Laser Ablation Inductively Coupled Plasma Spectrometry: Metal Imaging in Experimental and Clinical Wilson Disease
Abstract
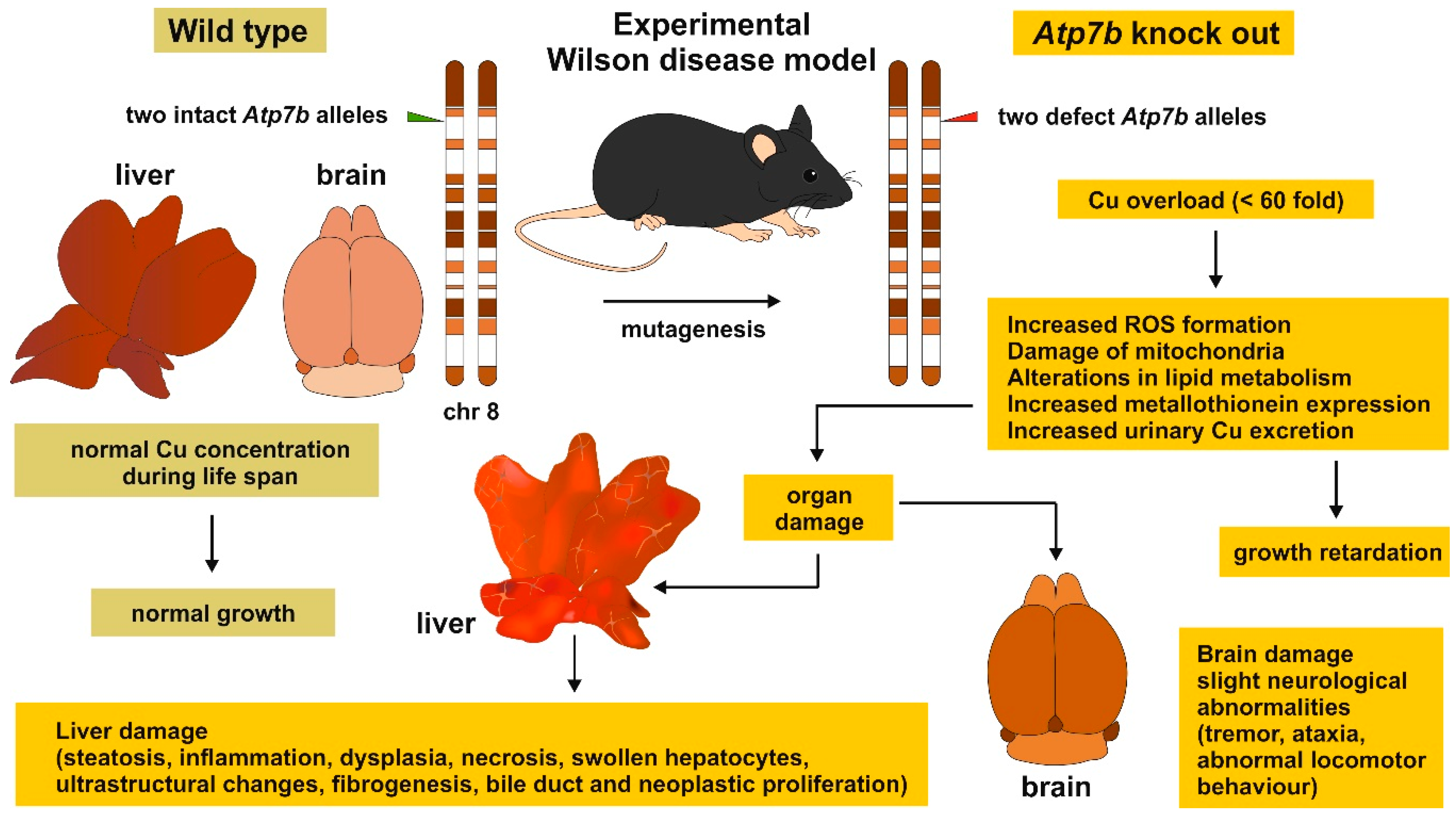
1. Introduction
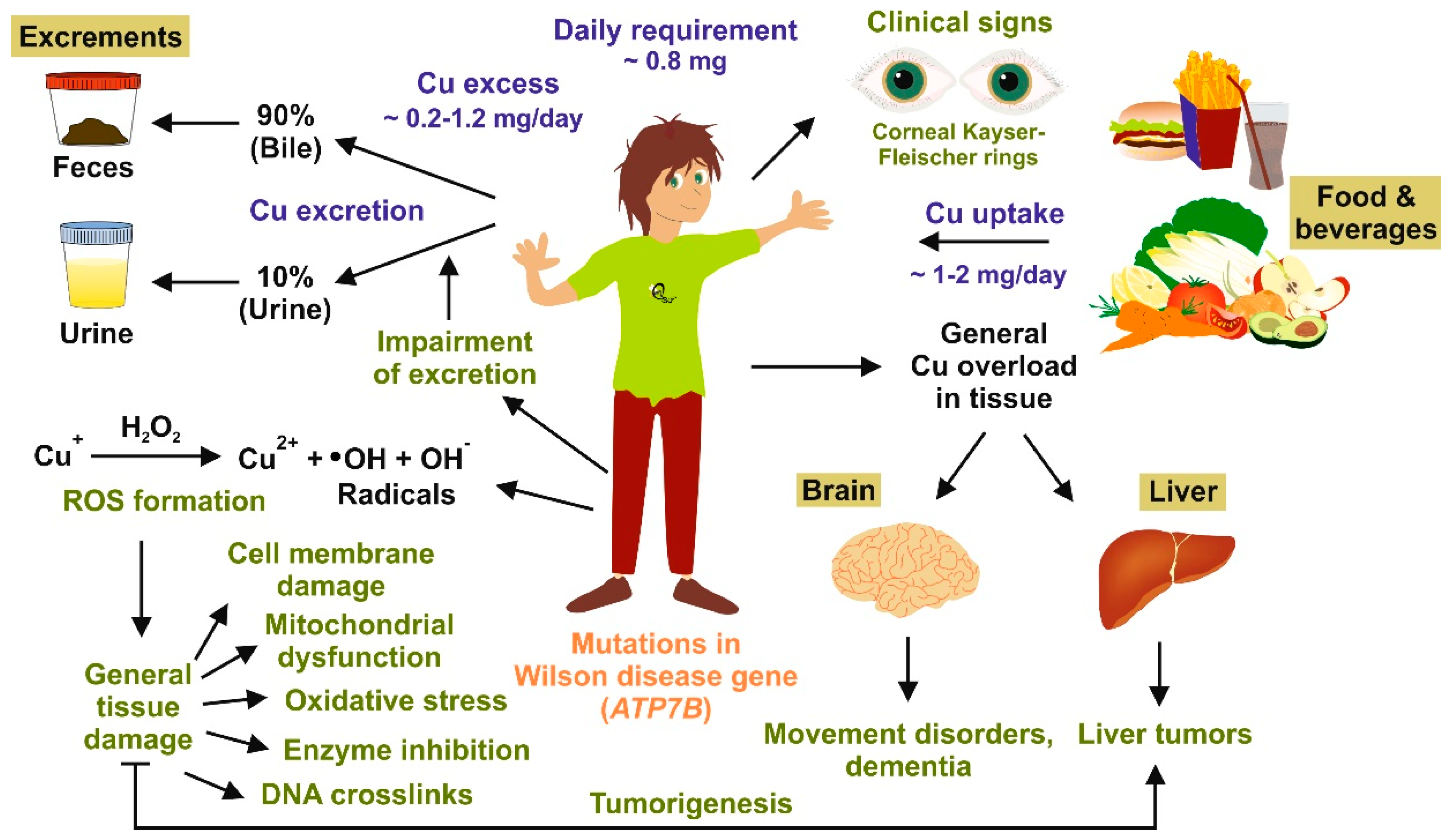
2. Copper Homeostasis and Overload
3. Copper Distribution in Wilson Disease Tissue
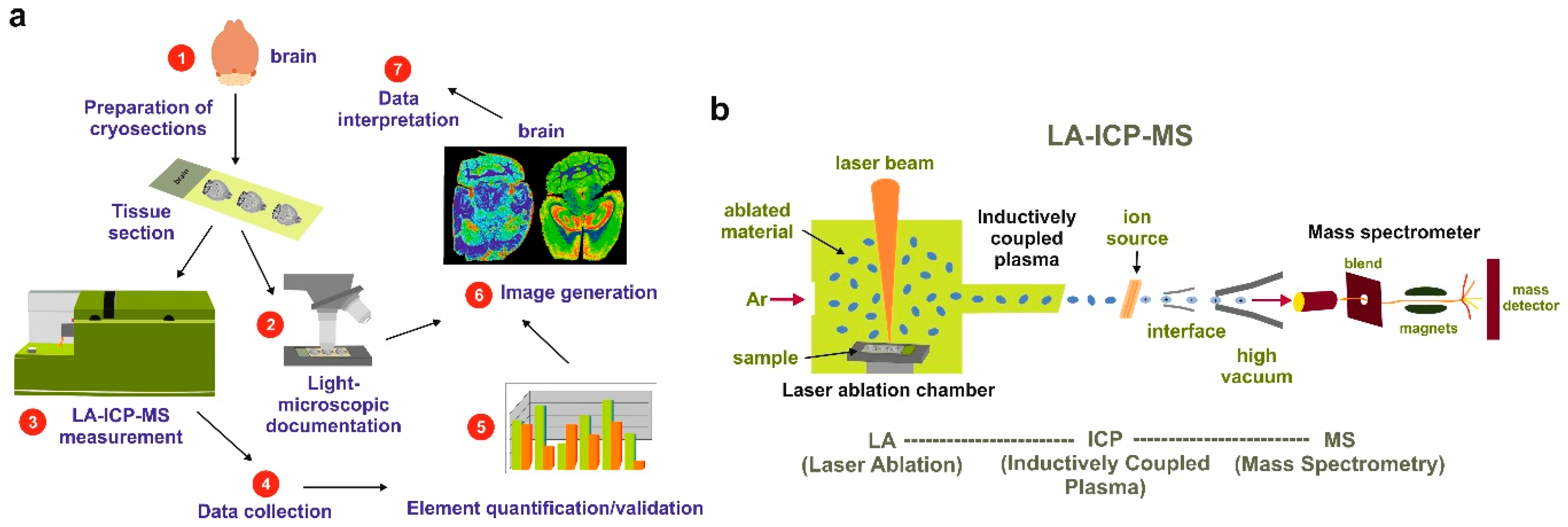
4. Laser Ablation Inductively Coupled Plasma Spectrometry Imaging
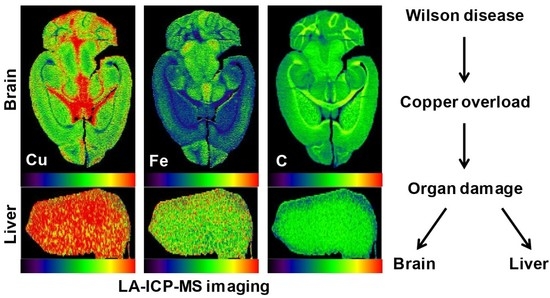
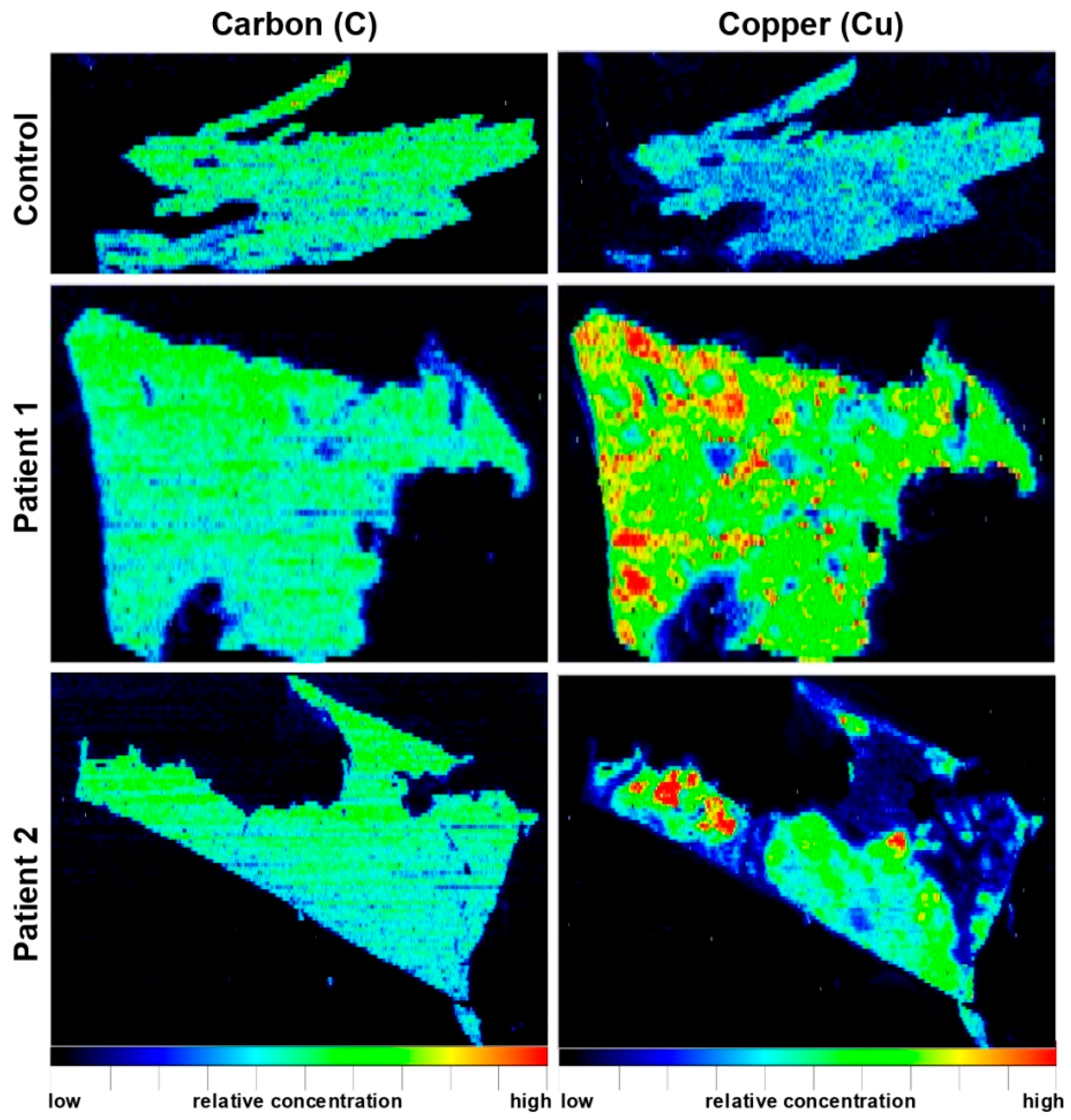
5. Wilson Disease Studies Using LA-ICP-MSI
6. Translational Aspects of LA-ICP-MSI
7. Materials and Methods
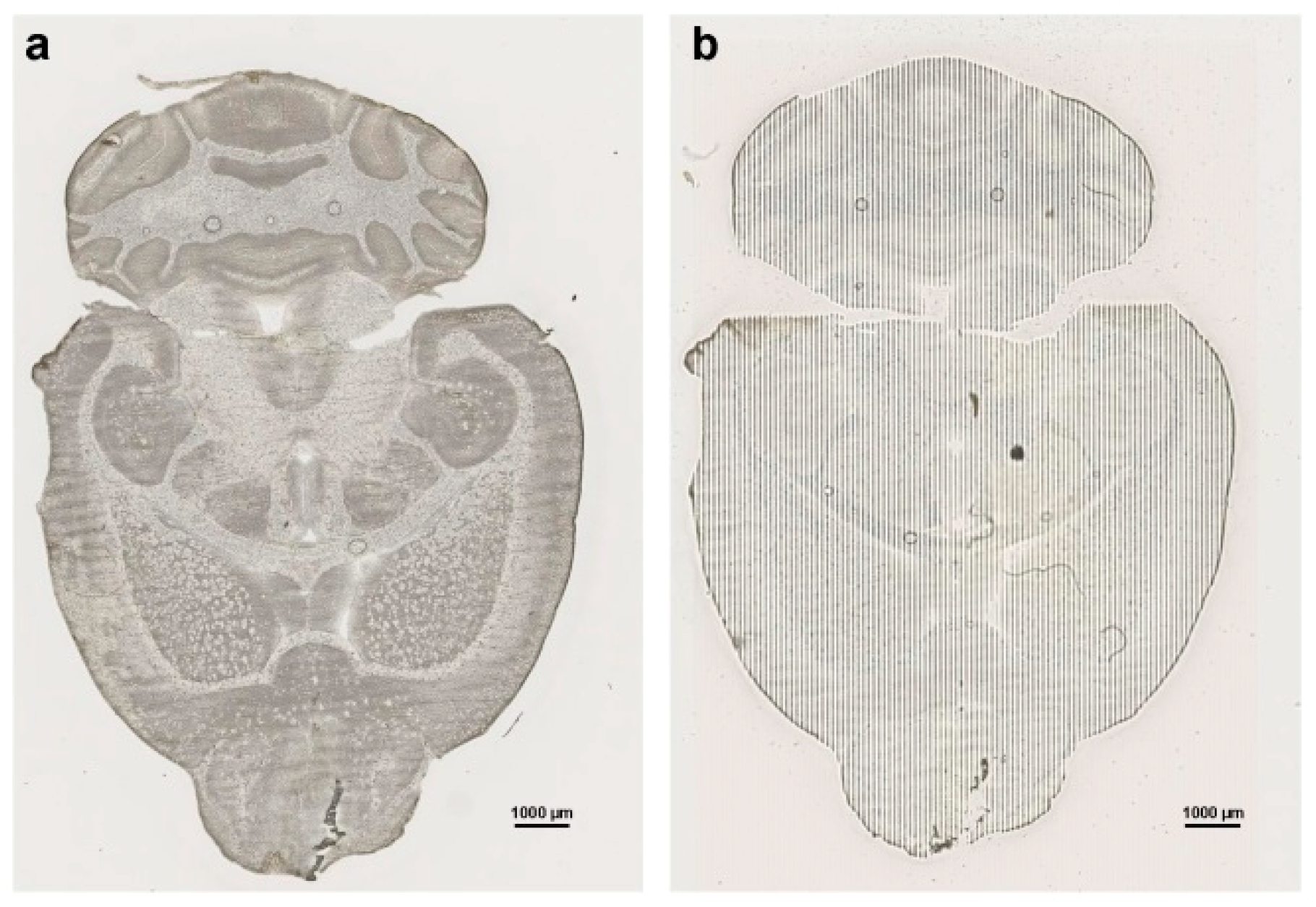
7.1. Images and Pictures
7.2. Animals
7.3. Human Samples
7.4. LA-ICP-MSI Measurements
7.5. Visualization of Data
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Merle, U.; Weiskirchen, R. Wilson’s disease: An inherited, silent, copper intoxication disease. Emj Neurol. 2016, 4, 74–83. [Google Scholar]
- Reilly, M.; Daly, L.; Hutchinson, M. An epidemiological study of Wilson’s disease in the Republic of Ireland. J. Neurol. Neurosurg. Psychiatry 1993, 56, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Cocoş, R.; Şendroiu, A.; Schipor, S.; Bohîlţea, L.C.; Şendroiu, I.; Raicu, F. Genotype-phenotype correlations in a mountain population community with high prevalence of Wilson’s disease: Genetic and clinical homogeneity. PLoS ONE 2014, 9, e98520. [Google Scholar] [CrossRef]
- European Association for Study of Liver. EASL Clinical Practice Guidelines: Wilson’s disease. J. Hepatol. 2012, 56, 671–685. [Google Scholar] [CrossRef]
- Stremmel, W.; Merle, U.; Weiskirchen, R. Clinical features of Wilson disease. Ann. Transl. Med. 2019. [Google Scholar] [CrossRef]
- Medici, V.; LaSalle, J.M. Genetics and epigenetic factors of Wilson disease. Ann. Transl. Med. 2019. [Google Scholar] [CrossRef]
- Roberts, E.A.; Schilsky, M.L.; American Association for Study of Liver Diseases (AASLD). Diagnosis and treatment of Wilson disease: An update. Hepatology 2008, 47, 2089–2111. [Google Scholar] [CrossRef]
