Anticancer Half-Sandwich Rhodium(III) Complexes
Abstract
1. Introduction
2. Mononuclear Complexes
2.1. Complexes with Three Monodentate Ligands
2.2. Complexes with Bidentate and Monodentate Ligands
2.2.1. Chlorido Complexes
2.2.2. Other Types of Monodentate Ligands
2.3. Complexes with a Tridentate Ligand
2.4. Other Types of Mononuclear Complexes
3. Multinuclear Complexes
3.1. Chlorido Complexes
3.2. Thiolato Complexes
3.3. Matallacages
3.4. Heterometallic Complexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.M.; Büsselberg, D. Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance and Induced Side Effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol. Rev. 2012, 64, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Kenny, R.G.; Marmion, C.J. Toward Multi-Targeted Platinum and Ruthenium Drugs—A New Paradigm in Cancer Drug Treatment Regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef]
- Štarha, P.; Vančo, J.; Trávníček, Z. Platinum iodido complexes: A comprehensive overview of anticancer activity and mechanisms of action. Coord. Chem. Rev. 2019, 380, 103–135. [Google Scholar] [CrossRef]
- Sigel, A.; Sigel, H.; Freisinger, E.; Sigel, R.K.O. (Eds.) Metallo-Drugs: Development and Action of Anticancer Agents, 1st ed.; Metal Ions in Life Sciences; De Gruyter: Berlin, Germany, 2018; Volume 18. [Google Scholar]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Meier-Menches, S.M.; Gerner, C.; Berger, W.; Hartinger, C.G.; Keppler, B.K. Structure–activity relationships for ruthenium and osmium anticancer agents—Towards clinical development. Chem. Soc. Rev. 2018, 47, 909–928. [Google Scholar] [CrossRef]
- Leung, C.H.; Zhong, H.J.; Chan, D.S.H.; Ma, D.L. Bioactive iridium and rhodium complexes as therapeutic agents. Coord. Chem. Rev. 2013, 257, 1764–1776. [Google Scholar] [CrossRef]
- Fanelli, M.; Formica, M.; Fusi, V.; Giorgi, L.; Micheloni, M.; Paoli, P. New trends in platinum and palladium complexes as antineoplastic agents. Coord. Chem. Rev. 2016, 310, 41–79. [Google Scholar] [CrossRef]
- Hanif, H.; Babak, M.V.; Hartinger, C.G. Development of anticancer agents: Wizardry with osmium. Drug Discov. Today 2014, 19, 1640–1648. [Google Scholar] [CrossRef]
- Štarha, P.; Trávníček, Z. Non-platinum complexes containing releasable biologically active ligands. Coord. Chem. Rev. 2019, 395, 130–145. [Google Scholar] [CrossRef]
- Alessio, E. Thirty Years of the Drug Candidate NAMI-A and the Myths in the Field of Ruthenium Anticancer Compounds: A Personal Perspective. Eur. J. Inorg. Chem. 2017, 2017, 1549–1560. [Google Scholar] [CrossRef]
- Wernitznig, D.; Kiakos, K.; Del Favero, G.; Harrer, N.; Machat, H.; Osswald, A.; Jakupec, M.A.; Wernitznig, A.; Sommergruber, W.; Keppler, B.K. First-in-class ruthenium anticancer drug (KP1339/IT-139) induces an immunogenic cell death signature in colorectal spheroids in vitro. Metallomics 2019, 11, 1044–1048. [Google Scholar] [CrossRef]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J., III; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Notaro, A.; Gasser, G. Monomeric and dimeric coordinatively saturated and substitutionally inert Ru(II) polypyridyl complexes as anticancer drug candidates. Chem. Soc. Rev. 2017, 46, 7317–7337. [Google Scholar] [CrossRef]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.S. The development of anticancer ruthenium(II) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef]
- Song, G.Y.; Li, X.W. Substrate Activation Strategies in Rhodium(III)-Catalyzed Selective Functionalization of Arenes. Acc. Chem. Rev. 2015, 48, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.J.; Li, M.W.; Allard, L.F.; Lee, S.S.; Flytzani-Stephanopoulos, M. Mild oxidation of methane to methanol or acetic acid on supported isolated rhodium catalysts. Nature 2017, 551, 605–608. [Google Scholar] [CrossRef]
- Chen, L.; Quan, H.; Xu, Z.; Wang, H.; Xia, Y.; Lou, L.; Yang, W. A modular biomimetic strategy for the synthesis of macrolide P-glycoprotein inhibitors via Rh-catalyzed C–H activation. Nat. Commun. 2020, 11, 2151. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, J.; Zhou, F.; Liu, Y.; You, J. Multicomponent Reactions of Pyridines To Give Ring-Fused Pyridiniums: In Situ Activation Strategy Using 1,2-Dichloroethane as a Vinyl Equivalent. Angew. Chem. Int. Ed. 2019, 58, 254–258. [Google Scholar] [CrossRef]
- Štarha, P. Multinuclear biologically active Ru, Rh, Os and Ir arene complexes. Coord. Chem. Rev. 2021, 431, 213690. [Google Scholar] [CrossRef]
- Soldevila-Barreda, J.J.; Sadler, P.J. Approaches to the design of catalytic metallodrugs. Curr. Opin. Chem. Biol. 2015, 25, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Soldevila-Barreda, J.J.; Metzler-Nolte, N. Intracellular Catalysis with Selected Metal Complexes and Metallic Nanoparticles: Advances toward the Development of Catalytic Metallodrugs. Chem. Rev. 2019, 119, 829–869. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.J.; Wang, W.; Mok, S.W.F.; Wu, C.; Law, B.Y.K.; Miao, X.M.; Wu, K.J.; Zhong, H.J.; Wong, C.Y.; Wong, V.K.W.; et al. Selective inhibition of lysine-specific demethylase 5A (KDM5A) using a rhodium (III) complex for triple-negative breast cancer therapy. Angew. Chem. Int. Ed. 2018, 57, 13091–13095. [Google Scholar] [CrossRef]
