Sinus Mucosa Thinning and Perforations after Sinus Lifting Performed with Different Xenografts: A Histological Analysis in Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. Study Design
2.3. Experimental Animals
2.4. Biomaterials
2.5. Sample Size
2.6. Randomization and Allocation Concealment
2.7. Clinical Procedures
2.8. Euthanasia
2.9. Housing and Husbandry
2.10. Histological Preparation
2.11. Calibration for Histometric Evaluations
2.12. Histological Analyses
2.13. Experimental Outcomes and Statistical Methods
3. Results
3.1. Clinical Outcomes
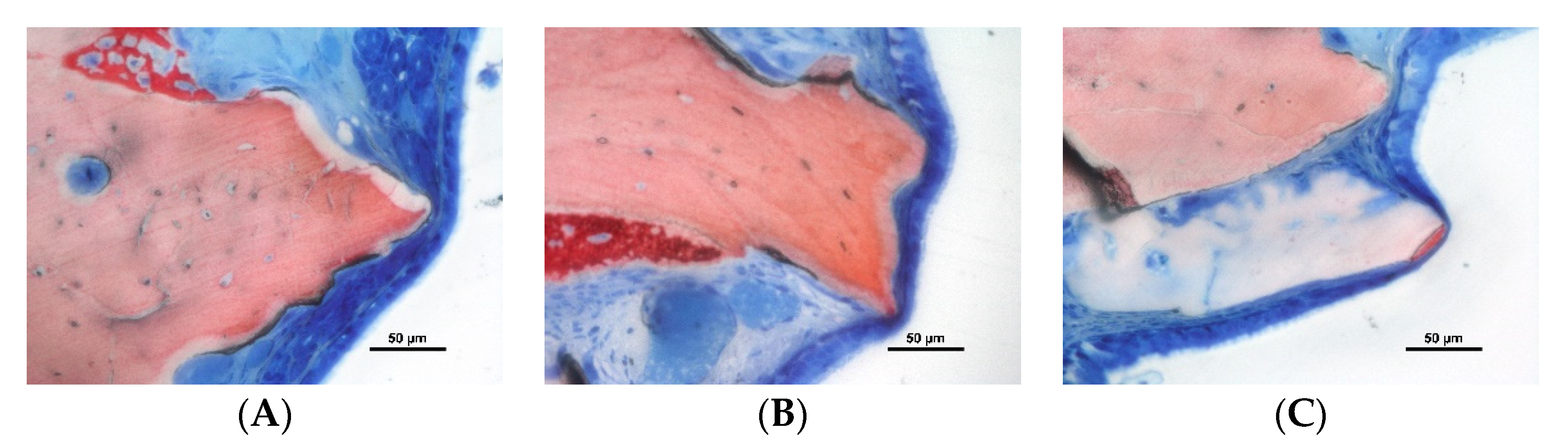
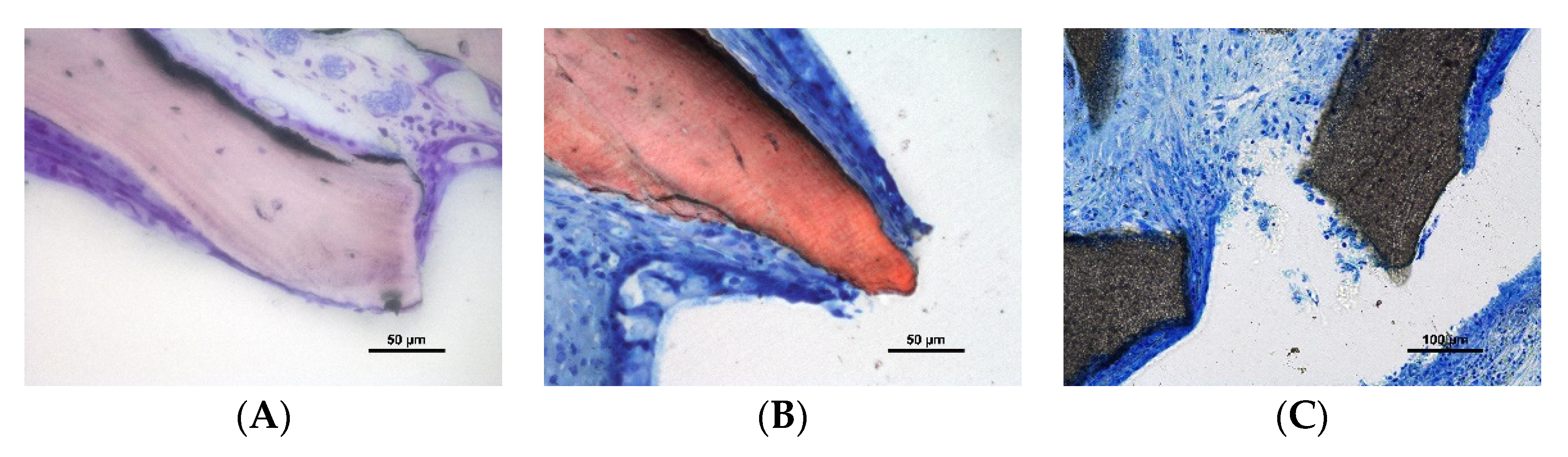
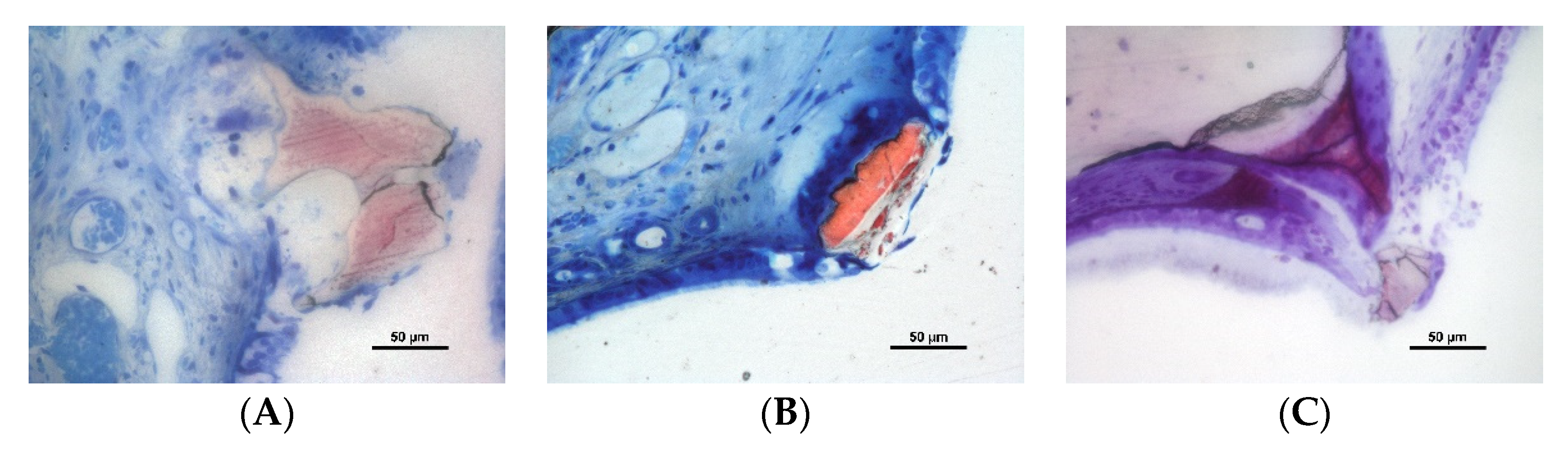
3.2. Descriptive Histological Evaluation
3.3. Histometric Assessments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pjetursson, B.E.; Tan, W.C.; Zwahlen, M.; Lang, N.P. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 216–240. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Wallace, S.S.; Testori, T. Long-Term Implant Survival in the Grafted Maxillary Sinus: A Systematic Review. Int. J. Periodontics Restor. Dent. 2013, 33, 773–783. [Google Scholar] [CrossRef]
- Kim, J.; Jang, H. A review of complications of maxillary sinus augmentation and available treatment methods. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Stacchi, C.; Andolsek, F.; Berton, F.; Perinetti, G.; Navarra, C.; Di Lenarda, R. Intraoperative Complications During Sinus Floor Elevation with Lateral Approach: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2017, 32, e107–e118. [Google Scholar] [CrossRef]
- Reiser, G.M.; Rabinovitz, Z.; Bruno, J.; Damoulis, P.D.; Griffin, T.J. Evaluation of maxillary sinus membrane response following elevation with the crestal osteotome technique in human cadavers. Int. J. Oral Maxillofac. Implant. 2001, 16, 833–840. [Google Scholar]
- Nkenke, E.; Schlegel, A.; Schultze-Mosgau, S.; Neukam, F.W.; Wiltfang, J. The endoscopically controlled osteotome sinus floor elevation: A preliminary prospective study. Int. J. Oral Maxillofac. Implant. 2002, 17, 557–566. [Google Scholar]
- Berengo, M.; Sivolella, S.; Majzoub, Z.; Cordioli, G. Endoscopic evaluation of the bone-added osteotome sinus floor elevation procedure. Int. J. Oral Maxillofac. Surg. 2004, 33, 189–194. [Google Scholar] [CrossRef]
