Clinical Efficacy and Safety of Lidocaine Tape for Topical Anesthesia of the Oral Mucosa: A Preliminary Controlled Trial
Abstract
:1. Introduction
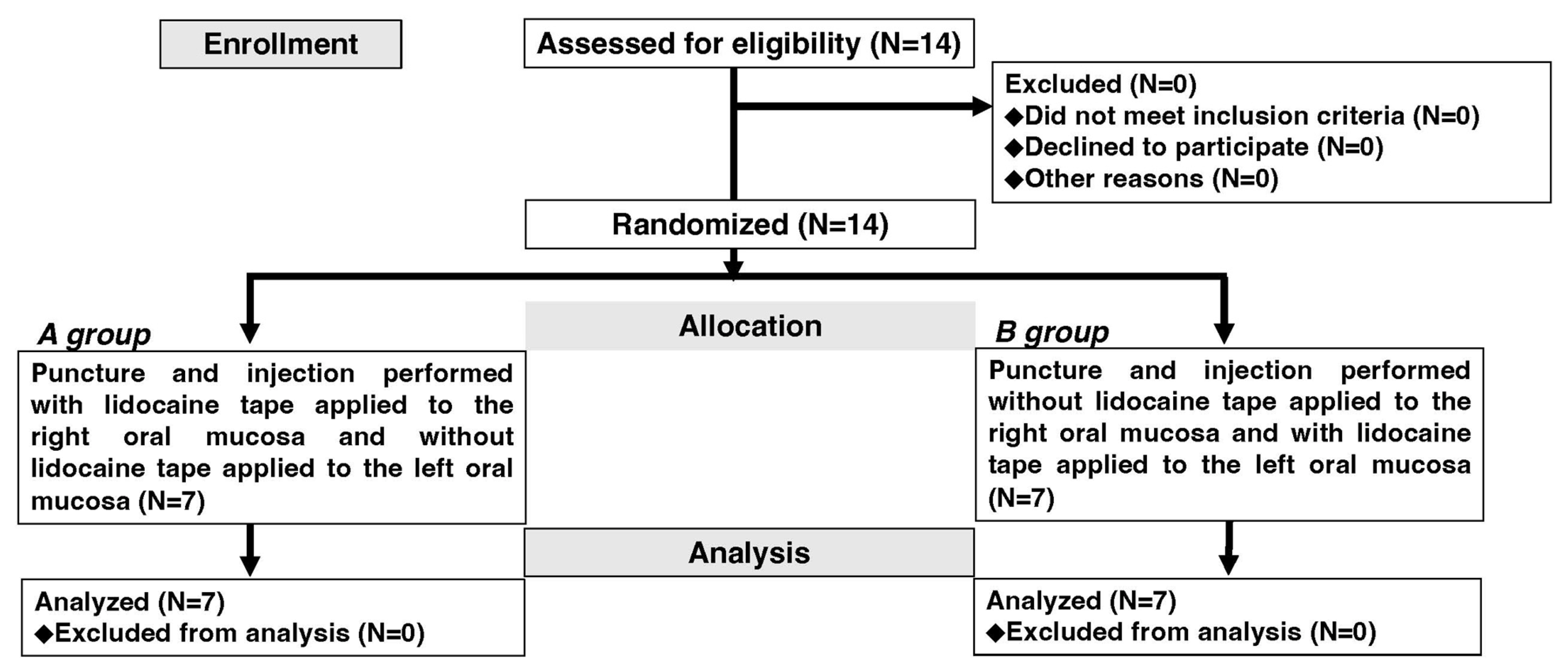
2. Materials and Methods
2.1. Study Design
2.2. Sample Size Estimation
2.3. Eligibility Criteria
- Age: ≥18 and <60 years at the time of obtaining consent.
- Sex: Any.
- No inflammation or other abnormal findings in the area to which the study drug will be applied (mucosa apical to the maxillary lateral incisor in the vestibular region of the oral cavity).
- Those who provided free and voluntary written informed consent.
2.4. Exclusion Criteria
- Individuals who could not make decisions regarding participation in this study.
- Drug-sensitive individuals who are allergic to any of the drugs used in this study, including Xylocaine dental cartridges.
- Pregnant women or women who may be pregnant.
- Those with a relationship with the Department of Oral and Maxillofacial Surgery of our hospital.
