Imaging Evaluation of Platelet-Rich Fibrin in Post-Exodontic Bone Regeneration: A Systematic Review
Abstract
:1. Introduction
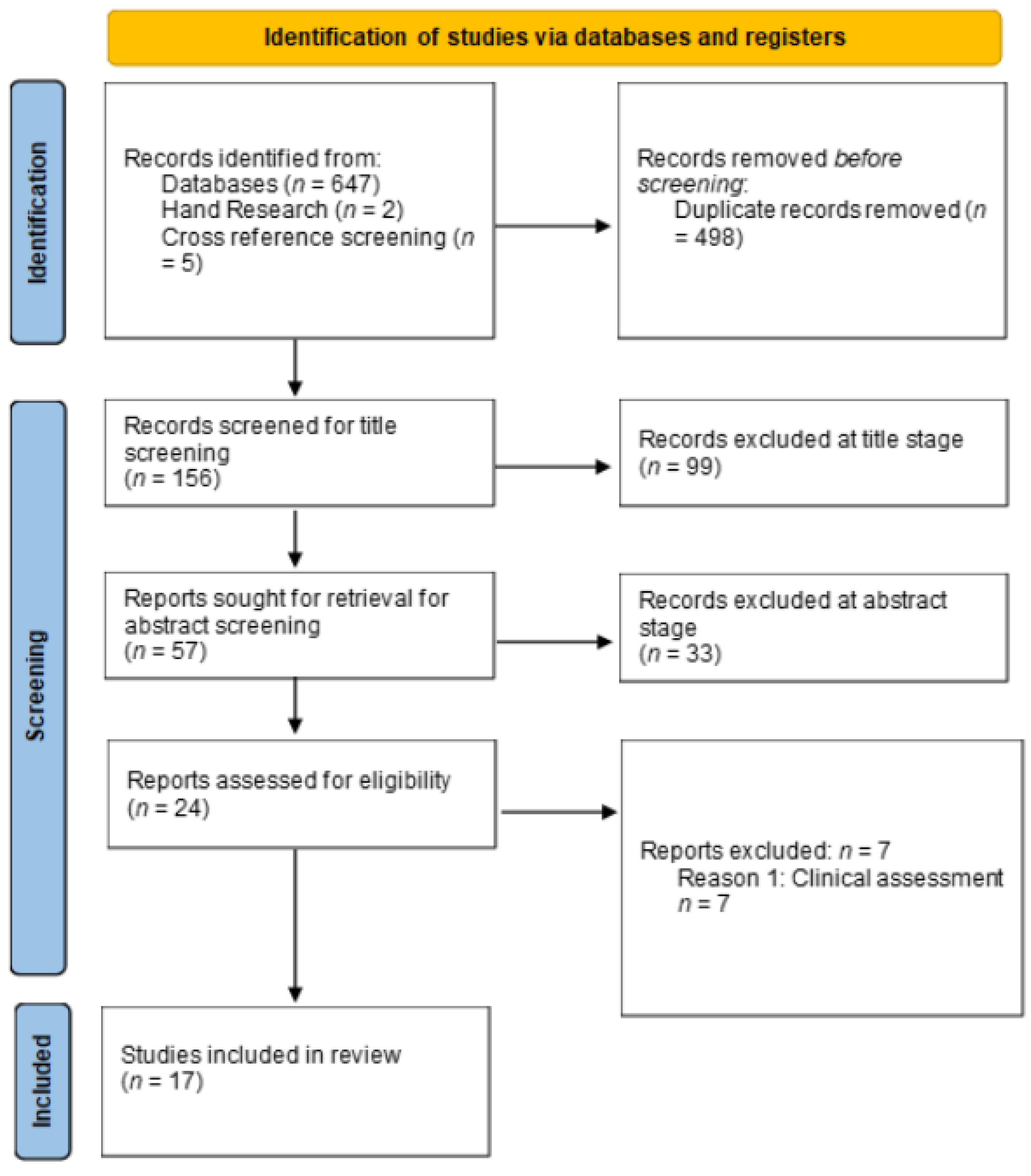
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Types of Included Studies
2.3. Search Strategy
2.4. Data Extraction and Quality Assessment
3. Results
3.1. Details of the Included Studies
3.2. Description of the Included Interventional Studies
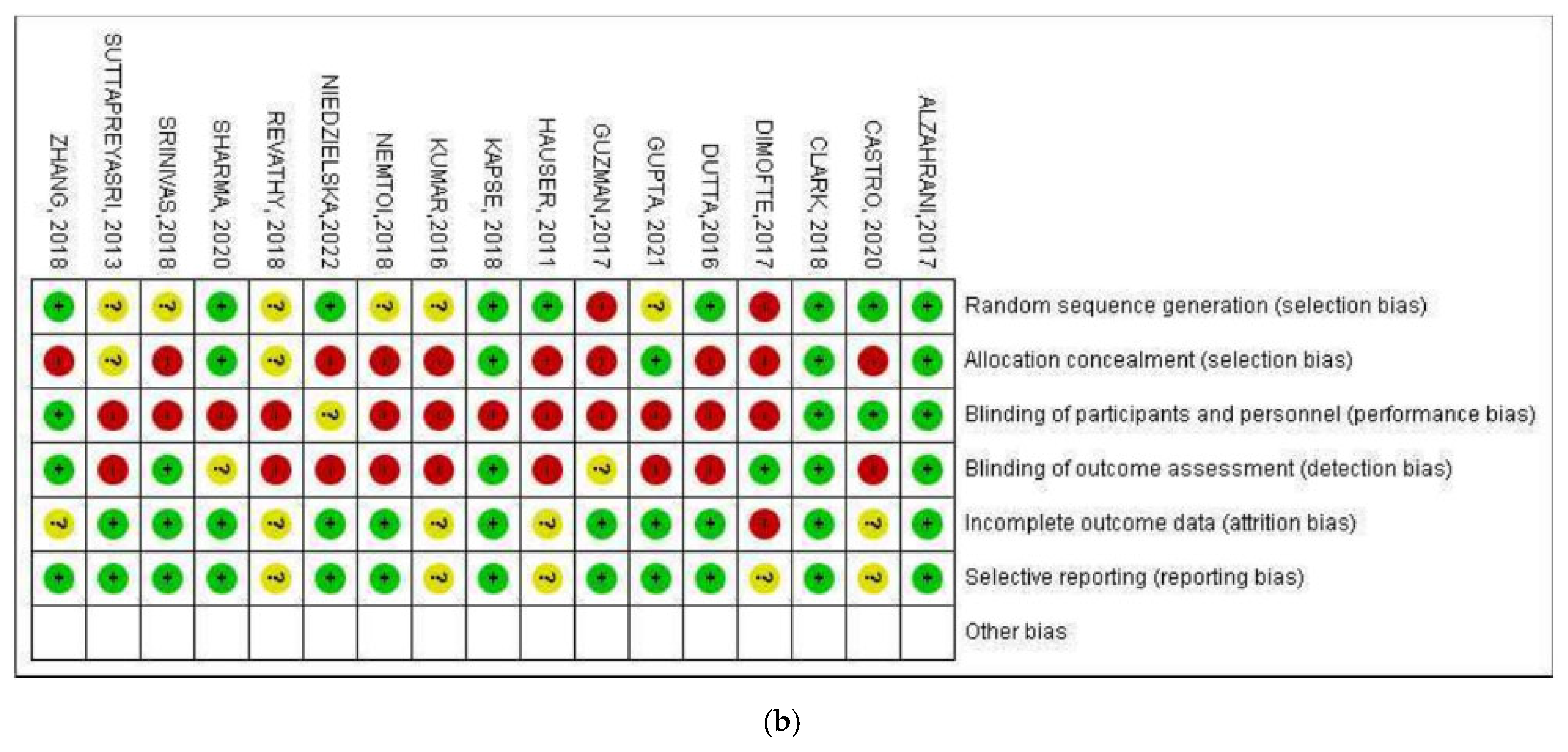
3.3. Quality Assessment of the Included Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Revathy, N.S.; Kannan, R.; Karthik, R.S.; Kumar, M.S.S.; Munshi, M.A.I.; Vijay, R. Comparative study on alveolar bone healing in postextraction socket versus healing aided with autologous platelet-rich fibrin following surgical removal of bilateral mandibular impacted third molar tooth: A radiographic evaluation. Natl. J. Maxillofac. Surg. 2018, 9, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Suttapreyasri, S.; Leepong, N. Influence of platelet-rich fibrin on alveolar ridge preservation. J. Craniofacial Surg. 2013, 24, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ruan, Z.; Shen, M.; Tan, L.; Huang, W.; Wang, L.; Huang, Y. Clinical effect of platelet-rich fibrin on the preservation of the alveolar ridge following tooth extraction. Exp. Ther. Med. 2018, 15, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Al-Maawi, S.; Becker, K.; Schwarz, F.; Sader, R.; Ghanaati, S. Efficacy of platelet-rich fibrin in promoting the healing of extraction sockets: A systematic review. Int. J. Implant Dent. 2021, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.R.; Mohanty, S.; Verma, M.; Kaur, R.R.; Bhatia, P.; Kumar, V.R.; Chaudhary, Z. Platelet-rich fibrin: The benefits. Br. J. Oral Maxillofac. Surg. 2016, 54, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Starzyńska, A.; Kaczoruk-Wieremczuk, M.; Lopez, M.A.; Passarelli, P.C.; Adamska, P. The growth factors in advanced platelet-rich fibrin (A-PRF) reduce postoperative complications after mandibular third molar odontectomy. Int. J. Environ. Res. Public Health 2021, 18, 13343. [Google Scholar] [CrossRef]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1998, 85, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Pawar, V.; Khurshid, Z.; Almasri, M.A.; Rudrappa, S.K.; Karthick, B.P.; Beshir, S.E.M.; Mokli, L.K. Influence of platelet concentrates on postextraction socket healing: A literature review. World J. Dent. 2022, 13, 176–180. [Google Scholar] [CrossRef]
- Arya, V.; Malhotra, V.L.; Rao, J.D.; Kirti, S.; Malhotra, S.; Sharma, R.S. Reduction in post extraction waiting period for dental implant patients using plasma rich in growth factors: An in vivo study using cone-beam computed tomography. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 285–293. [Google Scholar] [CrossRef]
- Castro, A.B.; Van Dessel, J.; Temmerman, A.; Jacobs, R.; Quirynen, M. Effect of different platelet-rich fibrin matrices for ridge preservation in multiple tooth extractions: A split-mouth randomized controlled clinical trial. J. Clin. Periodontol. 2021, 48, 984–995. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e56–e60. [Google Scholar] [CrossRef] [PubMed]
