Prevalence of Moderate to Severe Periodontitis in an 18–19th Century Sample—St. Bride’s Lower Churchyard (London, UK)
Abstract
:1. Introduction
2. Aim of the Study
3. Materials and Methods
3.1. The Sample
3.2. Inclusion/Exclusion Criteria
3.3. Data Collection
4. Reproducibility
5. Data Analysis/Case Definition
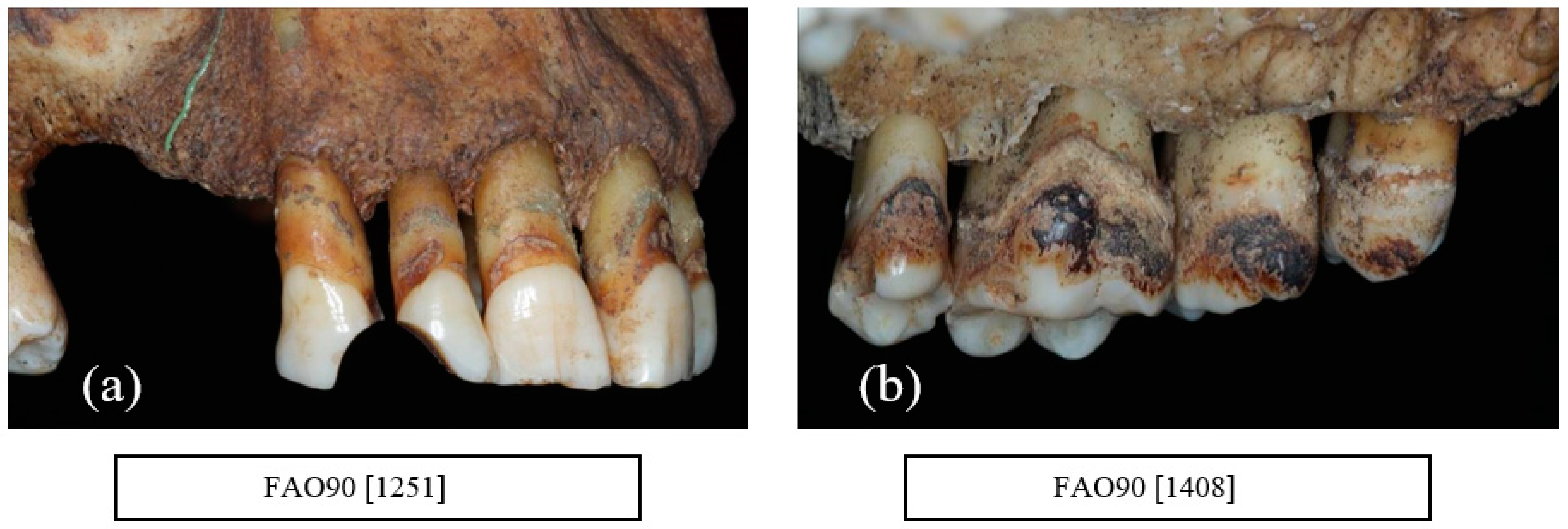
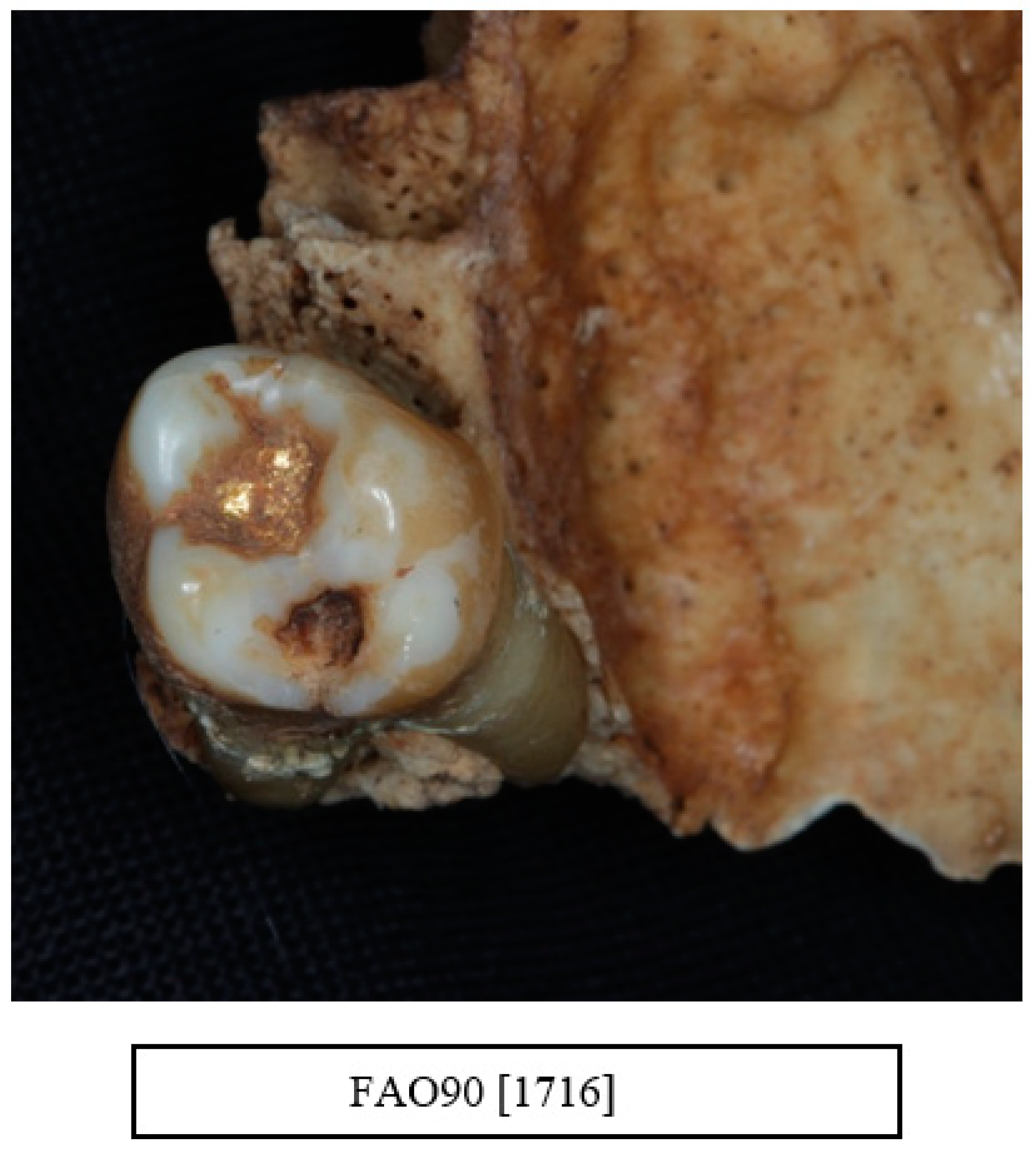
6. Periodontal Disease
6.1. Cases of Moderate to Severe Periodontitis
6.1.1. Case Definition I
6.1.2. Case Definition II
7. Results
Calibration/Reproducibility
8. Tooth Status
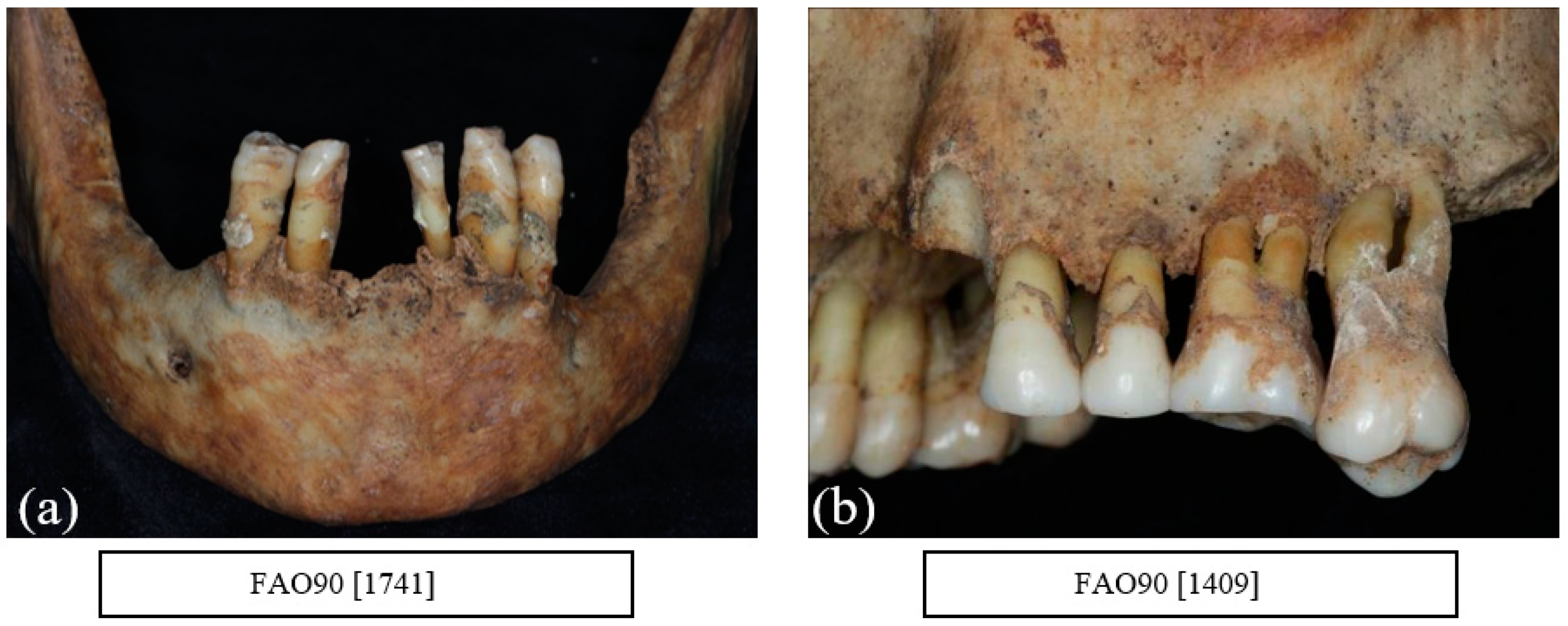
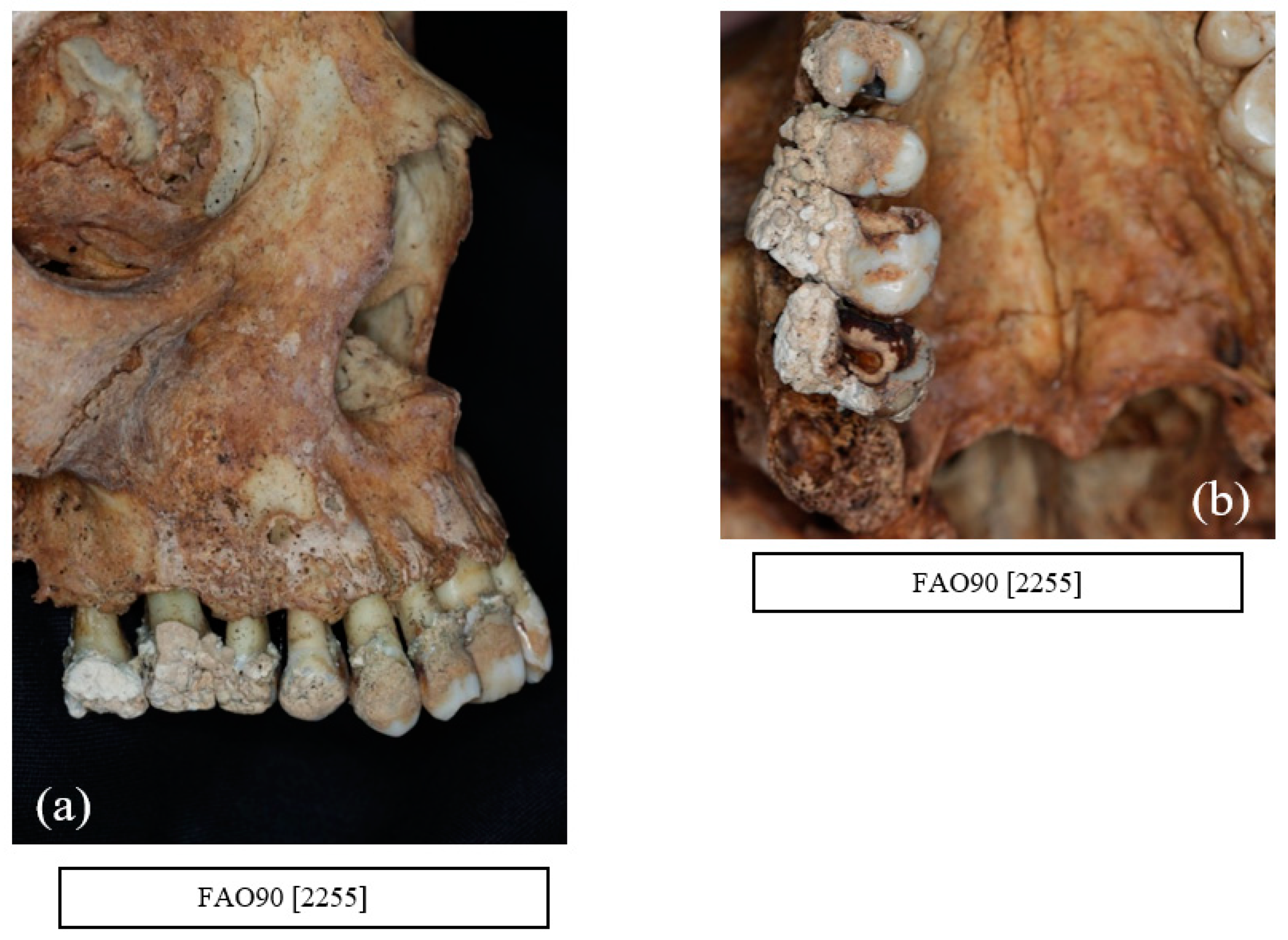
9. Alveolar Bone Loss
10. Horizontal Bone Loss
11. Vertical Bone Loss
12. Other Dental Pathologies and Anomalies
13. Evidence of Recognised Dental Treatment
14. Discussion
14.1. Overview
14.2. Methodological Issues When Determining the Prevalence of Periodontal Disease in Dried Skulls
14.3. The Impact of Ante-Mortem (AMTL) and Post-Mortem Damage (Including Anti-Mortem and Post-Mortem Tooth Loss) in Estimating the Level of Disease in Ancient Populations
14.4. Attempts to Overcome the Problem of Inaccurate Linear Measurement of the Distance between the CEJ and AC When Examining Dried Skulls
14.5. Problems When Comparing Disease Prevalence in Ancient and Modern Populations