- Palumbo, C.S.; Schilsky, M.L. Clinical practice guidelines in Wilson disease. Ann. Transl. Med. 2019. [Google Scholar] [CrossRef]
- Litwin, T.; Dzieżyc, K.; Członkowska, A. Wilson disease—Treatment perspectives. Ann. Transl. Med. 2019. [Google Scholar] [CrossRef]
- Medici, V.; Huster, D. Animal models of Wilson disease. Handb. Clin. Neurol. 2017, 142, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Reed, E.; Lutsenko, S.; Bandmann, O. Animal models of Wilson disease. J. Neurochem. 2018, 146, 356–373. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, L.; Chang, Q.; Xing, F.; Ma, Z.; Fang, Z.; Zhou, J.; Fu, L.; Wang, H.; Huang, X.; et al. Production of Wilson disease model rabbits with homology-directed precision point mutations in the ATP7B gene using the CRISPR/Cas9 system. Sci. Rep. 2018, 8, 1332. [Google Scholar] [CrossRef]
- Bourassa, M.W.; Miller, L.M. Metal imaging in neurodegenerative diseases. Metallomics 2012, 4, 721–738. [Google Scholar] [CrossRef] [PubMed]
- Susnea, I.; Weiskirchen, R. Trace metal imaging in diagnostic of hepatic metal disease. Mass Spectrom. Rev. 2016, 35, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef] [PubMed]
- van den Berghe, P.V.; Klomp, L.W. New developments in the regulation of intestinal copper absorption. Nutr. Rev. 2009, 67, 658–672. [Google Scholar] [CrossRef]
- Pierson, H.; Muchenditsi, A.; Kim, B.E.; Ralle, M.; Zachos, N.; Huster, D.; Lutsenko, S. The function of ATPase copper transporter ATP7B in intestine. Gastroenterology 2018, 154, 168–180. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Popiołek, Ł.; Kocot, J. The many “faces” of copper in medicine and treatment. Biometals 2014, 27, 611–621. [Google Scholar] [CrossRef]
- Ogra, Y.; Aoyama, M.; Suzuki, K.T. Protective role of metallothionein against copper depletion. Arch. Biochem. Biophys. 2006, 451, 112–118. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Lin, Z.; Han, M.; Cheng, H. Altered serum copper homeostasis suggests higher oxidative stress and lower antioxidant capability in patients with chronic hepatitis B. Medicine 2018, 97, e11137. [Google Scholar] [CrossRef]
