Phosphoric Acid Etch Partially Restores the Initial Bond Strength of Composite to Silver Diamine Fluoride–Treated Enamel Using Universal Adhesives
Abstract
1. Introduction
2. Materials and Methods
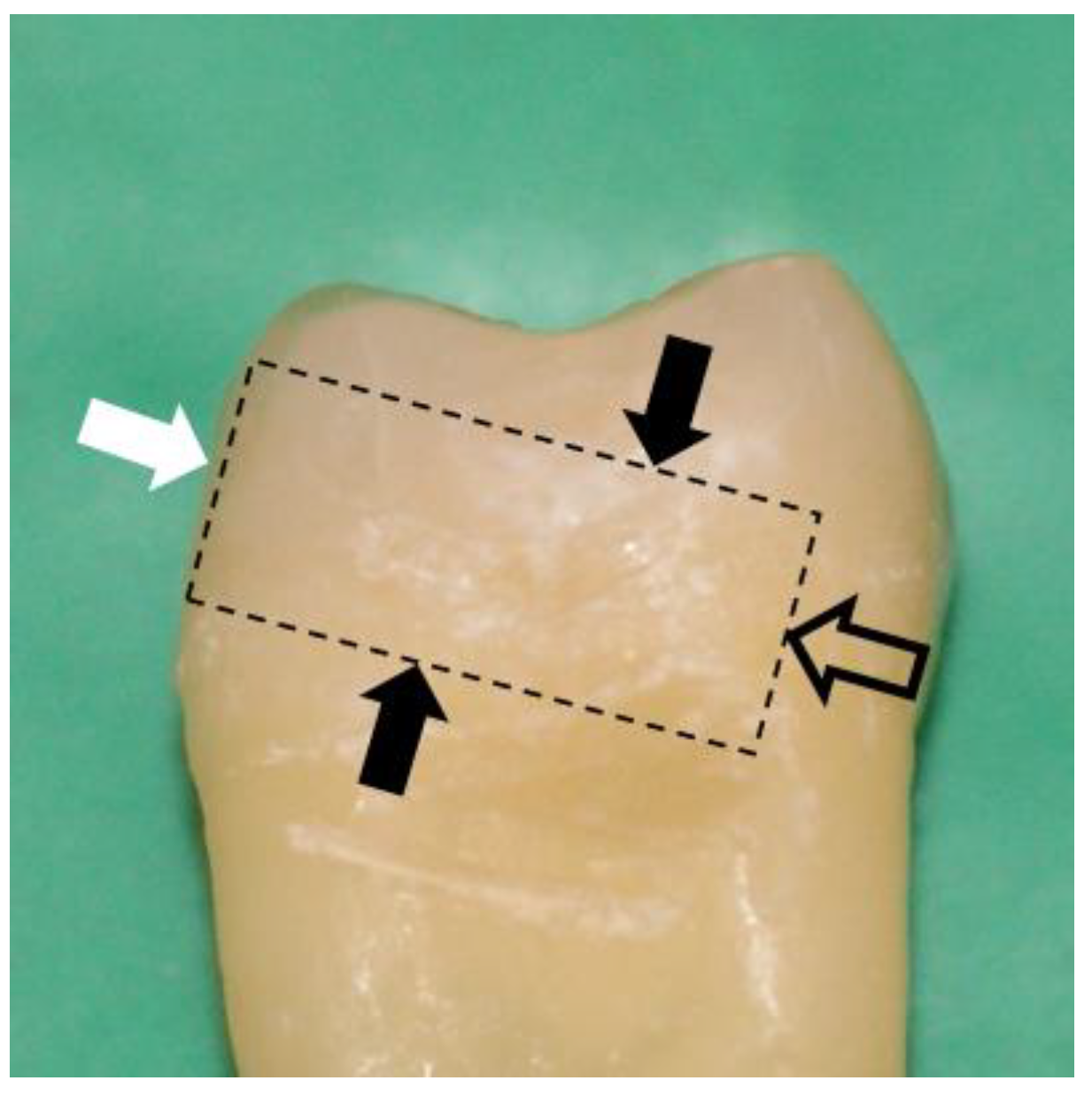
2.1. Sample Preparation
2.2. Micro-Tensile Bond Strength (μTBS) Test
2.3. Statistical Analyses
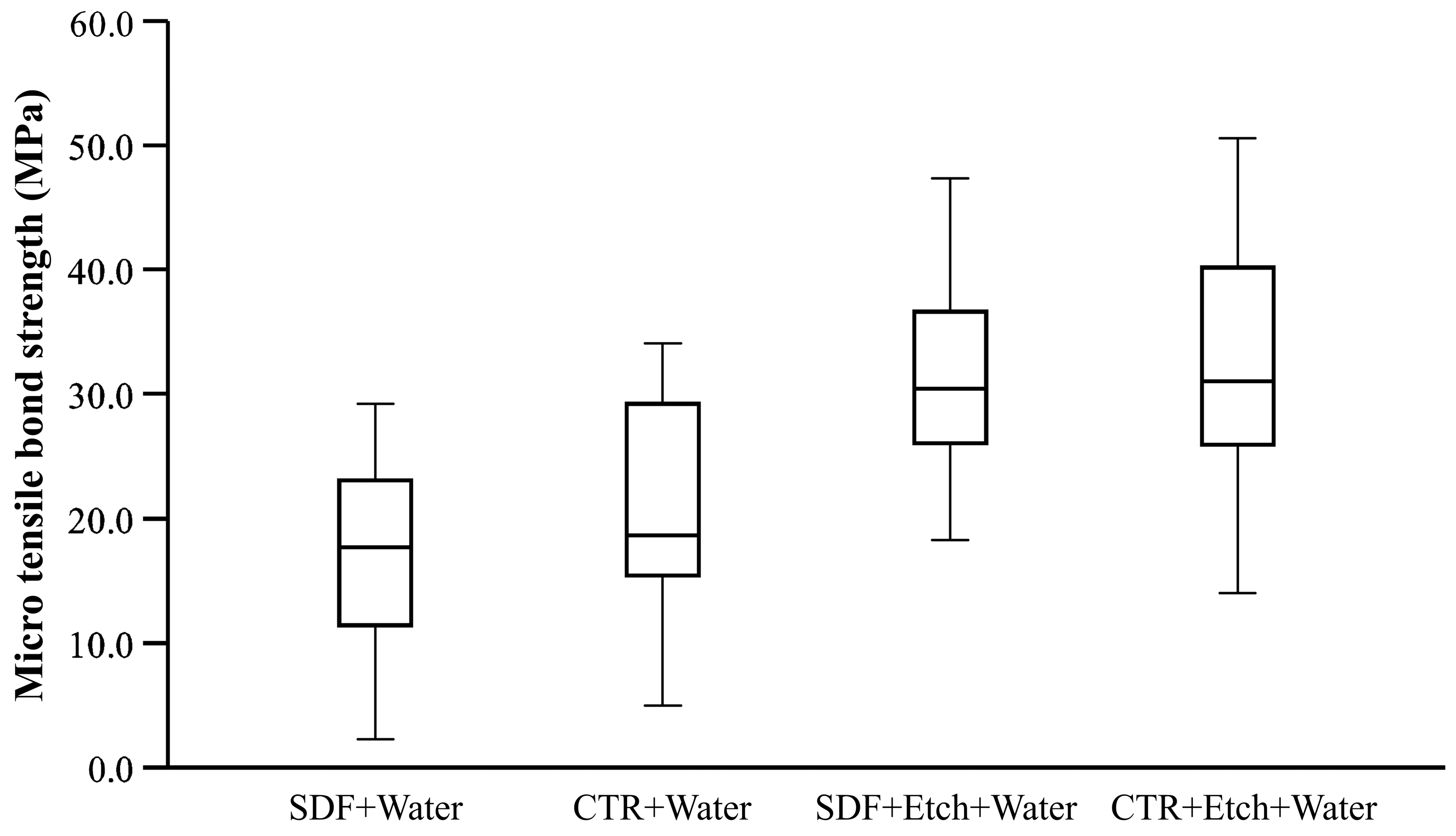
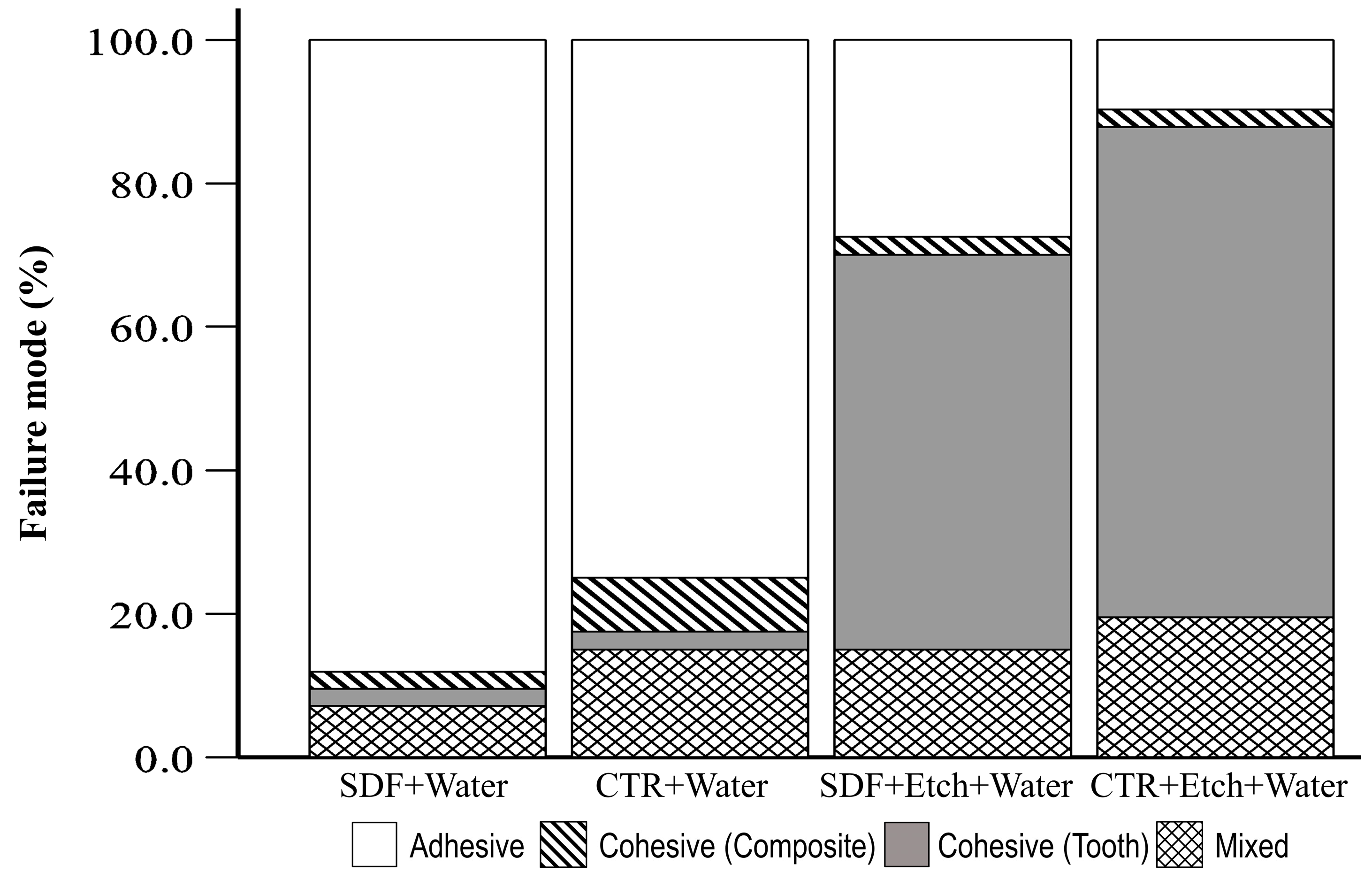
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, P.Y.F.; Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M. Global Burden and Inequality of Dental Caries, 1990 to 2019. J. Dent. Res. 2021, 101, 392–399. [Google Scholar] [CrossRef]
- Urquhart, O.; Tampi, M.P.; Pilcher, L.; Slayton, R.L.; Araujo, M.W.B.; Fontana, M.; Guzman-Armstrong, S.; Nascimento, M.M.; Novy, B.B.; Tinanoff, N.; et al. Nonrestorative Treatments for Caries: Systematic Review and Network Meta-analysis. J. Dent. Res. 2019, 98, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Baraka, M.; Tekeya, M.; Bakry, N.S.; Fontana, M. Twelve-month randomized controlled trial of 38% silver diamine fluoride with or without potassium iodide in indirect pulp capping of young permanent molars. J. Am. Dent. Assoc. 2022, 153, 1121–1133.e1. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, D.N.; Kidd, E.A.; Innes, N.; Clarkson, J. Complete or ultraconservative removal of decayed tissue in unfilled teeth. Cochrane Database Syst. Rev. 2006, 3, Cd003808. [Google Scholar] [CrossRef]
