Iron Sulfide Materials: Catalysts for Electrochemical Hydrogen Evolution
Abstract
1. Introduction
2. Iron Sulfide Phases as Electrocatalysts
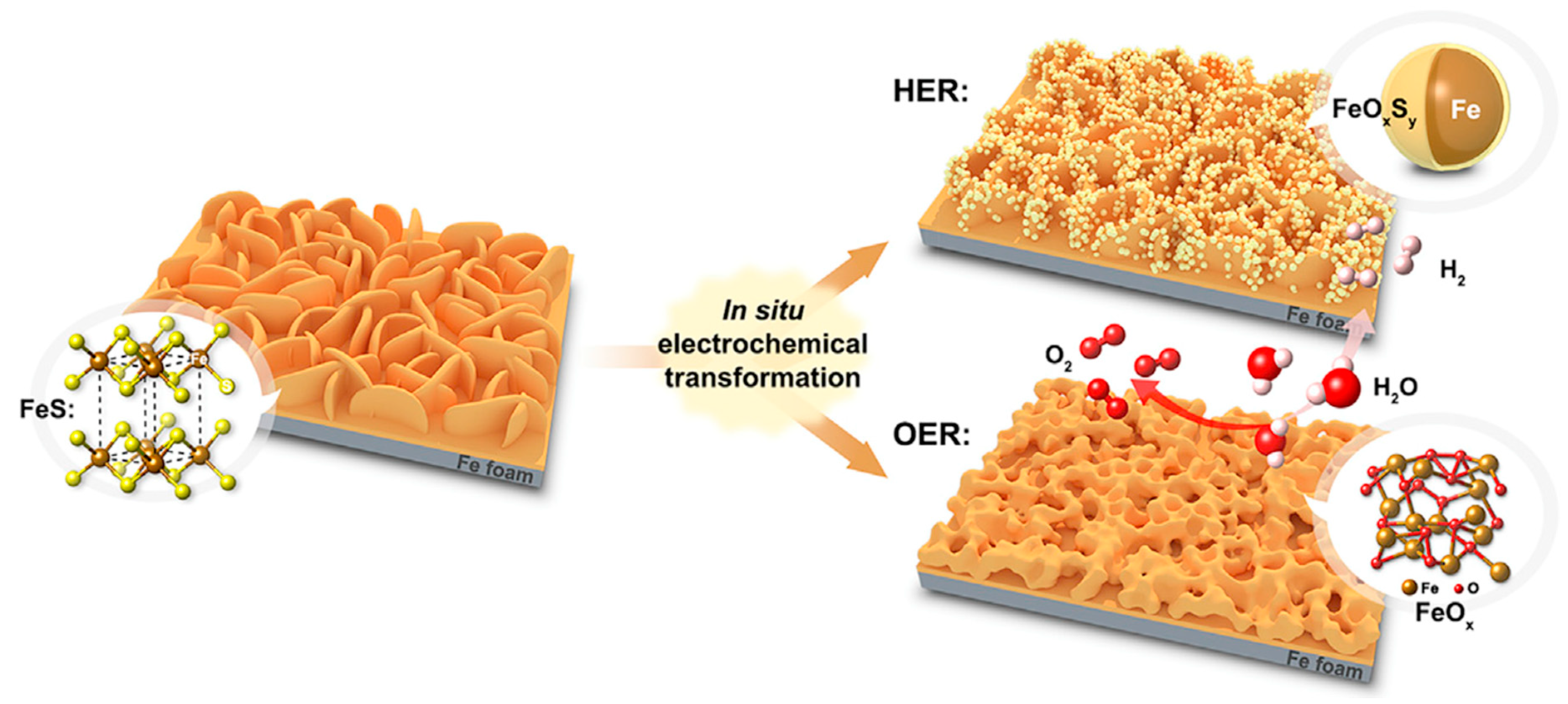
2.1. Iron Monosulfide, FeS
2.2. Iron Disulfide, FeS2
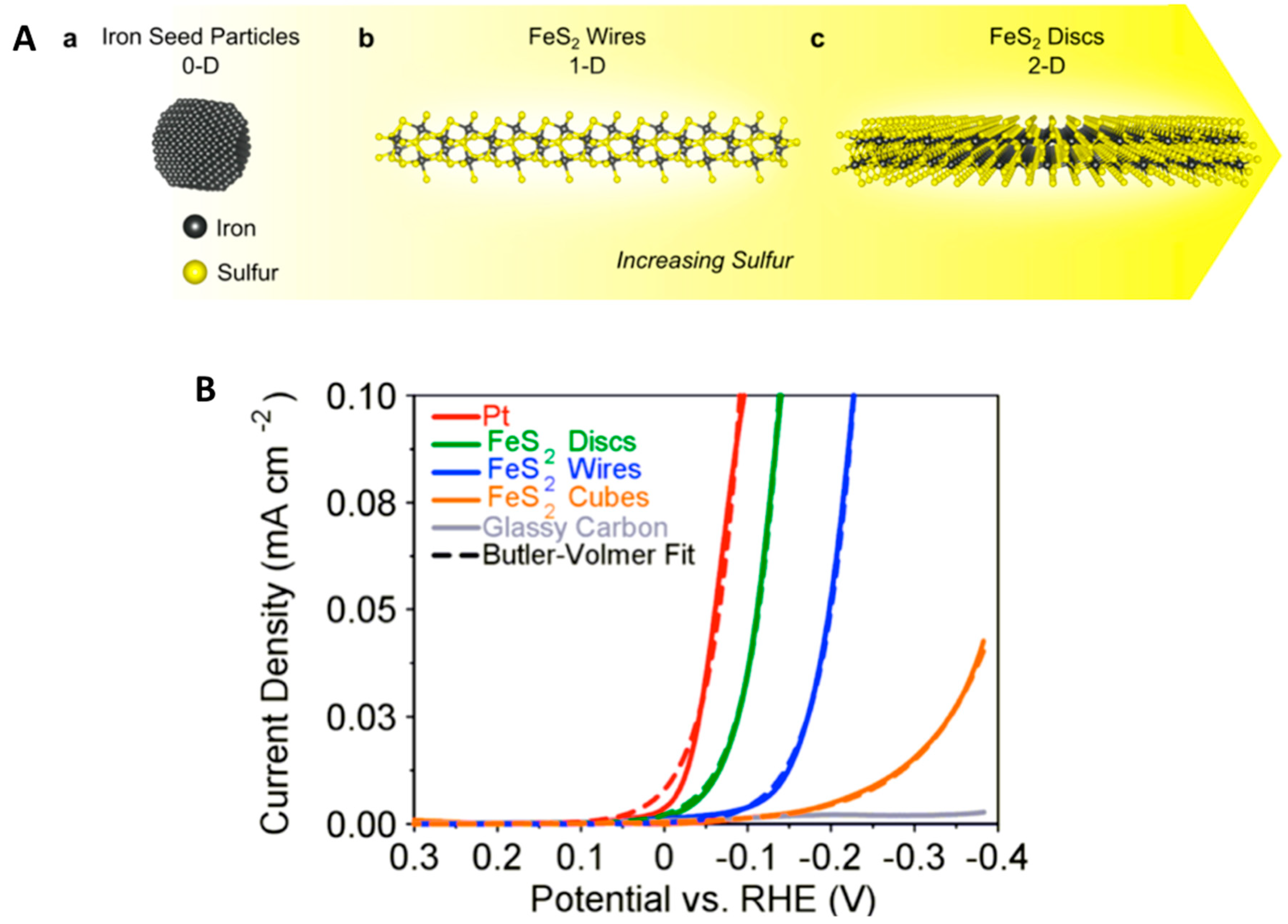
2.2.1. FeS2 Wires and Discs
2.2.2. Mesoporous FeS2 Nanoparticles
2.2.3. FeS2/C Electrode Coating
2.2.4. FeS2 Thin-Films
2.2.5. Resistance of FeS2 Catalysts to Sulfide Poisoning
2.3. pH Dependence of Iron Sulfide Electrocatalysts
2.4. HER Activity of Iron Sulfides in Comparison
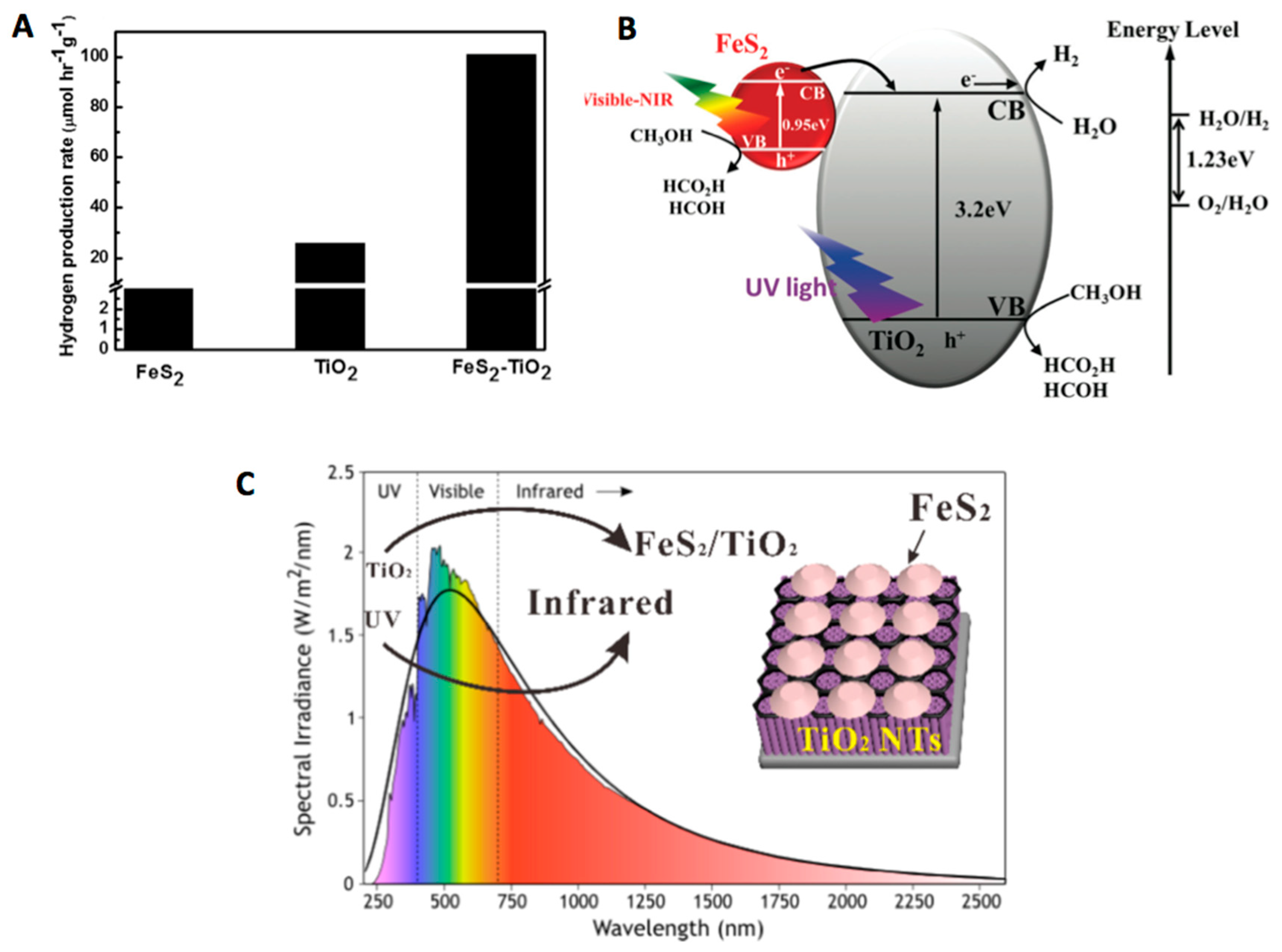
3. FeS2-TiO2 Composite Materials
4. Iron Sulfide-2D Carbon Hybrid Materials
4.1. FeS2/rGO Hybrid Catalysts
4.2. MoS2 and Ni Doped FeS2/rGO Hybrid Catalysts
4.3. Fe1−xS/GO Hybrid Catalyst
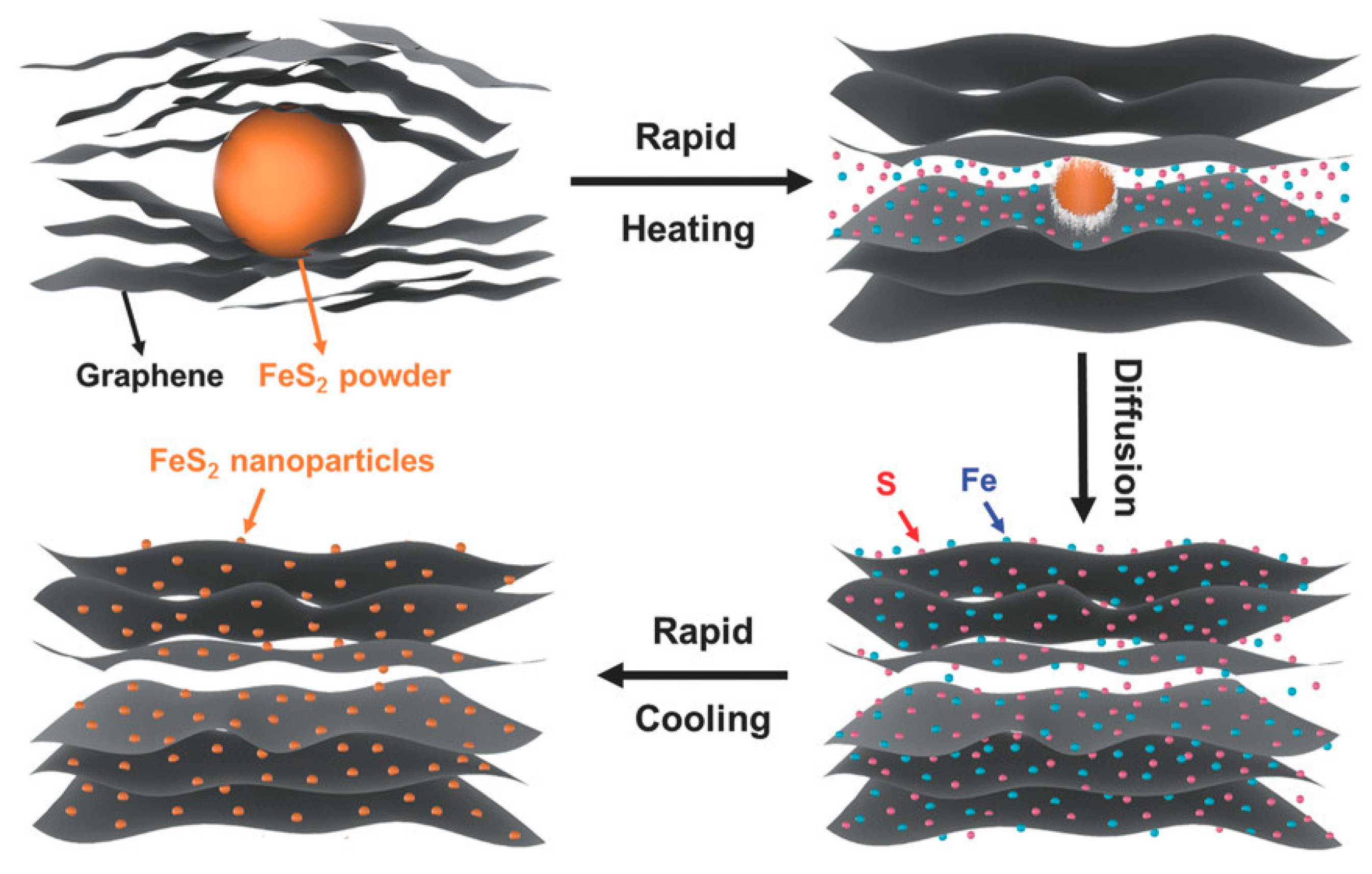
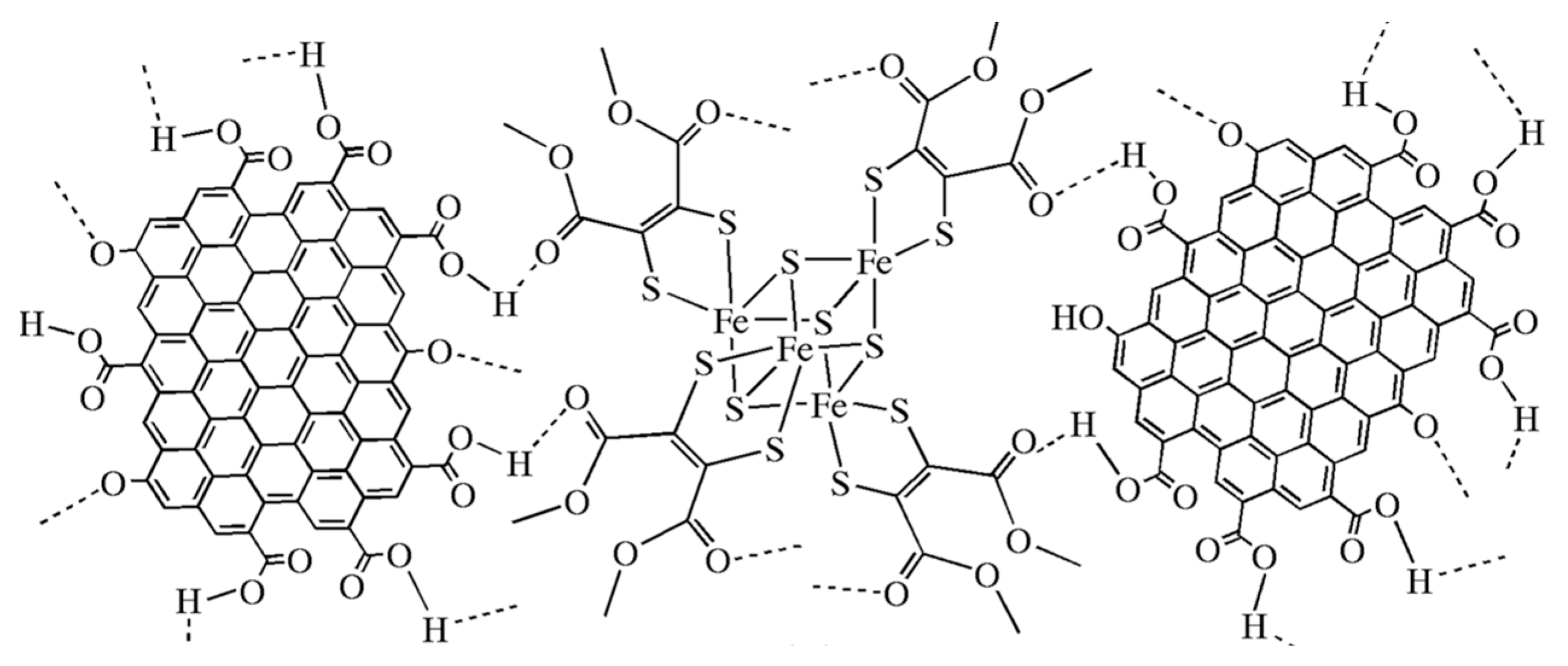
4.4. Fe4S4/Graphene Hybrid Catalyst
5. Metal Doping of Iron Sulfide Nanomaterials
5.1. Cobalt-Doped FeS2 Structures
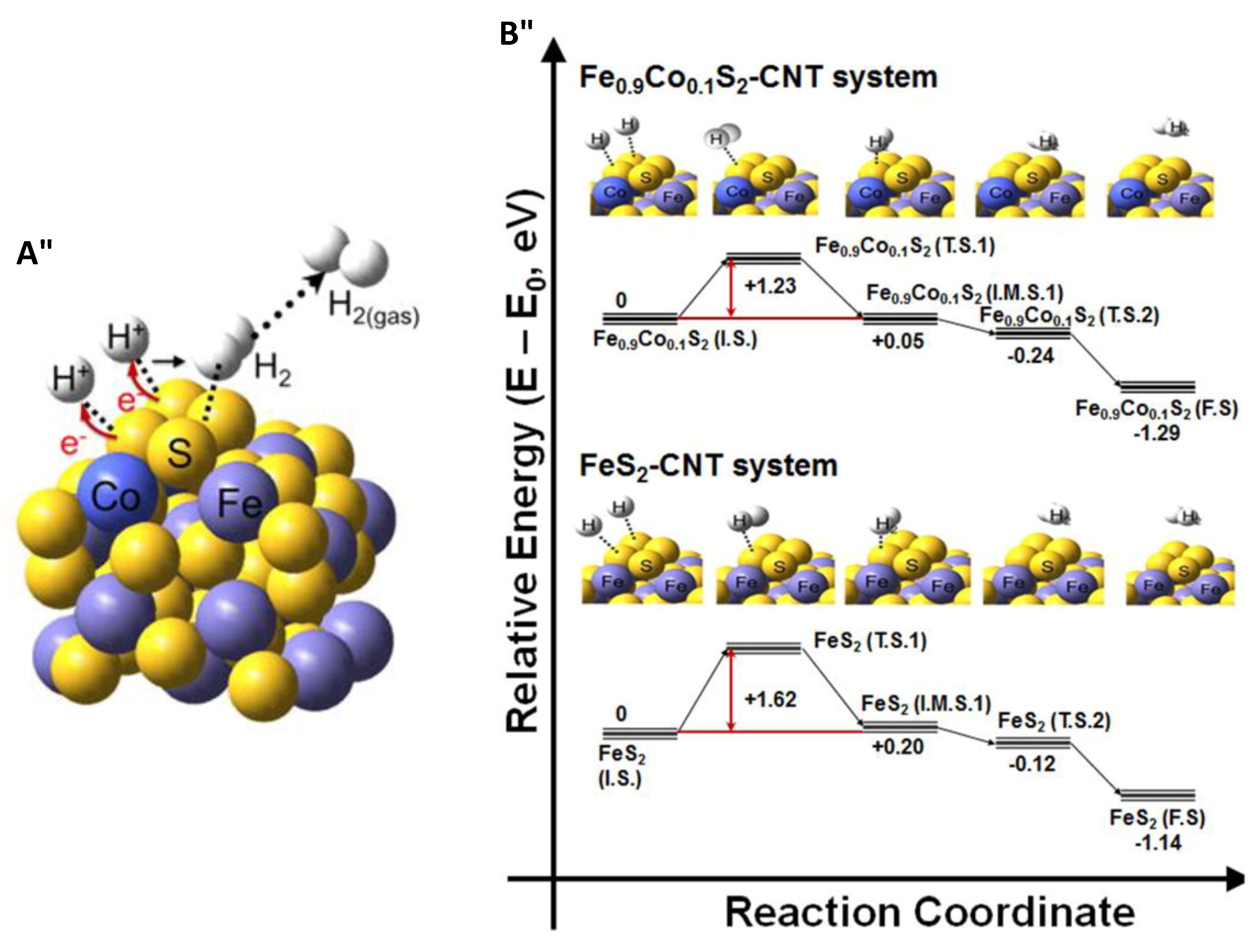
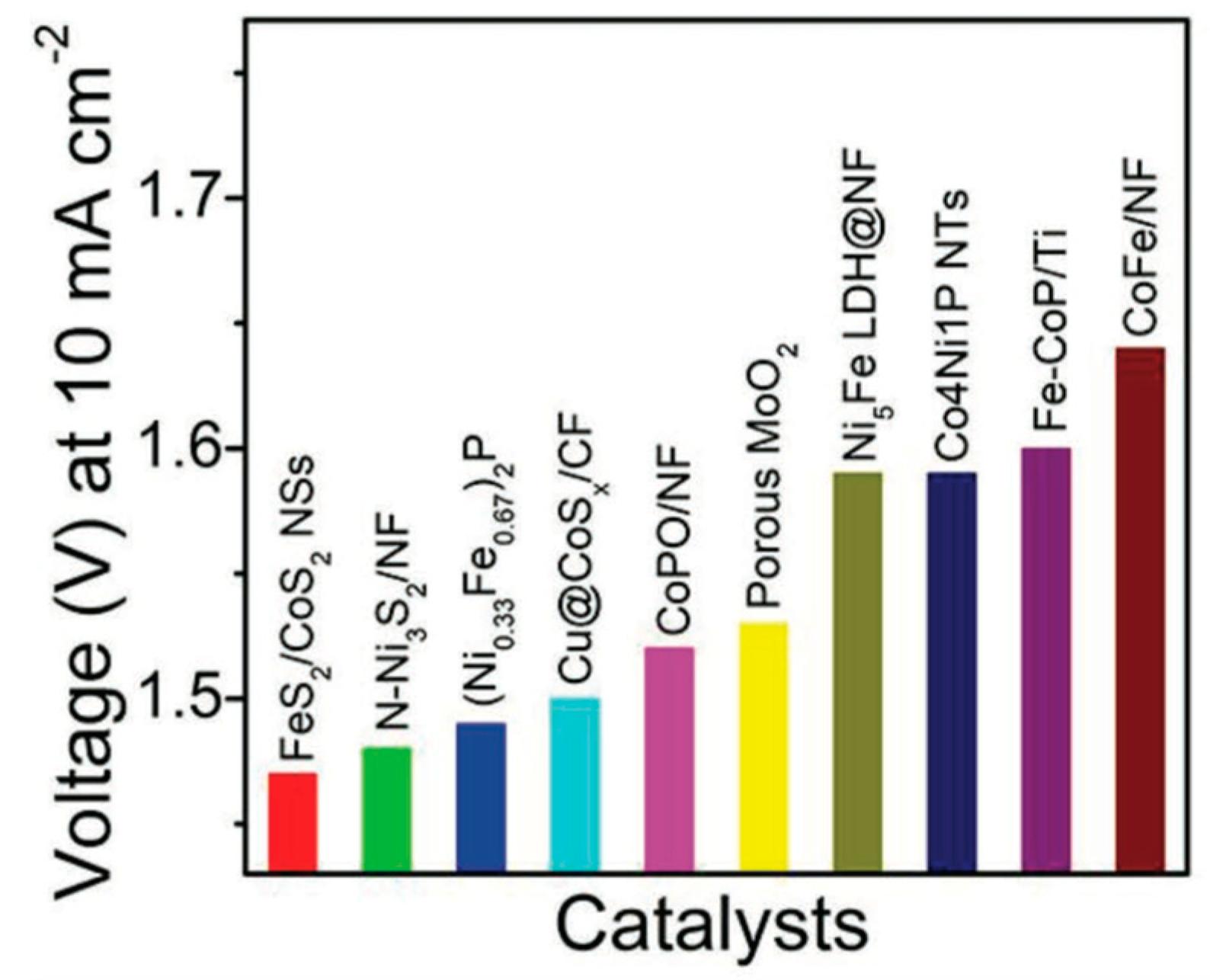
5.2. FeS2-CoS2 Hybrid Structures
5.3. Cobalt-Doped FeS2-CoS2 Hybrid Structures
5.4. FeS2-Doped MoS2 Nanoflowers
6. Conclusions and Outlook
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Summary Table of Discussed Iron Sulfide Materials
| Catalyst | Synthetic Procedure | Electrolyte | Tafel Slope | η HER (X mA cm−2) | Ref. |
|---|---|---|---|---|---|
| Iron sulfides | |||||
| Fe@FeOxSy | FeS nanosheets on iron foam | 1 M KOH | 77 | 243 (100) | [34] |
| FeS NPs on Nafion | Decomposition of Fe2S2(CO)6 | pH 7 | 150 | ~450 (0.71) | [35] |
| FeS2 discs FeS2 wires FeS2 cubes | Hot-injection procedure with FeI2 and S8 in different rations | pH 7 | 76 91 200 | - | [40] |
| FeS2/C NPs | Hydrothermal process from FeCl3·(H2O)6 and diethyl- dithio-carbamate trihydrate | 1 M KOH | 98 | 202 (10) | [44] |
| Mesoporous FeS2 | Conversion of mesoporous Fe2O3 with H2S and S8 into mesoporous FeS2 | pH 13 | 78 | 96(10) | [43] |
| FeS2 thin-films | Atomic Layer Deposition | - | - | - | [49] |
| Cathode in PEM electrolyzer FeS2/C Fe3S4/C Fe9S10/C | Polyol method from FeCl3 and thiourea | pH 7 | 204 224 234 | - | [55] |
| pH dependence of FeS2-catalyzed HER | Polyol method from FeCl3 and thiourea | pH | - | [54] | |
| 0.3 1 2 3 4 5 6 7 9 11 13 | 85 127 170 290 306 340 287 280 274 329 192 | ||||
| FeS2-TiO2 materials | |||||
| FeS2/TiO2 core–shell composites | Deposition of FeS2 NPs (solvothermal from FeCl3 and Na2S2O3) on nano-sized TiO2 | MeOH/H2O (1:1) | - | - | [60] |
| FeS2-TiO2 heterostructures | Injection of S8-oleylamine mixture in solution containing TiO2-octadecene and Fe-oleic acid precursors | MeOH EtOH H2O Na2S aq. Na2S/Na2SO3 solution | - | - | [61] |
| FeS2 sensitized TiO2 nanotubes | Deposition of FeS2 (from FeCl3 and thiourea) on TiO2 nanotubes | pH 7.4 | - | - | [62] |