- Kathawala, M.; Hirschfield, G.M. Insights into the management of Wilson’s disease. Ther. Adv. Gastroenterol. 2017, 10, 889–905. [Google Scholar] [CrossRef]
- Ferenci, P. Wilson’s Disease. Clin. Gastroenterol. Hepatol. 2005, 3, 726–733. [Google Scholar] [CrossRef]
- Hermann, W. Classification and differential diagnosis of Wilson’s disease. Ann. Transl. Med. 2019. [Google Scholar] [CrossRef]
- Faa, G.; Nurchi, V.; Demelia, L.; Ambu, R.; Parodo, G.; Congiu, T.; Sciot, R.; Van Eyken, P.; Silvagni, R.; Crisponi, G. Uneven hepatic copper distribution in Wilson’s disease. J. Hepatol. 1995, 22, 303–308. [Google Scholar] [CrossRef]
- Porter, H. Tissue copper proteins in Wilson’s disease. Intracellular distribution and chormatogrphic fractionation. Arch. Neurol. 1964, 11, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Goldfischer, S.; Popper, H.; Sternlieb, I. The significance of variations in the distribution of copper in liver disease. Am. J. Pathol. 1980, 99, 715–730. [Google Scholar] [PubMed]
- Pilloni, L.; Lecca, S.; Van Eyken, P.; Flore, C.; Demelia, L.; Pilleri, G.; Nurchi, A.M.; Farci, A.M.; Ambu, R.; Callea, F.; et al. Value of histochemical stains for copper in the diagnosis of Wilson’s disease. Histopathology 1998, 33, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Uerlings, R.; Weiskirchen, R. An at-a-glance visualization of regional metal accretion in tissue samples by image overlay in laser ablation inductively coupled plasma mass spectrometry. In Laser Ablation: Advances in Research and Applications Is Approaching; Bellucci, C., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 87–113. [Google Scholar]
- Bandura, D.R.; Baranov, V.I.; Tanner, S.D. Reaction chemistry and collisional processes in multiple devices for resolving isobaric interferences in ICP-MS. Freseniusj. Anal. Chem. 2001, 370, 454–470. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weiskirchen, S.; Kim, P.; Winkler, R. Software solutions for evaluation and visualization of laser ablation inductively coupled plasma mass spectrometry imaging (LA-ICP-MSI) data: A short overview. J. Cheminform. 2019, 11, 16. [Google Scholar] [CrossRef]
