Topographical and Ultrastructural Evaluation of Titanium Plates Coated with PLGA, Chitosan, and/or Meropenem: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
- A.
- Carriers: composed of copolymers PLGA and CH
- B.
- Antimicrobial agent: MEM
2.2. Bacteria Strains
2.3. Bacteria Cultivation
2.4. Sample Preparation for Scanning Electron Microscope “SEM”
2.5. SEM Protocol
2.6. SEM Image Analysis
2.7. Statistical Analysis
3. Results
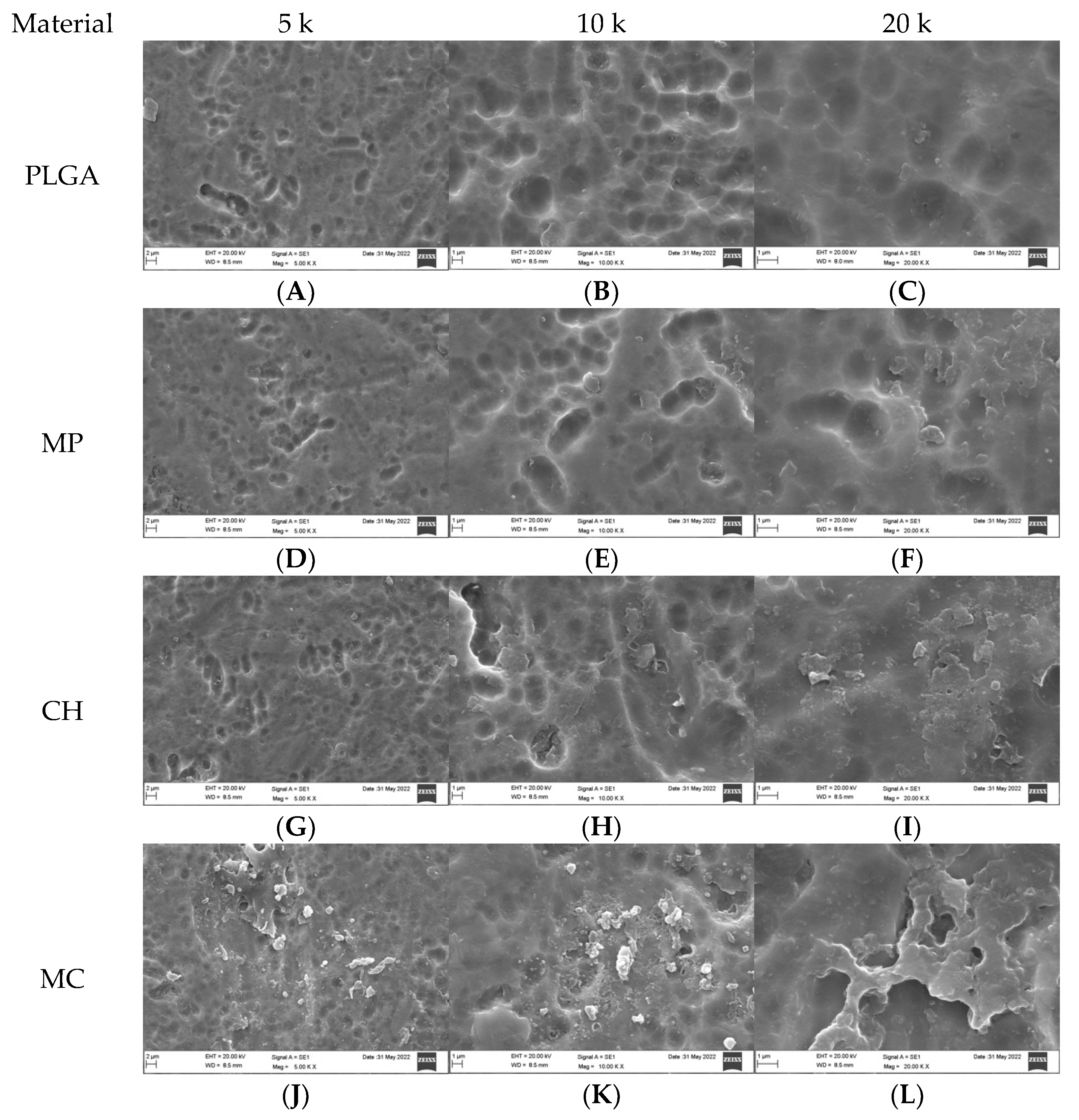
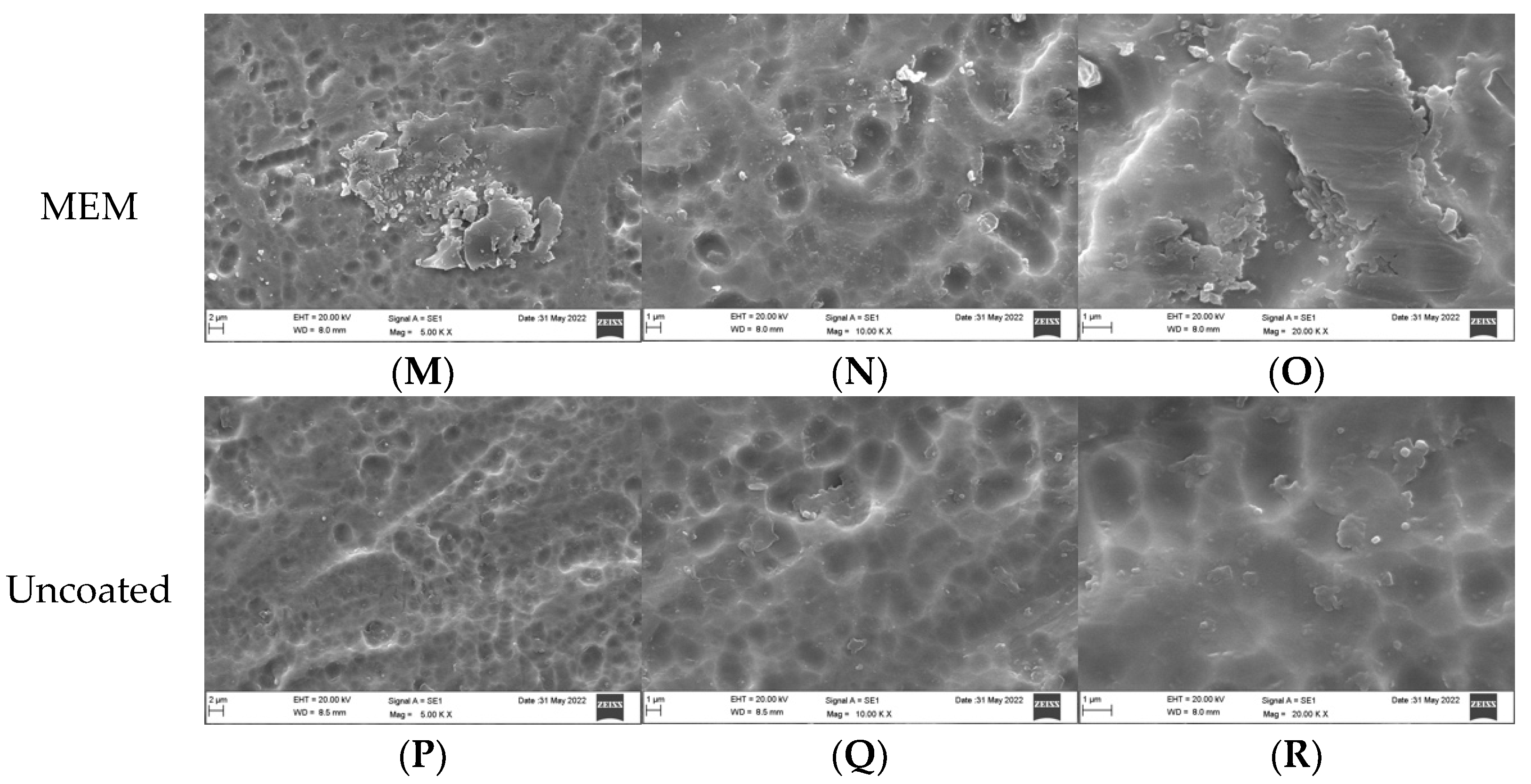
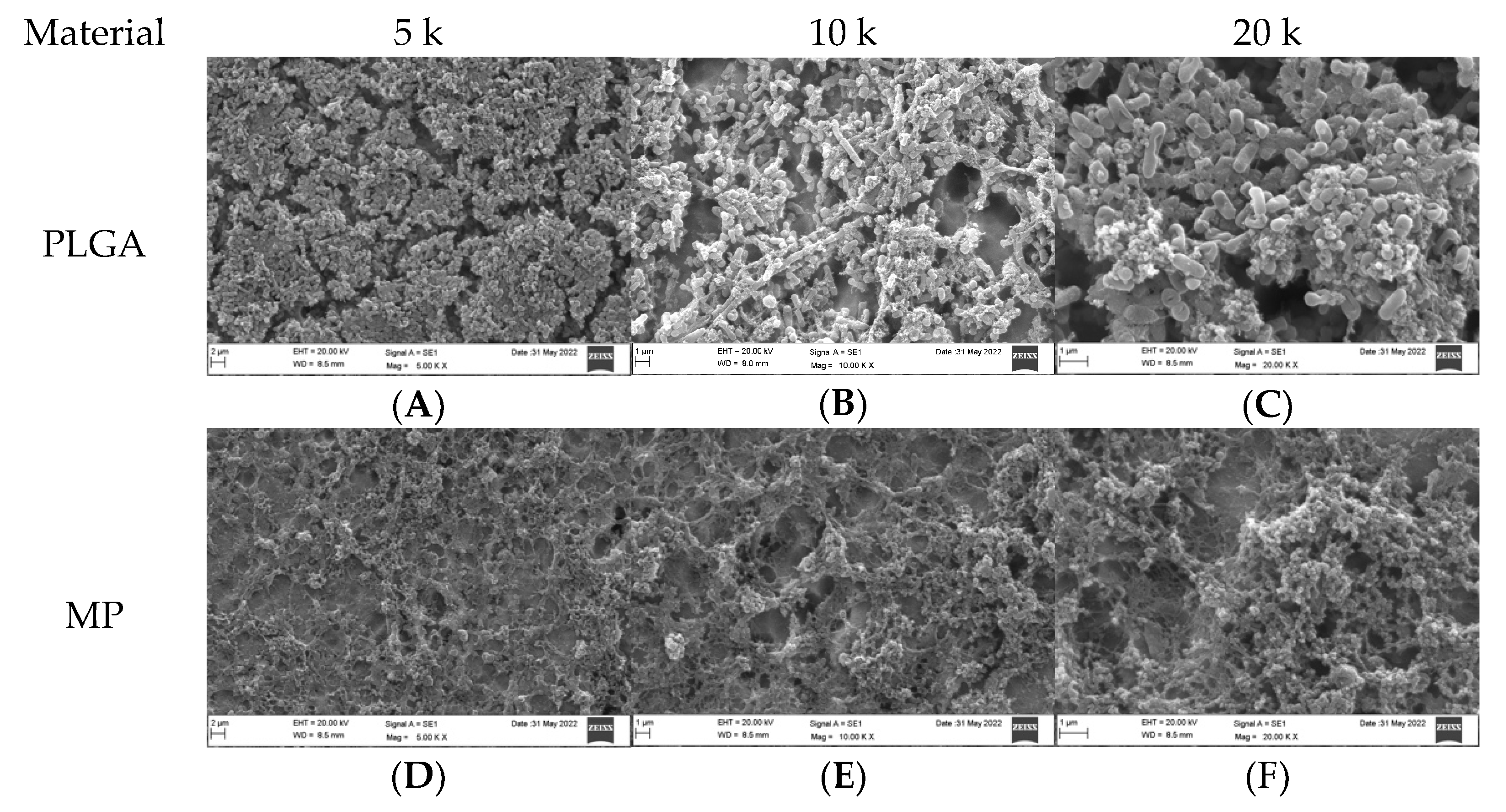
3.1. Qualitative Assessment of Coated Plates without Bacteria
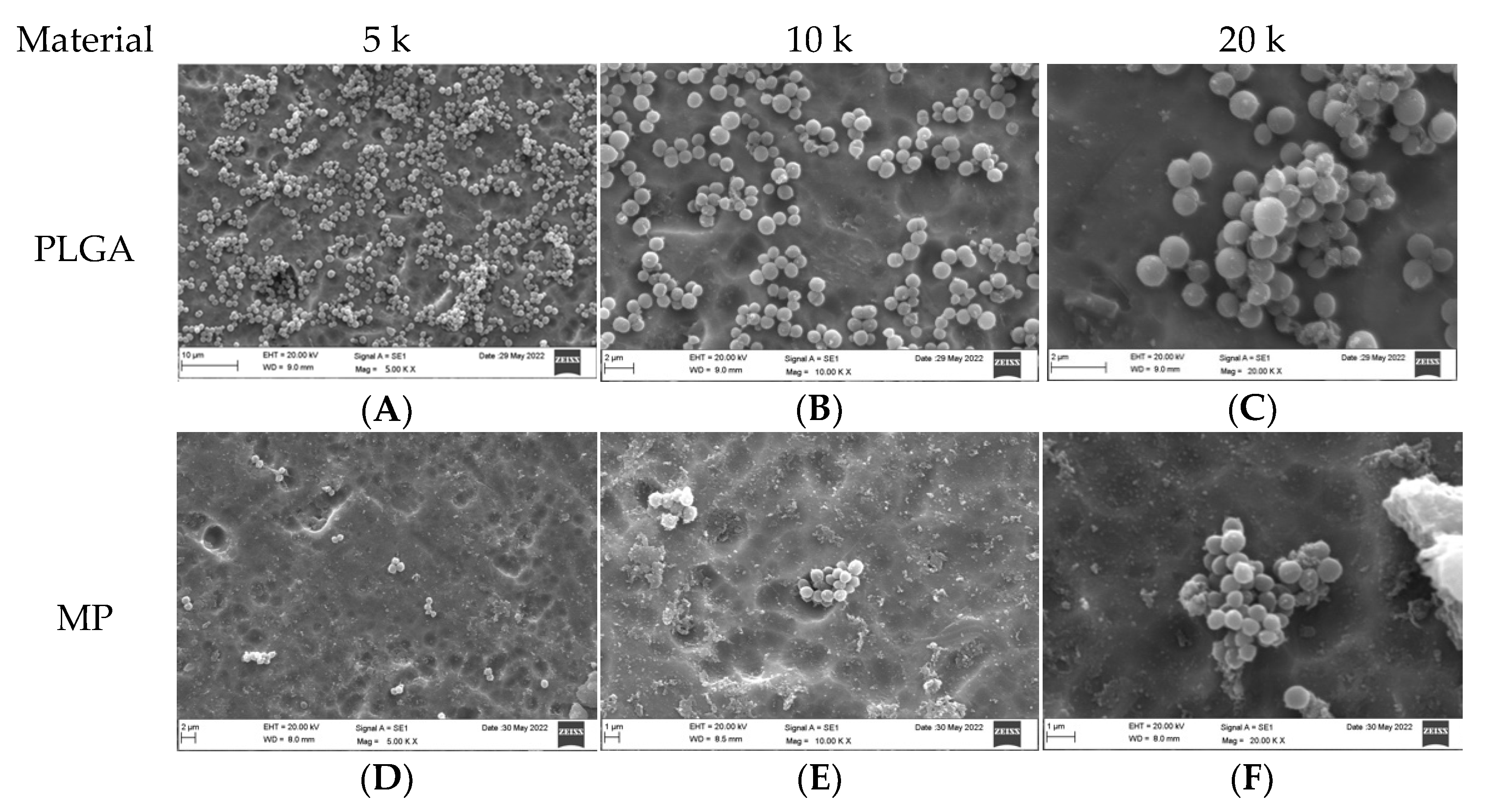
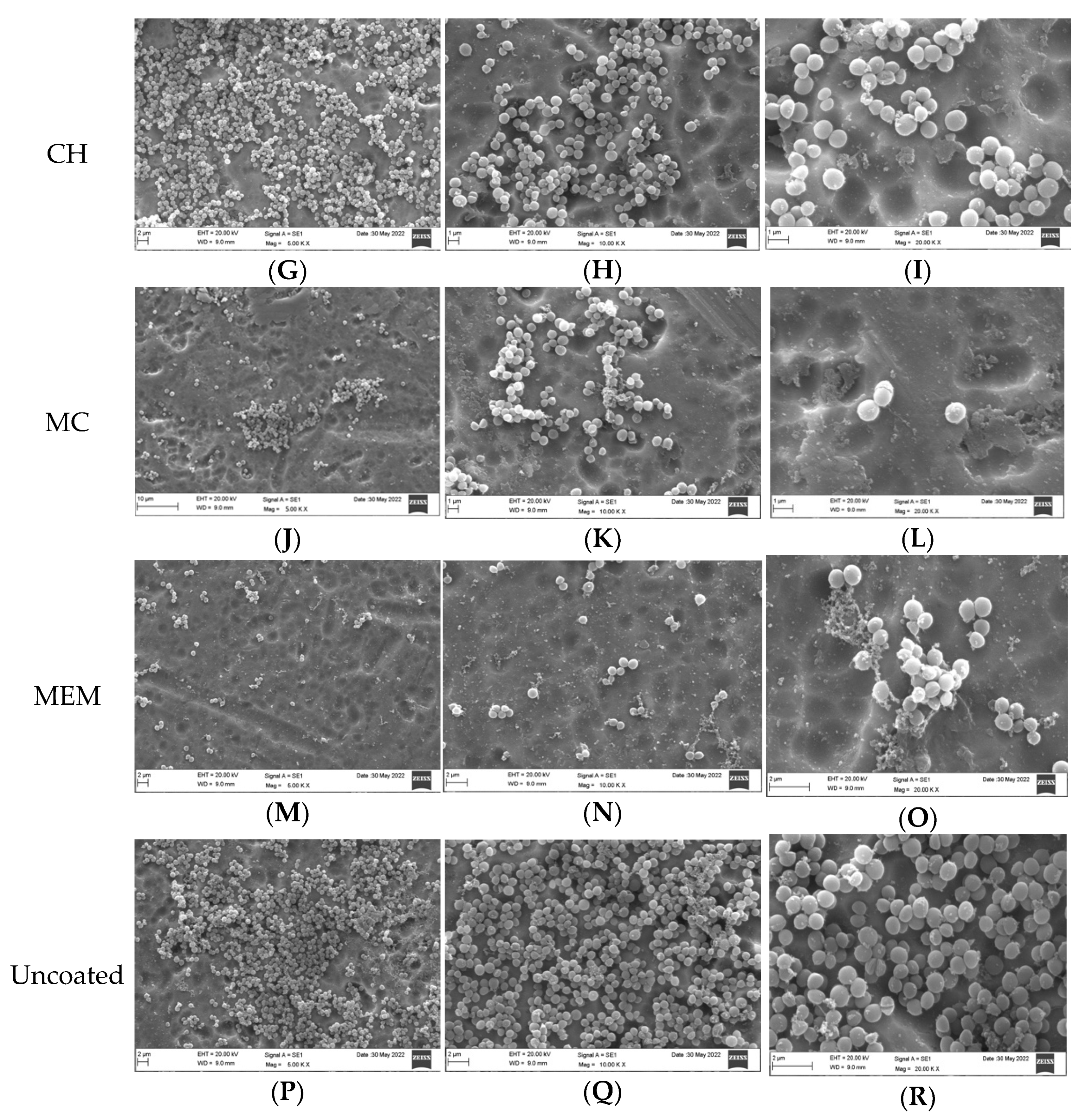
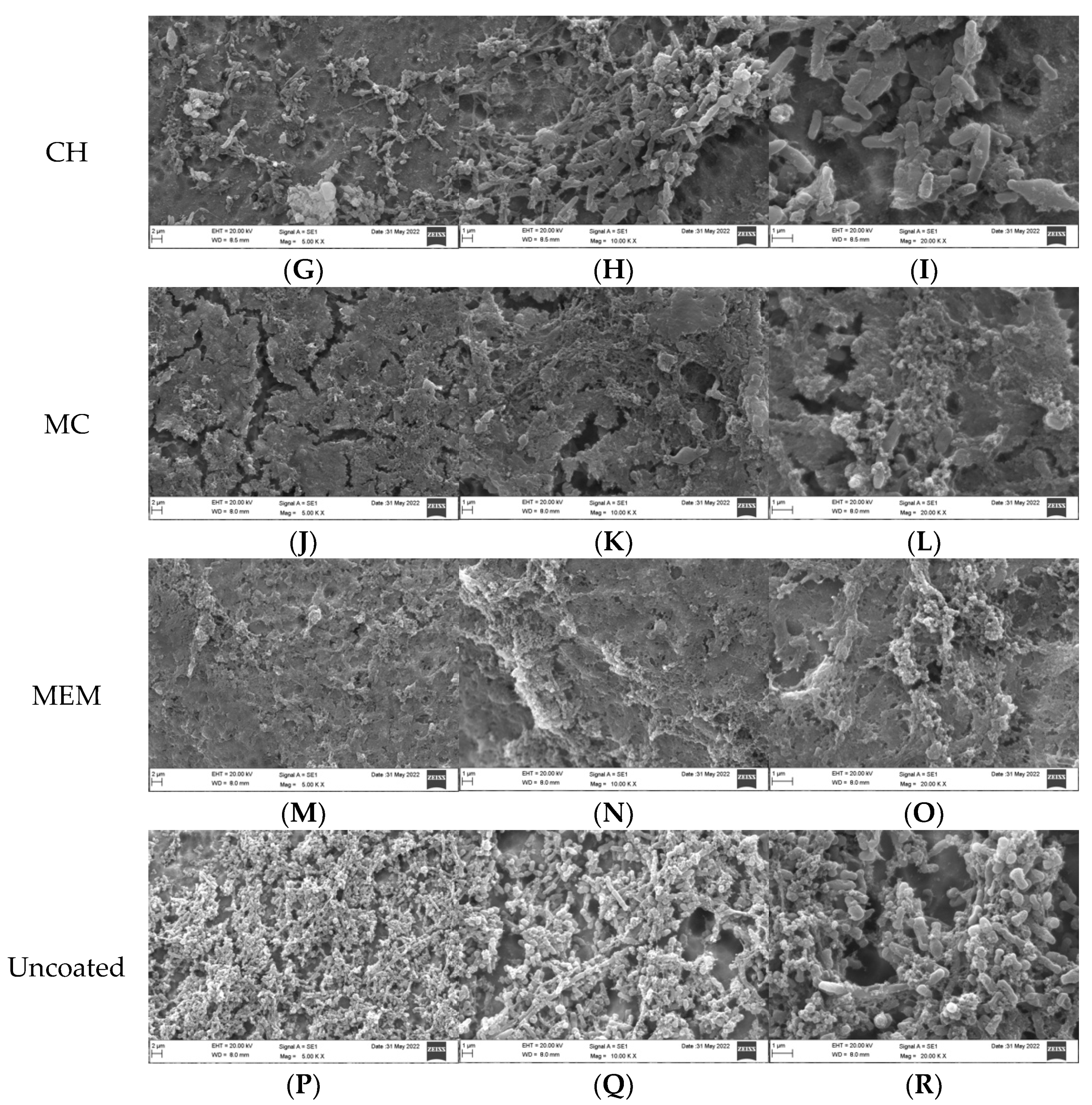
3.2. Qualitative Assessment of Coated Plates with Bacteria
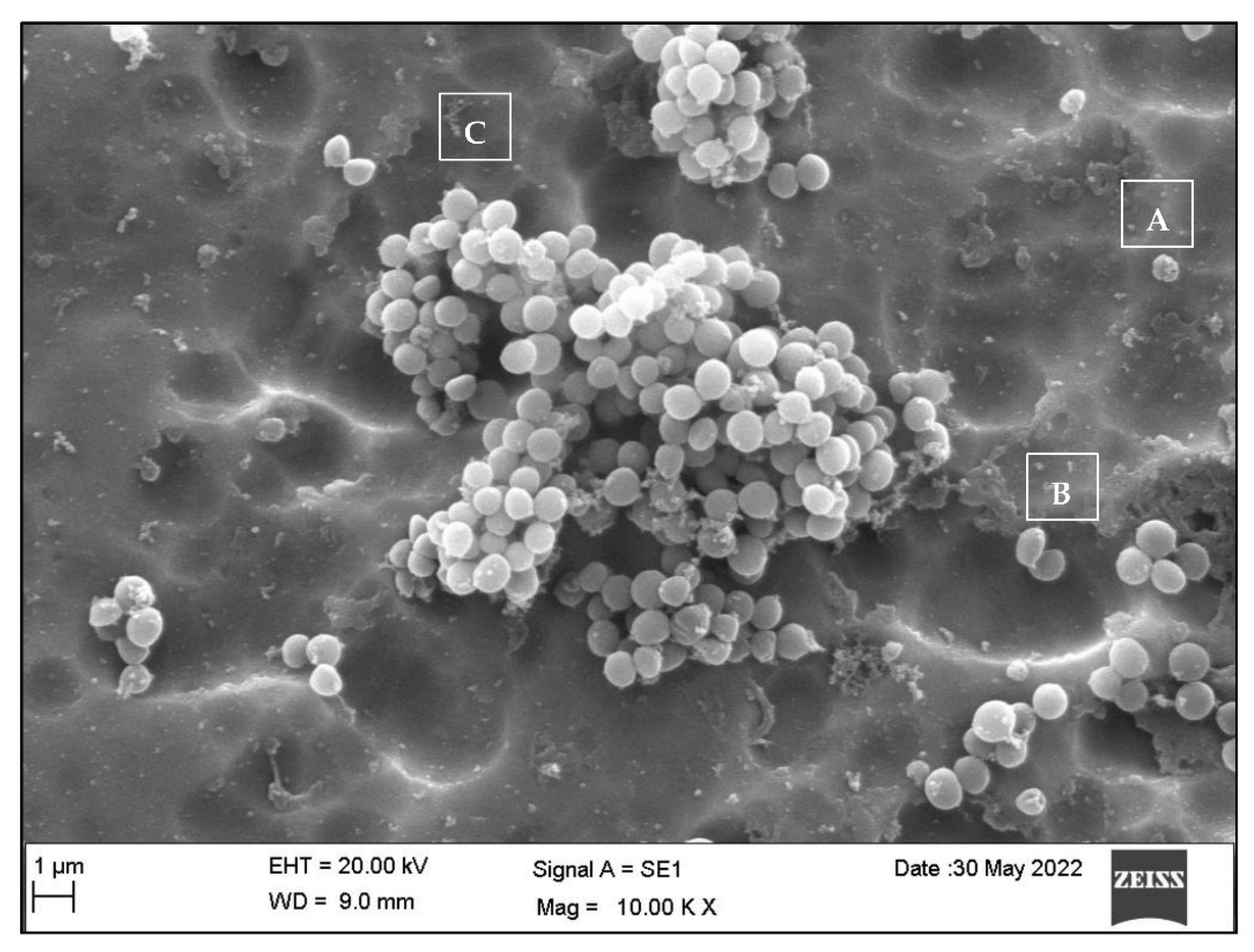
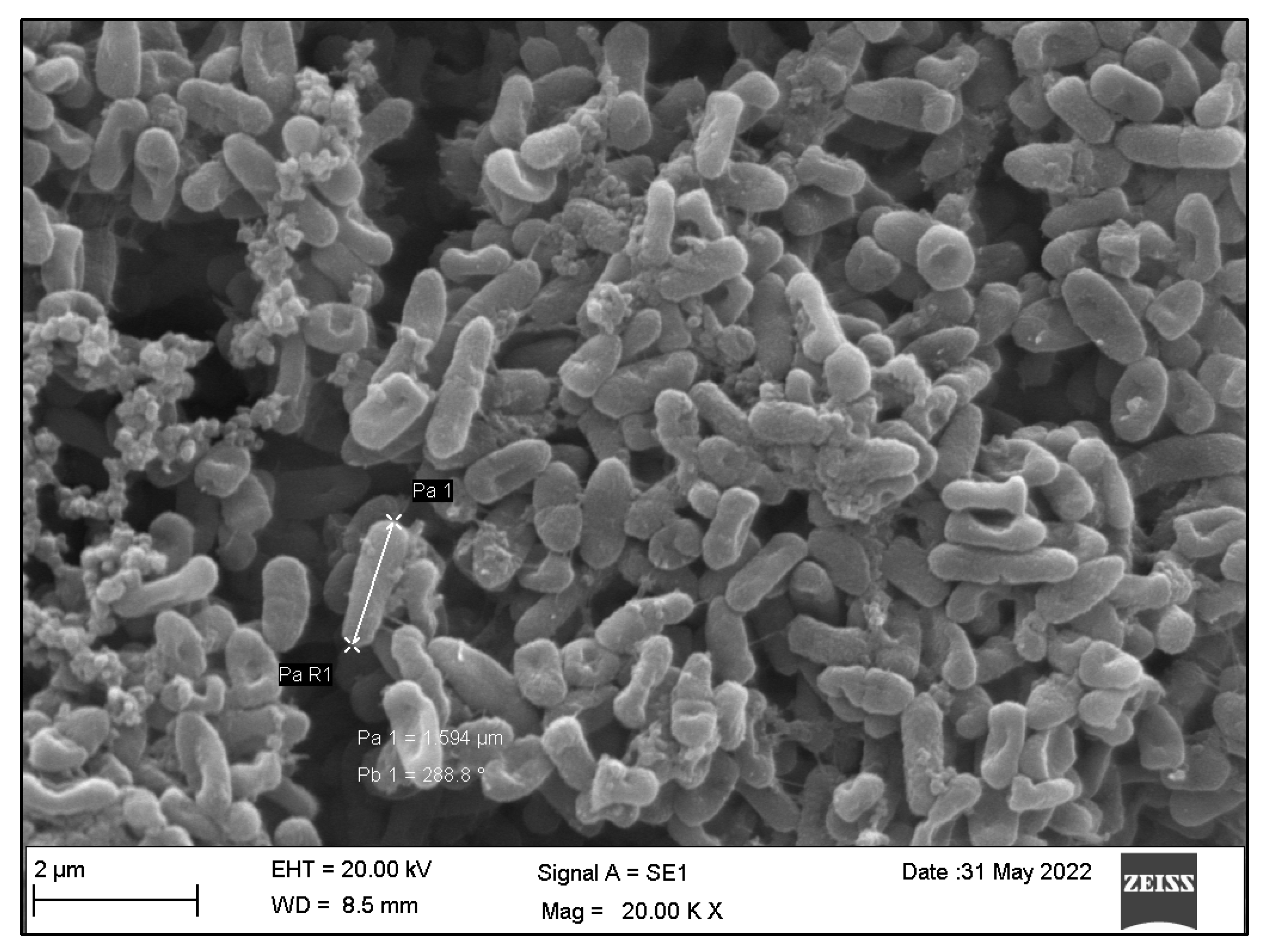
3.3. Qualitative Assessment of the SA and PA Bacteria
3.4. Quantitative Assessment of Coated Plates with Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haerle, F.; Champy, M.; Terry, B.C. Atlas of Craniomaxillofacial Osteosynthesis: Microplates, Miniplates, and Screws, 2nd ed.; Thieme: Stuttgart, Germany, 2009; ISBN 978-3-13-116492-6. [Google Scholar]