| Iron sulfide-2D carbon hybrid materials | |||||
| nano-FeS2-rGO | Current-induced high-temperature thermal shock process | 0.5 M H2SO4 | 66 | 139 (10) | [69] |
| FeS2-rGO | Hydrothermal reduction process | 0.5 M H2SO4 | 61 | 226 (10) | [71] |
| FeS2@MoS2/rGO | Autoclave reaction of FeCl3, phosphomolybdic acid hydrate and l-cysteine | 0.5 M H2SO4 | 38.4 | 123 (10) | [73] |
| FeNi0.05S2-rGO FeNi0.10S2-rGO FeNi0.15S2-rGO FeNi0.20S2-rGO FeNi0.25S2-rGO FeNi0.30S2-rGO | High-temperature hydrothermal method from GO, FeCl3, NiCl2, and thioacetamide | 0.5 M H2SO4 | 74.46 71.96 76.96 78.36 82.82 79.19 | 232 225 198 183 209 260 (10) | [74] |
| nano-micro Fe1−xS@S-GO | Autoclave reaction of aqueous solutions of FeCl3, GO, and thioacetamide | - | - | - | [75] |
| [PPh4]2[Fe4(μ3-S)4(DMET)4]@graphene | Ultrasonication of Fe4S4 cubane type cluster with functionalized graphene | p-toluene sulfonic acid | - | - | [76] |
| Metal-doped iron sulfide materials | |||||
| Fe0.50 Co0.50S2 on Ketjenblack oxidized with 10 N nitric acid | Chemical sulfurization of metal precursors using H2S | 0.5 M H2SO4 | 52 | 150 (10) | [79] |
| P/Co-FeS2 | Hydrothermal process of iron nitrate and cobalt nitrate followed by CVD treatment with S8 | 0.5 M H2SO4 | 41.5 | 90 (100) | [80] |
| Fe0.9Co0.1S2/CNT | Solvothermal approach | 0.5 M H2SO4 | 46 | 120 (20) | [81] |
| FeS2/CoS2 interface nanosheets | Annealing of CoFe2O4 NPs with sulfur | 1 M KOH | 44 | 78.2 (10) | [82] |
| Co-FeS2/CoS | Hydrothermal process from FeSO4, Co(NO3)2, thiourea, and sulfur | 0.5 M H2SO4 | 56 | 103 (10) | [83] |
| FeS2-doped MoS2 | Autoclave process of Na2Mo4·2H2O, thiourea, and Fe3O4 microspheres | 0.5 M H2SO4 | 82 | 136 (10) | [84] |
References
- Nandi, R.; Sengupta, S. Microbial production of hydrogen: An overview. Crit. Rev. Microbiol. 1998, 24, 61–84. [Google Scholar] [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H.W. Hydrogen—A sustainable energy carrier. Prog. Nat. Sci. Mater. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Ryabchuk, V.K.; Kuznetsov, V.N.; Emeline, A.V.; Artem’ev, Y.M.; Kataeva, G.V.; Horikoshi, S.; Serpone, N. Water Will Be the Coal of the Future-The Untamed Dream of Jules Verne for a Solar Fuel. Molecules 2016, 21, 1638. [Google Scholar] [CrossRef]
- Hacker, V.; Fankhauser, R.; Faleschini, G.; Fuchs, H.; Friedrich, K.; Muhr, M.; Kordesch, K. Hydrogen production by steam–iron process. J. Power Sources 2000, 86, 531–535. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Jin, F.M.; Zeng, X.; Yao, G.D.; Jing, Z.Z. A novel method for producing hydrogen from water with Fe enhanced by HS− under mild hydrothermal conditions. Int. J. Hydrogen Energy 2013, 38, 760–768. [Google Scholar] [CrossRef]
