The Effects of Functional Groups and Missing Linkers on the Adsorption Capacity of Aromatic Hydrocarbons in UiO-66 Thin Films
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. UiO-66-X Film Synthesis
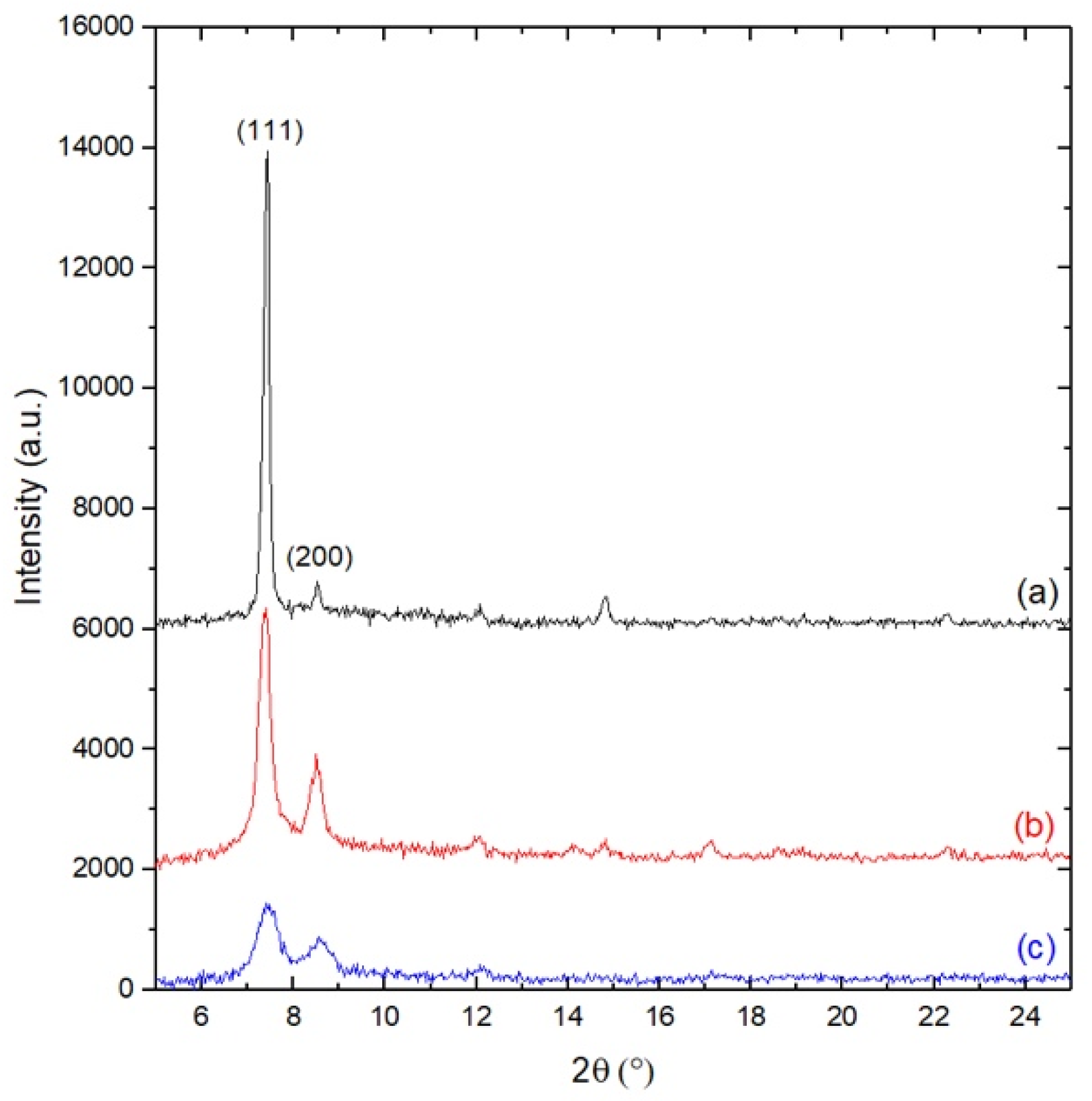
3.2. X-ray Diffraction
3.3. Reflection-Infrared Absorption Spectroscopy (RAIRS)
3.4. Raman Spectroscopy
3.5. Scanning Electron Microscopy (SEM)
3.6. X-ray Photoelectron Spectroscopy
3.7. Gas Doser
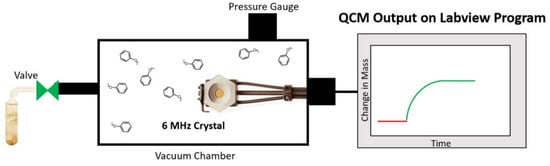
3.8. Quartz Crystal Microbalance Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal–organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Moghadam, P.Z.; Li, A.; Wiggin, S.B.; Tao, A.; Maloney, A.G.P.; Wood, P.A.; Ward, S.C.; Fairen-Jimenez, D. Development of a Cambridge Structural Database Subset: A Collection of Metal–Organic Frameworks for Past, Present, and Future. Chem. Mater. 2017, 29, 2618–2625. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Shearer, G.C.; Forselv, S.; Chavan, S.; Bordiga, S.; Mathisen, K.; Bjørgen, M.; Svelle, S.; Lillerud, K.P. In Situ Infrared Spectroscopic and Gravimetric Characterisation of the Solvent Removal and Dehydroxylation of the Metal Organic Frameworks UiO-66 and UiO-67. Top. Catal. 2013, 56, 770–782. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W.; Jasuja, H.; Glover, T.G.; Huang, Y.-G.; Walton, K.S. Stability and degradation mechanisms of metal–organic frameworks containing the Zr6O4(OH)4 secondary building unit. J. Mater. Chem. A 2013, 1, 5642–5650. [Google Scholar] [CrossRef]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Bordiga, S.; Nilsen, M.H.; Jakobsen, S.; Lillerud, K.P.; Lamberti, C. Disclosing the Complex Structure of UiO-66 Metal Organic Framework: A Synergic Combination of Experiment and Theory. Chem. Mater. 2011, 23, 1700–1718. [Google Scholar] [CrossRef]
- Øien, S.; Wragg, D.; Reinsch, H.; Svelle, S.; Bordiga, S.; Lamberti, C.; Lillerud, K.P. Detailed Structure Analysis of Atomic Positions and Defects in Zirconium Metal–Organic Frameworks. Cryst. Growth Des. 2014, 14, 5370–5372. [Google Scholar] [CrossRef]
- Lennox, M.J.; Düren, T. Understanding the Kinetic and Thermodynamic Origins of Xylene Separation in UiO-66(Zr) via Molecular Simulation. J. Phys. Chem. C 2016, 120, 18651–18658. [Google Scholar] [CrossRef]
- Finsy, V.; Verelst, H.; Alaerts, L.; De Vos, D.; Jacobs, P.A.; Baron, G.V.; Denayer, J.F.M. Pore-Filling-Dependent Selectivity Effects in the Vapor-Phase Separation of Xylene Isomers on the Metal−Organic Framework MIL-47. J. Am. Chem. Soc. 2008, 130, 7110–7118. [Google Scholar] [CrossRef]
- Gu, Z.-Y.; Jiang, D.-Q.; Wang, H.-F.; Cui, X.-Y.; Yan, X.-P. Adsorption and Separation of Xylene Isomers and Ethylbenzene on Two Zn–Terephthalate Metal–Organic Frameworks. J. Phys. Chem. C 2010, 114, 311–316. [Google Scholar] [CrossRef]
