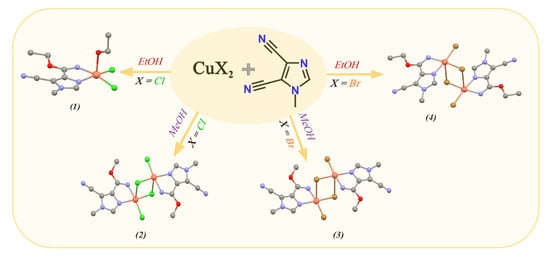
Activation of the Cyano Group at Imidazole via Copper Stimulated Alcoholysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Analysis
2.2. Computational Studies
2.3. IR Analysis
2.4. UV–Vis Analysis
3. Materials and Methods
3.1. General
3.2. Synthesis of Complexes 1–4
3.3. Crystal Structure Determination
3.4. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Satterfield, M.; Brodbelt, J.S. Relative Binding Energies of Gas-Phase Pyridyl Ligand/Metal Complexes by Energy-Variable Collisionally Activated Dissociation in a Quadrupole Ion Trap. Inorg. Chem. 2001, 40, 5393–5400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-M.; Lin, H.-K.; Zhu, S.-R.; Liu, T.-F.; Chen, Y.-T. Spectroscopy, cytotoxicity and DNA-binding of the lanthanum(III) complex of an l-valine derivative of 1,10-phenanthroline. J. Inorg. Biochem. 2002, 89, 97–106. [Google Scholar] [CrossRef]
- Masood, M.A.; Hodgson, D.J. Synthesis and characterization of the multidentate ligand 2,9-bis(N-pyrazolylmethyl)-1,10-phenanthroline (bpmp) and its copper(I) and copper(II) complexes. Inorg. Chem. 1993, 32, 4839–4844. [Google Scholar] [CrossRef]
- Erden, I.; Demirhan, N.; Avcıata, U. Synthesis and Characterization of a New Imidazole Ligand and its Complexes with Cobalt(II), Nickel(II) and Copper(II). Synth. React. Inorg. Met. Nano-Metal. Chem. 2007, 36, 559–562. [Google Scholar] [CrossRef]
- Handyside, T.M.; Lockhart, J.C.; McDonnell, M.B.; Rao, P.V.S. Ligands for the alkali metals. Part 6. Some bis(crown) Schiff bases which form pocket complexes with alkali-metal cations of appropriate size. J. Chem. Soc. Dalton Trans. 1982, 2331–2336. [Google Scholar] [CrossRef]
- Sandbhor, U.; Kulkarni, P.; Padhye, S.; Kundu, G.; MacKenzie, G.; Pritchard, R. Antimelanomal activity of the copper(II) complexes of 1-substituted 5-amino-imidazole ligands against B16F10 mouse melanoma cells. Bioorg. Med. Chem. Lett. 2004, 14, 2877–2882. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Rees, C.W. Comprehensive Heterocyclic Chemistry: The Structure, Reactions, Synthesis, and Uses OF Heterocyclic Compounds; Pergamon Press: Oxford, UK, 1984. [Google Scholar]
- Grimmett, M.R. Imidazole and Benzimidazole Synthesis; Academic Press: New York, NY, USA, 1997. [Google Scholar]
