A New Complex Borohydride LiAl(BH4)2Cl2
Abstract
1. Introduction
2. Results and Discussions
2.1. Initial Phase Analysis
2.2. Crystal Structure Refinement
2.3. NMR Characterization
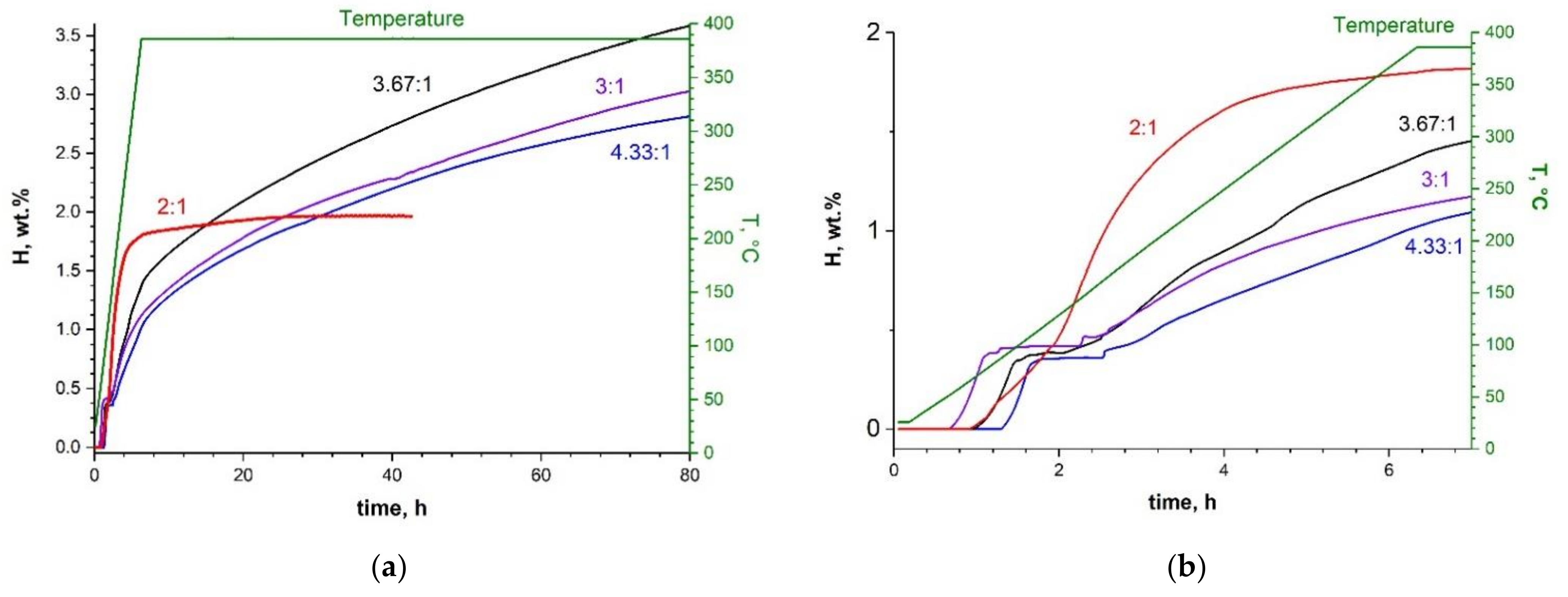
2.4. Dehydrogenation
3. Materials and Methods
3.1. Sample Preparation
3.2. Powder X-Ray Diffraction
3.3. Hydrogen Desorption
3.4. Solid-State NMR
3.5. Structural Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mauron, P.; Buchter, F.; Friedrichs, O.; Remhof, A.; Bielmann, M.; Zwicky, C.; Zuttel, A. Stability and Reversibility of LiBH4. J. Phys. Chem. B 2008, 112, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Barkhordarian, G.; Klassen, T.; Dornheim, M.; Bormann, R. Unexpected kinetic effect of MgB2 in reactive hydride composites containing complex borohydrides. J. Alloys Compd. 2007, 440, L18–L21. [Google Scholar] [CrossRef]
- Martelli, P.; Caputo, R.; Remhof, A.; Mauron, P.; Borgschulte, A.; Zuttel, A. Stability and Decomposition of NaBH4. J. Phys. Chem. C 2010, 114, 7173–7177. [Google Scholar] [CrossRef]