- Anwandter, A.; Bohmann, S.; Nally, M.; Castro, A.B.; Quirynen, M.; Pinto, N. Dimensional changes of the post extraction alveolar ridge, preserved with Leukocyte- and Platelet Rich Fibrin: A clinical pilot study. J. Dent. 2016, 52, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.A.; Murriky, A.; Shafik, S. Influence of platelet rich fibrin on post-extraction socket healing: A clinical and radiographic study. Saudi Dent. J. 2017, 29, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, B.; Das, P.; Rana, M.M.; Qureshi, A.Q.; Vaidya, K.C.; Ahmed Raziuddin, S.J. Wound healing and bone regeneration in postextraction sockets with and without platelet-rich fibrin. Ann. Maxillofac. Surg. 2018, 8, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.M.; Surma, S.; Romańczyk, M.; Wojtowicz, A.; Filipiak, K.J.; Czerniuk, M.R. Assessment of the effect of A-prf application during the surgical extraction of third molars on healing and the concentration of C-reactive protein. Pharmaceutics 2021, 13, 1471. [Google Scholar] [CrossRef]
- Inchingolo, F.; Hazballa, D.; Inchingolo, A.D.; Malcangi, G.; Marinelli, G.; Mancini, A.; Maggiore, M.E.; Bordea, I.R.; Scarano, A.; Farronato, M.; et al. Innovative Concepts and recent breakthrough for engineered graft and constructs for bone regeneration: A literature systematic review. Materials 2022, 15, 1120. [Google Scholar] [CrossRef]
- Kapse, S.; Surana, S.; Satish, M.; Hussain, S.E.; Vyas, S.; Thakur, D. Autologous platelet-rich fibrin: Can it secure a better healing? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 8–18. [Google Scholar] [CrossRef]
- Travezán-Moreyra, M.; Aguirre-Aguilar, A.; Arbildo-Vega, H. Effect of the platelet-rich fibrin on the healing of the soft tissues of sockets after atraumatic exodontics. A single-blind cross-randomized controlled clinical trial. Int. J. Odontostomatol. 2021, 15, 240–247. [Google Scholar] [CrossRef]
- Nemtoi, A.; Sirghe, A.; Nemtof, A.; Haba, D. The effect of a plasma with platelet-rich fibrin in bone regeneration and on rate of orthodontic tooth movement in adolescents. Rev. Chim. 2018, 69, 3727–3730. [Google Scholar] [CrossRef]
- Gupta, N.; Agarwal, S. Advanced–PRF: Clinical evaluation in impacted mandibular third molar sockets. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 43–49. [Google Scholar] [CrossRef]
- Hauser, F.; Gaydarov, N.; Badoud, I.; Vazquez, L.; Bernard, J.P.; Ammann, P. Clinical and histological evaluation of postextraction platelet-rich fibrin socket filling: A prospective randomized controlled study. Implant Dent. 2013, 22, 295–303. [Google Scholar] [CrossRef]
- Sharma, A.; Ingole, S.; Deshpande, M.; Ranadive, P.; Sharma, S.; Kazi, N.; Rajurkar, S. Influence of platelet-rich fibrin on wound healing and bone regeneration after tooth extraction: A clinical and radiographic study. J. Oral Biol. Craniofac. Res. 2020, 10, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. (Engl. Ed.) 2021, 74, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Santos, C.M.; de Mattos Pimenta, C.A.; Nobre, M.R. The PICO strategy for the Research Question Construction and Evidence Search. Rev. Lat. Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0; Centro Cochrane Iberoamericano: Barcelona, Spain, 2011. [Google Scholar]
- Clark, D.; Rajendran, Y.; Paydar, S.; Ho, S.; Cox, D.; Ryder, M.; Dollard, J.; Kao, R.T. Advanced platelet-rich fibrin and freeze-dried bone allograft for ridge preservation: A randomized controlled clinical trial. J. Periodontol. 2018, 89, 379–387. [Google Scholar] [CrossRef]
- Dutta, S.R.; Passi, D.; Singh, P.; Sharma, S.; Singh, M.; Srivastava, D. A randomized comparative prospective study of platelet-rich plasma, platelet-rich fibrin, and hydroxyapatite as a graft material for mandibular third molar extraction socket healing. Natl. J. Maxillofac. Surg. 2016, 7, 45–51. [Google Scholar]
