Effect of Photobiomodulation on Atrophic–Erosive Clinical Forms of Oral Lichen Planus: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of the Studies and Eligibility Criteria: Inclusion and Exclusion Criteria
2.2. Data Extraction
2.3. Quality Evaluation
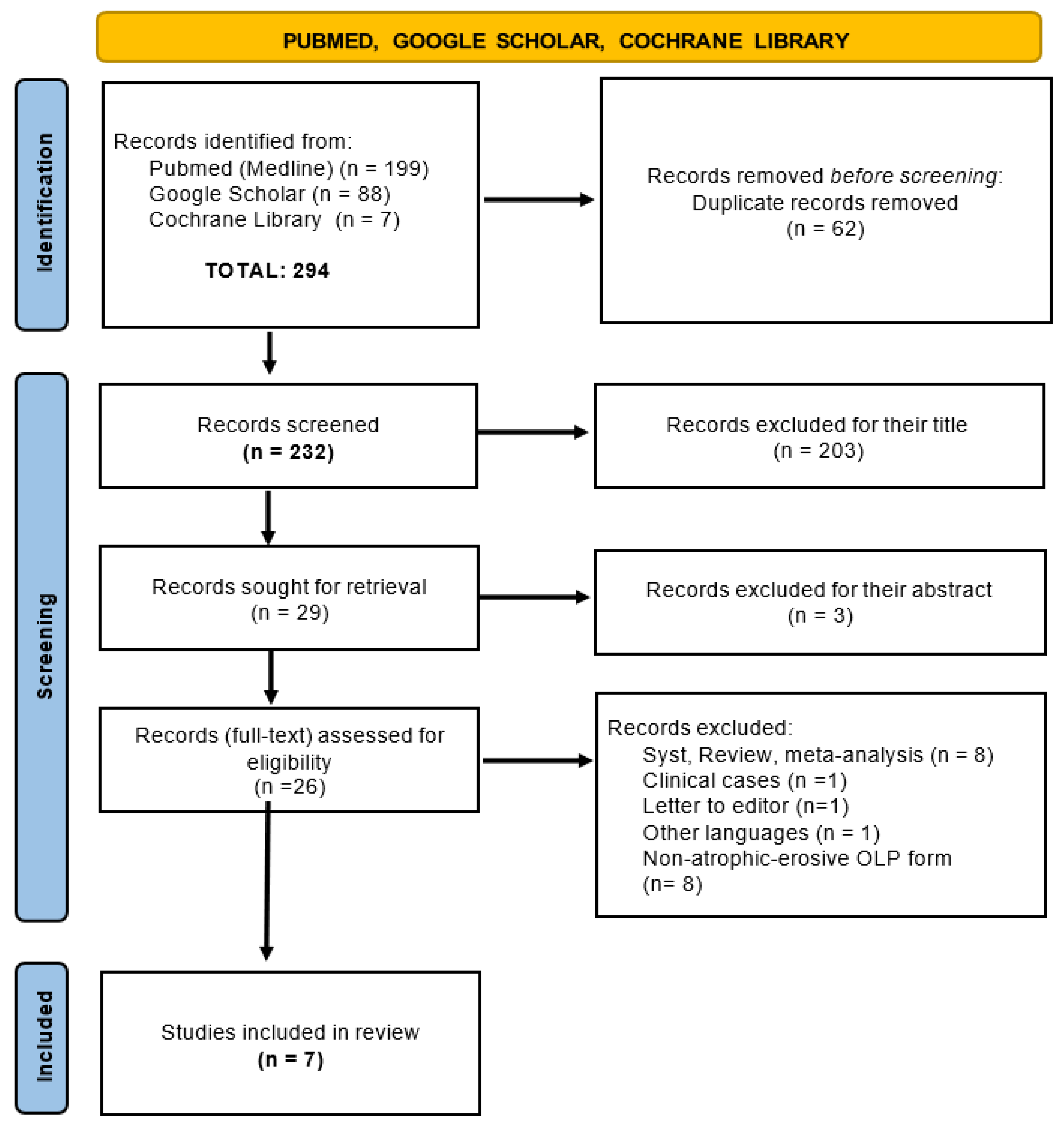
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sayáns, M.; Lorenzo-Pouso, A.I.; Chamorro-Petronacci, C.M.; Suárez-Peñaranda, J.M.; Padín-Iruegas, E.; González-Moles, M.A.; Marichalar-Mendía, X.; García-García, A.; Blanco-Carrión, A. Immunoexpression of apoptosis and cell-cycle arrest markers in oral lichen planus. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.L.P.V.; da Silva Simoura, J.A.; Cerqueira, J.D.M.; de Oliveira Lima-Arsati, Y.B.; Arsati, F.; Dos Santos, J.N.; Freitas, V.S. Relationship of psychological factors with salivary flow rate and cortisol levels in individuals with oral lichen planus: A case-control study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 675–680. [Google Scholar] [CrossRef]
- Shaw, H.; Konidena, A.; Malhotra, A.; Yumnam, N.; Farooq, F.; Bansal, V. Psychological status and uric acid levels in oral lichen planus patients–A case-control study. Indian J. Dent. Res. 2020, 31, 368. [Google Scholar] [PubMed]
- Li, K.; He, W.; Hua, H. Characteristics of the psychopathological status of oral lichen planus: A systematic review and meta-analysis. Aust. Dent. J. 2022, 2, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Fenoll, A.; Sánchez-Siles, M.; López-Jornet, P.; Camacho-Alonso, F.; Salazar-Sánchez, N. A retrospective clinicopatholo-gical study of 550 patients with oral lichen planus in south-eastern Spain. J. Oral Pathol. Med. 2010, 39, 491–496. [Google Scholar] [CrossRef]
- Nosratzehi, T. Oral lichen planus: An overview of potential risk factors, biomarkers and treatments. Asian Pac. J. CancerPrev. APJCP 2018, 19, 1161. [Google Scholar]
- Carrozzo, M.; Porter, S.; Mercadante, V.; Fedele, S. Oral lichen planus: A disease or a spectrum of tissue reactions? Types, causes, diagnostic algorhythms, prognosis, management strategies. Periodontology 2000 2019, 80, 105–125. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Johnson, N.W.; Van der Waal, I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Ruiz-Avila, I.; Gonzalez-Ruiz, L.; Ayen, A.; Gil-Montoya, J.A.; Ramos-Garcia, P. Malignant transformation risk of oral lichen planus: A systematic review and comprehensive meta-analysis. Oral Oncol. 2019, 96, 121–130. [Google Scholar] [CrossRef]
- Amin, N.R.; Yussif, N.; Ahmed, E. The effect of smoking on clinical presentation and expression of TLR-2 and CD34 in Oral lichen Planus patients: Clinical and immunohistochemical study. BMC Oral Health 2020, 20, 129. [Google Scholar] [CrossRef] [PubMed]
- Zotti, F.; Nocini, R.; Capocasale, G.; Bertossi, D.; Fior, A.; Peretti, M.; Manfrin, E.; Albanese, M. Oral Lichen Planus: Risk factors of malignant transformation and follow up. Ten years retrospective study. J. Clin. Exp. Dent. 2021, 13, e630. [Google Scholar] [CrossRef] [PubMed]