- Miwa, K.; Aoki, M.; Noritake, T.; Ohba, N.; Nakamori, Y.; Towata, S.-I.; Zuttel, A.; Orimo, S.-I. Thermodynamical stability of calcium borohydride Ca(BH4)2. Phys. Rev. B. 2006, 74, 155122. [Google Scholar] [CrossRef]
- Maekawa, H.; Matsuo, M.; Takamura, H.; Ando, M.; Noda, Y.; Karahashi, T.; Orimo, S.-I. Halide-Stabilized LiBH4, a Room-Temperature Lithium Fast-Ion Conductor. J. Am. Chem. Soc. 2009, 131, 894–895. [Google Scholar] [CrossRef]
- Mosegaard, L.; Moller, B.; Jorgensen, J.-E.; Filinchuk, Y.; Cerenius, Y.; Hanson, J.C.; Dimasi, E.; Besenbacher, F.; Jensen, T.R. Reactivity of LiBH4: In Situ Synchrotron Radiation Powder X-ray Diffraction Study. J. Phys. Chem. C 2008, 112, 1299–1303. [Google Scholar] [CrossRef]
- Arnbjerg, L.M.; Ravnsbæk, D.B.; Filinchuk, Y.; Vang, R.T.; Cerenius, Y.; Besenbacher, F.; Jorgensen, J.-E.; Jakobsen, H.J.; Jensen, T.R. Structure and Dynamics for LiBH4−LiCl Solid Solutions. Chem. Mater. 2009, 21, 5772–5782. [Google Scholar] [CrossRef]
- Rude, L.H.; Filinchuk, Y.; Sorby, M.H.; Hauback, B.C.; Besenbacher, F.; Jensen, T.R. Anion Substitution in Ca(BH4)2−CaI2: Synthesis, Structure and Stability of Three New Compounds. J. Phys. Chem. C 2010, 115, 7768–7777. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, Y.