- Niedzielska, I.; Ciapiński, D.; Bąk, M.; Niedzielski, D. The Assessment of the Usefulness of Platelet-Rich Fibrin in the Healing Process Bone Resorption. Coatings 2022, 12, 247. [Google Scholar] [CrossRef]
- Guzmán, C.G.F.; Paltas, M.M.E.; Benenaula, B.J.A.; Núñez, B.K.I.; Simbaña, G.D.V. Gingival and bone tissue healing in lower third molar surgeries. Comparative study between use of platelet rich fibrin versus physiological healing. Rev. Odontológica Mex. 2017, 21, 114–120. [Google Scholar] [CrossRef]
- Dimofte, A.M.; Forna, D.A.; Costan, V.V.; Popescu, E. The value of platelet-rich fibrin in bone regeneration following tooth extraction. Rom. J. Oral Rehabil. 2017, 9, 5–10. [Google Scholar]
- Cruz Molina, C.; Castro Rodríguez, Y. Results of platelet concentrates in guided bone regeneration. Rev. Cuba. Investig. Biomédicas 2020, 39, 1–20. [Google Scholar]
- Canellas, J.V.D.S.; Medeiros, P.J.D.; Figueredo, C.M.D.S.; Fischer, R.G.; Ritto, F.G. Platelet-rich fibrin in oral surgical procedures: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2019, 48, 395–414. [Google Scholar] [CrossRef] [PubMed]

| Inclusion | Exclusion |
|---|---|
| Patients age: 15–99 years | Animal studies |
| Treatment with PRF versus spontaneous healing (blood clots) or biomaterials, that is, bone-substitute materials, collagen membranes, and any other membrane of different origin | Treatment with PRF combined with biomaterials of different origins |
| Post-exodontic alveolus | Patients with periodontal or bone defects, including dehiscence or fenestrations |
| Treatments without any additional chemical or physical agents in the post-exodontic socket, except the use of suture materials | Patients with immediate implant placement |
| Patients not taking anticoagulants | Studies not reporting imaging data or unrelated to PRF |
| Pubmed P—I #1((((“Tooth Extraction/adverse effects”[Mesh] OR “Tooth Extraction/classification”[Mesh] OR “Tooth Extraction/methods”[Mesh]) OR Tooth Extractions OR Extractions, Tooth OR Extraction, Tooth) AND ((“Platelet-Rich Fibrin/cytology”[Mesh] OR “Platelet-Rich Fibrin/diagnostic imaging”[Mesh] OR “Platelet-Rich Fibrin/immunology”[Mesh] OR “Platelet-Rich Fibrin/physiology”[Mesh] OR “Platelet-Rich Fibrin/radiation effects”[Mesh]) OR Platelet Rich Fibrin OR Fibrin, Platelet-Rich)) AND C # 2((“Wound Healing”[Mesh]) AND (“[Mesh] OR “Wound Healing/immunology”[Mesh] OR “Wound Healing/radiation effects”[Mesh])OR Healing, Wound OR Healings, Wound OR Wound Healings)) AND O # 3 (“Radiologic evaluation/physiology” [Mesh] OR Regenerations, Bone OR Regeneration, Bone OR Bone Regenerations OR “Cone-Beam Computed Tomography” [Mesh] OR” (CBCT)) #1 AND #2 AND #3 |
| Scopus P-I #1 (TITLE-ABS-KEY (tooth extraction) OR TITLE-ABS-KEY (Extraction, Tooth)OR TITLE-ABS-KEY (Platelet-Rich Fibrin) OR TITLE-ABS-KEY (PRF)) C #2 (TITLE-ABS-KEY (Physiological healing) OR TITLE-ABS-KEY (Blood clot)OR TITLE-ABS-KEY (Wound Healing)) O #3 ((TITLE-ABS-KEY (bone regeneration) OR TITLE-ABS-KEY (Radiologic evaluation) OR TITLE-ABS-KEY(Cone beam computed tomography) OR TITLE-ABS-KEY (CBCT)) #1 AND #2 AND #3 |
| Science Direct P—I #1 Tooth Extraction, Platelet-Rich Plasma C # 2 Physiological healing, Blood clot O # 3 Bone Regeneration, Cone-Beam, Computed Tomography #1 AND #2 AND #3 |