- Threatt, S.D.; Synold, T.W.; Wu, J.; Barton, J.K. In vivo anticancer activity of a rhodium metalloinsertor in the HCT116 xenograft tumor model. Proc. Natl. Acad. Sci. USA 2020, 117, 17535–17542. [Google Scholar] [CrossRef]
- Liang, J.; Levina, A.; Jia, J.; Kappen, P.; Glover, C.; Johannessen, B.; Lay, P.A. Reactivity and Transformation of Antimetastatic and Cytotoxic Rhodium(III)−Dimethyl Sulfoxide Complexes in Biological Fluids: An XAS Speciation Study. Inorg. Chem. 2019, 58, 4880–4893. [Google Scholar] [CrossRef]
- Fan, R.; Bian, M.; Hu, L.; Wukun, L. A new rhodium(I) NHC complex inhibits TrxR: In vitro cytotoxicity and in vivo hepatocellular carcinoma suppression. Eur. J. Med. Chem. 2019, 183, 111721. [Google Scholar] [CrossRef]
- Dorcier, A.; Ang, W.H.; Bolano, S.; Gonsalvi, L.; Juillerat-Jeannerat, L.; Laurenczy, G.; Peruzzini, M.; Phillips, A.D.; Zanobini, F.; Dyson, P.J. In Vitro Evaluation of Rhodium and Osmium RAPTA Analogues: The Case for Organometallic Anticancer Drugs Not Based on Ruthenium. Organometallics 2006, 25, 4090–4096. [Google Scholar] [CrossRef]
- Pruchnik, H.; Latocha, M.; Zielinska, A.; Pruchnik, F.P. Rhodium(III) and iridium(III) pentamethylcyclopentadienyl complexes with tris(2-carboxyethyl)phosphine, properties and cytostatic activity. J. Organomet. Chem. 2016, 822, 74–79. [Google Scholar] [CrossRef]
- Adhikari, S.; Hussain, O.; Phillips, R.M.; Kollipara, M.R. Half-sandwich d6 metal complexes comprising of 2-substituted-1,8-naphthyridine ligands with unexpected bonding modes: Synthesis, structural and anti-cancer studies. J. Organomet. Chem. 2018, 854, 27–37. [Google Scholar] [CrossRef]
- Lucas, S.J.; Lord, R.M.; Basri, A.M.; Allison, S.J.; Phillips, R.M.; Blacker, A.J.; McGowan, P.C. Increasing anti-cancer activity with longer tether lengths of group 9 Cp* complexes. Dalton Trans. 2016, 45, 6812–6815. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.M.; Holmes, J.; Singer, F.N.; Frith, A.; Willans, C.E. Precious metal N-heterocyclic carbene-carbaboranyl complexes: Cytotoxic and selective compounds for the treatment of cancer. J. Organomet. Chem. 2020, 907, 121062. [Google Scholar] [CrossRef]
- Truong, D.; Sullivan, M.P.; Tong, K.K.H.; Steel, T.R.; Prause, A.; Lovett, J.H.; Andersen, J.W.; Jamieson, S.M.F.; Harris, H.H.; Ott, I.; et al. Potent Inhibition of Thioredoxin Reductase by the Rh Derivatives of Anticancer M(arene/Cp*)(NHC)Cl2 Complexes. Inorg. Chem. 2020, 59, 3281–3289. [Google Scholar] [CrossRef]
- Scharwitz, M.A.; Ott, I.; Geldmacher, Y.; Gust, R.; Sheldrick, W.S. Cytotoxic half-sandwich rhodium(III) complexes: Polypyridyl ligand influence on their DNA binding properties and cellular uptake. J. Organomet. Chem. 2008, 693, 2299–2309. [Google Scholar] [CrossRef]
- Geldmacher, Y.; Rubbiani, R.; Wefelmeier, P.; Prokop, A.; Ott, I.; Sheldrick, W.S. Synthesis and DNA-binding properties of apoptosis-inducing cytotoxic half-sandwich rhodium(III) complexes with methyl-substituted polypyridyl ligands. J. Organomet. Chem. 2011, 696, 1023–1031. [Google Scholar] [CrossRef]
- Soldevila-Barreda, J.J.; Habtemariam, A.; Romero-Canelón, I.; Sadler, P.J. Half-sandwich rhodium(III) transfer hydrogenation catalysts: Reduction of NAD+ and pyruvate, and antiproliferative activity. J. Inorg. Biochem. 2015, 153, 322–333. [Google Scholar] [CrossRef]
- Cross, J.M.; Blower, T.R.; Gallagher, N.; Gill, J.H.; Rockley, K.L.; Walton, J.W. Anticancer RuII and RhIII Piano-Stool Complexes that are Histone Deacetylase Inhibitors. ChemPlusChem 2016, 81, 1276–1280. [Google Scholar] [CrossRef]
- Shadap, L.; Tyagi, J.L.; Poluri, K.M.; Pinder, E.; Phillips, R.M.; Kaminsky, W.; Kollipara, M.R. Synthesis, structural and in-vitro functional studies of half-sandwich platinum group metal complexes having various bonding modes of benzhydrazone derivative ligands. Polyhedron 2020, 176, 114293. [Google Scholar] [CrossRef]
- Soldevila-Barreda, J.J.; Fawibe, K.B.; Azmanova, M.; Rafols, L.; Pitto-Barry, A.; Eke, U.B.; Barry, N.P.E. Synthesis, Characterisation and In Vitro Anticancer Activity of Catalytically Active Indole-Based Half-Sandwich Complexes. Molecules 2020, 25, 4540. [Google Scholar] [CrossRef]
- Kandioller, W.; Balsano, E.; Meier, S.M.; Jungwirth, U.; Göschl, S.; Roller, A.; Jakupec, M.A.; Berger, W.; Keppler, B.K.; Hartinger, C.G. Organometallic anticancer complexes of lapachol: Metal centre-dependent formation of reactive oxygen species and correlation with cytotoxicity. Chem. Commun. 2013, 49, 3348–3350. [Google Scholar] [CrossRef]
- Pettinari, R.; Marchetti, F.; Pettinari, C.; Condello, F.; Petrini, A.; Scopelliti, R.; Riedel, T.; Dyson, P.J. Organometallic rhodium(III) and iridium(III) cyclopentadienyl complexes with curcumin and bisdemethoxycurcumin co-ligands. Dalton Trans. 2015, 44, 20523–20531. [Google Scholar] [CrossRef] [PubMed]
- Kurzwernhart, A.; Mokesch, S.; Klapproth, E.; Adib-Ravazi, M.S.; Jakupec, M.A.; Hartinger, C.G.; Kandioller, W.; Keppler, B.K. Flavonoid-Based Organometallics with Different Metal Centers—Investigations of the Effects on Reactivity and Cytotoxicity. Eur. J. Inorg. Chem. 2016, 2016, 240–246. [Google Scholar] [CrossRef]
- Raja, N.; Devika, N.; Gupta, G.; Nayak, V.L.; Kamal, A.; Nagesh, N.; Therrien, B. Biological activities of pyrenyl-derived thiosemicarbazone half-sandwich complexes. J. Organomet. Chem. 2015, 794, 104–114. [Google Scholar] [CrossRef]