- Weinstein, A. Implant Retrieval: Material and Biological Analysis; Forgotten Books: London, UK, 1981; ISBN 0-364-60864-1. [Google Scholar]
- Nespoli, A.; Passaretti, F.; Szentmiklósi, L.; Maróti, B.; Placidi, E.; Cassetta, M.; Yada, R.Y.; Farrar, D.H.; Tian, K.V. Biomedical NiTi and β-Ti Alloys: From Composition, Microstructure and Thermo-Mechanics to Application. Metals 2022, 12, 406. [Google Scholar] [CrossRef]
- Schmidt, B.L.; Perrott, D.H.; Mahan, D.; Kearns, G. The Removal of Plates and Screws after Le Fort I Osteotomy. J. Oral Maxillofac. Surg. 1998, 56, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Turvey, T.A.; Proffit, W.P.; Phillips, C. Biodegradable Fixation for Craniomaxillofacial Surgery: A 10-Year Experience Involving 761 Operations and 745 Patients. Int. J. Oral Maxillofac. Surg. 2011, 40, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Li, Z.; Loh, X.J. Small Molecule Therapeutic-Loaded Liposomes as Therapeutic Carriers: From Development to Clinical Applications. RSC Adv. 2016, 6, 70592–70615. [Google Scholar] [CrossRef]