- Zou, X.X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, J.; Han, B. First-Principles Computational Screening of Highly Active Pyrites Catalysts for Hydrogen Evolution Reaction through a Universal Relation with a Thermodynamic Variable. J. Phys. Chem. C 2018, 122, 2107–2112. [Google Scholar] [CrossRef]
- Zhang, L.; Doyle-Davis, K.; Sun, X.L. Pt-Based electrocatalysts with high atom utilization efficiency: From nanostructures to single atoms. Energy Environ. Sci. 2019, 12, 492–517. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Lubitz, W.; Ogata, H.; Rüdiger, O.; Reijerse, E. Hydrogenases. Chem. Rev. 2014, 114, 4081–4148. [Google Scholar] [CrossRef]
- Simmons, T.R.; Berggren, G.; Bacchi, M.; Fontecave, M.; Artero, V. Mimicking hydrogenases: From biomimetics to artificial enzymes. Coord. Chem. Rev. 2014, 270, 127–150. [Google Scholar] [CrossRef]
- Hosseini, M.R.; Sarvi, M.N. Recent achievements in the microbial synthesis of semiconductor metal sulfide nanoparticles. Mater. Sci. Semicond. Process. 2015, 40, 293–301. [Google Scholar] [CrossRef]
- Kong, D.; Cha, J.J.; Wang, H.; Lee, H.R.; Cui, Y. First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Fu, H. Environmentally friendly and earth-abundant colloidal chalcogenide nanocrystals for photovoltaic applications. J. Mater. Chem. C 2018, 6, 414–445. [Google Scholar] [CrossRef]
- Faber, M.S.; Lukowski, M.A.; Ding, Q.; Kaiser, N.S.; Jin, S. Earth-Abundant Metal Pyrites (FeS2, CoS2, NiS2, and Their Alloys) for Highly Efficient Hydrogen Evolution and Polysulfide Reduction Electrocatalysis. J. Phys. Chem. C 2014, 118, 21347–21356. [Google Scholar] [CrossRef]
- Gao, M.R.; Zheng, Y.R.; Jiang, J.; Yu, S.H. Pyrite-Type Nanomaterials for Advanced Electrocatalysis. Acc. Chem. Res. 2017, 50, 2194–2204. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Zhu, J.X.; Yan, Q.Y.; Dou, S.X.; Sun, W.P. Nanostructured Metal Chalcogenides for Energy Storage and Electrocatalysis. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Wang, J.S.; Liu, J.; Zhang, B.; Ji, X.; Xu, K.; Chen, C.; Miao, L.; Jiang, J.J. The mechanism of hydrogen adsorption on transition metal dichalcogenides as hydrogen evolution reaction catalyst. Phys. Chem. Chem. Phys. 2017, 19, 10125–10132. [Google Scholar] [CrossRef]
- Khalid, S.; Ahmed, E.; Khan, Y.; Riaz, K.N.; Malik, M.A. Nanocrystalline Pyrite for Photovoltaic Applications. Chemistryselect 2018, 3, 6488–6524. [Google Scholar] [CrossRef]
- Bi, Y.; Yuan, Y.B.; Exstrom, C.L.; Darveau, S.A.; Huang, J.S. Air Stable, Photosensitive, Phase Pure Iron Pyrite Nanocrystal Thin Films for Photovoltaic Application. Nano Lett. 2011, 11, 4953–4957. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, H.; Qu, J.; Li, J. Two-dimensional layered MoS2: Rational design, properties and electrochemical applications. Energy Environ. Sci. 2016, 9, 1190–1209. [Google Scholar] [CrossRef]