- Adhikari, S.; Hussain, O.; Phillips, R.M.; Kaminsky, W.; Kollipara, M.R. Synthesis, structural and chemosensitivity studies of arene d6 metal complexes having N-phenyl-N′-(pyridyl/pyrimidyl)thiourea derivatives. Appl. Organomet. Chem. 2018, 32, e4362. [Google Scholar] [CrossRef]
- Tong, K.K.H.; Hanif, M.; Lovett, J.H.; Hummitzsch, K.; Harris, H.H.; Soehnel, T.; Jamieson, S.M.F.; Hartinger, C.G. Thiourea-derived chelating ligands and organometallic compounds and investigations into anticancer activity. Molecules 2020, 25, 3661. [Google Scholar] [CrossRef]
- Schmidlehner, M.; Flocke, L.S.; Roller, A.; Hejl, M.; Jakupec, M.A.; Kandioller, W.; Keppler, B.K. Cytotoxicity and preliminary mode of action studies of novel 2-aryl-4-thiopyrone-based organometallics. Dalton Trans. 2016, 45, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Hackl, C.M.; Legina, M.S.; Pichler, V.; Schmidlehner, M.; Roller, A.; Dömötör, O.; Enyedy, E.A.; Jakupec, M.A.; Kandioller, W.; Keppler, B.K. Thiomaltol-Based Organometallic Complexes with 1-Methylimidazole as Leaving Group: Synthesis, Stability, and Biological Behavior. Chem. Eur. J. 2016, 22, 17269–17281. [Google Scholar] [CrossRef]
- Harringer, S.; Happl, B.; Ozenil, M.; Kast, C.; Hejl, M.; Wernitznig, D.; Legin, A.A.; Schweikert, A.; Gajic, N.; Roller, A.; et al. Synthesis, Modification, and Biological Evaluation of a Library of Novel Water-Soluble Thiopyridone-Based Organometallic Complexes and Their Unexpected (Biological) Behavior. Chem. Eur. J. 2020, 26, 5419–5433. [Google Scholar] [CrossRef]
- Harringer, S.; Matzinger, M.; Gajic, N.; Hejl, M.; Jakupec, M.A.; Kandioller, W.; Keppler, B.K. First insights into the novel class of organometallic compounds bearing a bidentate selenopyridone coordination motif: Synthesis, characterization, stability and biological investigations. Inorg. Chim. Acta 2020, 513, 119919. [Google Scholar] [CrossRef]
- Ruiz, J.; Rodriguez, V.; Cutillas, N.; Samper, K.G.; Capdevila, M.; Palacios, O.; Espinosa, A. Novel C,N-chelate rhodium(III) and iridium(III) antitumor complexes incorporating a lipophilic steroidal conjugate and their interaction with DNA. Dalton Trans. 2012, 41, 12847–12856. [Google Scholar] [CrossRef]
- Rono, C.K.; Chu, W.K.; Darkwa, J.; Meyer, D.; Makhubela, B.C.E. Triazolyl RuII, RhIII, OsII, and IrIII Complexes as Potential Anticancer Agents: Synthesis, Structure Elucidation, Cytotoxicity, and DNA Model Interaction Studies. Organometallics 2019, 38, 3197–3211. [Google Scholar] [CrossRef]
- Yellol, G.S.; Donaire, A.; Yellol, J.G.; Vasylyeva, V.; Janiak, C.; Ruiz, J. On the antitumor properties of novel cyclometalated benzimidazole Ru(II), Ir(III) and Rh(III) complexes. Chem. Commun. 2013, 49, 11533–11535. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Gupta, R.K.; Paitandi, R.P.; Rana, N.K.; Sharma, G.; Koch, B.; Rana, L.K.; Hundal, M.S.; Pandey, D.S. Synthesis, Structure, DNA/Protein Binding, and Anticancer Activity of Some Half-Sandwich Cyclometalated Rh(III) and Ir(III) Complexes. Organometallics 2015, 34, 4491–4506. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Bridgewater, H.E.; Banerjee, S.; Soldevila-Barreda, J.J.; Clarkson, G.J.; Shi, H.; Imberti, C.; Sadler, P.J. Ligand-Controlled Reactivity and Cytotoxicity of Cyclometalated Rhodium(III) Complexes. Eur. J. Inorg. Chem. 2020, 2020, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Edafe, F.; Erlandsson, M.; Gonsalvi, L.; Ciancetta, A.; Re, N.; Ienco, A.; Messori, L.; Peruzzini, M.; Dyson, P.J. Rationalization of the inhibition activity of structurally related organometallic compounds against the drug target cathepsin B by DFT. Dalton Trans. 2010, 39, 5556–5563. [Google Scholar] [CrossRef] [PubMed]
- Dkhar, L.; Banothu, V.; Pinder, E.; Phillips, R.M.; Kaminsky, W.; Kollipara, M.R. Ru, Rh and Ir metal complexes of pyridyl chalcone derivatives: Their potent antibacterial activity, comparable cytotoxicity potency and selectivity to cisplatin. Polyhedron 2020, 185, 114606. [Google Scholar] [CrossRef]
- Li, Y.; de Kock, C.; Smith, P.J.; Guzgay, H.; Hendricks, D.T.; Naran, K.; Mizrahi, V.; Warner, D.F.; Chibale, K.; Smith, G.S. Synthesis, characterization, and pharmacological evaluation of silicon-containing aminoquinoline organometallic complexes as antiplasmodial, antitumor, and antimycobacterial agents. Organometallics 2013, 32, 141–150. [Google Scholar] [CrossRef]
- Su, W.; Li, Y.; Peng, B.; Xie, J.; Li, P.; Xiao, Q.; Huang, S. Half-sandwich (Cp*)RhCl2 core complexes containing sulfur donor thiosemicarbazones: Synthesis, cytotoxic activity and human serum albumin binding studies. J. Organomet. Chem. 2018, 868, 24–30. [Google Scholar] [CrossRef]
- Adhikari, S.; Hussain, O.; Phillips, R.M.; Kaminsky, W.; Kollipara, M.R. Neutral and cationic half-sandwich arene d6 metal complexes containing pyridyl and pyrimidyl thiourea ligands with interesting bonding modes: Synthesis, structural and anti-cancer studies. Appl. Organomet. Chem. 2018, 32, e4476. [Google Scholar] [CrossRef]