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapple, I.L.; Van der Weijden, F.; Doerfer, C.; Herrera, D.; Shapira, L.; Polak, D.; Madianos, P.; Louropoulou, A.; Machtei, E.; Donos, N.; et al. Primary prevention of periodontitis: Managing gingivitis. J. Clin. Periodontol. 2015, 42, S71–S76. [Google Scholar] [CrossRef] [Green Version]
- Kassebaum, N.J.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990-2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Raitapuro-Murray, T.; Molleson, T.I.; Hughes, F.J. The prevalence of periodontal disease in a Romano-British population c. 200-400 AD. Br. Dent. J. 2014, 217, 459–466. [Google Scholar] [CrossRef]
- Albandar, J.M.; Rise, J.; Gjermo, P.; Johansen, J.R. Radiographic quantification of alveolar bone level changes. A 2-year longitudinal study in man. J. Clin. Periodontal. 1986, 13, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Albandar, J.M. Validity and reliability of alveolar bone level measurements made on dry skulls. J. Clin. Periodontol. 1989, 16, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Brothwell, D.R. Digging Up Bones: The Excavation, Treatment, and Study of Human Skeletal Remains, 1st ed.; Trustees of the British Museum: London, UK, 1963. [Google Scholar]
- Kerr, N.W. A method of assessing periodontal status in archaeologically derived skeletal material. J. Periodontol. 1988, 2, 67–78. [Google Scholar]
- Davies, D.M.; Picton, D.C.A. A study of the periodontal state in two hundred and two skulls of primitive peoples. J. Periodontol. Res. 1969, 4, 230–234. [Google Scholar] [CrossRef]
