Abstract
Liquid crystals are among us, in living organisms and in electronic devices, and they have contributed to the development of our modern society. Traditionally developed by organic chemists, the field of liquid-crystalline materials is now involving chemists and physicists of all domains (computational, physical, inorganic, supramolecular, electro-chemistry, polymers, materials, etc.,). Such diversity in researchers confirms that the field remains highly active and that new applications can be foreseen in the future. In this review, liquid-crystalline materials developed around coordination complexes are presented, focusing on those showing thermotropic behavior, a relatively unexplored family of compounds.
1. Introduction
Liquid-crystals are known for over a century, and despite being part of our everyday life, basic research on liquid-crystalline materials remains highly attractive [1]. Indeed, new applications are emerging, in which the particular properties of liquid-crystals are exploited [2,3]. Accordingly, liquid crystals incorporating metal-based entities are showing great promises [4,5,6,7,8,9,10]. The introduction of additional intermolecular interactions, such as metal–metal or metal–ligand, into the supramolecular structure of the liquid-crystalline material, can modulate the properties, and ultimately, provide new opportunities.
Liquid-crystalline materials are classified into thermotropes and lyotropes [11]. In thermotropic liquid crystals, the mesophases are induced by the temperature, while in lyotropic liquid-crystals the mesophases are controlled by the concentrations of the different components in a single or mixture of solvents. Liquid-crystalline materials found in nature, such as lipids and membranes, are lyotropes. On the other hand, those involved in electronic devices are more likely to be thermotropes. In the case of metal-based liquid-crystalline materials, both types can be found in the literature [4,5,6,7,8,9,10], and some can even be amphotropes [11].
Among metal-based liquid-crystalline materials, those exploiting supramolecular coordination complexes have not yet found a commercial application, however, they are very interesting and they offer great potentials in biology [12,13,14,15,16] and material sciences [17,18,19]. Moreover, and despite having well-established synthetic strategies, the number of papers dealing with supramolecular coordination complexes as liquid-crystalline promoters remains low. This might be associated to the complexity of such systems, in which additional interactions are involved, thus increasing the difficulty of predicting and interpreting the corresponding mesophases. Nevertheless, owing to the development of our understanding on the molecular organization within mesophases, based on experiences as well as on new computational models, we should see more of these hybrid supramolecular liquid-crystalline materials in the future.
2. Lyotropic Liquid-Crystalline Self-Assembled Coordination Complexes
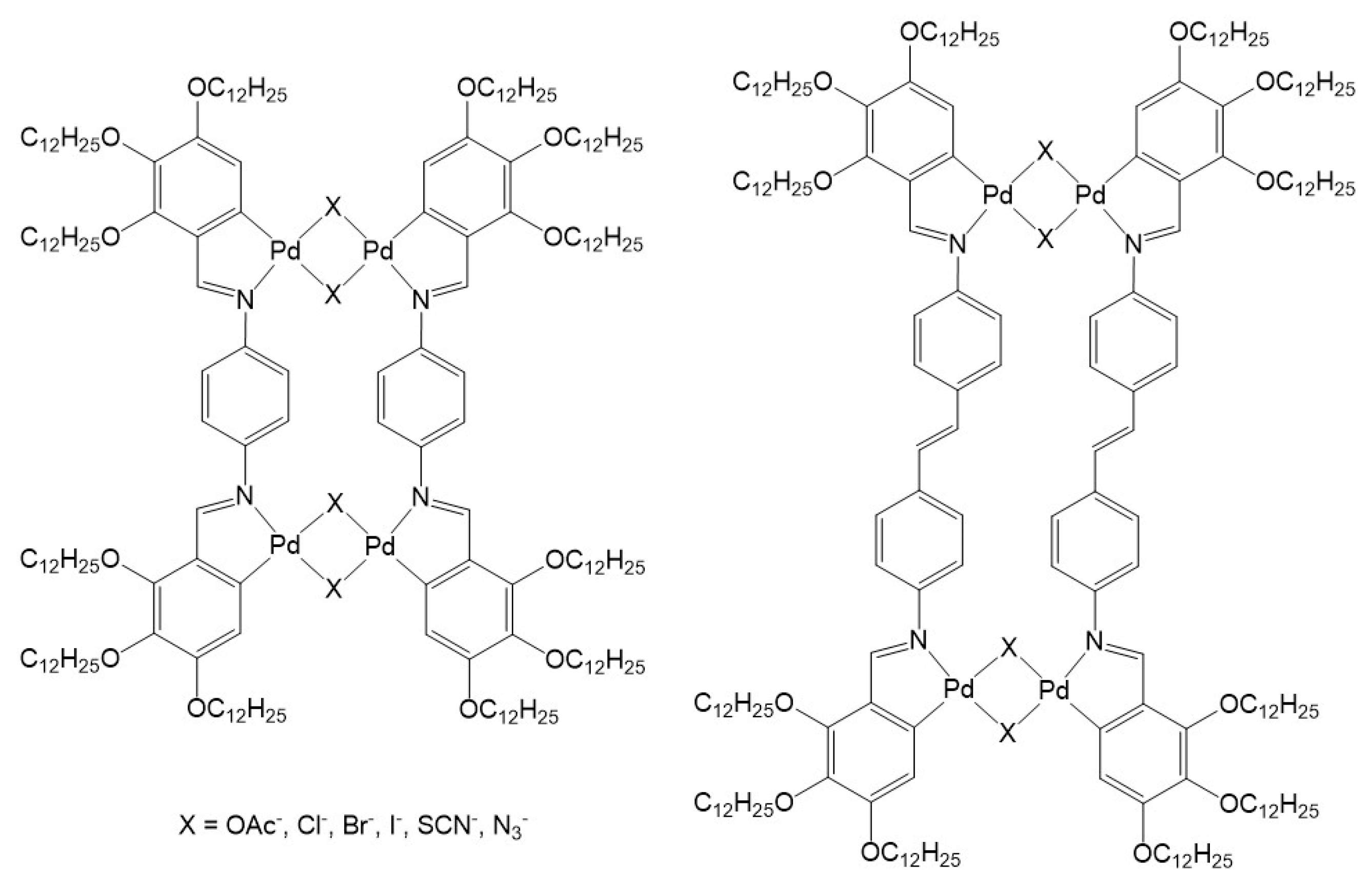
Incorporation of supramolecular coordination complexes in liquid-crystalline materials was first explored by Praefcke and Usol’tseva in the early 1990s. The lyomesomorphic behavior of large metalla-assemblies were showing nematic phases in alkanes [20]. In the case of the chloro-and bromo-bridged metalla-cycles (Figure 1), the nematic phase was stabilized upon addition of 2,4,7-trinitrofluorenone (TNF), suggesting intercalation of TNF between the disk-shaped columnar stacks. Following this initial study, analogous systems built from either palladium or platinum metal centers were prepared and their liquid-crystalline properties examined [21,22,23,24,25], showing similar organization in the lyotropic mesophases.
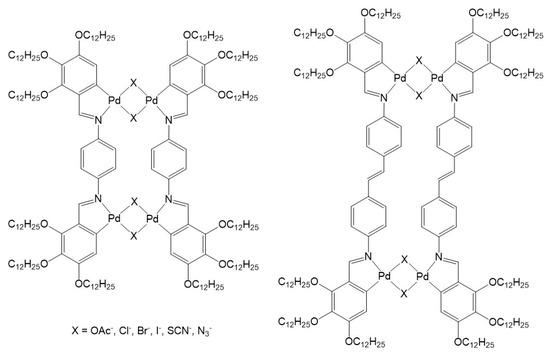
Figure 1.
Early supramolecular coordination complexes showing liquid-crystalline properties [20].