- Ye, C.; Chi, H. A Review of Recent Progress in Drug and Protein Encapsulation: Approaches, Applications and Challenges. Mater. Sci. Eng. C 2018, 83, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Jiang, S.; Li, Z.; Loh, X.J. Conjugation of Poly(Ethylene Glycol) to Poly(Lactide)-Based Polyelectrolytes: An Effective Method to Modulate Cytotoxicity in Gene Delivery. Mater. Sci. Eng. C 2017, 73, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, R.; Sepehri, N.; Manoochehri, S.; Rouhani, H.; Atyabi, F. Polylactide-Co-Glycolide Nanoparticles for Controlled Delivery of Anticancer Agents. Int. J. Nanomed. 2011, 6, 877–895. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the Marine Environment with Pharmacological, Cosmeceutical and Nutraceutical Potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Wiebe, R.; Dilay, L.; Thomson, K.; Rubinstein, E.; Hoban, D.J.; Noreddin, A.M.; Karlowsky, J.A. Comparative Review of the Carbapenems. Drugs 2007, 67, 1027–1052. [Google Scholar] [CrossRef]
- Guillaume, O.; Garric, X.; Lavigne, J.-P.; Van Den Berghe, H.; Coudane, J. Multilayer, Degradable Coating as a Carrier for the Sustained Release of Antibiotics: Preparation and Antimicrobial Efficacy In Vitro. J. Control. Release 2012, 162, 492–501. [Google Scholar] [CrossRef]
