Two Gingival Cell Lines Response to Different Dental Implant Abutment Materials: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Discs’ Preparation
2.2. Surface Roughness Measurements
2.3. SEM Surface Morphology Analysis
2.4. HGFB and HGKC Cell Culture Preparation
2.5. Cell Viability
2.6. Cytotoxicity
2.7. Statistical Analysis
3. Results
3.1. Surface Roughness
3.2. Surface Morphology Analysis
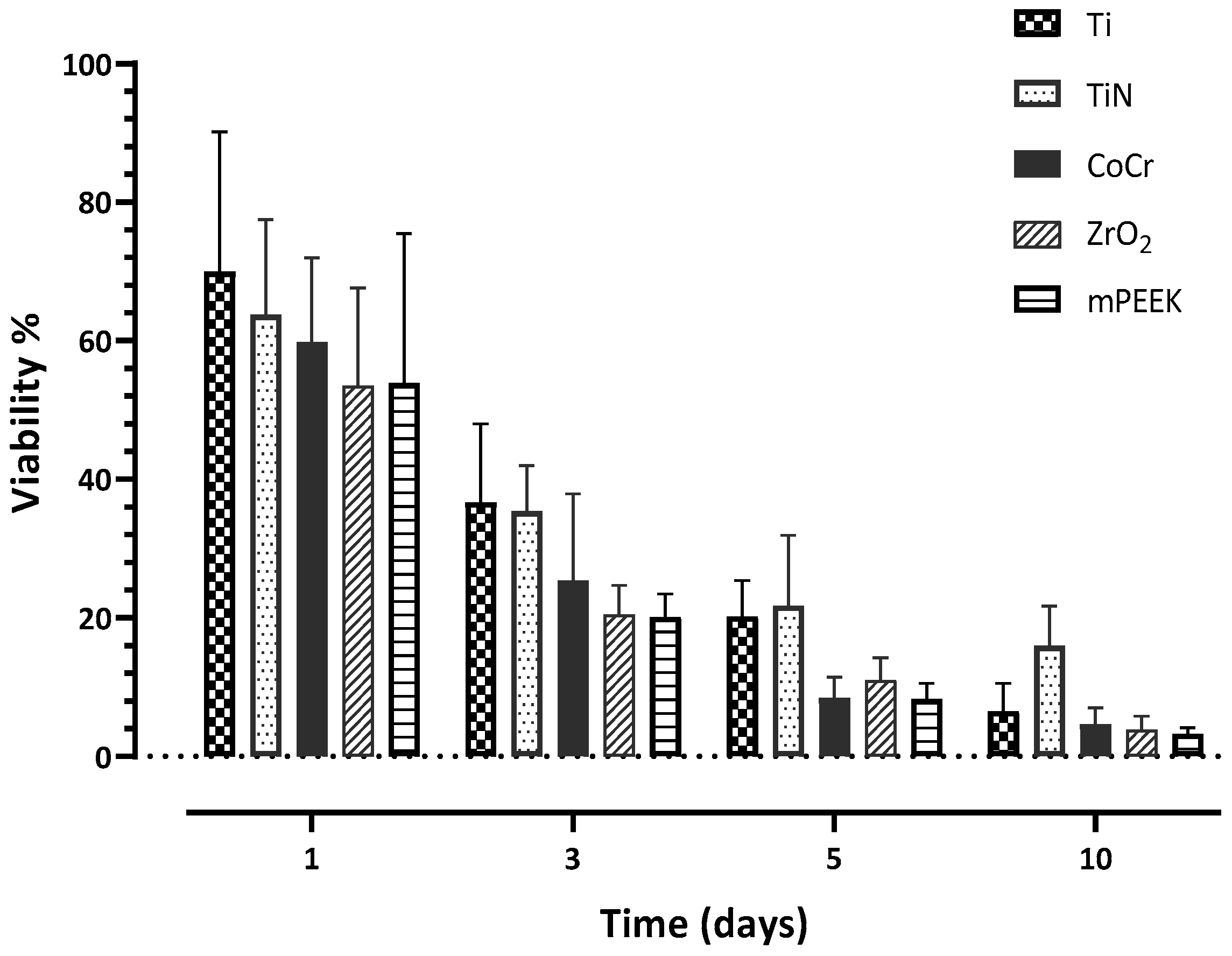
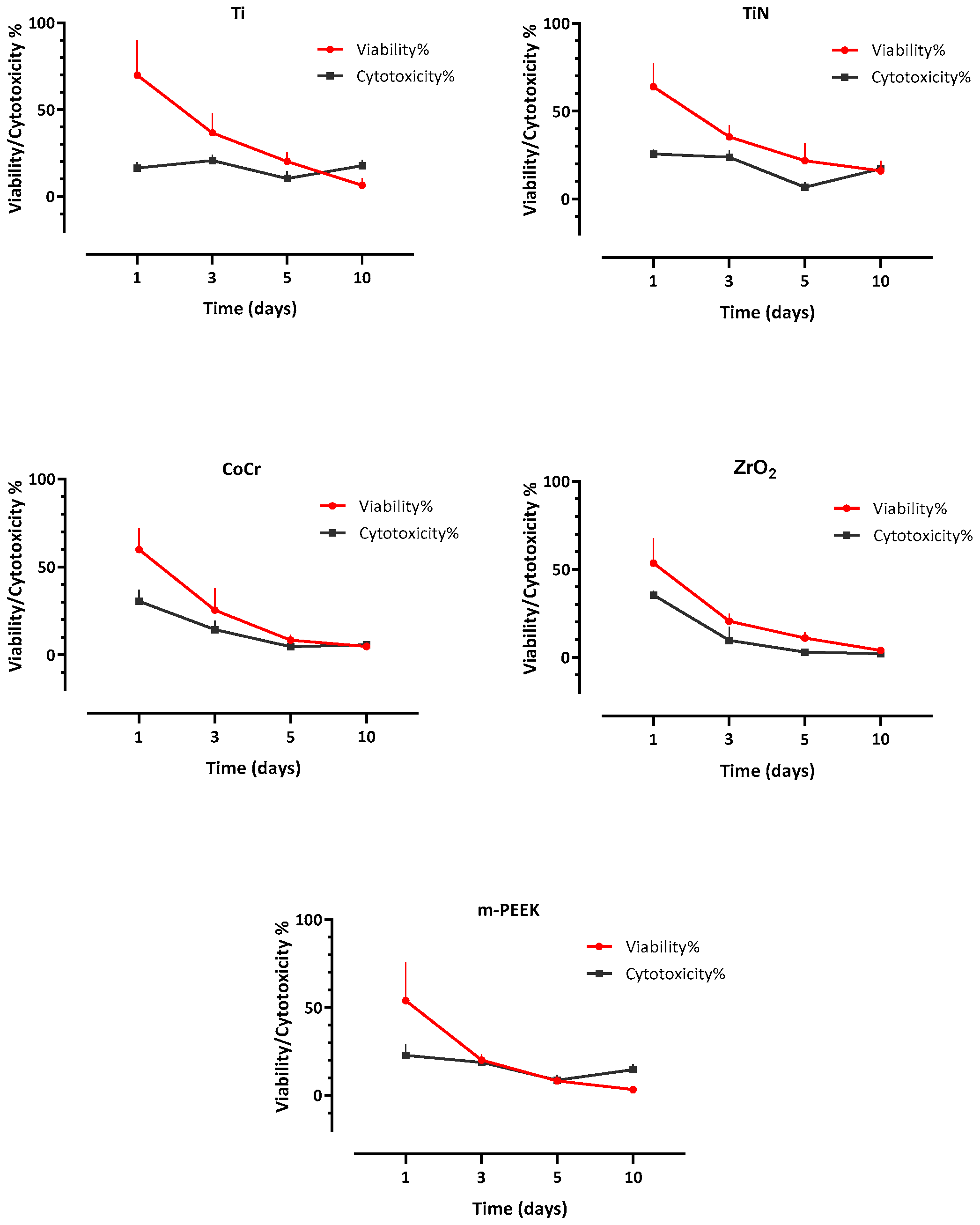
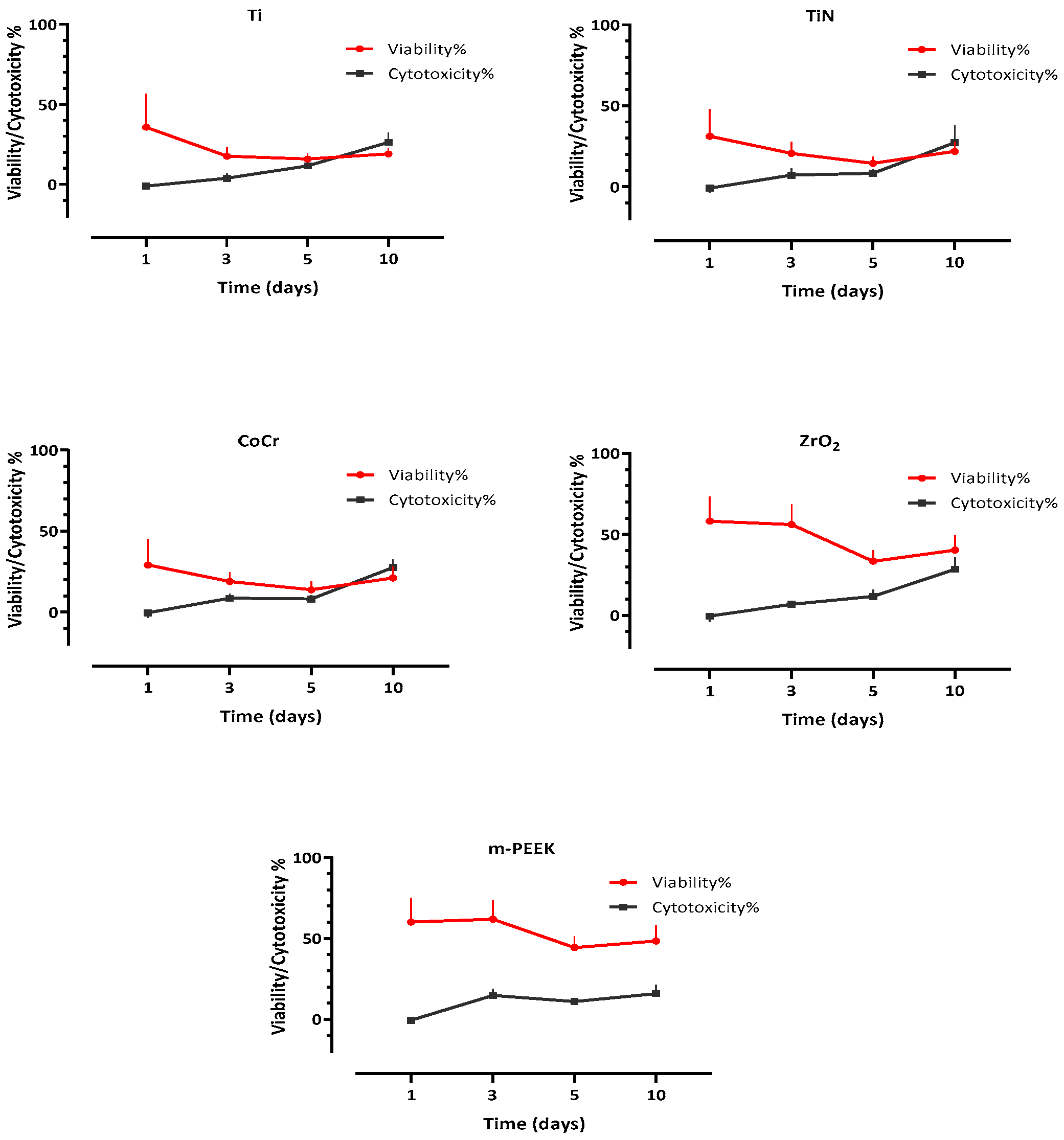
3.3. Cell Viability
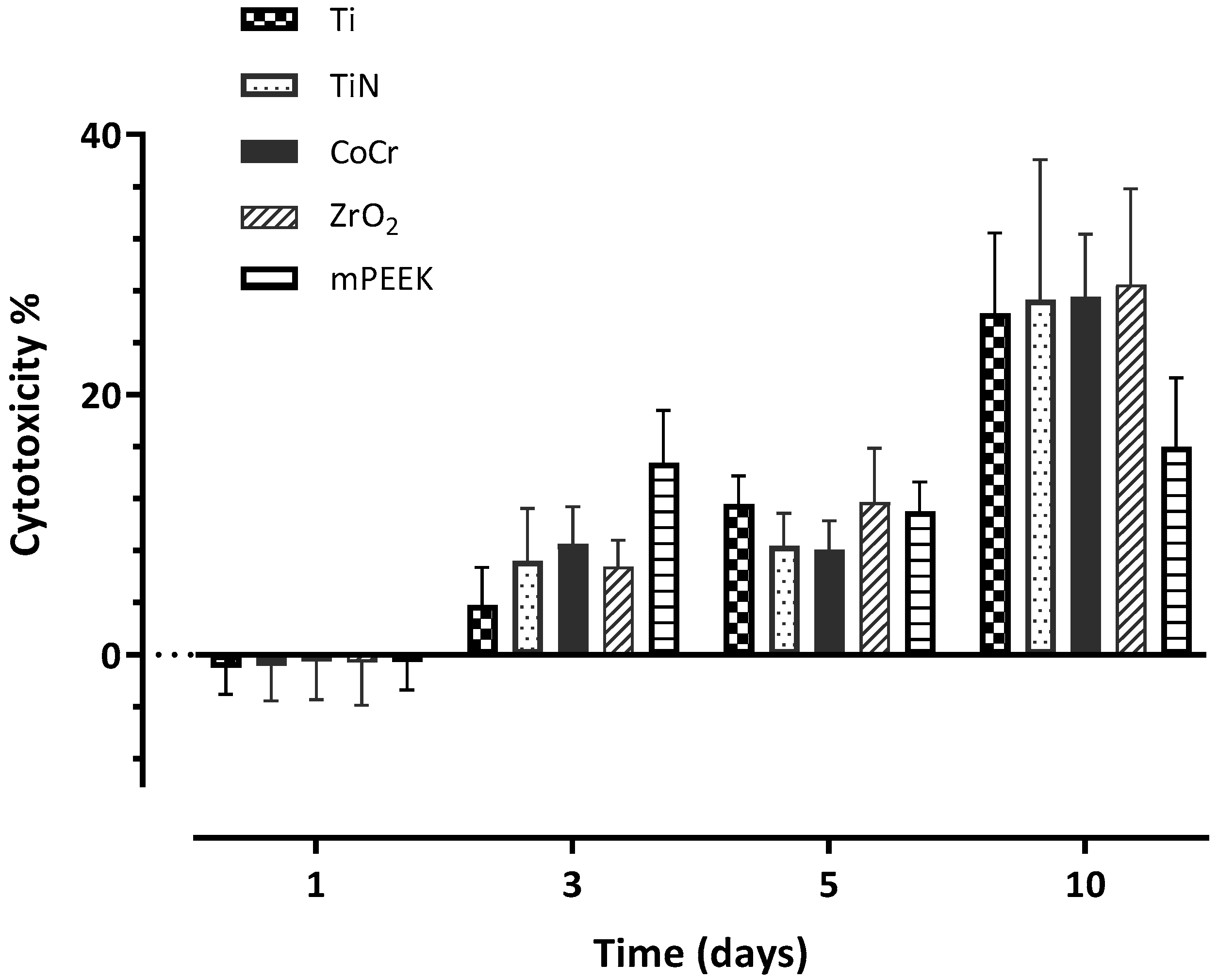
3.4. Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol. 2000 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Farawati, F.A.; Nakaparksin, P. What is the Optimal Material for Implant Prosthesis? Dent. Clin. N. Am. 2019, 63, 515–530. [Google Scholar] [CrossRef]
- Brodbeck, U. The ZiReal Post: A new ceramic implant abutment. J. Esthet. Restor. Dent. 2003, 15, 10–23, discussion 24. [Google Scholar] [CrossRef]
- Vigolo, P.; Fonzi, F.; Majzoub, Z.; Cordioli, G. An in vitro evaluation of ZiReal abutments with hexagonal connection: In original state and following abutment preparation. Int. J. Oral Maxillofac. Implant. 2005, 20, 108–114. [Google Scholar]
- Linkevicius, T.; Apse, P. Influence of abutment material on stability of peri-implant tissues: A systematic review. Int. J. Oral Maxillofac. Implant. 2008, 23, 449–456. [Google Scholar]
- Zembic, A.; Bosch, A.; Jung, R.E.; Hammerle, C.H.; Sailer, I. Five-year results of a randomized controlled clinical trial comparing zirconia and titanium abutments supporting single-implant crowns in canine and posterior regions. Clin. Oral Implant. Res. 2013, 24, 384–390. [Google Scholar] [CrossRef]