- Goncalves, P.C.; Griffiths, G.; Rawlinson, A. A study of the periodontal state of a late Medieval United Kingdom population. Arch. Oral. Biol. 2015, 60, 1797–1801. [Google Scholar] [CrossRef]
- Connell, B.; Rauxloh, P. A Rapid Method for Recording Human Skeletal Data; Museum of London: London, UK, 2003. [Google Scholar]
- Powers, N. Human Osteology Method Statement Published Online; Museum of London: London, UK, 2008; revised 2012. [Google Scholar]
- Miles, A.; Conheeney, J. A Post-Medieval Population from London: Excavations in the St Bride’s Lower Churchyard 75–82 Farringdon Street; EC4 MoLAS Studies Series; Museum of London: London, UK, 2005. [Google Scholar]
- Roberts, C.A.; Manchester, K. The Archaeology of Disease; The History Press: Cheltenham, UK, 2010. [Google Scholar]
- Whittaker, D.K.; Parker, J.H.; Jenkins, C. Tooth attrition and continuing eruption in a Romano-British population. Arch. Oral. Biol. 1982, 27, 405–409. [Google Scholar] [CrossRef]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Scheuer, J.; Bowman, J. Correlation of documentary and skeletal evidence in the St Brides crypt population. In Grave Reflections: Portraying the Past through Cemetery Studies; Canadian Scholar’s Press Inc.: Toronto, ON, Canada, 1995; pp. 49–70. [Google Scholar]
- Esclassan, R.; Grimoud, A.M.; Ruas, M.P.; Donat, R.; Sevin, A.; Astie, F.; Lucas, S.; Crubezy, E. Dental caries, tooth wear and diet in an adult medieval (12th–14th century) population from Mediterranean France. Arch. Oral. Biol. 2009, 54, 287–297. [Google Scholar] [CrossRef] [PubMed]