- Hilton, T.J. Keys to clinical success with pulp capping: A review of the literature. Oper. Dent. 2009, 34, 615–625. [Google Scholar] [CrossRef]
- Mertz-Fairhurst, E.J.; Curtis, J.W., Jr.; Ergle, J.W.; Rueggeberg, F.A.; Adair, S.M. Ultraconservative and cariostatic sealed restorations: Results at year 10. J. Am. Dent. Assoc. 1998, 129, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Turkistani, A.; Nakashima, S.; Shimada, Y.; Tagami, J.; Sadr, A. Microgaps and Demineralization Progress around Composite Restorations. J. Dent. Res. 2015, 94, 1070–1077. [Google Scholar] [CrossRef]
- Markham, M.D.; Tsujimoto, A.; Barkmeier, W.W.; Jurado, C.A.; Fischer, N.G.; Watanabe, H.; Baruth, A.G.; Latta, M.A.; Garcia-Godoy, F. Influence of 38% silver diamine fluoride application on bond stability to enamel and dentin using universal adhesives in self-etch mode. Eur. J. Oral Sci. 2020, 128, 354–360. [Google Scholar] [CrossRef]
- Camacho, K.J.; English, J.D.; Jacob, H.B.; Harris, L.M.; Kasper, F.K.; Bussa, H.I.; Quock, R.L. Silver diamine fluoride and bond strength to enamel in vitro: A pilot study. Am. J. Dent. 2018, 31, 317–319. [Google Scholar]
