Assessment of Oral Human Papillomavirus Prevalence in Pediatric and Adult Patients within a Multi-Ethnic Clinic Population
Abstract
:1. Introduction
2. Study Methods
2.1. Study Approval
2.2. Original Collection Protocol
2.3. DNA Isolation and Quantification
2.4. Real-Time qPCR Screening
- Glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
- GAPDH forward:
- 5′-ATCTTCCAGGAGCGAGATCC-3′; 20 nt, 55% GC, Tm: 66 °C
- GAPDH reverse:
- 5′-ACCACTGACACGTTGGCAGT-3′; 20 nt, 55% GC, Tm: 70 °C
- Optimal PCR Tm: 61 °C
- HPV16
- Forward primer:
- 5′-ATGTTTCAGGACCCACAGGA-3′; 20 nt; 50% GC: Tm = 66 °C
- Reverse primer:
- 5′-CCTCACGTCGCAGTAACTGT-3′; 20 nt; 55% Tm = 67 °C
- Optimal PCR Tm: 65 °C
- HPV18
- Forward primer:
- 5′-ATGGCGCGCTTTGAGGATCC-3′; 20 nt; 60% GC: Tm = 71 °C
- Reverse primer:
- 5′-GCATGCGGTATACTGTCTCT-3′; 20 nt; 50% GC: Tm = 64 °C
- Optimal PCR Tm: 63 °C
2.5. Statistical Analysis
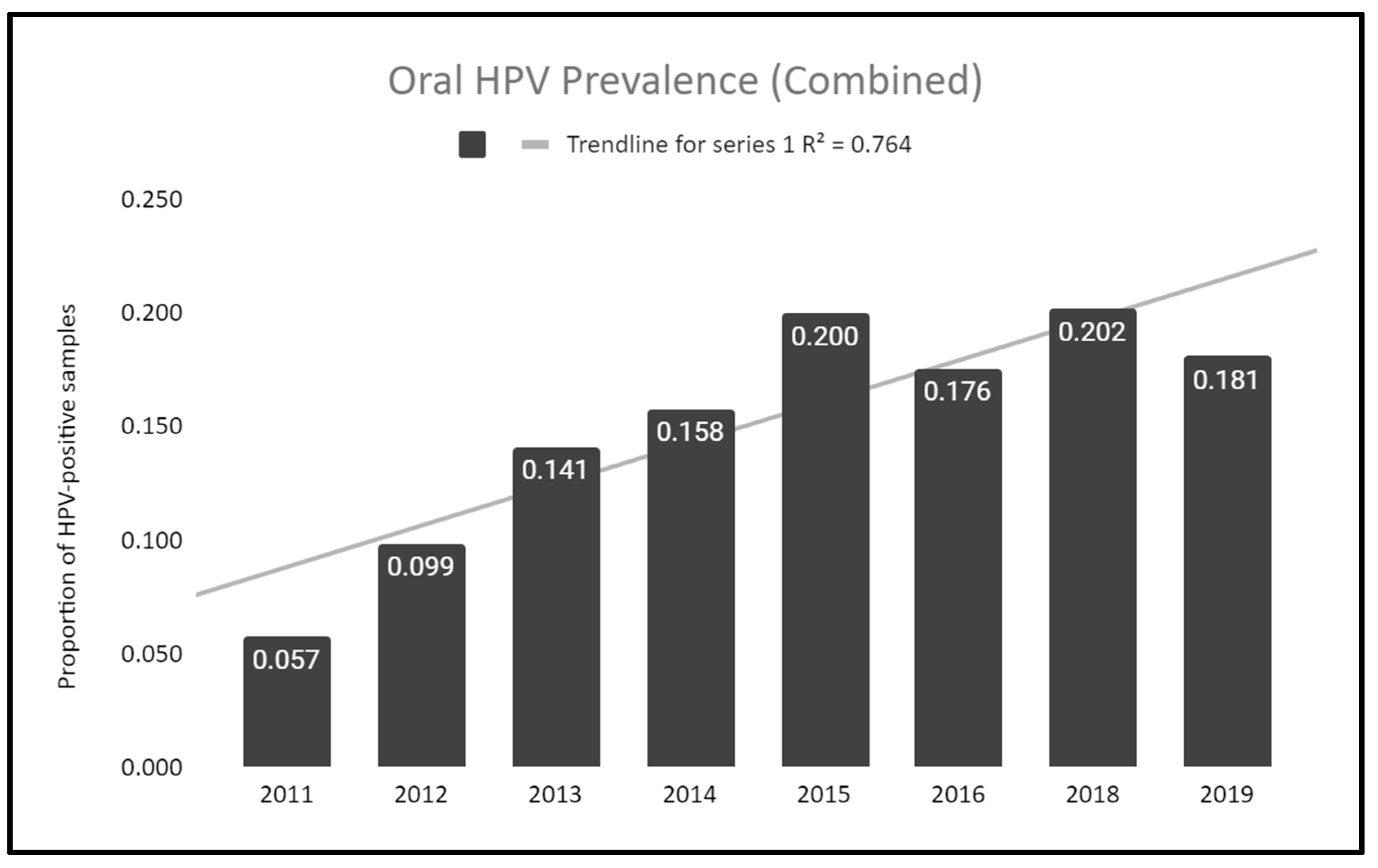
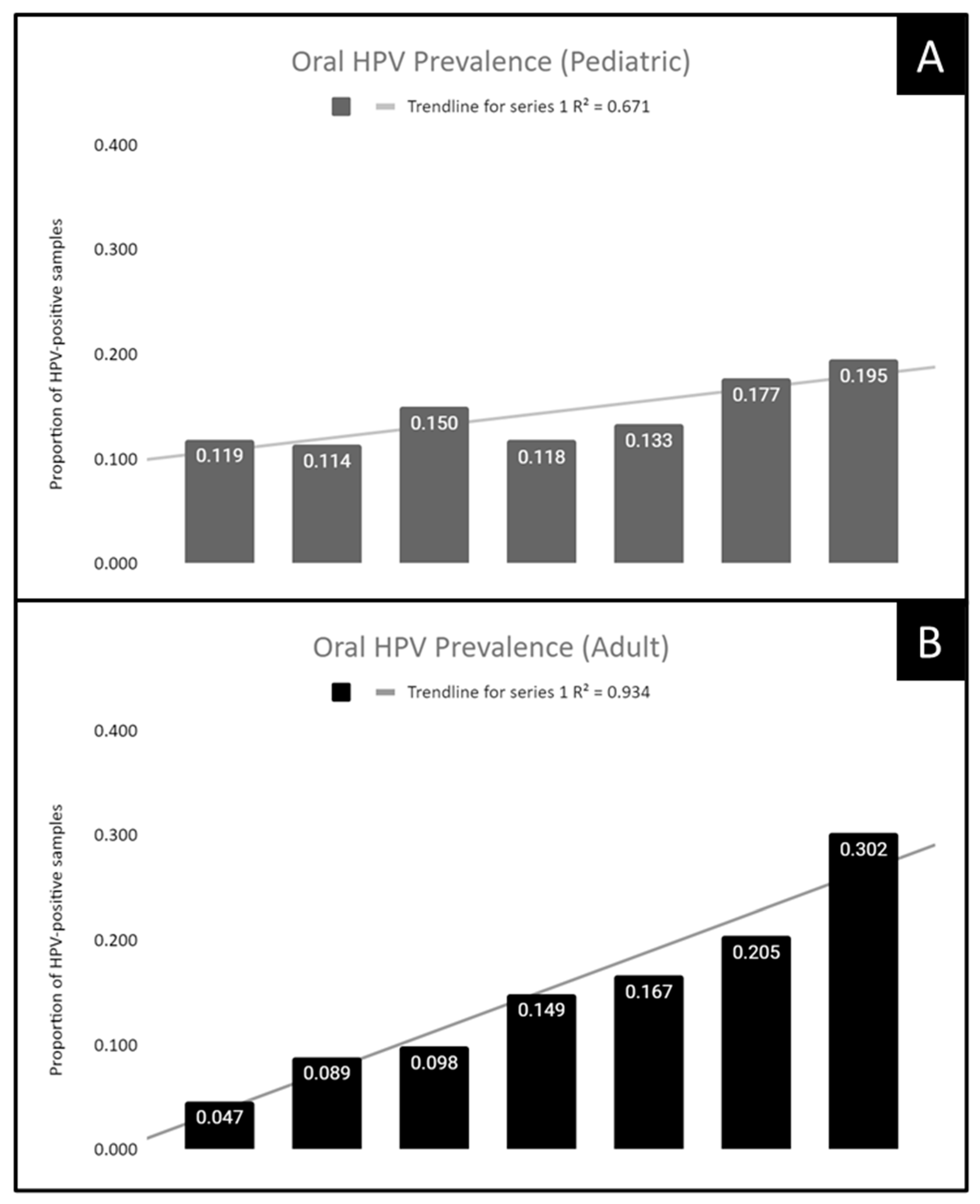
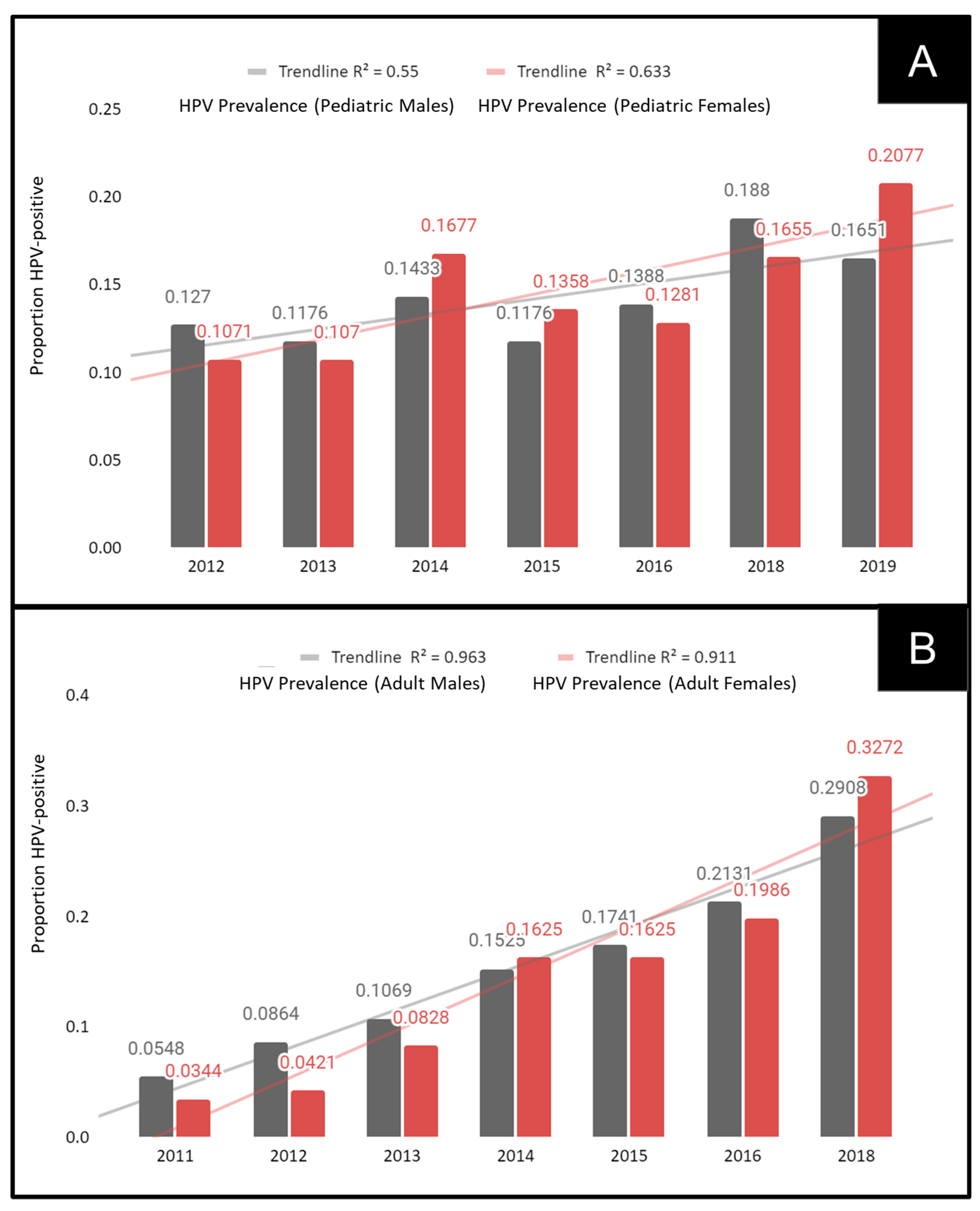
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Brianti, P.; De Flammineis, E.; Mercuri, S.R. Review of HPV-related diseases and cancers. New Microbiol. 2017, 40, 80–85. [Google Scholar] [PubMed]
- Jiang, S.; Dong, Y. Human papillomavirus and oral squamous cell carcinoma: A review of HPV-positive oral squamous cell carcinoma and possible strategies for future. Curr. Probl. Cancer 2017, 41, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Doorbar, J. The low-risk papillomaviruses. Virus Res. 2017, 231, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M. Pathology and epidemiology of HPV infection in females. Gynecol. Oncol. 2010, 117 (Suppl. S2), S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Yusupov, A.; Popovsky, D.; Mahmood, L.; Kim, A.S.; Akman, A.E.; Yuan, H. The nonavalent vaccine: A review of high-risk HPVs and a plea to the CDC. Am. J. Stem Cells 2019, 8, 52–64. [Google Scholar]
- De Sanjosé, S.; Brotons, M.; Pavon, M.A. The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Sabatini, M.E.; Chiocca, S. Human papillomavirus as a driver of head and neck cancers. Br. J. Cancer 2019, 122, 306–314. [Google Scholar] [CrossRef]
