Tripodal Oxazolidine-N-Oxyl Diradical Complexes of Dy3+ and Eu3+
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthetic Aspects and IR Spectra of the Complexes
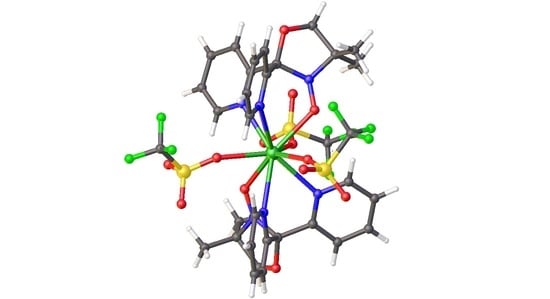
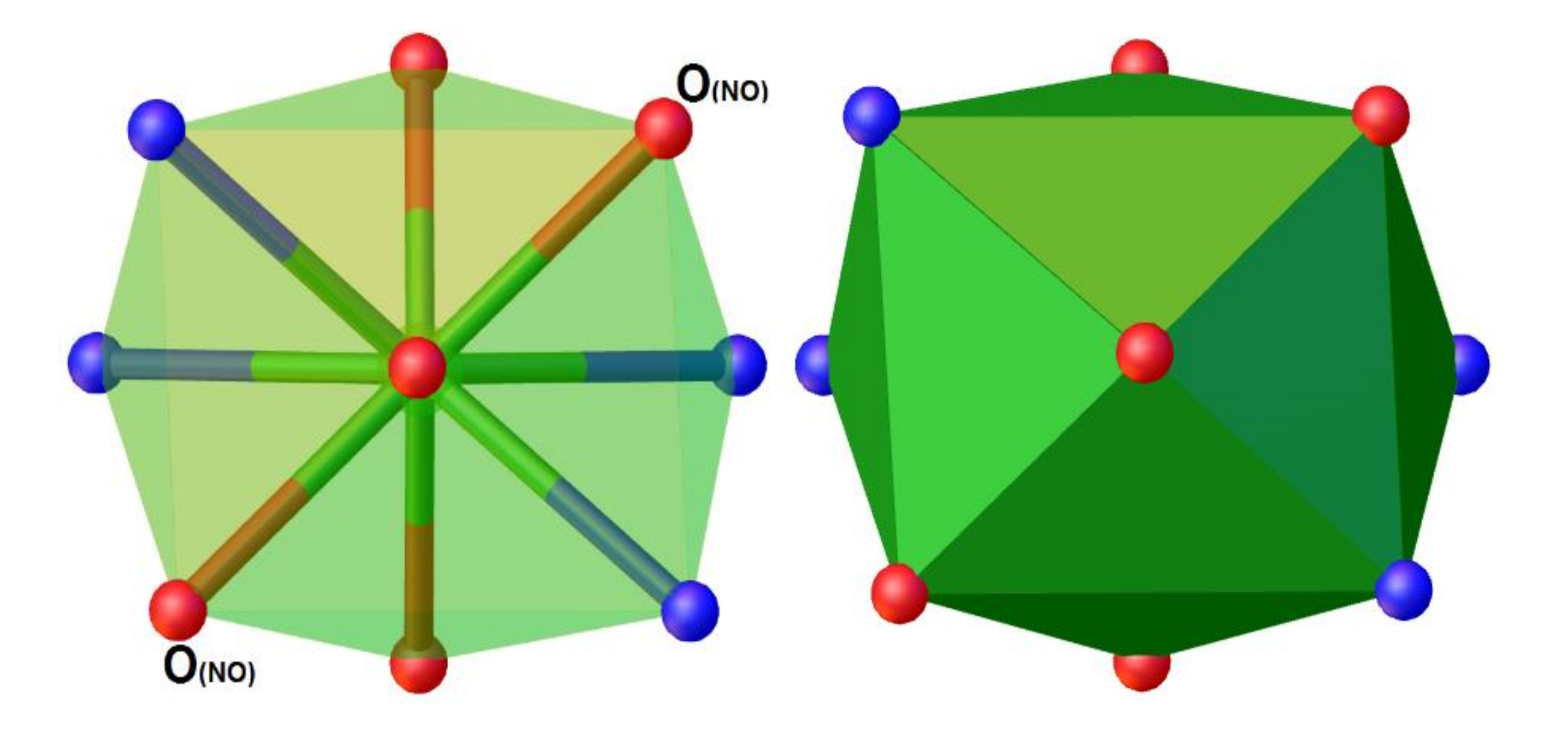
2.2. Description of the Crystal Structures 1–3
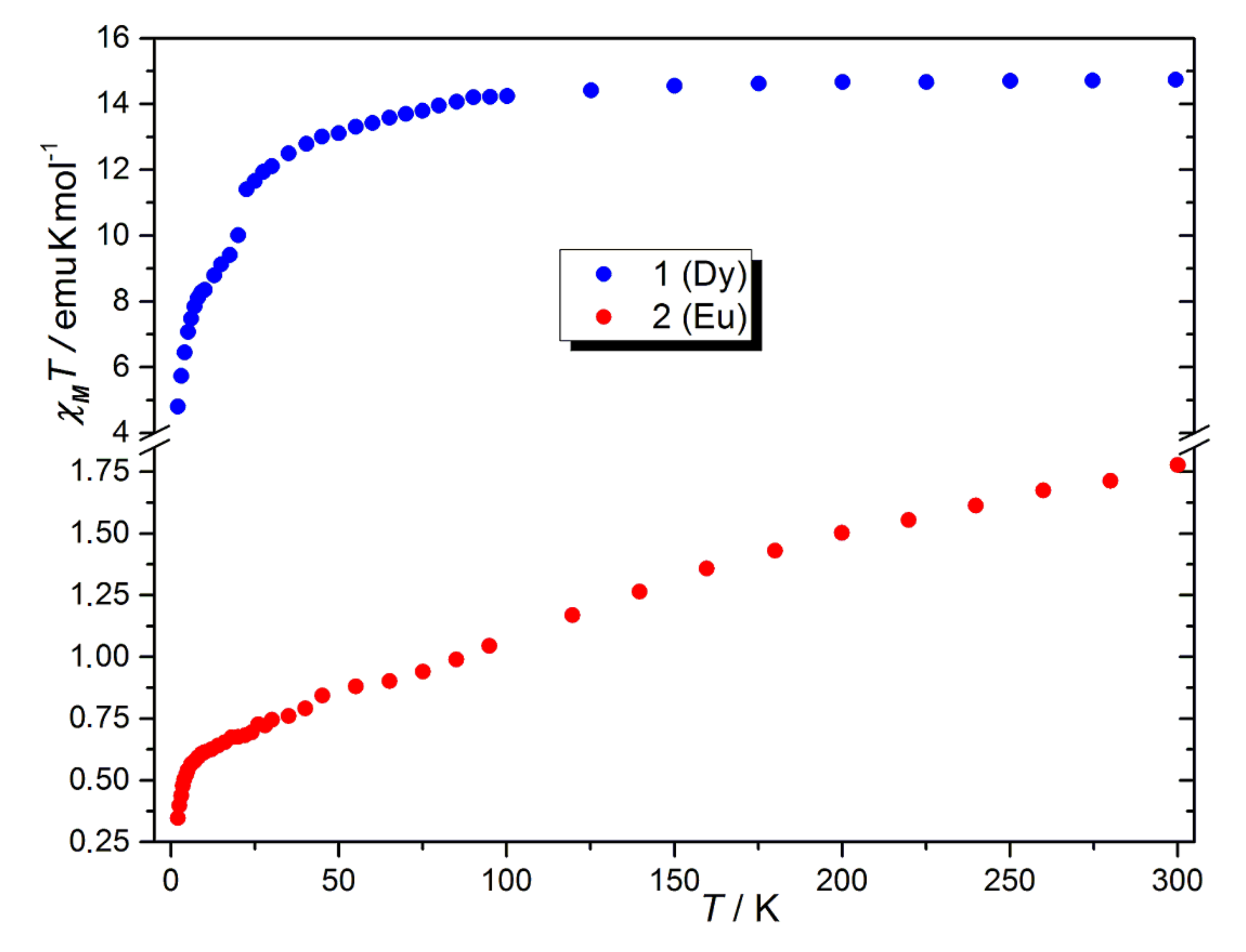
2.3. Magnetic Properties
3. Experimental Section
3.1. Materials and Physical Measurements
3.2. Synthesis of the Complexes
3.3. Single-Crystal X-ray Crystallography Data Collection and Refinement
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogani, L.; Wernsdorfer, W. Molecular Spintronics Using Single-Molecule Magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Shores, M.P.; Bockrath, M.; Long, J.R.; Park, H. Kondo Resonance in a Single-Molecule Transistor. Nature 2002, 417, 725–729. [Google Scholar] [CrossRef]
- Urdampilleta, M.; Nguyen, N.-V.; Cleuziou, J.-P.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W. Molecular Quantum Spintronics: Supramolecular Spin Valves Based on Single-Molecule Magnets and Carbon Nanotubes. Int. J. Mol. Sci. 2011, 12, 6656–6667. [Google Scholar] [CrossRef] [PubMed]
- Sanvito, S. Molecular Spintronics. Chem. Soc. Rev. 2011, 40, 3336. [Google Scholar] [CrossRef]
- Thiele, S.; Vincent, R.; Holzmann, M.; Klyatskaya, S.; Ruben, M.; Balestro, F.; Wernsdorfer, W. Electrical Readout of Individual Nuclear Spin Trajectories in a Single-Molecule Magnet Spin Transistor. Phys. Rev. Lett. 2013, 111, 037203. [Google Scholar] [CrossRef]
- Gobbi, M.; Novak, M.A.; Del Barco, E. Molecular Spintronics. J. Appl. Phys. 2019, 125, 240401. [Google Scholar] [CrossRef]
- Mannini, M.; Pineider, F.; Sainctavit, P.; Danieli, C.; Otero, E.; Sciancalepore, C.; Talarico, A.M.; Arrio, M.-A.; Cornia, A.; Gatteschi, D.; et al. Magnetic Memory of a Single-Molecule Quantum Magnet Wired to a Gold Surface. Nat. Mater. 2009, 8, 194–197. [Google Scholar] [CrossRef]