- Thoma, D.S.; Brandenberg, F.; Fehmer, V.; Buchi, D.L.; Hammerle, C.H.; Sailer, I. Randomized Controlled Clinical Trial of All-Ceramic Single Tooth Implant Reconstructions Using Modified Zirconia Abutments: Radiographic and Prosthetic Results at 1 Year of Loading. Clin. Implant. Dent. Relat. Res. 2016, 18, 462–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peñarrieta-Juanito, G.; Cruz, M.; Costa, M.; Miranda, G.; Marques, J.; Magini, R.; Mata, A.; Souza, J.C.; Caramês, J.; Silva, F.S. A novel gradated zirconia implant material embedding bioactive ceramics: Osteoblast behavior and physicochemical assessment. Materialia 2018, 1, 3–14. [Google Scholar] [CrossRef]
- Sailer, I.; Philipp, A.; Zembic, A.; Pjetursson, B.E.; Hammerle, C.H.; Zwahlen, M. A systematic review of the performance of ceramic and metal implant abutments supporting fixed implant reconstructions. Clin. Oral Implant. Res. 2009, 20 (Suppl. S4), 4–31. [Google Scholar] [CrossRef] [Green Version]
- Stimmelmayr, M.; Sagerer, S.; Erdelt, K.; Beuer, F. In vitro fatigue and fracture strength testing of one-piece zirconia implant abutments and zirconia implant abutments connected to titanium cores. Int. J. Oral Maxillofac. Implant. 2013, 28, 488–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mengel, R.; Buns, C.E.; Mengel, C.; Flores-de-Jacoby, L. An in vitro study of the treatment of implant surfaces with different instruments. Int. J. Oral Maxillofac. Implant. 1998, 13, 91–96. [Google Scholar]
- Kawashima, H.; Sato, S.; Kishida, M.; Yagi, H.; Matsumoto, K.; Ito, K. Treatment of titanium dental implants with three piezoelectric ultrasonic scalers: An in vivo study. J. Periodontol. 2007, 78, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.; Oliva, A.; Basile, M.A.; Giordano, M.; Mazzola, N.; Rizzo, A.; Lanza, A.; Guida, L. The effects of titanium nitride-coating on the topographic and biological features of TPS implant surfaces. J. Dent. 2011, 39, 720–728. [Google Scholar] [CrossRef]