- Yete, S.; D’Souza, W.; Saranath, D. High-Risk Human Papillomavirus in Oral Cancer: Clinical Implications. Oncology 2018, 94, 133–141. [Google Scholar] [CrossRef]
- Timbang, M.R.; Sim, M.W.; Bewley, A.F.; Farwell, D.G.; Mantravadi, A.; Moore, M.G. HPV-related oropharyngeal cancer: A review on burden of the disease and opportunities for prevention and early detection. Hum. Vaccines Immunother. 2019, 15, 1920–1928. [Google Scholar] [CrossRef]
- Simonidesová, S.; Hamšíková, E.; Klozar, J.; Tachezy, R. The prevalence of oral HPV infection in healthy populations: A systematic review with a focus on European populations. Epidemiol. Mikrobiol. Imunol. 2019, 67, 175–183. [Google Scholar]
- Rao, S.V.K.; Mejia, G.; Roberts-Thomson, K.; Logan, R. Epidemiology of Oral Cancer in Asia in the Past Decade—An Update (2000–2012). Asian Pac. J. Cancer Prev. 2013, 14, 5567–5577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aswathy, S.; Reshma, J.; Avani, D. Epidemiology of cervical cancer with special focus on India. Int. J. Women’s Health 2015, 7, 405–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, S.; Vuitton, L.; Sheyhidin, I.; Vuitton, D.A.; Zhang, Y.; Lu, X. Northwestern China: A place to learn more on oesophageal cancer. Part one: Behavioural and environmental risk factors. Eur. J. Gastroenterol. Hepatol. 2010, 22, 917–925. [Google Scholar] [CrossRef]
- Williamson, A.-L.; Passmore, J.-A.; Rybicki, E.; Marais, D.; Rybicki, E. Human Papillomavirus (HPV) Infection in Southern Africa: Prevalence, Immunity, and Vaccine Prospects. IUBMB Life 2002, 53, 253–258. [Google Scholar] [CrossRef]
- Colpani, V.; Falcetta, F.S.; Bidinotto, A.B.; Kops, N.L.; Falavigna, M.; Hammes, L.S.; Benzaken, A.S.; Maranhão, A.G.K.; Domingues, C.M.A.S.; Wendland, E.M. Prevalence of human papillomavirus (HPV) in Brazil: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0229154. [Google Scholar] [CrossRef] [Green Version]
