On the Aqueous Solution Behavior of C-Substituted 3,1,2-Ruthenadicarbadodecaboranes
Abstract
1. Introduction
2. Results and Discussion
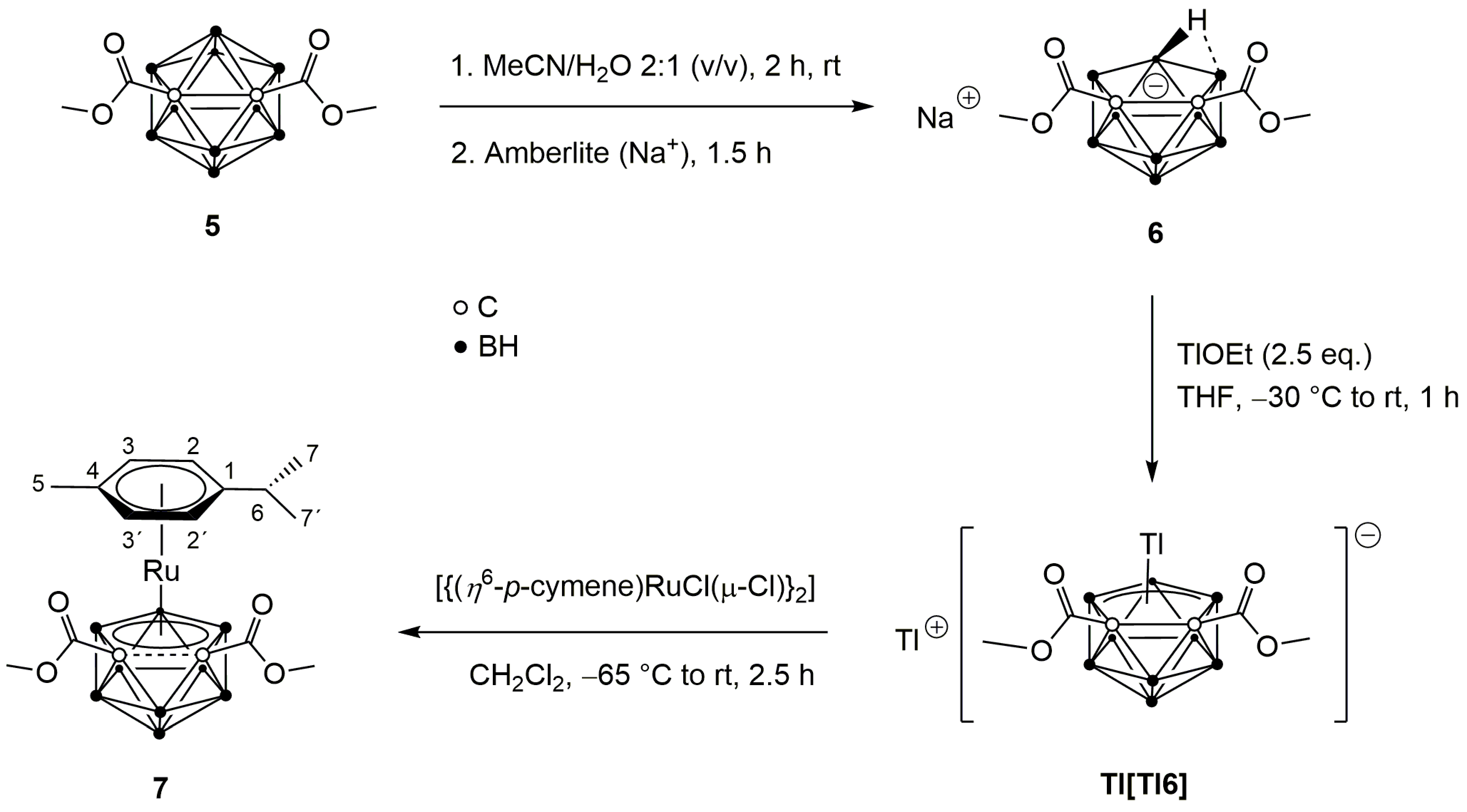
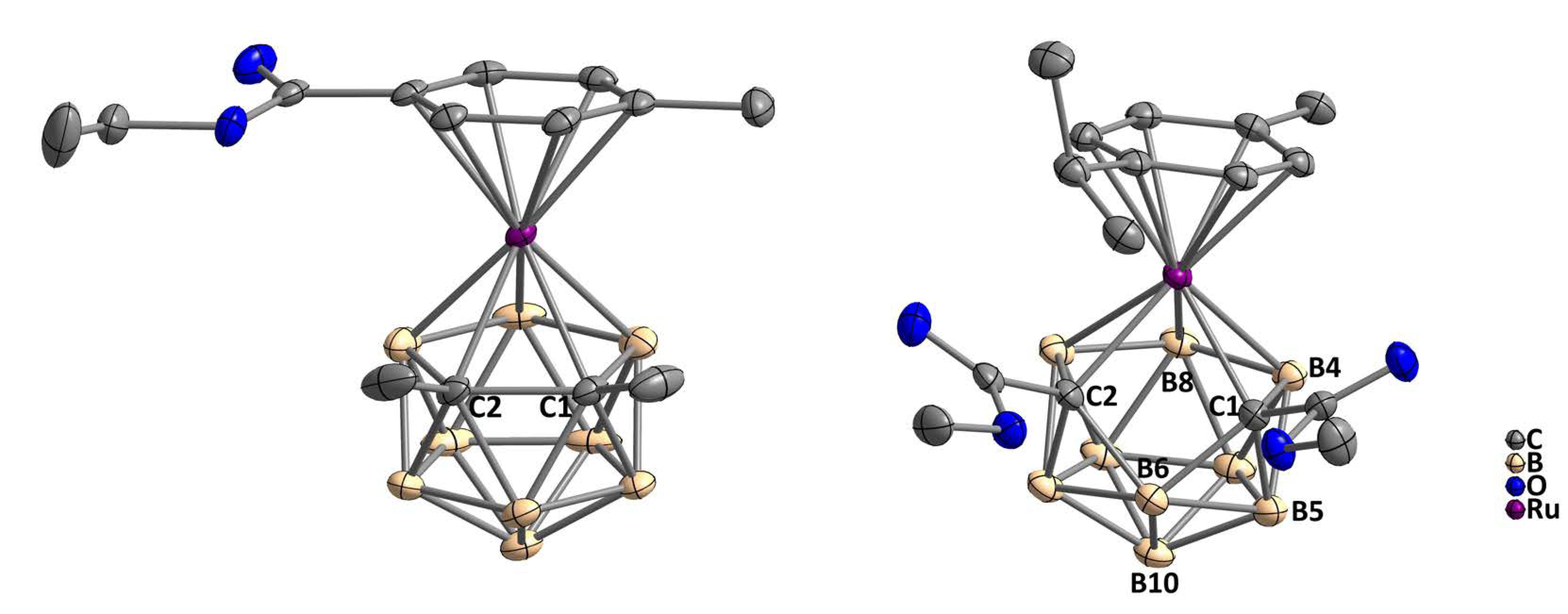
2.1. Synthesis and Characterization of Complexes 2–4 and 7
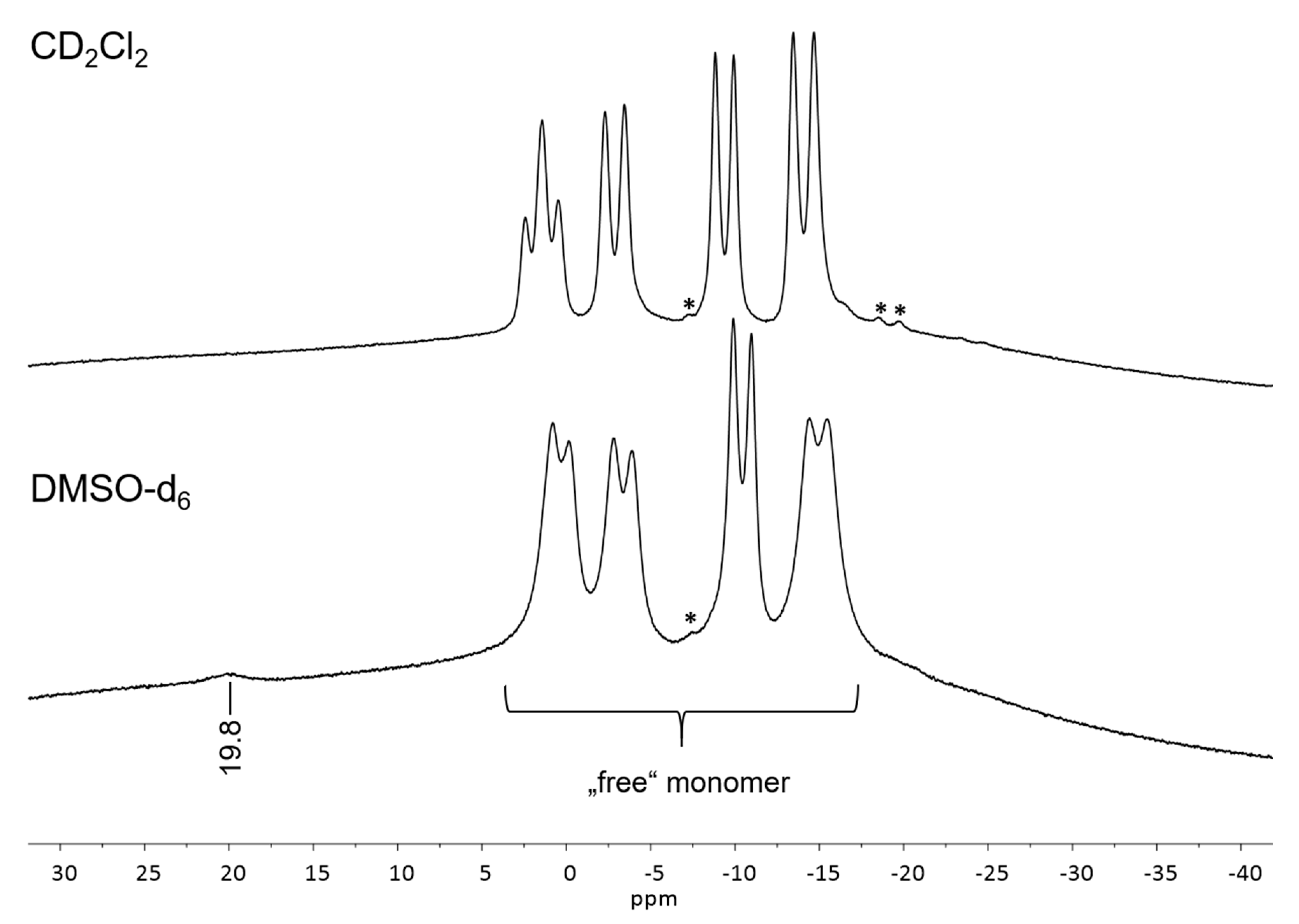
2.2. 11B NMR Spectra of Complex 3
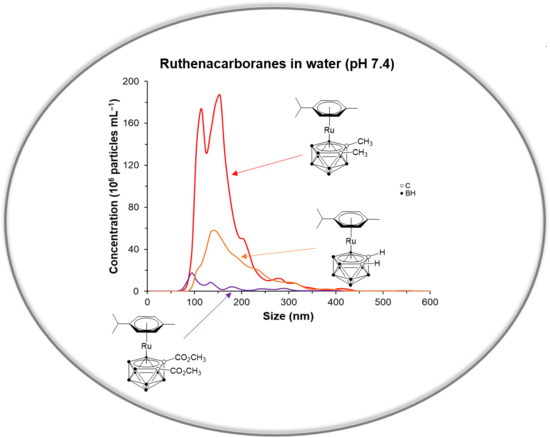
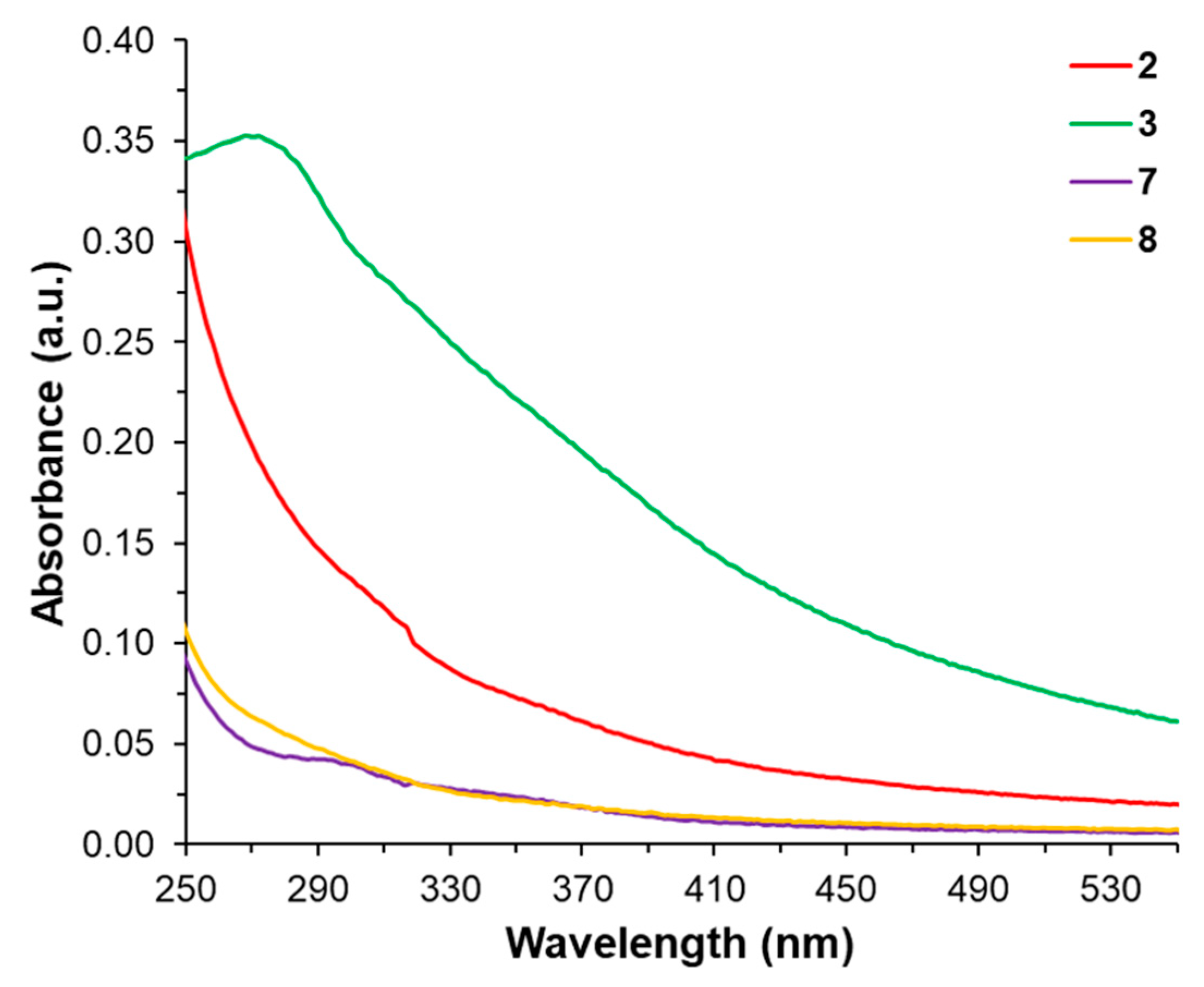
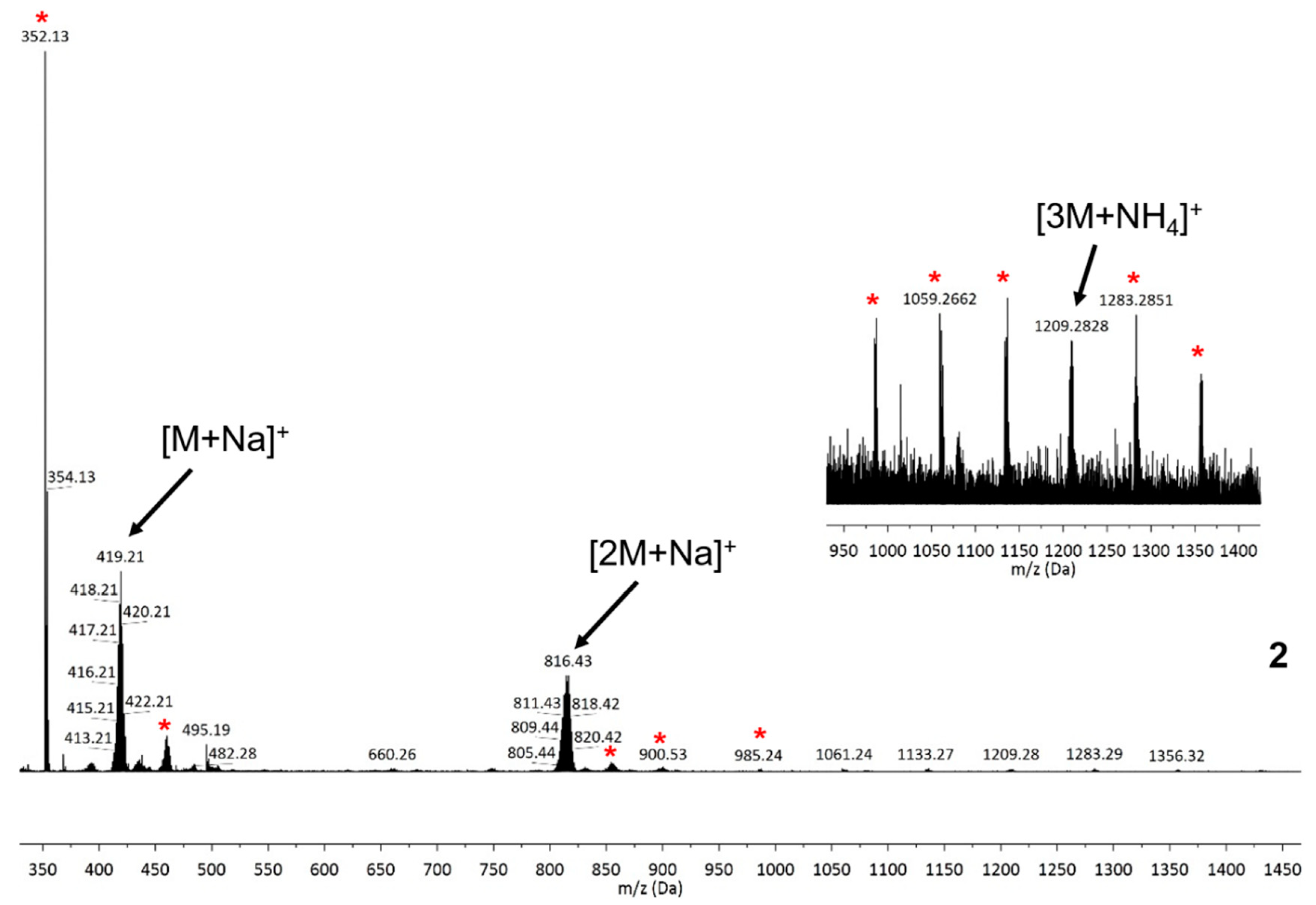
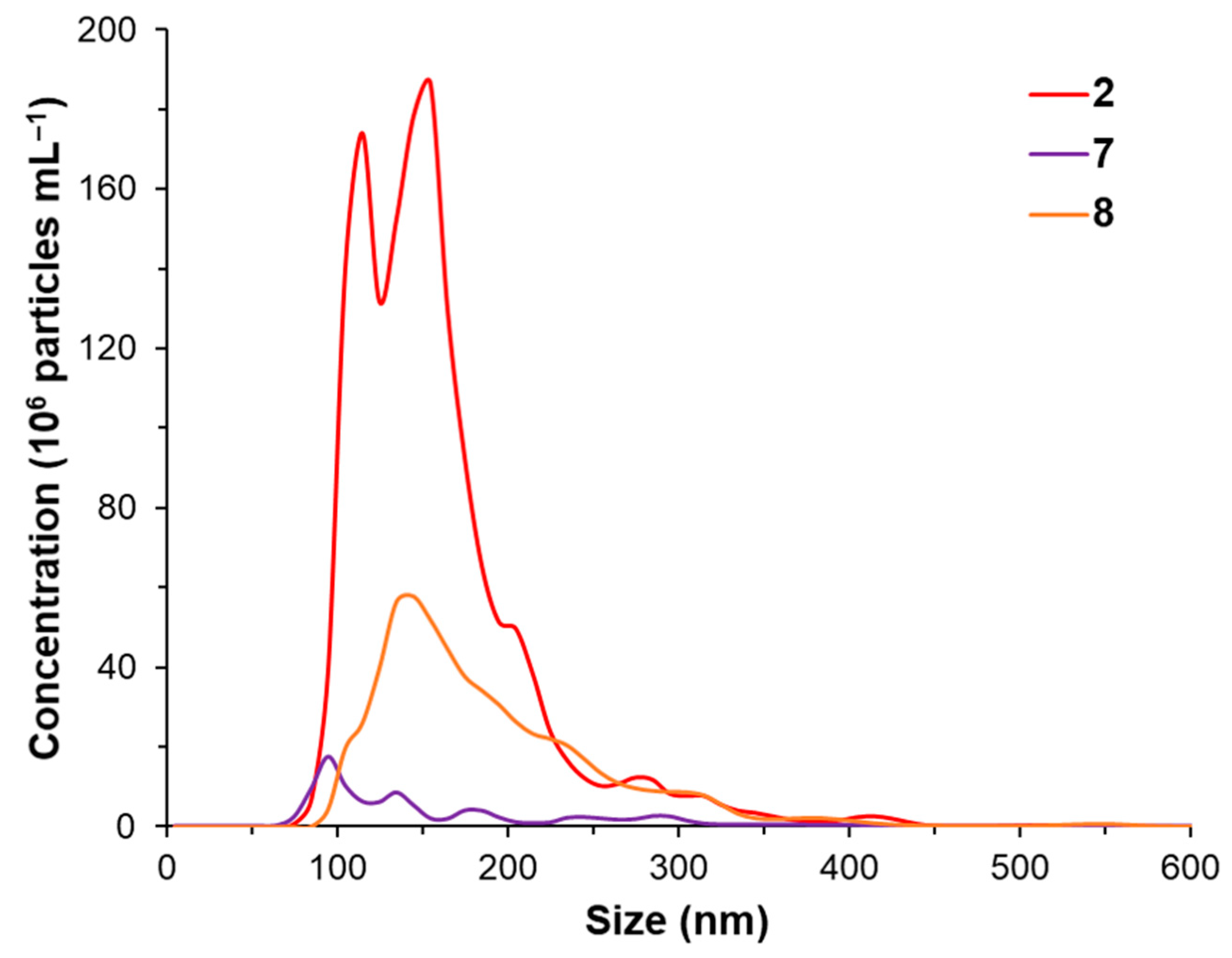
2.3. UV-Vis Spectroscopy, Mass Spectrometry and Nanoparticle Tracking Analysis (NTA)
3. Materials and Methods
3.1. General Procedures and Instrumentation
3.2. Syntheses
3.2.1. closo-[3-(η6-Biphenyl)-1,2-Me2-3,1,2-RuC2B9H9] (3)
3.2.2. closo-[3-(η6-(1-Me-4-COOEt-C6H4))-1,2-Me2-3,1,2-RuC2B9H9] (4)
3.2.3. pseudocloso-[3-(η6-p-Cymene)-1,2-(CO2Me)2-3,1,2-RuC2B9H9] (7)
3.3. Preparation of 2, 7, and 8 for UV-Vis Spectroscopy, Mass Spectrometry, and NTA Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
References
- Grimes, R.N. Metallacarboranes of the Transition and Lanthanide Elements. In Carboranes; Academic Press: Cambridge, MA, USA, 2016; pp. 711–903. [Google Scholar]
- Grimes, R.N. Structure and Bonding. In Carboranes; Academic Press: Cambridge, MA, USA, 2016; pp. 7–18. [Google Scholar]
- Brown, D.A.; Fanning, M.O.; Fitzpatrick, N.J. Molecular Orbital Theory of Organometallic compounds. 15. A Comparative Study of Ferrocene and π-Cyclopentadienyl-(3)-1,2-Dicarbollyliron. Inorg. Chem. 1978, 17, 1620–1623. [Google Scholar] [CrossRef]