- Su, J.Z.; Zhou, J.L.; Wang, L.; Liu, C.; Chen, Y.B. Synthesis and application of transition metal phosphides as electrocatalyst for water splitting. Sci. Bull. 2017, 62, 633–644. [Google Scholar] [CrossRef]
- Sosa, E.; Cabrera-Sierra, R.; Oropeza, M.T.; Gonzalez, I. Stability study of iron sulfide films, electrochemically grown on carbon steel, in different electrolytic media. Corrosion 2002, 58, 659–669. [Google Scholar] [CrossRef]
- Rickard, D.; Luther, G.W. Chemistry of Iron Sulfides. Chem. Rev. 2007, 107, 514–562. [Google Scholar] [CrossRef]
- Matamoros-Veloza, A.; Cespedes, O.; Johnson, B.R.G.; Stawski, T.M.; Terranova, U.; de Leeuw, N.H.; Benning, L.G. A highly reactive precursor in the iron sulfide system. Nat. Commun. 2018, 9, 3125. [Google Scholar] [CrossRef]
- Matamoros-Veloza, A.; Stawski, T.M.; Benning, L.G. Nanoparticle Assembly Leads to Mackinawite Formation. Cryst. Growth Des. 2018, 18, 6757–6764. [Google Scholar] [CrossRef]
- Heift, D.; Lacroix, L.-M.; Lecante, P.; Fazzini, P.-F.; Chaudret, B. Controlling the Sulfidation Process of Iron Nanoparticles: Accessing Iron−Iron Sulfide Core-Shell Structures. ChemNanoMat 2018, 4, 663–669. [Google Scholar] [CrossRef]
- Qin, H.; Jia, J.; Lin, L.; Ni, H.; Wang, M.; Meng, L. Pyrite FeS2 nanostructures: Synthesis, properties and applications. Mater. Sci. Eng. B 2018, 236, 104–124. [Google Scholar] [CrossRef]
- Raturi, A.K.; Waita, S.; Aduda, B.; Nyangonda, T. Photoactive iron pyrite films for photoelectrochemical (PEC) cells. Renew. Energy 2000, 20, 37–43. [Google Scholar] [CrossRef]
- Valand, T.; Burchardt, T.; van der Meer, S.F. The hydrogen evolution and corrosion of amorphous FeSx films. Corros. Sci. 2001, 43, 147–156. [Google Scholar] [CrossRef]
- Ennaoui, A.; Fiechter, S.; Pettenkofer, C.; Alonso-Vante, N.; Bueker, K.; Bronold, M.; Hoepfner, C.; Tributsch, H. Iron disulfide for solar energy conversion. Sol. Energy Mater. Sol. Cells 1993, 29, 289–370. [Google Scholar] [CrossRef]
- Dasbach, R.; Willeke, G.; Blenk, O. Iron sulfide for photovoltaics. MRS Bull. 1993, 18, 56–60. [Google Scholar] [CrossRef]
- Rui, X.; Tan, H.; Yan, Q. Nanostructured metal sulfides for energy storage. Nanoscale 2014, 6, 9889–9924. [Google Scholar] [CrossRef]
- Zou, X.; Wu, Y.; Liu, Y.; Liu, D.; Li, W.; Gu, L.; Liu, H.; Wang, P.; Sun, L.; Zhang, Y. In Situ Generation of Bifunctional, Efficient Fe-Based Catalysts from Mackinawite Iron Sulfide for Water Splitting. Chem 2018, 4, 1139–1152. [Google Scholar] [CrossRef]
- Di Giovanni, C.; Wang, W.-A.; Nowak, S.; Grenèche, J.-M.; Lecoq, H.; Mouton, L.; Giraud, M.; Tard, C. Bioinspired Iron Sulfide Nanoparticles for Cheap and Long-Lived Electrocatalytic Molecular Hydrogen Evolution in Neutral Water. ACS Catal. 2014, 4, 681–687. [Google Scholar] [CrossRef]
- Tard, C.; Giraud, M. Iron Sulfide Based Catalyst for Electrolytic Water Reduction into Hydrogen Gas. WO2014207156A1, 31 December 2014. [Google Scholar]