- Brown, E.G. Ring Nitrogen and Key Biomolecules: The Biochemistry of N-Heterocycles; Springer Science + Business Media: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Pozharskii, A.F.; Soldatenkov, A.T.; Katritzky, A.R. Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry and Biochemistry and the Role of Heterocycles in Science, Technology, Medicine, and Agriculture; John Wiley & Sons: Chichester, UK, 1997. [Google Scholar]
- Li, J.-X.; Du, Z.-X.; Zhou, J.; An, H.-Q.; Zhu, B.-L.; Wang, S.-R.; Zhang, S.-M.; Wu, S.-H.; Huang, W.-P. A potassium and cadmium coordination polymer connected by two kinds of coordination modes of 4,5-dicyanoimidazole ligands: Synthesis, crystal structure and fluorescent properties of {[K[Cd(dci)2(H2O)2]6]Cl}n. Inorg. Chem. Commun. 2010, 13, 127–130. [Google Scholar] [CrossRef]
- Hu, T.-P.; Wang, X.-X.; Zheng, B.-H.; Wang, X.-Q.; Hao, X.-N.; Liu, J.-F. Assembly of a 3D chiral Cu(I) metal–organic framework based on 4,5-dicyanoimidazole: CD spectrum, luminescence and selective gas adsorption. Inorg. Chem. Commun. 2016, 68, 17–20. [Google Scholar] [CrossRef]
- Wu, K.; Chang, T.; Wang, Y.; Hong, Y.; Wu, T. Interactions and mobility of copper(II)–Imidazole-containing copolymers. Eur. Polym. J. 2003, 39, 239–245. [Google Scholar] [CrossRef]
- Carella, A.; Centore, R.; Riccio, P.; Sirigu, A.; Quatela, A.; Palazzesi, C.; Casalboni, M. Polymethacrylate Copolymers Containing 4,5-Dicyanoimidazole-Based Chromophores and their Nonlinear Optical Behavior. Macromol. Chem. Phys. 2005, 206, 1399–1404. [Google Scholar] [CrossRef]
- Cocco, M.; Olla, C.; Onnis, V.; Schivo, M.; De Logu, A. 1-Acylamonoimidazoles synthesis and antimicrobial activity. Farmaco 1992, 47, 229–238. [Google Scholar] [PubMed]
- Sundberg, R.J.; Martin, R.B. Interactions of histidine and other imidazole derivatives with transition metal ions in chemical and biological systems. Chem. Rev. 1974, 74, 471–517. [Google Scholar] [CrossRef]
- Townsend, L.B.; Revankar, G.R. Benzimidazole nucleosides, nucleotides, and related derivatives. Chem. Rev. 1970, 70, 389–438. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Ye, B.-H.; Li, H.; Li, R.-H.; Zhou, J.-Y.; Ji, L.-N. Synthesis, electrochemical and spectroscopic properties of ruthenium(II) complexes containing 1,3-bis([1,10]phenanthroline-[5,6-d]imidazol-2-yl) benzene. Polyhedron 2000, 19, 1975–1983. [Google Scholar] [CrossRef]