- Leuenberger, M.N.; Loss, D. Quantum Computing in Molecular Magnets. Nature 2001, 410, 789–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krylov, A.I.; Doyle, J.; Ni, K.-K. Quantum Computing and Quantum Information Storage. Phys. Chem. Chem. Phys. 2021, 23, 6341–6343. [Google Scholar] [CrossRef]
- Winpenny, R.E.P. Quantum Information Processing Using Molecular Nanomagnets as Qubits. Angew. Chem. Int. Ed. 2008, 47, 7992–7994. [Google Scholar] [CrossRef]
- Waldmann, O. A Criterion for the Anisotropy Barrier in Single-Molecule Magnets. Inorg. Chem. 2007, 46, 10035–10037. [Google Scholar] [CrossRef] [PubMed]
- Neese, F.; Pantazis, D.A. What Is Not Required to Make a Single Molecule Magnet. Faraday Discuss. 2011, 148, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Harriman, K.L.M.; Errulat, D.; Murugesu, M. Magnetic Axiality: Design Principles from Molecules to Materials. Trends Chem. 2019, 1, 425–439. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular Magnetic Hysteresis at 60 Kelvin in Dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic Hysteresis up to 80 Kelvin in a Dysprosium Metallocene Single-Molecule Magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorace, L.; Gatteschi, D. Electronic Structure and Magnetic Properties of Lanthanide Molecular Complexes. In Lanthanides and Actinides in Molecular Magnetism; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 1–26. [Google Scholar]
- Liddle, S.T.; van Slageren, J. Improving F-Element Single Molecule Magnets. Chem. Soc. Rev. 2015, 44, 6655–6669. [Google Scholar] [CrossRef] [Green Version]
- Perfetti, M.; Bendix, J. The Multiple Faces, and Phases, of Magnetic Anisotropy. Inorg. Chem. 2019, 58, 11875–11882. [Google Scholar] [CrossRef]
- Dei, A.; Gatteschi, D.; Pécaut, J.; Poussereau, S.; Sorace, L.; Vostrikova, K. Crystal Field and Exchange Effects in Rare Earth Semiquinone Complexes. Comptes Rendus L’académie Sci. Ser. IIC-Chem. 2001, 4, 135–141. [Google Scholar] [CrossRef]
- Demir, S.; Jeon, I.-R.; Long, J.R.; Harris, T.D. Radical Ligand-Containing Single-Molecule Magnets. Coord. Chem. Rev. 2015, 289–290, 149–176. [Google Scholar] [CrossRef] [Green Version]