- Mengel, R.; Meer, C.; Flores-de-Jacoby, L. The treatment of uncoated and titanium nitride-coated abutments with different instruments. Int. J. Oral Maxillofac. Implant. 2004, 19, 232–238. [Google Scholar]
- Sawase, T.; Yoshida, K.; Taira, Y.; Kamada, K.; Atsuta, M.; Baba, K. Abrasion resistance of titanium nitride coatings formed on titanium by ion-beam-assisted deposition. J. Oral Rehabil. 2005, 32, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kassem, Y.M.; Alshimy, A.M.; El-Shabrawy, S.M. Mechanical evaluation of polyetheretherketone compared with zirconia as a dental implant material. Alex. Dent. J. 2019, 44, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Petit, C.; Montanaro, L.; Palmero, P. Functionally graded ceramics for biomedical application: Concept, manufacturing, and properties. Int. J. Appl. Ceram. Technol. 2018, 15, 820–840. [Google Scholar] [CrossRef]
- Santing, H.J.; Meijer, H.J.; Raghoebar, G.M.; Ozcan, M. Fracture strength and failure mode of maxillary implant-supported provisional single crowns: A comparison of composite resin crowns fabricated directly over PEEK abutments and solid titanium abutments. Clin. Implant Dent. Relat. Res. 2012, 14, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhao, X.; Zhao, J.; Zhao, Z.; Wang, Q.; Zhang, C. Biologically Modified Polyether Ether Ketone as Dental Implant Material. Front. Bioeng. Biotechnol. 2020, 8, 620537. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.; Archer, C.W.; Walker, P.S.; Blunn, G.W. Attachment and proliferation of osteoblasts and fibroblasts on biomaterials for orthopaedic use. Biomaterials 1995, 16, 287–295. [Google Scholar] [CrossRef]