- Rives, S.; Jobic, H.; Kolokolov, D.I.; Gabrienko, A.A.; Stepanov, A.G.; Ke, Y.; Frick, B.; Devic, T.; Férey, G.; Maurin, G. Diffusion of Xylene Isomers in the MIL-47(V) MOF Material: A Synergic Combination of Computational and Experimental Tools. J. Phys. Chem. C 2013, 117, 6293–6302. [Google Scholar] [CrossRef]
- Peralta, D.; Chaplais, G.; Paillaud, J.-L.; Simon-Masseron, A.; Barthelet, K.; Pirngruber, G.D. The separation of xylene isomers by ZIF-8: A demonstration of the extraordinary flexibility of the ZIF-8 framework. Microporous Mesoporous Mater. 2013, 173, 1–5. [Google Scholar] [CrossRef]
- Duerinck, T.; Bueno-Perez, R.; Vermoortele, F.; De Vos, D.E.; Calero, S.; Baron, G.V.; Denayer, J.F.M. Understanding Hydrocarbon Adsorption in the UiO-66 Metal–Organic Framework: Separation of (Un) saturated Linear, Branched, Cyclic Adsorbates, Including Stereoisomers. J. Phys. Chem. C 2013, 117, 12567–12578. [Google Scholar] [CrossRef]
- Ramsahye, N.A.; Gao, J.; Jobic, H.; Llewellyn, P.L.; Yang, Q.; Wiersum, A.D.; Koza, M.M.; Guillerm, V.; Serre, C.; Zhong, C.L.; et al. Adsorption and Diffusion of Light Hydrocarbons in UiO-66(Zr): A Combination of Experimental and Modeling Tools. J. Phys. Chem. C 2014, 118, 27470–27482. [Google Scholar] [CrossRef]
- Trens, P.; Belarbi, H.; Shepherd, C.; Gonzalez, P.; Ramsahye, N.A.; Lee, U.H.; Seo, Y.-K.; Chang, J.-S. Adsorption and separation of xylene isomers vapors onto the chromium terephthalate-based porous material MIL-101(Cr): An experimental and computational study. Microporous Mesoporous Mater. 2014, 183, 17–22. [Google Scholar] [CrossRef]
- Gonzalez, M.I.; Kapelewski, M.T.; Bloch, E.D.; Milner, P.J.; Reed, D.A.; Hudson, M.R.; Mason, J.A.; Barin, G.; Brown, C.M.; Long, J.R. Separation of Xylene Isomers through Multiple Metal Site Interactions in Metal–Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 3412–3422. [Google Scholar] [CrossRef]
- Grissom, T.G.; Sharp, C.H.; Usov, P.M.; Troya, D.; Morris, A.J.; Morris, J.R. Benzene, Toluene, and Xylene Transport through UiO-66: Diffusion Rates, Energetics, and the Role of Hydrogen Bonding. J. Phys. Chem. C 2018, 122, 16060–16069. [Google Scholar] [CrossRef]
- Driscoll, D.M.; Troya, D.; Usov, P.M.; Maynes, A.J.; Morris, A.J.; Morris, J.R. Characterization of Undercoordinated Zr Defect Sites in UiO-66 with Vibrational Spectroscopy of Adsorbed CO. J. Phys. Chem. C 2018, 122, 14582–14589. [Google Scholar] [CrossRef]
- Cui, W.-G.; Hu, T.-L.; Bu, X.-H. Metal–Organic Framework Materials for the Separation and Purification of Light Hydrocarbons. Adv. Mater. 2020, 32, 1806445. [Google Scholar] [CrossRef]