- Caruntu, C.; Mitran, M.; Mitran, C.; Sarbu, I.; Rusu, L.C.; Matei, C.; Constantin, C.; Neagu, M.; Georgescu, S.R. Markers of oral lichen planus malignant transformation. Dis. Markers 2018, 2018, 1959506. [Google Scholar]
- García-Pola, M.J.; González-Álvarez, L.; Garcia-Martin, J.M. Tratamiento del liquen plano oral. Revisión sistemática y protocol de actuación. Med. Clin. 2017, 149, 351–362. [Google Scholar] [CrossRef]
- Suvarna, C.; Chaitanya, N.C.; Ameer, S.; Mannava, H.; Bontala, P.; Alyami, J.S.; Samreen, H.; Kondapaneni, J. A comparative evaluation on the effect of oral zinc 50 mg with or without 0.1% triamcinolone orabase on oral lichen planus. Int. J. Appl. Basic Med. Res. 2020, 10, 54. [Google Scholar]
- Chiang, C.P.; Chang, J.Y.F.; Wang, Y.P.; Wu, Y.H.; Lu, S.Y.; Sun, A. Oral lichen planus–differential diagnoses, serum autoantibodies, hematinic deficiencies, and management. J. Formos. Med. Assoc. 2018, 117, 756–765. [Google Scholar] [CrossRef]
- Piñas, L.; García-García, A.; Pérez-Sayáns, M.; Suárez-Fernández, R.; Alkhraisat, M.H.; Anitua, E. The use of topical cortices-teroides in the treatment of oral lichen planus in Spain: A national survey. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e264. [Google Scholar]
- Del Vecchio, A.; Palaia, G.; Grassotti, B.; Tenore, G.; Ciolfi, C.; Podda, G.; Impellizzeri, A.; Mohsen, A.; Galluccio, G.; Romeo, U. Effects of laser photobiomodulation in the management of oral lichen planus: A literature review. La Clin. Ter. 2021, 172, 464–467. [Google Scholar]
- Cafaro, A.; Arduino, P.G.; Massolini, G.; Romagnoli, E.; Broccoletti, R. Clinical evaluation of the efficiency of low-level laser therapy for oral lichen planus: A prospective case series. Lasers Med. Sci. 2014, 29, 185–190. [Google Scholar] [CrossRef][Green Version]
- Cronshaw, M.; Parker, S.; Anagnostaki, E.; Mylona, V.; Lynch, E.; Grootveld, M. Photobiomodulation dose parameters in dentistry: A systematic review and meta-analysis. Dent. J. 2020, 8, 114. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Benedicenti, S.; Amaroli, A.; Sălăgean, T.; Pop, I.D.; Todea, D.; Bordea, I.R. Photobiomodulation therapy in oral mucositis and potentially malignant oral lesions: A therapy towards the future. Cancers 2020, 12, 1949. [Google Scholar] [CrossRef] [PubMed]
- Rola, P.; Doroszko, A.; Derkacz, A. The Use of Low-Level Energy Laser Radiation in Basic and Clinical Research. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2014, 23, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, T.; Xing, D.; Wang, F.; Pei, Y.; Wei, X. Single cell analysis of PKC activation during proliferation and apoptosis induced by laser irradiation. J. Cell. Physiol. 2006, 206, 441–448. [Google Scholar] [CrossRef]
- de Carvalho, M.M.; Hidalgo, M.A.R.; Scarel-Caminaga, R.M.; Ribeiro Junior, N.V.; Sperandio, F.F.; Pigossi, S.C.; de Carli, M.L. Photobiomodulation of gingival lesions resulting from autoimmune diseases: Systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 3949–3964. [Google Scholar] [CrossRef] [PubMed]
- Al-Maweri, S.A.; Kalakonda, B.; Al-Soneidar, W.A.; Al-Shamiri, H.M.; Alakhali, M.S.; Alaizari, N. Efficacy of low-level laser therapy in management of symptomatic oral lichen planus: A systematic review. Lasers Med. Sci. 2017, 32, 1429–1437. [Google Scholar] [CrossRef]