| Web of Science (Core Collection) P—I #1 TS = (Tooth Extraction OR Extraction tooth OR Platelet-Rich Fibrin OR PRF) Indexes = SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, ESCI, CCR-EXPANDED, IC Timespan = All years C #2 TS = (Wound Healing OR Physiological healing OR Blood Clot) Indexes = SCI EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, ESCI, CCR-EXPANDED, IC Timespan = All years O #3 TS = (Bone regeneration OR) Indexes = SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, ESCI, CCR-EXPANDED, IC Timespan = All years #1 AND #2 AND #3 |
| New York Academy of Medicine Grey Literature Tooth Extraction, Platelet-Rich Fibrin, Wound Healing, Physiological Healing, Bone regeneration, Radiologic evaluation, Cone Beam. |
| First Author/Country, Geographic Region | Study Design | Sample Size | Age Range | Number of Patients | Intervention | Control Time | Reason for the Extraction | ||
|---|---|---|---|---|---|---|---|---|---|
| Test | Control | Type Test | Control | ||||||
| Clark et al., 2018, United States, North America [26] | RCT | 40 | Median age 58 years | 10 | 10 | PRF | A-PRF + FDBA; FDBA; Blood clot | 15 weeks | Uniradicular pieces in need of replacement with dental implants |
| Revathy et al., 2018, India, Asia [1] | RCT | 25 | Between 18 and 35 years | 25 | 25 | PRF | Blood clot | 4, 12, and 24 weeks | Impacted mandibular third molar |
| Castro et al., 2018, Belgium, Europe [10] | RCT | 21 | Over 18 years | 30 | 30 | PRF | L-PRF; Blood clot | 12 weeks | Non-treatable uniradicular pieces located in esthetic areas |
| Suttapreyasri and Leepong, 2013, Thailand, Asia [2] | RCT | 8 | Between 22 and 44 years | 10 | 10 | PRF | Blood clot | 1, 2, 4, 6, and 8 weeks | Symmetrical extraction premolars |
| Hauser et al., 2011, Switzerland, Europe [21] | RCT | 23 | Between 22 and 75 years | 9 PRF–6 PRF—FLAP | 8 | PRF | Blood clot; PRF FLAP | 1, 2, 5, and 8 weeks | Premolars for implant replacement due to: Endodontic treatment failures, root fractures, advanced carious lesions and periodontal compromise |
| Sharma et al., 2020, India, Asia [22] | RCT | 30 | Between 18 and 45 years | 30 | 30 | PRF | Blood clot | 3, 7, and 24 days and 12 weeks | Bilateral exodontia of mandibular first or second molars |
| Kumar et al., 2016, India, Asia [5] | RCT | 34 | Between 18 and 40 years | 34 | 34 | PRF | Blood clot | 2, 4, and 6 months | Impacted mandibular third molar |
| Zhang et al., 2018, China, Asia [3] | RCT | 28 | No details on age | 14 | 14 | PRF | Blood clot | 1, 16, and 48 weeks | Fractured teeth or root remnants |
| Kapse et al., 2018, India, Asia [17] | RCT | 30 | Between 18 and 40 years | 30 | 30 | PRF | Blood clot | 1, 4, 7, and 14 days; and 8 y 16 weeks | Bilateral impacted third molars |
| Gupta and Agarwal, 2021, UK, Europe [20] | RCT | 20 | Between 18 and 35 years | 20 | 20 | PRF | Blood clot | 1, 3 days; and 1, 4, and 24 weeks | Bilateral impacted third molars |
| Alzahrani et al., 2017, Saudi Arabia, Asia [13] | RCT | 24 | Between 25 and 50 years | 12 | 12 | PRF | Blood clot | 1, 4, and 8 weeks | Exodontia of a tooth due to root fracture, poor periodontal prognosis, failure of endodontic treatment, advanced caries |
| Srinivas et al., 2018, India, Asia [14] | RCT | 30 | Between 20 and 50 years | 30 | 30 | PRF | Blood clot | 24 h and 3 months | Upper or lower teeth with/without chronic periodontal disease |
| Dutta et al., 2016, India, Asia [27] | RCT | 40 | Between 17 and 36 years | 40 | 10 | PRF | Blood clot; PRF + HA | 1, 2, and 6 months | Extraction of mandibular third molars |
| Niedzielska et al., 2022, Poland, Europe [28] | RCT | 50 | No details on age | 48 | 41 | PRF | Blood clot | Immediate postoperative and 6 months | Exodontia of 2 homonymous maxillary or mandibular teeth: endodontic failure, coronary fracture |