- Morrison, C.; Macnair, R.; MacDonald, C.; Wykman, A.; Goldie, I.; Grant, M.H. In vitro biocompatibility testing of polymers for orthopaedic implants using cultured fibroblasts and osteoblasts. Biomaterials 1995, 16, 987–992. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Gittens, R.A.; Schneider, J.M.; Hyzy, S.L.; Haithcock, D.A.; Ullrich, P.F.; Schwartz, Z.; Boyan, B.D. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012, 12, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torstrick, F.B.; Lin, A.S.P.; Potter, D.; Safranski, D.L.; Sulchek, T.A.; Gall, K.; Guldberg, R.E. Porous PEEK improves the bone-implant interface compared to plasma-sprayed titanium coating on PEEK. Biomaterials 2018, 185, 106–116. [Google Scholar] [CrossRef]
- Abrahamsson, I.; Berglundh, T.; Glantz, P.O.; Lindhe, J. The mucosal attachment at different abutments. An experimental study in dogs. J. Clin. Periodontol. 1998, 25, 721–727. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Abrahamsson, I.; Berglundh, T.; Lindhe, J. Soft tissue reactions to plaque formation at implant abutments with different surface topography. An experimental study in dogs. J. Clin. Periodontol. 2002, 29, 456–461. [Google Scholar] [CrossRef] [PubMed]
- de Val, J.E.M.S.; Gomez-Moreno, G.; Perez-Albacete Martinez, C.; Ramirez-Fernandez, M.P.; Granero-Marin, J.M.; Gehrke, S.A.; Calvo-Guirado, J.L. Peri-implant tissue behavior around non-titanium material: Experimental study in dogs. Ann. Anat. 2016, 206, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Gheisarifar, M.; Thompson, G.A.; Drago, C.; Tabatabaei, F.; Rasoulianboroujeni, M. In