-S.; Suh, J.-Y.; Shim, J.H.; Cho, Y.W. Metal halide doped metal borohydrides for hydrogen storage: The case of Ca (BH4)2–CaX2 (X = F, Cl) mixture. J. Alloys Compds. 2009, 506, 721–727. [Google Scholar] [CrossRef]
- Nakamori, Y.; Miwa, K.; Ninomiya, A.; Li, H.; Ohba, N.; Towata, S.-I.; Zuttel, A.; Orimo, S.-I. Correlation between thermodynamical stabilities of metal borohydrides and cation electronegativites: First-principles calculations and experiments. Phys. Rev. B 2006, 74, 045126. [Google Scholar] [CrossRef]
- Nickels, E.A.; Jones, M.O.; David, W.I.F.; Johnson, S.R.; Lowton, R.L.; Sommariva, M.; Edwards, P.P. Tuning the decomposition temperature in complex hydrides: Synthesis of a mixed alkali metal borohydrides. Angew. Chem. Int. Ed. 2008, 120, 2859–2861. [Google Scholar] [CrossRef]
- Hagemann, H.; Longhini, M.; Kaminski, J.W.; Wesolowski, T.A.; Cerny, R.; Penin, N.; Sorby, M.; Hauback, B.; Severa, G.; Jensen, C. LiSc(BH4)4: A Novel Salt of Li+ and Discrete Sc(BH4)4− Complex Anions. J. Phys. Chem. A 2008, 112, 7551–7555. [Google Scholar] [CrossRef]