- Favaro, J.C.; de Mello Peixoto, Y.C.T.; Geha, O.; Dias, F.A.; Guiraldo, R.D.; Lopes, M.B.; Berger, S.B. Can silver diamine fluoride or silver nanoparticle-based anticaries agents to affect enamel bond strength? Restor. Dent. Endod. 2021, 46, e7. [Google Scholar] [CrossRef]
- Fröhlich, T.T.; Botton, G.; Rocha, R.O. Bonding of Glass-Ionomer Cement and Adhesives to Silver Diamine Fluoride-treated Dentin: An Updated Systematic Review and Meta-Analysis. J. Adhes. Dent. 2022, 24, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, T.T.; Rocha, R.O.; Botton, G. Does previous application of silver diammine fluoride influence the bond strength of glass ionomer cement and adhesive systems to dentin? Systematic review and meta-analysis. Int. J. Paediatr. Dent. 2020, 30, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Quock, R.L.; Barros, J.A.; Yang, S.W.; Patel, S.A. Effect of silver diamine fluoride on microtensile bond strength to dentin. Oper. Dent. 2012, 37, 610–616. [Google Scholar] [CrossRef] [PubMed]
- El Mourad, A.M. Assessment of Bonding Effectiveness of Adhesive Materials to Tooth Structure using Bond Strength Test Methods: A Review of Literature. Open Dent. J. 2018, 12, 664–678. [Google Scholar] [CrossRef]