- Gozzi, M.; Schwarze, B.; Hey-Hawkins, E. Half- and Mixed-Sandwich Metallacarboranes in Catalysis. In Handbook of Boron Science with Applications in Organometallics, Catalysis, Material Science and Medicine; Hosmane, N.S., Eagling, R., Eds.; World Scientific, Ltd.: London, UK, 2018; pp. 27–80. ISBN 978-1786344410. [Google Scholar]
- Gozzi, M.; Schwarze, B.; Hey-Hawkins, E. Half- and Mixed-Sandwich Metallacarboranes for Potential Applications in Medicine. Pure Appl. Chem. 2019, 91, 563–573. [Google Scholar] [CrossRef]
- Núñez, R.; Tarrés, M.; Ferrer-Ugalde, A.; de Biani, F.F.; Teixidor, F. Electrochemistry and Photoluminescence of Icosahedral Carboranes, Boranes, Metallacarboranes, and Their Derivatives. Chem. Rev. 2016, 116, 14307–14378. [Google Scholar] [CrossRef]
- Grishin, I.D.; Kiseleva, N.E.; Markin, A.V.; Chizhevsky, I.T.; Grishin, D.F. Synthesis of Functional Polymers Based on Methacrylic Monomers Using Ruthenium Carborane Complexes. Polym. Sci. Ser. B 2015, 57, 1–8. [Google Scholar] [CrossRef]
- Louie, A.S.; Vasdev, N.; Valliant, J.F. Preparation, Characterization, and Screening of a High Affinity Organometallic Probe for α-Adrenergic Receptors. J. Med. Chem. 2011, 54, 3360–3367. [Google Scholar] [CrossRef]
- Gozzi, M.; Schwarze, B.; Sárosi, M.-B.; Lönnecke, P.; Drača, D.; Maksimović-Ivanić, D.; Mijatović, S.; Hey-Hawkins, E. Antiproliferative Activity of (η6-Arene)ruthenacarborane Sandwich Complexes Against HCT116 and MCF-7 Cell Lines. Dalton Trans. 2017, 46, 12067–12080. [Google Scholar] [CrossRef]
- Schwarze, B.; Sobottka, S.; Schiewe, R.; Sarkar, B.; Hey-Hawkins, E. Spectroscopic and Electronic Properties of Molybdacarborane Complexes with Non-innocently acting Ligands. Chem. Eur. J. 2019, 25, 8550–8559. [Google Scholar] [CrossRef]