- Storhoff, B.N.; Lewis, H.C., Jr. Organonitrile complexes of transition metals. Coord. Chem. Rev. 1977, 23, 1–29. [Google Scholar] [CrossRef]
- Rasmussen, P.; Rongguang, L.; Butler, W.; Bayón, J. Dicyanoimidazole complexes of Cu(I): A carbonyl assisted alcoholysis of nitrile. Inorg. Chim. Acta 1986, 118, 7–13. [Google Scholar] [CrossRef]
- Du, M.; Zhao, X.-J.; Batten, S.R.; Ribas, J. From 1-D Coordination Polymers to 3-D Hydrogen-Bonding Networks: Crystal Engineering and Magnetism of CuII–dca–Cyanopyridine Supramolecular Systems (dca = Dicyanamide, N(CN)2−). Cryst. Growth Des. 2005, 5, 901–909. [Google Scholar] [CrossRef]
- Du, M.; Wang, Q.; Wang, Y.; Zhao, X.-J.; Ribas, J. Metal dicyanamide layered coordination polymers with cyanopyridine co-ligands: Synthesis, crystal structures and magnetism. J. Solid State Chem. 2006, 179, 3926–3936. [Google Scholar] [CrossRef]
- Mei, L.; Jia, X.; Cheng, Z.J. Synthesis and catalytic activity of metallo-organic complexes bearing 5-amino 2-ethylpyridine-2-carboximidate. J. Chem. Sci. 2016, 128, 855–860. [Google Scholar] [CrossRef]
- Devi, R.B.; Devi, S.P.; Singh, R.B.; Singh, R.H.; Swu, T.; Devi, W.R.; Singh, C.B. Synthesis, spectroscopic, and biological studies on copper(II) complexes containing equatorial–apical chloride bridges: Crystal structure of [Cu2(μ-Cl)2(O-2-butoxyethylpyridine-2-carboximidate)2Cl2]. J. Coord. Chem. 2014, 67, 891–909. [Google Scholar] [CrossRef]
- Xuan, R.-C.; Xu, M.; Cheng, D.-P. Dichloro (O-ethyl 3-methylpyridine-2-carboximidic acid-κ2N,N′) copper(II). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2006, 62, m587–m589. [Google Scholar] [CrossRef] [PubMed]
- Gurbanov, A.V.; Mahmoudi, G.; Da Silva, M.F.C.G.; Zubkov, F.I.; Mahmudov, K.T.; Pombeiro, A.J. Cyanosilylation of aldehydes catalyzed by mixed ligand copper(II) complexes. Inorg. Chim. Acta 2018, 471, 130–136. [Google Scholar] [CrossRef]
- Kelley, S.P.; Nuss, J.S.; Rogers, R.D. Nonstoichiometric, Protic Azolium Azolate Ionic Liquids Provide Unique Environments for N-Donor Coordination Chemistry. Chem. Eur. J. 2015, 21, 17196–17199. [Google Scholar] [CrossRef] [PubMed]