- Nolan, P.J.; Freeman, K.; Kraut, R.A. Correlation Between Schneiderian Membrane Perforation and Sinus Lift Graft Outcome: A Retrospective Evaluation of 359 Augmented Sinus. J. Oral Maxillofac. Surg. 2014, 72, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Han, J.-Y.; Kang, P.; Momen-Heravi, F. The clinical and radiographic outcomes of Schneiderian membrane perforation without repair in sinus elevation surgery. Clin. Implant. Dent. Relat. Res. 2019, 21, 931–937. [Google Scholar] [CrossRef]
- Galli, S.K.D.; Lebowitz, R.A.; Giacchi, R.J.; Glickman, R.; Jacobs, J.B. Chronic sinusitis complicating sinus lift surgery. Am. J. Rhinol. 2001, 15, 181–186. [Google Scholar] [CrossRef]
- Kato, S.; Botticelli, D.; De Santis, E.; Kanayama, M.; Ferreira, S.; Jr, I.R.-G. Sinus mucosa thinning and perforation after sinus augmentation. A histological study in rabbits. Oral Maxillofac. Surg. 2021, 25, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Miki, M.; Botticelli, D.; Silva, E.R.; Xavier, S.; Baba, S. Incidence of Sinus Mucosa Perforations During Healing After Sinus Elevation Using Deproteinized Bovine Bone Mineral as Grafting Material: A Histologic Evaluation in a Rabbit Model. Int. J. Oral Maxillofac. Implant. 2021, 36, 660–668. [Google Scholar] [CrossRef]
- Omori, Y.; Botticelli, D.; Ferri, M.; Delgado-Ruiz, R.; Balan, V.F.; Xavier, S.P. Argon Bioactivation of Implants Installed Simultaneously to Maxillary Sinus Lifting without Graft. An Experimental Study in Rabbits. Dent. J. 2021, 9, 105. [Google Scholar] [CrossRef]
- Corbella, S.; Taschieri, S.; Weinstein, R.L.; Del Fabbro, M. Histomorphometric outcomes after lateral sinus floor elevation procedure: A systematic review of the literature and meta-analysis. Clin. Oral Implant. Res. 2016, 27, 1106–1122. [Google Scholar] [CrossRef]
- Balan, V.V.F.; Botticelli, D.; Peñarrocha-Oltra, D.; Masuda, K.; Godoy, E.P.; Xavier, S.P. Sinus augmentation using bovine xenografts processed at either low or high temperatures. An experimental study in rabbits. Dent. J. 2021; under processing. [Google Scholar]
- Lee, J.H.; Yi, G.S.; Lee, J.W.; Kim, D.J. Physicochemical characterization of porcine bone-derived grafting material and comparison with bovine xenografts for dental applications. J. Periodontal Implant. Sci. 2017, 47, 388–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trajkovski, B.; Jaunich, M.; Müller, W.-D.; Beuer, F.; Zafiropoulos, G.-G.; Houshmand, A. Hydrophilicity, Viscoelastic, and Physicochemical Properties Variations in Dental Bone Grafting Substitutes. Materials 2018, 11, 215. [Google Scholar] [CrossRef] [Green Version]
