Abstract
In this paper we report the binding properties, by combined 1H NMR, optical absorption, and fluorescence studies, of a molecular tweezer composed of two Zn(salen)-type Schiff-base units connected by a flexible spacer, towards a series of ditopic diamines having a strong Lewis basicity, with different chain length and rigidity. Except for the 1,2-diaminoethane, in all other cases the formation of stable 1:1 Lewis acid-base adducts with large binding constants is demonstrated. For α,ω-aliphatic diamines, binding constants progressively increase with the increasing length of the alkyl chain, thanks to the flexible nature of the spacer and the parallel decreased conformational strain upon binding. Stable adducts are also found even for short diamines with rigid molecular structures. Given their preorganized structure, these latter species are not subjected to loss of degrees of freedom. The binding characteristics of the tweezer have been exploited for the colorimetric and fluorometric selective and sensitive detection of piperazine.
1. Introduction
“Molecular tweezers” refer to bifunctional molecular receptors characterized by the presence of two binding sites connected with a more or less rigid spacer [1,2]. They have the ability to form complexes with a substrate molecule, resembling a tweezer holding an object. Depending on the nature of the binding sites and on the conformational rigidity of the spacer, they find various applications such as in molecular recognition [1,3], including biomolecules [4,5,6], or fullerenes [7], enzyme inhibition or prevention of protein aggregation [8,9,10], catalysis [11], switchable molecular tweezers [12,13], electrochemical switches [14,15], and as building blocks for supramolecular nanostructures [16,17].
Various molecular tweezers have been synthesized as hosts for guest molecules. Among them, glycoluril- [1,2] or porphyrin-based [3] tweezers are those most studied. The latter have been involved in various investigations for their ability to bind ditopic species, e.g., for configuration [18,19] and chirogenesis [20,21,22,23] studies, and for the development of sensors targeting specific molecules [24], including chiral species [25,26]. Moreover, the binding behavior with ditopic guests of different length and rigidities has also been explored [27,28,29,30].
Zn(salen)-type Schiff-base complexes have recently been investigated for their sensing properties [31,32,33], mostly related to their Lewis acidic character [34]. These complexes easily coordinate Lewis bases with formation of Lewis acid-base adducts, and this process is accompanied by relevant changes of their spectroscopic properties. Among them, derivatives from the 2,3-diaminomaleonitrile, Zn(salmal) [35,36], are those mostly studied for sensing a variety of Lewis bases [37,38,39,40,41,42,43,44].
Recently, a dinuclear Zn(salmal) Schiff-base complex (1, Scheme 1) whose units are connected with a non-conjugated, flexible spacer, has been synthesized and characterized [45]. It has been found that in non-coordinating solvents 1 is stabilized by the formation of intramolecular aggregates, which hardly deaggregate by addition of monotopic Lewis bases. However, in the presence of strong ditopic Lewis bases, such as diamines, the complex easily deaggregates with formation of 1:1 adducts, thus acting as a “molecular tweezer”. Deaggregation is accompanied by relevant optical absorption changes and a substantial enhancement of the fluorescence. Therefore, 1 has been investigated for the selective and sensitive detection of some biogenic diamines [46].
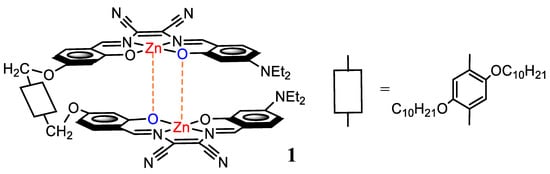
Scheme 1.
Structure of the dinuclear complex 1.
As the dinuclear aggregate complex 1 acts as a “molecular tweezer” upon deaggregation, this is an unusual feature compared to conventional tweezers characterized by binding sites kept separate by a spacer. Thus, starting from the defined aggregate 1, in the formation of Lewis acid-base adducts, it will not be subjected to binding as a consequence of a specific conformation of the Lewis base. Rather, the ability of the Lewis base to bind the aggregate could be related to its basicity and to the stability of the adduct. It is thus interesting to investigate the features affecting the binding interactions between the aggregate molecular tweezer 1, having a flexible spacer, and the structure of the ditopic Lewis bases.
The aim of this work is to study, through 1H NMR, UV/vis, and fluorescence spectroscopies, the binding interactions of the tweezer 1 with diamines of different chain length and rigidity, to better understand their Lewis acid-base interactions.
2. Results and Discussion
To study the binding of various diamines to the molecular tweezer 1, either aliphatic, alicyclic, and aromatic diamines were considered (Scheme 2). In particular, the flexible primary α,ω-aliphatic diamines, NH2(CH2)nNH2 (n = 2–12), were studied, and the results compared with those related to the rigid diamines, piperazine (PZ), 1,4-diazabicyclo[2.2.2]octane (DABCO), and 4,4′-bipyridine (BPY), and the semi-rigid 1,2-bis(4-pyridyl)ethane (DPE).
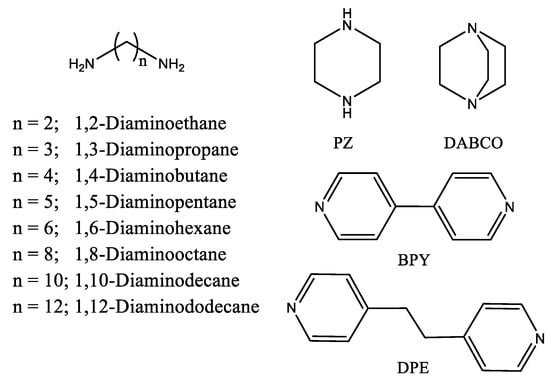
Scheme 2.
Structure of investigated diamines.
UV/vis optical absorption and fluorescence spectral data and binding constants for the formation of (1:1) 1·diamine adducts (equilibrium (1)) are reported in Table 1. Spectrophotometric and spectrofluorometric titrations for the representative 1,10-diaminodecane are reported in Figure 1 and Figure 2.
1 (soln) + diamine (soln) ⇋ 1·diamine (soln)

Table 1.
Binding constants and optical spectroscopic data for investigated 1·diamine adducts a in chloroform solution.