- Jaaskelainen, S.; Appiah, N.S.; Koshevoy, I.; Hirva, P.; Appiah, N.S.; Koshevoy, I. Impacts of the counter ions on 4,5-dicyano-1-methylimidazole silver coordination. New J. Chem. 2018, 42, 3363–3370. [Google Scholar] [CrossRef] [Green Version]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper (II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane] copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Jamnicky, M.; Segl’A, P.; Koman, M. Methanolysis of pyridine-2-carbonitrile in the coordination sphere of copper(II), cobalt(II) and nickel(II). The structure of [Ni(o-methylpyridine-2-carboximidate)3]Br2·4H2O. Polyhedron 1995, 14, 1837–1847. [Google Scholar] [CrossRef]
- Godlewska, S.; Jezierska, J.; Baranowska, K.; Augustin, E.; Dołęga, A. Copper(II) complexes with substituted imidazole and chlorido ligands: X-ray, UV–Vis, magnetic and EPR studies and chemotherapeutic potential. Polyhedron 2013, 65, 288–297. [Google Scholar] [CrossRef]
- Sun, Y.; Lemaur, V.; Beltrán, J.I.; Cornil, J.; Huang, J.; Zhu, J.; Wang, Y.; Fröhlich, R.; Wang, H.; Jiang, L.; et al. Neutral Mononuclear Copper(I) Complexes: Synthesis, Crystal Structures, and Photophysical Properties. Inorg. Chem. 2016, 55, 5845–5852. [Google Scholar] [CrossRef]
- Du, H.; Sivappa, R.; Lovely, C.J.; He, Y. New Methods of Imidazole Functionalization—From Imidazole to Marine Alkaloids. Synlett 2006, 2006, 965–992. [Google Scholar] [CrossRef]
- Gulli, S.; Poli, R.; Daran, J.-C.; Daran, J. Synthesis and Structure of Four-Coordinate Copper(II) Complexes Stabilized by β-Ketiminato Ligands and Application in the Reverse Atom-Transfer Radical Polymerization of Styrene. Eur. J. Inorg. Chem. 2011, 2011, 1666–1672. [Google Scholar] [CrossRef]
- Sreekanth, A.; Kurup, M.R.P. Structural and spectral studies on four coordinate copper(II) complexes of 2-benzoylpyridine N(4),N(4)-(butane-1,4-diyl)thiosemicarbazone. Polyhedron 2003, 22, 3321–3332. [Google Scholar] [CrossRef]
- Yang, L.; Chen, W.; Chen, Y.; Liu, W.; Lei, T.; Li, L.; Lin, M.; Wu, J.; Cao, Y.; Li, W.; et al. Copper(II) and Cadmium(II) Complexes Based on N,N-Bis(3,5-dimethyl-2-hydroxybenzyl)-N-(2-pyridylmethyl) amine Ligand: Syntheses, Structures, Magnetic, and Luminescent Properties. Z. Anorg. Allg. Chem. 2012, 638, 1833–1838. [Google Scholar] [CrossRef]