- Guillaume, O.; Lavigne, J.-P.; Lefranc, O.; Nottelet, B.; Coudane, J.; Garric, X. New Antibiotic-Eluting Mesh Used for Soft Tissue Reinforcement. Acta Biomater. 2011, 7, 3390–3397. [Google Scholar] [CrossRef]
- Borowicz, S.; Van Scoyk, M.; Avasarala, S.; Rathinam, M.K.K.; Tauler, J.; Bikkavilli, R.K.; Winn, R.A. The Soft Agar Colony Formation Assay. JoVE 2014, 1, 51998. [Google Scholar] [CrossRef]
- An, Y.H.; Friedman, R.J. Laboratory Methods for Studies of Bacterial Adhesion. J. Microbiol. Methods 1997, 30, 141–152. [Google Scholar] [CrossRef]
- Kammoun, R.; Zmantar, T.; Ghoul, S. Scanning Electron Microscopy Approach to Observe Bacterial Adhesion to Dental Surfaces. Methods X 2020, 7, 101107. [Google Scholar] [CrossRef] [PubMed]
- Vögeling, H.; Duse, L.; Seitz, B.S.; Plenagl, N.; Wojcik, M.; Pinnapireddy, S.R.; Bakowsky, U. Multilayer Bacteriostatic Coating for Surface Modified Titanium Implants. Phys. Status Solidi A 2018, 215, 1700844. [Google Scholar] [CrossRef]