vitro study of surface alterations to polyetheretherketone and titanium and their effect upon human gingival fibroblasts. J. Prosthet. Dent. 2021, 125, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Grossner-Schreiber, B.; Herzog, M.; Hedderich, J.; Duck, A.; Hannig, M.; Griepentrog, M. Focal adhesion contact formation by fibroblasts cultured on surface-modified dental implants: An in vitro study. Clin. Oral Implant Res. 2006, 17, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Kasaj, A.; Reichert, C.; Gotz, H.; Rohrig, B.; Smeets, R.; Willershausen, B. In vitro evaluation of various bioabsorbable and nonresorbable barrier membranes for guided tissue regeneration. Head Face Med. 2008, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Pae, A.; Lee, H.; Kim, H.S.; Kwon, Y.D.; Woo, Y.H. Attachment and growth behaviour of human gingival fibroblasts on titanium and zirconia ceramic surfaces. Biomed. Mater. 2009, 4, 025005. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Scheideler, L.; Rehbein, D.; Axmann, D.; Geis-Gerstorfer, J. Roughness induced dynamic changes of wettability of acid etched titanium implant modifications. Biomaterials 2004, 25, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Polski Komitet Normalizacyjny: Warsaw, Poland, 2009.
- Scientific, T.F. alamarBlue®Assay—Thermo Fisher Scientific Inc. 2018. Available online: http://tools.thermofisher.com/content/sfs/manuals/PI-DAL1025-1100_TI%20alamarBlue%20Rev%201.1.pdf (accessed on 10 August 2020).
- Scientific, T.F. Thermo Fisher Scientific. 2014. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/MAN0011851_Pierce_LDH_Cytotoxicity_Asy_UG.pdf (accessed on 10 August 2020).