- McKeown, A.H.; Bennett, J.L. A preliminary investigation of post-mortem tooth loss. J. Forensic. Sci. 1995, 40, 755–757. [Google Scholar] [CrossRef] [PubMed]
- de Faria Vasconcelos, K.; Evangelista, K.M.; Rodrigues, C.D.; Estrela, C.; de Sousa, T.O.; Silva, M.A.G. Detection of periodontal bone loss using cone beam CT and intraoral radiography. Dentomaxillofacial Radiol. 2012, 41, 64–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, B.K.J.; Leichter, J.W.; Chandler, N.P.; Cullinan, M.P.; Holborow, D.W. Radiographic study of ethnic variation in alveolar bone height among New Zealand dental students. J. Periodontol. 2007, 78, 1070–1074. [Google Scholar] [CrossRef]
- Persson, R.E.; Hollender, L.G.; Laurell, L.; Persson, G.R. Horizontal alveolar bone loss and vertical bone defects in an adult patient population. J. Periodontol. 1998, 69, 348–356. [Google Scholar] [CrossRef]
- Smith, B.G.; Knight, J.K. An index for measuring the wear of teeth. B Dent. J. 1984, 156, 435–438. [Google Scholar] [CrossRef]
- Larsen, C.S. Bioarchaeology: Interpreting Behaviour from the Human Skeleton; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A.; Genco, R.J. Periodontitis in US adults: National Health and Nutrition Examination Survey 2009–2014. J. Am. Dent. Assoc. 2018, 149, 576–588.e6. [Google Scholar] [CrossRef]
