–201. [Google Scholar] [CrossRef]
- Ahi, Z.B.; Renkler, N.Z.; Gul Seker, M.; Tuzlakoglu, K. Biodegradable polymer films with a natural antibacterial extract as novel periodontal barrier membranes. Int. J. Biomater. 2019, 2019, 7932470. [Google Scholar] [CrossRef]
- Son, J.S.; Hwang, E.J.; Kwon, L.S.; Ahn, Y.G.; Moon, B.K.; Kim, J.; Kim, D.H.; Kim, S.G.; Lee, S.Y. Antibacterial Activity of Propolis-Embedded Zeolite Nanocomposites for Implant Application. Materials 2021, 14, 1193. [Google Scholar] [CrossRef]
- Bruschi, M.L.; Rosseto, H.C.; de Francisco, L.M.B.; de Toledo, L.A.S.; Raphaela, R.R. Nanostructured propolis as therapeutic systems with antimicrobial activity. In Nano-and Microscale Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 377–391. [Google Scholar]
- Al-Bayaty, F.H.; bin Ismail, I.H.; binti Nasruddin, N.A.; binti Suradi, N.F. Formulation and Evaluation of new biodegradable periodontal chips from Malaysian propolis in chitosan base. J. Int. Dent. Med. Res. 2017, 10, 292. [Google Scholar]
- de Alcântara Sica de Toledo, L.; Rosseto, H.C.; Dos Santos, R.S.; Spizzo, F.; Del Bianco, L.; Montanha, M.C.; Esposito, E.; Kimura, E.; de Souza Bonfim-Mendonça, P.; Estivalet Svidzinski, T.I.; et al. Thermal magnetic field activated propolis release from liquid crystalline system based on magnetic nanoparticles. AAPS. Pharm. 2018, 19, 3258–3271. [Google Scholar] [CrossRef] [PubMed]
- Asawahame, C.; Sutjarittangtham, K.; Eitssayeam, S.; Tragoolpua, Y.; Sirithunyalug, B.; Sirithunyalug, J. Antibacterial activity and inhibition of adherence of Streptococcus mutans by propolis electrospun fibers. AAPS Pharm. 2015, 16, 182–191. [Google Scholar] [CrossRef] [PubMed]
- de Souza Ferreira, S.B.; de Assis Dias, B.R.; Obregón, C.S.; Gomes, C.C.; de Araújo Pereira, R.R.; Ribeiro Godoy, J.S.; Svidzinski, T.I.E.; Bruschi, M.L. Microparticles containing propolis and metronidazole: In vitro characterization, release study and antimicrobial activity against periodontal pathogens. Pharm. Dev. Technol. 2014, 19, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.G.; De Carvalho, R.A. Orally disintegrating films containing propolis: Properties and release profile. J. Pharm. Sci. 2015, 104, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Meimandi-Parizi, A.; Oryan, A.; Sayahi, E.; Bigham-Sadegh, A. Propolis extract a new reinforcement material in improving bone healing: An in vivo study. Int. J. Surg. 2018, 56, 94–101. [Google Scholar] [CrossRef]
- Kresnoadi, U.; Rahayu, R.P.; Ariani, M.D.; Soesanto, S. The potential of natural propolis extract combined with bovine bone graft in increasing heat shock protein 70 and osteocalcin on socket preservation. Eur. J. Dent. 2020, 14, 031–037. [Google Scholar] [CrossRef]
- Cardile, V.; Panico, A.; Gentile, B.; Borrelli, F.; Russo, A. Effect of propolis on human cartilage and chondrocytes. Life Sci. 2003, 73, 1027–1035. [Google Scholar] [CrossRef]
- Balata, G.F.; Abdelhady, M.I.; Mahmoud, G.M.; Matar, M.A.; Abd El-Latif, A.N. Formulation of saudi propolis into biodegradable chitosan chips for vital pulpotomy. Curr. Drug Deliv. 2018, 15, 97–109. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 88, 105906. [Google Scholar]
- Delgado, A.H.; Sauro, S.; Lima, A.F.; Loguercio, A.D.; Della Bona, A.; Mazzoni, A.; Collares, F.M.; Staxrud, F.; Ferracane, J.; Tsoi, J.; et al. RoBDEMAT: A risk of bias tool and guideline to support reporting of pre-clinical dental materials research and assessment of systematic reviews. J. Dent. 2022, 127, 104350. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, J.; Qazi, F.; Farooqui, W.; Ahmed, S.; Zehra, T.; Khurshid, Z. Effect of Chinese propolis as an intracanal medicament on post-operative endodontic pain: A double-blind randomized controlled trial. Int. J. Environm. Res. Public Health 2020, 17, 445. [Google Scholar] [CrossRef] [PubMed]
- Zenouz, A.T.; Mehdipour, M.; Abadi, R.T.A.; Shokri, J.; Rajaee, M.; Aghazadeh, M.; Golizadeh, N. Effect of use of propolis on serum levels of il-17 and clinical symptoms and signs in patients with ulcerative oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Rad. 2015, 119, e166–e167. [Google Scholar] [CrossRef]
- Aslani, A.; Malekpour, N. Design, formulation, and physicochemical evaluation of periodontal propolis mucoadhesive gel. Dent. Res. J. 2016, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Samet, N.; Laurent, C.; Susarla, S.M.; Samet-Rubinsteen, N. The effect of bee propolis on recurrent aphthous stomatitis: A pilot study. Clin. Oral Investig. 2005, 11, 143–147. [Google Scholar] [CrossRef]