- Allen Brain Reference Atlases. Available online: http://atlas.brain-map.org/ (accessed on 18 April 2019).
- Hare, D.J.; Kysenius, K.; Paul, B.; Knauer, B.; Hutchinson, R.W.; O’Connor, C.; Fryer, F.; Hennessey, T.P.; Bush, A.I.; Crouch, P.J.; et al. Imaging metals in brain tissue by laser ablation—Inductively coupled plasma—Mass spectrometry (LA-ICP-MS). J. Vis. Exp. 2017, 119. [Google Scholar] [CrossRef] [PubMed]
- Hare, D.J.; Raven, E.P.; Roberts, B.R.; Bogeski, M.; Portbury, S.D.; McLean, C.A.; Masters, C.L.; Connor, J.R.; Bush, A.I.; Crouch, P.J.; et al. Laser ablation-inductively coupled plasma-mass spectrometry imaging of white and gray matter iron distribution in Alzheimer’s disease frontal cortex. Neuroimage 2016, 137, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Brar, S.; Henderson, D.; Schenck, J.; Zimmerman, E.A. Iron accumulation in the substantia nigra of patients with Alzheimer disease and Parkinsonism. Arch. Neurol. 2009, 66, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Matusch, A.; Depboylu, C.; Palm, C.; Wu, B.; Höglinger, G.U.; Schäfer, M.K.; Becker, J.S. Cerebral bioimaging of Cu, Fe, Zn, and Mn in the MPTP mouse model of Parkinson’s disease using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). J. Am. Soc. Mass Spectrom. 2010, 21, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.S.; Matusch, A.; Palm, C.; Salber, D.; Morton, K.A.; Becker, J.S. Bioimaging of metals in brain tissue by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and metallomics. Metallomics 2010, 2, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Pornwilard, M.-M.; Merle, U.; Weiskirchen, R.; Becker, J.S. Bioimiaging of copper deposition in Wilson’s disease mouse liver by laser ablation inductively coupled plasma mass spectrometry imaging (LA-ICP-MS). Int. J. Mass Spectrom. 2013, 354–355, 281–287. [Google Scholar] [CrossRef]