- Other individuals deemed inappropriate as research participants by the principal investigator.
2.5. Participants
2.6. Interventions
2.7. Management and Procedures for Research Drugs
2.8. Preparation of Case Reports
2.9. Specific Methods for Protecting Personal Information
2.10. Endpoints
2.11. Statistical Analysis
2.12. Monitoring
2.13. Preservation and Handling of Recorded Documents
2.13.1. Storage of Information
2.13.2. Retention of Documents
2.14. Conflicts of Interest of the Research Organization and Researchers
3. Results
3.1. Participants’ Characteristics
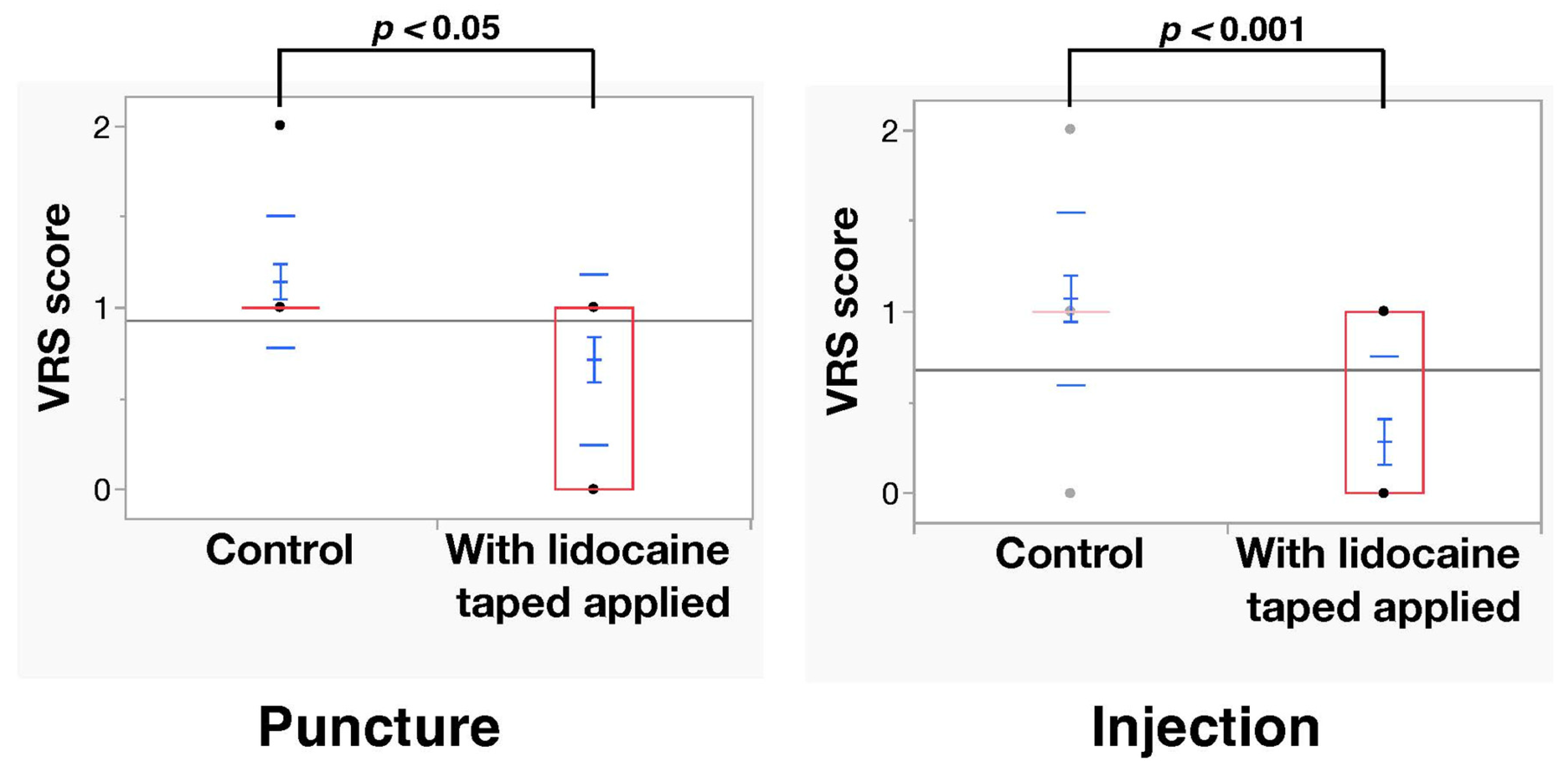
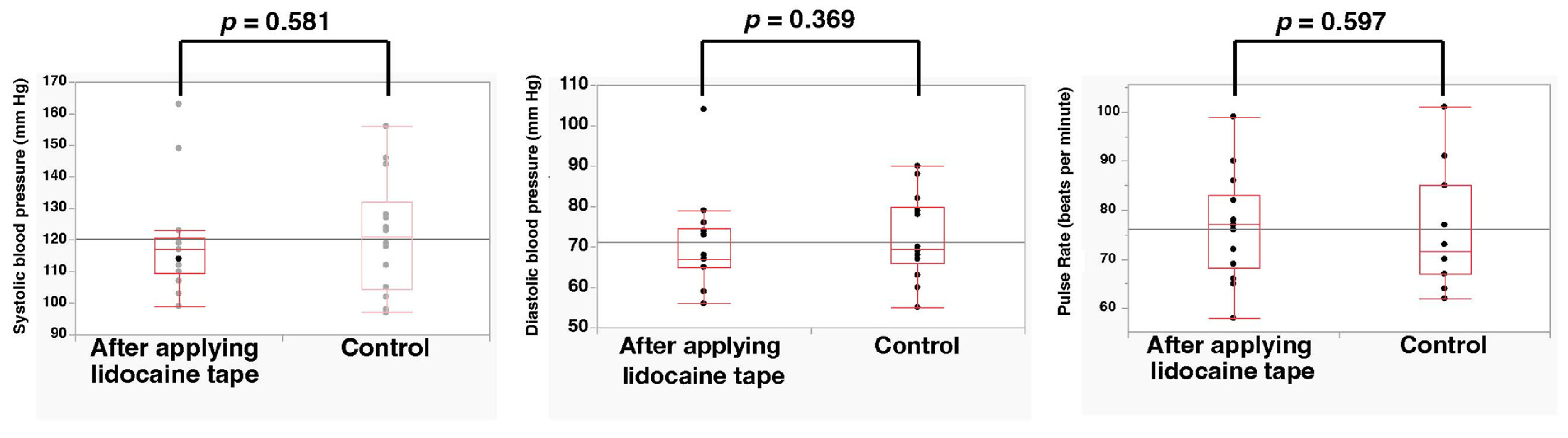
3.2. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obata, K.; Naito, H.; Yakushiji, H.; Obara, T.; Ono, K.; Nojima, T.; Tsukahara, K.; Yamada, T.; Sasaki, A.; Nakao, A. Incidence and characteristics of medical emergencies related to dental treatment: A retrospective single-center study. Acute Med. Surg. 2021, 8, e651. [Google Scholar] [CrossRef]
- Shimoda, H.; Yamauchi, K.; Takahashi, T. Transient asystole associated with vasovagal reflex in an oral surgery patient: A case report. SAGE Open Med. Case Rep. 2023, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- McMillan, A.S.; Walshaw, D.; Meechan, J.G. The efficacy of Emla and 5% lignocaine gel for anaesthesia of