- O’Connor, C.J.; Eduok, E.E.; Owens, J.W.; Stevens, E.D.; Klein, C.L. The synthesis, magnetic properties, and crystal structure of two copper(II) complexes prepared from 2-aminomethylpyridine. Inorg. Chim. Acta 1986, 117, 175–181. [Google Scholar] [CrossRef]
- Helis, H.M.; Goodman, W.H.; Wilson, R.B.; Morgan, J.A.; Hodgson, D.J. A novel halogen-bridged system: Synthesis and structures of dibromo [2-(2-aminomethyl) pyridine] copper(II) and dibromo (2-methyl-1, 2-diaminopropane) copper(II). Inorg. Chem. 1977, 16, 2412–2416. [Google Scholar] [CrossRef]
- Žilić, D.; Maity, D.; Cetina, M.; Molčanov, K.; Džolić, Z.; Herak, M. Magnetostructural Characterization of Oxalamide Dihalo-Bridged Copper Dimers: Intra- and Interdimer Interactions Studied by Single-Crystal Electron Spin Resonance Spectroscopy. ChemPhysChem 2017, 18, 2397–2408. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-P.; Hu, H.-P.; Cheng, Z.-Y.; Qiu, X.-J.; Wang, C.-X. Structural insights into the coordination and selective extraction of copper(II) by tertiary amine ligands derived from 2-aminomethylpyridine. Polyhedron 2017, 128, 76–84. [Google Scholar] [CrossRef]
- Gentschev, P.; Möller, N.; Krebs, B. New functional models for catechol oxidases. Inorg. Chim. Acta 2000, 300–302, 442–452. [Google Scholar] [CrossRef]
- Grigereit, T.E.; Ramakrishna, B.L.; Place, H.; Willett, R.D.; Pellacani, G.C.; Manfredini, T.; Menabue, L.; Bonomartini-Corradi, A.; Battaglia, L.P. Structures and magnetic properties of trinuclear copper(II) halide salts. Inorg. Chem. 1987, 26, 2235–2243. [Google Scholar] [CrossRef]
- Kapoor, R.; Kataria, A.; Venugopalan, P.; Kapoor, P.; Corbella, M.; Rodríguez, M.; Romero, I.; Llobet, A. New Tetranuclear Cu(II) Complexes: Synthesis, Structure, and Magnetic Properties. Inorg. Chem. 2004, 43, 6699–6706. [Google Scholar] [CrossRef]
- Macías, B.; Villa, M.V.; Chicote, E.; Martín-Velasco, S.; Castiñeiras, A.; Borrás, J. Copper complexes with dithiocarbamates derived from natural occurring amino acids. Crystal and molecular structure of [Cu(en)(EtOH)(H2O)3][Cu(dtc-pro)2]. Polyhedron 2002, 21, 1899–1904. [Google Scholar] [CrossRef]