- Arafa, M.G.; Ghalwash, D.; El-Kersh, D.M.; Elmazar, M.M. Propolis-based niosomes as oromuco-adhesive films: A randomized clinical trial of a therapeutic drug delivery platform for the treatment of oral recurrent aphthous ulcers. Sci. Rep. 2018, 8, 18056. [Google Scholar] [CrossRef]
- Delavarian, Z.; Paakfetrat, A.; Nazari, F.; Tonekaboni, A.; Shakeri, M.T. An investigation of the effects propolis on the recurrent oral aphthous ulcers. Avicen. J. Phytomed. 2015, 5, 113. [Google Scholar]
- Ali, H.S.; Abdul Rasool, B.K. Propolis buccal paste in treatment of aphthous ulceration: Formulation and clinical evaluation. Asian J. Pharm. Clin. Res. 2011, 4, 29–33. [Google Scholar]
- Madhubala, M.M.; Srinivasan, N.; Ahamed, S. Comparative evaluation of propolis and triantibiotic mixture as an intracanal medicament against Enterococcus faecalis. J. Endod. 2011, 37, 1287–1289. [Google Scholar] [CrossRef]
- Şenel, S.; Aksoy, E.A.; Akca, G. Application of chitosan based scaffolds for drug delivery and tissue engineering in dentistry. In Marine-Derived Biomaterials for Tissue Engineering Applications; Springer: Singapore, 2019; pp. 157–178. [Google Scholar]
- Rahman, H.S.; Othman, H.H.; Hammadi, N.I.; Yeap, S.K.; Amin, K.M.; Samad, N.A.; Alitheen, N.B. Novel drug delivery systems for loading of natural plant extracts and their biomedical applications. Int. J. Nanomed. 2020, 15, 2439–2483. [Google Scholar] [CrossRef] [PubMed]
- De Rezende, G.P.D.S.R.; Costa, L.R.D.R.S.D.; Pimenta, F.C.; Baroni, D.A. In vitro antimicrobial activity of endodontic pastes with propolis extracts and calcium hydroxide: A preliminary study. Braz. Dent. J. 2008, 19, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Andrade, Â.L.; Manzi, D.; Domingues, R.Z. Tetracycline and propolis incorporation and release by bioactive glassy compounds. J. Non-Cryst. Solids 2006, 352, 3502–3507. [Google Scholar] [CrossRef]
- Parolia, A.; Kundabala, M.; Rao, N.N.; Acharya, S.R.; Agrawal, P.; Mohan, M.; Thomas, M. A comparative histological analysis of human pulp following direct pulp capping with Propolis, mineral trioxide aggregate and Dycal. Aust. Dent. J. 2010, 55, 59–64. [Google Scholar] [CrossRef]
- Ozório, J.E.V.; de Oliveira, D.A.; de Sousa-Neto, M.D.; Perez, D.E.D.C. Standardized propolis extract and calcium hydroxide as pulpotomy agents in primary pig teeth. J. Dent. Child. 2012, 79, 53–58. [Google Scholar]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Sabir, A. Using propolis as A therapeutic agent in dentistry. Cakradonya Dent. J. 2012, 4, 480–486. [Google Scholar]
| Electronic Database Search and Terms |
| PubMed (MEDLINE) #1 Propolis [MeSH] OR Bee Glue OR Glue, Bee OR Bee Bread OR Bread, Bee #2 Tissue Scaffolds [MeSH] OR Scaffold, Tissue OR Scaffolds, Tissue OR Tissue Scaffold OR Tissue Scaffolding OR Scaffolding, Tissue OR Scaffoldings, Tissue OR Tissue Scaffoldings OR Scaffold OR Drug Delivery Systems [MeSH] OR Delivery System, Drug OR Delivery Systems, Drug OR Drug Delivery System OR System, Drug Delivery OR Systems, Drug Delivery OR Drug Targeting OR Drug Targetings OR Targeting, Drug OR Targetings, Drug OR Nanofibers [MeSH] OR Nanofiber OR Nanospheres [MeSH] OR Nanosphere OR Hydrogels [MeSH] OR Hydrogel OR Injectable #3 Guided Tissue Regeneration [MeSH] OR Tissue Regeneration, Guided OR Regeneration, Guided Tissue OR Dentistry OR Dental OR Regenerative Dentistry OR Dental, Regenerative OR Periodontics [MeSH] OR Periodontic OR Periodontal Medicine OR Medicine, Periodontal OR Medicines, Periodontal OR Periodontal Medicines OR Periodontal OR Periodontal Regeneration OR Periodontal Engineering OR Oral Bone Regeneration OR Periapical Tissue OR Regenerative Endodontics [MeSH] OR Endodontic, Regenerative OR Endodontics, Regenerative OR Regenerative Endodontic OR Aphthous Stomatitis OR Mucosal Lesions #1 AND #2 AND #3 |