- Bárcia, P.S.; Guimarães, D.; Mendes, P.A.P.; Silva, J.A.C.; Guillerm, V.; Chevreau, H.; Serre, C.; Rodrigues, A.E. Reverse shape selectivity in the adsorption of hexane and xylene isomers in MOF UiO-66. Microporous Mesoporous Mater. 2011, 139, 67–73. [Google Scholar] [CrossRef]
- Chang, N.; Yan, X.-P. Exploring reverse shape selectivity and molecular sieving effect of metal–organic framework UIO-66 coated capillary column for gas chromatographic separation. J. Chromatogr. A 2012, 1257, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.A.; Martins, V.D.; Ferreira, A.F.P.; Rodrigues, A.E. Adsorption of xylene isomers in MOF UiO-66 by molecular simulation. Microporous Mesoporous Mater. 2014, 190, 165–170. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef] [PubMed]
- Shearer, G.C.; Chavan, S.; Bordiga, S.; Svelle, S.; Olsbye, U.; Lillerud, K.P. Defect Engineering: Tuning the Porosity and Composition of the Metal–Organic Framework UiO-66 via Modulated Synthesis. Chem. Mater. 2016, 28, 3749–3761. [Google Scholar] [CrossRef]
- Morris, W.; Wang, S.; Cho, D.; Auyeung, E.; Li, P.; Farha, O.K.; Mirkin, C.A. Role of Modulators in Controlling the Colloidal Stability and Polydispersity of the UiO-66 metal–Organic framework. ACS Appl. Mater. Interfaces 2017, 9, 33413–33418. [Google Scholar] [CrossRef]
- Atzori, C.; Shearer, G.C.; Maschio, L.; Civalleri, B.; Bonino, F.; Lamberti, C.; Svelle, S.; Lillerud, K.P.; Bordiga, S. Effect of Benzoic Acid as a Modulator in the Structure of UiO-66: An Experimental and Computational Study. J. Phys. Chem. C 2017, 121, 9312–9324. [Google Scholar] [CrossRef]
- Wu, H.; Chua, Y.S.; Krungleviciute, V.; Tyagi, M.; Chen, P.; Yildirim, T.; Zhou, W. Unusual and Highly Tunable Missing-Linker Defects in Zirconium Metal–Organic Framework UiO-66 and Their Important Effects on Gas Adsorption. J. Am. Chem. Soc. 2013, 135, 10525–10532. [Google Scholar] [CrossRef]
- Cliffe, M.J.; Wan, W.; Zou, X.; Chater, P.A.; Kleppe, A.K.; Tucker, M.G.; Wilhelm, H.; Funnell, N.P.; Coudert, F.-X.; Goodwin, A.L. Correlated defect nanoregions in a metal–organic framework. Nat. Commun. 2014, 5, 4176. [Google Scholar] [CrossRef]
- Shearer, G.C.; Chavan, S.; Ethiraj, J.; Vitillo, J.G.; Svelle, S.; Olsbye, U.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. Tuned to Perfection: Ironing Out the Defects in Metal–Organic Framework UiO-66. Chem. Mater. 2014, 26, 4068–4071. [Google Scholar] [CrossRef]
- Vandichel, M.; Hajek, J.; Vermoortele, F.; Waroquier, M.; De Vos, D.E.; Van Speybroeck, V. Active site engineering in UiO-66 type metal–organic frameworks by intentional creation of defects: A theoretical rationalization. CrystEngComm 2015, 17, 395–406. [Google Scholar] [CrossRef]
- Liang, W.; Coghlan, C.J.; Ragon, F.; Rubio-Martinez, M.; D’Alessandro, D.M.; Babarao, R. Defect engineering of UiO-66 for CO2 and H2O uptake–A combined experimental and simulation study. Dalton Trans. 2016, 45, 4496–4500. [Google Scholar] [CrossRef] [PubMed]