- Tarrés, M.; Viñas, C.; González-Cardoso, P.; Hänninen, M.M.; Sillanpää, R.; Dorďovič, V.; Uchman, M.; Teixidor, F.; Matějíček, P. Aqueous Self-Assembly and Cation Selectivity of Cobaltabisdicarbollide Dianionic Dumbbells. Eur. J. Chem. 2014, 20, 6786–6794. [Google Scholar] [CrossRef]
- Uchman, M.; Ďorďovič, V.; Tošner, Z.; Matějíček, P. Classical Amphiphilic Behavior of Nonclassical Amphiphiles: A Comparison of Metallacarborane Self-Assembly with SDS Micellization. Angew. Chem. Int. Ed. Engl. 2015, 54, 14113–14117. [Google Scholar] [CrossRef]
- Zaulet, A.; Teixidor, F.; Bauduin, P.; Diat, O.; Hirva, P.; Ofori, A.; Viñas, C. Deciphering the Role of the Cation in Anionic Cobaltabisdicarbollide Clusters. J. Organomet. Chem. 2018, 865, 214–225. [Google Scholar] [CrossRef]
- Fernandez-Alvarez, R.; Ďorďovič, V.; Uchman, M.; Matějíček, P. Amphiphiles without Head-and-Tail Design: Nanostructures Based on the Self-Assembly of Anionic Boron Cluster Compounds. Langmuir 2018, 34, 3541–3554. [Google Scholar] [CrossRef]
- Coan, K.E.D.; Shoichet, B.K. Stoichiometry and Physical Chemistry of Promiscuous Aggregate-Based Inhibitors. J. Am. Chem. Soc. 2008, 130, 9606–9612. [Google Scholar] [CrossRef]
- Bould, J.; Kennedy, J.D. An Assessment of the Intercarbon Stretching Phenomenon in C-substituted “pseudocloso” {3,1,2-RuC2B9} Metalladicarbaboranes. J. Organomet. Chem. 2014, 749, 163–173. [Google Scholar] [CrossRef]