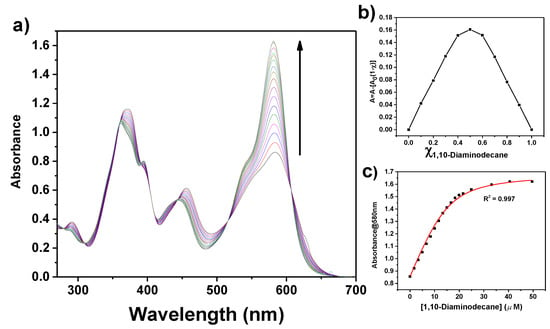
Figure 1.
(a) Optical absorption titration curves of 1 (15 µM solution in CHCl3) with addition of 1,10-diaminodecane. The concentration of 1,10-diaminodecane added varied from 0 to 50 µM. (b) Job’s plot for the binding of 1 with 1,10-diaminodecane. The total concentration of 1 and 1,10-diaminodecane is 15 μM. (c) Variation of the absorbance at 580 nm as a function of the concentration of 1,10-diaminodecane added and fit of the binding isotherm with Equation (1) (red line).
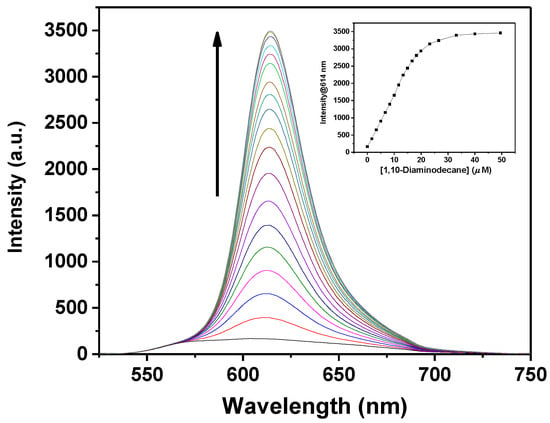
Figure 2.
Fluorescence titration curves of 1 (15 µM solution in CHCl3; λexc = 516 nm) with addition of 1,10-diaminodecane. The concentration of 1,10-diaminodecane added varied from 0 to 50 µM. Inset: variation of the fluorescence intensity at 614 nm as a function of the concentration of 1,10-diaminodecane added.
In all instances, the binding of diamines to 1 involves an increase of the optical absorption band centered at λmax = 580 nm and an enhancement of fluorescence intensity at λmax ≅ 614 nm (e.g., the fluorescence quantum yield, ϕ, increases from 0.03 to 0.29 on switching from 1 to the adduct with 1,10-diaminodecane). Moreover, with the exception of diaminoethane, optical absorption spectra show the presence of multiple isosbestic points, indicative of the formation of species with a defined stoichiometry. Job’s plot analyses clearly indicate the formation of 1:1 adducts.
2.1. α,ω-Aliphatic Diamines
As is shown in Table 1, a substantial variation of the binding constants with the chain length is observed. Despite the analogous Lewis basicity [47] along this investigated series of aliphatic, linear primary diamines, binding constants span over more than three orders of magnitude. While shorter diamines are characterized by relatively low binding constants, the increasing of the chain length parallels an increase of binding constant values. Largest binding constants are reached with the 1,8-diaminooctane, and then remain almost unchanged, even if slightly smaller, on further increase of the chain length.
1H NMR titration studies further support the formation of 1:1 adducts. The titration for the representative 1,10-diaminodecane is reported in Figure 3. In particular, after the addition of half stoichiometric amount of 1,10-diaminodecane to a CDCl3 solution of 1, the 1H NMR spectrum shows some changes with the appearance of new broad signals. A complete variation of the 1H NMR spectrum, with the presence of broad signals, is observed after the addition of a stoichiometric amount of 1,10-diaminodecane. Finally, upon the addition of 4-fold molar excess of 1,10-diaminodecane the spectrum evolves towards a set of sharp signals, except for the down-field shifted H3 and H3′ protons which remain slightly broad, indicative of the formation of a defined 1:1: adduct. Moreover, the doublet of doublet benzylic proton signals, H5, of the aggregate complex 1 becomes a sharp singlet, indicating that the restricted rotation around the benzylic bonds is no longer operating in the adduct.
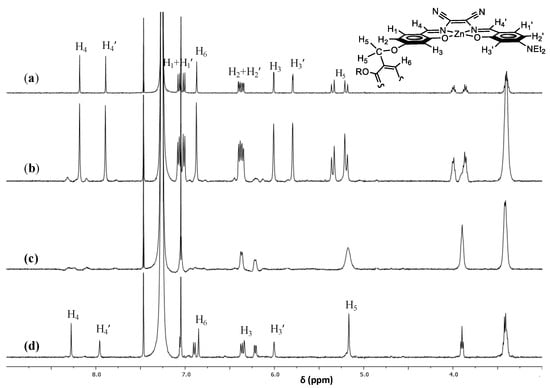
Figure 3.
1H NMR titration spectra of 1 (50 μM in CDCl3 (a)) with addition of 1,10-diaminodecane. The concentration of 1,10-diaminodecane added was 25 μM (b), 50 μM (c), and 200 μM (d). For assignment of 1H NMR signals of the aggregate 1. Adapted from ref. [45].
1,2-Diaminoethane behaves quite differently. In fact, starting from 10 μM solutions of 1 a detectable variation of optical absorption or fluorescence spectra occurs after the addition of ca. 2-fold molar excess of diaminoethane, while the saturation point is reached by the addition of ca. 800-fold molar excess, unlike longer diamines which form 1:1 adducts with 1 by addition of stoichiometric amounts. Moreover, in contrast with longer diamines, optical absorption spectra do not show any isosbestic point. These observations suggest the formation of multiple, instead of single, adducts (e.g., 1:2 adducts), likely favored by the presence of the large stoichiometric excess of diamine.