- Lapasam, A.; Hussain, O.; Phillips, R.M.; Kaminsky, W.; Kollipara, M.R. Synthesis, characterization and chemosensitivity studies of half-sandwich ruthenium, rhodium and iridium complexes containing κ1(S) and κ2(N,S) aroylthiourea ligands. J. Organomet. Chem. 2019, 880, 272–280. [Google Scholar] [CrossRef]
- Geldmacher, Y.; Splith, K.; Kitanovic, I.; Alborzinia, H.; Can, S.; Rubbiani, R.; Nazif, M.A.; Wefelmeier, P.; Prokop, A.; Ott, I.; et al. Cellular impact and selectivity of half-sandwich organorhodium(III) anticancer complexes and their organoiridium(III) and trichloridorhodium(III) counterparts. J. Biol. Inorg. Chem. 2012, 17, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Sharma, G.; Koch, B.; Park, S.; Lee, S.S.; Kim, J. Syntheses, characterization and molecular structures of novel Ru(II), Rh(III) and Ir(III) complexes and their possible roles as antitumour and cytotoxic agents. New J. Chem. 2013, 37, 2573–2581. [Google Scholar] [CrossRef]
- Liu, Z.; Habtemariam, A.; Pizarro, A.M.; Fletcher, S.A.; Kisova, A.; Vrana, O.; Salassa, L.; Bruijnincx, P.C.A.; Clarkson, G.J.; Brabec, V.; et al. Organometallic half-sandwich iridium anticancer complexes. J. Med. Chem. 2011, 54, 3011–3026. [Google Scholar] [CrossRef]
- Matsheku, A.C.; Chen, M.Y.H.; Jordaan, S.; Prince, S.; Smith, G.S.; Makhubela, B.C.E. Acridine-containing RuII, OsII, RhIII and IrIII Half-Sandwich Complexes: Synthesis, Structure and Antiproliferative Activity. Appl. Organomet. Chem. 2017, 31, e3852. [Google Scholar] [CrossRef]
- Gras, M.; Therrien, B.; Suss-Fink, G.; Casini, A.; Edafe, F.; Dyson, P.J. Anticancer activity of new organo-ruthenium, rhodium and iridium complexes containing the 2-(pyridine-2-yl)thiazole N,N-chelating ligand. J. Organomet. Chem. 2010, 695, 1119–1125. [Google Scholar] [CrossRef]
- Gupta, R.K.; Pandey, R.; Sharma, G.; Prasad, R.; Koch, B.; Srikrishna, S.; Li, P.Z.; Xu, Q.; Pandey, D.S. DNA Binding and Anti-Cancer Activity of Redox-Active Heteroleptic Piano-Stool Ru(II), Rh(III), and Ir(III) Complexes Containing 4-(2-Methoxypyridyl)phenyldipyrromethene. Inorg. Chem. 2013, 52, 3687–3698. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Sharma, G.; Pandey, R.; Kumar, A.; Koch, B.; Li, P.Z.; Xu, Q.; Pandey, D.S. DNA/Protein Binding, Molecular Docking, and in Vitro Anticancer Activity of Some Thioether-Dipyrrinato Complexes. Inorg. Chem. 2013, 52, 13984–13996. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.; Govender, P.; Therrien, B.; Clavel, C.M.; Dyson, P.J.; Smith, G.S. Neutral and cationic multinuclear half-sandwich rhodium and iridium complexes coordinated to poly(propyleneimine) dendritic scaffolds: Synthesis and cytotoxicity. J. Organomet. Chem. 2013, 729, 20–27. [Google Scholar] [CrossRef]
- Almodares, Z.; Lucas, S.J.; Crossley, B.D.; Basri, A.M.; Pask, C.M.; Hebden, A.J.; Phillips, R.M.; McGowan, P.C. Rhodium, Iridium, and Ruthenium Half-Sandwich Picolinamide Complexes as Anticancer Agents. Inorg. Chem. 2014, 53, 727–736. [Google Scholar] [CrossRef]
- Kalidasan, M.; Forbes, S.; Mozharivskyj, Y.; Ahmadi, M.; Ahmadihosseini, Z.; Phillips, R.M.; Kollipara, M.R. Mononuclear half-sandwich cyclic-p-perimeter platinum group metal complexes having bithiazole ligands: Synthesis, molecular and anti-cancer studies. Inorg. Chim. Acta 2014, 421, 349–358. [Google Scholar] [CrossRef]
- Palepu, N.R.; Nongbri, S.L.; Premkumar, J.R.; Verma, A.K.; Bhattacharjee, K.; Joshi, S.R.; Forbes, S.; Mozharivskyj, Y.; Thounaojam, R.; Aguan, K.; et al. Synthesis and evaluation of new salicylaldehyde-2-picolinylhydrazone Schiff base compounds of Ru(II), Rh(III) and Ir(III) as in vitro antitumor, antibacterial and fluorescence imaging agents. J. Biol. Inorg. Chem. 2015, 20, 619–638. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Gupta, R.K.; Paitandi, R.P.; Singh, K.B.; Trigun, S.K.; Hundal, M.S.; Pandey, D.S. Cationic Ru(II), Rh(III) and Ir(III) complexes containing cyclic p-perimeter and 2-aminophenyl benzimidazole ligands: Synthesis, molecular structure, DNA and protein binding, cytotoxicity and anticancer activity. J. Organomet. Chem. 2016, 801, 68–79. [Google Scholar] [CrossRef]
- Thangavel, S.; Paulpandi, M.; Friedrich, H.B.; Murugan, K.; Kalva, S.; Skelton, A.A. Synthesis, characterization, antiproliferative and molecular docking study of new half sandwich Ir(III), Rh(III) and Ru(II) complexes. J. Inorg. Biochem. 2016, 159, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Palepu, N.R.; Sutradhar, D.; Shepherd, S.L.; Phillips, R.M.; Kaminsky, W.; Chandra, A.K.; Kollipara, M.R. Neutral and cationic half-sandwich arene ruthenium, Cp*Rh and Cp*Ir oximato and oxime complexes: Synthesis, structural, DFT and biological studies. J. Organomet. Chem. 2016, 820, 70–81. [Google Scholar] [CrossRef]
- Adhikari, S.; Sutradhar, D.; Shepherd, S.L.; Phillips, R.M.; Chandra, A.K.; Kollipara, M.R. Synthesis, structural, DFT calculations and biological studies of rhodium and iridium complexes containing azine Schiff-base ligands. Polyhedron 2016, 117, 404–414. [Google Scholar] [CrossRef]
- Deo, C.; Wang, H.; Bogliotti, N.; Zang, Y.; Retailleau, P.; He, X.P.; Li, J.; Xie, J. Photoswitchable arene ruthenium and pentamethylcyclopentadienyl rhodium complexes containing osulfonamide azobenzene ligands: Synthesis, characterization and cytotoxicity. J. Organomet. Chem. 2016, 820, 111–119. [Google Scholar] [CrossRef]