- Nasman, A.; Du, J.; Dalianis, T. A global epidemic increase of an HPV induced tonsil and tongue-base cancer-potential benefit from a pan-gender use of HPV vaccine. J. Intern. Med. 2019, 287, 134–152. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.A.; Mehta, V. The Growing Epidemic of HPV-Positive Oropharyngeal Carcinoma: A Clinical Review for Primary Care Providers. J. Am. Board Fam. Med. 2015, 28, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Cleveland, J.L.; Junger, M.L.; Saraiya, M.; Markowitz, L.E.; Dunne, E.F.; Epstein, J.B. The connection between human papillomavirus and oropharyngeal squamous cell carcinomas in the United States. J. Am. Dent. Assoc. 2011, 142, 915–924. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Pharr, J.R.; Kachen, A.; Cross, C. Health Disparities Among Sexual Gender Minority Women in the United States: A Population-Based Study. J. Community Health 2019, 44, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Pabayo, R.; Cook, D.M.; Harling, G.; Gunawan, A.; Rosenquist, N.A.; Muennig, P.; Pabayo, R.; Cook, D.M.; Harling, G.; Gunawan, A.; et al. State-level income inequality and mortality among infants born in the United States 2007–2010: A Cohort Study. BMC Public Health 2019, 19, 1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingsley, K.; O’Malley, S.; Ditmyer, M.; Chino, M. Analysis of oral cancer epidemiology in the US reveals state-specific trends: Implications for oral cancer prevention. BMC Public Health 2008, 8, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunnell, A.; Pettit, N.; Reddout, N.; Sharma, K.; O’Malley, S.; Chino, M.; Kingsley, K. Analysis of primary risk factors for oral cancer from select US states with increasing rates. Tob. Induc. Dis. 2010, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Callahan, K.E.; Pinheiro, P.S.; Cvijetic, N.; Kelly, R.E.; Ponce, C.P.; Kobetz, E.N. Worse Breast Cancer Outcomes for Southern Nevadans, Filipina and Black Women. J. Immigr. Minor. Health 2016, 19, 1330–1337. [Google Scholar] [CrossRef]
- Siegel, R.L.; Fedewa, S.A.; Miller, K.D.; Goding-Sauer, A.; Pinheiro, P.S.; Martinez-Tyson, D.; Jemal, A. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J. Clin. 2015, 65, 457–480. [Google Scholar] [CrossRef] [Green Version]