- Biguetti, C.C.; Cavalla, F.; Fonseca, A.C.; Tabanez, A.P.; Siddiqui, D.A.; Wheelis, S.E.; Taga, R.; Fakhouri, W.D.; Silva, R.M.; Rodrigues, D.C.; et al. Effects of Titanium Corrosion Products on In Vivo Biological Response: A Basis for the Understanding of Osseointegration Failures Mechanisms. Front. Mater. 2021, 8, 651970. [Google Scholar] [CrossRef]
- Dutt, Y.; Dhiman, R.; Singh, T.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Raj, V.S.; Chang, C.-M.; Priyadarshini, A. The Association between Biofilm Formation and Antimicrobial Resistance with Possible Ingenious Bio-Remedial Approaches. Antibiotics 2022, 11, 930. [Google Scholar] [CrossRef]
- Fitzgerald, J.R. Evolution of Staphylococcus Aureus during Human Colonization and Infection. Infect. Genet. Evol. 2014, 21, 542–547. [Google Scholar] [CrossRef]
- Nandakumar, V.; Chittaranjan, S.; Kurian, V.M.; Doble, M. Characteristics of Bacterial Biofilm Associated with Implant Material in Clinical Practice. Polym. J. 2013, 45, 137–152. [Google Scholar] [CrossRef]
- Archer, N.K.; Mazaitis, M.J.; Costerton, J.W.; Leid, J.G.; Powers, M.E.; Shirtliff, M.E. Staphylococcus Aureus Biofilms: Properties, Regulation, and Roles in Human Disease. Virulence 2011, 2, 445–459. [Google Scholar] [CrossRef]