| Scopus #1 ALL(“Propolis” OR “Bee Glue” OR “Glue, Bee” OR “Bee Bread” OR “Bread, Bee”) #2 ALL(“Tissue Scaffolds” OR “Scaffold, Tissue” OR “Scaffolds, Tissue” OR “Tissue Scaffold” OR “Tissue Scaffolding” OR “Scaffolding, Tissue” OR “Scaffoldings, Tissue” OR “Tissue Scaffoldings” OR “Scaffold” OR “Drug Delivery Systems” OR “Delivery System, Drug” OR “Delivery Systems, Drug” OR “Drug Delivery System” OR “System, Drug Delivery” OR “Systems, Drug Delivery” OR “Drug Targeting” OR “Drug Targetings” OR “Targeting, Drug” OR “Targetings, Drug” OR “Nanofibers” OR “Nanofiber” OR “Nanospheres” OR “Nanosphere” OR “Hydrogels” OR “Hydrogel” OR “Injectable”) #3 ALL(“Guided Tissue Regeneration” OR “Tissue Regeneration, Guided” OR “Regeneration, Guided Tissue” OR “Dentistry” OR “Dental” OR “Regenerative Dentistry” OR “Dental, Regenerative” OR “Periodontics” OR “Periodontic” OR “Periodontal Medicine” OR “Medicine, Periodontal” OR “Medicines, Periodontal” OR “Periodontal Medicines” OR “Periodontal” OR “Periodontal Regeneration” OR “Periodontal Engineering” OR “Oral Bone Regeneration” OR “Periapical Tissue” OR “Regenerative Endodontics” OR “Endodontic, Regenerative” OR “Endodontics, Regenerative” OR “Regenerative Endodontic” OR “Aphthous Stomatitis” OR “Mucosal Lesions”) #1 AND #2 AND #3 |
| Embase #1 ‘Propolis’ OR ‘Bee Glue’ OR ‘Glue, Bee’ OR ‘Bee Bread’ OR ‘Bread, Bee’ #2 ‘Tissue Scaffolds’ OR ‘Scaffold, Tissue’ OR ‘Scaffolds, Tissue’ OR ‘Tissue Scaffold’ OR ‘Tissue Scaffolding’ OR ‘Scaffolding, Tissue’ OR ‘Scaffoldings, Tissue’ OR ‘Tissue Scaffoldings’ OR ‘Scaffold’ OR ‘Drug Delivery Systems’ OR ‘Delivery System, Drug’ OR ‘Delivery Systems, Drug’ OR ‘Drug Delivery System’ OR ‘System, Drug Delivery’ OR ‘Systems, Drug Delivery’ OR ‘Drug Targeting’ OR ‘Drug Targetings’ OR ‘Targeting, Drug’ OR ‘Targetings, Drug’ OR ‘Nanofibers’ OR ‘Nanofiber’ OR ‘Nanospheres’ OR ‘Nanosphere’ OR ‘Hydrogels’ OR ‘Hydrogel’ OR ‘Injectable’ #3 ‘Guided Tissue Regeneration’ OR ‘Tissue Regeneration, Guided’ OR ‘Regeneration, Guided Tissue’ OR ‘Dentistry’ OR ‘Dental’ OR ‘Regenerative Dentistry’ OR ‘Dental, Regenerative’ OR ‘Periodontics’ OR ‘Periodontic’ OR ‘Periodontal Medicine’ OR ‘Medicine, Periodontal’ OR ‘Medicines, Periodontal’ OR ‘Periodontal Medicines’ OR ‘Periodontal’ OR ‘Periodontal Regeneration’ OR ‘Periodontal Engineering’ OR ‘Oral Bone Regeneration’ OR ‘Periapical Tissue’ OR ‘Regenerative Endodontics’ OR ‘Endodontic, Regenerative’ OR ‘Endodontics, Regenerative’ OR ‘Regenerative Endodontic’ OR ‘Aphthous Stomatitis’ OR ‘Mucosal Lesions’ #1 AND #2 AND #3 |
| Web of Science TS = (Propolis OR Bee Glue OR Glue, Bee OR Bee Bread OR Bread, Bee) TS = (Tissue Scaffolds OR Scaffold, Tissue OR Scaffolds, Tissue OR Tissue Scaffold OR Tissue Scaffolding OR Scaffolding, Tissue OR Scaffoldings, Tissue OR Tissue Scaffoldings OR Scaffold OR Drug Delivery Systems OR Delivery System, Drug OR Delivery Systems, Drug OR Drug Delivery System OR System, Drug Delivery OR Systems, Drug Delivery OR Drug Targeting OR Drug Targetings OR Targeting, Drug OR Targetings, Drug OR Nanofibers OR Nanofiber OR Nanospheres OR Nanosphere OR Hydrogels OR Hydrogel OR Injectable) TS = (Guided Tissue Regeneration OR Tissue Regeneration, Guided OR Regeneration, Guided Tissue OR Dentistry OR Dental OR Regenerative Dentistry OR Dental, Regenerative OR Periodontics OR Periodontic OR Periodontal Medicine OR Medicine, Periodontal OR Medicines, Periodontal OR Periodontal Medicines OR Periodontal OR Periodontal Regeneration OR Periodontal Engineering OR Oral Bone Regeneration OR Periapical Tissue OR Regenerative Endodontics OR Endodontic, Regenerative OR Endodontics, Regenerative OR Regenerative Endodontic OR Aphthous Stomatitis OR Mucosal Lesions) #1 AND #2 AND #3 |
| Cochrane #1 Propolis OR Bee Glue OR Glue, Bee OR Bee Bread OR Bread, Bee #2 Tissue Scaffolds OR Scaffold, Tissue OR Scaffolds, Tissue OR Tissue Scaffold OR Tissue Scaffolding OR Scaffolding, Tissue OR Scaffoldings, Tissue OR Tissue Scaffoldings OR Scaffold OR Drug Delivery Systems OR Delivery System, Drug OR Delivery Systems, Drug OR Drug Delivery System OR System, Drug Delivery OR Systems, Drug Delivery OR Drug Targeting OR Drug Targetings OR Targeting, Drug OR Targetings, Drug OR Nanofibers OR Nanofiber OR Nanospheres OR Nanosphere OR Hydrogels OR Hydrogel OR Injectable #3 Guided Tissue Regeneration OR Tissue Regeneration, Guided OR Regeneration, Guided Tissue OR Dentistry OR Dental OR Regenerative Dentistry OR Dental, Regenerative OR Periodontics OR Periodontic OR Periodontal Medicine OR Medicine, Periodontal OR Medicines, Periodontal OR Periodontal Medicines OR Periodontal OR Periodontal Regeneration OR Periodontal Engineering OR Oral Bone Regeneration OR Periapical Tissue OR Regenerative Endodontics OR Endodontic, Regenerative OR Endodontics, Regenerative OR Regenerative Endodontic OR Aphthous Stomatitis OR Mucosal Lesions #1 AND #2 AND #3 |