- Garcia, M.P.; Green, M.; Stone, F.G.A.; Somerville, R.G.; Welch, A.J.; Briant, C.E.; Cox, D.N.; Mingos, D.M.P. Metallaborane Chemistry. Part 14. Icosahedral η6-Arene Carbametallaboranes of Iron and Ruthenium: Molecular Structures of closo-[1-(η6-C6H5Me)-2,4-Me-1,2,4-FeC2B9H9] and closo-[3-(η6-C6H6)-3,1,2-RuC2B9H11]. Dalton Trans. 1985, 11, 2343–2348. [Google Scholar] [CrossRef]
- Hanusa, T.P.; Huffman, J.C.; Todd, L.J. Synthesis of π-(Arene)metallocarboranes Containing Iron and Ruthenium. Crystal Structure of 3,1,2-(η6-1,3,5-(CH3)3C6H3)FeC2B9H11. Polyhedron 1982, 1, 77–82. [Google Scholar] [CrossRef]
- Shaw, K.F.; Reid, B.D.; Welch, A.J. Synthesis and Characterisation of Metal Complexes of Ether Carbaboranes. Molecular Structures of d6 ML3, d8 ML2 and d10 ML Complexes of Mono- and Di-ether C2B9 Carbaborane Ligands, Showing the Progressive Importance of Secondary M···O Bonding. J. Organomet. Chem. 1994, 482, 207–220. [Google Scholar] [CrossRef]
- Genady, A.R.; Tan, J.; El-Zaria, M.; Zlitni, A.; Janzen, N.; Valliant, J.F. Synthesis, Characterization and Radiolabeling of Carborane-Functionalized Tetrazines for Use in Inverse Electron Demand Diels–Alder Ligation Reactions. J. Organomet. Chem. 2015, 791, 204–213. [Google Scholar] [CrossRef]
- Safronov, A.V.; Hawthorne, M.F. Novel Synthesis of 3-Iodo-ortho-Carborane. Inorg. Chim. Acta 2011, 375, 308–310. [Google Scholar] [CrossRef]
- Brain, P.T.; Bühl, M.; Cowie, J.; Lewis, Z.G.; Welch, A.J. Synthesis and Characterisation of pseudocloso Iridium and Ruthenium Diphenyl Carbaboranes. Molecular Structures of 1,2-Ph2-3-(η6-C6H6)-3,1,2-pseudocloso-RuC2B9H9 and 1,2-Ph2-3-(cym)-3,1,2-pseudocloso-RuC2B9H9 (cym = p-cymene) and Individual Gauge for Localised Orbitals Calculations on Carbametallaboranes. Dalton Trans. 1996, 2, 231–237. [Google Scholar]