- Cerny, R.; Severa, G.; Ravnsbaek, D.; Filinchuk, Y.; d’Anna, V.; Hagemann, H.; Haase, D.; Jensen, C.M.; Jensen, T.R. NaSc(BH4)4: A Novel Scandium-Based Borohydride. J. Phys. Chem. C 2010, 114, 1357–1364. [Google Scholar] [CrossRef]
- Ravnsbaek, D.; Filinchuk, Y.; Cerenius, Y.; Jakobsen, H.J.; Besenbacher, F.; Skibsted, J.; Jensen, T.R. A Series of Mixed-Metal Borohydrides. Angew. Chem. Int. Ed. 2009, 48, 6659–6663. [Google Scholar]
- Lindemann, I.; Domenech, F.R.; Dunsch, L.; Filinchuk, Y.; Cerny, R.; Hagemann, H.; D’Anna, V.; Daku, L.M.L.; Schultz, L.; Gutfleisch, O. Al3Li4(BH4)13: A Complex Double-Cation Borohydride with a New Structure. Chem. Euro. J. 2010, 16, 8707–8712. [Google Scholar]
- Lindemann, I.; Borgschulte, A.; Callini, E.; Zuttel, A.; Schultz, L.; Gutfleisch, O. Insight into the decomposition pathway of the complex hydride Al3Li4 (BH4)13. Int. J. Hydrogen Energy 2013, 38, 2790–2795. [Google Scholar] [CrossRef]
- Li, H.-W.; Yan, Y.; Orimo, S.-I.; Zuttel, A.; Jensen, C.M. Recent progress in metal borohydrides for hydrogen storage. Energies 2011, 4, 185–214. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Ley, M.B.; Lee, Y.-S.; Hagemann, H.; D’Anna, V.; Cho, Y.W.; Filinchuk, Y.; Jensen, T.R. A mixed-cation mixed-anion borohydride NaY(BH4)2Cl2. Int. J. Hydrogen Energy 2012, 37, 8428–8438. [Google Scholar]