- O’Turner, D.; Williams-Cocks, S.J.; Bullen, R.; Catmull, J.; Falk, J.; Martin, D.; Mauer, J.; Barber, A.E.; Wang, R.C.; Gerstenberger, S.L.; et al. High-risk human papillomavirus (HPV) screening and detection in healthy patient saliva samples: A pilot study. BMC Oral Health 2011, 11, 28. [Google Scholar] [CrossRef] [Green Version]
- Flake, C.; Arafa, J.; Hall, A.; Ence, E.; Howard, K.; Kingsley, K. Screening and detection of human papillomavirus (HPV) high-risk strains HPV16 and HPV18 in saliva samples from subjects under 18 years old in Nevada: A pilot study. BMC Oral Health 2012, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Tiku, V.; Todd, C.J.; Kingsley, K. Assessment of Oral Human Papillomavirus Prevalence in a Multi-ethnic Pediatric Clinic Population. Compend. Contin. Educ. Dent. 2016, 37, e1–e4. [Google Scholar]
- Brouwer, A.F.; Campredon, L.P.; Walline, H.M.; Marinelli, B.M.; Goudsmit, C.M.; Thomas, T.B.; Delinger, R.L.; Lau, Y.K.; Andrus, E.C.; Nair, T.; et al. Incidence and clearance of oral and cervicogenital HPV infection: Longitudinal analysis of the MHOC cohort study. BMJ Open 2022, 12, e056502. [Google Scholar] [CrossRef]
- Giuliani, E.; Rollo, F.; Donà, M.G.; Garbuglia, A.R. Human Papillomavirus Oral Infection: Review of Methodological Aspects and Epidemiology. Pathogens 2021, 10, 1411. [Google Scholar] [CrossRef] [PubMed]
- Morais, E.; Kothari, S.; Roberts, C.; Yen, G.; Chen, Y.-T.; Lynam, M.; Pedrós, M.; Mirghani, H.; Alemany, L.; Pavon, M.A.; et al. Oral human papillomavirus (HPV) and associated factors among healthy populations: The design of the PROGRESS (PRevalence of Oral hpv infection, a Global aSSessment) study. Contemp. Clin. Trials 2021, 25, 106630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; D’Souza, G.; Fakhry, C.; O’Bigelow, E.; Usyk, M.; Burk, R.D.; Zhao, N. Oral HPV associated with differences in oral microbiota beta diversity and microbiota abundance. J. Infect. Dis. 2022, jiac010. [Google Scholar] [CrossRef] [PubMed]
- Morán-Torres, A.; Pazos-Salazar, N.G.; Téllez-Lorenzo, S.; Jiménez-Lima, R.; Lizano, M.; Reyes-Hernández, D.O.; Marin-Aquino, J.D.J.; Manzo-Merino, J. HPV oral and oropharynx infection dynamics in young population. Braz. J. Microbiol. 2021, 52, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Löffler, P. Review: Vaccine Myth-Buster—Cleaning Up With Prejudices and Dangerous Misinformation. Front. Immunol. 2021, 12, 2220. [Google Scholar] [CrossRef]