- Gaines, D.F.; Nelson, C.K.; Kunz, J.C.; Morris, J.H.; Reed, D. Solvent Effects on the Boron-11 and Proton NMR Spectra of Decaborane(14), B10H14. Inorg. Chem. 1984, 23, 3252–3254. [Google Scholar] [CrossRef]
- Crociani, B.; Antonaroli, S.; Marini, A.; Matteoli, U.; Scrivanti, A. Mechanistic Study on the Coupling Reaction of Aryl Bromides with Arylboronic Acids Catalyzed by (Iminophosphine) palladium(0) Complexes. Detection of a Palladium(II) Intermediate with a Coordinated Boron Anion. Dalton Trans. 2006, 22, 2698–2705. [Google Scholar] [CrossRef][Green Version]
- Deore, B.A.; Yu, I.; Woodmass, J.; Freund, M.S. Conducting Poly(anilineboronic acid) Nanostructures: Controlled Synthesis and Characterization. Macromol. Chem. Phys. 2008, 209, 1094–1105. [Google Scholar] [CrossRef]
- Bonechi, C.; Ristori, S.; Martini, S.; Panza, L.; Martini, G.; Rossi, C.; Donati, A. Solution Behavior of a Sugar-Based Carborane for Boron Neutron Capture Therapy: A Nuclear Magnetic Resonance Investigation. Biophys. Chem. 2007, 125, 320–327. [Google Scholar] [CrossRef]
- He, T.; Misuraca, J.C.; Musah, R.A. “Carboranyl-cysteine”-Synthesis, Structure and Self-Assembly Behavior of a Novel α-Amino Acid. Sci. Rep. 2017, 7, 16995. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles, 1st ed.; Wiley-VCH: Weinheim, Germany, 1983; ISBN 0-47-1-29340-7. [Google Scholar]
- Carr, B.; Wright, M. Nanoparticle Tracking Analysis: A Review of Applications and Usage in the Analysis of Exosomes and Microvesicles 2010–2012; Nanosight: Salisbury, UK, 2013; pp. 1–33. [Google Scholar]