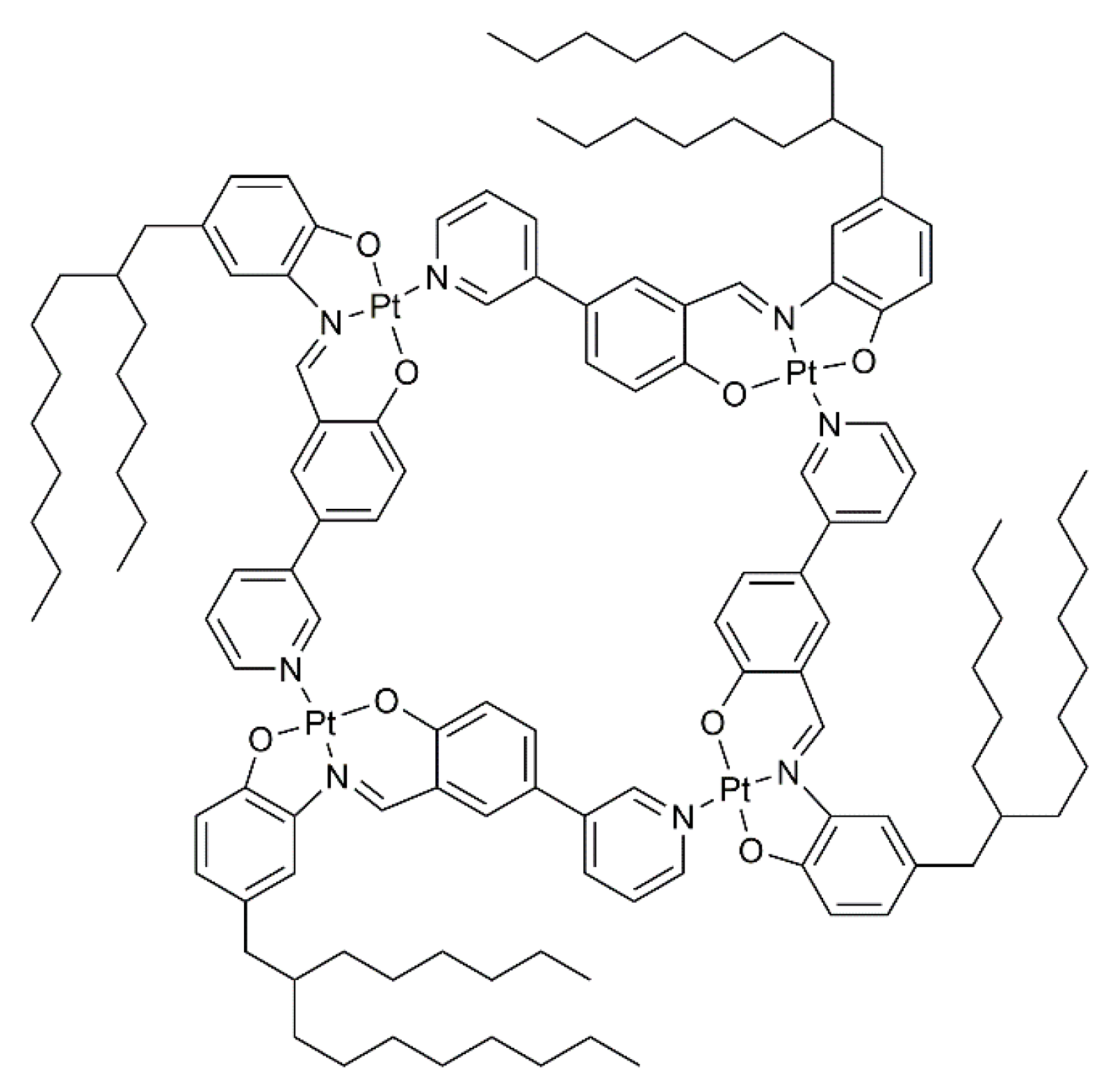
Later on, analogous platinum Schiff base pyridyl type metalla-cycles were synthesized by MacLachlan and his coworkers [26]. In the series, the 2-hexyldecyl derivative (Figure 2) showed the most interesting liquid-crystalline properties. In non-polar organic solvents, lyotropic mesophases were observed for the tetranuclear metalla-cycle. As demonstrated by the authors, a supramolecular aggregation of individual Pt-based metalla-cycle into columnar arrays was responsible for the liquid-crystalline properties.
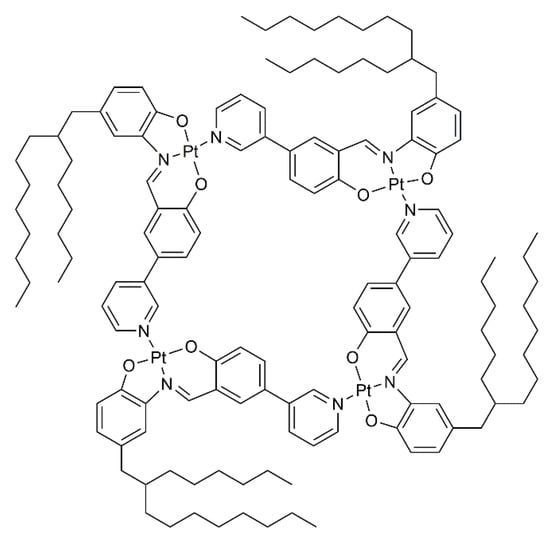
Figure 2.
A tetranuclear metalla-cycle with lyotropic liquid-crystalline behavior [26].
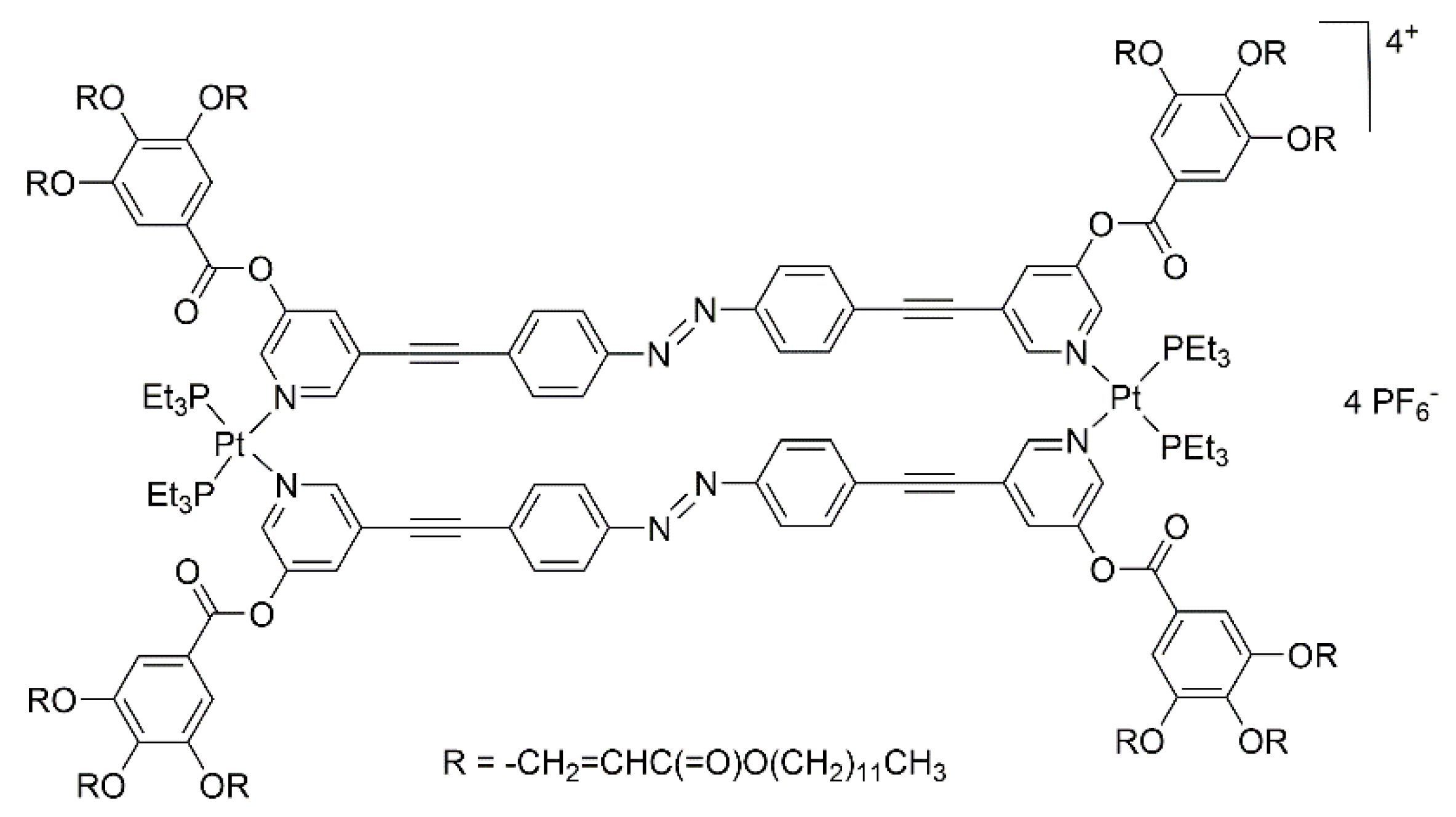
In a similar manner, Gin and co-workers have prepared dinuclear-Pt mesogenic metalla-cycles incorporating bis-pyridyl linkers [27]. The hexafluorophosphate salt (Figure 3) possesses a thermotropic columnar hexagonal (ColH) liquid-crystalline phase, which can be swollen by polar solvents, to generate a lyotropic liquid-crystalline phase. Upon irradiation, conversion of the azo group to the cis isomer was observed, thus provoking a disorder in the liquid-crystalline phase. Photo-polymerization of neighboring metalla-cycles increases the stability of the mesophases, with however, a similar rate of conversion of the azo groups.
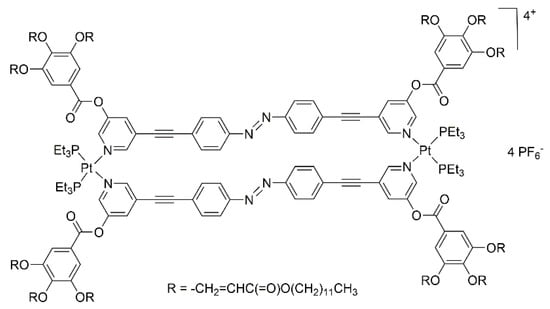
Figure 3.