human gingival mucosa. Br. J. Oral Maxillofac. Surg. 2000, 38, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Leopold, A.; Wilson, S.; Weaver, J.S.; Moursi, A.M. Pharmacokinetics of lidocaine delivered from a transmucosal patch in children. Anesth. Prog. 2002, 49, 82–87. [Google Scholar] [PubMed]
- Stecker, S.S.; Swift, J.Q.; Hodges, J.S.; Erickson, P.R. Should a mucoadhesive patch (DentiPatch) be used for gingival anesthesia in children? Anesth. Prog. 2002, 49, 3–8. [Google Scholar]
- Fukayama, H.; Suzuki, N.; Umino, M. Comparison of topical anesthesia of 20% benzocaine and 60% lidocaine gel. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 157–161. [Google Scholar] [CrossRef]
- Martin, M.D.; Ramsay, D.S.; Whitney, C.; Fiset, L.; Weinstein, P. Topical anesthesia: Differentiating the pharmacological and psychological contributions to efficacy. Anesth. Prog. 1994, 41, 40–47. [Google Scholar]
- Kawano, T.; Shiraishi, S.; Nakamura, T.; Yokoo, H.; Takasaki, M. Comparison of analgesic effect of lidocaine tape versus eutectic mixture of lidocaine and tetracaine during infiltration of local anesthetics before epidural block. Masui 1996, 45, 1074–1077. (In Japanese) [Google Scholar]
- Common Terminology Criteria for Adverse Events, version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed on 10 October 2022).