- Luo, P.; Roca, A.; Tiede, K.; Privett, K.; Jiang, J.; Pinkstone, J.; Ma, G.; Veinot, J.; Boxall, A. Application of Nanoparticle Tracking Analysis for Characterising the Fate of Engineered Nanoparticles in Sediment-Water Systems. J. Environ. Sci. (China) 2018, 64, 62–71. [Google Scholar] [CrossRef]
- Jarzębski, M.; Bellich, B.; Białopiotrowicz, T.; Śliwa, T.; Kościński, J.; Cesàro, A. Particle Tracking Analysis in Food and Hydrocolloids Investigations. Food Hydrocoll. 2017, 68, 90–101. [Google Scholar] [CrossRef]
- Gercel-Taylor, C.; Atay, S.; Tullis, R.H.; Kesimer, M.; Taylor, D.D. Nanoparticle Analysis of Circulating Cell-derived Vesicles in Ovarian Cancer Patients. Anal. Biochem. 2012, 428, 44–53. [Google Scholar] [CrossRef]
- Gross, J.; Sayle, S.; Karow, A.R.; Bakowsky, U.; Garidel, P. Nanoparticle Tracking Analysis of Particle Size and Concentration Detection in Suspensions of Polymer and Protein Samples: Influence of Experimental and Data Evaluation Parameters. Eur. J. Pharm. Biopharm. 2016, 104, 30–41. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical Evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the Measurement of Nanoparticles and Protein Aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Spencer, J.L.; Green, M.; Stone, F.G.A. Metallocarboranes: New Syntheses. Chem. Commun. 1972, 1178–1179. [Google Scholar] [CrossRef]
- Kazantsev, A.V.; Akhmetov, S.F.; Tverdokhlebov, A.I. Synthesis and Study of o- and m-Carborane Derivatives. Izvestiya Akademii Nauk SSSR Seriya Khimicheskaya 1973, 23, 73–77. [Google Scholar]