A dinuclear platinum-based metalla-cycle with ColH liquid-crystalline properties in polar solvents [27].
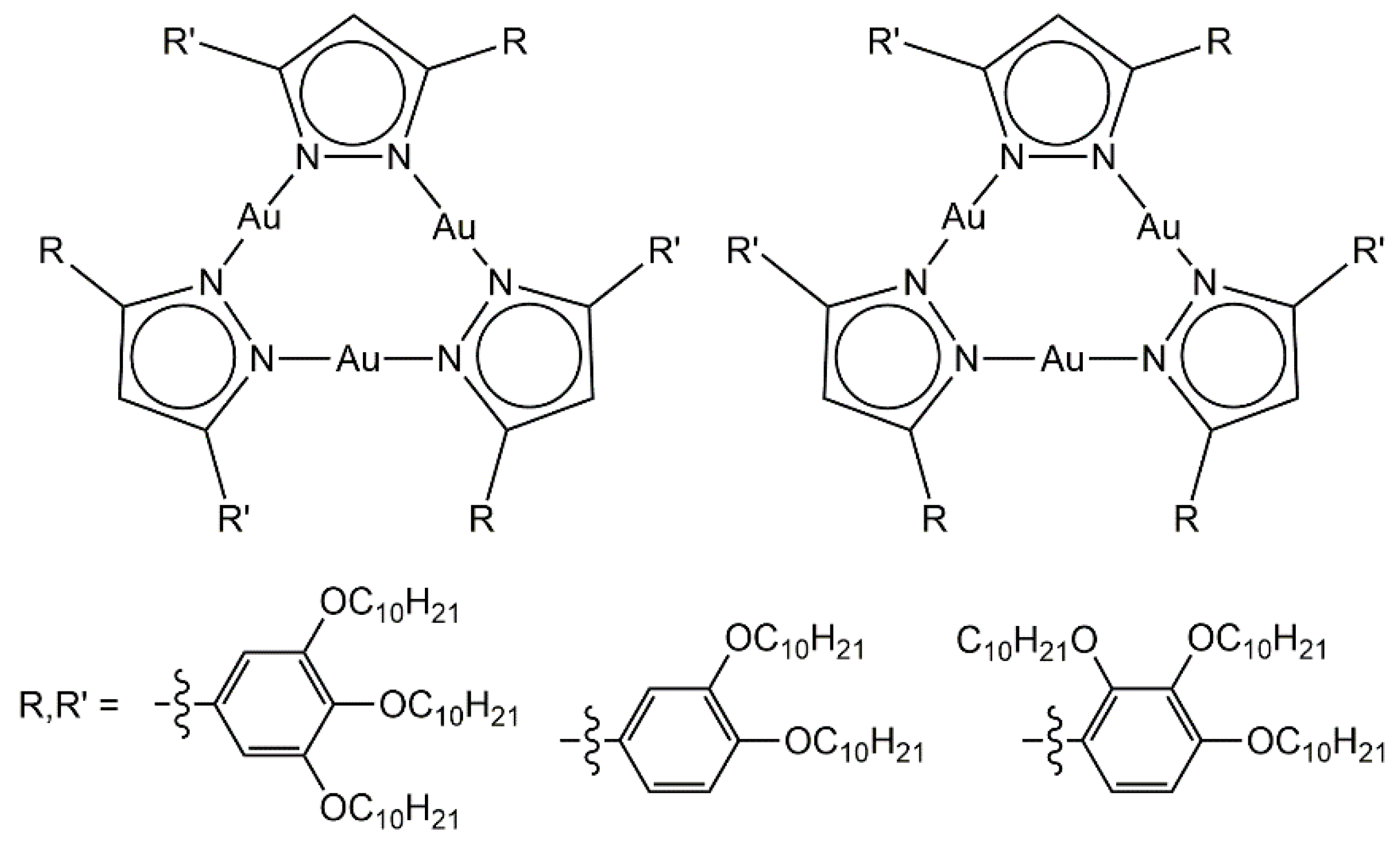
Trinuclear gold metalla-cycles have been used to generate columnar liquid-crystalline materials [28]. The presence of six alkyl chains at the periphery of the pyrazolato-gold complex (Figure 4) controls the formation of columnar stacks in the solid and liquid-crystalline states; Both showing an hexagonal symmetry. Interestingly, the functional groups (R, R’) can be equivalent or not, thus allowing the formation of two isomers. The proportion and nature of the two isomers influence the transition temperatures, and accordingly, the arrangement of the trinuclear assemblies in the lyotropic mesophases.
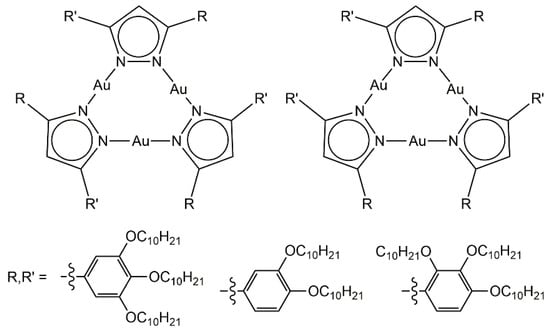
Figure 4.
Trinuclear pyrazolato-gold metalla-cycles with columnar arrangement [28].
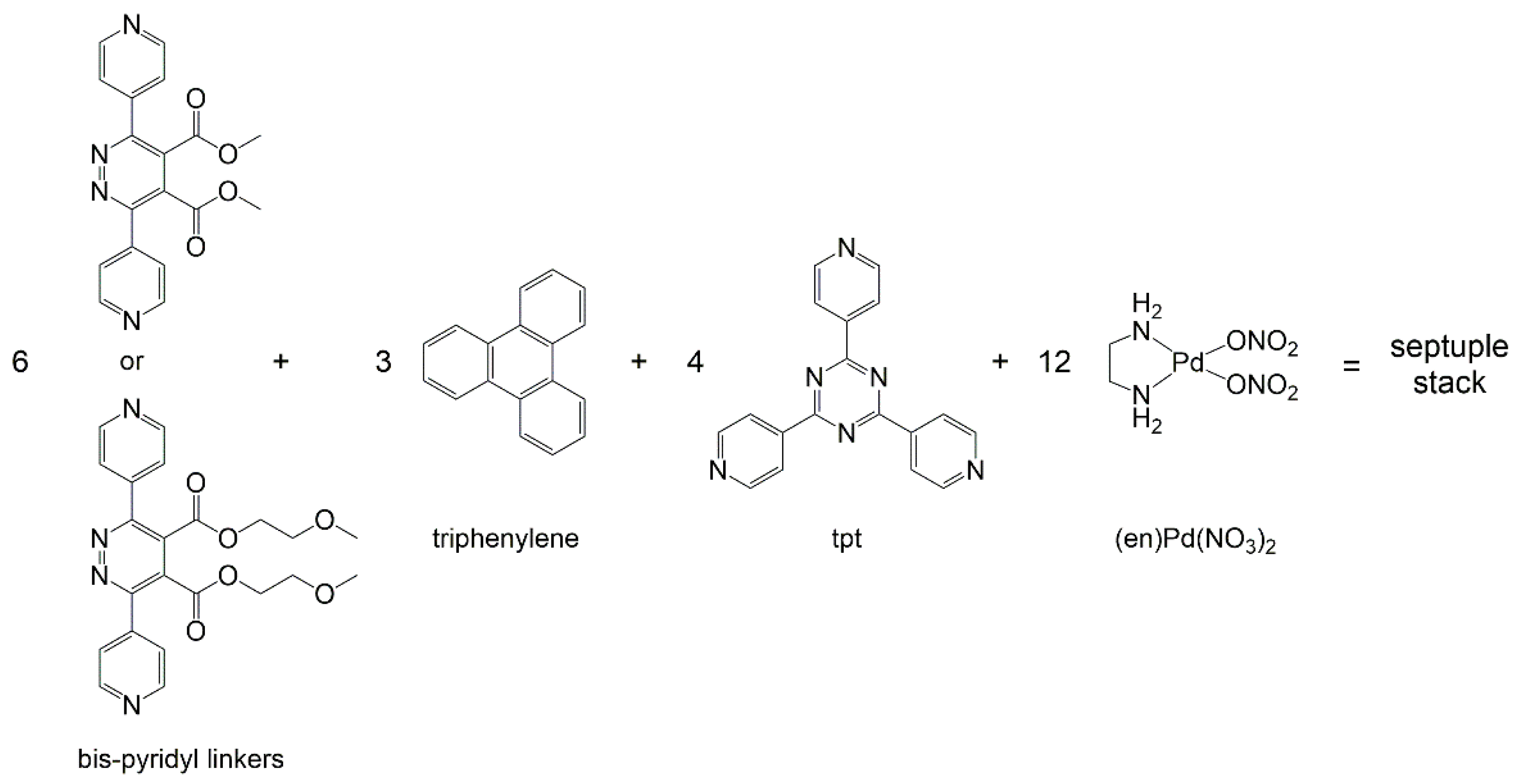
Addition of water to discrete septuple columnar stacks can induce lyotropic liquid-crystalline mesophases [29]. The columnar stacks were synthesized in solution by the self-assembly of a bis-pyridyl linker, tris(4-pyridyl)-2,4,6-triazine (tpt), triphenylene, and the palladium complex (en)Pd(NO3)2 (en = ethylenediamine), see Scheme 1. Assemblies composed of six bis-pyridyl linkers, four tpt panels, twelve (en)Pd corners, and three triphenylene intercalated guests, were isolated. The presence of water-soluble side chains on the bis-pyridyl linkers was crucial for the generation of the mesophases and for keeping fluidity to the supramolecular system.