As binding constants for the formation of 1·diamine adducts reflect the relative stability of the adducts with respect to the aggregate [48], given the analogous Lewis basicity along the series of diamines and the entropic cost upon binding the diamine to 1, the increasing binding constants with the increased length of diamines can be related to an increased stability of the intramolecular cyclic adducts. In view of the flexibility of the spacer in the tweezer 1, which in principle can accommodate almost any ditopic Lewis base, the different binding constants along the series could be attributed to a larger entropic cost of the loss of degrees of freedom for diamines with a shorter alkyl chain, while the involved longer α,ω-diamines are not subjected to conformational strain upon binding, resulting in larger intramolecular cyclic adducts, also with gain of degrees of freedom of the flexible spacer of the tweezer.
In comparison to host–guest studies involving glycoluril- or porphyrin-based tweezers with ditopic guests of different length, 1 behaves quite differently, being characterized by increasing binding constants with increasing chain length of diamines. In fact, it has been found that most of these investigated tweezers, having rigid or semi-flexible spacers, show a preference for a particular guest, rather than for shorter or longer ones. This has been associated with the guest best matching the distance between the binding sites of the tweezer [27,28,29,30,49,50,51].
2.2. Rigid Diamines
The binding interaction for the formation of 1·diamine adducts is also affected by the rigidity of the diamine. PZ is a cyclic secondary diamine with a N-N distance comparable to that of 1,2-diaminoethane. In spite of this, PZ forms stable 1:1 adducts with 1, with a large binding constant (Table 1), especially if compared to that of aliphatic diamines with shorter chain length (n ≤ 4). DABCO is a bicyclic tertiary diamine whose N-N distance is comparable to that of PZ. It has been widely used to study the binding characteristics of porphyrin-based tweezers [22,30,52,53,54]. Again, DABCO binds easily with 1 with a binding constant slightly higher than that of PZ. This is consistent with the greater Lewis basicity of the tertiary alicyclic DABCO with respect to the secondary alicyclic PZ [47].
It therefore turns out that the preorganized structure of PZ and DABCO favors the formation of stable adducts because, except for the entropic cost upon binding the diamine to 1, these species are not subjected to loss of degrees of freedom, contrary to aliphatic diamines with short alkyl chain.
1H NMR titrations using DABCO as titrant suggest the formation of stable 1:1 adducts even for alicyclic diamines (Figure 4). In this case, however, when half stoichiometric amount of DABCO is added to CDCl3 solution of 1, two sets of signals are evident in the spectrum. This indicates the presence in solution of the aggregate complex 1 and its adduct with DABCO, in a slow equilibrium on the NMR time scale. Moreover, from the comparison of the signal of H5 protons, a sharper singlet is observed for the adduct with 1,10-diaminodecane, with respect to the broad signal for that with DABCO. This suggests a greater mobility of benzyl hydrogens in the larger intramolecular cyclic 1·diaminodecane adduct compared to those of the 1·DABCO adduct (Figure 5).
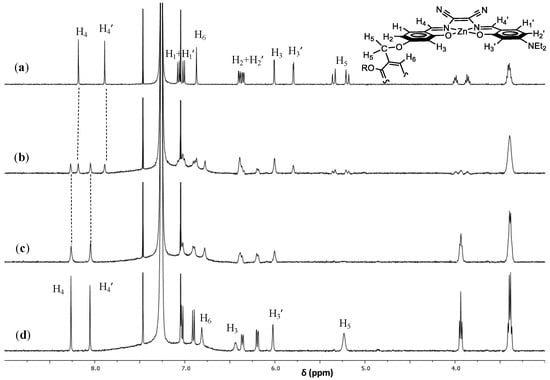
Figure 4.
1H NMR titration spectra of 1 (50 μM in CDCl3 (a)) with addition of DABCO. The concentration of DABCO added was 25 μM (b), 100 μM (c), and 200 μM (d).
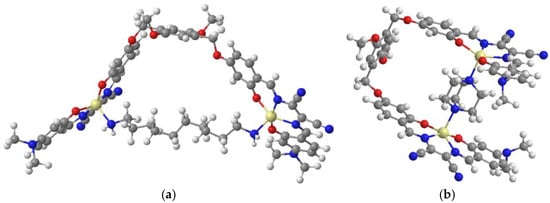
Figure 5.
(a) Modeling (PM3, using the HyperChem Software (8.0)) of the 1·diaminodecane adduct and (b) modeling of the 1·DABCO adduct.
The structure of DPE is more rigid than that of α,ω-aliphatic diamines because of the presence of two heterocyclic aromatic rings linked by an ethyl group. In the anti-conformation, a N-N distance of 9.3 Å can be estimated, slightly longer than that of 1,6-diaminohexane (8.7 Å). However, the binding constant of DPE results are one order of magnitude lower than that of 1,6-diaminohexane. This can be attributed to a higher conformational strain upon binding DPE to 1, in comparison with the flexible diaminohexane.
BPY was also investigated as rigid diamine. Spectrophotometric titrations again indicate the formation of a defined species; by the presence of multiple isosbestic points, however, the saturation point is reached with ca. a 500-fold molar excess of BPY. In this case the binding isotherm is fitted with a model involving a 1:2 adduct (equilibria (2)), instead of a 1:1 adduct (equilibrium (1)).
1 (soln) + BPY (soln) ⇋ 1·BPY
1·BPY (soln) + BPY (soln) ⇋ 1·(BPY)2 (soln)
Even if the ditopic BPY is expected to possess a strong Lewis basicity comparable to pyridine, it behaves as a monotopic species. Deaggregation of 1 with pyridine gave the same results (Table 1) [45].
2.3. Sensing Piperazine
The molecular tweezer 1 can be used as a suitable chemodosimeter for the detection of piperazine. PZ possesses important pharmacological properties and is used, together with its salts, as an anthelmintic [55], in industrial gas treatments such as CO2 capture system [56,57], and also as the precursor for a class of psychogenic drugs [58,59]. Piperazine may cause allergic dermatitis [60], and it has been demonstrated that, although not extremely toxic, it has a low biodegradability [61]. Detection of PZ is thus relevant for environment monitoring and protection [62].