- Peng, T.Y.; Lin, D.J.; Mine, Y.; Tasi, C.Y.; Li, P.J.; Shih, Y.H.; Chiu, K.C.; Wang, T.H.; Hsia, S.M.; Shieh, T.M. Biofilm Formation on the Surface of (Poly)Ether-Ether-Ketone and In Vitro Antimicrobial Efficacy of Photodynamic Therapy on Peri-Implant Mucositis. Polymers 2021, 13, 940. [Google Scholar] [CrossRef]
- Peng, T.Y.; Shih, Y.H.; Hsia, S.M.; Wang, T.H.; Li, P.J.; Lin, D.J.; Sun, K.T.; Chiu, K.C.; Shieh, T.M. In Vitro Assessment of the Cell Metabolic Activity, Cytotoxicity, Cell Attachment, and Inflammatory Reaction of Human Oral Fibroblasts on Polyetheretherketone (PEEK) Implant-Abutment. Polymers 2021, 13, 2995. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Sundriyal, P.; Sahu, M.; Prakash, O.; Bhattacharya, S. Long-term surface modification of PEEK polymer using plasma and PEG silane treatment. Surf. Interfaces 2021, 25, 101253. [Google Scholar] [CrossRef]
- Rozeik, A.S.; Chaar, M.S.; Sindt, S.; Wille, S.; Selhuber-Unkel, C.; Kern, M.; El-Kholy, S.; Dorfer, C.; Fawzy El-Sayed, K.M. Cellular properties of human gingival fibroblasts on novel and conventional implant-abutment materials. Dent. Mater. 2022, 38, 540–548. [Google Scholar] [CrossRef]
- Ramenzoni, L.L.; Attin, T.; Schmidlin, P.R. In Vitro Effect of Modified Polyetheretherketone (PEEK) Implant Abutments on Human Gingival Epithelial Keratinocytes Migration and Proliferation. Materials 2019, 12, 1401. [Google Scholar] [CrossRef] [Green Version]
- Bagchi, P.; Alfawzan, A.A.; Magar, S.M.; Priya, R.; Kochhar, A.S.; Agrawal, S.; AlMutairi, F.J. An In vitro Evaluation of Effect of Implant Abutment on Human Gingival Epithelial Keratinocytes. Ann. Afr. Med. 2021, 21, 217. [Google Scholar]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Ma, R.; Tang, T. Current strategies to improve the bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-S.; Ko, Y.; Kye, S.-B.; Yang, S.-M. Human gingival fibroblast (HGF-1) attachment and proliferation on several abutment materials with various colors. Int. J. Oral Maxillofac. Implant. 2014, 29, 969–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhurakivska, K.; Ciacci, N.; Troiano, G.; Caponio, V.C.A.; Scrascia, R.; Pallecchi, L.; Lo Muzio, L.; Arena, F. Nitride-Coated and Anodic-Oxidized Titanium Promote a Higher Fibroblast and Reduced Streptococcus gordonii Proliferation Compared to the Uncoated Titanium. Prosthesis 2020, 2, 333–339. [Google Scholar] [CrossRef]
- Nothdurft, F.P.; Fontana, D.; Ruppenthal, S.; May, A.; Aktas, C.; Mehraein, Y.; Lipp, P.; Kaestner, L. Differential behavior of fibroblasts and epithelial cells on structured implant abutment materials: A comparison of materials and surface topographies. Clin. Implant. Dent. Relat. Res. 2015, 17, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- van Brakel, R.; Cune, M.S.; van Winkelhoff, A.J.; de Putter, C.; Verhoeven, J.W.; van der Reijden, W. Early bacterial colonization and soft tissue health around zirconia and titanium abutments: An in vivo study in man. Clin. Oral Implant. Res. 2011, 22, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Gatewood, R.R.; Cobb, C.M.; Killoy, W.J. Microbial colonization on natural tooth structure compared with smooth and plasma-sprayed dental implant surfaces. Clin. Oral Implant. Res. 1993, 4, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Piattelli, A.; Polimeni, A.; Di Iorio, D.; Carinci, F. Bacterial adhesion on commercially pure titanium and anatase-coated titanium healing screws: An in vivo human study. J. Periodontol. 2010, 81, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.V.; Reddy, M.S.; Reddy, C.R.; Pithani, P.; Kumar, R.S.; Kulkarni, G. The influence of implant abutment surface roughness and the type of cement on retention of implant supported crowns. J. Clin. Diagn. Res. 2015, 9, ZC05–ZC07. [Google Scholar] [CrossRef] [PubMed]
- Maness, P.C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal activity of photocatalytic TiO2 reaction: Toward an understanding of its killing mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [CrossRef] [Green Version]
- Cicciu, M.; Fiorillo, L.; Herford, A.S.; Crimi, S.; Bianchi, A.; D’Amico, C.; Laino, L.; Cervino, G. Bioactive Titanium Surfaces: Interactions of Eukaryotic and Prokaryotic Cells of Nano Devices Applied to Dental Practice. Biomedicines 2019, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhola, R.; Su, F.; Krull, C.E. Functionalization of titanium based metallic biomaterials for implant applications. J. Mater. Sci. Mater. Med. 2011, 22, 1147–1159. [Google Scholar] [CrossRef]
- Ananth, H.; Kundapur, V.; Mohammed, H.S.; Anand, M.; Amarnath, G.S.; Mankar, S. A Review on Biomaterials in Dental Implantology. Int. J. Biomed. Sci. 2015, 11, 113–120. [Google Scholar]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef]
- Moura, C.E.B.; Queiroz Neto, M.F.; Braz, J.; de Medeiros Aires, M.; Silva Farias, N.B.; Barboza, C.A.G.; Cavalcanti Junior, G.B.; Rocha, H.A.O.; Alves Junior, C. Effect of plasma-nitrided titanium surfaces on the differentiation of pre-osteoblastic cells. Artif. Organs 2019, 43, 764–772. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-1:2018; Biological Evaluation of Medical Devices. BSI Standards Limited: London, UK, 2009.