- Barnard, B.F.B. Metal-promoted reactions of 2-cyanopyridine: Iron(II), cobalt(II), nickel(II), and copper(II) complexes of O-methylpyridine-2-carboximidate. J. Chem. Soc. A Inorg. Phys. Theor. 1969, 2140–2144. [Google Scholar] [CrossRef]
- Cini, R.; Caputo, P.A.; Intini, F.P.; Natile, G. Mechanistic and Stereochemical Investigation of Imino Ethers Formed by Alcoholysis of Coordinated Nitriles: X-ray Crystal Structures of cis-and trans-Bis(1-imino-1-methoxyethane)dichloroplatinum(II). Inorg. Chem. 1995, 34, 1130–1137. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: Chichester, UK, 2001. [Google Scholar]
- Pavia, D.L.; Lapman, G.M.; Kriz, G.S. Introduction to Spectroscopy, 3rd ed.; Saunders Golden Sunburst Series; Thomson Brooks/Cole: Belmont, CA, USA, 1996. [Google Scholar]
- Bruker AXS. APEX2—Software Suite for Crystallographic Programs; Bruker AXS, Inc.: Madison, WI, USA, 2010. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C: Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS-2008/1—Bruker AXS Area Detector Scaling and Absorption Correction; Bruker AXS: Madison, WI, USA, 2008. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Keith, T.A. AIMAll (Version 12.06.03), TK Gristmill Software; AIMAll: Overland Park, KS, USA, 2003. [Google Scholar]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
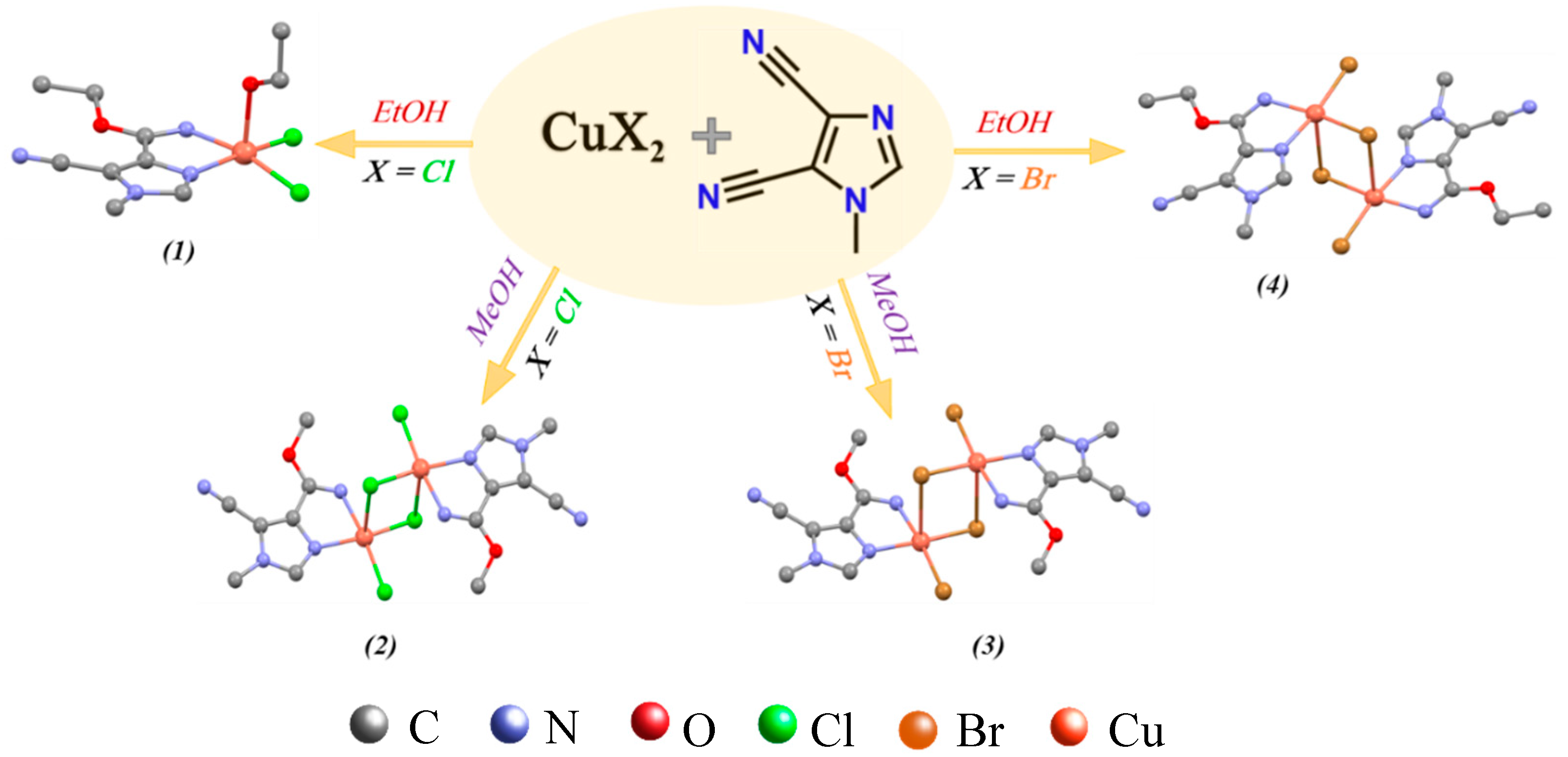
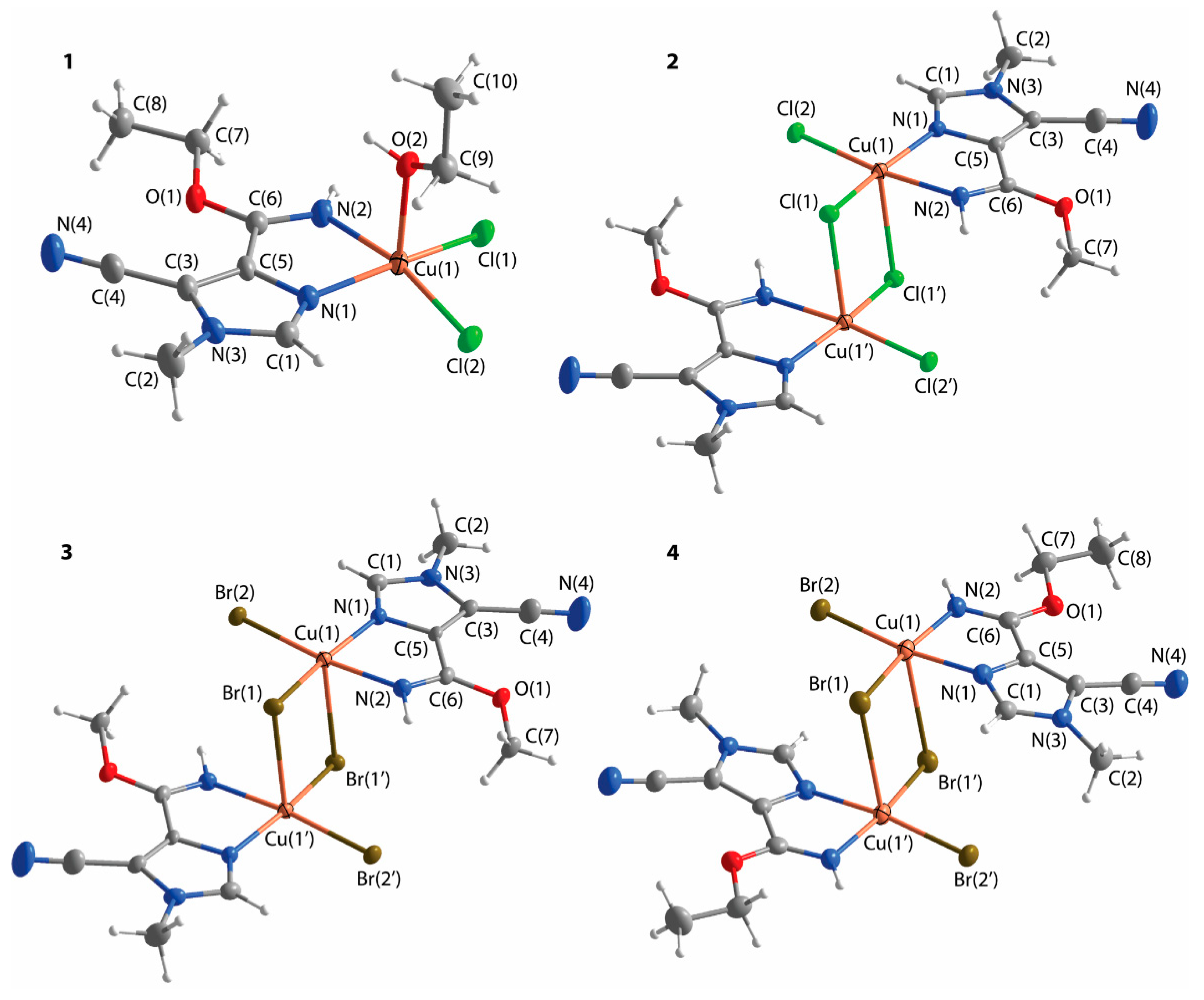
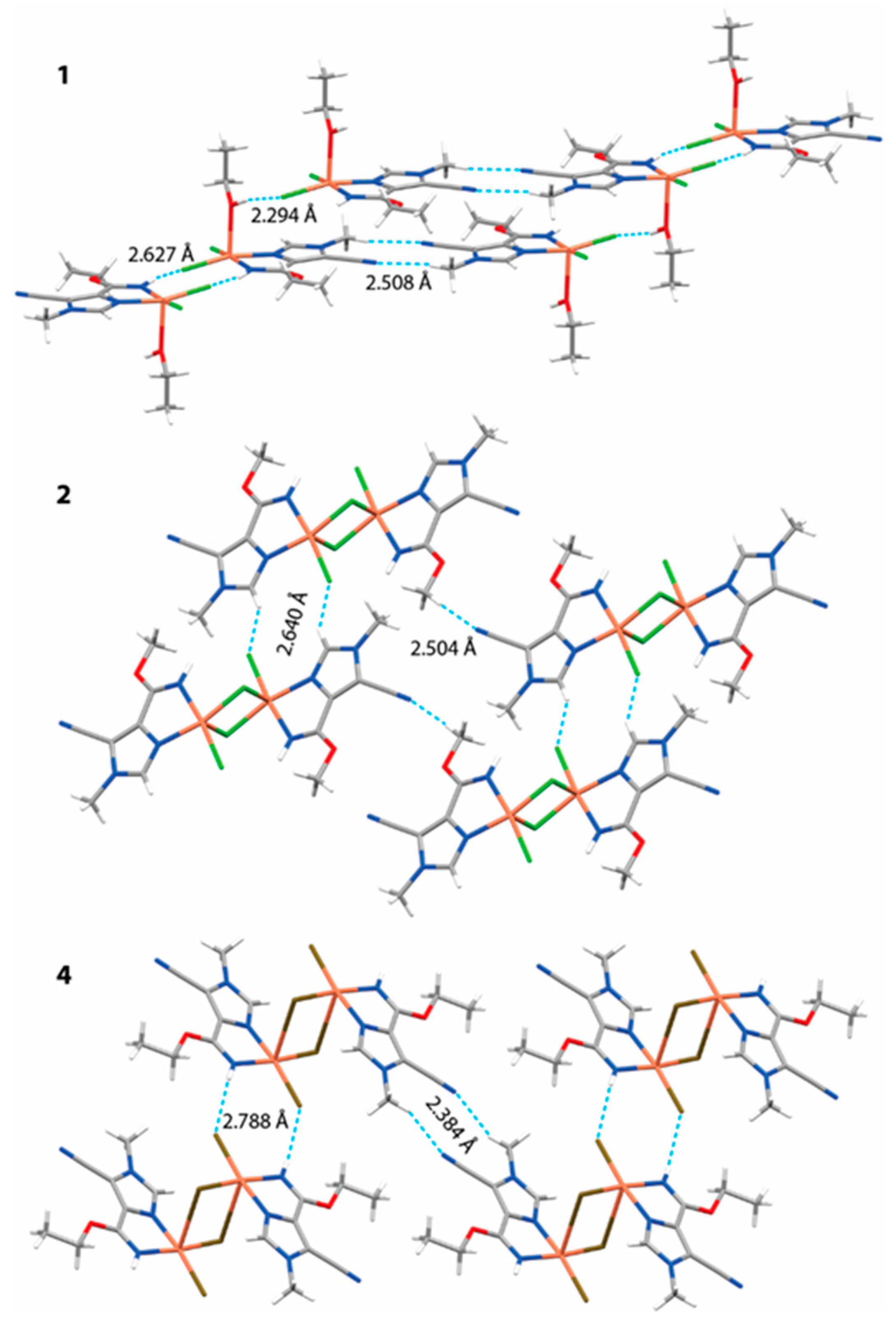
| Parameter | Complex 1 | Complex 2 | Complex 3 | Complex 4 |
|---|---|---|---|---|
| Bond distance (Å) | ||||
| Cu1–N1 | 2.024 | 2.016 | 2.016 | 1.989 |
| Cu1–N2 | 2.038 | 2.010 | 2.012 | 2.032 |
| Cu1–O2/X’ (ax) | 2.275 | 2.781 | 2.944 | 3.139 |
| Cu1–X1 | 2.265 | 2.250 | 2.380 | 2.395 |
| Cu1–X2 | 2.252 | 2.250 | 2.388 | 2.387 |
| Cu1–Cu1′ | - | 3.395 | 3.526 | 3.860 |
| Angle (o) | ||||
| O2/X’–Cu1–X1 | 100.4 | 95.8 | 97.8 | 92.6 |
| O2/X’–Cu1–X2 | 97.1 | 100.7 | 99.7 | 105.6 |
| O2/X’–Cu1–N1 | 91.0 | 89.8 | 88.2 | 78.8 |
| O2/X’–Cu1–N2 | 92.2 | 82.5 | 83.0 | 88.7 |
| X1–Cu1–N2 | 89.8 | 89.9 | 90.3 | 170.0 |
| X1–Cu1–N1 | 165.2 | 167.9 | 168.2 | 90.3 |
| X1–Cu1–X2 | 96.4 | 95.8 | 95.2 | 95.8 |
| X2–Cu1–N1 | 91.5 | 93.7 | 93.8 | 172.3 |
| X2–Cu1–N2 | 167.7 | 173.1 | 173.4 | 93.3 |
| N1–Cu1–N2 | 80.3 | 80.2 | 80.3 | 80.3 |