- Armstrong, S.; Geraldeli, S.; Maia, R.; Raposo, L.H.A.; Soares, C.J.; Yamagawa, J. Adhesion to tooth structure: A critical review of “micro” bond strength test methods. Dent. Mater. 2010, 26, e50–e62. [Google Scholar] [CrossRef]
- Armstrong, S.; Breschi, L.; Ozcan, M.; Pfefferkorn, F.; Ferrari, M.; Van Meerbeek, B. Academy of Dental Materials guidance on in vitro testing of dental composite bonding effectiveness to dentin/enamel using micro-tensile bond strength (muTBS) approach. Dent. Mater. 2017, 33, 133–143. [Google Scholar] [CrossRef]
- Sadek, F.T.; Monticelli, F.; Muench, A.; Ferrari, M.; Cardoso, P.E.C. A novel method to obtain microtensile specimens minimizing cut flaws. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 78B, 7–14. [Google Scholar] [CrossRef]
- Elevate Oral Care LLC. Safety Data Sheet. Available online: https://www.elevateoralcare.com/site/images/AASDS082415.pdf (accessed on 18 June 2023).
- BISCO Dental Inc. Safety Data Sheet. Available online: https://www.bisco.com/assets/1/22/All_Bond_Universal_SDS_US_English2.pdf (accessed on 18 June 2023).
- Ultradent Products Inc. Safety Data Sheet. Available online: https://www.ultradent.com/Resources/GetSds?key=7-001-20-en-us (accessed on 18 June 2023).
- Elevate Oral Care LLC. Advantage Arrest Instructions. Available online: https://www.elevateoralcare.com/site/images/AABottle_DFU_031218.pdf (accessed on 18 June 2023).