| Study | Country of Study | Study Design | Objective | Application | Biomaterial | Type of Propolis | Propolis Origin | Characterization of Propolis | Drug Release | Toxicity | Controls | Simple Size | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [8] | Romania | In vitro study | Designed a composite biomaterial based on a high viscosity soft propolis extract (70% propolis) and shell clam, with antiseptic and osteoinductive qualifies for hard tissue regeneration. | Mineralized tissue engineering that can be used in dentistry | Bioactive cement with antiseptic and osteoinductive qualities | Aqueous extract 70% | Commercial 70% soft propolis extract, Bioremed, Romania. | Scanning electronic microscopy (SEM), reversed-phase liquid chromatography (HPLC) with diode array detection, proliferation assay, and differentiation assay | Not available | Cell viability and adhesion level with human dental papilla cell line. | The cells cultured in normal culture condition (DMEM-F12 (Sigma-Aldrich) supplemented with 10% FCS (Hyclone), 1% Antibiotic-antimycotic (Sigma-Aldrich) | n = 3 | The shell-propolis-based biomaterial promoted and sustained human stem cells attachment, proliferation, and differentiation, presenting an important osteoinductive effect essential for the mineralized tissue reparation process. |
| [9] | Brazil | In vitro study | Development and characterization of semisolid systems containing propolis or sildenafil prepared from Carbopol 934P and poloxamer 407 | Endodontics in pulp protection | Binary polymeric systems containing poloxamer 407 (P407) and Carbopol 934P (C934P) were designed to deliver propolis extract (PE) | Ethanolic extract | Propolis was collected from an experimental apiary in the farm of the State University of Maringa (Parana State, Brazil). | Rheological analysis by ViscoStar− Plus R controlled shear rate rotating viscometer | 50% in 500 min | Not available | Sildenafil citrate was purchased from Pfizer (Dongcheng District, Beijing, China) | n = 3 for all tests, except bioadhesive strength (n = 5) | The data obtained in these formulations indicated a potentially useful role in pulp protection, however, clinical evaluation is necessary. |
| [11] | Italy | In vitro study | The optimization of the electrospinning process to fabricate zein electrospun nanofibres (ZN) loaded with propolis (PZN). | Tissue inflammation in the presence of oral lesions | Electrospun fibers | Hydroalcoholic solution | Informed only the location Erbaflor, Italy | Not available | 70% in 24 h | Not available | Pure propolis | n = 20 | The zein nanofibers can guarantee a sustained release of propolis directly to the target, providing a more efficient solution for treatments based on the administration of ‘one shot’ of the active ingredient, minimizing side effects. |
| [14] | Malaysia | In vitro study | The antifungal activity of propolis, triple antibiotic paste (TAP), 2% chlorhexidine gel, and calcium hydroxide with propylene glycol was evaluated on root canal dentinal tubules. | Candida albicans-infected root canal dentinal tubules | Intracanal medicaments | Ethanolic extract | Stakich, Royal Oak, Michigan, USA | Not available | Not available | Not available | Triple antibiotic paste (TAP), 2% chlorhexidine gel and calcium hydroxide with propylene glycol | n = 18 | Propolis demonstrated comparable efficacy to triple antibiotic paste, 2% chlorhexidine gel, and calcium hydroxide with propylene glycol in inhibiting the growth of C. albicans at both depths over a period of 7 days. |
| [15] | Brazil | In vitro study | Antimicrobial activity of sustained-release propolis-based chitosan varnish useful on dental cariogenic biofilm prevention | Anti-cariogenic agent | Propolis—based chitosan varnish | Ethanolic extract | Green propolis was collected from commercial beekeeping named Pharmanéctar® in Minas Gerais State, Brazil. | Provided by fabricant PharmaNectar, Brazil, 2007, not described the techniques was used | 20% in 24 h | Not available | Chlorhexidine 0.12%, chitosan-based varnish and nystatin | n = 5 | Sustained-release chitosan-based propolis varnishes (5%, 10%, and 15%) inhibited all tested microorganisms, deserving clinical studies to confirm it is in vivo activity. |