- Kaczmarczyk, K.H.; Yusuf, H. The impact of HPV vaccination on the prevention of oropharyngeal cancer: A scoping review. Community Dent. Health, 2021; Epub ahead of print. [Google Scholar]
- McClure, C.C.; Cataldi, J.R.; O’Leary, S.T. Vaccine Hesitancy: Where We Are and Where We Are Going. Clin. Ther. 2017, 39, 1550–1562. [Google Scholar] [CrossRef] [Green Version]
- Stahl, J.-P.; Cohen, R.; Denis, F.; Gaudelus, J.; Martinot, A.; Lery, T.; Lepetit, H. The impact of the web and social networks on vaccination. New challenges and opportunities offered to fight against vaccine hesitancy. Med. Mal. Infect. 2016, 46, 117–122. [Google Scholar] [CrossRef]
- Mammas, I.N.; Dalianis, T.; Doukas, S.G.; Zaravinos, A.; Achtsidis, V.; Thiagarajan, P.; Theodoridou, M.; Spandidos, D.A. Paediatric virology and human papillomaviruses: An update. Exp. Ther. Med. 2019, 17, 4337–4343. [Google Scholar] [CrossRef]
- Dean, T.C.; Gilliland, A.E.; Cameron, J.E. Parents’ receptiveness to oral health clinic-based vaccination. Vaccine 2020, 38, 4226–4229. [Google Scholar] [CrossRef]
- Alawi, F. Oral health care providers should be administering vaccines. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 131, 267–268. [Google Scholar] [CrossRef]
- Geoghegan, S.; O’Callaghan, K.P.; Offit, P.A. Vaccine Safety: Myths and Misinformation. Front. Microbiol. 2020, 11, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, S.K.; Kingsley, K. Human Papillomavirus (HPV) Vaccine Knowledge, Awareness and Acceptance among Dental Students and Post-Graduate Dental Residents. Dent. J. 2020, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Rutkoski, H.; Tay, D.L.; Dixon, B.L.; Pinzon, L.M.; Mooney, R.; Winkler, J.R.; Kepka, D. A Multi-state Evaluation of Oral Health Students’ Knowledge of Human Papillomavirus-Related Oropharyngeal Cancer and HPV Vaccination. J. Cancer Educ. 2020, 35, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Kepka, D.; Rutkoski, H.; Pappas, L.; Tay, D.L.; Winkler, J.R.; Dixon, B.; Velazquez, A.; Pinzon, L.M. US oral health students’ willingness to train and administer the HPV vaccine in dental practices. Prev. Med. Rep. 2019, 15, 100957. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.L.; Tay, D.; Kaiser, D.; Praag, A.; Rutkoski, H.; Dixon, B.L.; Pinzon, L.M.; Winkler, J.R.; Kepka, D. The perspectives, barriers, and willingness of Utah dentists to engage in human papillomavirus (HPV) vaccine practices. Hum. Vaccines Immunother. 2019, 16, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.K.; Jackson, R.D.; Sommariva, S.; Neelamegam, M.; Desch, J. USA dental health providers’ role in HPV vaccine communication and HPV-OPC protection: A systematic review. Hum. Vaccines Immunother. 2019, 15, 1863–1869. [Google Scholar] [CrossRef]
- Daley, E.M.; Thompson, E.L.; Beckstead, J.; Driscoll, A.; Vamos, C.; Piepenbrink, R.P.; Desch, J.; Merrell, L.; Cayama, M.B.R.; Owens, H.; et al. Discussing HPV and oropharyngeal cancer in dental settings: Gender and provider-type matter. Hum. Vaccines Immunother. 2021, 17, 5454–5459. [Google Scholar] [CrossRef]
- Murphy, C.C.; Yang, Y.C. Use of Age-Period-Cohort Analysis in Cancer Epidemiology Research. Curr. Epidemiol. Rep. 2018, 5, 418–431. [Google Scholar] [CrossRef]
- Foote, K.; Foote, D.; Kingsley, K. Surveillance of the Incidence and Mortality of Oral and Pharyngeal, Esophageal, and Lung Cancer in Nevada: Potential Implications of the Nevada Indoor Clean Air Act. Int. J. Environ. Res. Public Health 2021, 18, 7966. [Google Scholar] [CrossRef]
- La Fauci, V.; Squeri, R.; Genovese, C.; Anzalone, C.; Fedele, F.; Squeri, A.; Alessi, V. An observational study of university students of healthcare area: Knowledge, attitudes and behaviour towards vaccinations. Clin. Ter. 2019, 170, e448–e453. [Google Scholar] [CrossRef]
- Marti, M.; de Cola, M.; Macdonald, N.E.; Dumolard, L.; Duclos, P. Assessments of global drivers of vaccine hesitancy in 2014—Looking beyond safety concerns. PLoS ONE 2017, 12, e0172310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, S.; MacDonald, N.E.; Marti, M.; Dumolard, L. Vaccine hesitancy around the globe: Analysis of three years of WHO/UNICEF Joint Reporting Form data-2015–2017. Vaccine 2018, 36, 3861–3867. [Google Scholar] [CrossRef]