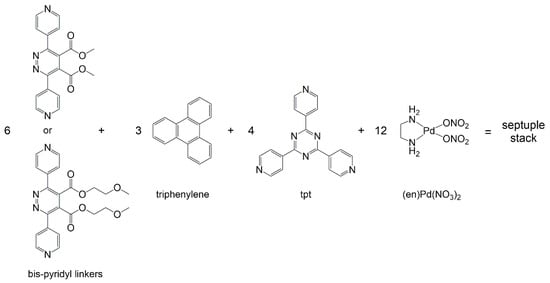
Scheme 1.
Building-blocks used to synthesize septuple columnar stacks in solution [29].
3. Thermotropic Liquid-Crystalline Self-Assembled Coordination Complexes
Metal-based clusters have been used as central cores to generate mesomorphic materials. Functionalization of the peripheral ligands has allowed a bottom-up approach to prepare liquid-crystalline materials. The choice of the cluster dictates the type of the functionalized ligands to be used. For the manganese cluster, [Mn12O12(RCO2)16(H2O)4], functionalized carboxylic acid derivatives are needed (Figure 5A). The Mn12O12 clusters show mesophases with cubic or smectic phases. The magnetic properties of the cluster are retained in the mesophases, thus providing magnetic liquid-crystalline materials [30]. Similarly, to insert mesogenic arms to sawhorse-type dinuclear ruthenium complexes, carboxylic acid derivatives are needed (Figure 5B). In these systems, the cyanobiphenyl-based poly(arylester) dendron was used to induce mesomorphic properties [31]. Smectic A and nematic phases were observed, according to the generation of the dendrimers.
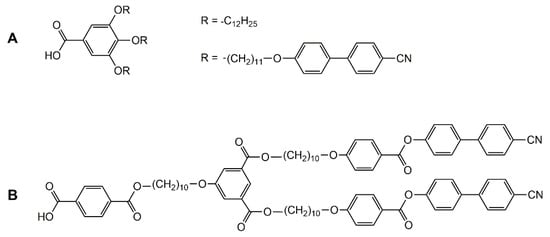
Figure 5.
Functionalized carboxylic acid derivatives grafted to manganese (A) and ruthenium (B) metallic-cores [30,31].
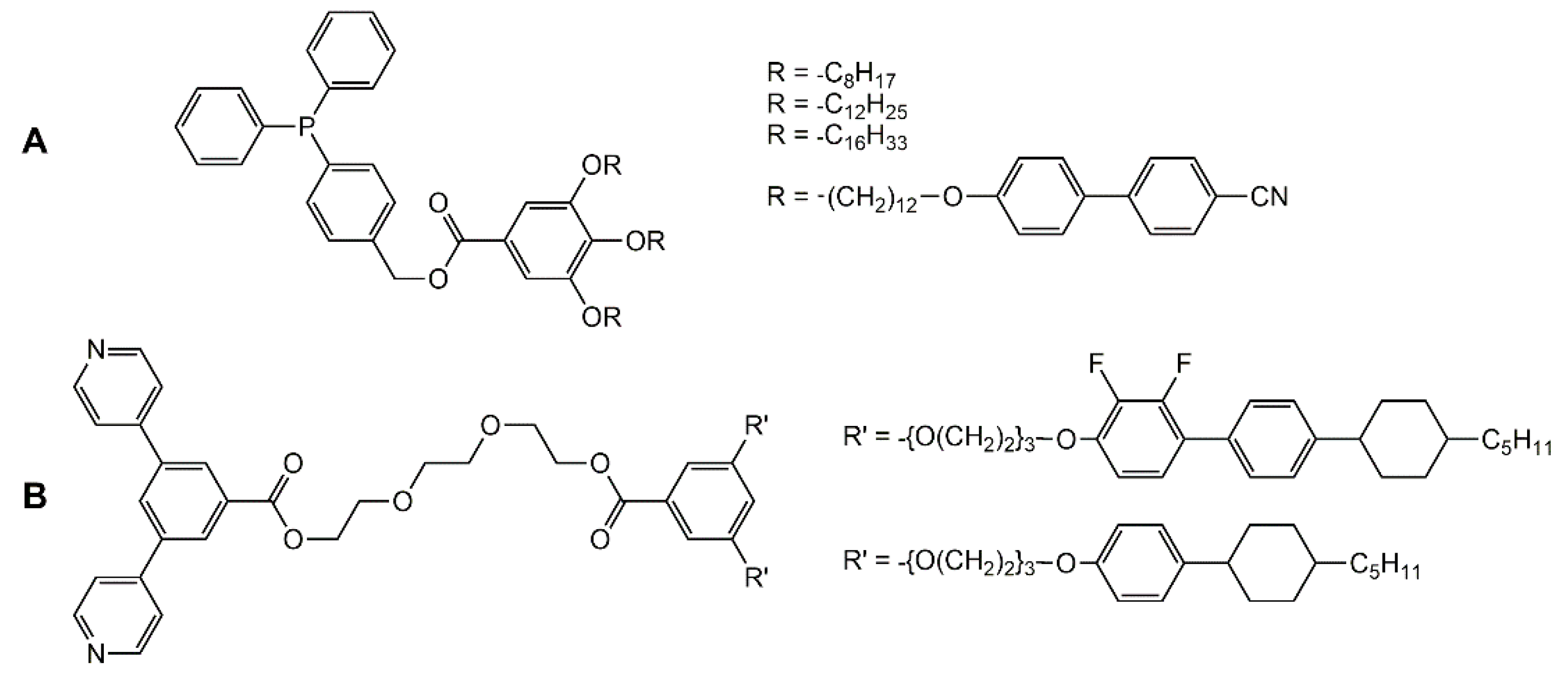
In the case of the cupper cluster [Cu4I4], diphenyl-phosphine ligands (Figure 6A) were linked to the cubic tetrametallic core, thus introducing four mesogenic side-chains [32]. The supramolecular system shows a smectic A phase from room temperature to 100 °C. In the case of the spherical Pd12L24 framework (L = bis-pyridyl ligand), functionalized bis-pyridyl connectors (Figure 6B) were used to ensure stability of the Pd12 core, and to insert mesogenic arms [33]. Interestingly, the free ligands show thermotropic behavior, while the cluster-based systems have lyotropic properties.
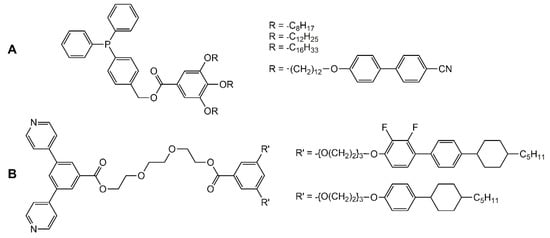
Figure 6.
Functionalized diphenyl-phosphine (A) and bis-pyridyl (B) ligands coordinated to clusters [32,33].
Rectangular columnar arrangement has been observed for nickel, palladium, and copper tetranuclear metalla-cycles (Figure 7). In these systems, a large inner cavity (≈9 Å in diameter) is observed in the metalla-cycle [34]. The isotropic temperature of the metalla-cycles was higher than the metal free cycle, and well below the temperature of decomposition. The nature of the peripheral chains and the choice of the metal influence the thermotropic behavior [35]. Moreover, the cavity can be exploited to accommodate guest molecules, and accordingly, to offer another alternative for fine tuning the liquid-crystalline properties.
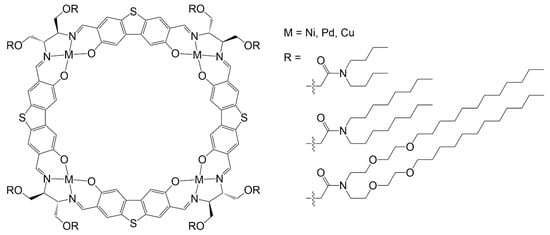
Figure 7.
Rectangular columnar systems with thermotropic liquid-crystalline properties [34].