| Cu1–N1–C5–C3 | −173.6 | 173.9 | −172.8 | −168.2 |
| τ ** | 0.042 | 0.087 | 0.085 | 0.038 |
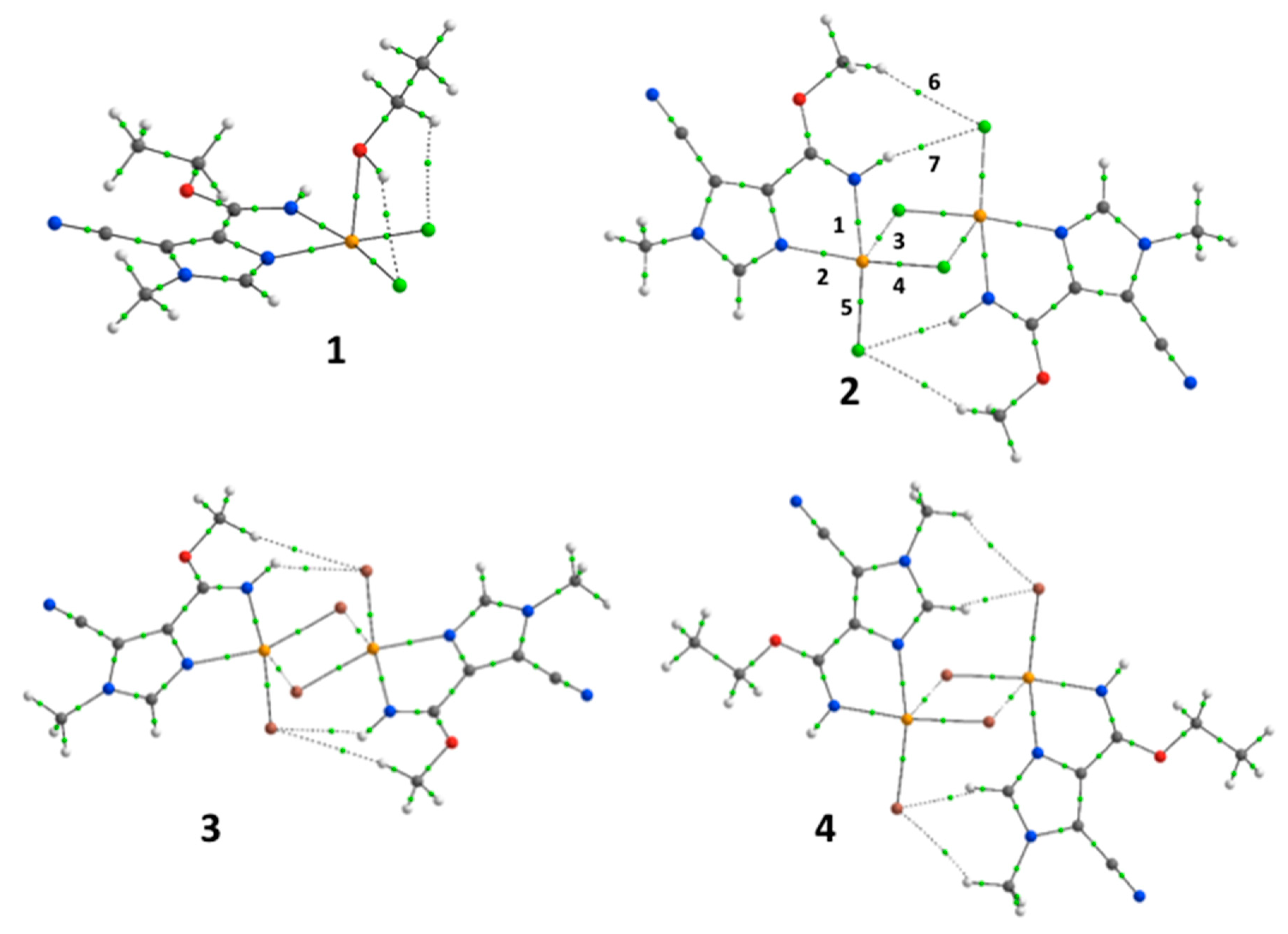
| BCP# | Type | ρ(eÅ−3) | |V|/G | EINT (kJ·mol−1) |
|---|---|---|---|---|
| [CuCl2(5-cyano-4-C(OEt)N-1-methylimidazole)(EtOH)] (1) | ||||
| 1 | Cu1–N2 | 0.496 | 1.20 | −144 |
| 2 | Cu1–N1 | 0.473 | 1.18 | −138 |
| 3 | Cu1–Cl1 | 0.546 | 1.34 | −136 |
| 4′ | Cu1–O2 | 0.206 | 0.97 | −41 |
| 5 | Cu1–Cl2 | 0.493 | 1.31 | −119 |
| [Cu2(µ-Cl)2Cl2(5-cyano-4-C(OMe)N-1-methylimidazole)2] (2) | ||||
| 1 | Cu1–N2 | 0.525 | 1.22 | −153 |
| 2 | Cu1–N1 | 0.505 | 1.20 | −150 |
| 3 | Cu1–Cl1 | 0.494 | 1.31 | −121 |
| 4 | Cu1–Cl1′ | 0.162 | 0.99 | −25 |
| 5 | Cu1–Cl2 | 0.497 | 1.31 | −121 |
| [Cu2(µ-Br)2Br2(5-cyano-4-C(OMe)N-1-methylimidazole)2] (3) | ||||
| 1 | Cu1–N2 | 0.525 | 1.22 | −153 |
| 2 | Cu1–N1 | 0.491 | 1.20 | −144 |
| 3 | Cu1–Br1 | 0.434 | 1.34 | −90 |
| 4 | Cu1–Br1′ | 0.160 | 1.03 | −23 |
| 5 | Cu1–Br2 | 0.440 | 1.36 | −91 |
| [Cu2(µ-Br)2Br2(5-cyano-4-C(OEt)N-1-methylimidazole)2] (4) | ||||
| 1 | Cu1–N2 | 0.526 | 1.22 | −155 |
| 2 | Cu1–N1 | 0.496 | 1.20 | −144 |
| 3 | Cu1–Br2 | 0.428 | 1.34 | −89 |
| 4 | Cu1–Br1′ | 0.144 | 1.01 | −20 |
| 5 | Cu1–Br1 | 0.440 | 1.35 | −91 |
| υ(N2–H) | υ(C1–H) | υ(C≡N) | υ(C6=N2) | υ(C5=C3) | υ(C–O) | υas(C–O) | |
|---|---|---|---|---|---|---|---|
| υ(C5=C3) | υ(C6=N2) | β(N2–H) β(C1–H) υ(C5–N1) υ(N3–C2) | ρ(Me)/OEt skeletal | ||||
| - | 3126 | 2240(vs) | - | - | 1316(m) * | - | |
| 1 | 3283 | 3123 | 2236(w) | 1644(s) | 1587(vs) | 1304(s) | 1007 |
| 2 | 3359 | 3100 | 2242(w) | 1653(vs) | 1593(s) | 1298(s) | 1145 |
| 3 | 3347 | 3097 | 2242(w) | 1651(vs) | 1591(s) | 1296(s) | 1144 |
| 4 | 3289 | 3153 | 2245(w) | 1639(s) | 1586(vs) | 1302(s) | 1005 |
| 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|
| 731 (742) | dd+M(X)LCT | 802 (807) | dd+Cl(p)+CN(π) | 683 (698) | dd+LM(X)CT | 722 (687) | dd+LM(X)CT |
| 399 (380) | dd+Cl(p)+CN(π) | 406 (380) | dd+Cl(p)+CN(π) | 511 (483) | dd+Br(p)+CN(π) | 499 (483) | dd+Br(p)+CN(π) |
| 315 (303) | dd+LM(X)CT | 314 (303) | dd+LM(X)CT | ||||
| 255 (256) | LM(X)CT | 255 (255) | LM(X)CT | 275 (259) | M(X)LCT+LL | 269 (259) | M(X)LCT+LL |
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Empirical formula | C10H16Cl2CuN4O2 | C14H16Cl4Cu2N8O2 | C14H16Br4Cu2N8O2 | C8H10Br2CuN4O |