- Johnston, C. Bonding to molars—The effect of etch time (an in vitro study). Eur. J. Orthod. 1998, 20, 195–199. [Google Scholar] [CrossRef]
- Eliades, G.; Watts, D.C.; Eliades, T. Dental Hard Tissues and Bonding Interfacial Phenomena and Related Properties, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Daugherty, M.M.; Lien, W.; Mansell, M.R.; Risk, D.L.; Savett, D.A.; Vandewalle, K.S. Effect of high-intensity curing lights on the polymerization of bulk-fill composites. Dent. Mater. 2018, 34, 1531–1541. [Google Scholar] [CrossRef]
- BISCO Dental Inc. All-Bond-Universal Instructions. Available online: https://www.bisco.com/assets/1/22/All-Bond_Universal_Rev_10-21.pdf (accessed on 18 June 2023).
- Kim, M.; Kim, R.H.; Lee, S.C.; Lee, T.K.; Hayashi, M.; Yu, B.; Jo, D.W. Evaluation of Tensile Bond Strength between Self-Adhesive Resin Cement and Surface-Pretreated Zirconia. Materials 2022, 15, 3089. [Google Scholar] [CrossRef]
- Lutgen, P.; Chan, D.; Sadr, A. Effects of silver diammine fluoride on bond strength of adhesives to sound dentin. Dent. Mater. J. 2018, 37, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Eckert, G.J.; Platt, J.A. A statistical evaluation of microtensile bond strength methodology for dental adhesives. Dent. Mater. 2007, 23, 385–391. [Google Scholar] [CrossRef]
- Yee, R.; Holmgren, C.; Mulder, J.; Lama, D.; Walker, D.; van Palenstein Helderman, W. Efficacy of silver diamine fluoride for Arresting Caries Treatment. J. Dent. Res. 2009, 88, 644–647. [Google Scholar] [CrossRef]
- Moszner, N.; Salz, U.; Zimmermann, J. Chemical aspects of self-etching enamel–dentin adhesives: A systematic review. Dent. Mater. 2005, 21, 895–910. [Google Scholar] [CrossRef]
- Yoshihara, K.; Yoshida, Y.; Nagaoka, N.; Fukegawa, D.; Hayakawa, S.; Mine, A.; Nakamura, M.; Minagi, S.; Osaka, A.; Suzuki, K. Nano-controlled molecular interaction at adhesive interfaces for hard tissue reconstruction. Acta Biomater. 2010, 6, 3573–3582. [Google Scholar] [CrossRef]
- Ko, A.K.O.; Matsui, N.; Nakamoto, A.; Ikeda, M.; Nikaido, T.; Burrow, M.F.; Tagami, J. Effect of silver diammine fluoride application on dentin bonding performance. Dent. Mater. J. 2020, 39, 407–414. [Google Scholar] [CrossRef]
- Yoshihara, K.; Nagaoka, N.; Okihara, T.; Kuroboshi, M.; Hayakawa, S.; Maruo, Y.; Nishigawa, G.; De Munck, J.; Yoshida, Y.; Van Meerbeek, B. Functional monomer impurity affects adhesive performance. Dent. Mater. 2015, 31, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, H.; Hamama, H.H.; Burrow, M.F. Effect of a silver diamine fluoride and potassium iodide-based desensitizing and cavity cleaning agent on bond strength to dentine. Int. J. Adhes. Adhes. 2016, 68, 54–61. [Google Scholar] [CrossRef]
- Wang, C.; Ou, Y.; Zhang, L.; Zhou, Z.; Li, M.; Xu, J.; Fan, J.; Fu, B.; Hannig, M. Effects of regional enamel and prism orientations on bovine enamel bond strength and cohesive strength. Eur. J. Oral Sci. 2018, 126, 334–342. [Google Scholar] [CrossRef] [PubMed]
- BISCO Dental Inc. Bisedent Globe. Available online: https://www.bisco.com/assets/1/18/bisdent_globe__universal_adhesives.pdf (accessed on 18 June 2023).