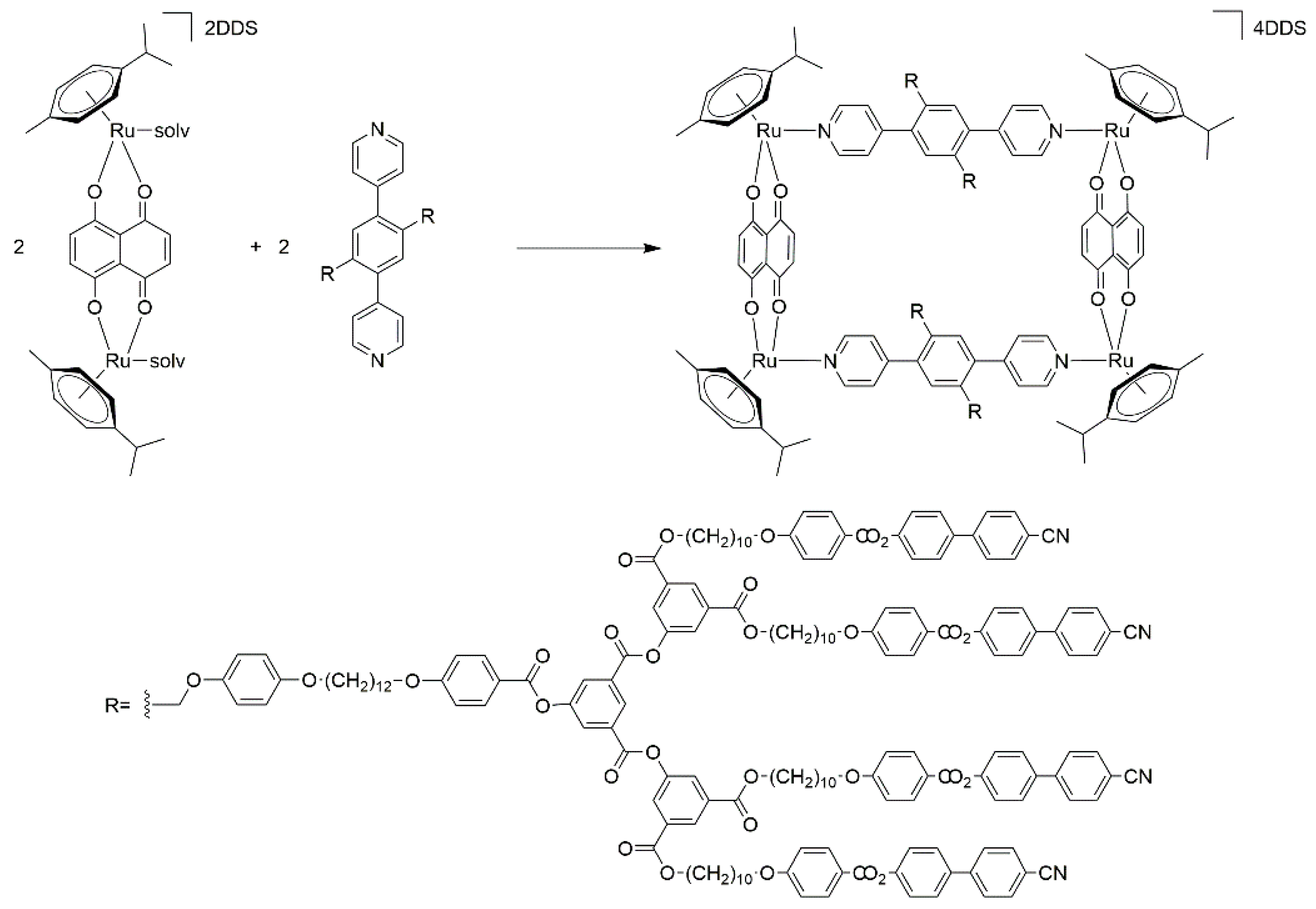
Bis-pyridyl linkers can also be used to assemble arene-ruthenium metalla-rectangles [36]. Addition of 1,4-di(4-pyridinyl)-benzene poly(arylester) derivative to the dinuclear arene-ruthenium complex [Ru2(p-cymene)2(donq)][DDS]2 (donq = dihydroxynaphthoquinone, DDS = dodecyl sulfate) generates a tetranuclear metalla-cycle, isolated as its DDS salt (Scheme 2). The presence of four dendritic arms bearing cyano-biphenyl end-groups ensures mesomorphic properties above 50 °C. Both compounds, the bis-pyridyl linker and the metalla-cycle, possess smectic phases with a multilayered organization [37].
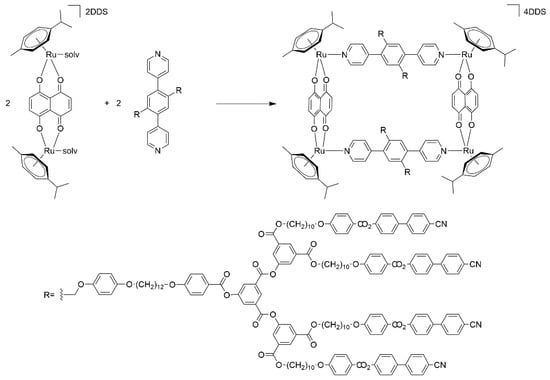
Scheme 2.
Synthesis of an arene-ruthenium metalla-cycle from a functionalized dendritic bis-pyridyl linker [37].
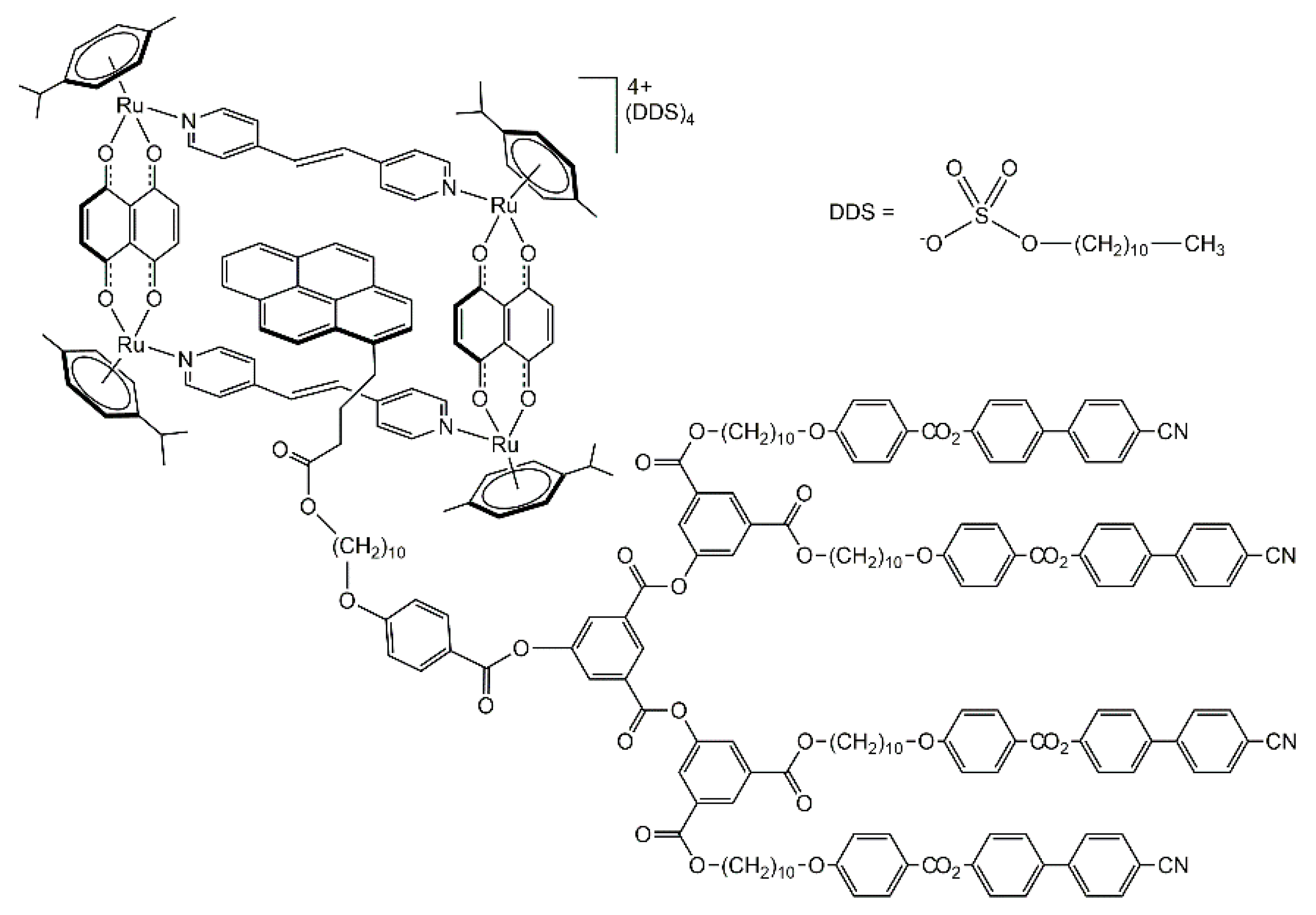
The host-guest chemistry of arene-ruthenium metalla-assemblies is well-established [38,39], and arene-ruthenium metalla-prisms and metalla-rectangles have been used to encapsulate pyrenyl-functionalized guests [40]. In the case of the pyrenyl-functionalized dendromesogenic guest encapsulated in a tetranuclear arene-ruthenium metalla-cycle (Figure 8), thermotropic liquid-crystalline properties were observed [41]. The guest alone is showing a smectic A phase, while the host-guest system possesses a cubic phase. The multi-component arrangement is highly segregated, suggesting a multi-layered structure involving metalla-cycles, dodecyl sulfates, and the side-arms of the pyrenyl-functionalized dendrons.