- Boaru, S.G.; Merle, U.; Uerlings, R.; Zimmermann, A.; Weiskirchen, S.; Matusch, A.; Stremmel, W.; Weiskirchen, R. Simultaneous monitoring of cerebral metal accumulation in an experimental model of Wilson’s disease by laser ablation inductively coupled plasma mass spectrometry. Bmc Neurosci. 2014, 15, 98. [Google Scholar] [CrossRef]
- Boaru, S.G.; Merle, U.; Uerlings, R.; Zimmermann, A.; Flechtenmacher, C.; Willheim, C.; Eder, E.; Ferenci, P.; Stemmel, W.; Weiskirchen, R. Laser ablation inductively coupled plasma mass spectrometry imaging of metals in experimental and clinical Wilson’s disease. J. Cell. Mol. Med. 2015, 19, 806–814. [Google Scholar] [CrossRef]
- Hachmöller, O.; Aichler, M.; Schwamborn, K.; Lutz, L.; Werner, M.; Sperling, M.; Walch, A.; Karst, U. Element bioimaging of liver needle biopsy specimens from patients with Wilson’s disease by laser ablation-inductively coupled plasma-mass spectrometry. J. Trace Elem. Med. Biol. 2016, 35, 97–102. [Google Scholar] [CrossRef]
- Hachmöller, O.; Zibert, A.; Zischka, H.; Sperling, M.; Groba, S.R.; Grünewald, I.; Wardelmann, E.; Schmidt, H.H.; Karst, U. Spatial investigation of the elemental distribution in Wilson’s disease liver after d-penicillamine treatment by LA-ICP-MS. J. Trace Elem. Med. Biol. 2017, 44, 26–31. [Google Scholar] [CrossRef]
- Hachmöller, O.; Aichler, M.; Schwamborn, K.; Lutz, L.; Werner, M.; Sperling, M.; Walch, A.; Karst, U. Investigating the influence of standard staining procedures on the copper distribution and concentration in Wilson’s disease liver samples by laser ablation-inductively coupled plasma-mass spectrometry. J. Trace Elem. Med. Biol. 2017, 44, 71–75. [Google Scholar] [CrossRef]
- Moreno, D.; Murillo, O.; Gazquez, C.; Hernández-Alcoceba, R.; Uerlings, R.; González-Aseguinolaza, G.; Weiskirchen, R. Visualization of the therapeutic efficacy of a gene correction approach in Wilson’s disease by laser-ablation inductively coupled mass spectrometry. J. Hepatol. 2018, 68, 1088–1090. [Google Scholar] [CrossRef]