- Donlan, R. Biofilms and Device-Associated Infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef]
- Davis, C.M.; Gregoire, C.E.; Steeves, T.W.; Demsey, A. Prevalence of Surgical Site Infections Following Orthognathic Surgery: A Retrospective Cohort Analysis. J. Oral Maxillofac. Surg. 2016, 74, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, V.; Chhabra, P.; Dover, M.S. Removal of Miniplates in Maxillofacial Surgery: A Follow-Up Study. J. Oral Maxillofac. Surg. 2005, 63, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic Resistance of Bacterial Biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Amato, S.M.; Fazen, C.H.; Henry, T.C.; Mok, W.W.K.; Orman, M.A.; Sandvik, E.L.; Volzing, K.G.; Brynildsen, M.P. The Role of Metabolism in Bacterial Persistence. Front. Microbiol. 2014, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Cerioli, M.; Batailler, C.; Conrad, A.; Roux, S.; Perpoint, T.; Becker, A.; Triffault-Fillit, C.; Lustig, S.; Fessy, M.-H.; Laurent, F.; et al. Pseudomonas Aeruginosa Implant-Associated Bone and Joint Infections: Experience in a Regional Reference Center in France. Front. Med. 2020, 7, 513242. [Google Scholar] [CrossRef] [PubMed]