- Briganti, M.; Lucaccini, E.; Chelazzi, L.; Ciattini, S.; Sorace, L.; Sessoli, R.; Totti, F.; Perfetti, M. Magnetic Anisotropy Trends along a Full 4f-Series: The fn+7 Effect. J. Am. Chem. Soc. 2021, 143, 8108–8115. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, M.; Li, X.-L.; Tang, J. Molecular Magnetism of Lanthanide: Advances and Perspectives. Coord. Chem. Rev. 2019, 378, 350–364. [Google Scholar] [CrossRef]
- Parmar, V.S.; Mills, D.P.; Winpenny, R.E.P. Mononuclear Dysprosium Alkoxide and Aryloxide Single-Molecule Magnets. Chem. A Eur. J. 2021, 27, 7625–7645. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Wu, S.; Chen, Y.; Wan, R.; Huang, G.; Liu, Y.; Liu, J.; Reta, D.; Giansiracusa, M.J.; et al. Opening Magnetic Hysteresis by Axial Ferromagnetic Coupling: From Mono-Decker to Double-Decker Metallacrown. Angew. Chem. Int. Ed. 2021, 60, 5299–5306. [Google Scholar] [CrossRef]
- Canaj, A.B.; Dey, S.; Martí, E.R.; Wilson, C.; Rajaraman, G.; Murrie, M. Insight into D 6 h Symmetry: Targeting Strong Axiality in Stable Dysprosium(III) Hexagonal Bipyramidal Single-Ion Magnets. Angew. Chem. Int. Ed. 2019, 58, 14146–14151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, M.A.; Hansen, U.B.; Perfetti, M.; Pedersen, K.S.; Bartolomé, E.; Simeoni, G.G.; Mutka, H.; Rols, S.; Jeong, M.; Zivkovic, I.; et al. Chemical Tunnel-Splitting-Engineering in a Dysprosium-Based Molecular Nanomagnet. Nat. Commun. 2018, 9, 1292. [Google Scholar] [CrossRef]
- Perfetti, M.; Caneschi, A.; Sukhikh, T.S.; Vostrikova, K.E. Lanthanide Complexes with a Tripodal Nitroxyl Radical Showing Strong Magnetic Coupling. Inorg. Chem. 2020, 59, 16591–16598. [Google Scholar] [CrossRef]
- Ito, A.; Nakano, Y.; Urabe, M.; Tanaka, K.; Shiro, M. Structural and Magnetic Studies of Copper(II) and Zinc(II) Coordination Complexes Containing Nitroxide Radicals as Chelating Ligands. Eur. J. Inorg. Chem. 2006, 2006, 3359–3368. [Google Scholar] [CrossRef]