| [18] | Turkey | In vitro study | Produce barrier membranes from biodegradable polymers, namely, PLLA and PCL, with an antibacterial feature promoted by propolis. | Guided tissue regeneration in periodontology | Biodegradable polymer films | Ethyl alcoholic extract | Not informed | Not available | Not available | Not available | Antibiotic disk including 30 μgr chloramphenicol (C30) (HIMEDIA) | n = 5 | Propolis has a positive influence on the thermal, mechanical, and degradation properties of the blend films to achieve the required values for GTR. Also, films with propolis showed antibacterial activity against Gram (+) bacteria. |
| [19] | Korea | In vitro study | The potential of propolis-embedded zeolite nanocomposites for dental implant application. | Dental implants | Propolis-embedded zeolite nanocomposites | Aqueous extract | Propolis extracts were purchased from Rapha Propolis Co., (Jeonju, Korea). | Fourier transform-infrared spectra (FT-IR) | 90% in 30 days | MTT cell cytotoxicity assay | PLA/PCL pellets containing propolis-embedded zeolite nanocomposites | Not informed | Eluted propolis solution from PLA/PCL pellets showed significant antibacterial efficacy against C. albicans, S. mutans, and S. sobrinus. |
| [20] | Brazil | In vitro study | Development and characterization of semisolid systems containing propolis prepared from carbomer 934P and poloxamer 407 (P407) | Periodontal pocket for the treatment of periodontitis | Semisolid Systems | Ethanolic extract | Propolis was collected from an experimental apiary in the farm of the State University of Maringa (Parana State, Brazil) | Not available | 80% in 168 h | Not available | Formulations without propolis microparticles | n = 5 | The release profile studies showed that propolis could be released from the systems for an extended period (more than 7 days). The properties of the candidate formulations indicate a potential advantageous role in the treatment of periodontal diseases. |
| [21] | Malaysia | In vitro study | Formulated periodontal chips from Malaysian propolis in chitosan base and to evaluate the physical, biological and antibacterial properties. | Treatment of chronic periodontitis | Biodegradable periodontal chips | Ethanolic extract | Raw propolis purchased from Ayer Keroh, Malacca, Malaysia | Not available | 80% in 6 days | Not available | Chlorhexidine (0.2%, w/v) and ethanol (20%, w/v) | n = 15 for all tests, except surface morphology and thickness (n = 35) | Malaysian propolis can be evaluated into a chip and be used in treating patients with periodontal disease. It was found to be biodegradable have a high release rate, and have antimicrobial activity against gram-positive and gram-negative bacteria. |
| [22] | Brazil | In vitro study | Development of a novel liquid crystalline system containing MNPs and propolis | Periodontal pockets | A liquid crystalline system containing iron oxide magnetic nanoparticles (MNPs) | Ethanolic extract | Propolis was acquired from the Iguatemi Experimental Farm of the State University of Maringa, Parana state, Brazil. | Folin-Ciocalteu method | 36% in 120 h | Cytotoxicity by micro-crustacean Artemia salina and fibroblasts cell line. | A system without propolis | n = 6 | The system containing propolis and magnetic nanoparticles displays important in vitro fungicide activity, which was increased when an alternating external magnetic field was applied, indicating a potential alternative therapy for the treatment of periodontal disease. |
| [23] | Thailand | In vitro study | Antibacterial activity against Streptococcus mutans and the inhibition of adhesion on a smooth glass surface during the biofilm formation was tested. | Mouth-dissolving dosage form and as an anti-cariogenic agent | Propolis-PVP electrospun fibers | Ethanolic extract 5% (w/v) | Propolis was obtained from Chiangmai Healthy Product Co., Ltd. (Chiangmai, Thailand). | Not available | Not available | Not available | Chlorhexidine mouthwash solution (0.12%) 1 mg/mL | n = 8 (SEM); n = 3 (antimicrobial assays) | The results indicated the potential of electrospun fibers to be used as mouth-dissolving fibers for effective antibacterial activity in the oral cavity. |
| [24] | Brazil | In vitro study | The antimicrobial activity of microparticles was evaluated against some microorganisms of periodontal importance. | Periodontal pockets | Ethylcellulose microparticles | Ethanolic extract | Three samples of propolis from Apis mellifera L. beehives were collected at apiaries in the Northeast of Paraná state, Brazil. | Determination of total flavonoid content and, determination of total phenol content by Folin-Ciocalteu method | 20% in 32 h | Not available | Metronidazole | n = 3 for all tests, except the determination of total flavonoid content and total phenol content (n = 6) | The strains of Enterococcus faecalis, Streptococcus pyogenes, and Streptococcus mutans were more susceptible to the propolis and E. faecalis to the metronidazole. |