- Nammour, S.; El Mobadder, M.; Brugnera, A.J.; Namour, M.; Houeis, S.; Heysselaer, D.; Vanheusden, A.; Namour, A. Photobiomodulation Therapy vs. Corticosteroid for the Management of Erosive/Ulcerative and Painful Oral Lichen Planus. Assessment of Success Rate during One-Year Follow-Up: A Retrospective Study. Healthcare 2021, 9, 1137. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Robertson, J.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2013. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 21 November 2022).
- Gambino, A.; Cabras, M.; Cafaro, A.; Broccoletti, R.; Carossa, S.; Hopper, C.; Conrotto, D.; Porter, S.R.; Arduino, P.G. Preliminary evaluation of the utility of optical coherence tomography in detecting structural changes during photobiomodulation treatment in patients with atrophic-erosive oral lichen planus. Photodiagnosis Photodyn. Ther. 2021, 34, 102255. [Google Scholar] [CrossRef]
- Mirza, S.; Rehman, N.; Alrahlah, A.; Vohra, F. Efficacy of photodynamic therapy or low level laser therapy against steroid therapy in the treatment of erosive-atrophic oral lichen planus. Photodiagnosis Photodyn. Ther. 2018, 21, 404–408. [Google Scholar] [CrossRef]
- Mutafchieva, M.Z.; Draganova-Filipova, M.N.; Zagorchev, P.I.; Tomov, G.T. Effects of low level laser therapy on erosive-atrophic oral lichen planus. Folia Med. 2018, 60, 417–424. [Google Scholar] [CrossRef]
- Lavaee, F.; Shadmanpour, M. Comparison of the effect of photodynamic therapy and topical corticosteroid on oral lichen planus lesions. Oral Dis. 2019, 25, 1954–1963. [Google Scholar] [CrossRef]
- Khater, M.M.; Khattab, F.M. Efficacy of 1064 Q switched Nd: YAG laser in the treatment of oral lichen planus. J. Dermatol. Treat. 2020, 31, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Tarasenko, S.; Stepanov, M.; Morozova, E.; Unkovskiy, A. High-level laser therapy versus scalpel surgery in the treatment of oral lichen planus: A randomized control trial. Clin. Oral Investig. 2021, 25, 5649–5660. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, A.; Jacomacci, W.; Quinto, J.; Veltrini, V.; Iwaki, L.; Tolentino, E. Potential Malignant Transformation of Oral Lichen Planus: Retrospective Stue y. Int. J. Odontostomat. 2015, 9, 511–517. [Google Scholar] [CrossRef][Green Version]
- Sadeghian, R.; Rohani, B.; Golestannejad, Z.; Sadeghian, S.; Mirzaee, S. Comparison of therapeutic effect of mucoadhesive nano-triamcinolone gel and conventional triamcinolone gel on oral lichen planus. Dent. Res. J. 2019, 16, 277. [Google Scholar]
- Humbert, P.; Guichard, A. The topical corticosteroid classification called into question: Towards a new approach. Exp. Dermatol. 2015, 24, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Boñar-Alvarez, P.; Sayáns, M.P.; Garcia-Garcia, A.; Chamorro-Petronacci, C.; Gándara-Vila, P.; Luces-González, R.; Rey, E.O.; Blanco-Carrión, A.; Suárez-Peñaranda, J. Correlation between clinical and pathological features of oral lichen planus: A retrospective observational study. Medicine 2019, 98, e14614. [Google Scholar] [CrossRef]