- Dolotko, O.; Gupta, S.; Kobayashi, T.; McDonald, E.; Hlova, I.; Majzoub, E.; Balema, V.P.; Pruski, M.; Pecharsky, V.K. Mechanochemical reactions and hydrogen storage capacities in MBH4–SiS2 systems. Int. J. Hydrogen Energy 2019, 44, 7381–7391. [Google Scholar] [CrossRef]
- Kobayashi, T.; Dolotko, O.; Gupta, S.; Pecharsky, V.K.; Pruski, M. Mechanochemistry of the LiBH4–AlCl3 System: Structural Characterization of the Products by Solid-State NMR. J. Phys. Chem. C 2018, 122, 1955–1962. [Google Scholar] [CrossRef]
- Lesage, A.; Sakellariou, D.; Steuernagel, S.; Emsley, L. Carbon−proton chemical shift correlation in solid-state NMR by through-bond multiple-quantum spectroscopy. J. Am. Chem. Soc. 1998, 120, 13194–13201. [Google Scholar]
- Fung, B.M.; Khitrin, A.K.; Ermolaev, K. An improved broadband decoupling sequence for liquid crystals and solids. J. Magn. Reson. 2000, 142, 97–101. [Google Scholar] [CrossRef]
- Eriksson, L.; Westdahl, M. TREOR, a semi-exhaustive trial-and-error powder indexing program for all symmetries. J. Appl. Crystallogr. 1985, 18, 367–370. [Google Scholar]