- Hawthorne, M.F.; Young, D.C.; Garrett, P.M.; Owen, D.A.; Schwerin, S.G.; Tebbe, F.N.; Wegner, P.A. Preparation and Characterization of the (3)-1,2- and (3)-1,7-Dicarbadodecahydroundecaborate(−1) Ions. J. Am. Chem. Soc. 1968, 90, 862–868. [Google Scholar] [CrossRef]
- Harris, R.K.; Becker, E.D.; Cabral de Menezes, S.M.; Goodfellow, R.; Granger, P. NMR Nomenclature: Nuclear Spin Properties and Conventions for Chemical Shifts. IUPAC Recommendations 2001. Solid State Nucl. Magn. Reson. 2002, 22, 458–483. [Google Scholar] [CrossRef] [PubMed]
- CrysAlis-Pro. Empirical Absorption Correction; Oxford Diffraction Ltd.: Abingdon, UK, 2014. [Google Scholar]
- Sheldrick, G.M. SHELXT-integrated Space-group and Crystal-structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.C.; Park, T. Materials Crystal Chemistry; Marcel Dekker: New York, NY, USA, 1997; ISBN 0824797981. [Google Scholar]
- Jutzi, P.; Wegener, D.; Hursthouse, M.B. Kristallines Tl2B9H9C2Me2: Synthese und Festkörperstruktur; ein Beitrag zum Problem “attraktive Tl(I)–Tl(I)-Wechselwirkungen”. Chem. Ber. 1991, 124, 295–299. [Google Scholar] [CrossRef]
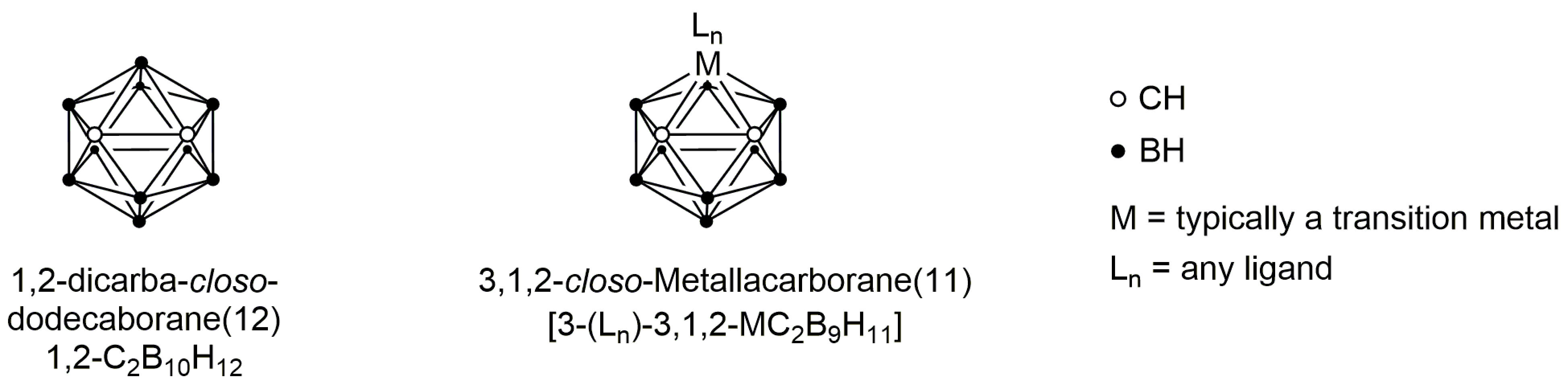

| [3-(η6-p-cymene)-3,1,2-RuC2B9H11] (8) a | 7 | [3-{η6-(4-Me-1-COOEt-C6H4)}-3,1,2-RuC2B9H11] (9) a | 4 | |
|---|---|---|---|---|
| Ru–Ctd1 b | 1.714(4) | 1.768(1) | 1.708(2) | 1.738(1) |
| Ru–Ctd2 b | 1.619(4) | 1.485(1) | 1.623(2) | 1.598(1) |
| Ru–B(C2B3 face) c | 2.203(3) | 2.216(2) | 2.205(8) | 2.195(5) |
| Ru–C(C2B3 face) c | 2.171(2) | 2.127(2) | 2.166(5) | 2.171(3) |
| Ru–C(arene) c | 2.224(3) | 2.265(2) | 2.217(7) | 2.237(3) |