- Ferri, E.P.; de Barros Gallo, C.; Abboud, C.S.; Yanaguizawa, W.H.; Horliana, A.C.R.T.; da Silva, D.d.F.T.; Pavani, C.; Bussadori, S.K.; Nunes, F.D.; Mesquita-Ferrari, R.A.; et al. Efficacy of photobiomodulation on oral lichen planus: A protocol study for a double-blind, randomised controlled clinical trial. BMJ Open 2018, 8, e024083. [Google Scholar] [CrossRef]
| PICO Question | Characteristics |
|---|---|
| Patient | Patients with atrophic–erosive OLP |
| Intervention | PBM treatment with laser |
| Comparison | Drugs or laser off |
| Outcome | Remission of symptoms |
| Study | Selection | Comparability | Exposition | Note |
|---|---|---|---|---|
| Gambino et al., 2018 [28] | ⋆⋆ | ⋆ | ⋆⋆ | 5 |
| Mirza et al., 2018 [29] | ⋆⋆ | ⋆⋆ | ⋆⋆ | 6 |
| Mutafchieva et al., 2018 [30] | ⋆⋆ | ⋆⋆ | ⋆ | 5 |
| Lavaee et al., 2019 [31] | ⋆⋆⋆ | ⋆⋆ | ⋆⋆ | 7 |
| Khater et al., 2019 [32] | ⋆⋆⋆ | ⋆⋆ | ⋆ | 6 |
| Nammour et al., 2021 [26] | ⋆⋆ | ⋆⋆ | ⋆ | 5 |
| Tarasenko et al., 2021 [33] | ⋆⋆ | ⋆⋆ | ⋆⋆⋆ | 7 |
| Author, Year, Country | Type of Study | Sample Size (n) | Loc. of Lesions | PBM Type | Laser Parameters | Control Group | Follow- Up Type |
|---|---|---|---|---|---|---|---|
| Gambino et al., 2018 [28], Italy | Clinical trial | 40 | Oral mucosa | Diode laser gallium arsenide and aluminum (AlGaAs) | 980 nm; 400 mW; 8 J/cm2; 10 s -point size 0.5 cm2; 8 sessions/1 week for 8 weeks | Twice daily propionate clobetasol to 0.05% gel with aqueous of hydroxyethyl-cellulos at 4% (100 g), in equal parts (50:50) for 8 weeks | After treatment (unspecified) |
| Mirza et al., 2018 [29] Saudi Arabia | Randomized controlled clinical trial | 45 | Oral mucosa, Tongue | Diode laser (unspecified) | 630 nm; 10 mW/cm2 1.5 J/cm2; 2.5 min; 1 cm2. 2 times per week, each 3 days. Max. 10 sessions | Topical; corticosteroids in mouthwash: Dexamethasone (0.5 mg in 5 mL of water for 5 min); 30 min later: Nystatin (30 drops during 5 min) 4 times per day for 1 month | Control group: weekly follow-up during intake. Once or twice a week and a year |
| Mutafchieva et al., 2018 [30] Bulgaria | Open clinical trial | 12 | Oral mucosa, Gum, Tongue, Labial mucosa, Palate | Diodelaser (unspecified) | 810 nm; 0.5 W; 1.2 J/cm2; 30 s 3 times in a week | No control group | A month after treatment |
| Khater et al., 2019 [32], Egypt | Open clinical trial | 24 | Buccal mucosa, Gum, Tongue, Labial mucosa, Palate | Nd-YAG (neodymium) laser with Q shift | 1064 nm; 0.5 W; 1.2 J/cm2; 30 s 3 times per week for 1 month | No control group | After treatment (unspecified) |
| Lavaee et al., 2019 [31], Iran | Double- blind ran- dom- ized clinical trial | 8 | Buccal mucosa | Diode laser InGaAlP (Indium Gallium Aluminum Phosphorus) | 660 nm; 25 mW; 19.23 J/cm2 | Stimulated laser + Topical corticosteroids (triamcinolone acetonide 0.10% 3 times in a day and 40 drops of 0.1% nystatin oral suspension for 4 min | 3rd and 7th week of treatment |
| Nammour et al., 2021 [26], Belgium | Clinical trial | 96 | Buccal mucosa | Red light helium–neon (He–Ne) laser | 635 nm; 0.1 W; 1415 J/ cm2; 40 s Every 48 hours for 6 weeks | Topical cortisone (0.05% clobetasol propionate gel); 3 times per day for 6 weeks | 6 weeks, 1 month, 6 months and 12 months after treatment |