- Roisnel, T.; Rodríquez-Carvajal, J. WinPLOTR: A Windows Tool for Powder Diffraction Pattern Analysis. Mat. Sci. Forum 2001, 378–381, 118–123. [Google Scholar] [CrossRef]
- Favre-Nicolin, V.; Cerny, R. FOX, free objects for crystallography’: A modular approach to ab initio structure determination from powder diffraction. J. Appl. Crystallogr. 2002, 35, 734–743. [Google Scholar] [CrossRef]
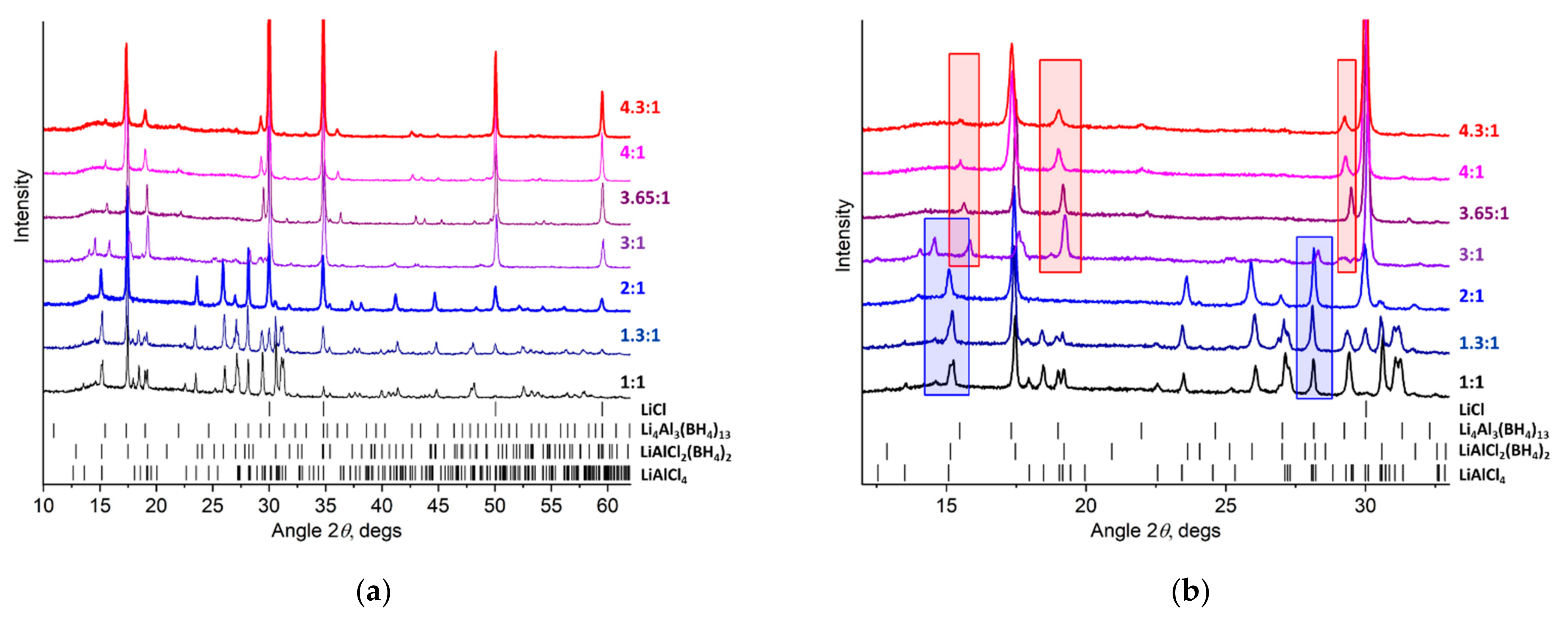
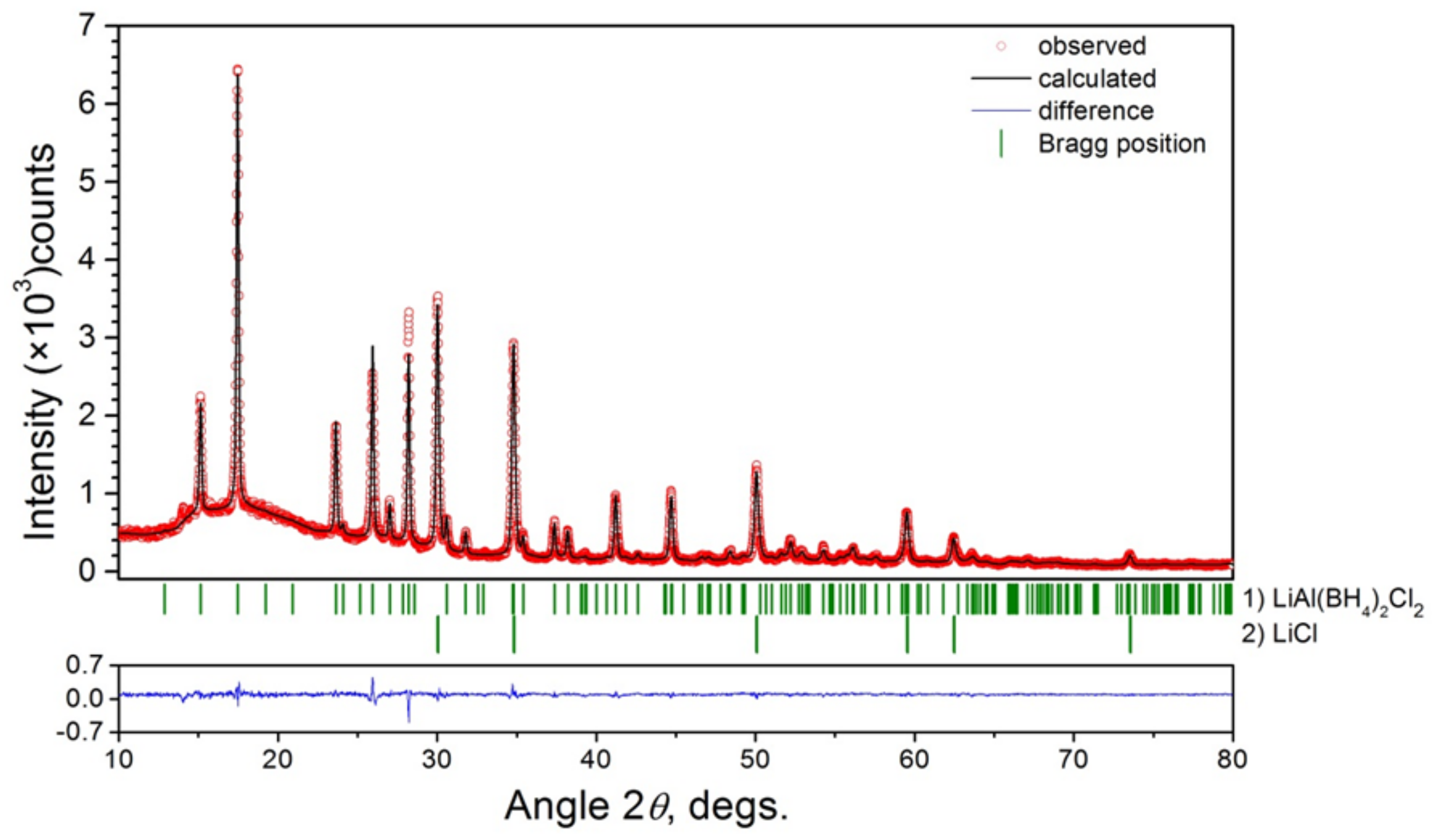
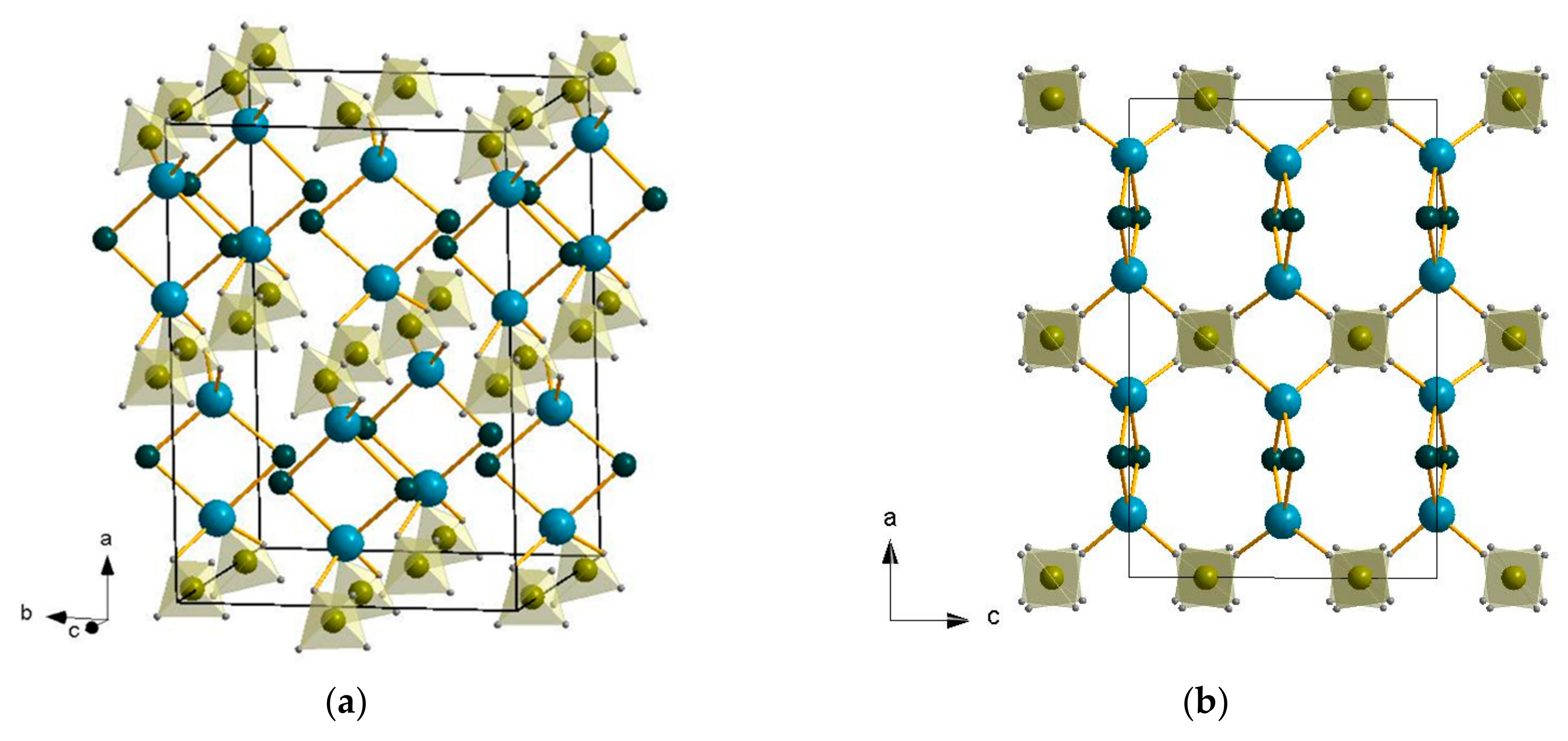
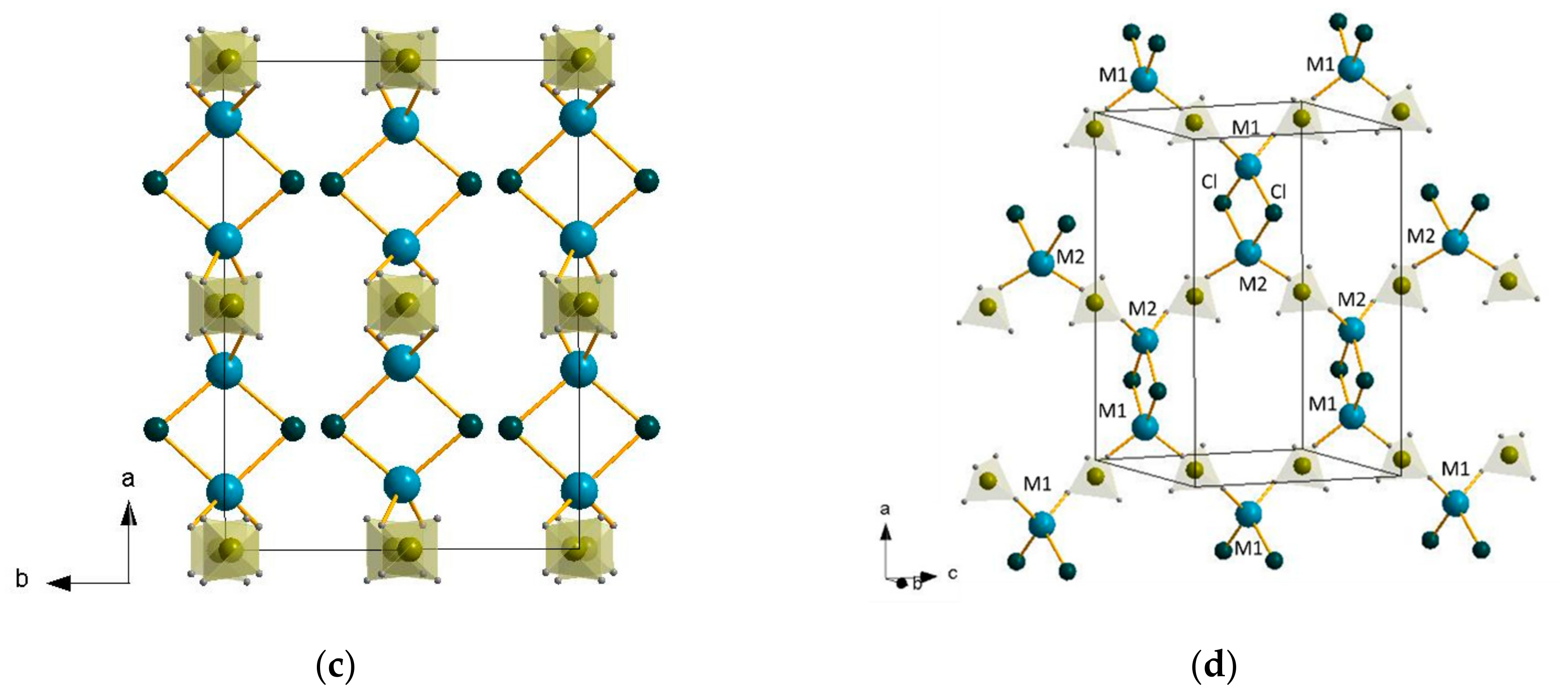

| LiBH4:AlCl3 Molar Ratio | Li4Al3(BH4)13 | LiAlCl2(BH4)2 | LiAlCl4 | LiCl |
|---|---|---|---|---|
| 4.3:1 | a = 11.390(1) Å | not present | not present | present |
| 4:1 | a = 11.367(1) Å | not present | not present | present |
| 3.65:1 | a = 11.350(1) Å | not present | not present | present |
| 3:1 | a = 11.291(2) Å | a = 12.159(4) Å, b = 8.493(4) Å, c = 7.377(3) Å | not present | present |
| 2:1 | not present | a = 11.6698(5) Å, b = 8.4724(4) Å, c = 7.5116(3) Å | not present | present |
| 1.3:1 | not present | a = 11.577(1) Å, b = 8.436(1) Å, c = 7.553(1) Å | present | present |