- Uerlings, R.; Moreno, D.; Murillo, O.; Gazquez, C.; Hernández-Alcoceba, R.; González-Aseguinolaza, G.; Weiskirchen, R. Brain copper storage after genetic long-term correction in a mouse model of Wilson disease. Neurol. Genet. 2018, 4, e243. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.C.; Lichtmannegger, J.; Zischka, H.; Sperling, M.; Karst, U. High spatial resolution LA-ICP-MS demonstrates massive liver copper depletion in Wilson disease rats upon Methanobactin treatment. J. Trace Elem. Med. Biol. 2018, 49, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Deng, J.; Borjigin, J. A new strain of rat for functional analysis of PINA. Brain Res. Mol. Brain Res. 2005, 137, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Buiakova, O.I.; Xu, J.; Lutsenko, S.; Zeitlin, S.; Das, K.; Das, S.; Ross, B.M.; Mekios, C.; Scheinberg, I.H.; Gilliam, T.C. Null mutation of the murine ATP7B (Wilson disease) gene results in intracellular copper accumulation and late-onset hepatic nodular transformation. Hum. Mol. Genet. 1999, 8, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Huster, D.; Purnat, T.D.; Burkhead, J.L.; Ralle, M.; Fiehn, O.; Stuckert, F.; Olson, N.E.; Teupser, D.; Lutsenko, S. High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of Wilson disease. J. Biol. Chem. 2007, 282, 8343–8355. [Google Scholar] [CrossRef] [PubMed]
- Murillo, O.; Luqui, D.M.; Gazquez, C.; Martinez-Espartosa, D.; Navarro-Blasco, I.; Monreal, J.I.; Guembe, L.; Moreno-Cermeno, A.; Corrales, F.J.; Prieto, J.; et al. Long-term metabolic correction of Wilson’s disease in a murine model by gene therapy. J. Hepatol. 2016, 64, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Ralle, M.; Huster, D.; Vogt, S.; Schirrmeister, W.; Burkhead, J.L.; Capps, T.R.; Gray, L.; Lai, B.; Maryon, E.; Lutsenko, S. Wilson disease at a single cell level: Intracellular copper trafficking activates compartment-specific responses in hepatocytes. J. Biol. Chem. 2010, 285, 30875–30883. [Google Scholar] [CrossRef]