- White, D.A.; Tsakos, G.; Pitts, N.B.; Fuller, E.; Douglas, G.V.A.; Murray, J.J.; Steele, J.G. Adult dental health survey 2009: Common oral health conditions and their impact on the population. Br. Dent. J. 2012, 213, 567–572. [Google Scholar] [CrossRef]
- Frohlich, B.; Littleton, J. An Analysis of dental pathology and diet on historic Bahrain. Paléorient 1989, 15, 59–75. [Google Scholar]
- Albandar, J.M.; Streckfus, C.F.; Adesanya, M.R.; Winn, D.M. Cigar, pipe, and cigarette smoking as risk factors for periodontal disease and tooth loss. J. Periodontol. 2000, 71, 1874–1881. [Google Scholar] [CrossRef]
- Tonetti, M.S. Cigarette smoking and periodontal diseases: Etiology and management of disease. Ann. Periodontol. 1998, 3, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Mant, M.; Roberts, C. Diet and dental caries in post-medieval London. Int. J. Hist. Archaeol. 2015, 19, 188–207. [Google Scholar] [CrossRef] [Green Version]
- Hillson, S. Recording dental caries in archaeological human remains. Int. J. Osteoarchaeol. 2001, 11, 249–289. [Google Scholar] [CrossRef]
- Smith, J.S. Archaeological oral health: A comparison of post-medieval and modern-day dental caries exposure of adults in East London. Br. Dent. J. 2019, 227, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Angulo, E.K.; Bernabé, E.; Marcenes, W. Ethnic inequalities in dental caries among adults in East London. J. Public Health 2016, 38, e55–e62. [Google Scholar] [CrossRef] [Green Version]