- Ando, T.; Shimoo, Y.; Nakasato, M.; Yoshida, H. Pain-relief effect of surface anesthetics on the alveolar pain at the local anesthetic injection. J. Jpn. Soc. Hosp. Pharm. 2010, 46, 780–782. [Google Scholar]
- Cho, S.Y.; Kim, E.; Park, S.H.; Roh, B.D.; Lee, C.Y.; Lee, S.J.; Jung, I.Y. Effect of topical anesthesia on pain from needle insertion and injection and its relationship with anxiety in patients awaiting apical surgery: A randomized double-blind clinical trial. J. Endod. 2017, 43, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Gernandt, S.; Scolozzi, P. The use of equimolar mixtures of nitrous oxide and oxygen in oral surgery-a retrospective study of patients in a Swiss university hospital setting. J. Clin. Med. 2023, 12, 4117. [Google Scholar] [CrossRef] [PubMed]
- Hindocha, N.; Manhem, F.; Bäckryd, E.; Bågesund, M. Ice versus lidocaine 5% gel for topical anaesthesia of oral mucosa—A randomized cross-over study. BMC Anesthesiol. 2019, 19, 227. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, H.; Ali, F.; Aslam, H.; Khan, M.S.; Waqas, M.; Lal, A. Cryoanesthesia with ethyl chloride spray versus 5% lidocaine gel in alleviating oral local anesthetic injection pain for buccal anaesthesia: A randomized clinical (controlled) trial. J. Dent. Res. Dent. Clin. Dent. Prospect. 2023, 17, 40–46. [Google Scholar] [CrossRef]
- Hemavathi, U.; Aymen, S.; Shetty, A.; Cherian, R.; Garg, S.; Jeevan, B.S. Comparative evaluation of efficacy of EMLA versus ice as topical anesthetic in prior to needle prick in palatine nerve blocks-a randomized split mouth study EMLA versus ice as topical anesthetic in dental nerve blocks. J. Maxillofac. Oral Surg. 2023, 22, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, N.; Mathur, S.; Malik, M. Effectiveness of cryotherapy, sucrose solution and a combination therapy for pain control during local anesthesia in children: A split mouth study. J. Clin. Pediatr. Dent. 2022, 46, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Almugait, M.; AbuMostafa, A. Comparison between the analgesic effectiveness and patients’ preference for virtual reality vs. topical anesthesia gel during the administration of local anesthesia in adult dental patients: A randomized clinical study. Sci. Rep. 2021, 11, 23608. [Google Scholar] [CrossRef] [PubMed]
- Felemban, O.M.; Alshamrani, R.M.; Aljeddawi, D.H.; Bagher, S.M. Effect of virtual reality distraction on pain and anxiety during infiltration anesthesia in pediatric patients: A randomized clinical trial. BMC Oral Health 2021, 21, 321. [Google Scholar] [CrossRef]
- Alshatrat, S.M.; Sabarini, J.M.; Hammouri, H.M.; Al-Bakri, I.A.; Al-Omari, W.M. Effect of immersive virtual reality on pain in different dental procedures in children: A pilot study. Int. J. Paediatr. Dent. 2022, 32, 264–272. [Google Scholar] [CrossRef]
- Hochman, M.; Chiarello, D.; Hochman, C.B.; Lopatkin, R.; Pergola, S. Computerized local anesthetic delivery vs. traditional syringe technique. Subjective pain response. N. Y. State Dent. J. 1997, 63, 24–29. [Google Scholar]
- Primosch, R.E.; Brooks, R. Influence of anesthetic flow rate delivered by the Wand Local Anesthetic System on pain response to palatal injections. Am. J. Dent. 2002, 15, 15–20. [Google Scholar] [PubMed]
- Johnson, J.; Primosch, R.E. Influence of site preparation methods on the pain reported during palatal infiltration using the Wand Local Anesthetic System. Am. J. Dent. 2003, 16, 165–169. [Google Scholar] [PubMed]