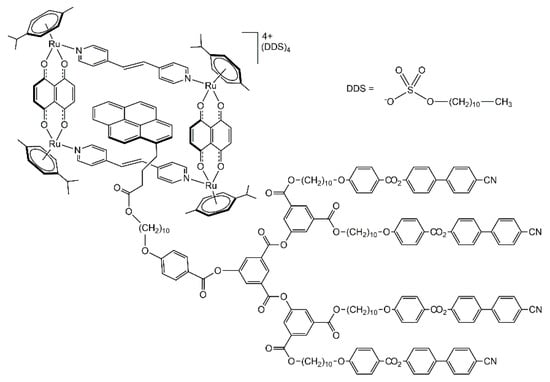
Figure 8.
Pyrenyl-functionalized dendrimer encapsulated in an arene ruthenium metalla-cycle [41].
4. Suppression of Liquid-Crystalline Properties by Self-Assembled Coordination Complexes
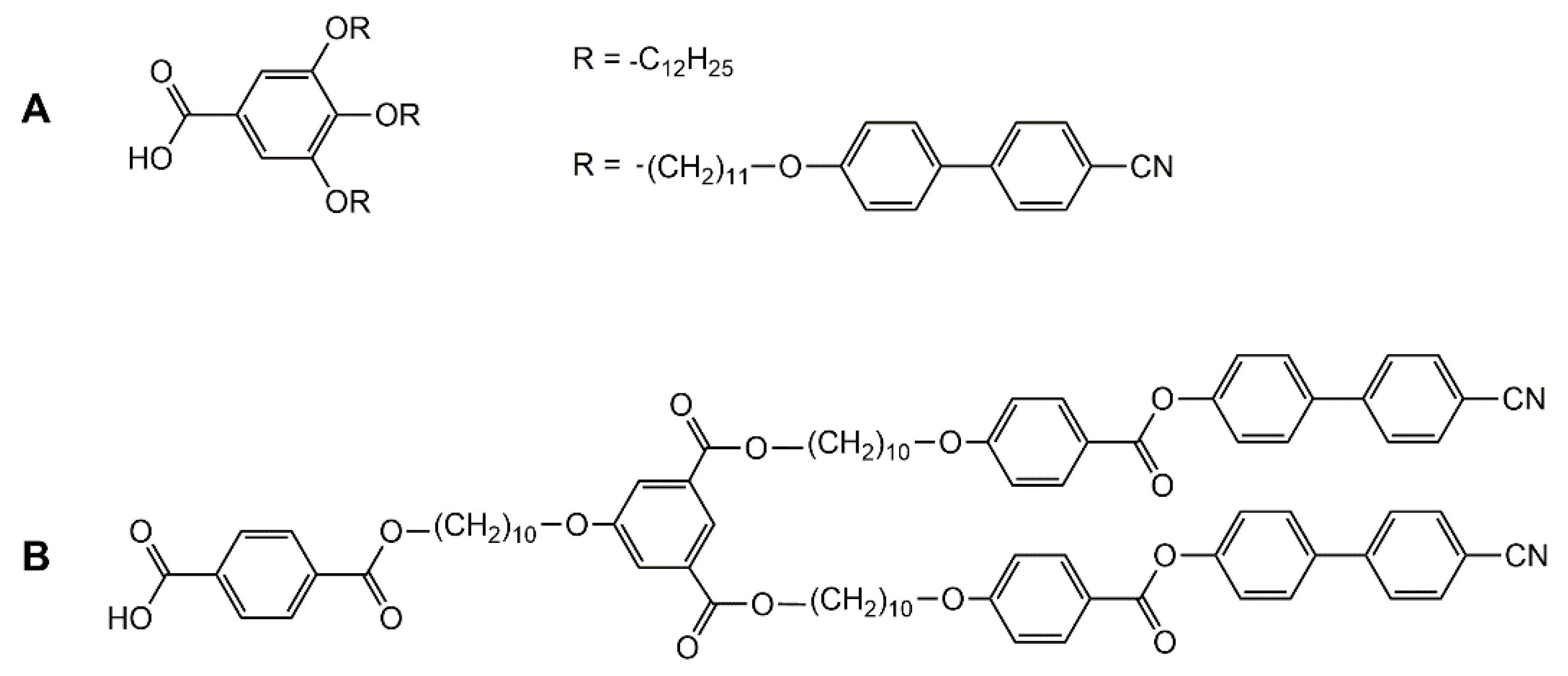
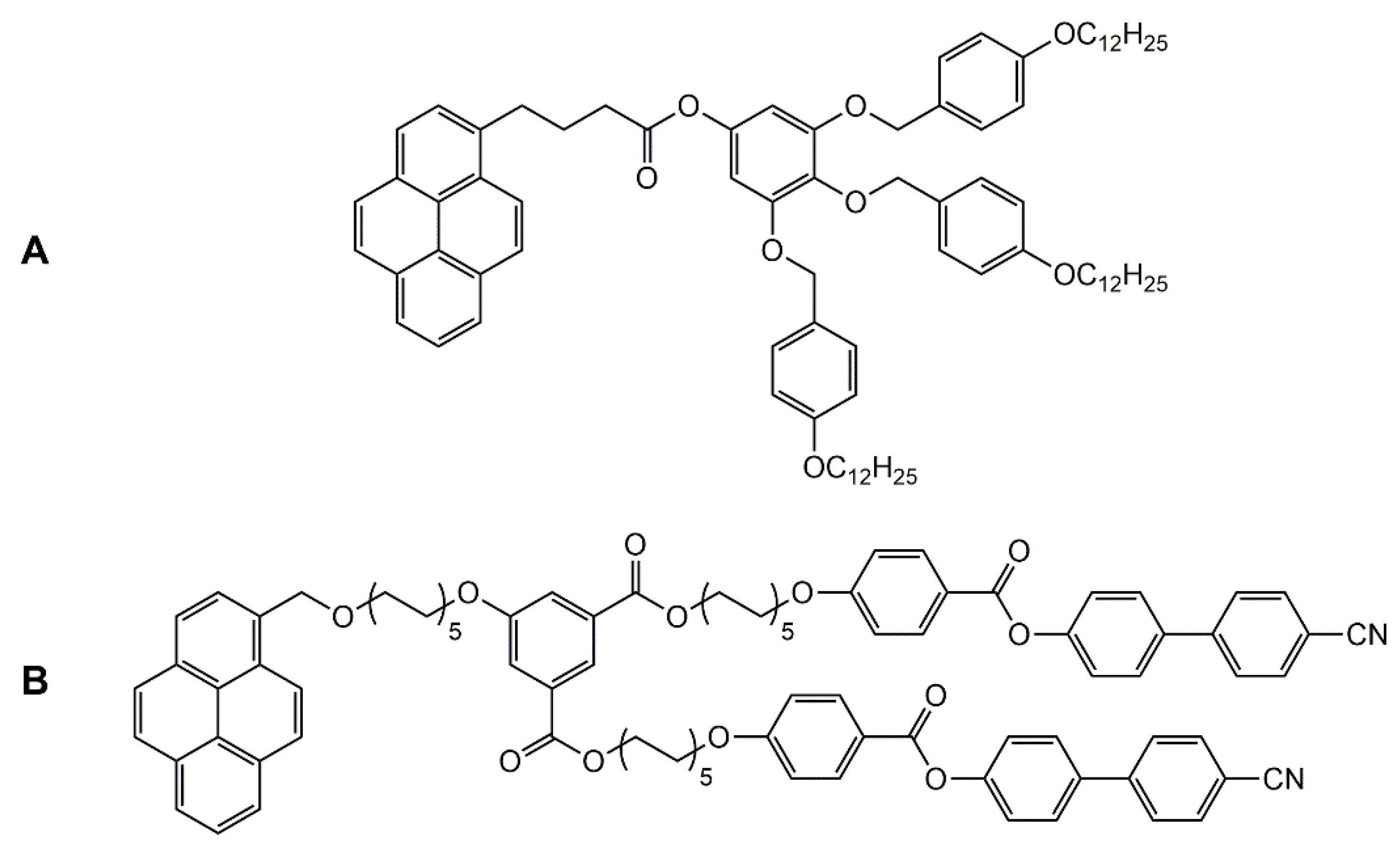
Encapsulation of guest molecules in metalla-assemblies offers great potentials in molecular recognition [42], transport [43], and protection of guest molecules [44], as well as in cavity-controlled reaction (molecular flask) [45]. Moreover, the presence of guest molecules in the cavity of a supramolecular coordination complex can modify its geometry and properties. Accordingly, liquid-crystalline behavior can be modulated upon host–guest interactions. Indeed, encapsulation of pyrenyl-functionalized dendrimers in the cavity of a hexanuclear arene ruthenium metalla-prism has showed the suppression of the liquid-crystalline properties of the organic compound (guest) [46]. The pyrenyl-functionalized poly (arylester) dendron (Figure 9A) shows an unidentified liquid-crystalline behavior, and no cytotoxicity to cancer cells (A2780 and A2780cisR). On the other hand, the host–guest system is cytotoxic, with IC50 < 3 μM on these two cancer cell lines, with however, no liquid-crystalline behavior.