| Tarasenko et al. 2021 [33], Germany | Randomized controlled clinical trial with parallel arms and blinded examiner | 75 | Buccal mucosa, tongue, alveolar crest, palate, floor of mouth, lips | Nd–YAG (neodymium aluminum garnet laser) | 1064 nm; 1.5 W or 3 W; 40 Hz; 15 s Postoperative 5.5 min | Scalpel with 5–10, size 5.0 microfilament sutures no painkillers | 14, 30 days and 2 years after the operation |
| Author, Year | Intervention Type | Scales or Test to Measure Effectiveness | Results |
|---|---|---|---|
| Gambino et al., 2018 [28] | Therapy | OCT 1 | The corticosteroid is more effective in the short term while PBM is better in the long-term. |
| Mirza et al. 2018 [29] | Therapy | EI 2 Thongprasom VAS 3 | Control is significantly better at relieving pain, but PBM improved clinical signs. No side effects. |
| Mutafchieva et al., 2018 [30] | Therapy | Thongprasom VAS EI | General relief of symptoms, most with minor discomfort. Clinical improvement in 59.3% of the lesions. Moderate improvement in all cases, except for one that was cured. |
| Khater et al. 2019 [32] | Therapy | EI VAS Thongprasom | Clinical signs improved in 37.3% of the lesions with severe pain and discomfort presented by most patients before PBM; only mild discomfort remained. In almost all cases, there was a moderate recovery, which was complete in only one case. |
| Lavaee et al. 2019 [31] | Therapy | VAS EI Thongprasom SI 4 | No statistically significant differences between intervention group and control group. |
| Nammour et al. 2021 [26] | Therapy | VAS REU 5 | The treatments were beneficial in the absence of pain and recurrence but without significant differences between intervention group and con trol group. |
| Tarasenko et al., 2021 [33] | Therapy | VAS Pearson’s coeffi cient | Laser s more effective at the end of the first postoperative month. The com- bination LLLT + HLLT produced superior clinical performance compared to conventional surgical excision. However, the pain reduction was more signific-ant in HLLT than in LLLT. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz Roca, J.A.; López Jornet, P.; Gómez García, F.J.; Marcos Aroca, P. Effect of Photobiomodulation on Atrophic–Erosive Clinical Forms of Oral Lichen Planus: A Systematic Review. Dent. J. 2022, 10, 221. https://doi.org/10.3390/dj10120221
Ruiz Roca JA, López Jornet P, Gómez García FJ, Marcos Aroca P. Effect of Photobiomodulation on Atrophic–Erosive Clinical Forms of Oral Lichen Planus: A Systematic Review. Dentistry Journal. 2022; 10(12):221. https://doi.org/10.3390/dj10120221
Chicago/Turabian StyleRuiz Roca, Juan Antonio, Pía López Jornet, Francisco José Gómez García, and Paula Marcos Aroca. 2022. "Effect of Photobiomodulation on Atrophic–Erosive Clinical Forms of Oral Lichen Planus: A Systematic Review" Dentistry Journal 10, no. 12: 221. https://doi.org/10.3390/dj10120221
APA StyleRuiz Roca, J. A., López Jornet, P., Gómez García, F. J., & Marcos Aroca, P. (2022). Effect of Photobiomodulation on Atrophic–Erosive Clinical Forms of Oral Lichen Planus: A Systematic Review. Dentistry Journal, 10(12), 221. https://doi.org/10.3390/dj10120221