- Caglar, E.; Kuscu, O.O.; Sandalli, N.; Ari, I. Prevalence of dental caries and tooth wear in a Byzantine population (13th c. A.D.) from northwest Turkey. Arch. Oral. Biol. 2007, 52, 1136–1145. [Google Scholar] [CrossRef]
- Hillam, C. Dental Practice in Europe at the end of the Eighteenth Century; Editions Rodopi B.V.: Amsterdam, The Netherlands; New York, NY, USA, 2003. [Google Scholar]
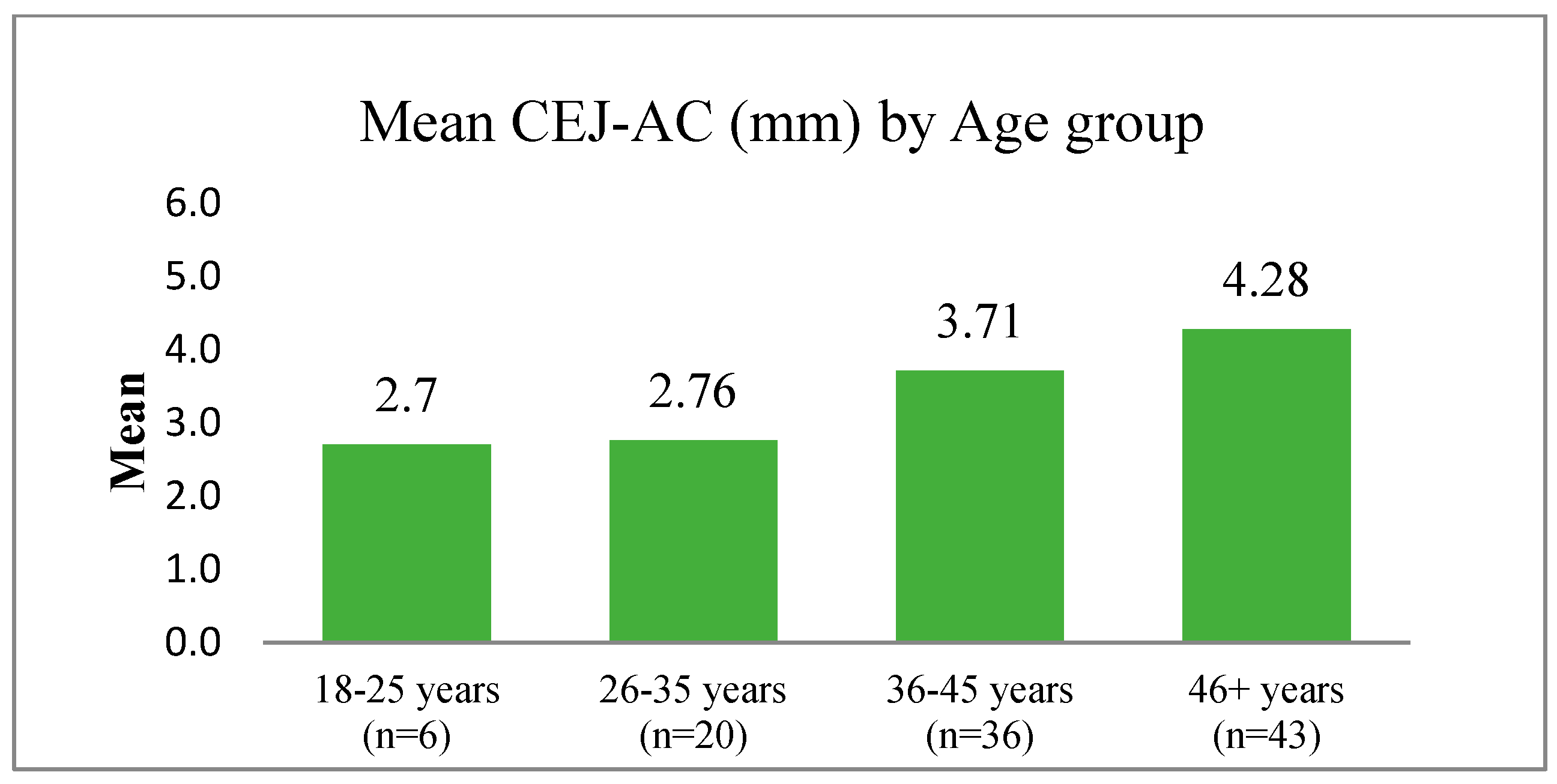
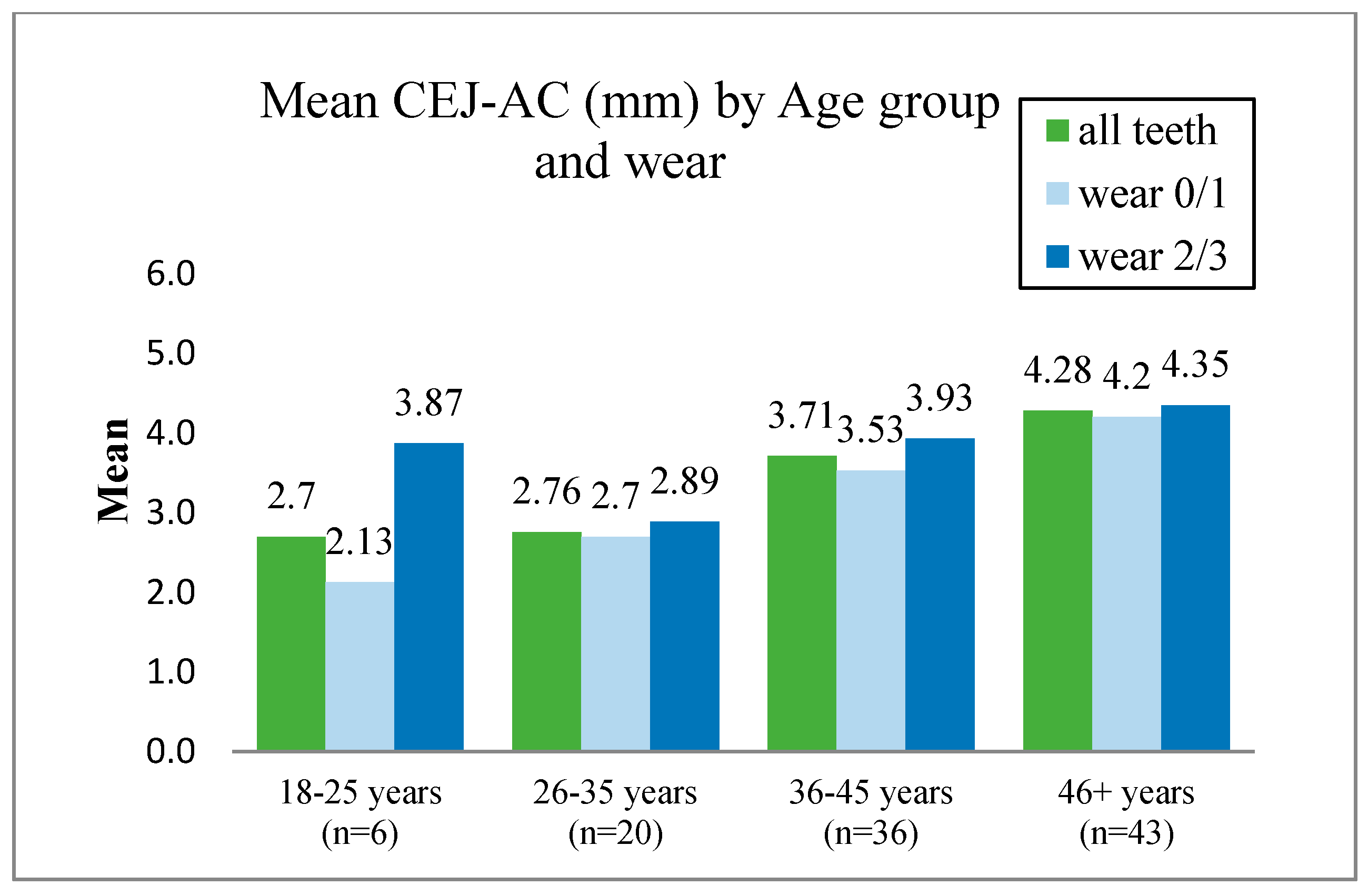

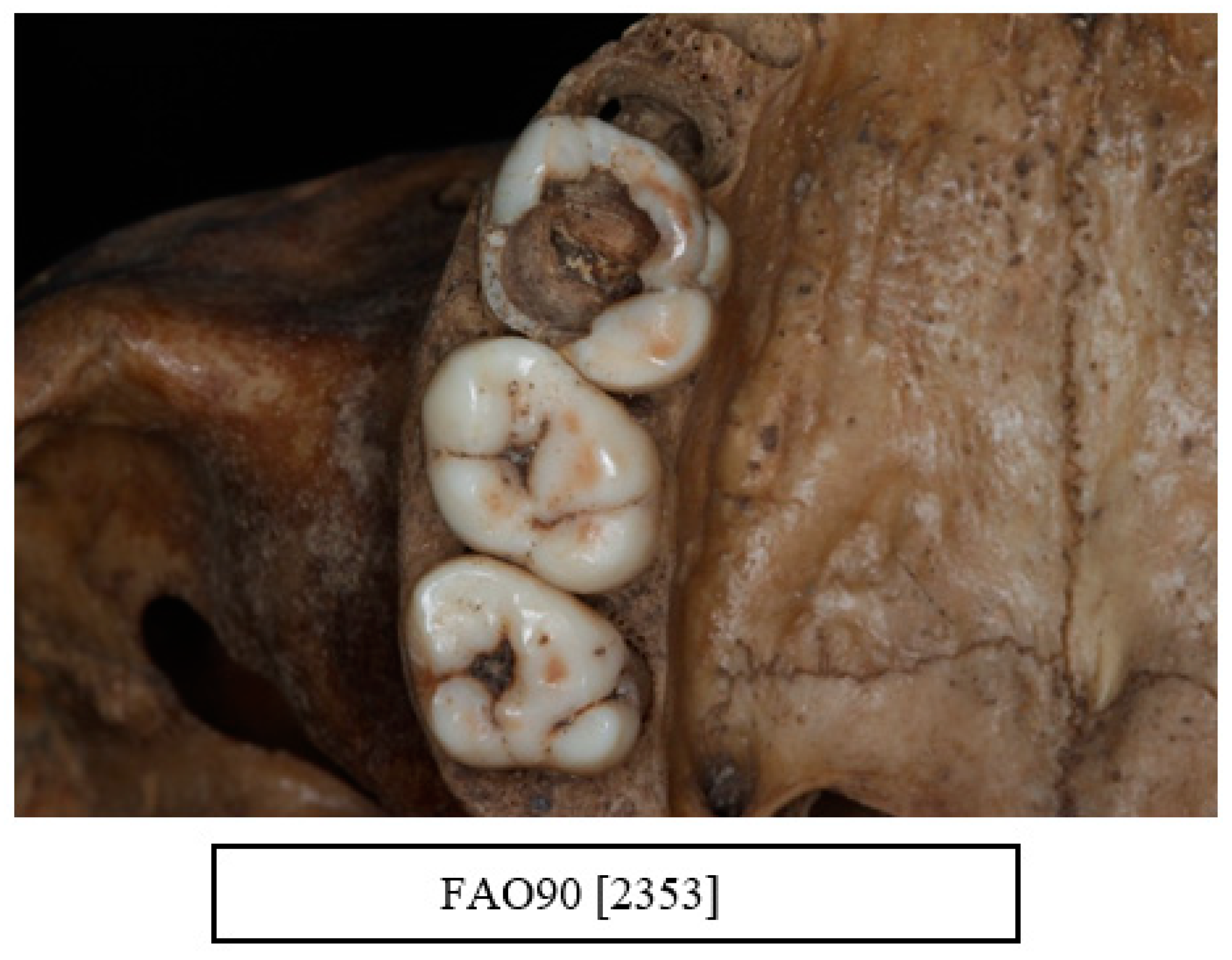
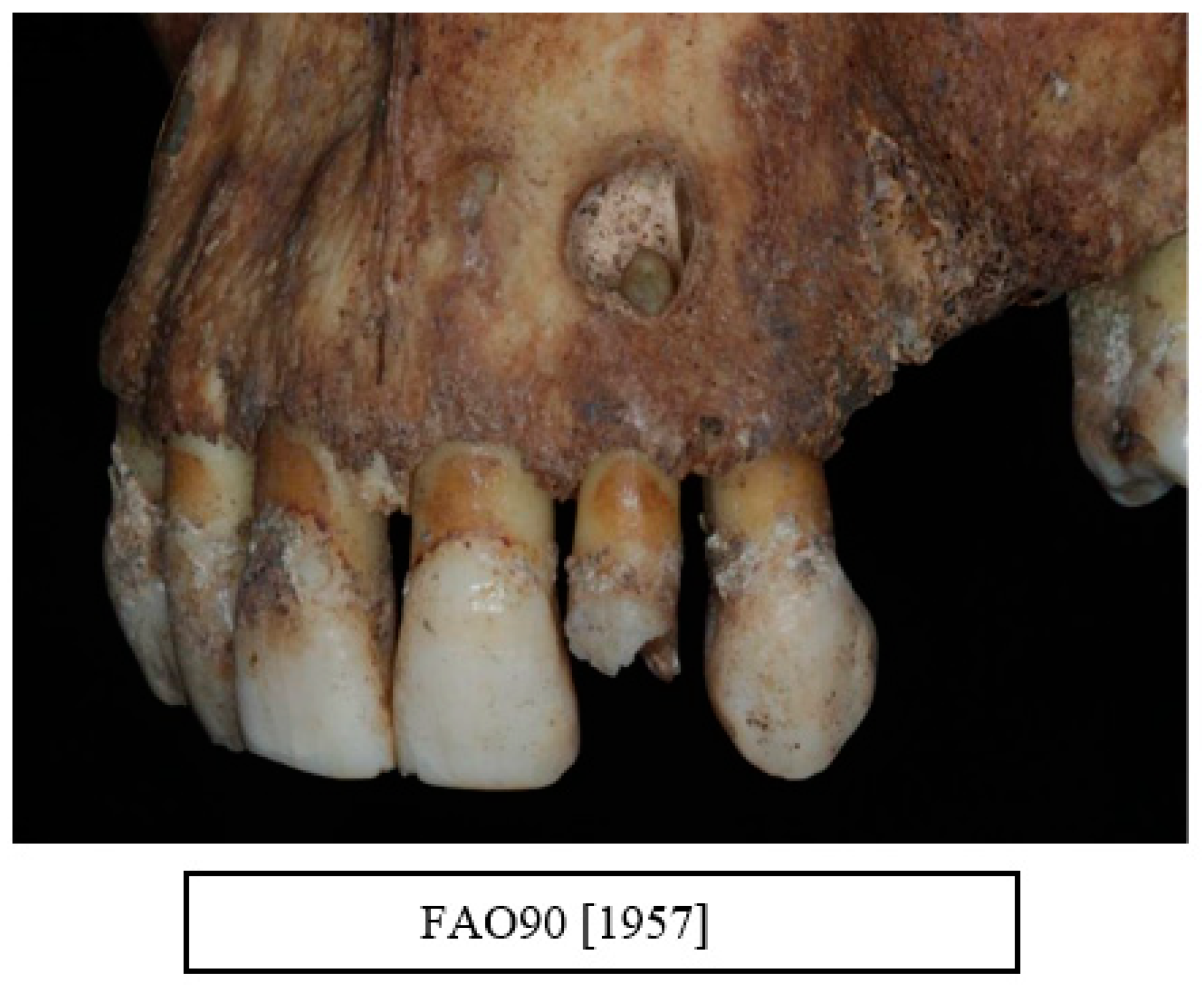
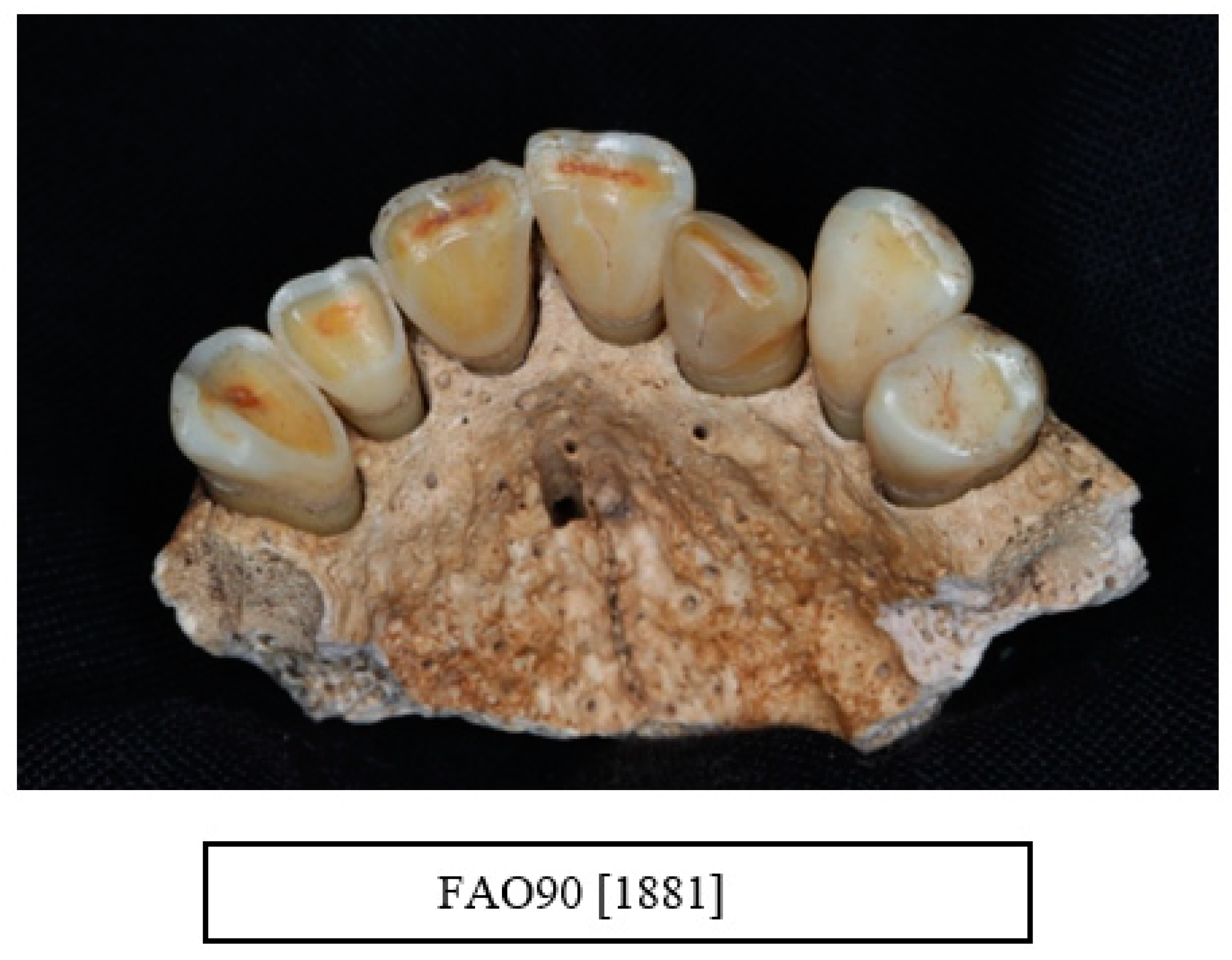
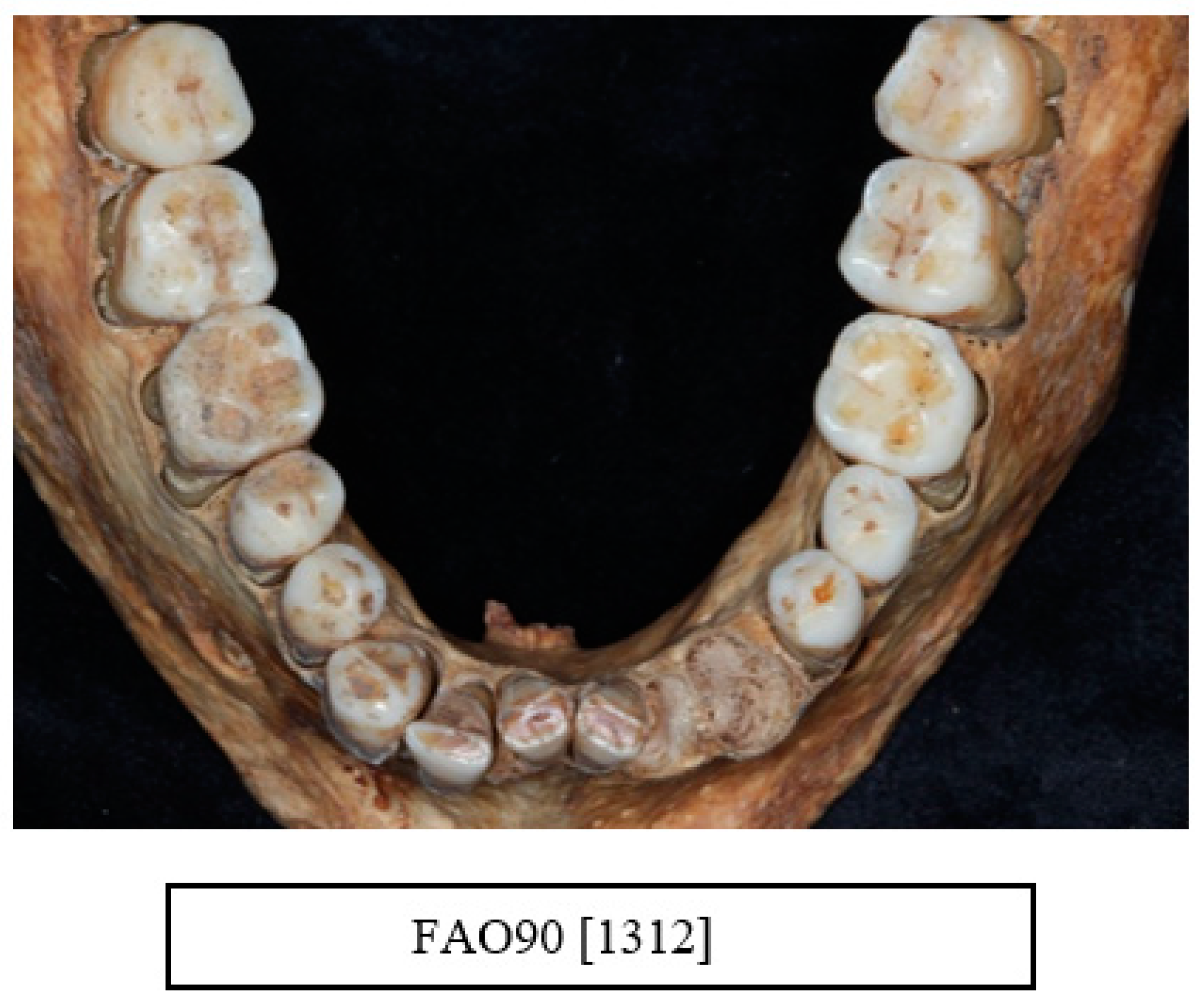
| Data Collected from Each Skull |
|---|
| Age |
| Sex |
| Bone present (mandible/maxilla) |
| Teeth present |
| Teeth lost ante-mortem (AMTL) |
| Teeth lost post-mortem |
| Tooth surface loss |
| Bone loss |
| Pattern of bone loss (horizontal or vertical) |
| Bone defect |
| Calculus |
| Caries |
| Other pathologies/anomalies (furcation, enamel hypoplasia, enamel projection) |
| Dental Pathology/Anomalies | Recorded as |
|---|---|
| Caries | Present or absent per tooth |
| Calculus | Present or absent per tooth |
| Furcation of the tooth | Present or absent per tooth |
| Periapical lesion | Present or absent per tooth |
| Pulp exposure | Present or absent per tooth |
| Fracture | Present or absent per tooth |
| Dehiscence | Present or absent per tooth |
| Fenestration | Present or absent per tooth |
| Enamel hypoplasia | Present or absent per skull |