- Christian, J.S. The Quinolone Antibiotics. Prim. Care Update OB/GYNS 1996, 3, 87–92. [Google Scholar] [CrossRef]
| Bacteria | Materials | Mean ± SD | Median | Range | Mean Rank | K.W p-Value ** |
|---|---|---|---|---|---|---|
| SA | PLGA | 39.67 ± 1.53 | 40.00 | 3.00 | 5.000 | 0.005 * |
| MP | 18.33 ± 1.16 | 19.00 | 2.00 | 2.000 | ||
| CH | 119.67 ± 1.16 | 119.00 | 2.00 | 8.000 | ||
| MC | 632.00 ± 10.15 | 634.00 | 20.00 | 14.000 | ||
| MEM | 440.33 ± 1.53 | 440.00 | 3.00 | 11.000 | ||
| Uncoated | 1061.00 ± 3.61 | 1060.00 | 7.00 | 17.000 | ||
| PA | PLGA | 3.33 ± 0.58 | 3.00 | 1.00 | 4.333 | 0.006 * |
| MP | 12.67 ± 1.16 | 12.00 | 2.00 | 8.000 | ||
| CH | 2.67 ± 0.58 | 3.00 | 1.00 | 2.667 | ||
| MC | 1261.67 ± 6.81 | 1264.00 | 13.00 | 11.000 | ||
| MEM | 2108.00 ± 8.54 | 2107.00 | 17.00 | 14.000 | ||
| Uncoated | 2923.33 ± 4.04 | 2924.00 | 8.00 | 17.000 |
| Compound | Bacteria | Sum of Ranks | p-value |
|---|---|---|---|
| PLGA | SA | 15.00 | 0.04 * |
| PA | 6.00 | ||
| MP | SA | 15.00 | 0.04 * |
| PA | 6.00 | ||
| CH | SA | 15.00 | 0.04 * |
| PA | 6.00 | ||
| MC | SA | 6.00 | 0.05 |
| PA | 15.00 | ||
| MEM | SA | 6.00 | 1.05 |
| PA | 15.00 | ||