- Kulkarni, S.; Harvey, B.; Prybylski, D.; Jalloh, M.F. Trends in classifying vaccine hesitancy reasons reported in the WHO/UNICEF Joint Reporting Form, 2014–2017: Use and comparability of the Vaccine Hesitancy Matrix. Hum. Vaccines Immunother. 2021, 17, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xue, H.; Calabrese, C.; Chen, H.; Dang, J.H.T. Understanding Human Papillomavirus Vaccine Promotions and Hesitancy in Northern California through Examining Public Facebook Pages and Groups. Front. Digit. Health 2021, 3, 62. [Google Scholar] [CrossRef] [PubMed]

| Demographics | Study Sample | Clinic | Statistical Analysis |
|---|---|---|---|
| Sex | |||
| Adult—Males | N = 269 (48.5%) | 50.9% | χ2 = 2.305, d.f. = 1 p = 0.1290 |
| Adult—Females | N = 286 (51.5%) | 49.1% | |
| Race/Ethnicity | |||
| White | N = 226 (40.7%) | 34.6% | χ2 = 16.444, d.f. = 1 p = 0.0001 |
| Minority | N = 329 (59.3%) | 65.4% | |
| Hispanic | N = 130 (23.4%) | 58.6% | |
| Black | N = 108 (19.5%) | 10.2% | |
| Asian/Other | N = 91 (16.4%) | 6.6% | |
| Age | |||
| Average/Range Median | 42.80 yrs. 42 yrs. | 42.31 yrs. 41 yrs. | Two-tailed t-test p = 0.411 |
| Range | 18–88 yrs. | 18–89 yrs. | |
| Sex | |||
| Pediatric—Males | N = 174 (46.4%) | 47.2% | χ2 = 0.257, d.f. = 1 p = 0.6123 |
| Pediatric—Females | N = 201 (53.6%) | 52.8% | |
| Race/Ethnicity | |||
| White | N = 81 (21.6%) | 24.7% | χ2 = 0.480, d.f. = 1 p = 0.4884 |
| Minority | N = 294 (78.4%) | 75.3% | |
| Hispanic | N = 118 (31.5%) | 52.1% | |
| Black | N = 102 (27.2%) | 11.8% | |
| Asian/Other | N = 75 (20.0%) | 11.4% | |
| Age | |||
| Average/Range Median | 13.03 yrs. 12 yrs. | 10.44 yrs. 10 yrs. | Two-tailed t-test p = 0.019 |
| Range | 5–17 yrs. | 0–17 yrs. |
| Study Sample | DNA Concentration | DNA Purity (A260:A80) |
|---|---|---|
| Pediatric samples N = 470 | Average: 218.4 ng/uL ± 88.2 Range: 110–631.1 ng/uL | Average: 1.74 Range: 1.70–1.79 |
| Adult samples N = 460 | Average: 334.1 ng/uL ± 91.1 Range: 135–815.1 ng/uL | Average: 1.76 Range: 1.70–1.81 |
| Year | HPV-Positive Samples | HPV-Negative Samples | Total Sample Number | Proportion of HPV-Positives |
|---|---|---|---|---|
| 2011 | n = 5 | n = 82 | n = 87 | 0.057 |