- Gass, I.A.; Gartshore, C.J.; Lupton, D.W.; Moubaraki, B.; Nafady, A.; Bond, A.M.; Boas, J.F.; Cashion, J.D.; Milsmann, C.; Wieghardt, K.; et al. Anion Dependent Redox Changes in Iron Bis-Terdentate Nitroxide {NNO} Chelates. Inorg. Chem. 2011, 50, 3052–3064. [Google Scholar] [CrossRef] [PubMed]
- Gass, I.A.; Tewary, S.; Nafady, A.; Chilton, N.F.; Gartshore, C.J.; Asadi, M.; Lupton, D.W.; Moubaraki, B.; Bond, A.M.; Boas, J.F.; et al. Observation of Ferromagnetic Exchange, Spin Crossover, Reductively Induced Oxidation, and Field-Induced Slow Magnetic Relaxation in Monomeric Cobalt Nitroxides. Inorg. Chem. 2013, 52, 7557–7572. [Google Scholar] [CrossRef]
- Gass, I.A.; Tewary, S.; Rajaraman, G.; Asadi, M.; Lupton, D.W.; Moubaraki, B.; Chastanet, G.; Létard, J.-F.; Murray, K.S. Solvate-Dependent Spin Crossover and Exchange in Cobalt(II) Oxazolidine Nitroxide Chelates. Inorg. Chem. 2014, 53, 5055–5066. [Google Scholar] [CrossRef]
- Gass, I.A.; Asadi, M.; Lupton, D.W.; Moubaraki, B.; Bond, A.M.; Guo, S.-X.; Murray, K.S. Manganese(II) Oxazolidine Nitroxide Chelates: Structure, Magnetism, and Redox Properties. Aust. J. Chem. 2014, 67, 1618. [Google Scholar] [CrossRef]
- Pedersen, A.H.; Geoghegan, B.L.; Nichol, G.S.; Lupton, D.W.; Murray, K.S.; Martínez-Lillo, J.; Gass, I.A.; Brechin, E.K. Hexahalorhenate(IV) Salts of Metal Oxazolidine Nitroxides. Dalt. Trans. 2017, 46, 5250–5259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gass, I.A.; Lu, J.; Asadi, M.; Lupton, D.W.; Forsyth, C.M.; Geoghegan, B.L.; Moubaraki, B.; Cashion, J.D.; Martin, L.L.; Bond, A.M.; et al. Use of the TCNQF42− Dianion in the Spontaneous Redox Formation of [FeIII(L−)2][TCNQF4•−]. Chempluschem 2018, 83, 658–668. [Google Scholar] [CrossRef]
- Gass, I.A.; Lu, J.; Ojha, R.; Asadi, M.; Lupton, D.W.; Geoghegan, B.L.; Moubaraki, B.; Martin, L.L.; Bond, A.M.; Murray, K.S. [FeII(L•)2][TCNQF4•−]2: A Redox-Active Double Radical Salt. Aust. J. Chem. 2019, 72, 769–777. [Google Scholar] [CrossRef]
- Liu, B.-C.; Ge, N.; Zhai, Y.-Q.; Zhang, T.; Ding, Y.-S.; Zheng, Y.-Z. An Imido Ligand Significantly Enhances the Effective Energy Barrier of Dysprosium(III) Single-Molecule Magnets. Chem. Commun. 2019, 55, 9355–9358. [Google Scholar] [CrossRef] [PubMed]