- Idrees, K.B.; Chen, Z.; Zhang, X.; Mian, M.R.; Drout, R.J.; Islamoglu, T.; Farha, O.K. Tailoring Pore Aperture and Structural Defects in Zirconium-Based Metal–Organic Frameworks for Krypton/Xenon Separation. Chem. Mater. 2020, 32, 3776–3782. [Google Scholar] [CrossRef]
- Winarta, J.; Shan, B.; McIntyre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A Decade of UiO-66 Research: A Historic Review of Dynamic Structure, Synthesis Mechanisms, and Characterization Techniques of an Archetypal Metal–Organic Framework. Cryst. Growth Des. 2020, 20, 1347–1362. [Google Scholar] [CrossRef]
- Virmani, E.; Rotter, J.M.; Mähringer, A.; von Zons, T.; Godt, A.; Bein, T.; Wuttke, S.; Medina, D.D. On-Surface Synthesis of Highly Oriented Thin Metal–Organic Framework Films through Vapor-Assisted Conversion. J. Am. Chem. Soc. 2018, 140, 4812–4819. [Google Scholar] [CrossRef]
- Semrau, A.L.; Wannapaiboon, S.; Pujari, S.P.; Vervoorts, P.; Albada, B.; Zuilhof, H.; Fischer, R.A. Highly Porous Nanocrystalline UiO-66 Thin Films via Coordination Modulation Controlled Step-by-Step Liquid-Phase Growth. Cryst. Growth Des. 2019, 19, 1738–1747. [Google Scholar] [CrossRef]
- Miyamoto, M.; Kohmura, S.; Iwatsuka, H.; Oumi, Y.; Uemiya, S. In situ solvothermal growth of highly oriented Zr-based metal organic framework UiO-66 film with monocrystalline layer. CrystEngComm 2015, 17, 3422–3425. [Google Scholar] [CrossRef]
- Hashem, T.; Valdez Sanchez, E.P.; Weidler, P.; Gliemann, H.; Alkordi, M.H.; Wöll, C. Liquid-Phase Quasi-Epitaxial Growth of Highly Stable, Monolithic UiO-66-NH2 MOF thin Films on Solid Surfaces. ChemistryOpen 2020, 9, 524–527. [Google Scholar] [CrossRef]
- Cmarik, G.E.; Kim, M.; Cohen, S.M.; Walton, K.S. Tuning the Adsorption Properties of UiO-66 via Ligand Functionalization. Langmuir 2012, 28, 15606–15613. [Google Scholar] [CrossRef]
- Jasuja, H.; Peterson, G.W.; Decoste, J.B.; Browe, M.A.; Walton, K.S. Evaluation of MOFs for air purification and air quality control applications: Ammonia removal from air. Chem. Eng. Sci. 2015, 124, 118–124. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Browe, M.A.; Wagner, G.W.; Rossin, J.A.; Peterson, G.W. Removal of chlorine gas by an amine functionalized metal–organic framework via electrophilic aromatic substitution. Chem. Commun. 2015, 51, 12474–12477. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.W.; DeCoste, J.B.; Fatollahi-Fard, F.; Britt, D.K. Engineering UiO-66-NH2 for Toxic Gas Removal. Ind. Eng. Chem. Res. 2014, 53, 701–707. [Google Scholar] [CrossRef]
- Rada, Z.H.; Abid, H.R.; Sun, H.; Shang, J.; Li, J.; He, Y.; Liu, S.; Wang, S. Effects of –NO2 and –NH2 functional groups in mixed-linker Zr-based MOFs on gas adsorption of CO2 and CH4. Prog. Nat. Sci. Mater. Int. 2018, 28, 160–167. [Google Scholar] [CrossRef]