- Yersak, T.A.; Macpherson, H.A.; Kim, S.C.; Le, V.-D.; Kang, C.S.; Son, S.-B.; Kim, Y.-H.; Trevey, J.E.; Oh, K.H.; Stoldt, C.; et al. Solid State Enabled Reversible Four Electron Storage. Adv. Energy Mater. 2013, 3, 120–127. [Google Scholar] [CrossRef]
- Murphy, R.; Strongin, D.R. Surface reactivity of pyrite and related sulfides. Surf. Sci. Rep. 2009, 64, 1–45. [Google Scholar] [CrossRef]
- Huerta-Flores, A.M.; Torres-Martinez, L.M.; Moctezuma, E.; Singh, A.P.; Wickman, B. Green synthesis of earth-abundant metal sulfides (FeS2, CuS, and NiS2) and their use as visible-light active photocatalysts for H2 generation and dye removal. J. Mater. Sci. Mater. Electron. 2018, 29, 11613–11626. [Google Scholar] [CrossRef]
- Jasion, D.; Barforoush, J.M.; Qiao, Q.; Zhu, Y.; Ren, S.; Leonard, K.C. Low-Dimensional Hyperthin FeS2 Nanostructures for Efficient and Stable Hydrogen Evolution Electrocatalysis. ACS Catal. 2015, 5, 6653–6657. [Google Scholar] [CrossRef]
- Ren, S.; Leonard, K.C.; Barforoush, J.M.; Jasion, D. Low-dimensional Hyperthin FeS2 Nanostructures for Electrocatalysis. US20180222767A1, 9 August 2018. [Google Scholar]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.H.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the Characterization of Porous Solids. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Miao, R.; Dutta, B.; Sahoo, S.; He, J.; Zhong, W.; Cetegen, S.A.; Jiang, T.; Alpay, S.P.; Suib, S.L. Mesoporous Iron Sulfide for Highly Efficient Electrocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2017, 139, 13604–13607. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, M.; Zhou, Y.; Zhang, D.; Wang, H.; Liu, X.; Wang, D.; Wang, W. Pyrite FeS2/C nanoparticles as an efficient bi-functional catalyst for overall water splitting. Dalton Trans. 2018, 47, 14917–14923. [Google Scholar] [CrossRef]
- Anantharaj, S.; Karthik, P.E.; Kundu, S. Self-assembled IrO2 nanoparticles on a DNA scaffold with enhanced catalytic and oxygen evolution reaction (OER) activities. J. Mater. Chem. A 2015, 3, 24463–24478. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.H.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Liu, H.F.; Chi, D.Z. Synthesis of iron sulfide and iron oxide nanocrystal thin films for green energy applications. Procedia Eng. 2016, 141, 32–37. [Google Scholar] [CrossRef]
- Dasgupta, N.P.; Meng, X.; Elam, J.W.; Martinson, A.B.F. Atomic Layer Deposition of Metal Sulfide Materials. Acc. Chem. Res. 2015, 48, 341–348. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, X. Atomic Layer Deposition of the Metal Pyrites FeS2, CoS2, and NiS2. Angew. Chem. Int. Ed. 2018, 57, 5898–5902. [Google Scholar] [CrossRef]