| Supernumerary teeth | Present or absent per tooth |
| Enamel projection | Present or absent per tooth |
| Age Group | Number of Individuals | % of Individuals | Number of Teeth |
|---|---|---|---|
| 18–25 years | 5 | 83.3% | 4.8 |
| 26–35 years | 19 | 95.0% | 4.1 |
| 36–45 years | 31 | 86.1% | 3.9 |
| 46+ years | 40 | 93.0% | 8.8 |
| Total | 95 | 90.5% | 6.0 |
| Age Group | Number of Individuals | % of Individuals | Average Number of Teeth |
|---|---|---|---|
| 18–25 years | 5 | 83.3% | 6.8 |
| 26–35 years | 18 | 90.0% | 5.5 |
| 36–45 years | 28 | 77.8% | 5.0 |
| 46+ years | 34 | 79.1% | 3.6 |
| Total | 85 | 81.0% | 4.6 |
| Age Group | Number of Adults | % Adults with Bone Loss |
|---|---|---|
| 18–26 years | 2 | 33.3 |
| 26–35 years | 7 | 35.0 |
| 36–45 years | 13 | 36.1 |
| 46+ years | 17 | 39.5 |
| Total | 39 | 37.1 |
| Age Group | No. of Affected Adults (Case Definition I) | % Affected Adults (Case Definition I) | No. of Affected Adults (Case Definition II) | % Affected Adults (Case Definition II) |
| 18–25 years | 2 | 33.3 | 2 | 33.3 |
| 26–35 years | 2 | 10.0 | 3 | 15.0 |
| 36–45 years | 0 | 0.0 | 9 | 25.0 |
| 46+ years | 18 | 41.9 | 12 | 27.9 |
| Total | 22 | 21.0 | 26 | 24.8 |
| Gender | ||||
| Sex | No. of Affected Adults (Case Definition I) | % Adults (Case Definition I) | No. of Affected Adults (Case Definition II) | % Adults (Case Definition II) |
| Male | 15 | 22.7 | 22 | 33.3 |
| Female | 7 | 17.9 | 4 | 10.3 |
| Total | 22 | 21.0 | 26 | 24.8 |