- DeCou, J.M.; Abrams, R.S.; Hammond, J.H.; Lowder, L.R.; Gauderer, M.W. Iontophoresis: A needle-free, electrical system of local anesthesia delivery for pediatric surgical office procedures. J. Pediatr. Surg. 1999, 34, 946–949. [Google Scholar] [CrossRef]
- Cubayachi, C.; Couto, R.O.; de Gaitani, C.M.; Pedrazzi, V.; Freitas, O.; Lopez, R.F. Needle-free buccal anesthesia using iontophoresis and amino amide salts combined in a mucoadhesive formulation. Colloids Surf. B Biointerfaces 2015, 136, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- do Couto, R.O.; Cubayachi, C.; Calefi, P.L.; Lopez, R.F.V.; Pedrazzi, V.; De Gaitani, C.M.; de Freitas, O. Combining amino amide salts in mucoadhesive films enhances needle-free buccal anesthesia in adults. J. Control. Release 2017, 266, 205–215. [Google Scholar] [CrossRef] [PubMed]
- do Couto, R.O.; Cubayachi, C.; Duarte, M.P.F.; Lopez, R.F.V.; Pedrazzi, V.; De Gaitani, C.M.; de Freitas, O. Towards the advance of a novel iontophoretic patch for needle-free buccal anesthesia. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111778. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Fernandes, S.; Haq, M.A.; Bafna, Y.; Bhatt, R.; Sinha, S.; Gajjar, S.; Kumar, S.; Haque, M. Iontophoresis-infused deep topical anesthesia and injectable local anesthesia for dental procedures among pediatric patients: Performances and consequences. Cureus 2023, 15, e43748. [Google Scholar] [CrossRef] [PubMed]
- Hjermstad, M.J.; Fayers, P.M.; Haugen, D.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H.; Fainsinger, R.; Aass, N.; Kaasa, S.; European Palliative Care Research Collaborative (EPCRC). Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: A systematic literature review. J. Pain Symptom Manag. 2011, 41, 1073–1093. [Google Scholar] [CrossRef] [PubMed]
- Hino, H.; Baba, N.; Unozawa, S.; Tokuda, S.; Kawaharada, Y.; Nakamura, K.; Kani, T. Efficacy and safety of Penles® Tape 18 mg (lidocaine tape) for pain relief during molluscum contagiosum removal—Special drug use investigation in children. J. Jpn. Organ. Clin. Dermatol. 2015, 32, 202–218. (In Japanese) [Google Scholar] [CrossRef]
- Kawaharada, Y.; Sato, F.; Kani, T. Examination of the safety and effectiveness of Penless® Tape 18 mg (lidocaine) for pain relief during skin laser irradiation therapy (use results survey). Jpn. J. Laser Med. 2020, 40, 320–329. (In Japanese) [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tani, R.; Yamasaki, S.; Hamada, A.; Higaki, M.; Asada, Y.; Yanamoto, S. Clinical Efficacy and Safety of Lidocaine Tape for Topical Anesthesia of the Oral Mucosa: A Preliminary Controlled Trial. Dent. J. 2023, 11, 276. https://doi.org/10.3390/dj11120276
Tani R, Yamasaki S, Hamada A, Higaki M, Asada Y, Yanamoto S. Clinical Efficacy and Safety of Lidocaine Tape for Topical Anesthesia of the Oral Mucosa: A Preliminary Controlled Trial. Dentistry Journal. 2023; 11(12):276. https://doi.org/10.3390/dj11120276
Chicago/Turabian StyleTani, Ryouji, Sachiko Yamasaki, Atsuko Hamada, Mirai Higaki, Yasuyuki Asada, and Souichi Yanamoto. 2023. "Clinical Efficacy and Safety of Lidocaine Tape for Topical Anesthesia of the Oral Mucosa: A Preliminary Controlled Trial" Dentistry Journal 11, no. 12: 276. https://doi.org/10.3390/dj11120276
APA StyleTani, R., Yamasaki, S., Hamada, A., Higaki, M., Asada, Y., & Yanamoto, S. (2023). Clinical Efficacy and Safety of Lidocaine Tape for Topical Anesthesia of the Oral Mucosa: A Preliminary Controlled Trial. Dentistry Journal, 11(12), 276. https://doi.org/10.3390/dj11120276