| Uncoated | SA | 6.00 | 0.05 |
| PA | 15.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qubaisey, M.; Khounganian, R.; Al-Badah, A.; Ali, R. Topographical and Ultrastructural Evaluation of Titanium Plates Coated with PLGA, Chitosan, and/or Meropenem: An In Vitro Study. Dent. J. 2022, 10, 220. https://doi.org/10.3390/dj10120220
Al-Qubaisey M, Khounganian R, Al-Badah A, Ali R. Topographical and Ultrastructural Evaluation of Titanium Plates Coated with PLGA, Chitosan, and/or Meropenem: An In Vitro Study. Dentistry Journal. 2022; 10(12):220. https://doi.org/10.3390/dj10120220
Chicago/Turabian StyleAl-Qubaisey, Mohammad, Rita Khounganian, Abdulhakim Al-Badah, and Raisuddin Ali. 2022. "Topographical and Ultrastructural Evaluation of Titanium Plates Coated with PLGA, Chitosan, and/or Meropenem: An In Vitro Study" Dentistry Journal 10, no. 12: 220. https://doi.org/10.3390/dj10120220
APA StyleAl-Qubaisey, M., Khounganian, R., Al-Badah, A., & Ali, R. (2022). Topographical and Ultrastructural Evaluation of Titanium Plates Coated with PLGA, Chitosan, and/or Meropenem: An In Vitro Study. Dentistry Journal, 10(12), 220. https://doi.org/10.3390/dj10120220