- Riachi, F.; Naaman, N.; Tabarani, C.; Aboelsaad, N.; Aboushelib, M.N.; Berberi, A.; Salameh, Z. Influence of Material Properties on Rate of Resorption of Two Bone Graft Materials after Sinus Lift Using Radiographic Assessment. Int. J. Dent. 2012, 2012, 737262. [Google Scholar] [CrossRef] [PubMed]
- Gargallo-Albiol, J.; Tattan, M.; Sinjab, K.H.; Chan, H.-L.; Wang, H.-L. Schneiderian membrane perforation via transcrestal sinus floor elevation: A randomized ex vivo study with endoscopic validation. Clin. Oral Implant. Res. 2019, 30, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Gargallo-Albiol, J.; Sinjab, K.H.; Barootchi, S.; Chan, H.-L.; Wang, H.-L. Microscope and micro-camera assessment of Schneiderian membrane perforation via transcrestal sinus floor elevation: A randomized ex vivo study. Clin. Oral Implant. Res. 2019, 30, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Lang, N.P.; Iida, T.; Ferri, M.; Alccayhuaman, K.A.A.; Botticelli, D. Influence of the position of the antrostomy in sinus floor elevation assessed with cone-beam computed tomography: A randomized clinical trial. J. Investig. Clin. Dent. 2018, 9, e12362. [Google Scholar] [CrossRef]
- Kawakami, S.; Lang, N.; Ferri, M.; Alccayhuaman, K.; Botticelli, D. Influence of the Height of the Antrostomy in Sinus Floor Elevation Assessed by Cone Beam Computed Tomography: A Randomized Clinical Trial. Int. J. Oral Maxillofac. Implant. 2019, 34, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Hirota, A.; Lang, N.P.; Ferri, M.; Mesa, N.F.; Alccayhuaman, K.A.A.; Botticelli, D. Tomographic evaluation of the influence of the placement of a collagen membrane subjacent to the sinus mucosa during maxillary sinus floor augmentation: A randomized clinical trial. Int. J. Implant. Dent. 2019, 5, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Lang, N.P.; Ferri, M.; Hirota, A.; Alccayhuaman, A.A.A.; Botticelli, D. Tomographic assessment on the in-fluence on dimensional variations of the use of a collagen membrane to protect the antrostomy after maxillary sinus floor augmentation. A randomized clinical trial. Int. J. Oral Maxillofac. Implant. 2020, 35, 350–356. [Google Scholar] [CrossRef]
- Cricchio, G.; Faria, P.E.; Lundgren, S.; Sennerby, L.; Salata, L.A. Histological Findings Following the Use of a Space-Making Device for Bone Reformation and Implant Integration in the Maxillary Sinus of Primates. Clin. Implant. Dent. Relat. Res. 2009, 11, e14–e22. [Google Scholar] [CrossRef] [PubMed]