- Palepu, N.R.; Adhikari, S.; Premkumar, R.J.; Verma, A.K.; Shepherd, S.L.; Phillips, R.M.; Kaminsky, W.; Kollipara, M.R. Half-sandwich ruthenium, rhodium and iridium complexes featuring oxime ligands: Structural studies and preliminary investigation of in vitro and in vivo anti-tumour activities. Appl. Organomet. Chem. 2017, 31, e3640. [Google Scholar] [CrossRef]
- Palepu, N.R.; Premkumar, R.J.; Verma, A.K.; Bhattacharjee, K.; Joshi, S.R.; Forbes, S.; Mozharivskyj, Y.; Kollipara, M.R. Antibacterial, in vitro antitumor activity and structural studies of rhodium and iridium complexes featuring the two positional isomers of pyridine carbaldehyde picolinic hydrazone ligand. Arab. J. Chem. 2018, 11, 714–728. [Google Scholar] [CrossRef]
- Štarha, P.; Dvořák, Z.; Trávníček, Z. Half-sandwich Ir(III) and Rh(III) 2,2′-dipyridylamine complexes: Synthesis, characterization and in vitro cytotoxicity against the ovarian carcinoma cells. J. Organomet. Chem. 2018, 872, 114–122. [Google Scholar] [CrossRef]
- Gilewska, A.; Barszcz, B.; Masternak, J.; Kazimierczuk, K.; Sitkowski, J.; Wietrzyk, J.; Turlej, E. Similarities and differences in d6 low-spin ruthenium, rhodium and iridium half-sandwich complexes: Synthesis, structure, cytotoxicity and interaction with biological targets. J. Biol. Inorg. Chem. 2019, 24, 591–606. [Google Scholar] [CrossRef]
- Lapasam, A.; Pinder, E.; Phillips, R.M.; Kaminsky, W.; Kollipara, M.R. Synthesis, structure and bonding modes of pyrazine based ligands of Cp*Rh and Cp*Ir complexes: The study of in-vitro cytotoxicity against human cell lines. J. Organomet. Chem. 2019, 899, 120887. [Google Scholar] [CrossRef]
- Sliwinska, U.; Pruchnik, F.P.; Ulaszewski, S.; Latocha, M.; Nawrocka-Musial, D. Properties of η5-pentamethylcyclopentadienyl rhodium(III) and iridium(III) complexes with quinolin-8-ol and their cytostatic activity. Polyhedron 2010, 29, 1653–1659. [Google Scholar] [CrossRef]
- Dömötör, O.; Pape, V.F.S.; May, N.V.; Szakacs, G.; Enyedy, E.A. Comparative solution equilibrium studies of antitumor ruthenium(η6-p-cymene) and rhodium(η5-C5Me5) complexes of 8-hydroxyquinolines. Dalton Trans. 2017, 46, 4382–4396. [Google Scholar] [CrossRef]
- Wirth, S.; Rohbogner, C.J.; Cieslak, M.; Kazmierczak-Baranska, J.; Donevski, S.; Nawrot, B.; Lorenz, I.P. Rhodium(III) and iridium(III) complexes with 1,2-naphthoquinone-1-oximate as a bidentate ligand: Synthesis, structure, and biological activity. J. Biol. Inorg. Chem. 2010, 15, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Enyedy, E.A.; Dömötör, O.; Hackl, C.M.; Roller, A.; Novak, M.S.; Jakupec, M.A.; Keppler, B.K.; Kandioller, W. Solution equilibria and antitumor activities of pentamethylcyclopentadienyl rhodium complexes of picolinic acid and deferiprone. J. Coord. Chem. 2015, 68, 1583–1601. [Google Scholar] [CrossRef]
- Burgoyne, A.R.; Kaschula, C.H.; Parker, M.I.; Smith, G.S. Synthesis and anticancer evaluation of mono- and trinuclear half-sandwich rhodium(III) and iridium(III) complexes based on N,O-salicylaldiminato-sulfonated scaffolds. J. Organomet. Chem. 2017, 846, 100–104. [Google Scholar] [CrossRef]
- Dömötör, O.; Hackl, C.M.; Bali, K.; Roller, A.; Hejl, M.; Jakupec, M.A.; Keppler, B.K.; Kandioller, W.; Enyedy, E.A. Comparative equilibrium and structural studies of new pentamethylcyclopentadienyl rhodium complexes bearing (O,N) donor bidentate ligands. J. Organomet. Chem. 2017, 846, 287–295. [Google Scholar] [CrossRef][Green Version]
- Markham, J.; Liang, J.; Levina, A.; Mak, R.; Johannessen, B.; Kappen, P.; Glover, C.J.; Lai, B.; Vogt, S.; Lay, P.A. (Pentamethylcyclopentadienato)rhodium Complexes for Delivery of the Curcumin Anticancer Drug. Eur. J. Inorg. Chem. 2017, 2017, 1812–1823. [Google Scholar] [CrossRef]
- Meszaros, J.P.; Poljarevic, J.M.; Gal, G.T.; May, N.V.; Spengler, G.; Enyedy, E.A. Comparative solution and structural studies of half-sandwich rhodium and ruthenium complexes bearing curcumin and acetylacetone. J. Inorg. Biochem. 2019, 195, 91–100. [Google Scholar] [CrossRef]
- Su, W.; Wang, X.; Lei, X.; Xiao, Q.; Huang, S.; Li, P. Synthesis, characterization, cytotoxic activity of half-sandwich rhodium(III), and iridium(III) complexes with curcuminoids. J. Organomet. Chem. 2017, 833, 54–60. [Google Scholar] [CrossRef]
- Mokesch, S.; Novak, M.S.; Roller, A.; Jakupec, M.A.; Kandioller, W.; Keppler, B.K. 1,3-Dioxoindane-2-carboxamides as bioactive ligand scaffolds for the development of novel organometallic anticancer drugs. Organometallics 2015, 34, 848–857. [Google Scholar] [CrossRef]
- Domotor, O.; Aicher, S.; Schmidlehner, M.; Novak, M.S.; Roller, A.; Jakupec, M.A.; Kandioller, W.; Hartinger, C.G.; Keppler, B.K.; Enyedy, E.A. Antitumor pentamethylcyclopentadienyl rhodium complexes of maltol and allomaltol: Synthesis, solution speciation and bioactivity. J. Inorg. Biochem. 2014, 134, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Schmidlehner, M.; Pichler, V.; Roller, A.; Jakupec, M.A.; Kandioller, W.; Keppler, B.K. Organometallic complexes of (thio)allomaltol-based Mannich-products: Synthesis, stability and preliminary biological investigations. J. Organomet. Chem. 2015, 782, 69–76. [Google Scholar] [CrossRef]