| [25] | Brazil | In vitro study | Production and characterization of orally disintegrating films from gelatin and hydrolyzed collagen containing the ethanol extract of propolis. | Control oral infection | Films of gelatin and hydrolyzed collagen | Ethanolic extract | 12-type resin (Star Rigel Raf- ~ fard, Sao Paulo, Brazil) | Folin–Ciocalteau method, Fourier transform infrared spectroscopy (FTIR) and, scanning electron microscopy | 80% in 15 min | Not available | Films without the ethanol extract of propolis | n = 10 for all tests, except SEM (n = 16); in vitro release and antimicrobial assay (n = 3) | The ethanol extract of propolis produced the antimicrobial activity in the film as well as provided a better resistance matrix and increased mucoadhesiveness. |
| [29] | Egypt | In vitro study and animal model | The formulation of commercial Saudi propolis into biodegradable chitosan chips and evaluation of its effectiveness as a pulpotomy agent. | Treatment of vital pulpotomy | Saudi Propolis into biodegradable chitosan chips | Ethanolic extract | Propolis (El Akbr)® was obtained from a honey bee market located in Jeddah, Saudi Arabia (El Maher shop, Wadi El Nahil Co., Taeif, Saudi Arabia). | Determination of total phenolic content, determination of total flavonoid content, determination of the antioxidant activity of the extract, quantification of polyphenolic constituents | 35% in 7 days | Histopathological evaluation | Formocresol | n = 6 (in vitro); n = 18 (animal model) | Formulation of propolis extract as chitosan biodegradable chips can be used effectively for local sustained propolis delivery into the infected periodontal pockets, as it results in the production of higher quality secondary dentin with the less inflammatory response of the pulp. |
| [34] | Pakistan | Randomized clinical trial | Assessing the effect of Chinese propolis paste as an intracanal medicament on postoperative endodontic pain intensity | Endodontics treat in necrotic teeth with periapical radiolucency | Paste | Propolis powder | FMBP. Henan Fumei Biotechnology Co., Ltd., Changge, China, reg no.: 411082100010933 | Not available | Not available | Not available | Calcium hydroxide 20%. | n = 40 | The effect of propolis was found to be comparable to the calcium hydroxide group in managing postoperative endodontic pain, with no reported adverse effects. |
| [35] | India | Randomized clinical trial | Assess the effectiveness of applying topical propolis for the treatment of oral lichen planus. | Oral lichen planus: | Topical propolis | Not informed | Not informed | Not available | Not available | Not available | Triamcinolone acetonide 0.1% | n = 27 | Topical propolis demonstrated comparable effectiveness to triamcinolone acetonide 0.1% in managing oral lichen planus (OLP). |
| [36] | Iran | In vitro study | A mucoadhesive gel formulation incorporating a concentrated extract of propolis was developed for the treatment of periodontitis. | Periodontitis | Mucoadhesive gel | Propolis particles | Agricultural Research Center (Isfahan, Iran) | Folin–Ciocalteu method for determination of polyphenol contents. Aluminum chloride colorimetric method was used to determine flavonoid content | 80% em 7 days | Not available | Tetracycline disc (30 µg/mL) | n = 3 | Drug release assay demonstrated that propolis exhibited a prolonged release from the system, lasting more than 7 days. Additionally, propolis exhibited a substantial growth inhibition zone against Porphyromonas gingivalis. |
| [37] | USA | Randomized clinical trial | Daily ingestion of one 500-mg capsule of propolis will reduce the frequency of outbreaks of recurrent aphthous stomatitis | Aphthous ulcers | 500-mg capsule of propolis | Incapsuled | Vitamin World | Not available | Not available | Not available | Placebo capsule of a calcium-based food supplement | n = 10 (propolis group) n = 9 (placebo group) | Daily ingestion of 500 mg of propolis can potentially reduce the frequency of aphthous ulcer episodes, particularly those who have not found relief through alternative treatment methods. |