- Wu, L.; Dzade, N.Y.; Gao, L.; Scanlon, D.O.; Öztürk, Z.; Hollingsworth, N.; Weckhuysen, B.M.; Hensen, E.J.M.; de Leeuw, N.H.; Hofmann, J.P. Enhanced Photoresponse of FeS2 Films: The Role of Marcasite–Pyrite Phase Junctions. Adv. Mater. 2016, 28, 9602–9607. [Google Scholar] [CrossRef]
- Protopopoff, E.; Marcus, P. Poisoning of the Cathodic Hydrogen Evolution Reaction by Sulfur Chemisorbed on Platinum (110). J. Electrochem. Soc. 1988, 135, 3073–3075. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Susceptibility of FeS2 hydrogen evolution performance to sulfide poisoning. Electrochem. Commun. 2015, 58, 29–32. [Google Scholar] [CrossRef]
- Zakaria, S.N.A.; Hollingsworth, N.; Islam, H.U.; Roffey, A.; Santos-Carballal, D.; Roldan, A.; Bras, W.; Sankar, G.; Hogarth, G.; Holt, K.B.; et al. Insight into the Nature of Iron Sulfide Surfaces During the Electrochemical Hydrogen Evolution and CO2 Reduction Reactions. ACS Appl. Mater. Interfaces 2018, 10, 32078–32085. [Google Scholar] [CrossRef]
- Villalba, M.; Peron, J.; Giraud, M.; Tard, C. pH-dependence on HER electrocatalytic activity of iron sulfide pyrite nanoparticles. Electrochem. Commun. 2018, 91, 10–14. [Google Scholar] [CrossRef]
- Giovanni, C.D.; Reyes-Carmona, Á.; Coursier, A.; Nowak, S.; Grenèche, J.M.; Lecoq, H.; Mouton, L.; Rozière, J.; Jones, D.; Peron, J.; et al. Low-Cost Nanostructured Iron Sulfide Electrocatalysts for PEM Water Electrolysis. ACS Catal. 2016, 6, 2626–2631. [Google Scholar] [CrossRef]
- Roldan, A.; de Leeuw, N.H. Catalytic water dissociation by greigite Fe3S4 surfaces: Density functional theory study. Proc. R. Soc. A 2016. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Xu, H.; Ouyang, S.X.; Liu, L.Q.; Reunchan, P.; Umezawa, N.; Ye, J.H. Recent advances in TiO2-based photocatalysis. J. Mater. Chem. A 2014, 2, 12642–12661. [Google Scholar] [CrossRef]
- Wan, D.; He, Q.; Zhang, L.; Jia, Q.; Zhang, R.; Zhang, H.; Wang, B.; Wei, L. Study on pyrite FeS2 films deposited on Si(100) substrate by synchrotron radiation surface x-ray diffraction method. J. Cryst. Growth 2004, 268, 222–226. [Google Scholar] [CrossRef]
- Lee, G.; Kang, M. Physicochemical properties of core/shell structured pyrite FeS2/anatase TiO2 composites and their photocatalytic hydrogen production performances. Curr. Appl. Phys. 2013, 13, 1482–1489. [Google Scholar] [CrossRef]
- Kuo, T.R.; Liao, H.J.; Chen, Y.T.; Wei, C.Y.; Chang, C.C.; Chen, Y.C.; Chang, Y.H.; Lin, J.C.; Lee, Y.C.; Wen, C.Y.; et al. Extended visible to near-infrared harvesting of earth-abundant FeS2-TiO2 heterostructures for highly active photocatalytic hydrogen evolution. Green Chem. 2018, 20, 1640–1647. [Google Scholar] [CrossRef]
- Xin, Y.M.; Li, Z.Z.; Wu, W.L.; Fu, B.H.; Zhang, Z.H. Pyrite FeS2 Sensitized TiO2 Nanotube Photoanode for Boosting Near-Infrared Light Photoelectrochemical Water Splitting. ACS Sustain. Chem. Eng. 2016, 4, 6659–6667. [Google Scholar] [CrossRef]