| C–C(cluster) | 1.627(4) | 2.243(2) | 1.623(3) | 1.680(5) |
| B–B d | 1.774(7) | 1.799(3) | 1.778(7) | 1.772(7) |
| B–C(cluster) c | 1.720(5) | 1.662(3) | 1.719(3) | 1.722(6) |
| C(cluster)–C(exo) c | – | 1.497(1) | – | 1.517(5) |
| Ru–B(6) | 3.494(1) | 2.979(2) | – | – |
| B(6)–B(10) | 1.759(1) | 1.885(2) | – | – |
| B(4)–B(5) | 1.797(1) | 1.838(3) | – | – |
| C(1)–B(4) | 1.718(1) | 1.636(2) | – | – |
| C(1)–B(5) | 1.696(1) | 1.614(2) | ||
| Deviation from coplanarity e | 5.11(9) | 2.5(1) | 2.3(5) | 6.3(1) |
| Ru–C(1)–B(6) | 126.79(3) | 100.12(9) | – | – |
| C(1)–B(6)–C(2) | 55.99(2) | 88.7(1) | – | – |
| B(6)–C(2)–Ru | 126.49(5) | 100.14(9) | – | – |
| C(2)–Ru–C(1) | 44.02(4) | 69.75(6) | – | – |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gozzi, M.; Schwarze, B.; Coburger, P.; Hey-Hawkins, E. On the Aqueous Solution Behavior of C-Substituted 3,1,2-Ruthenadicarbadodecaboranes. Inorganics 2019, 7, 91. https://doi.org/10.3390/inorganics7070091
Gozzi M, Schwarze B, Coburger P, Hey-Hawkins E. On the Aqueous Solution Behavior of C-Substituted 3,1,2-Ruthenadicarbadodecaboranes. Inorganics. 2019; 7(7):91. https://doi.org/10.3390/inorganics7070091
Chicago/Turabian StyleGozzi, Marta, Benedikt Schwarze, Peter Coburger, and Evamarie Hey-Hawkins. 2019. "On the Aqueous Solution Behavior of C-Substituted 3,1,2-Ruthenadicarbadodecaboranes" Inorganics 7, no. 7: 91. https://doi.org/10.3390/inorganics7070091
APA StyleGozzi, M., Schwarze, B., Coburger, P., & Hey-Hawkins, E. (2019). On the Aqueous Solution Behavior of C-Substituted 3,1,2-Ruthenadicarbadodecaboranes. Inorganics, 7(7), 91. https://doi.org/10.3390/inorganics7070091