| [38] | Egypt | Clinical and in vitro studies | Treating aphthous ulceration by maintaining a therapeutic level of the active ingredient in the mouth for a prolonged period of time and enhancing drug absorption | Aphthous ulcers | Niosomal oromuco-adhesive films | Commercial propolis | Imtenan Health Co., Egypt | The content of total flavonoid compounds was determined by an aluminum chloride colorimetric assay. The content of total phenolic compounds was determined by the Folin-Ciocalteau assay | 64% in 8 h | Not available | Placebo group | n = 3 (in vitro); n = 24 (clinical study) | In the group receiving medication, the reduction in ulcer size was observed as early as the second and third day of treatment. Complete healing was achieved within the first 10 days, and the pain relief lasted for more than 4–5 h, which was in stark contrast to the placebo group. |
| [39] | Iran | Randomized clinical trial | Assess the potential impact of this product in reducing the occurrence of recurrent aphthous ulcers. | Aphthous ulcers | 500-mg capsule of propolis | Incapsuled | Not informed | Not available | Not available | Not available | Placebo group | n = 22 | Propolis group exhibited a lower number of relapses compared to the placebo group. Moreover, significant reductions in the number and size of lesions, pain levels, and recovery time were observed in the propolis group. |
| [40] | United Arab Emirates | Clinical and in vitro studies | Formulations of buccal pastes containing propolis were developed and subjected to both pharmaceutical and clinical evaluations for the treatment of recurrent aphthous stomatitis. | Aphthous ulcers | Buccal paste | Ethanolic extract | Hajj Seed local farms (Dubai, UAE) | Not available | Not available | Not available | Control formula (placebo) | n = 3 (in vitro); n = 120 (clinical study) | The healing rate of aphthous ulcers was significantly higher compared to the placebo group. Furthermore, the size of the ulcers decreased within the first day of application. Patients in the propolis groups experienced a significant reduction in pain intensity within the initial 24 h, with 90% of patients reporting relief, compared to only 35% in the placebo group. |
| D1. Bias in Planning and Allocation | D2. Bias in Sample/Specimen Preparation | D3. Bias in Outcome Assessment | D4. Bias in Data Treatment and Outcome Reporting | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Authors, Year | 1.1 Control Group | 1.2 Randomizatio n of Samples | 1.3 Sample Size Rationale and Reporting | 2.1 Standardizatio n of Samples and Materials | 2.2 Identical Experimental Conditions across Groups | 3.1 Adequate and Standardized Testing Procedures and Outcomes | 3.2 Blinding of the Testing Operator | 4.1 Statistical Analysis | 4.2 Reporting Study Outcomes |
| [8] Simu et al., 2018 | Sufficiently Reported | Not reported | Not reported | Insufficiently reported | Insufficiently reported | Sufficiently reported | Not reported | Insufficiently reported | Insufficiently reported |
| [9] Fabri et al., 2011 | Sufficiently Reported | Not reported | Not reported | Insufficiently Reported | Sufficiently reported | Sufficiently reported | Not reported | Sufficiently reported | Sufficiently reported |
| [11] Bonadies et al., 2019 | Sufficiently reported | Not reported | Not reported | Sufficiently reported | Sufficiently reported | Sufficiently reported | Not reported | Not reported | Insufficiently reported |
| [14] Chua et al., 2014 | Sufficiently Reported | Not reported | Not reported | Sufficiently Reported | Sufficiently reported | Insufficiently reported | Not reported | Sufficiently reported | Sufficiently reported |
| [15] Franca et al., 2014 | Sufficiently Reported | Not reported | Not reported | Sufficiently Reported | Sufficiently reported | Sufficiently reported | Not reported | Insufficiently reported | Insufficiently reported |
| [18] Ahi et al., 2019 | Sufficiently Reported | Not reported | Not reported | Sufficiently reported | Sufficiently reported | Sufficiently reported | Not reported | Not reported | Insufficiently reported |
| [19] Son et al., 2021 | Sufficiently reported | Not reported | Not reported | Sufficiently reported | Insufficiently reported | Sufficiently reported | Not reported | Not reported | Insufficiently reported |
| [20] Bruschi et al., 2013 | Sufficiently Reported | Not reported | Not reported | Sufficiently Reported | Sufficiently reported | Sufficiently reported | Not reported | Sufficiently reported | Sufficiently reported |