- ISO 10993-12:2021; Biological Evaluation of Medical Devices. Sample Preparation and Reference Materials: Geneva, Switzerland, 2007.
- Pabst, A.; Walter, C.; Bell, A.; Weyhrauch, M.; Schmidtmann, I.; Scheller, H.; Lehmann, K. Influence of CAD/CAM zirconia for implant-abutment manufacturing on gingival fibroblasts and oral keratinocytes. Clin. Oral Investig. 2016, 20, 1101–1108. [Google Scholar] [CrossRef]
- Pendegrass, C.; Fontaine, C.; Chan, G.; Hosseini, P.; Blunn, G. Intraosseous transcutaneous amputation prostheses vs. dental implants: A comparison between keratinocytes and gingival cell adhesion in vitro. Eur. Cells Mater. 2015, 29, 237–249. [Google Scholar] [CrossRef]
- Corvino, E.; Pesce, P.; Mura, R.; Marcano, E.; Canullo, L. Influence of Modified Titanium Abutment Surface on Peri-implant Soft Tissue Behavior: A Systematic Review of In Vitro Studies. Int. J. Oral Maxillofac. Implant. 2020, 35, 503–519. [Google Scholar] [CrossRef]
- Krithikadatta, J.; Gopikrishna, V.; Datta, M. CRIS Guidelines (Checklist for Reporting In-vitro Studies): A concept note on the need for standardized guidelines for improving quality and transparency in reporting in-vitro studies in experimental dental research. J. Conserv. Dent. 2014, 17, 301–304. [Google Scholar] [CrossRef] [PubMed]


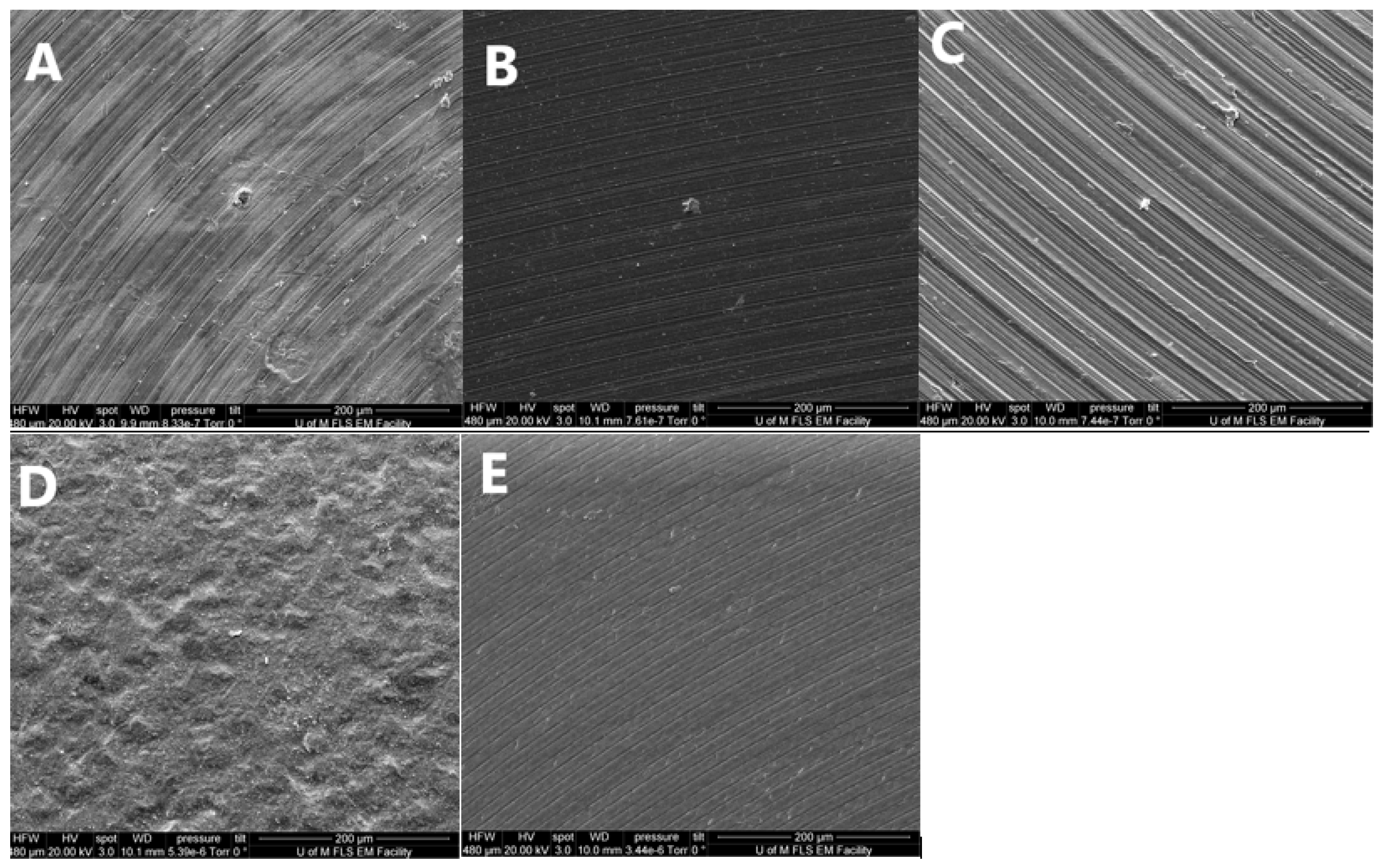


| Abutment Material | Ti | TiN | CoCr | ZrO2 | m-PEEK |
|---|---|---|---|---|---|
| Savalue: mean µm (SD) | 0.233 µm (0.018) | 0.235 µm (0.012) | 0.349 µm (0.014) | 0.363 µm (0.013) | 0.232 µm (0.021) |
| MinimumSavalue | 0.212 µm | 0.222 µm | 0.340 µm | 0.213 µm | 0.212 µm |
| MaximumSavalue | 0.247 µm | 0.247 µm | 0.366 µm | 0.373 µm | 0.255 µm |
| Materials | Ti | TiN | CoCr | ZrO2 | m-PEEK |
|---|---|---|---|---|---|
| Time | |||||
| Day 1 | 69.96 (20.17) A | 63.80 (13.69) A | 59.81 (12.13) A | 53.50 (14.15) A | 53.9 (21.55) A |
| Day 3 | 30.69 (11.3) A | 35.42 (6.57) A | 25.39 (12.53) A,B | 20.48 (4.19) B | 20.15 (3.29) B |
| Day 5 | 20.24 (5.18) A | 21.79 (10.12) A | 8.43 (2.99) B | 10.99 (3.25) B | 8.27 (2.28) B |
| Day 10 | 6.53 (4.03) A | 16.01 (5.70) B | 4.69 (2.35) A | 3.81 (2.03) A | 3.3 (0.87) A |
| Materials | Ti | TiN | CoCr | ZrO2 | m-PEEK |