- Cricchio, G.; Palma, V.C.; Faria, P.E.; De Olivera, J.A.; Lundgren, S.; Sennerby, L.; Salata, L.A. Histological Outcomes on the Development of New Space-making Devices for Maxillary Sinus Floor Augmentation. Clin. Implant. Dent. Relat. Res. 2011, 13, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Schweikert, M.; Botticelli, D.; de Oliveira, J.A.; Scala, A.; Salata, L.A.; Lang, N.P. Use of a titanium device in lateral sinus floor elevation: An experimental study in monkeys. Clin. Oral Implant. Res. 2012, 23, 100–105. [Google Scholar] [CrossRef]
- Scala, A.; Botticelli, D.; Faeda, R.S.; Rangel, I.G., Jr.; de Oliveira, J.A.; Lang, N.P. Lack of influence of the Schneiderian membrane in forming new bone apical to implants simultaneously installed with sinus floor elevation: An experimental study in monkeys. Clin. Oral Implant. Res. 2012, 23, 175–181. [Google Scholar] [CrossRef]
- Scala, A.; Botticelli, D.; Rangel, I.G.; De Oliveira, J.A.; Okamoto, R.; Lang, N.P. Early healing after elevation of the maxillary sinus floor applying a lateral access: A histological study in monkeys. Clin. Oral Implant. Res. 2010, 21, 1320–1326. [Google Scholar] [CrossRef]
- Asai, S.; Shimizu, Y.; Ooya, K. Maxillary sinus augmentation model in rabbits: Effect of occluded nasal ostium on new bone formation. Clin. Oral Implant. Res. 2002, 13, 405–409. [Google Scholar] [CrossRef]
- Xu, H.; Shimizu, Y.; Asai, S.; Ooya, K. Grafting of deproteinized bone particles inhibits bone resorption after maxillary sinus floor elevation. Clin. Oral Implant. Res. 2004, 15, 126–133. [Google Scholar] [CrossRef]
- Caneva, M.; Lang, N.P.; Rangel, I.J.G.; Ferreira, S.; Caneva, M.; De Santis, E.; Botticelli, D. Sinus mucosa elevation using Bio-Oss® or Gingistat® collagen sponge: An experimental study in rabbits. Clin. Oral Implant. Res. 2017, 28, e21–e30. [Google Scholar] [CrossRef]
- Sakuma, S.; Ferri, M.; Imai, H.; Mesa, N.F.; Victorio, D.J.B.; Alccayhuaman, K.A.A.; Botticelli, D. Involvement of the maxillary sinus ostium (MSO) in the edematous processes after sinus floor augmentation: A cone-beam computed tomographic study. Int. J. Implant. Dent. 2020, 6, 35. [Google Scholar] [CrossRef]
- Aimetti, M.; Massei, G.; Morra, M.; Cardesi, E.; Romano, F. Correlation between gingival phenotype and Schneiderian membrane thickness. Int. J. Oral Maxillofac. Implant. 2008, 23, 1128–1132. [Google Scholar]