| Nemtoi et al., 2018, Romania, Europe [19] | RCT | 40 | Between 12 and 20 years | 20 | 20 | PRF | Blood clot | Immediate postoperative and 6 months | Exodontia of upper or lower teeth |
| Guzmán et al., 2017, Ecuador, South America [29] | RCT | 30 | Between 16 and 27 years | 30 | 30 | PRF | Blood clot | 60 days | Extraction of mandibular third molars |
| Dimofte et al., 2017, Romania, Europe [30] | RCT | 63 | Between 18 and 58 years | No details | No details | PRF | Blood clot | 7 days; and 12 weeks | Bilateral extraction, presence of retained roots, non-restorable caries |
| Author | Variables | Evaluation Method |
|---|---|---|
| Clark et al. [26] | Loss of ridge height Loss of ridge width (coronal) Loss of ridge width (central) Loss of ridge width (apical) | Radiographic evaluation (micro-CT) Histomorphometric evaluation |
| Revathy et al. [1] | Bone healing (osteoblastic activity) | Radiographic evaluation: Panoramic X-ray |
| Castro et al. [10] | Change in horizontal ridge level of 1 mm Change in horizontal ridge level between −3 and −5 mm vertical bone resorption in the vestibular and palatal table | Radiographic evaluation: (CBCT) |
| Suttapreyasri and Leepong [2] | Bone resorption of the alveolar ridge Soft tissue healing | Clinical evaluation Radiographic evaluation |
| Hauser et al. [21] | Bone tissue healing Soft tissue healing | Clinical evaluation Radiographic evaluation (periapical parallelism technique) Histological evaluation (micro-CT) |
| Sharma et al. [22] | Bone tissue healing Soft tissue healing | Clinical evaluation Radiographic evaluation: panoramic X-ray |
| Kumar et al. [5] | Bone tissue healing Healing of soft tissues Pain | Clinical evaluation Radiographic evaluation: periapical |
| Zhang et al. [3] | Changes in alveolar ridge height, width, bone density Bone density | Clinical evaluation Radiographic evaluation Histological evaluation |
| Kapse et al. [17] | Bone regeneration (lamina dura, bone density, and trabecular pattern). Pain Edema | Clinical evaluation (VAS, edematization) Radiographic evaluation (periapical) |
| Gupta and Agarwal [20] | Soft tissue healing Pain assessment Consumption of analgesics Edematization Soft tissue healing Trismus | Clinical evaluation: (VAS, edematization, trismus) Radiographic evaluation: (periapical) |
| Alzahrani et al. [13] | Alveolar ridge width Bone regeneration | Clinical evaluation Radiographic evaluation: periapical |
| Srinivas et al. [14] | Alveolar bone height Bone density | Clinical evaluation Histological evaluation |
| Dutta et al. [27] | Soft tissue healing Bone regeneration | Clinical evaluation Radiographic evaluation: periapical |
| Niedzielska et al. [28] | Alveolar bone height Width Bone density | Clinical radiographic evaluation (CBCT) |
| Nemtoi et al. [19] | Bone regeneration Tooth movement | Clinical evaluation radiographic evaluation (CBCT) |
| Guzmán et al. [29] | Soft tissue healing Bone quality | Clinical evaluation Radiographic evaluation (panoramic X-ray) |
| Dimofte et al. [30] | Bone density | Clinical evaluation Radiographic evaluation (CBCT and panoramic X-ray) |
| Author | PRF Result on Alveolar Ridge Preservation (Width, Length, Depth) and/or Bone Tissue Quality | Result of Physiological Healing and/or Biomaterials in Preservation of the Alveolar Ridge (Width, Length, Depth) and/or Quality of Bone Tissue | Statistical Significance Yes/No | Effect of Platelet Concentrate Reported in the Study |
|---|---|---|---|---|