|---|---|---|---|---|---|
| Time | |||||
| Day 1 | 35.78 (20.99) A,C | 31.16 (16.79) A | 29.00 (16.18) A | 58.18 (15.2) B,C | 60.01 (15.06) B |
| Day 3 | 17.65 (5.37) A | 20.65 (7.04) A | 18.18 (5.7) A | 56.09 (12.6) B | 61.81 (11.96) B |
| Day 5 | 15.84 (3.52) A | 14.49 (4.22) A | 13.77 (5.12) A | 33.40 (6.82) C | 44.38 (6.80) B |
| Day 10 | 19.09 (3.27) A | 21.87 (6.06) A | 21.05 (6.92) A | 40.27 (9.30) B | 48.38 (9.40) B |
| Materials | Ti | TiN | CoCr | ZrO2 | m-PEEK |
|---|---|---|---|---|---|
| Time | |||||
| Day 1 | 16.34 (3.45) A | 25.62 (2.47) B,D | 30.33 (6.57) B,C | 35.36 (2.49) C | 22.85 (6.06) D |
| Day 3 | 20.71 (3.47) A,B,D | 23.80 (4.06) A,D | 14.33 (5.17) B,C,E | 9.56 (7.69) C | 18.86 (2.82) D,E |
| Day 5 | 10.34 (4.13) A,D | 6.75 (2.66) A,D | 4.66 (2.04) B | 2.91 (1.01) C | 8.72 (2.93) D |
| Day 10 | 17.86 (3.32) A,C | 17.23 (2.97) A,C,E | 5.68 (1.15) B,E | 1.96 (0.48) B | 14.77 (2.93) C |
| Materials | Ti | TiN | CoCr | ZrO2 | m-PEEK |
|---|---|---|---|---|---|
| Time | |||||
| Day 1 | −1.02 (2.03) A | −0.84 (2.70) A | −0.51 (2.92) A | −0.55 (3.33) A | −0.52 (2.16) A |
| Day 3 | 3.79 (2.90) A | 7.22 (4.03) A,C | 8.51 (2.89) B,C | 6.80 (2.01) A,B | 14.75 (4.01) D |
| Day 5 | 11.61 (2.15) A | 8.36 (2.51) A | 8.07 (2.24) A | 11.72 (4.12) A | 11.01 (2.27) A |
| Day 10 | 26.25 (6.17) A | 27.29 (10.77) A | 27.51 (4.82) A | 28.46 (7.35) A | 15.99 (5.27) B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, M.A.; Kushnerev, E.; Alamoush, R.A.; Seymour, K.G.; Yates, J.M. Two Gingival Cell Lines Response to Different Dental Implant Abutment Materials: An In Vitro Study. Dent. J. 2022, 10, 192. https://doi.org/10.3390/dj10100192
Osman MA, Kushnerev E, Alamoush RA, Seymour KG, Yates JM. Two Gingival Cell Lines Response to Different Dental Implant Abutment Materials: An In Vitro Study. Dentistry Journal. 2022; 10(10):192. https://doi.org/10.3390/dj10100192
Chicago/Turabian StyleOsman, Muataz A., Evgeny Kushnerev, Rasha A. Alamoush, Kevin. G. Seymour, and Julian M. Yates. 2022. "Two Gingival Cell Lines Response to Different Dental Implant Abutment Materials: An In Vitro Study" Dentistry Journal 10, no. 10: 192. https://doi.org/10.3390/dj10100192