The tweezer 1 allows both the colorimetric and fluorometric selective and sensitive detection of PZ. A calculated limit of detection (LOD) down to 0.76 µM and 0.33 µM is obtained from the spectrophotometric and spectrofluorometric data, respectively, with a linear dynamic range up to 10 μM (Figure 6 and Figure 7). These values are better than those reported in the literature using spectrophotometric methods [63,64,65,66]. Various other techniques have been developed for the detection and quantitation of piperazine, such as HPLC [67], voltammetry [68], or capillary electrophoresis [69]; most of them, however, require time-consuming procedures. Therefore, the development of simple and direct methods for sensing piperazine is highly desirable. In this regard, only a few optical chemosensors are reported in the literature for the selective detection of PZ [70,71,72].
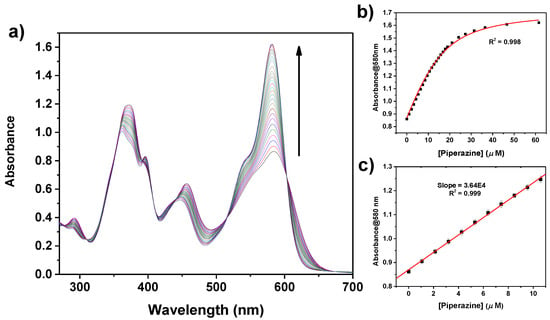
Figure 6.
(a) Optical absorption titration curves of 1 (15 µM solution in CHCl3) with addition of PZ. The concentration of PZ added varied from 0 to 60 µM. (b) Variation of the absorbance at 580 nm as a function of the concentration of PZ added and fit of the binding isotherm with Equation (1) (red line). (c) Linear best fit in the linear dynamic range (weight given by data error bars).
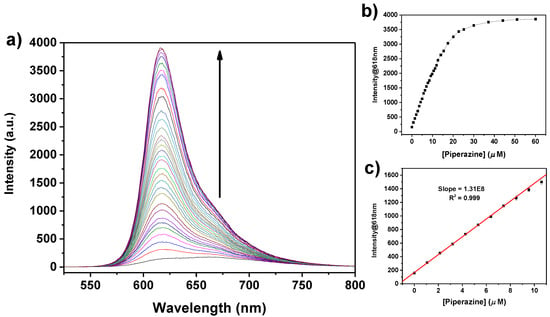
Figure 7.
(a) Fluorescence titration curves of 1 (15 µM solution in CHCl3; λexc = 515 nm) with addition of PZ. The concentration of PZ added varied from 0 to 60 µM. (b) Variation of the fluorescence intensity at 618 nm as a function of the concentration of PZ added. (c) Linear best fit in the linear dynamic range (weight given by data error bars).
The selectivity of 1 towards PZ was proven by performing competitive experiments. These were conducted by mixing a solution of 1 with PZ (1:1 molar ratio) and a 10-fold molar excess of interferent (Figure 8). These results were then compared with those obtained by adding to 1 either PZ in an equimolar amount, or the interferent in a 10-fold molar excess. As potential interferents, some monotopic species with a strong Lewis basicity were considered. Pyridine was chosen as the heterocyclic aromatic amine, while isopropylamine, diethylamine, and triethylamine were chosen as prototype compounds of primary, secondary, and tertiary amines. Moreover, the heteroditopic 4-amino-1-butanol, bearing two different coordinating sites with a different Lewis basicity, was also considered. As shown in Figure 8, very small or no changes of the absorbance at λmax = 580 nm are observed after the addition of each potential interferent, especially for diethylamine and triethylamine. Therefore, these data suggest that 1 can be considered a selective receptor towards PZ, even in the presence of common aliphatic or aromatic monotopic or heteroditopic amines.
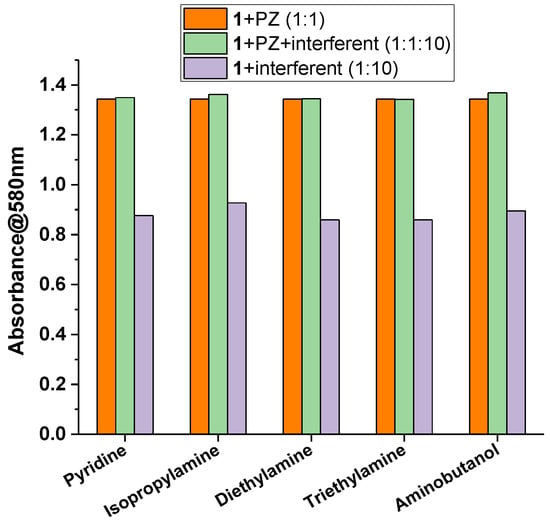
Figure 8.
Absorbance of 1 at 580 nm upon the addition of an equimolar amount (15 µM) of piperazine (orange bars); upon the addition of an equimolar amount (15 µM) of piperazine with the presence of 10-fold molar excess (150 µM) of interferent (green bars); upon the addition of 10-fold molar excess (150 µM) of interferent (violet bars).
3. Experimental Section
3.1. Materials and General Procedures
All the chemicals used were purchased from Sigma-Aldrich (Darmstadt, Germany) and used as received. Complex 1 was synthesized and purified as previously reported [45]. Chloroform stabilized with amylene was used for optical absorption and fluorescence titrations. Before being used, it was purified as follows: dried on anhydrous K2CO3 for 2 h, filtered and stored over molecular sieves (3 Å) in the dark under argon atmosphere. Chloroform solutions of 1 were prepared by dissolving the compound in chloroform and filtering it through a 0.2 μm Teflon membrane filter. CDCl3 was stored over molecular sieves (3 Å).
3.2. Physical Measurements
1H NMR measurements were run at 27 °C on a Varian Unity S 500 (499.88 MHz for 1H) spectrometer. Tetramethylsilane was used as internal reference for all NMR experiments. Optical absorption spectra were recorded at room temperature with an Agilent Cary 60 spectrophotometer. Fluorescence spectra were recorded at room temperature using a JASCO FP-8200 spectrofluorometer (JASCO Europe). Spectrophotometric and fluorometric titrations were performed with a 1 cm path cell using 15 µM chloroform solutions of 1. Chloroform solutions of involved Lewis bases were added to the solution of 1 using Rainin (METTLER TOLEDO, Columbus, OH, USA) positive displacements pipettes. At least three replicate titrations were performed for each diamine. In fluorometric titrations, the wavelength of excitation was chosen in an isosbestic point. The fluorescence quantum yield was obtained using fluorescein (ϕF = 0.925) in 0.1 M NaOH as a standard. The absorbance value of the samples at and above the excitation wavelength was lower than 0.1 for 1 cm path length cuvettes.