| 1:1 | not present | a = 11.575(2) Å, b = 8.417(1) Å, c = 7.541(1) Å | present | not present |
| Atom | Site | x | y | z | Uiso, Å2 | Occupancy |
|---|---|---|---|---|---|---|
| M1 (Al1) | 4a | 0.633(2) | 0 | 0 | 0.081(2) | 0.45(2) |
| M1 (Li1) | 4a | 0.633(2) | 0 | 0 | 0.081(2) | 0.55(2) |
| M2 (Al2) | 4a | −0.119(2) | 0 | 0 | 0.081(2) | 0.55(2) |
| M2 (Li2) | 4a | −0.119(2) | 0 | 0 | 0.081(2) | 0.45(2) |
| Cl | 8c | 0.752(1) | 0.8070(2) | 0.0306(3) | 0.098(1) | 1 |
| B1 | 4b | 0 | 0.479(1) | ¼ | 0.052(3) | 1 |
| H11 | 8c | 0.063 | 0.401(1) | 0.327 | 0.052(3) | 1 |
| H12 | 8c | 0.050 | 0.558(1) | 0.152 | 0.052(3) | 1 |
| B2 | 4b | 0 | 0.983(1) | ¼ | 0.052(3) | 1 |
| H21 | 8c | 0.065 | 1.061(1) | 0.175 | 0.052(3) | 1 |
| H22 | 8c | 0.048 | 0.904(1) | 0.350 | 0.052(3) | 1 |
| M1–H11 | 2.7202(1) | 2x |
| M1–H11 | 2.7628(1) | 2x |
| M1–H12 | 1.5758(1) | 2x |
| M1–B1 | 2.4411(1) | 2x |
| M1–Cl | 2.1613(1) | 2x |
| M2–H21 | 2.5683(1) | 2x |
| M2–H21 | 2.5715(1) | 2x |
| M2–H22 | 1.6158(1) | 2x |
| M2–B2 | 2.3385(1) | 2x |
| M2–Cl | 2.2337(1) | 2x |
| M1–Cl–M2 | 82.515(4) | 2x |
| B1–H11 | 1.1503(1) | 2x |
| B1–H12 | 1.1508(1) | 2x |