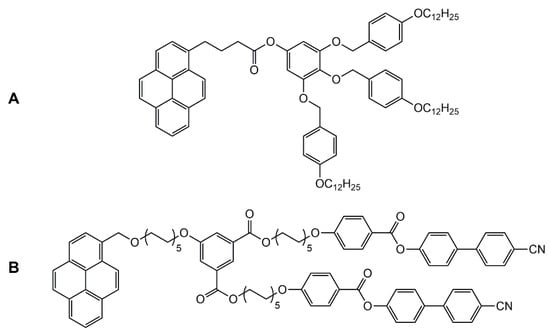
Figure 9.
Two pyrenyl-functionalized liquid-crystalline compounds (A,B) showing suppressed liquid-crystalline properties after encapsulation in a coordination complex [46,47].
Similarly, a porphyrin-based tetragonal prism has been used to encapsulate a pyrenyl-functionalized guest compound [47]. The pyrenyl derivative (Figure 9B) possesses between 23 and 107 °C a smectic A phase. Upon addition of the platinum-based tetragonal prismatic host, the mesomorphic properties of the guest are lost. However, adding coronene or pyrene as competing guests, re-established the liquid-crystalline properties of the pyrenyl-functionalized compound, thus offering a switch-on switch-off control over the liquid-crystalline properties.
5. Conclusions
The field of liquid-crystalline materials is shifting toward more complexed systems, in which multiple components are used to generate highly organized supramolecular arrangements. These sophisticated materials are expected to have an impact in various fields, such as in engineering (molecular electronics, photonics, high mechanical strength fibers, light modulators, lasers), energy (battery electrolytes), healthcare (artificial membranes, drug delivery, gene therapy), environment (biocompatible plastics), chemistry (surfactants, detergents, elastomers, gels), separation technology (sensors), informatics (intelligent switches), catalysis, and others [1,2,3,4,48,49,50,51,52,53,54,55,56,57,58,59].
A pillar of supramolecular chemistry is coordination chemistry [60], and therefore, it is not surprising to see more and more supramolecular coordination complexes being incorporated within liquid-crystalline materials. However, as illustrated in this short review, examples dealing with thermotropic liquid-crystalline coordination complexes remain scarce. Nevertheless, when considering the number of active groups in the field of coordination-driven self-assemblies [61,62,63,64], and the exciting applications that can be foreseen for the next generation of liquid-crystalline materials, this trend should be reversed in a few years.
Funding
B.T. thanks the Swiss National Science Foundation (grant No 200021-162361) for financial support.
Conflicts of Interest
The author declares no conflict of interest.
References
- Kato, T.; Uchida, J.; Ichikawa, T.; Sakamoto, T. Functional liquid crystals towards the next generation of materials. Angew. Chem. Int. Ed. 2018, 57, 4355–4371. [Google Scholar] [CrossRef] [PubMed]
- Khokhlov, A.R.; Emelyanenko, A.V. Nanostructures liquid crystal systems and applications. Beilstein J. Nanotechnol. 2018, 9, 2644–2645. [Google Scholar] [CrossRef] [PubMed]
- Goodby, J.W. Editorial – liquid crystals. Chem. Soc. Rev. 2007, 36, 1855–1856. [Google Scholar] [CrossRef] [PubMed]
- Donnio, B.; Guillon, D.; Bruce, D.W.; Deschenaux, R. Comprehensive Coordination Chemistry II: From Biology to Nanotechnology; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier: Oxford, UK, 2003; Volume 7, pp. 357–627. [Google Scholar]
- Amijs, C.H.M.; van Klink, G.P.M.; van Koten, G. Metallasupramolecular architectures, an overview of functional properties and applications. Dalton Trans. 2006, 2006, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Saalfrank, R.W.; Maid, H.; Scheurer, A. Supramolecular coordination chemistry: The synergistic effect of serendipity and rational design. Angew. Chem. Int. Ed. 2008, 47, 8794–8824. [Google Scholar] [CrossRef] [PubMed]
- Zangrando, E.; Casanova, M.; Alessio, E. Trinuclear metallacycles: Metallatriangles and much more. Chem. Rev. 2008, 108, 4979–5013. [Google Scholar] [CrossRef]
- Pucci, D.; Donnio, B. Metal-containing liquid crystals. In Handbook of Liquid Crystals; Non-Conventional, Supramolecular, Chromonic and Amphiphilic Liquid Crystals; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H., Raynes, P., Eds.; Wiley-VCH: Weinheim, Germany, 2014; Volume 5. [Google Scholar]
- Dzhardimalieva, G.I.; Uflyand, I.E. Metal chelate monomers as precursors of polymeric materials. J. Inorg. Organomet. Chem. 2016, 26, 1112–1173. [Google Scholar] [CrossRef]
- Uchida, J.; Kato, T. Liquid-crystalline fork-like dendrons. Liq. Cryst. 2017, 44, 1816–1829. [Google Scholar] [CrossRef]
- Tschierske, C. Amphotropic liquid crystals. Curr. Opin. Colloid Interface Sci. 2002, 7, 355–370. [Google Scholar] [CrossRef]
- Goodby, J.W. Liquid crystals and life. Liq. Cryst. 1998, 24, 25–38. [Google Scholar] [CrossRef]
- Stewart, G.T. Liquid crystals in biology I. Historical, biological and medical aspects. Liq. Cryst. 2003, 30, 541–557. [Google Scholar] [CrossRef]
- Stewart, G.T. Liquid crystals in biology II. Origins and processes of life. Liq. Cryst. 2004, 31, 443–471. [Google Scholar] [CrossRef]
- Woltman, S.J.; Jay, G.D.; Crawford, G.P. Liquid-crystal materials find a new order in biomedical applications. Nat. Mater. 2007, 6, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Hirst, L.S.; Charras, G. Liquid crystals in living tissue. Nature 2017, 544, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, Y.; Yang, K.; Wei, J.; Guo, J. Stabilization and optical switching of liquid crystal blue phase doped with azobenzene-based bent-shaped hydrogen-bonded assemblies. RSC Adv. 2015, 5, 67357–67364. [Google Scholar] [CrossRef]
- Jau, H.-C.; Li, Y.; Li, C.-C.; Chen, C.-W.; Wang, C.-T.; Bisoyi, H.K.; Lin, T.-H.; Bunning, T.J.; Li, Q. Light-driven wide-range nonmechanical beam steering and spectrum scanning based on a self-organized liquid crystal grating enabled by a chiral molecular switch. Adv. Opt. Mater. 2015, 3, 166–170. [Google Scholar] [CrossRef]
- Bukusoglu, E.; Bedolla Pantoja, M.; Mushenheim, P.C.; Wang, X.; Abbott, N.L. Design of responsive and active (soft) materials using liquid crystals. Ann. Rev. Chem. Biomol. Eng. 2016, 7, 163–196. [Google Scholar] [CrossRef]
- Usol’tseva, N.; Praefcke, K.; Singer, D.; Gündogan, B. Lyotropic phase behaviour of disc-shaped tetra-palladium organyls in apolar organic solvents. Liq. Cryst. 1994, 16, 601–616. [Google Scholar] [CrossRef]
- Usol’tseva, N.; Praefcke, K.; Singer, D.; Gündogan, B. The first case of a lyotropic twisted nematic (N∗) phase induced by a chiral charge transfer complex. Liq. Cryst. 1994, 16, 617–623. [Google Scholar] [CrossRef]
- Praefcke, K.; Dielde, S.; Pickardt, J.; Gündogan, B.; Nütz, U.; Singer, D. On the molecular and mesophase structures of disc-like tetrapalladium liquid crystals. Liq. Cryst. 1995, 18, 857–865. [Google Scholar] [CrossRef]
- Usol’tseva, N.; Hauck, G.; Koswing, H.D.; Praefcke, K.; Heinrich, B. On the nematic-nematic phase transition in mixtures composed of sheet-shaped palladium organyls and apolar organic solvents. Liq. Cryst. 1996, 20, 731–739. [Google Scholar] [CrossRef]
- Heinrich, B.; Praefcke, K.; Guillon, D.J. Structural study of columnar liquid-crystalline phases in homologous series of tetrapalladium organyls. J. Mater. Chem. 1997, 7, 1363–1372. [Google Scholar] [CrossRef]
- Praefcke, K.; Holbrey, J.D.; Usol’tseva, N.; Blunk, D. Amphotropic properties of multi-palladium and -platinum liquid crystals. Mol. Cryst. Liq. Cryst. 1997, 292, 123–139. [Google Scholar] [CrossRef]
- Frischmann, P.D.; Guieu, S.; Tabeshi, R.; MacLachlan, M.J. Columnar organization of head-to-tail self-assembled Pt4 rings. J. Am. Chem. Soc. 2010, 132, 7668–7675. [Google Scholar] [CrossRef] [PubMed]
- Pecinovsky, C.S.; Hatakeyama, E.S.; Gin, D.L. Polymerizable photochromic macrocyclic metallomesogens: Design of supramolecular polymers with responsive nanopores. Adv. Mater. 2008, 20, 174–178. [Google Scholar] [CrossRef]