- Benamara, N.; Diop, M.; Leuvrey, C.; Lenertz, M.; Gilliot, P.; Gallart, M.; Bolvin, H.; Setifi, F.; Rogez, G.; Rabu, P.; et al. Octahedral Hexachloro Environment of Dy3+ with Slow Magnetic Relaxation and Luminescent Properties. Eur. J. Inorg. Chem. 2021, 2021, 2099–2107. [Google Scholar] [CrossRef]
- Andrews, P.C.; Beck, T.; Forsyth, C.M.; Fraser, B.H.; Junk, P.C.; Massi, M.; Roesky, P.W. Templated Assembly of a µ6-CO32− Dodecanuclear Lanthanum Dibenzoylmethanide Hydroxido Cluster with Concomitant Formation of Phenylglyoxylate. Dalt. Trans. 2007, 5651–5654. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Kolybalov, D.S.; Pylova, E.K.; Bashirov, D.A.; Komarov, V.Y.; Kuratieva, N.V.; Smolentsev, A.I.; Fitch, A.N.; Konchenko, S.N. A Fresh Look at the Structural Diversity of Dibenzoylmethanide Complexes of Lanthanides. New J. Chem. 2019, 43, 9934–9942. [Google Scholar] [CrossRef]
- Johnston, D.H.; Shriver, D.F. Vibrational Study of the Trifluoromethanesulfonate Anion: Unambiguous Assignment of the Asymmetric Stretching Modes. Inorg. Chem. 1993, 32, 1045–1047. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Chilton, N.F.; Vettese, G.F.; Pineda, E.M.; Crowe, I.F.; Ziller, J.W.; Winpenny, R.E.P.; Evans, W.J.; Mills, D.P. Physicochemical Properties of Near-Linear Lanthanide(II) Bis(Silylamide) Complexes (Ln = Sm, Eu, Tm, Yb). Inorg. Chem. 2016, 55, 10057–10067. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, Version 2.1, Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; Universitat de Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Long, J.; Lyubov, D.M.; Mahrova, T.V.; Lyssenko, K.A.; Korlyukov, A.A.; Fedorov, Y.V.; Chernikova, E.Y.; Guari, Y.; Larionova, J.; Trifonov, A.A. Heteroleptic Lanthanide Complexes Coordinated by Tripodal Tetradentate Ligand: Synthesis, Structure, and Magnetic and Photoluminescent Properties. Cryst. Growth Des. 2020, 20, 5184–5192. [Google Scholar] [CrossRef]