- Petrini, A.; Pettinari, R.; Marchetti, F.; Pettinari, C.; Therrien, B.; Galindo, A.; Scopelliti, R.; Riedel, T.; Dyson, P.J. Cytotoxic Half-Sandwich Rh(III) and Ir(III) b-Diketonates. Inorg. Chem. 2017, 56, 13600–13612. [Google Scholar] [CrossRef]
- Meszaros, J.P.; Geisler, H.; Poljarevic, J.M.; Roller, A.; Legina, M.S.; Hejl, M.; Jakupec, M.A.; Keppler, B.K.; Kandioller, W.; Enyedy, E.A. Naphthoquinones of natural origin: Aqueous chemistry and coordination to half-sandwich organometallic cations. J. Organomet. Chem. 2020, 907, 121070. [Google Scholar] [CrossRef]
- Su, W.; Luo, Z.; Dong, S.; Chen, X.; Xiao, J.; Peng, B.; Li, P. Novel half-sandwich rhodium(III) and iridium(III) photosensitizers for dual chemo- and photodynamic therapy. Photodiagn. Photodyn. Ther. 2019, 26, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Hackl, C.M.; Schoenhacker-Alte, B.; Klose, M.H.M.; Henke, H.; Legina, M.S.; Jakupec, M.A.; Berger, W.; Keppler, B.K.; Bruggemann, O.; Teasdale, I.; et al. Synthesis and in vivo anticancer evaluation of poly(organo)phosphazene-based metallodrug conjugates. Dalton Trans. 2017, 46, 12114–12124. [Google Scholar] [CrossRef]
- Aradhyula, B.P.R.; Kalidasan, M.; Gangele, K.; Deb, D.K.; Shepherd, S.L.; Phillips, R.M.; Poluri, K.M.; Kollipara, M.R. Synthesis, Structural and Biological Studies of Some Half-Sandwich d6-Metal Complexes with Pyrimidine-Based Ligands. ChemistrySelect 2017, 2, 2065–2076. [Google Scholar] [CrossRef]
- Burgoyne, A.R.; Kaschula, C.H.; Parker, M.I.; Smith, G.S. Tripodal Half-Sandwich Rhodium and Iridium Complexes Containing Sulfonate and Pyridinyl Entities as Antitumor Agents. Eur. J. Inorg. Chem. 2017, 2017, 5379–5386. [Google Scholar] [CrossRef]
- Tremlett, W.D.J.; Tong, K.K.H.; Steel, T.R.; Movassaghi, S.; Hanif, M.; Jamieson, S.M.F.; Sohnel, T.; Hartinger, C.G. Hydroxyquinoline-derived anticancer organometallics: Introduction of amphiphilic PTA as an ancillary ligand increases their aqueous solubility. J. Inorg. Biochem. 2019, 199, 110768. [Google Scholar] [CrossRef]
- Patalenszki, J.; Biro, L.; Benyei, A.C.; Radosova-Muchova, T.; Kasparkova, J.; Buglyo, P. Half-sandwich complexes of ruthenium, osmium, rhodium and iridium with DL-methionine or S-methyl-L-cysteine: A solid state and solution equilibrium study. RSC Adv. 2015, 5, 8094–8107. [Google Scholar] [CrossRef]
- Aboura, W.; Batchelor, L.K.; Garci, A.; Dyson, P.J.; Therrien, B. Reactivity and biological activity of N,N,S-Schiff-base rhodium pentamethylcyclopentadienyl complexes. Inorg. Chim. Acta 2020, 501, 119265. [Google Scholar] [CrossRef]
- Amouri, H.; Moussa, J.; Renfrew, A.K.; Dyson, P.J.; Rager, M.N.; Chamoreau, L.M. Discovery, Structure, and Anticancer Activity of an Iridium Complex of Diselenobenzoquinone. Angew. Chem. Int. Ed. 2010, 49, 7530–7533. [Google Scholar] [CrossRef]
- Rubner, G.; Bensdorf, K.; Wellner, A.; Bergemann, S.; Ott, I.; Gust, R. [Cyclopentadienyl]metalcarbonyl complexes of acetylsalicylic acid as neo-anticancer agents. Eur. J. Med. Chem. 2010, 45, 5157–5163. [Google Scholar] [CrossRef]
- Parveen, S.; Hanif, M.; Leung, E.; Tong, K.K.H.; Yang, A.; Astin, J.; De Zoysa, G.H.; Steel, T.R.; Goodman, D.; Movassaghi, S.; et al. Anticancer organorhodium and -iridium complexes with low toxicity in vivo but high potency in vitro: DNA damage, reactive oxygen species formation, and haemolytic activity. Chem. Commun. 2019, 55, 12016–12019. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Kumar, J.M.; Garci, A.; Rangaraj, N.; Nagesh, N.; Therrien, B. Anticancer Activity of Half-Sandwich Rh(III) and Ir(III) Metalla-Prisms Containing Lipophilic Side Chains. ChemPlusChem 2014, 79, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Garci, A.; Murray, B.S.; Dyson, P.J.; Fabre, G.; Trouillas, P.; Giannini, F.; Furrer, J.; Suss-Fink, G.; Therrien, B. Synthesis, molecular structure, computational study and in vitro anticancer activity of dinuclear thiolatobridged pentamethylcyclopentadienyl Rh(III) and Ir(III) complexes. Dalton Trans. 2013, 42, 15457–15463. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Kumar, J.M.; Garci, A.; Nagesh, N.; Therrien, B. Exploiting natural products to build metalla-assemblies: The anticancer activity of embelin-derived Rh(III) and Ir(III) metalla-rectangles. Molecules 2014, 19, 6031–6046. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Oggu, G.S.; Nagesh, N.; Bokara, K.K.; Therrien, B. Anticancer activity of large metalla-assemblies built from half-sandwich complexes. CrystEngComm 2016, 18, 4952–4957. [Google Scholar] [CrossRef]
- Govender, P.; Riedel, T.; Dyson, P.J.; Smith, G.S. Regulating the anticancer properties of organometallic dendrimers using pyridylferrocene entities: Synthesis, cytotoxicity and DNA binding studies. Dalton Trans. 2016, 45, 9529–9539. [Google Scholar] [CrossRef]
- Wenzel, M.; de Almeida, A.; Bigaeva, E.; Kavanagh, P.; Picquet, M.; Le Gendre, P.; Bodio, E.; Casini, A. New Luminescent Polynuclear Metal Complexes with Anticancer Properties: Toward Structure-Activity Relationships. Inorg. Chem. 2016, 55, 2544–2557. [Google Scholar] [CrossRef]
- Chellan, P.; Land, K.M.; Shokar, A.; Au, A.; An, S.H.; Taylor, D.; Smith, P.J.; Riedel, T.; Dyson, P.J.; Chibale, K.; et al. Synthesis and evaluation of new polynuclear organometallic Ru(II), Rh(III) and Ir(III) pyridyl ester complexes as in vitro antiparasitic and antitumor agents. Dalton Trans. 2014, 43, 513–526. [Google Scholar] [CrossRef]