3.3. Calculation of Binding Constants and Limit of Detection
Binding constants, K, for the formation of 1 diamine adducts (equilibrium 1) were calculated by fitting the binding isotherms, obtained from the plot of A vs. cA from spectrophotometric titration data, with Equation (1) [73,74].
where A0 is the initial absorbance of the solution having a concentration c0, A is the absorbance intensity after addition of a given amount of diamine (DA) at a concentration cDA, and Alim is the limiting absorbance reached in the presence of an excess of DA. Further details are reported elsewhere [46,48]. These calculated binding constants are comparable to those previously obtained by a multivariate analysis from spectrophotometric titrations of 1 with PZ, DPE, and 1,4-diaminobutane [45]. In the case of BPY, binding constants K1 and K2 for a 1:2 adduct (equilibria (2)) were calculated by fitting the binding isotherm using Equation (35) of Ref. [73].
The limit of detection (LOD) was estimated, both from optical absorption or fluorescence data, according to IUPAC recommendations (Equation (2)) [75,76].
where K = 3, Sb is the standard deviation of the blank solution, i.e., the absorbance or fluorescence signal of 1, and S is the slope of the calibration curve obtained from the plot of the absorbance or fluorescence intensity of 1 vs. the concentration of the DA added. Each point is related to the mean value obtained from at least three replicate measurements. Twenty blank replicates were considered.
LOD = K × Sb/S
4. Conclusions
The binding properties of a molecular tweezer, composed of two Zn(salmal) units connected by a flexible spacer, towards a series of ditopic diamines having strong Lewis basicity have been explored by means of combined 1H NMR, optical absorption, and fluorescence studies. The formation of stable 1:1 Lewis acid-base adducts with large binding constants is demonstrated. For α,ω-aliphatic diamines, binding constants progressively increase with the increasing length of the alkyl chain, thanks to the flexible nature of the spacer and there is a parallel decrease of the conformational strain upon binding for longer diamines, reaching the largest value for the 1,8-diaminooctane. Stable adducts are also found even for short diamines with rigid molecular structures. The preorganized structure of these ditopic species which, except for the entropic cost upon binding the diamine to 1, are not subjected to loss of degrees of freedom, accounts for the large binding constants.
These binding characteristics can be exploited for the detection of ditopic strong Lewis bases. The colorimetric and fluorometric selective and sensitive detection has been demonstrated for piperazine.
Author Contributions
All experimental work was performed by G.M., G.C. and S.F. Data analysis was performed by G.M., S.F. and S.D.B. The manuscript was written by S.D.B., with contributions from the other authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
This work was supported by the University of Catania, PIACERI 2020/2022, Linea di Intervento 2.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hardouin–Lerouge, M.; Hudhomme, P.; Sallé, M. Molecular clips and tweezers hosting neutral guests. Chem. Soc. Rev. 2011, 40, 30–43. [Google Scholar] [CrossRef]
- Leblond, J.; Petitjean, A. Molecular Tweezers: Concepts and Applications. ChemPhysChem 2011, 12, 1043–1051. [Google Scholar] [CrossRef]
- Valderrey, V.; Aragay, G.; Ballester, P. Porphyrin tweezer receptors: Binding studies, conformational properties and applications. Co-Ord. Chem. Rev. 2014, 258-259, 137–156. [Google Scholar] [CrossRef]
- Klärner, F.-G.; Schrader, T. Aromatic Interactions by Molecular Tweezers and Clips in Chemical and Biological Systems. Acc. Chem. Res. 2012, 46, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Zhou, W.; Li, D.; Chai, Y.; Xiang, Y.; Yuan, R. RNA-regulated molecular tweezers for sensitive fluorescent detection of microRNA from cancer cells. Biosens. Bioelectron. 2015, 71, 98–102. [Google Scholar] [CrossRef]
- Fokkens, M.; Schrader, T.; Klärner, F.-G. A Molecular Tweezer for Lysine and Arginine. J. Am. Chem. Soc. 2005, 127, 14415–14421. [Google Scholar] [CrossRef] [PubMed]
- Pérez, E.M.; Martín, N. Molecular tweezers for fullerenes. Pure Appl. Chem. 2010, 82, 523–533. [Google Scholar] [CrossRef]
- Mbarek, A.; Moussa, G.; Chain, J.L. Pharmaceutical Applications of Molecular Tweezers, Clefts and Clips. Molecules 2019, 24, 1803. [Google Scholar] [CrossRef]
- Hadrovic, I.; Rebmann, P.; Klärner, F.-G.; Bitan, G.; Schrader, T. Molecular Lysine Tweezers Counteract Aberrant Protein Aggregation. Front. Chem. 2019, 7, 657. [Google Scholar] [CrossRef]
- Schrader, T.; Bitan, G.; Klärner, F.-G. Molecular tweezers for lysine and arginine—Powerful inhibitors of pathologic protein aggregation. Chem. Commun. 2016, 52, 11318–11334. [Google Scholar] [CrossRef]
- Gianneschi, N.C.; Cho, S.-H.; Nguyen, S.; Mirkin, C.A. Reversibly Addressing an Allosteric Catalyst In Situ: Catalytic Molecular Tweezers. Angew. Chem. Int. Ed. 2004, 43, 5503–5507. [Google Scholar] [CrossRef]