- Hamada, D.; Fujinami, T.; Yamauchi, S.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatsuki, Y.; Tsuchimoto, M.; Coletti, C.; Re, N. Luminescent DyIII Single Ion Magnets with Same N6O3 Donor Atoms but Different Donor Atom Arrangements, ‘fac’-[DyIII(HLDL-Ala)3]·8H2O and ‘mer’-[DyIII(HLDL-Phe)3]·7H2O. Polyhedron 2016, 109, 120–128. [Google Scholar] [CrossRef]
- Murakami, R.; Nakamura, T.; Ishida, T. Doubly TEMPO-Coordinated Gadolinium(III), Lanthanum(III), and Yttrium(III) Complexes. Strong Superexchange Coupling across Rare Earth Ions. Dalt. Trans. 2014, 43, 5893–5898. [Google Scholar] [CrossRef]
- Petrochenkova, N.V.; Tkachenko, I.A.; Mirochnik, A.G.; Karasev, V.E.; Kavun, V.Y. Luminescence and Magnetic Properties of Europium(III) Carboxylatodibenzoylmethanates. Opt. Spectrosc. 2010, 109, 917–920. [Google Scholar] [CrossRef]
- Kahn, M.L.; Sutter, J.-P.; Golhen, S.; Guionneau, P.; Ouahab, L.; Kahn, O.; Chasseau, D. Systematic Investigation of the Nature of the Coupling between a Ln(III) Ion (Ln = Ce(III) to Dy(III)) and Its Aminoxyl Radical Ligands. Structural and Magnetic Characteristics of a Series of {Ln(Radical)2} Compounds and the Related {Ln(Nitrone)2} Derivat. J. Am. Chem. Soc. 2000, 122, 3413–3421. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rey, P.; Caneschi, A.; Sukhikh, T.S.; Vostrikova, K.E. Tripodal Oxazolidine-N-Oxyl Diradical Complexes of Dy3+ and Eu3+. Inorganics 2021, 9, 91. https://doi.org/10.3390/inorganics9120091
Rey P, Caneschi A, Sukhikh TS, Vostrikova KE. Tripodal Oxazolidine-N-Oxyl Diradical Complexes of Dy3+ and Eu3+. Inorganics. 2021; 9(12):91. https://doi.org/10.3390/inorganics9120091
Chicago/Turabian StyleRey, Philippe, Andrea Caneschi, Taisiya S. Sukhikh, and Kira E. Vostrikova. 2021. "Tripodal Oxazolidine-N-Oxyl Diradical Complexes of Dy3+ and Eu3+" Inorganics 9, no. 12: 91. https://doi.org/10.3390/inorganics9120091
APA StyleRey, P., Caneschi, A., Sukhikh, T. S., & Vostrikova, K. E. (2021). Tripodal Oxazolidine-N-Oxyl Diradical Complexes of Dy3+ and Eu3+. Inorganics, 9(12), 91. https://doi.org/10.3390/inorganics9120091