| 2012 | n = 15 | n = 137 | n = 152 | 0.099 |
| 2013 | n = 11 | n = 67 | n = 78 | 0.141 |
| 2014 | n = 21 | n = 112 | n = 133 | 0.158 |
| 2015 | n = 13 | n = 52 | n = 65 | 0.200 |
| 2016 | n = 13 | n = 74 | n = 87 | 0.176 |
| 2018 | n = 40 | n = 199 | n = 229 | 0.202 |
| 2019 | n = 15 | n = 83 | n = 98 | 0.181 |
| Total | n = 133 | n = 806 | N = 930 |
| Year | Pediatric | Adult |
|---|---|---|
| 2011 | N/A | Average: 20.8 years Range (18–26 years) |
| 2012 | Average: 16.0 years Range (15–17 years) | Average: 26.25 years Range (18–55 years) |
| 2013 | Average: 16.0 years Range (15–17 years) | Average: 21.3 years Range (18–27 years) |
| 2014 | Average: 15.7 years Range (14–17 years) | Average: 26.9 years Range (18–41 years) |
| 2015 | Average: 15.6 years Range (13–17 years) | Average: 29.1 years Range (18–66 years) |
| 2016 | Average: 14.3 years Range (12–16 years) | Average: 34.3 years Range (23–52 years) |
| 2018 | Average: 14.68 years Range (13–17 years) | Average: 28.9 years Range (18–49 years) |
| 2019 | Average: 14.81 years Range (14–17 years) | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornhaber, M.S.; Florence, T.; Davis, T.; Kingsley, K. Assessment of Oral Human Papillomavirus Prevalence in Pediatric and Adult Patients within a Multi-Ethnic Clinic Population. Dent. J. 2022, 10, 54. https://doi.org/10.3390/dj10040054
Kornhaber MS, Florence T, Davis T, Kingsley K. Assessment of Oral Human Papillomavirus Prevalence in Pediatric and Adult Patients within a Multi-Ethnic Clinic Population. Dentistry Journal. 2022; 10(4):54. https://doi.org/10.3390/dj10040054
Chicago/Turabian StyleKornhaber, Melissa Solomon, Taylor Florence, Trexton Davis, and Karl Kingsley. 2022. "Assessment of Oral Human Papillomavirus Prevalence in Pediatric and Adult Patients within a Multi-Ethnic Clinic Population" Dentistry Journal 10, no. 4: 54. https://doi.org/10.3390/dj10040054
APA StyleKornhaber, M. S., Florence, T., Davis, T., & Kingsley, K. (2022). Assessment of Oral Human Papillomavirus Prevalence in Pediatric and Adult Patients within a Multi-Ethnic Clinic Population. Dentistry Journal, 10(4), 54. https://doi.org/10.3390/dj10040054