- Doistau, B.; Benda, L.; Cantin, J.-L.; Cador, O.; Pointillart, F.; Wernsdorfer, W.; Chamoreau, L.-M.; Marvaud, V.; Hasenknopf, B.; Vives, G. Dual switchable molecular tweezers incorporating anisotropic MnIII–salphen complexes. Dalton Trans. 2020, 49, 8872–8882. [Google Scholar] [CrossRef]
- Doistau, B.; Tron, A.; Denisov, S.A.; Jonusauskas, G.; McClenaghan, N.D.; Gontard, G.; Marvaud, V.; Hasenknopf, B.; Vives, G. Terpy(Pt-salphen)2 Switchable Luminescent Molecular Tweezers. Chem. A Eur. J. 2014, 20, 15799–15807. [Google Scholar] [CrossRef]
- Doistau, B.; Benda, L.; Cantin, J.-L.; Chamoreau, L.-M.; Ruiz, E.; Marvaud, V.; Hasenknopf, B.; Vives, G. Six States Switching of Redox-Active Molecular Tweezers by Three Orthogonal Stimuli. J. Am. Chem. Soc. 2017, 139, 9213–9220. [Google Scholar] [CrossRef]
- Krykun, S.; Dekhtiarenko, M.; Canevet, D.; Carré, V.; Aubriet, F.; Levillain, E.; Allain, M.; Voitenko, Z.; Sallé, M.; Goeb, S. Metalla-Assembled Electron-Rich Tweezers: Redox-Controlled Guest Release Through Supramolecular Dimerization. Angew. Chem. Int. Ed. 2020, 59, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Han, Y.; Gao, Z.; Wang, F. Multicomponent Assembled Systems Based on Platinum(II) Terpyridine Complexes. Acc. Chem. Res. 2018, 51, 2719–2729. [Google Scholar] [CrossRef]
- Han, Y.; Tian, Y.; Li, Z.; Wang, F. Donor–acceptor-type supramolecular polymers on the basis of preorganized molecular tweezers/guest complexation. Chem. Soc. Rev. 2018, 47, 5165–5176. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tanasova, M.; Vasileiou, C.; Borhan, B. Fluorinated Porphyrin Tweezer: A Powerful Reporter of Absolute Configuration for erythro and threo Diols, Amino Alcohols, and Diamines. J. Am. Chem. Soc. 2008, 130, 1885–1893. [Google Scholar] [CrossRef]
- Huang, X.; Rickman, B.H.; Borhan, B.; Berova, N.; Nakanishi, K. Zinc Porphyrin Tweezer in Host−Guest Complexation: Determination of Absolute Configurations of Diamines, Amino Acids, and Amino Alcohols by Circular Dichroism. J. Am. Chem. Soc. 1998, 120, 6185–6186. [Google Scholar] [CrossRef]
- Borovkov, V.V.; Lintuluoto, J.M.; Hembury, G.A.; Sugiura, M.; Arakawa, R.; Inoue, Y. Supramolecular Chirogenesis in Zinc Porphyrins: Interaction with Bidentate Ligands, Formation of Tweezer Structures, and the Origin of Enhanced Optical Activity. J. Org. Chem. 2003, 68, 7176–7192. [Google Scholar] [CrossRef]
- Brahma, S.; Ikbal, S.A.; Rath, S.P. Synthesis, Structure, and Properties of a Series of Chiral Tweezer–Diamine Complexes Consisting of an Achiral Zinc(II) Bisporphyrin Host and Chiral Diamine Guest: Induction and Rationalization of Supramolecular Chirality. Inorg. Chem. 2014, 53, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Blom, M.; Norrehed, S.; Andersson, C.-H.; Huang, H.; Light, M.E.; Bergquist, J.; Grennberg, H.; Gogoll, A. Synthesis and Properties of Bis-Porphyrin Molecular Tweezers: Effects of Spacer Flexibility on Binding and Supramolecular Chirogenesis. Molecules 2015, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Norrehed, S.; Johansson, H.; Grennberg, H.; Gogoll, A. Improved Stereochemical Analysis of Conformationally Flexible Diamines by Binding to a Bisporphyrin Molecular Clip. Chem. A Eur. J. 2013, 19, 14631–14638. [Google Scholar] [CrossRef]
- Lubian, E.; Baldini, F.; Giannetti, A.; Trono, C.; Carofiglio, T. Solid-supported Zn(II) porphyrin tweezers as optical sensors for diamines. Chem. Commun. 2010, 46, 3678–3680. [Google Scholar] [CrossRef]
- Li, X.; Burrell, C.E.; Staples, R.J.; Borhan, B. Absolute Configuration for 1,n-Glycols: A Nonempirical Approach to Long-Range Stereochemical Determination. J. Am. Chem. Soc. 2012, 134, 9026–9029. [Google Scholar] [CrossRef]
- Li, X.; Borhan, B. Prompt Determination of Absolute Configuration for Epoxy Alcohols via Exciton Chirality Protocol. J. Am. Chem. Soc. 2008, 130, 16126–16127. [Google Scholar] [CrossRef]
- Crossley, M.J.; Hambley, T.W.; Mackay, L.G.; Try, A.C.; Walton, R. Porphyrin analogues of Tröger’s base: Large chiral cavities with a bimetallic binding site. J. Chem. Soc. Chem. Commun. 1995, 1077–1079. [Google Scholar] [CrossRef]
- Hayashi, T.; Nonoguchi, M.; Aya, T.; Ogoshi, H. Molecular recognition of α,ω-diamines by metalloporphyrin dimer. Tetrahedron Lett. 1997, 38, 1603–1606. [Google Scholar] [CrossRef]
- Carofiglio, T.; Lubian, E.; Menegazzo, I.; Saielli, G.; Varotto, A. Melamine-Bridged Bis(porphyrin-ZnII) Receptors: Molecular Recognition Properties. J. Org. Chem. 2009, 74, 9034–9043. [Google Scholar] [CrossRef]
- Norrehed, S.; Polavarapu, P.; Yang, W.; Gogoll, A.; Grennberg, H. Conformational restriction of flexible molecules in solution by a semirigid bis-porphyrin molecular tweezer. Tetrahedron 2013, 69, 7131–7138. [Google Scholar] [CrossRef]
- Consiglio, G.; Oliveri, I.P.; Failla, S.; Di Bella, S. On the Aggregation and Sensing Properties of Zinc(II) Schiff-Base Complexes of Salen-Type Ligands. Molecules 2019, 24, 2514. [Google Scholar] [CrossRef] [PubMed]