| H–B1–H | 109.1(1)–109.8(1) | |
| B2–H21 | 1.1502(1) | 2x |
| B2–H22 | 1.1502(1) | 2x |
| H–B2–H | 109.4(1)–109.6(1) |
| LiBH4:AlCl3 Ratio | H2 Release (wt. %) 1 | H2 (vol. %) | B2H6 (vol. %) |
|---|---|---|---|
| 4.33:1 | 2.8 | 84.0 | 16.0 |
| 3.67:1 | 3.5 | 97.0 | 3.0 |
| 3:1 | 3.0 | 99.7 | 0.3 |
| 2:1 | 2.0 | 99.8 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolotko, O.; Kobayashi, T.; Hlova, I.Z.; Gupta, S.; Pecharsky, V.K. A New Complex Borohydride LiAl(BH4)2Cl2. Inorganics 2021, 9, 35. https://doi.org/10.3390/inorganics9050035
Dolotko O, Kobayashi T, Hlova IZ, Gupta S, Pecharsky VK. A New Complex Borohydride LiAl(BH4)2Cl2. Inorganics. 2021; 9(5):35. https://doi.org/10.3390/inorganics9050035
Chicago/Turabian StyleDolotko, Oleksandr, Takeshi Kobayashi, Ihor Z. Hlova, Shalabh Gupta, and Vitalij K. Pecharsky. 2021. "A New Complex Borohydride LiAl(BH4)2Cl2" Inorganics 9, no. 5: 35. https://doi.org/10.3390/inorganics9050035
APA StyleDolotko, O., Kobayashi, T., Hlova, I. Z., Gupta, S., & Pecharsky, V. K. (2021). A New Complex Borohydride LiAl(BH4)2Cl2. Inorganics, 9(5), 35. https://doi.org/10.3390/inorganics9050035