- Meng, X.C.; Zhang, Z.S. Two dimensional graphitic materials for photoelectrocatalysis: A short review. Catal. Today 2018, 315, 2–8. [Google Scholar] [CrossRef]
- Xu, G.R.; Hui, J.J.; Huang, T.; Chen, Y.; Lee, J.M. Platinum nanocuboids supported on reduced graphene oxide as efficient electrocatalyst for the hydrogen evolution reaction. J. Power Sources 2015, 285, 393–399. [Google Scholar] [CrossRef]
- Wang, S.N.; Liao, L.; Shi, Z.P.; Xiao, J.J.; Gao, Q.S.; Zhang, Y.H.; Liu, B.H.; Tang, Y. Mo2C/Reduced-Graphene-Oxide Nanocomposite: An Efficient Electrocatalyst for the Hydrogen Evolution Reaction. Chemelectrochem 2016, 3, 2110–2115. [Google Scholar] [CrossRef]
- Johnson, D.W.; Dobson, B.P.; Coleman, K.S. A manufacturing perspective on graphene dispersions. Curr. Opin. Colloid Interface Sci. 2015, 20, 367–382. [Google Scholar] [CrossRef]
- Clancy, A.J.; Bayazit, M.K.; Hodge, S.A.; Skipper, N.T.; Howard, C.A.; Shaffer, M.S.P. Charged Carbon Nanomaterials: Redox Chemistries of Fullerenes, Carbon Nanotubes, and Graphenes. Chem. Rev. 2018, 118, 7363–7408. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Chen, Y.N.; Xu, S.M.; Li, Y.C.; Jacob, R.J.; Kuang, Y.D.; Liu, B.Y.; Wang, Y.L.; Pastel, G.; Salamanca-Riba, L.G.; Zachariah, M.R.; et al. FeS2 Nanoparticles Embedded in Reduced Graphene Oxide toward Robust, High-Performance Electrocatalysts. Adv. Energy Mater. 2017, 7, 1700482. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Chen, H.; Sun, Y.; Qian, W.; Lin, H.; Han, S. Highly active and stable electrocatalysts of FeS2–reduced graphene oxide for hydrogen evolution. J. Mater. Sci. 2019, 54, 1422–1433. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, R.; Zhang, J.F.; Shi, Y.M.; Zhang, B. Anion-exchange synthesis of nanoporous FeP nanosheets as electrocatalysts for hydrogen evolution reaction. Chem. Commun. 2013, 49, 6656–6658. [Google Scholar] [CrossRef]
- Guo, Y.X.; Shang, C.S.; Zhang, X.Y.; Wang, E.K. Electrocatalytic hydrogen evolution using the MS2@MoS2/rGO (M = Fe or Ni) hybrid catalyst. Chem. Commun. 2016, 52, 11795–11798. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Chen, H.; Sun, Y.; Lin, H.; Han, S. Effect of nickel-doped FeS2 nanoparticles-reduced graphene oxide electrocatalysts for efficient hydrogen evolution. J. Alloys Compd. 2019, 775, 1293–1300. [Google Scholar] [CrossRef]
- Li, Y.; Yin, J.; Chu, C.; Sui, N.; Shi, S.; Wei, J.; Di, F.; Guo, J.; Wang, C.; Xu, W.; et al. Earth-abundant Fe1−xS@S-doped graphene oxide nano–micro composites as high-performance cathode catalysts for green solar energy utilization: Fast interfacial electron exchange. RSC Adv. 2018, 8, 4340–4347. [Google Scholar] [CrossRef]
- Begum, A.; Sheikh, A.H.; Moula, G.; Sarkar, S. Fe4S4 Cubane Type Cluster Immobilized on a Graphene Support: A High Performance H2 Evolution Catalysis in Acidic Water. Sci. Rep. 2017, 7, 16948. [Google Scholar] [CrossRef]