- Iida, T.; Neto, E.C.M.; Botticelli, D.; Alccayhuaman, K.A.A.; Lang, N.P.; Xavier, S. Influence of a collagen membrane positioned subjacent the sinus mucosa following the elevation of the maxillary sinus. A histomorphometric study in rabbits. Clin. Oral Implant. Res. 2017, 28, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, D.; Lang, N.P. Dynamics of osseointegration in various human and animal models - a comparative analysis. Clin. Oral Implant. Res. 2017, 28, 742–748. [Google Scholar] [CrossRef] [PubMed]


| 2 Weeks | 10 Weeks | |||||
|---|---|---|---|---|---|---|
| low-T | high-T | p value | low-T | high-T | p value | |
| Pristine mucosa in µm | 61 ± 21 | 63 ± 14 | 0.617 | 62 ± 12 | 54 ± 8 | 0.107 |
| Thinned mucosa in µm | 26 ± 3.2 | 26 ± 5.3 | 0.789 | 19 ± 3.0 | 21 ± 4.5 | 0.065 |
| No. sinus with thinned mucosa | 8 | 9 | >0.9999 | 10 | 10 | NA |
| No. thinned mucosa zones | 118 | 149 | 0.191 | 237 | 195 | 0.090 |
| No. of sinuses with perforations | 6 | 3 | 0.375 | 9 | 7 | 0.625 |
| No. of perforations | 8 | 3 | 0.188 | 19 | 14 | 0.898 |
| <40 | <30 | <20 | <10 | |
|---|---|---|---|---|
| 2 weeks low-T (n = 9) | 118 | 73 | 38 | 11 |
| 2 weeks high-T (n = 9) | 149 | 98 | 58 | 16 |
| 10 weeks low-T (n = 10) | 237 | 198 | 137 | 46 |
| 10 weeks high-T (n = 10) | 195 | 148 | 96 | 44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favero, R.; Apaza Alccayhuaman, K.A.; Botticelli, D.; Xavier, S.P.; Ferreira Balan, V.; Macchi, V.; De Caro, R. Sinus Mucosa Thinning and Perforations after Sinus Lifting Performed with Different Xenografts: A Histological Analysis in Rabbits. Dent. J. 2022, 10, 2. https://doi.org/10.3390/dj10010002
Favero R, Apaza Alccayhuaman KA, Botticelli D, Xavier SP, Ferreira Balan V, Macchi V, De Caro R. Sinus Mucosa Thinning and Perforations after Sinus Lifting Performed with Different Xenografts: A Histological Analysis in Rabbits. Dentistry Journal. 2022; 10(1):2. https://doi.org/10.3390/dj10010002
Chicago/Turabian StyleFavero, Riccardo, Karol Alí Apaza Alccayhuaman, Daniele Botticelli, Samuel Porfirio Xavier, Vitor Ferreira Balan, Veronica Macchi, and Raffaele De Caro. 2022. "Sinus Mucosa Thinning and Perforations after Sinus Lifting Performed with Different Xenografts: A Histological Analysis in Rabbits" Dentistry Journal 10, no. 1: 2. https://doi.org/10.3390/dj10010002
APA StyleFavero, R., Apaza Alccayhuaman, K. A., Botticelli, D., Xavier, S. P., Ferreira Balan, V., Macchi, V., & De Caro, R. (2022). Sinus Mucosa Thinning and Perforations after Sinus Lifting Performed with Different Xenografts: A Histological Analysis in Rabbits. Dentistry Journal, 10(1), 2. https://doi.org/10.3390/dj10010002