| Dental Pathologies and Anomalies | Number of Adults | % of Adults | Mean Teeth Affected |
|---|---|---|---|
| Tooth wear (attrition) | 105 | 100 | 16.7 |
| Calculus | 105 | 100 | 19 |
| Furcation of the tooth | 92 | 87.6 | 3.6 |
| Caries | 94 | 89.5 | 4.8 |
| Periapical lesion/bone loss | 42 | 40 | 0.7 |
| Pulp exposure | 53 | 50.5 | 1 |
| Enamel projection | 17 | 16.2 | 0.3 |
| Dehiscence | 22 | 21 | 0.4 |
| Fenestration | 55 | 52.4 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mutairi, R.; Liversidge, H.; Gillam, D.G. Prevalence of Moderate to Severe Periodontitis in an 18–19th Century Sample—St. Bride’s Lower Churchyard (London, UK). Dent. J. 2022, 10, 56. https://doi.org/10.3390/dj10040056
Al-Mutairi R, Liversidge H, Gillam DG. Prevalence of Moderate to Severe Periodontitis in an 18–19th Century Sample—St. Bride’s Lower Churchyard (London, UK). Dentistry Journal. 2022; 10(4):56. https://doi.org/10.3390/dj10040056
Chicago/Turabian StyleAl-Mutairi, Ruqayah, Helen Liversidge, and David Geoffrey Gillam. 2022. "Prevalence of Moderate to Severe Periodontitis in an 18–19th Century Sample—St. Bride’s Lower Churchyard (London, UK)" Dentistry Journal 10, no. 4: 56. https://doi.org/10.3390/dj10040056
APA StyleAl-Mutairi, R., Liversidge, H., & Gillam, D. G. (2022). Prevalence of Moderate to Severe Periodontitis in an 18–19th Century Sample—St. Bride’s Lower Churchyard (London, UK). Dentistry Journal, 10(4), 56. https://doi.org/10.3390/dj10040056