- Yuhas, B.D.; Smeigh, A.L.; Samuel, A.P.S.; Shim, Y.; Bag, S.; Douvalis, A.P.; Wasielewski, M.R.; Kanatzidis, M.G. Biomimetic Multifunctional Porous Chalcogels as Solar Fuel Catalysts. J. Am. Chem. Soc. 2011, 133, 7252–7255. [Google Scholar] [CrossRef]
- Shim, Y.; Young, R.M.; Douvalis, A.P.; Dyar, S.M.; Yuhas, B.D.; Bakas, T.; Wasielewski, M.R.; Kanatzidis, M.G. Enhanced Photochemical Hydrogen Evolution from Fe4S4-Based Biomimetic Chalcogels Containing M2+ (M = Pt, Zn, Co, Ni, Sn) Centers. J. Am. Chem. Soc. 2014, 136, 13371–13380. [Google Scholar] [CrossRef]
- Huang, S.Y.; Sodano, D.; Leonard, T.; Luiso, S.; Fedkiw, P.S. Cobalt-Doped Iron Sulfide as an Electrocatalyst for Hydrogen Evolution. J. Electrochem. Soc. 2017, 164, F276–F282. [Google Scholar] [CrossRef]
- Kuo, T.-R.; Chen, W.-T.; Liao, H.-J.; Yang, Y.-H.; Yen, H.-C.; Liao, T.-W.; Wen, C.-Y.; Lee, Y.-C.; Chen, C.-C.; Wang, D.-Y. Improving Hydrogen Evolution Activity of Earth-Abundant Cobalt-Doped Iron Pyrite Catalysts by Surface Modification with Phosphide. Small 2017, 13, 1603356. [Google Scholar] [CrossRef]
- Wang, D.Y.; Gong, M.; Chou, H.L.; Pan, C.J.; Chen, H.A.; Wu, Y.P.; Lin, M.C.; Guan, M.Y.; Yang, J.; Chen, C.W.; et al. Highly Active and Stable Hybrid Catalyst of Cobalt-Doped FeS2 Nanosheets-Carbon Nanotubes for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2015, 137, 1587–1592. [Google Scholar] [CrossRef]
- Li, Y.X.; Yin, J.; An, L.; Lu, M.; Sun, K.; Zhao, Y.Q.; Gao, D.Q.; Cheng, F.Y.; Xi, P.X. FeS2/CoS2 Interface Nanosheets as Efficient Bifunctional Electrocatalyst for Overall Water Splitting. Small 2018, 14, 1801070. [Google Scholar] [CrossRef]
- Wang, K.; Guo, W.L.; Yan, S.C.; Song, H.Z.; Shi, Y. Hierarchical Co-FeS2/CoS2 heterostructures as a superior bifunctional electrocatalyst. RSC Adv. 2018, 8, 28684–28691. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, X.; Lu, Q.Q.; Li, Q.; Han, C.; Xing, Z.C.; Yang, X.R. FeS2-doped MoS2 nanoflower with the dominant 1T-MoS2 phase as an excellent electrocatalyst for high-performance hydrogen evolution. Electrochim. Acta 2017, 249, 72–78. [Google Scholar] [CrossRef]

© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heift, D. Iron Sulfide Materials: Catalysts for Electrochemical Hydrogen Evolution. Inorganics 2019, 7, 75. https://doi.org/10.3390/inorganics7060075
Heift D. Iron Sulfide Materials: Catalysts for Electrochemical Hydrogen Evolution. Inorganics. 2019; 7(6):75. https://doi.org/10.3390/inorganics7060075
Chicago/Turabian StyleHeift, Dominikus. 2019. "Iron Sulfide Materials: Catalysts for Electrochemical Hydrogen Evolution" Inorganics 7, no. 6: 75. https://doi.org/10.3390/inorganics7060075
APA StyleHeift, D. (2019). Iron Sulfide Materials: Catalysts for Electrochemical Hydrogen Evolution. Inorganics, 7(6), 75. https://doi.org/10.3390/inorganics7060075