- Barberá, J.; Elduque, A.; Giménez, R.; Lahoz, F.J.; López, J.A.; Oro, L.A.; Serrano, J.L. (Pyrazolato) gold complexes showing room-temperature columnar mesophases. Synthesis, properties, and structural characterization. Inorg. Chem. 1998, 37, 2960–2967. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Hanaoka, Y.; Yoshizawa, M.; Akita, M.; Ichikawa, T.; Yoshio, M.; Kato, T.; Fujita, M. m x n Stacks of discrete aromatic stacks in solution. J. Am. Chem. Soc. 2010, 132, 9555–9557. [Google Scholar] [CrossRef]
- Terazzi, E.; Bourgogne, C.; Welter, R.; Gallani, J.-L.; Guillon, D.; Rogez, G.; Donnio, B. Single-molecule magnets with mesomorphic lamellar ordering. Angew. Chem. Int. Ed. 2008, 47, 490–495. [Google Scholar] [CrossRef]
- Frein, S.; Auzias, M.; Sondenecker, A.; Vieille-Petit, L.; Guintchin, B.; Maringa, N.; Süss-Fink, G.; Barberá, J.; Deschenaux, R. Mesomorphic metallo-dendrimers based on the metal-metal bonded Ru2(CO)4 sawhorse unit. Chem. Mater. 2008, 20, 1340–1343. [Google Scholar] [CrossRef]
- Huitorel, B.; Benito, Q.; Fargues, A.; Garcia, A.; Gacoin, T.; Boilot, J.-P.; Perruchas, S.; Camerel, F. Mechanochromic luminescence and liquid crystallinity of molecular copper clusters. Chem. Mater. 2016, 28, 8190–8200. [Google Scholar] [CrossRef]
- Uchida, J.; Yoshio, M.; Sato, S.; Yokoyama, H.; Fujita, M.; Kato, T. Self-assembly of giant spherical liquid-crystalline complexes and formation of nanostructured dynamic gels that exhibit self-healing properties. Angew. Chem. Int. Ed. 2017, 56, 14085–14089. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Hamazaki, T.; Suzuki, A.; Kurahashi, K.; Tanaka, K. Metal-ion-induced switch of liquid-crystalline orientation of metallomacrocycles. Chem. Eur. J. 2016, 22, 15674–15683. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Ishida, Y.; Tanaka, K. Columnar liquid-crystalline metallomacrocycles. J. Am. Chem. Soc. 2015, 137, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Barry, N.P.E.; Furrer, J.; Therrien, B. In- and out-cavity interactions by modulating the size of ruthenium metallarectangles. Helv. Chim. Acta 2010, 93, 1313–1328. [Google Scholar] [CrossRef]
- Alvariño, C.; Heinrich, B.; Donnio, B.; Deschenaux, R.; Therrien, B. Supramolecular arene-ruthenium metallacycle with thermotropic liquid-crystalline properties. Inorg. Chem. 2019, 58, 9505–9512. [Google Scholar] [CrossRef] [PubMed]
- Therrien, B. Biologically relevant arene ruthenium metalla-assemblies. CrystEngComm 2015, 17, 484–491. [Google Scholar] [CrossRef]
- Therrien, B. The role of the second coordination sphere in the biological activity of arene ruthenium metalla-assemblies. Front. Chem. 2018, 6, art602. [Google Scholar] [CrossRef]
- Mattsson, J.; Zava, O.; Renfrew, A.K.; Sei, Y.; Yamaguchi, K.; Dyson, P.J.; Therrien, B. Drug delivery of lipophilic pyrenyl derivatives by encapsulation in a water soluble metalla-cage. Dalton Trans. 2010, 39, 8248–8255. [Google Scholar] [CrossRef]
- Pitto-Barry, A.; Barry, N.P.E.; Russo, V.; Heinrich, B.; Donnio, B.; Therrien, B.; Deschenaux, R. Designing supramolecular liquid-crystalline hybrids from pyrenyl-containing dendrimers and arene ruthenium metallacycles. J. Am. Chem. Soc. 2014, 136, 17616–17625. [Google Scholar] [CrossRef]
- Stang, P.J.; Olenyuk, B. Self-assembly, symmetry, and molecular architecture: Coordination as the motif in the rational design of supramolecular metallacyclic polygons and polyhedral. Acc. Chem. Res. 1997, 30, 502–518. [Google Scholar] [CrossRef]
- Therrien, B.; Süss-Fink, G.; Govindaswamy, P.; Renfrew, A.K.; Dyson, P.J. The “complex-in-a-complex” cations [(acac)2M⊂Ru6(p-iPrC6H4Me)6(tpt)2(dhbq)3]6+: A Trojan horse for cancer cells. Angew. Chem. Int. Ed. 2008, 47, 3773–3776. [Google Scholar] [CrossRef] [PubMed]
- Amouri, H.; Desmarets, C.; Moussa, J. Confined nanospaces in metallocages: Guest molecules, weakly encapsulated anions, and catalyst sequestration. Chem. Rev. 2012, 112, 2015–2041. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, M.; Klosterman, J.K.; Fujita, M. Functional molecular flasks: New properties and reactions within discrete, self-assembled hosts. Angew. Chem. Int. Ed. 2009, 48, 3418–3438. [Google Scholar] [CrossRef] [PubMed]
- Pitto-Barry, A.; Barry, N.P.E.; Zava, O.; Deschenaux, R.; Therrien, B. Encapsulation of pyrene-functionalized poly(benzyl ether) dendrons into a water-soluble organometallic cage. Chem. Asian J. 2011, 6, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Ye, Y.; Tong, Z.; Yang, J.; Li, Z.; Hua, B.; Shao, L.; Li, S. A porphyrin-based discrete tetragonal prismatic cage: Host-guest complexation and its application in tuning liquid-crystalline behavior. Macromol. Rapid Commun. 2016, 37, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Bader, K.; Wöhrle, T.; Öztürk, E.; Baro, A.; Laschat, S. Encapsulating propeller-like columnar liquid crystals with an aromatic outer shell: Influence of phenoxy-terminated side chains on the phase behavior of triphenylbenzenes. Soft Matter 2018, 14, 6409–6414. [Google Scholar] [CrossRef]
- Ren, Y.-Y.; Xu, Z.; Li, G.; Huang, J.; Fan, X.; Xu, L. Hierarchical self-assembly of a fluorescence emission-enhanced organogelator and its multiple stimuli-responsive behaviors. Dalton Trans. 2017, 46, 333–337. [Google Scholar] [CrossRef]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153–161. [Google Scholar] [CrossRef]
- Zheludev, N.; Kivshar, Y.S. From metamaterials to metadevices. Nat. Mater. 2012, 11, 917–924. [Google Scholar] [CrossRef]
- Ohm, C.; Brehmer, M.; Zentel, R. Liquid crystalline elastomers as actuators and sensors. Adv. Mater. 2010, 22, 3366–3387. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, Q.; Tian, H. Photochromic materials: More than meets the eye. Adv. Mater. 2013, 25, 378–399. [Google Scholar] [CrossRef] [PubMed]
- Mercs, L.; Albrecht, M. Beyond catalysis: N-heterocyclic carbene complexes as components for medicinal luminescent, and functional materials applications. Chem. Soc. Rev. 2010, 39, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Priimagi, A.; Cavallo, G.; Metrangolo, P.; Resnati, G. The halogen bond in the design of functional supramolecular materials: Recent advances. Acc. Chem. Res. 2013, 46, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Broer, D.J. Programmable and adaptive mechanics with liquid crystal polymer networks and elastomers. Nat. Mater. 2015, 14, 1087–1098. [Google Scholar] [CrossRef]
- Coles, H.; Morris, S. Liquid-crystal lasers. Nat. Photonics 2010, 4, 676–685. [Google Scholar] [CrossRef]
- O’Neill, M.; Kelly, S.M. Ordered materials for organic electronics and photonics. Adv. Mater. 2011, 23, 566–584. [Google Scholar] [CrossRef]
- Tschierske, C. Development of structural complexity by liquid-crystal self-assembly. Angew. Chem. Int. Ed. 2013, 52, 8828–8878. [Google Scholar] [CrossRef]
- Lehn, J.-M. Towards self-organization and complex matter. Science 2002, 295, 2400–2403. [Google Scholar] [CrossRef]
- Holliday, B.J.; Mirkin, C.A. Strategies for the coordination of supramolecular compounds through coordination chemistry. Angew. Chem. Int. Ed. 2001, 40, 2022–2043. [Google Scholar] [CrossRef]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal−organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis, and functionality of metal−organic materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Y.-J.; Li, Z.-H.; Jin, G.-X. Rational design of polynuclear organometallic assemblies from a simple heteromultifunctional ligand. J. Am. Chem. Soc. 2015, 137, 13670–13678. [Google Scholar] [CrossRef] [PubMed]
- Sepehrpour, H.; Fu, W.; Sun, Y.; Stang, P.J. Biomedically relevant self-assembled metallacycles and metallacages. J. Am. Chem. Soc. 2019, 141, 14005–14020. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).