- Vogt, S.; Ralle, M. Opportunities in multidimensional trace metal imaging: Taking copper-associated disease research to the next level. Anal. Bioanal. Chem. 2013, 405, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Weiskirchen, S.; Uerlings, R.; Kueppers, A.; Stellmacher, F.; Viveiros, A.; Zoller, H.; Weiskirchen, R. Quantification of liver iron overload disease with laser ablation inductively coupled plasma mass spectrometry. Bmc Med. Imaging 2018, 18, 51. [Google Scholar] [CrossRef]
- M-M, P.; Weiskirchen, R.; Gassler, N.; Bosserhoff, A.K.; Becker, J.S. Novel bioimaging techniques of metals by laser ablation inductively coupled plasma mass spectrometry for diagnosis of fibrotic and cirrhotic liver disorders. PLoS ONE 2013, 8, e58702. [Google Scholar] [CrossRef] [PubMed]
- Vašinová Galiová, M.; Copjaková, R.; Škoda, R.; Štepánková, K.; Vanková, M.; Kuta, J.; Prokeš, L.; Kynický, J.; Kanický, V. 2D elemental mapping of sections of human kidney stones using laser ablation inductively-coupled plasma-mass spectrometry: Possibilities and limitations. Spectrochim. Acta Part B Atom. Spectrosc. 2014, 100, 105–115. [Google Scholar] [CrossRef]
- Niedzwiecki, M.M.; Austin, C.; Remark, R.; Merad, M.; Gnjatic, S.; Estrada-Gutierrez, G.; Espejel-Nuñez, A.; Borboa-Olivares, H.; Guzman-Huerta, M.; Wright, R.J.; et al. A multimodal imaging workflow to visualize metal mixtures in the human placenta and explore colocalization with biological response markers. Metallomics 2016, 8, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Bonta, M.; Gonzalez, J.J.; Derrick Quarles, C.; Russo, R.E.; Hegedus, B.; Limbeck, A. Elemental mapping of biological samples by the combined use of LIBS and LA-ICP-MS. J. Anal. Atom. Spectrom. 2016, 31, 252–258. [Google Scholar] [CrossRef]
- Queipo Abad, S.; Rodríguez-González, P.; García Alonso, J.I. Evidence of the direct adsorption of mercury in human hair during occupational exposure to mercury vapour. J. Trace Elem. Med. Biol. 2016, 36, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Su, X.; Xu, W.; Zhang, S.; Zhuo, X.; Ma, D. Determination of arsenic and lead in single hair strands by laser ablation inductively coupled plasma mass spectrometry. Sci. Rep. 2017, 7, 3426. [Google Scholar] [CrossRef]