| [21] Al-Bayaty et al., 2017 | Sufficiently Reported | Not reported | Not reported | Sufficiently Reported | Insufficiently reported | Sufficiently reported | Not reported | Insufficiently reported | Insufficiently reported |
| [22] de Alcântara Sica de Toledo et al., 2018 | Sufficiently Reported | Not reported | Not reported | Sufficiently reported | Sufficiently reported | Sufficiently reported | Not reported | Sufficiently reported | Sufficiently reported |
| [23] Asawahame et al., 2014 | Sufficiently Reported | Not reported | Not reported | Insufficiently Reported | Sufficiently reported | Insufficiently reported | Not reported | Not reported | Insufficiently reported |
| [24] de Souza Ferreira et al., 2013 | Sufficiently Reported | Not reported | Not reported | Sufficiently Reported | Insufficiently reported | Insufficiently reported | Not reported | Not reported | Insufficiently reported |
| [25] Borges et al., 2015 | Sufficiently Reported | Not reported | Not reported | Insufficiently Reported | Sufficiently reported | Sufficiently reported | Not reported | Not reported | Insufficiently reported |
| [29] Balata et al., 2018 | Sufficiently Reported | Not reported | Not reported | Sufficiently Reported | Sufficiently reported | Insufficiently reported | Not reported | Not reported | Insufficiently reported |
| [37] Arafa et al., 2018 | Not reported | Not reported | Not reported | Inufficiently reported | Sufficiently reported | Sufficiently reported | Not reported | Sufficiently reported | Sufficiently reported |
| [39] Ali and Abdul Rasool, 2011 | Sufficiently reported | Not reported | Not reported | Sufficiently reported | Sufficiently reported | Sufficiently reported | Not reported | Insufficiently reported | Insufficiently reported |
| [41] Aslani and Malekpour, 2016 | Sufficiently reported | Not reported | Not reported | Sufficiently reported | Sufficiently reported | Sufficiently reported | Not reported | Not reported | Insufficiently reported |
| Author, Year | 1.1 Bias due to Counfounding | 1.2 Bias in Selection of Participants into the Study | 2.1 Bias in Classification of Interventions | 3.1 Bias due to Deviations from Intended Interventions | 3.2 Bias due to Missing Data | 3.3 Bias in Measurement of Outcomes | 3.4 Bias in Selection of the Reported Result |
|---|---|---|---|---|---|---|---|
| [39] Ali and Rassol, 2011 | Low | Low | Low | Low | Low | Low | Low |
| Author, Year | 1. Randomization Process | 2. Deviations from Intended Interventions | 3. Missing Outcome Data | 4. Measurement of the Outcome | 5. Selection of the Reported Result | Overall Risk of Bias |
|---|---|---|---|---|---|---|
| [34] Shabbir et al., 2020 | Low | Some concerns | Some concerns | Low | Low | Some concerns |
| [35] Zenouz et al., 2015 | Low | Some concerns | Some concerns | Low | Low | Some concerns |
| [36] Samet et al., 2005 | Some concerns | Some concerns | Some concerns | Low | Low | Some concerns |
| [37] Arafa et al., 2018 | Low | Some concerns | Some concerns | Low | Low | Some concerns |
| [38] Delavarian et al., 2020 | Some concerns | Some concerns | Some concerns | Low | Low | Some concerns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barboza, A.d.S.; Ribeiro de Andrade, J.S.; Ferreira, M.L.; Peña, C.L.D.; da Costa, J.S.; Fajardo, A.R.; Lund, R.G. Propolis Controlled Delivery Systems for Oral Therapeutics in Dental Medicine: A Systematic Review. Dent. J. 2023, 11, 162. https://doi.org/10.3390/dj11070162
Barboza AdS, Ribeiro de Andrade JS, Ferreira ML, Peña CLD, da Costa JS, Fajardo AR, Lund RG. Propolis Controlled Delivery Systems for Oral Therapeutics in Dental Medicine: A Systematic Review. Dentistry Journal. 2023; 11(7):162. https://doi.org/10.3390/dj11070162
Chicago/Turabian StyleBarboza, Andressa da Silva, Juliana Silva Ribeiro de Andrade, Monika Lamas Ferreira, Carla Lucía David Peña, Juliê Silveira da Costa, André Ricardo Fajardo, and Rafael Guerra Lund. 2023. "Propolis Controlled Delivery Systems for Oral Therapeutics in Dental Medicine: A Systematic Review" Dentistry Journal 11, no. 7: 162. https://doi.org/10.3390/dj11070162
APA StyleBarboza, A. d. S., Ribeiro de Andrade, J. S., Ferreira, M. L., Peña, C. L. D., da Costa, J. S., Fajardo, A. R., & Lund, R. G. (2023). Propolis Controlled Delivery Systems for Oral Therapeutics in Dental Medicine: A Systematic Review. Dentistry Journal, 11(7), 162. https://doi.org/10.3390/dj11070162