- Burgoyne, A.R.; Kaschula, C.H.; Parker, M.I.; Smith, G.S. In vitro Cytotoxicity of Half-Sandwich Platinum Group Metal Complexes of a Cationic Alkylated Phosphaadamantane Ligand. Eur. J. Inorg. Chem. 2016, 2016, 1267–1273. [Google Scholar] [CrossRef]
- Rao, A.B.P.; Uma, A.; Chiranjeevi, T.; Bethu, M.S.; Rao, J.V.; Deb, D.K.; Sarkar, B.; Kaminsky, W.; Kollipara, M.R. The in vitro antitumor activity of oligonuclear polypyridyl rhodium and iridium complexes against cancer cells and human pathogens. J. Organomet. Chem. 2016, 824, 131–139. [Google Scholar] [CrossRef]
- Sudding, L.C.; Payne, R.; Govender, P.; Edafe, F.; Clavel, C.M.; Dyson, P.J.; Therrien, B.; Smith, G.S. Evaluation of the in vitro anticancer activity of cyclometalated half-sandwich rhodium and iridium complexes coordinated to naphthaldimine-based poly(propyleneimine) dendritic scaffolds. J. Organomet. Chem. 2014, 774, 79–85. [Google Scholar] [CrossRef]
- Burgoyne, A.R.; Makhubela, B.C.E.; Meyer, M.; Smith, G.S. Trinuclear Half-Sandwich RuII, RhIII and IrIII Polyester Organometallic Complexes: Synthesis and in vitro Evaluation as Antitumor Agents. Eur. J. Inorg. Chem. 2015, 2015, 1433–1444. [Google Scholar] [CrossRef]
- Furrer, J.; Süss-Fink, G. Thiolato-bridged dinuclear arene ruthenium complexes and their potential as anticancer drugs. Coord. Chem. Rev. 2016, 309, 36–50. [Google Scholar] [CrossRef]
- Johnpeter, J.P.; Gupta, G.; Kumar, J.M.; Srinivas, G.; Nagesh, N.; Therrien, B. Biological Studies of Chalcogenolato-Bridged Dinuclear Half-Sandwich Complexes. Inorg. Chem. 2013, 52, 13663–13673. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Murray, B.S.; Dyson, P.J.; Therrien, B. Highly cytotoxic trithiolato-bridged dinuclear Rh(III) and Ir(III) complexes. J. Organomet. Chem. 2014, 767, 78–82. [Google Scholar] [CrossRef]
- Gupta, G.; Murray, B.S.; Dyson, P.J.; Therrien, B. Synthesis, Molecular Structure and Cytotoxicity of Molecular Materials Based on Water Soluble Half-Sandwich Rh(III) and Ir(III) Tetranuclear Metalla-Cycles. Materials 2013, 6, 5352–5366. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Denoyelle-Di-Muro, E.; Mbakidi, J.P.; Leroy-Lhez, S.; Sol, V.; Therrien, B. Delivery of porphin to cancer cells by organometallic Rh(III) and Ir(III) metalla-cages. J. Organomet. Chem. 2015, 787, 44–50. [Google Scholar] [CrossRef]
- Van Niekerk, A.; Chellan, P.; Mapolie, S.F. Heterometallic Multinuclear Complexes as Anti-Cancer Agents-An Overview of Recent Developments. Eur. J. Inorg. Chem. 2019, 2019, 3432–3455. [Google Scholar] [CrossRef]
- Nkoana, W.; Nyoni, D.; Chellan, P.; Stringer, T.; Taylor, D.; Smith, P.J.; Hutton, A.T.; Smith, G.S. Heterometallic half-sandwich complexes containing a ferrocenyl motif: Synthesis, molecular structure, electrochemistry and antiplasmodial evaluation. J. Organomet. Chem. 2014, 752, 67–75. [Google Scholar] [CrossRef]
- Paitandi, R.P.; Gupta, R.K.; Singh, R.S.; Sharma, G.; Koch, B.; Pandey, D.S. Interaction of ferrocene appended Ru(II), Rh(III) and Ir(III) dipyrrinato complexes with DNA/protein, molecular docking and antitumor activity. Eur. J. Med. Chem. 2014, 84, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Cassells, I.; Stringer, T.; Hutton, A.T.; Prince, S.; Smith, G.S. Impact of various lipophilic substituents on ruthenium(II), rhodium(III) and iridium(III) salicylaldimine-based complexes: Synthesis, in vitro cytotoxicity studies and DNA interactions. J. Biol. Inorg. Chem. 2018, 23, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Askari, B.; Rudbari, H.A.; Micale, N.; Schirmeister, T.; Maugeri, A.; Navarra, M. Anticancer study of heterobimetallic platinum(II)-ruthenium(II) and platinum(II)-rhodium(III) complexes with bridging dithiooxamide ligand. J. Organomet. Chem. 2019, 900, 120918. [Google Scholar] [CrossRef]
- Steckhan, E.; Herrmann, S.; Ruppert, R.; Dietz, E.; Frede, M.; Spika, E. Analytical Study of a Series of Substituted (2,2′-Bipyridyl) (Pentamethylcyclopentadienyl) Rhodium and -Iridium Complexes with Regard to Their Effectiveness as Redox Catalysts for the Indirect Electrochemical and Chemical Reduction of NAD(P)+. Organometallics 1991, 10, 1568–1577. [Google Scholar] [CrossRef]
- Buriez, O.; Kerr, J.; Fish, R. Regioselective reduction of NAD+ models with [Cp*Rh(Bpy)H]+: Structure–activity relationships and mechanistic aspects in the formation of the 1,4-NADH derivatives. Angew. Chem. Int. Ed. 1999, 38, 1997–2000. [Google Scholar] [CrossRef]
- Canivet, J.; Süss-Fink, G.; Štěpnička, P. Water-soluble phenanthroline complexes of rhodium, iridium and ruthenium for the regeneration of NADH in the enzymatic reduction of ketones. Eur. J. Inorg. Chem. 2007, 2007, 4736–4742. [Google Scholar] [CrossRef]
- Betanzos-Lara, S.; Liu, Z.; Habtemariam, A.; Pizarro, A.M.; Qamar, B.; Sadler, P.J. Organometallic Ruthenium and Iridium Transfer-Hydrogenation Catalysts Using Coenzyme NADH as a Cofactor. Angew. Chem. Int. Ed. 2012, 51, 3897–3900. [Google Scholar] [CrossRef]