- Leoni, L.; Dalla Cort, A. The Supramolecular Attitude of Metal–Salophen and Metal–Salen Complexes. Inorganics 2018, 6, 42. [Google Scholar] [CrossRef]
- Yin, H.-Y.; Tang, J.; Zhang, J.-L. Introducing Metallosalens into Biological Studies: The Renaissance of Traditional Coordination Complexes. Eur. J. Inorg. Chem. 2017, 2017, 5085–5093. [Google Scholar] [CrossRef]
- Di Bella, S. Lewis acidic zinc(II) salen-type Schiff-base complexes: Sensing properties and responsive nanostructures. Dalton Trans. 2021, 50, 6050–6063. [Google Scholar] [CrossRef]
- Consiglio, G.; Failla, S.; Finocchiaro, P.; Oliveri, I.P.; Purrello, R.; Di Bella, S. Supramolecular Aggregation/Deaggregation in Amphiphilic Dipolar Schiff-Base Zinc(II) Complexes. Inorg. Chem. 2010, 49, 5134–5142. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Di Bella, S.; Failla, S.; Malandrino, G. New molecular architectures by aggregation of tailored zinc(II) Schiff-base complexes. New J. Chem. 2011, 35, 2826–2831. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Di Bella, S. Sensitive Fluorescent Detection and Lewis Basicity of Aliphatic Amines. J. Phys. Chem. A 2011, 115, 14325–14330. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Di Bella, S. Highly sensitive fluorescent probe for detection of alkaloids. Tetrahedron 2011, 67, 9446–9449. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Di Bella, S. Lewis basicity of relevant monoanions in a non-protogenic organic solvent using a zinc(II) Schiff-base complex as a reference Lewis acid. Dalton Trans. 2017, 46, 11608–11614. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Malandrino, G.; Di Bella, S. Phase Transition and Vapochromism in Molecular Assemblies of a Polymorphic Zinc(II) Schiff-Base Complex. Inorg. Chem. 2014, 53, 9771–9777. [Google Scholar] [CrossRef]
- Mirabella, S.; Oliveri, I.P.; Ruffino, F.; Maccarrone, G.; Di Bella, S. Low-cost chemiresistive sensor for volatile amines based on a 2D network of a zinc(II) Schiff-base complex. Appl. Phys. Lett. 2016, 109, 143108. [Google Scholar] [CrossRef]
- Tang, J.; Cai, Y.-B.; Jing, J.; Zhang, J.-L. Unravelling the correlation between metal induced aggregation and cellular uptake/subcellular localization of Znsalen: An overlooked rule for design of luminescent metal probes. Chem. Sci. 2015, 6, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xie, D.; Yin, H.-Y.; Jing, J.; Zhang, J.-L. Cationic sulfonium functionalization renders Znsalens with high fluorescence, good water solubility and tunable cell-permeability. Org. Biomol. Chem. 2016, 14, 3360–3368. [Google Scholar] [CrossRef]
- Strianese, M.; Guarnieri, D.; Lamberti, M.; Landi, A.; Peluso, A.; Pellecchia, C. Fluorescent salen-type Zn(II) Complexes As Probes for Detecting Hydrogen Sulfide and Its Anion: Bioimaging Applications. Inorg. Chem. 2020, 59, 15977–15986. [Google Scholar] [CrossRef]
- Consiglio, G.; Oliveri, I.P.; Cacciola, S.; Maccarrone, G.; Failla, S.; Di Bella, S. Dinuclear zinc(II) salen-type Schiff-base complexes as molecular tweezers. Dalton Trans. 2020, 49, 5121–5133. [Google Scholar] [CrossRef]
- Munzi, G.; Failla, S.; Di Bella, S. Highly selective and sensitive colorimetric/fluorometric dual mode detection of relevant biogenic amines. Analyst 2021, 146, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Maccarrone, G.; Di Bella, S. A Lewis Basicity Scale in Dichloromethane for Amines and Common Nonprotogenic Solvents Using a Zinc(II) Schiff-Base Complex as Reference Lewis Acid. J. Org. Chem. 2011, 76, 8879–8884. [Google Scholar] [CrossRef]
- Forte, G.; Oliveri, I.P.; Consiglio, G.; Failla, S.; Di Bella, S. On the Lewis acidic character of bis(salicylaldiminato)zinc(II) Schiff-base complexes: A computational and experimental investigation on a series of compounds varying the bridging diimine. Dalton Trans. 2017, 46, 4571–4581. [Google Scholar] [CrossRef]
- She, N.; Moncelet, D.; Gilberg, L.; Lu, X.; Sindelar, V.; Briken, V.; Isaacs, L. Glycoluril-Derived Molecular Clips are Potent and Selective Receptors for Cationic Dyes in Water. Chem. A Eur. J. 2016, 22, 15270–15279. [Google Scholar] [CrossRef] [PubMed]
- Burnett, C.A.; Witt, D.; Fettinger, J.C.; Isaacs, L. Acyclic Congener of Cucurbituril: Synthesis and Recognition Properties. J. Org. Chem. 2003, 68, 6184–6191. [Google Scholar] [CrossRef]
- Heilmann, M.; Tiefenbacher, K. A Modular Phosphorylated Glycoluril-Derived Molecular Tweezer for Potent Binding of Aliphatic Diamines. Chem. A Eur. J. 2019, 25, 12900–12904. [Google Scholar] [CrossRef]
- Solladié, N.; Aziat, F.; Bouatra, S.; Rein, R. Bis-porphyrin tweezers: Rigid or flexible linkers for better adjustment of the cavity to bidentate bases of various size. J. Porphyr. Phthalocyanines 2008, 12, 1250–1260. [Google Scholar] [CrossRef]
- Murphy, R.B.; Pham, D.-T.; White, J.M.; Lincoln, S.F.; Johnston, M.R. Molecular tweezers with a rotationally restricted linker and freely rotating porphyrin moieties. Org. Biomol. Chem. 2018, 16, 6206–6223. [Google Scholar] [CrossRef] [PubMed]