| Clark et al. [26] | PRF: Ridge height: 1.8 ± 2.1 mm; Coronal width: 2.9 ± 1.7 mm Medial width: 1.8 ± 1.3 mm Apical width: 1.5 ± 1.6 mm Bone quality: 46 ± 18% | Blood clot: Ridge height: 3.8 ± 2.0 mm. Coronal width: 2.9 ± 1.7 mm Medial width: 1.8 ± 1.3 mm Apical width: 1.5 ± 1.6 mm Bone quality: 487 ± 48 mg/cm3 *. PRF + FDBA: Ridge height: 1.0 ± 2.3 mm. Coronal width: 1.9 ± 1.1 mm Median width: 1.7 ± 1.2 mm Apical width: 1.6 ± 1.5 mm Bone quality: 3 ± 3%. FDBA: Crest height: 2.2 ± 1.8 mm Coronal width: 2.5 ± 1.1 mm Medial width: 1.5 ± 1.2 mm Apical width: 1.2 ± 1.3 mm Bone quality: 29 ± 14%. | Height: Yes (p < 0.005) Width: No details Bone quality: No details | PRF produced more vital bone compared to FDBA, and also preserved the bone crest similar to FDBA and better than the blood clot; in relation to A-PRF + FDBA there is no statistical significance in bone formation. |
| Revathy et al. [1] | PRF: First month: 11.28650 UH third month: 17.08300 UH sixth month: 20.21800 UH | No details | First month: (p = 0.061) Third month: (p = 0.000, <1%) Sixth month: (p = 0.000, <1%) | PRF improves bone healing and bone formation compared to the control side, with significant bone gain at 1, 3, and 6 months after surgery. |
| Castro et al. [10] | PRF: Coronal width: −2.2 ± 0.9 mm Medial width: −1.6 ± 0.9 mm Apical width: −1.2 ± 0.8 mm Buccal wall height: 0.2 ± 1.1 mm P/L wall height: −1 ± 0.8 mm Bone quality: 54.5 ± 5.6% | L-PRF: Coronal width: −2 ± 1.0 mm; Medial width: −1.8 ± −1.7 mm Apical width: −1.2 ± 0.8 mm; Buccal wall height: 0.2 ± 1.2 mm P/L wall height: −1.1 ± 0.9 mm Bone quality: 47.7 ± 7.9%. Blood clot: Coronal width: −2.2 ± 1.0 mm Medial width: −1.7 ± 0.8 mm Apical width: −1.4 ± 0.8 mm; Apical width: −1.4 ± 0.8 mm. Buccal wall height: −0.2 ± 0.8 mm; P/L wall height: −0.2 ± 0.8 mm. P/L wall height: −1.0 ± 0.9 mm Bone quality: 34.7 ± 6.9%. | Width: No (p > 0.05) Buccal height: No (p = 0.3) P/L:(p = 0.8 Bone quality: L-PRF vs. PRF: No (p > 0.05); PRF vs. blood clot Yes (p < 0.05) | Horizontal and vertical changes at 1 mm below the alveolar ridge (vestibular and palatal) are similar in the three sites. Higher values were reported with L-PRF (85.2%) and PRF (83.8%) filling in relation to the control group (67.9%). Histological and imaging analysis showed bone neoformation for the PRF groups but not in the control group. |
| Suttapreyasri and Leepong [2] | PRF: 0–8 weeks Height M-D: 0.7 ± 1.33 mm. Width: No details Quality: No details | Blood clot: 0–8 weeks Height M-D: 1.23 ± 1.14 mm. Width: No details Quality: No details | Height M-D: No (p > 0.005) Width: No details Quality: No details | PRF can stimulate bone regeneration in situ without waiting for a normal body response, however, due to the minimal number of cytokines in PRF, the effect of bone regeneration is limited and cannot maintain the shape of the alveolar ridge post-exodontia, being statistically insignificant at 1, 2, 4, 6, and 8 weeks. |
| Hauser et al. [21] | PRF: 8 weeks Height: M: −1.2 ± 0.40 mm D: −0.76 ± 0.25 mm | Blood clot: 8-week height: M: −0.77 ± 0.17 mm. D: −2.07 ± 0.81. PRF-FLAP: 8-week height: M: −0.86 ± 0.34 D: −2.15 ± 1.05 | Height M-D: Yes mesial wall in the blood clot group (p < 0.05) | Use of PRF to fill the alveolus without a flap following tooth extraction is associated with improved healing of alveolar bone tissue and preservation of ridge width and bone architecture. |