- Coverdale, J.P.C.; Romero-Canelón, I.; Sanchez-Cano, C.; Clarkson, G.J.; Habtemariam, A.; Wills, M.; Sadler, P.J. Asymmetric Transfer Hydrogenation by Synthetic Catalysts in Cancer Cells. Nat. Chem. 2018, 10, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Soldevila-Barreda, J.J.; Romero-Canelón, I.; Habtemariam, A.; Sadler, P.J. Transfer hydrogenation catalysis in cells as a new approach to anticancer drug design. Nat. Commun. 2015, 6, 6582. [Google Scholar] [CrossRef] [PubMed]
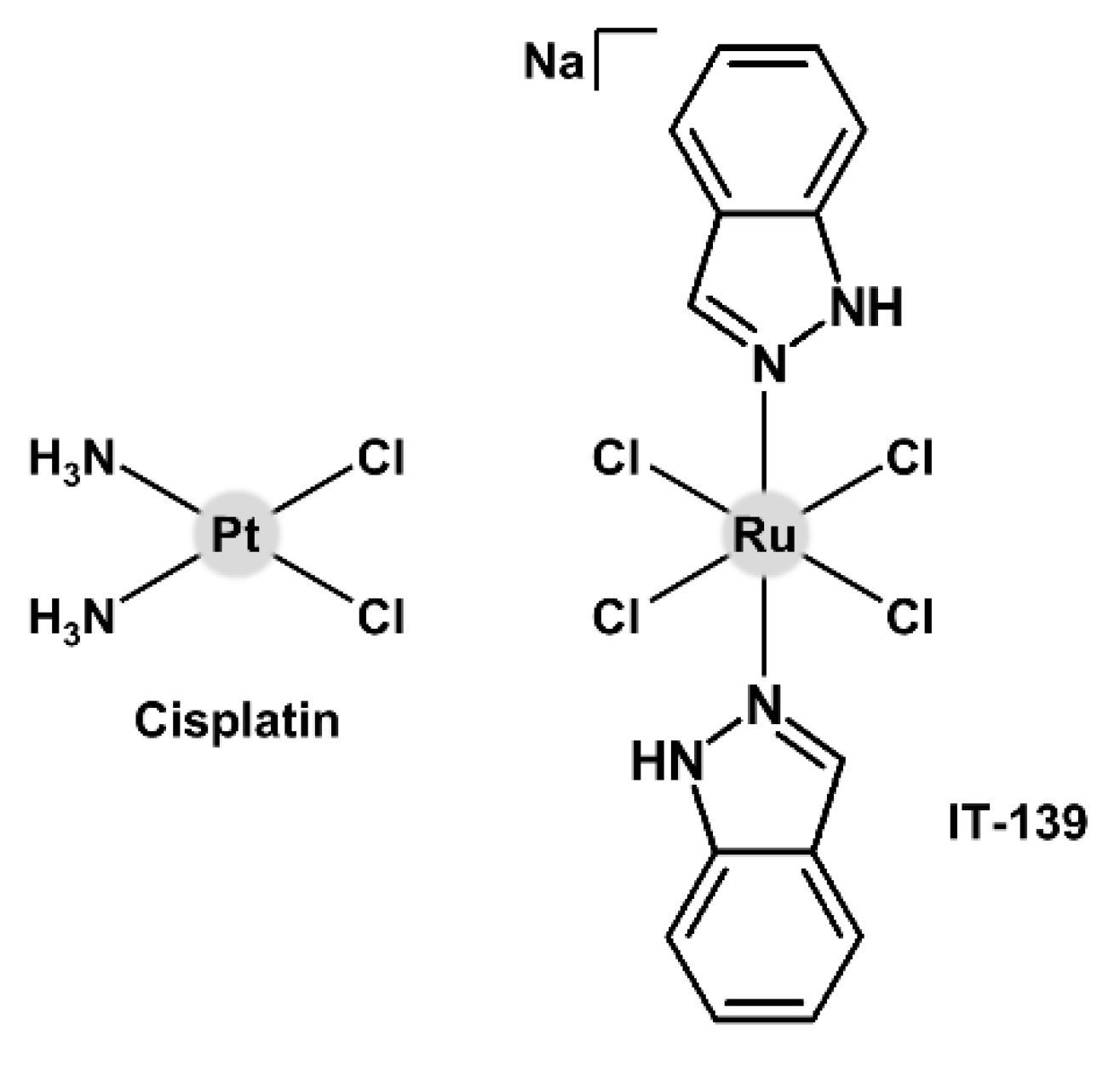
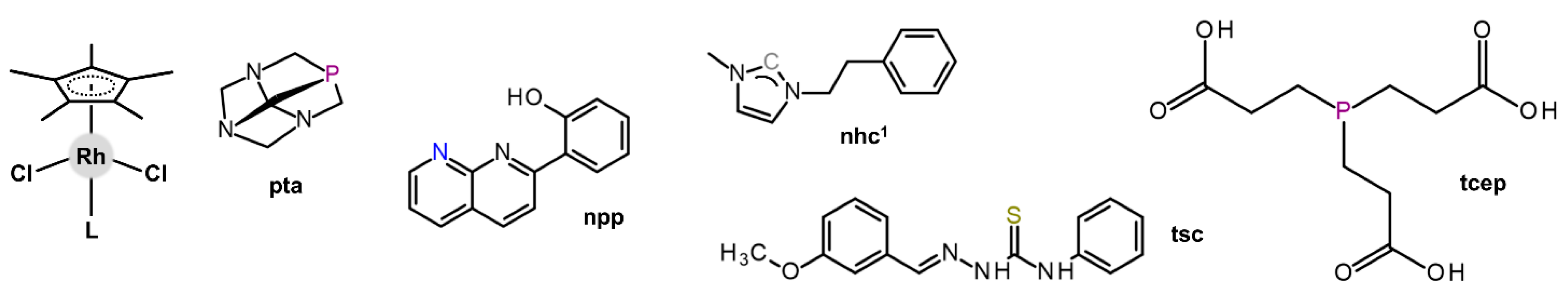


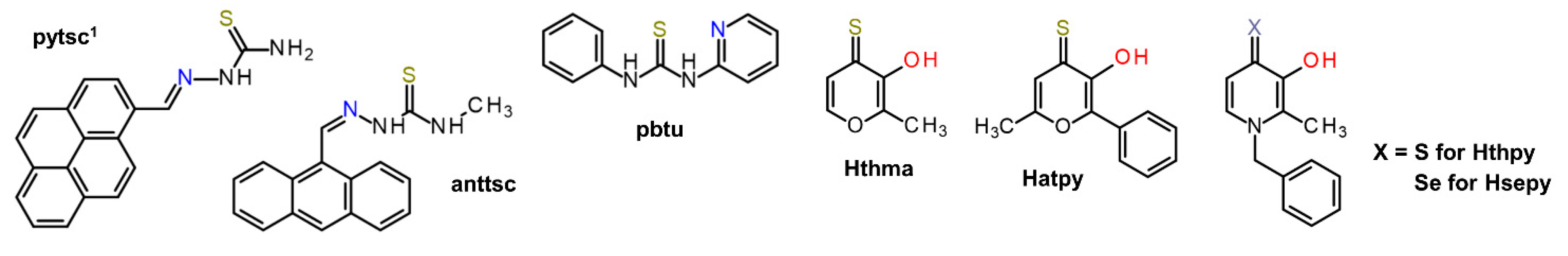
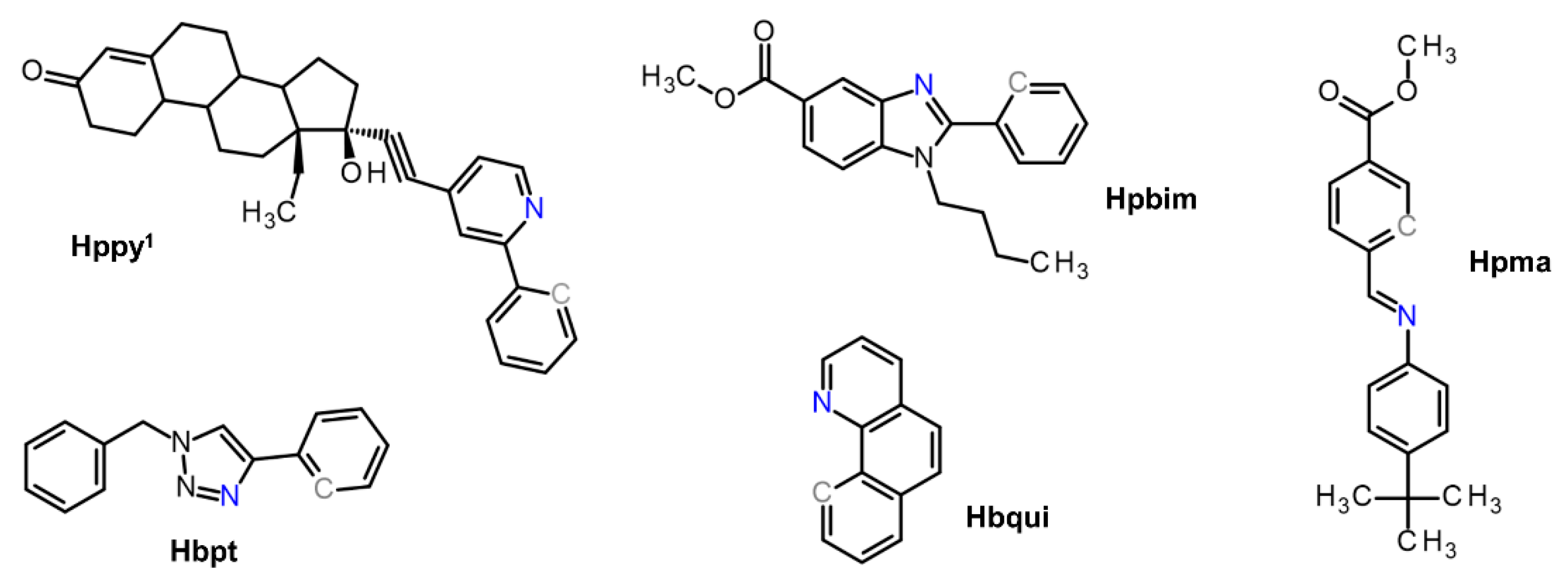
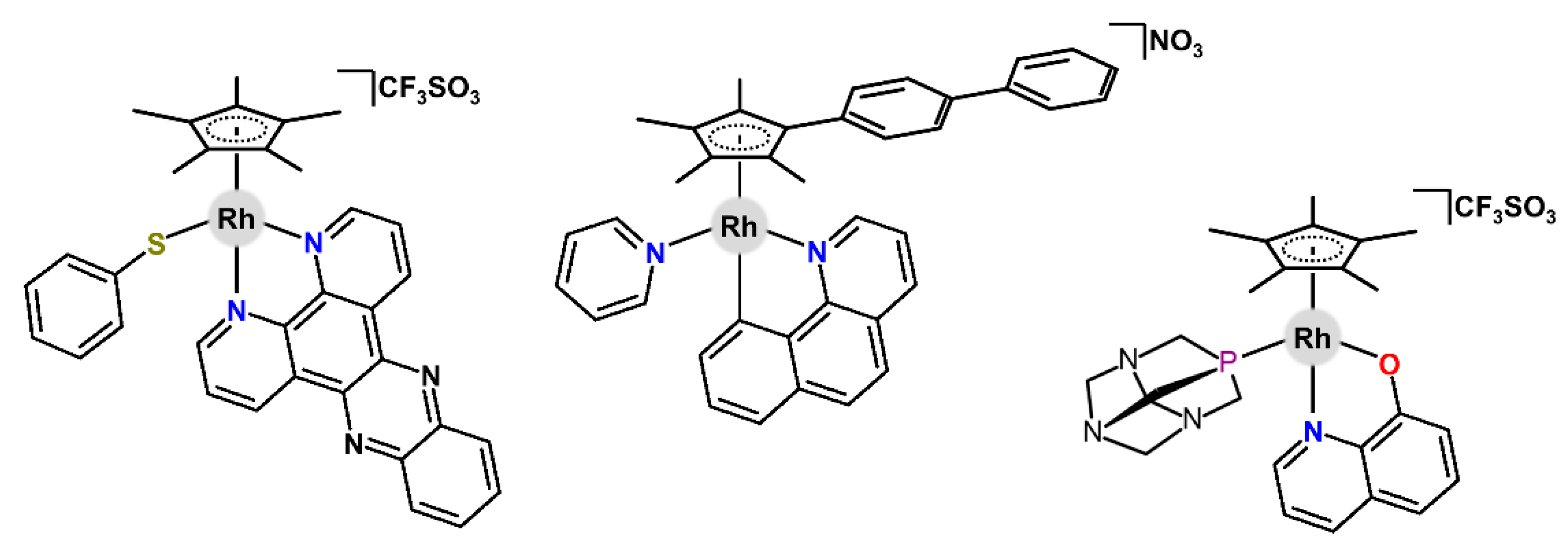
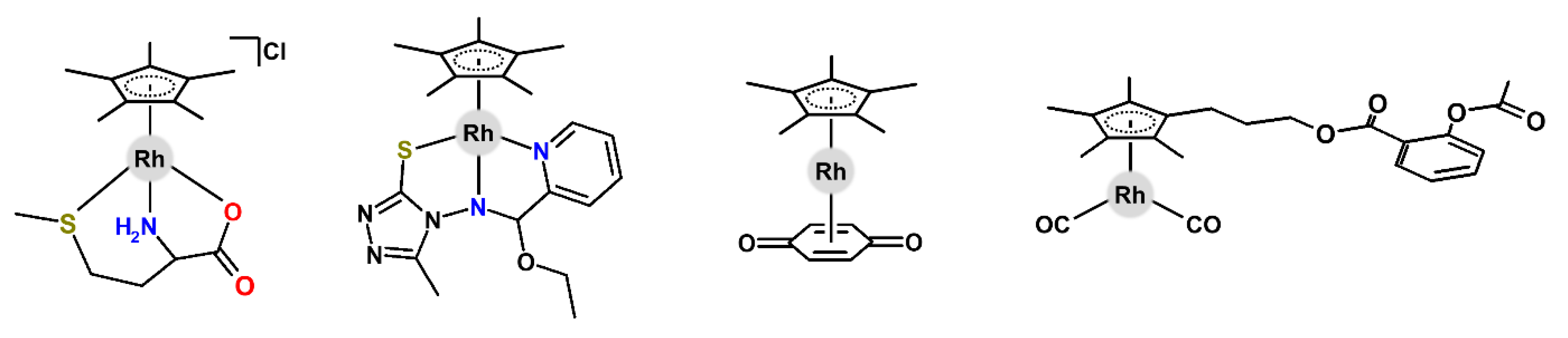
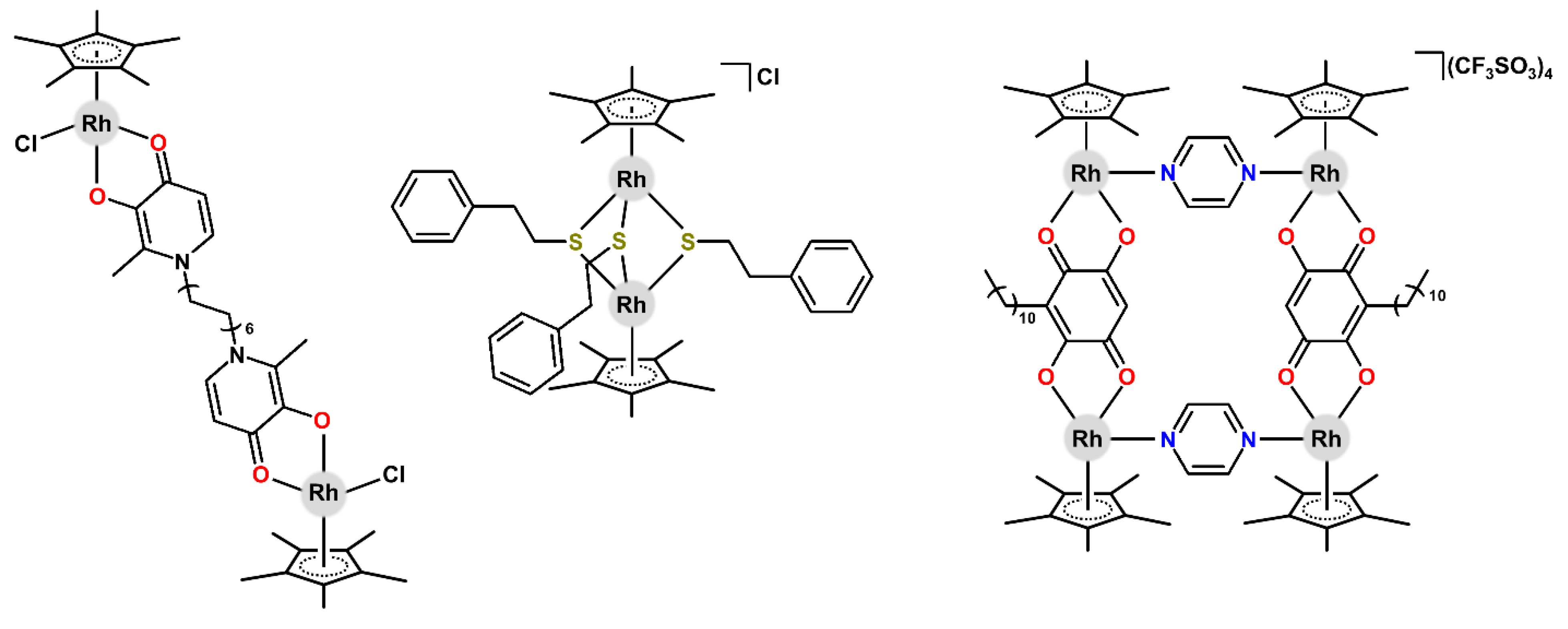
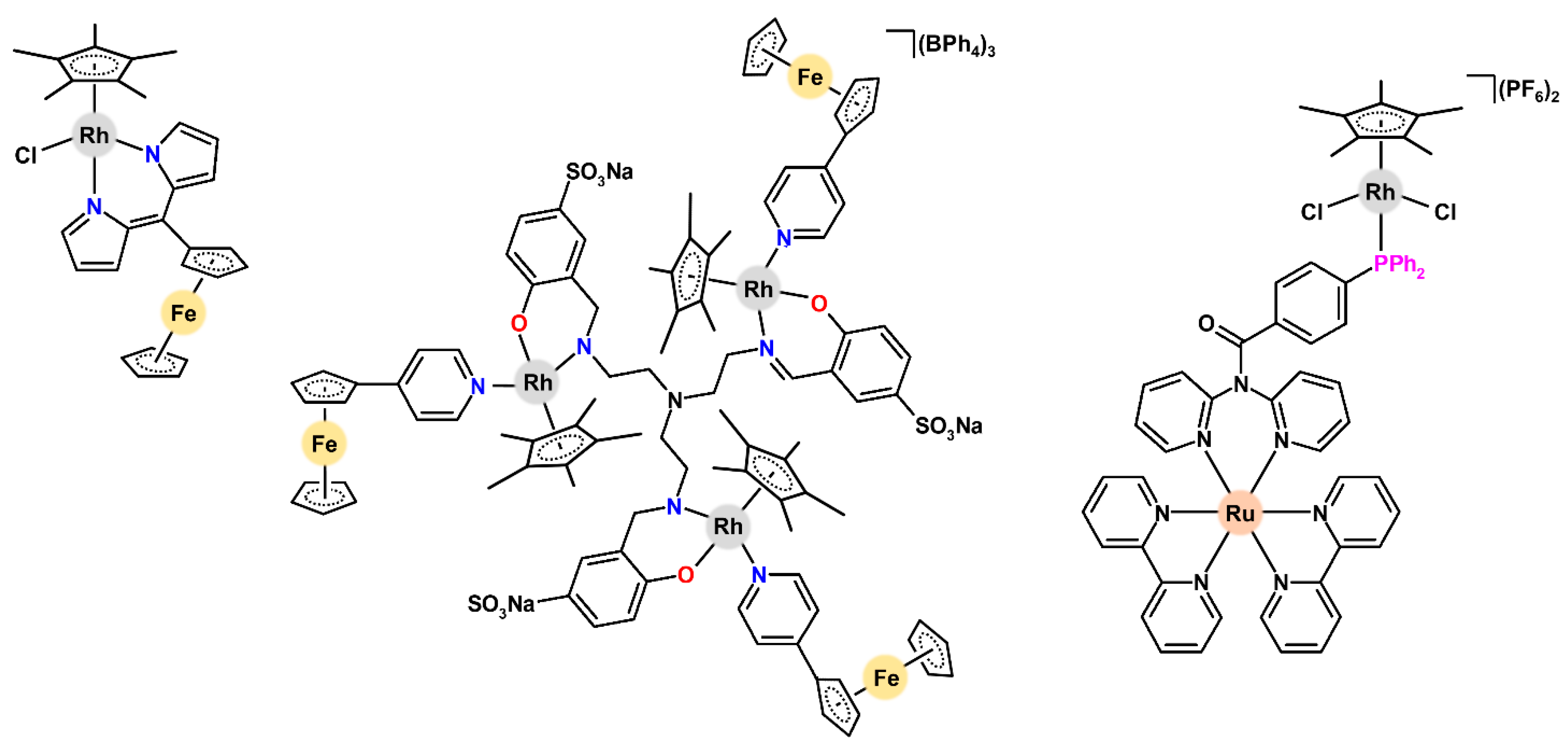
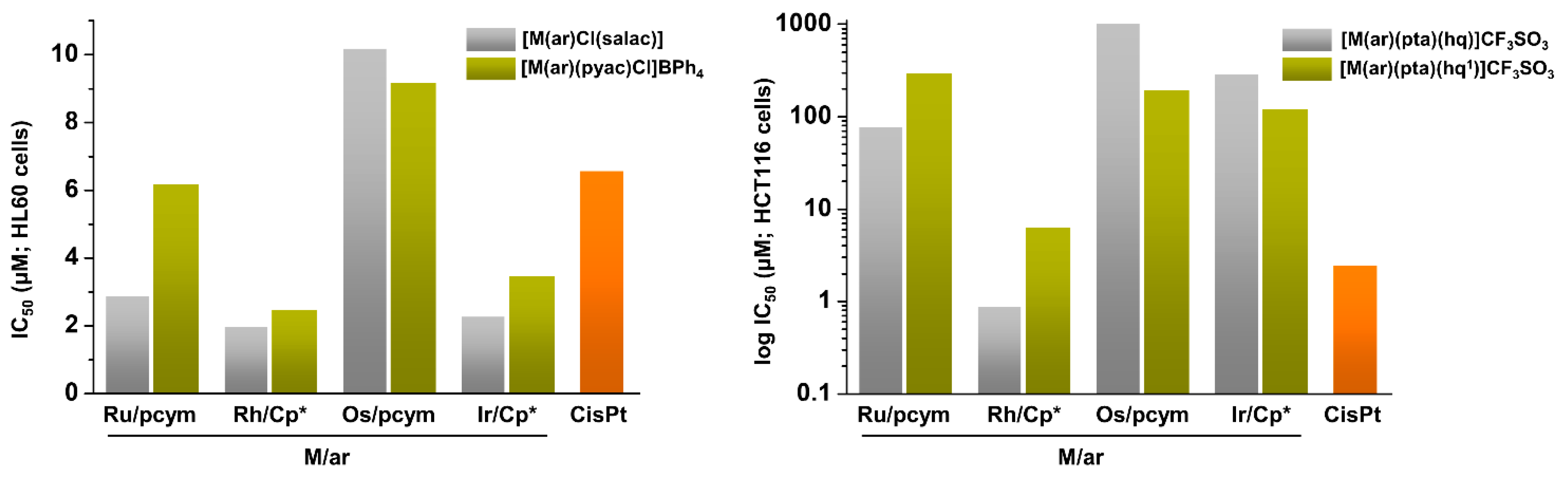
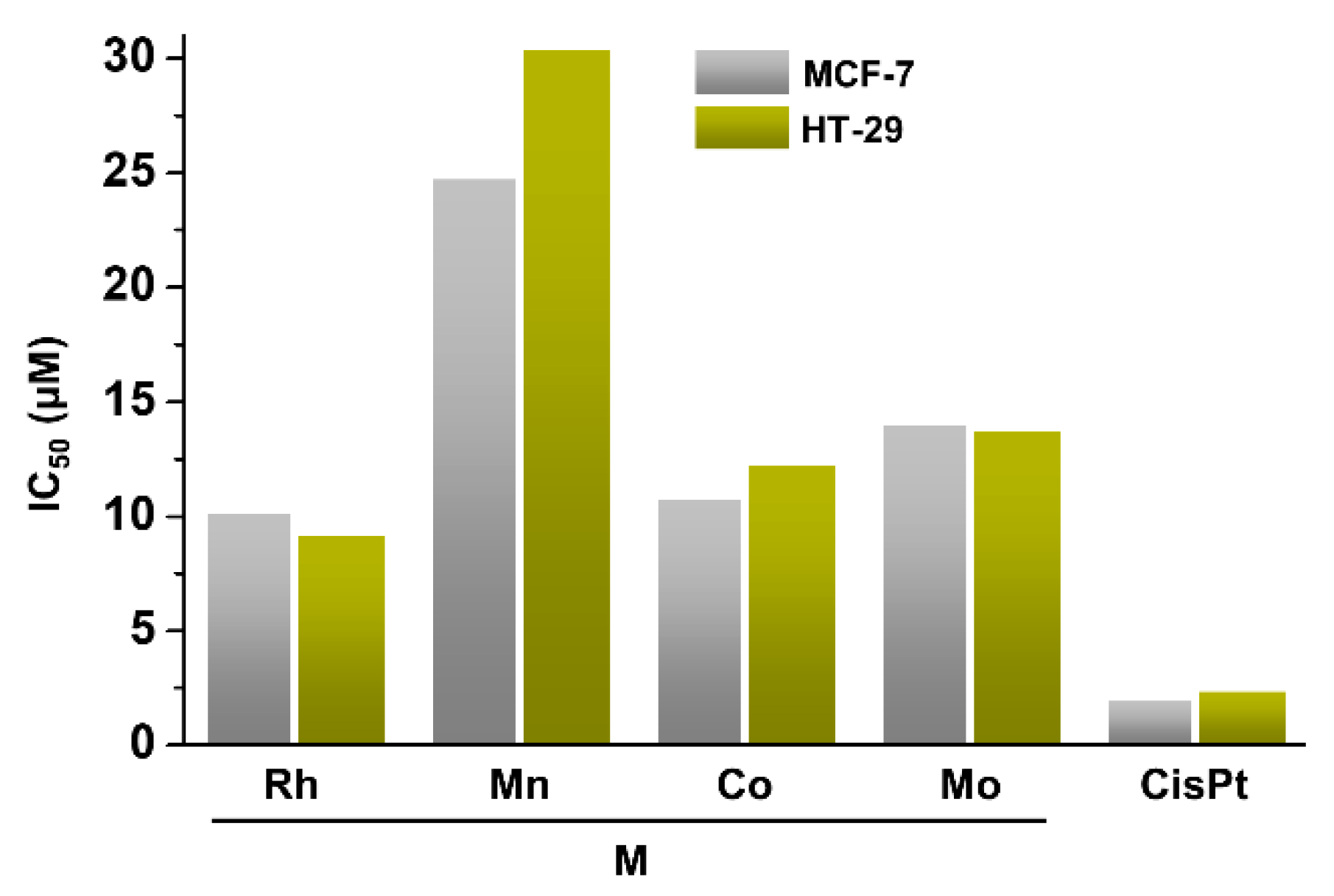
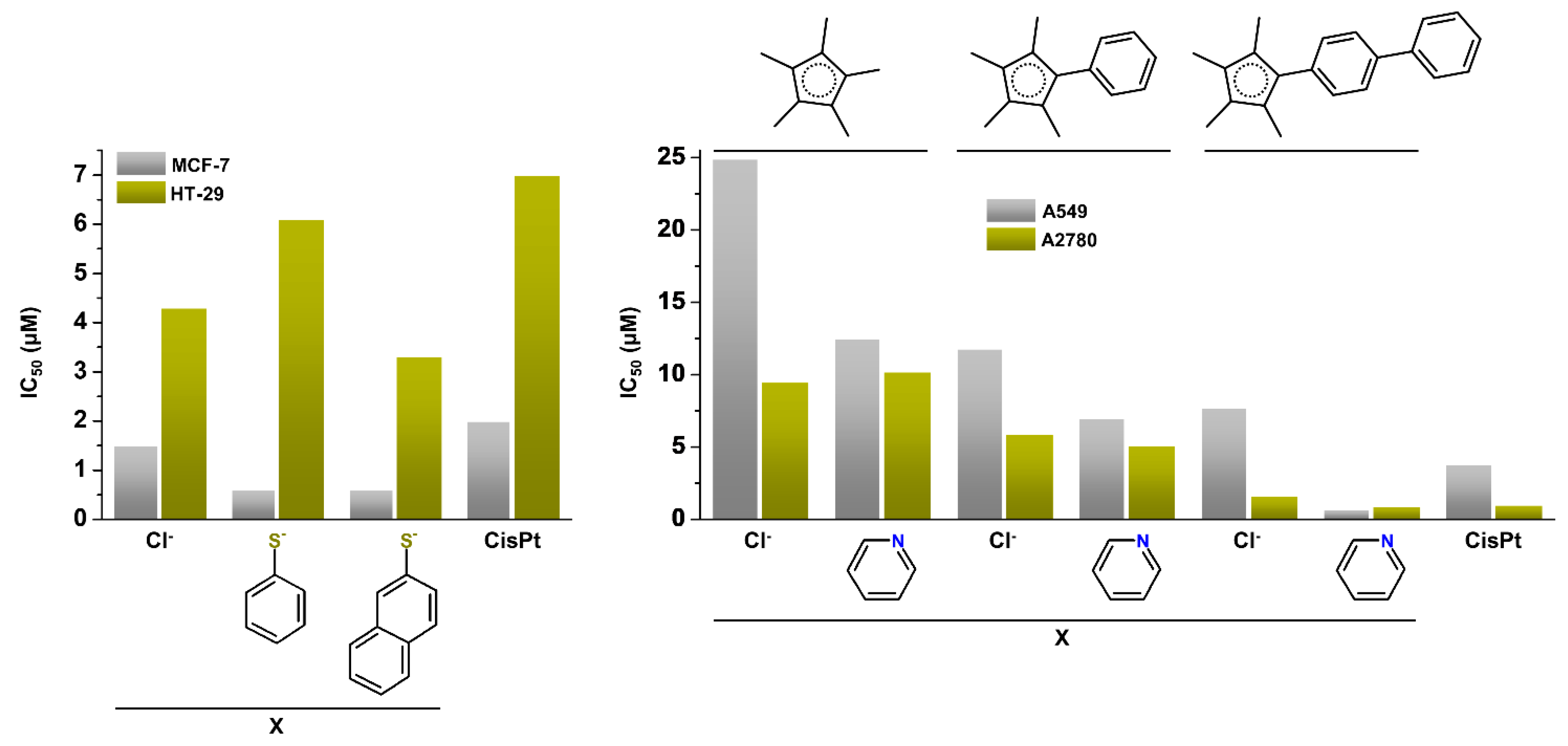
| Complex | Ovarian a | Lung d | Breast h | Colon j | Normal m | Ref. |
|---|---|---|---|---|---|---|
| [Rh(η5-Cp*)(pta)Cl2] | – | 584 | 512 i | 380 | – | [30] |
| [Rh(η5-Cp*)(tcep)Cl2] | – | – | 450 (65.0) | – | – | [31] |
| [Rh(η5-Cp*)(npp)Cl2] | – | – | – | 61.3 k (2.8) | >100 n (3.4) | [32] |
| [Rh(η5-Cp1)(py)Cl2] | – | – | – | 132.0 (2.5) | – | [33] |
| [Rh(η5-Cp*)(nhc1)Cl2] | 5.6 (1.3) | – | 65.0 (1.5) | >100 (1.5) | 87.0 o (8.5) | [34] |
| [Rh(η5-Cp*)(nhc2)Cl2] | – | 23.0 e | – | 11.0 k | – | [35] |
| [Rh(η5-Cp*)(dppn)Cl]CF3SO3 | – | – | 0.8 (2.0) | 3.2 (7.0) | – | [36] |
| [Rh(η5-Cp*)(dppz)Cl]CF3SO3 | – | – | 1.5 (2.0) | 4.3 (7.0) | 2.8 | [36,37] |
| [Rh(η5-Cp*)(phen)Cl]CF3SO3 | – | – | 4.7 | 8.0 | – | [37] |
| [Rh(η5-Cp*)(phen)Cl]PF6 | 17.8 | – | – | – | – | [38] |
| [Rh(η5-Cp*)(phen-SAHA)Cl]Cl | – | 4.1 e | – | – | – | [39] |
| [Rh(η5-Cp*)(bzhyOH)Cl]PF6 | – | – | – | 2.0 k (3.5) | >100 n (3.4) | [40] |
| [Rh(η5-Cp*)Cl(pyin)] | 13.0 (10.3) | – | – | – | 34.7 o (43.0) | [41] |
| [Rh(η5-Cp*)Cl(lap)] | 7.3 b (0.1) | 91.0 (1.3) | – | 93.0 k (2.7) | – | [42] |
| [Rh(η5-Cp*)Cl(cur)] | 14.9 | – | – | – | 13.7 | [43] |
| [Rh(η5-Cp*)Cl(fla)] | 3.1 b | 15.0 | – | 7.9 l | – | [44] |
| [Rh(η5-Cp*)(pytsc1)Cl]Cl | – | 5.1 (1.8) f | 13.5 (2.8) f | – | 166.5 (0.5) f | [45] |
| [Rh(η5-Cp*)(pbtu)Cl]Cl | – | – | – | 9.7 (2.8) | 19.5 n (3.4) | [46] |
| [Rh(η5-Cp*)(bzit)Cl]PF6 | – | >100 | – | 76.0 k | – | [47] |
| [Rh(η5-Cp*)(atpy)Cl] | 0.8 b (50.0) c | 3.8 (156) c | – | 1.0 l (62.0) c | – | [48] |
| [Rh(η5-Cp*)Cl(thma)] | 1.0 b (0.2) | 5.9 (1.3) | – | 1.0 l (3.5) | – | [49] |
| [Rh(η5-Cp*)Cl(thpy)] | 0.4 b (0.2) | 0.7 (1.3) | – | 0.3 l (3.5) | – | [50] |
| [Rh(η5-Cp*)Cl(sepy1)] | 25.0 b (0.2) | 99.0 (1.3) | – | 27.0 l (3.5) | – | [51] |
| [Rh(η5-Cp*)Cl(ppy1)] | 6.0 (1.4) | – | 9.9 i (60.0) | – | – | [52] |
| [Rh(η5-Cp*)(bpt)Cl] | – | 6.0 (4.7) g | – | – | – | [53] |
| [Rh(η5-Cp*)Cl(pbim)] | 7.1 (1.5) | – | 6.4 i (38.0) | 7.8 (9.5) | – | [54] |
| [Rh(η5-Cp*)Cl(pma)] | – | 8.0 (20.0) | – | – | – | [55] |
| [Rh(η5-Cpbph)(bqui)Cl] | 1.6 (1.0) | 7.7 (3.8) | – | – | – | [56] |
| Complex | Ovarian a | Lung c | Breast f | Colon g | Normal j | Ref. |
|---|---|---|---|---|---|---|
| [Rh(η5-Cp*)(dppz)(npth)]CF3SO3 | – | – | 0.6 (2.0) | 3.3 (7.0) | – | [36] |
| [Rh(η5-Cp*)(pyth-N,S)(pyth-S)] | – | – | – | 20.3 (0.3) | 39.5 (6.4) k | [100] |
| [Rh(η5-Cpbph)(py)(bqui)]NO3 | 0.9 (1.0) | 0.7 (3.8) | – | – | – | [56] |
| [Rh(η5-Cp*)(meim)(thma)]PF6 | 0.9 b (0.2) | 2.6 (1.3) | – | 0.5 (3.5) h | – | [49] |
| [Rh(η5-Cp*)(pta)(hq)]CF3SO3 | – | 2.0 (0.8) d | – | 0.9 (2.5) i | – | [102] |
| [Rh(η5-Cp*)(pta)(cur)]CF3SO3 | 12.5 | – | – | – | 17.2 | [43] |
| [Rh(η5-Cp*)(dpa)I]PF6 | 70.1 (5.9) | – | – | – | – | [81] |
| [Rh(η5-Cp*)(met)]Cl | >50.0 (3.4) | – | – | – | – | [103] |
| [Rh(η5-Cp*)(patt1)]Cl | 21.0 (2.3) | – | – | – | >200 (8.4) | [104] |
| [Rh(η5-Cp*)(η4-bqn)] | >400 (3.0) | – | – | – | – | [105] |
| [Rh(η5-Cpas)(CO)2] | – | – | 10.2 (2.0) | 9.2 (2.4) | – | [106] |
| [Rh2(µ-mal1)(η5-Cp*)2Cl2] | – | 0.1 (0.8) d | – | 0.2 (2.5) i | – | [107] |
| [Rh2(µ-dhbq)(η5-Cp*)2Cl2] | – | 0.7 (1.0) e | 0.8 (0.9) e | – | 1.1 l (1.2) e | [108] |
| [Rh2(µ-SR1)2(η5-Cp*)2Cl2] | 1.1 (1.6) | – | – | – | 1.0 (8.6) | [109] |
| [Rh2(µ-SR1)3(η5-Cp*)2]Cl | 0.1 (1.6) | – | – | – | 0.1 (8.6) | [109] |
| [Rh4(µ-dhbq)2(µ-pyz)2(η5-Cp*)4](CF3SO3)4 | – | 0.5 (0.9) e | – | – | 62.0 | [110] |
| [Rh6(µ-dhbq)3(µ-tpt)2(η5-Cp*)6](CF3SO3)6 | – | 0.5 (1.0) e | 0.5 (0.9) e | – | 1.0 l (1.2) e | [108] |
| [Rh8(µ-dhbq)4(µ-tpp)2(η5-Cp*)8](CF3SO3)8 | – | 70.0 | 72.0 | – | 98.0 m | [111] |
| [Rh(η5‑Cp*)(pyFc)(saea)]PF6 | 59.0 (1.5) | – | – | – | 65.5 (10.0) | [112] |
| [Rh8(μ-saea1)(η5‑Cp*)8(pyFc)8](PF6)8 | 10.7 (1.5) | – | – | – | 9.4 (10.0) | [112] |
| [Cl2(η5-Cp*)Rh(µ-bpyPPh3)Ru(bpy)2](PF6)2 | >50 (2.5) | >50 (8.0) | – | – | – | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Máliková, K.; Masaryk, L.; Štarha, P. Anticancer Half-Sandwich Rhodium(III) Complexes. Inorganics 2021, 9, 26. https://doi.org/10.3390/inorganics9040026
Máliková K, Masaryk L, Štarha P. Anticancer Half-Sandwich Rhodium(III) Complexes. Inorganics. 2021; 9(4):26. https://doi.org/10.3390/inorganics9040026
Chicago/Turabian StyleMáliková, Klaudia, Lukáš Masaryk, and Pavel Štarha. 2021. "Anticancer Half-Sandwich Rhodium(III) Complexes" Inorganics 9, no. 4: 26. https://doi.org/10.3390/inorganics9040026
APA StyleMáliková, K., Masaryk, L., & Štarha, P. (2021). Anticancer Half-Sandwich Rhodium(III) Complexes. Inorganics, 9(4), 26. https://doi.org/10.3390/inorganics9040026