- Ballester, P.; Costa, A.; Castilla, A.M.; Deyà, P.M.; Frontera, A.; Gomila, R.M.; Hunter, C.A.; Costa, A. DABCO-Directed Self-Assembly of Bisporphyrins (DABCO=1,4-Diazabicyclo[2.2.2]octane). Chem. A Eur. J. 2005, 11, 2196–2206. [Google Scholar] [CrossRef]
- Martin, R. Modes of action of anthelmintic drugs. Veter. J. 1997, 154, 11–34. [Google Scholar] [CrossRef]
- Feron, P.H.M.; Cousins, A.; Jiang, K.; Zhai, R.; Garcia, M. An update of the benchmark post-combustion CO2-capture technology. Fuel 2020, 273, 117776. [Google Scholar] [CrossRef]
- Mazari, S.; Ali, B.S.; Jan, B.M.; Saeed, I.M. Degradation study of piperazine, its blends and structural analogs for CO2 capture: A review. Int. J. Greenh. Gas Control. 2014, 31, 214–228. [Google Scholar] [CrossRef]
- Kumar, R.R.; Sahu, B.; Pathania, S.; Singh, P.K.; Akhtar, M.J.; Kumar, B. Piperazine, a Key Substructure for Antidepressants: Its Role in Developments and Structure-Activity Relationships. ChemMedChem 2021. [Google Scholar] [CrossRef]
- Katz, D.P.; Deruiter, J.; Bhattacharya, D.; Ahuja, M.; Bhattacharya, S.; Clark, C.; Suppiramaniam, V.; Dhanasekaran, M. Benzylpiperazine: “A messy drug”. Drug Alcohol Depend. 2016, 164, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L.; Yiannias, J.A. Contact Dermatitis to Medications and Skin Products. Clin. Rev. Allergy Immunol. 2018, 56, 41–59. [Google Scholar] [CrossRef]
- Eide-Haugmo, I.; Brakstad, O.G.; Hoff, K.A.; Sørheim, K.R.; da Silva, E.F.; Svendsen, H.F. Environmental impact of amines. Energy Procedia 2009, 1, 1297–1304. [Google Scholar] [CrossRef]
- European Chemicals Bureau; Institute for Health and Consumer Protection E.U. Risk Assessment Report-Piperazine; Munn, S.J.; Allanou, R.; Aschberger, K.; Cosgrove, O.; Pakalin, S.; Paya-Perez, A.; Pellegrini, G.; Schwarz-Schulz, B.; et al. Office for Official Publications of the European Communities, Luxemburg. 2005. Available online: https://echa.europa.eu/documents/10162/35f9602c-cb84-448f-9383-250e1a5ad350 (accessed on 11 April 2021).
- El-Shabouri, S.R.; Mohamed, F.A.; Mohamed, A.M.I. A rapid spectrophotometric method for determination of piperazine. Talanta 1987, 34, 968–970. [Google Scholar] [CrossRef]
- Wahbi, A.-A.M.; Abounassif, M.A.; Gad-Kariem, E.A. Colorimetric determination of piperazine with p-benzoquinone. Talanta 1986, 33, 179–181. [Google Scholar] [CrossRef]
- Wahbi, A.-A.M.; Abounassif, M.; Kariem, E.A. Spectrophometric determination of piperazine with chloranil. Analyst 1984, 109, 1513–1514. [Google Scholar] [CrossRef]
- Muralikrishna, U.; Krishnamurthy, M.; Rao, N.S. Analytical uses of charge-transfer complexes: Determination of pure and dosage forms of piperazine. Analyst 1984, 109, 1277–1279. [Google Scholar] [CrossRef]
- Bu, X.; Pang, M.; Wang, B.; Zhang, Y.; Xie, K.; Zhao, X.; Wang, Y.; Guo, Y.; Liu, C.; Wang, R.; et al. Determination of Piperazine in Eggs Using Accelerated Solvent Extraction (ASE) and Solid Phase Extraction (SPE) with High-Performance Liquid Chromatography—Fluorescence Detection (HPLC-FLD) and Pre-Column Derivatization with Dansyl Chloride. Anal. Lett. 2019, 53, 53–71. [Google Scholar] [CrossRef]
- Hadi, M.; Ahmadvand, E.; Ehsani, A. Electroanalytical Sensing of Piperazine at Carbon Nanotubes/Nafion Composite-modified Glassy Carbon and Screen-printed Carbon Electrodes in Human Plasma. J. Anal. Chem. 2020, 75, 238–245. [Google Scholar] [CrossRef]
- Denis, C.M.; Baryla, N.E. Determination of piperazine in pharmaceutical drug substances using capillary electrophoresis with indirect UV detection. J. Chromatogr. A 2006, 1110, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Guanais Goncalves, C.; Dini, F.; Martinelli, E.; Catini, A.; Lundström, I.; Paolesse, R.; Di Natale, C. Detection of diverse potential threats in water with an array of optical sensors. Sens. Actuators B Chem. 2016, 236, 997–1004. [Google Scholar] [CrossRef]
- Lu, G.; Grossman, J.E.; Lambert, J.B. General but Discriminating Fluorescent Chemosensor for Aliphatic Amines. J. Org. Chem. 2006, 71, 1769–1776. [Google Scholar] [CrossRef]
- McGrier, P.L.; Solntsev, K.M.; Miao, S.; Tolbert, L.M.; Miranda, O.R.; Rotello, V.M.; Bunz, U.H.F. Hydroxycruciforms: Amine-Responsive Fluorophores. Chem. A Eur. J. 2008, 14, 4503–4510. [Google Scholar] [CrossRef]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2010, 40, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
- Bourson, J.; Pouget, J.; Valeur, B. Ion-responsive fluorescent compounds. 4. Effect of cation binding on the photophysical properties of a coumarin linked to monoaza- and diaza-crown ethers. J. Phys. Chem. 1993, 97, 4552–4557. [Google Scholar] [CrossRef]
- Currie, L.A. Detection and quantification limits: Origins and historical overview. Anal. Chim. Acta 1999, 391, 127–134. [Google Scholar] [CrossRef]
- Analytical Methods Committee, Recommendations for the definition, estimation and use of the detection limit. Analyst 1987, 112, 199–204. [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).