- Horst, J.A.; Ellenikiotis, H.; Milgrom, P.L. UCSF Protocol for Caries Arrest Using Silver Diamine Fluoride: Rationale, Indications and Consent. J. Calif. Dent. Assoc. 2016, 44, 16–28. [Google Scholar] [CrossRef]
- Jabbour, Z.; Esmaeili, M.; Hayashi, M.; Kim, R. Radiographic Changes to Silver Diamine Fluoride Treated Carious Lesions after a Rinsing Step. Dent. J. 2022, 10, 149. [Google Scholar] [CrossRef] [PubMed]

| Product | pH | Ingredients | Manufacturer |
|---|---|---|---|
| Advantage Arrest (SDF 38%) | 10–11 | Silver diamine fluoride (Ag(NH3)2F) (30–50%); FD&C blue #1 (<1%); deionized water (≤62.5%) | Elevate Oral Care, West Palm Beach, FL, USA |
| All-Bond Universal (unit dose) | 3.2 | Bisphenol A diglycidylmethacrylate (20–50%); ethanol (30–50%); MDP (5–25%); 2-hydroxyethyl methacrylate (5–25%) | BISCO, Schaumburg, IL, USA |
| Ultra-Etch (Phosphoric acid etch 35%) | <1 | Phosphoric acid (≥25–<40%); polyethylene glycol (1–10%); dimethicone (≥0.1–<10%); other | Ultradent, San Fernando, CA, USA |
| Group | Premolar | Molar | Total Beams | Pre-Test Failure | Glue Failure | Manipulation Error | Total Beams Included |
|---|---|---|---|---|---|---|---|
| SDF+Water | 2 | 4 | 43 | 4 | 1 | 42 | |
| CTR+Water | 2 | 4 | 41 | 1 | 1 | 40 | |
| SDF+Etch+Water | 2 | 4 | 41 | 41 | |||
| CTR+Etch+Water | 2 | 4 | 43 | 2 | 41 |
| Group | Estimated | Standard Error | 95% Confidence Interval | |
|---|---|---|---|---|
| Mean | Lower Bound | Upper Bound | ||
| SDF+Water a | 16.34 | 1.14 | 14.08 | 18.60 |
| CTR+Water b | 20.61 | 1.17 | 18.30 | 22.92 |
| SDF+Etch+Water c | 31.90 | 1.16 | 29.62 | 34.18 |
| CTR+Etch+Water c | 32.21 | 1.16 | 29.91 | 34.50 |
| Group | Tooth Type | Adhesive | Cohesive (Composite) | Cohesive (Tooth) | Mixed | Total | p |
|---|---|---|---|---|---|---|---|
| SDF+Water | Premolar | 11 (91.7) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 12 (100) | 0.836 |
| Molar | 26 (86.7) | 1 (3.3) | 1 (3.3) | 2 (6.7) | 30 (100) | ||
| CTR+Water | Premolar | 11 (78.6) | 0 (0.0) | 0 (0.0) | 3 (21.4) | 14 (100) | 0.426 |
| Molar | 19 (73.1) | 3 (11.5) | 1 (3.8) | 3 (11.5) | 26 (100) | ||
| SDF+Etch+Water | Premolar | 3 (25.0) | 0 (0.0) | 5 (41.7) | 4 (33.3) | 12 (100) | 0.319 |
| Molar | 8 (27.6) | 1 (3.4) | 17 (58.6) | 3 (10.3) | 29 (100) | ||
| CTR+Etch+Water | Premolar | 1 (7.7) | 1 (7.7) | 9 (69.2) | 2 (15.4) | 13 (100) | 0.493 |
| Molar | 3 (10.7) | 0 (0.0) | 19 (67.9) | 6 (21.4) | 28 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabbour, Z.; Kim, M.; Hayashi, M.; Kim, R. Phosphoric Acid Etch Partially Restores the Initial Bond Strength of Composite to Silver Diamine Fluoride–Treated Enamel Using Universal Adhesives. Dent. J. 2023, 11, 161. https://doi.org/10.3390/dj11070161
Jabbour Z, Kim M, Hayashi M, Kim R. Phosphoric Acid Etch Partially Restores the Initial Bond Strength of Composite to Silver Diamine Fluoride–Treated Enamel Using Universal Adhesives. Dentistry Journal. 2023; 11(7):161. https://doi.org/10.3390/dj11070161
Chicago/Turabian StyleJabbour, Zaher, Mijoo Kim, Marc Hayashi, and Reuben Kim. 2023. "Phosphoric Acid Etch Partially Restores the Initial Bond Strength of Composite to Silver Diamine Fluoride–Treated Enamel Using Universal Adhesives" Dentistry Journal 11, no. 7: 161. https://doi.org/10.3390/dj11070161
APA StyleJabbour, Z., Kim, M., Hayashi, M., & Kim, R. (2023). Phosphoric Acid Etch Partially Restores the Initial Bond Strength of Composite to Silver Diamine Fluoride–Treated Enamel Using Universal Adhesives. Dentistry Journal, 11(7), 161. https://doi.org/10.3390/dj11070161