| Sharma et al. [22] | PRF: Bone quality: Immediate: 87.816 ± 33.318 16 weeks: 91,980 ± 33,728 | Blood clot: Bone quality: Immediate: 85.378 ± 28.211 16 weeks: 88.689 ± 28.5847 | Bone quality: No (p > 0.05) | Bone generation was not statistically significant at week 16 in relation to the control group; however, it accelerated the neoformation of bone tissue in the alveolus. |
| Kumar et al. [5] | PRF: 2 months: 0.11± 0.10 4 months: 0.16 ± 0.11 6 months: 0.16 ± 0.11 | Blood clot: 2 months: 0.13 ± 0.12 4 months: 0.19 ± 0.13 6 months: 0.23 ± 0.12 | Bone quality: No (p = 0.24) | Bone tissues show no significant difference in relation to the control group at 2, 4, and 6 months (p: 0.10). |
| Zhang et al. [3] | PRF: 3 months Buccal ridge: 1.6000 ± 1.46416 Lingual ridge: 1.0000 ± 0.70711 Width: 1.0500 ± 0.77862 | Blood clot: 3 months Buccal crest: 2.8000 ± 1.81487 Lingual crest: 2.0500 ± 1.29180 Width: 2.0760 ± 1.67149 | No statistical differences | Significantly greater bone neoformation in the PRF group (p < 0.001) No statistically significant differences in the mean value of vestibular alveolar ridge height, lingual/palatal alveolar ridge height, and alveolar ridge width). Advantageous PRF in alveolar ridge preservation. |
| Kapse et al. [17] | PRF: Lamina dura: 8 weeks: 1.23 ± 0.10 16 weeks: 1.80 ± 0.07 Bone density: 8 weeks: 1.23 ± 0.09 16 weeks: 1.83 ± 0.07 Trabecular pattern 8 weeks: 1.20 ± 0.11 16 weeks: 1.87 ± 0.06 | Blood clot: Lamina dura: 8 weeks: 0.40 ± 0.009; 16 weeks: 0.90 ± 0.12; Bone density: 8 weeks: 0.27 ± 0.08 16 weeks: 0.63 ± 0.09 Trabecular pattern 8 weeks: 0.30 ± 0.09 16 weeks: 0.50 ± 0.09 | Lamina dura—8 and 16 weeks: Yes (p < 0.001) Bone density—8 and 16 weeks: Yes (p < 0.001) Trabecular pattern —8 and 16 weeks: Yes (p < 0.001) | Regarding bone healing (lamina dura, bone density, and trabecular pattern) (p < 0.001) was higher at week 16 in relation to week 8 in sockets with PRF. |
| Gupta and Agarwal [20] | PRF: 1 month: 18.75% ± 5.12 3 months: 51.47% ± 3.93 6 months: 77.63% ± 6.97 | Blood clot: 1 month: 13.58% ± 4.87 3 months: 47.58% ± 3.17 6 months: 70.54% ± 5.76 | 1 month: Yes (p = 0.0023) 3 months: Yes (p= 0.0014) 6 months: Yes (p = 0.0012) | Bone regeneration in sites with PRF at the first, third, and sixth months is statistically significant in relation to the control group (p < 0.005). |
| Alzahrani et al. [13] | PRF: Alveolar ridge width: 1 week: 11.70 ± 2.37 4 weeks: 11.33 ± 2.30 8 weeks: 10.97 ± 2.33 Bone fill: 1 week: 68.82 ± 1.07%. 4 weeks: 74.03 ± 1.22%. 8 weeks: 80.35 ± 2.61% | Blood clot: Alveolar ridge width: 1 week: 13.01 ± 3.00 mm 1 week: 13.01 ± 3.00 mm 4 weeks: 12.04 ± 2.50 mm. 8 weeks: 11.54 ± 2.42 mm. Bone filling: 1 week: 74.05 ± 1.66%. 4 weeks: 81.54 ± 3.33%. 8 weeks: 88.81 ± 1.53%. | 1- 4 weeks: Yes (p = 0.012) 1- 8 weeks: Yes (p = 0.036) 4–8 weeks: No (p = 0.37) | Alveolar ridge width loss in the PRF group (−0.97 mm—8.58%) was significantly lower compared to the control group (−1.92—13.54%) at 4 and 8 weeks; PRF increases the efficiency of cell proliferation thus decreasing long-term bone loss. |
| Srinivas et al. [14] | PRF: Bone height: 24 h: 13.93 ± 3.56 mm 3 months: 12.28 ± 3.84 mm Bone density (alveolus): 24 h: 319.79 ± 95.472 3 months: 564.76 ± 94.856 Periapical region: 24 h: 530.39 ± 203.289 3 months: 748.02 ± 202.878 | Blood clot: Bone height: 24 h: 14.68 ± 4.32 mm 3 months: 12.78 ± 3.82 mm Bone density (alveolus): 24 h: 194.82 ± 78.986 3 months: 295.87 ± 87.217 Periapical region: 24 h: 518.84 ± 266.518 3 months: 613.15 ± 237.926 | 24 h—3 months—Without PRF Alveolar height Yes (p < 0.001) Bone density: Yes (p < 0.003 Periapical region: Yes (p < 0.043) 24 h—3 months—With PRF Alveolar height: Yes (p <0.001) Bone density: Yes (p < 0.001) Periapical region: Yes (p < 0.05) | Improved bone density was reported. |