| Fw (g·mol–1) | 358.71 | 597.23 | 775.07 | 401.56 |
| Crystal system | Triclinic | Triclinic | Triclinic | Triclinic |
| Space group | ||||
| a (Å) | 5.6535(5) | 7.1887(4) | 7.3978(2) | 7.3737(9) |
| b (Å) | 10.3149(8) | 7.4368(5) | 7.6151(2) | 8.4147(11) |
| c (Å) | 13.5498(10) | 10.5356(7) | 10.7043(3) | 10.6554(14) |
| α (o) | 108.336(3) | 82.585(3) | 81.023(2) | 83.614(5) |
| β (o) | 95.921(3) | 89.776(3) | 88.684(2) | 76.079(5) |
| γ (o) | 98.615(3) | 84.799(3) | 84.835(2) | 75.706(5) |
| Z | 2 | 1 | 1 | 2 |
| ρcalc (Mg·m–3) | 1.627 | 1.783 | 2.170 | 2.148 |
| μ(Mo Kα) (mm–1) | 1.859 | 2.421 | 8.556 | 8.178 |
| F(000) | 366 | 298 | 370 | 386 |
| θ range for data collection (o) | 1.604 to 29.000 | 1.949 to 29.999 | 1.926 to 28.998 | 1.972 to 26.998 |
| Refl. collected | 28668 | 24042 | 20109 | 18426 |
| Independent refl. | 3882 [R(int) = 0.0514] | 3229 [R(int) = 0.0466] | 3143 [R(int) = 0.0703] | 2728 [R(int) = 0.0767] |
| GOOF on F2 | 1.023 | 1.050 | 1.079 | 1.024 |
| Final R indices [I>2σ(I)] a | R1 = 0.0363, wR2 = 0.0813 | R1 = 0.0295, wR2 = 0.0707 | R1 = 0.0546, wR2 = 0.1000 | R1 = 0.0349, wR2 = 0.0668 |
| R indices (all data) | R1 = 0.0541, wR2 = 0.0878 | R1 = 0.0438, wR2 = 0.0753 | R1 = 0.0724, wR2 = 0.1068 | R1 = 0.0709, wR2 = 0.0771 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gayfullina, R.; Jääskeläinen, S.; Koshevoy, I.O.; Hirva, P. Activation of the Cyano Group at Imidazole via Copper Stimulated Alcoholysis. Inorganics 2019, 7, 87. https://doi.org/10.3390/inorganics7070087
Gayfullina R, Jääskeläinen S, Koshevoy IO, Hirva P. Activation of the Cyano Group at Imidazole via Copper Stimulated Alcoholysis. Inorganics. 2019; 7(7):87. https://doi.org/10.3390/inorganics7070087
Chicago/Turabian StyleGayfullina, Rezeda, Sirpa Jääskeläinen, Igor O. Koshevoy, and Pipsa Hirva. 2019. "Activation of the Cyano Group at Imidazole via Copper Stimulated Alcoholysis" Inorganics 7, no. 7: 87. https://doi.org/10.3390/inorganics7070087
APA StyleGayfullina, R., Jääskeläinen, S., Koshevoy, I. O., & Hirva, P. (2019). Activation of the Cyano Group at Imidazole via Copper Stimulated Alcoholysis. Inorganics, 7(7), 87. https://doi.org/10.3390/inorganics7070087