- Vellingiri, K.; Kumar, P.; Deep, A.; Kim, K.-H. Metal–organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure. Chem. Eng. J. 2017, 307, 1116–1126. [Google Scholar] [CrossRef]
- Demir, H.; Walton, K.S.; Sholl, D.S. Computational Screening of Functionalized UiO-66 Materials for Selective Contaminant Removal from Air. J. Phys. Chem. C 2017, 121, 20396–20406. [Google Scholar] [CrossRef]
- Akpinar, I.; Drout, R.J.; Isamoglu, T.; Kato, S.; Lyu, J.; Farha, O.K. Exploiting π–π interactions to design an efficient sorbent for atrazine removal from water. ACS Appl. Mater. Interfaces 2019, 11, 6097–6103. [Google Scholar] [CrossRef]





| Name | Defect Free | 1 Missing Linker | 2 Missing Linker | 3 Missing Linker | 4 Missing Linker | Adsorption Capacity at 20 °C |
|---|---|---|---|---|---|---|
| UiO-66-NH2 | ||||||
| Benzene | 13.50% | 15.00% | 16.60% | 18.20% | 20.00% | 18.10% |
| Toluene | 15.90% | 17.70% | 19.60% | 21.50% | 23.60% | 23.20% |
| Ethyl benzene | 18.40% | 20.40% | 22.60% | 24.80% | 27.20% | 21.10% |
| p-xylene | 18.40% | 20.40% | 22.60% | 24.80% | 27.20% | 19.20% |
| m-xylene | 18.40% | 20.40% | 22.60% | 24.80% | 27.20% | 18.10% |
| o-xylene | 18.40% | 20.40% | 22.60% | 24.80% | 27.20% | 22.00% |
| UiO-66-NO2 | ||||||
| Benzene | 12.20% | 13.60% | 15.10% | 16.60% | 18.30% | 21.60% |
| Toluene | 14.40% | 16.10% | 17.80% | 19.60% | 21.60% | 21.80% |
| Ethyl benzene | 16.60% | 18.50% | 20.50% | 22.60% | 24.80% | 23.60% |
| p-xylene | 16.60% | 18.50% | 20.50% | 22.60% | 24.80% | 20.80% |
| m-xylene | 16.60% | 18.50% | 20.50% | 22.60% | 24.80% | 24.00% |
| o-xylene | 16.60% | 18.50% | 20.50% | 22.60% | 24.80% | 22.30% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shankwitz, J.; Speed, D.; Sinanan, D.; Szulczewski, G. The Effects of Functional Groups and Missing Linkers on the Adsorption Capacity of Aromatic Hydrocarbons in UiO-66 Thin Films. Inorganics 2021, 9, 1. https://doi.org/10.3390/inorganics9010001
Shankwitz J, Speed D, Sinanan D, Szulczewski G. The Effects of Functional Groups and Missing Linkers on the Adsorption Capacity of Aromatic Hydrocarbons in UiO-66 Thin Films. Inorganics. 2021; 9(1):1. https://doi.org/10.3390/inorganics9010001
Chicago/Turabian StyleShankwitz, Jennifer, Daniel Speed, Dillon Sinanan, and Greg Szulczewski. 2021. "The Effects of Functional Groups and Missing Linkers on the Adsorption Capacity of Aromatic Hydrocarbons in UiO-66 Thin Films" Inorganics 9, no. 1: 1. https://doi.org/10.3390/inorganics9010001
APA StyleShankwitz, J., Speed, D., Sinanan, D., & Szulczewski, G. (2021). The Effects of Functional Groups and Missing Linkers on the Adsorption Capacity of Aromatic Hydrocarbons in UiO-66 Thin Films. Inorganics, 9(1), 1. https://doi.org/10.3390/inorganics9010001