| Dutta et al. [27] | PRF: Bone quality: Hard laminin: 1 month: −0.6 ± 0.16, 2 months: 0.4 ± 0.16 6 months: 1.1 ± 0.10 Overall bone density: 1 month: −0.4 ± 0.16 2 months: 0.4 ± 0.16 6 months: 1.2 ± 0.13 Trabecular pattern: 1 month: −0.6 ± 0.16 2 months: 0.3 ± 0.15 6 months: 1.3 ± 0.15 | Blood clot Bone quality: Hard lamella: 1 month: −1.9 ± 0.1 2 months: −1 ± 0.14 6 months: 0.1 ± 0.10 Overall bone density: 1 month: −1.9 ± 0.10 2 months: −1.3 ± 0.21 6 months: 0.1 ± 0.23 Trabecular pattern: 1 month: −1.9 ± 0.10 2 months: −1.3 ± 0.21 6 months: 0.1 ± 0.17 | Statistical significance: Bone regeneration with PRF at 1, 2, and 6 months (p < 0.05) in relation to the control | Bone healing of the lamina dura. |
| Niedzielska et al. [28] | PRF: Width: 9.43 ± 1.74 mm Height: 1.49 ± 0.84 mm Bone quality: A: 308.16 ± 128.15 | Blood clot: Width: 9.15 ± 1.51 Height: 1.85 ± 0.86 Bone quality: A: 279.40 ± 136.23 | No statistical significance immediate post-exodontic: width and height of alveolar process. There is statistical significance 6 months post-exodontic: width and height of alveolar process (p = 0.0085) | Changes in the alveolar process. Changes in alveolar process height. |
| Nemtoi et al. [19] | PRF Height: Immediate: 5 mm 6 months: 1.9 mm Bone quality—14 weeks: D1 (1250 HU) | Blood Clot: Height: Immediate: 4.8 mm 6 months: 2.9 mm Bone quality—8 weeks: D1 (1250 HU) | There is statistical significance in bone regeneration | Changes in the alveolar process. Changes in alveolar process height. |
| Guzmán et al. [29] | PRF: 60 days: 163.86 UH | Blood Clot: 60 days: 159.31 UH | There is a statistically significant difference at 60 days (p < 0.015) | Bone healing. |
| Dimofte et al. [30] | PRF: Monoradicular teeth with PRF, density increased (p = 0.00484) compared to the control side. Pluriradicular teeth. Bone density increased in mesial (p < <0.001) and distal (p = 0.00304) roots for the mandible. The same results were obtained for the maxilla where PRF was used: mesiovestibular (p < 0.001) disto vestibular (p < 0.001) palatal (p < 0.001) roots. The ridge preservation (width, length, depth): no details | Blood Clot: No details | There is a statistically significant difference (0.000484) | Improved bone healing. Improved bone density. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Barahona, M.; Delgado-Gaete, B.; Morales-Navarro, D.; Urbizo-Vélez, J.; Avecillas-Rodas, R. Imaging Evaluation of Platelet-Rich Fibrin in Post-Exodontic Bone Regeneration: A Systematic Review. Dent. J. 2023, 11, 277. https://doi.org/10.3390/dj11120277
Molina-Barahona M, Delgado-Gaete B, Morales-Navarro D, Urbizo-Vélez J, Avecillas-Rodas R. Imaging Evaluation of Platelet-Rich Fibrin in Post-Exodontic Bone Regeneration: A Systematic Review. Dentistry Journal. 2023; 11(12):277. https://doi.org/10.3390/dj11120277
Chicago/Turabian StyleMolina-Barahona, Magdalena, Bolívar Delgado-Gaete, Denia Morales-Navarro, Joaquín Urbizo-Vélez, and Renata Avecillas-Rodas. 2023. "Imaging Evaluation of Platelet-Rich Fibrin in Post-Exodontic Bone Regeneration: A Systematic Review" Dentistry Journal 11, no. 12: 277. https://doi.org/10.3390/dj11120277
APA StyleMolina-Barahona, M., Delgado-Gaete, B., Morales-Navarro, D., Urbizo-Vélez, J., & Avecillas-Rodas, R. (2023). Imaging Evaluation of Platelet-Rich Fibrin in Post-Exodontic Bone Regeneration: A Systematic Review. Dentistry Journal, 11(12), 277. https://doi.org/10.3390/dj11120277