- Eastman, R.R.; Jursa, T.P.; Benedetti, C.; Lucchini, R.G.; Smith, D.R. Hair as a biomarker of environmental manganese exposure. Environ. Sci. Technol. 2013, 47, 1629–1637. [Google Scholar] [CrossRef]
- Konz, I.; Fernández, B.; Fernández, M.L.; Pereiro, R.; González-Iglesias, H.; Coca-Prados, M.; Sanz-Medel, A. Quantitative bioimaging of trace elements in the human lens by LA-ICP-MS. Anal. Bioanal. Chem. 2014, 406, 2343–2348. [Google Scholar] [CrossRef]
- Roberts, B.R.; Doecke, J.D.; Rembach, A.; Yévenes, L.F.; Fowler, C.J.; McLean, C.A.; Lind, M.; Volitakis, I.; Masters, C.L.; Bush, A.I.; et al. AIBL research group. Rubidium and potassium levels are altered in Alzheimer’s disease brain and blood but not in cerebrospinal fluid. Acta Neuropathol. Commun. 2016, 4, 119. [Google Scholar] [CrossRef] [PubMed]
- Birka, M.; Wentker, K.S.; Lusmöller, E.; Arheilger, B.; Wehe, C.A.; Sperling, M.; Stadler, R.; Karst, U. Diagnosis of nephrogenic systemic fibrosis by means of elemental bioimaging and speciation analysis. Anal. Chem. 2015, 87, 3321–3328. [Google Scholar] [CrossRef]
- Doble, P.A.; Miklos, G.L.G. Distributions of manganese in diverse human cancers provide insights into tumour radioresistance. Metallomics 2018, 10, 1191–1210. [Google Scholar] [CrossRef]
- Aramendía, M.; Rello, L.; Bérail, S.; Donnard, A.; Pécheyran, C.; Resano, M. Direct analysis of dried blood spots by femtosecond-laser ablation-inductively coupled plasma-mass spectrometry. Feasibility of split-flow laser ablation for simultaneous trace element and isotopic analysis. J. Anal. At. Spectrom. 2015, 30, 296–309. [Google Scholar] [CrossRef]
- Nischkauer, W.; Vanhaecke, F.; Limbeck, A. Self-aliquoting micro-grooves in combination with laser ablation-ICP-mass spectrometry for the analysis of challenging liquids: Quantification of lead in whole blood. Anal. Bioanal. Chem. 2016, 408, 5671–5676. [Google Scholar] [CrossRef]
- Kröger, S.; Sperling, M.; Karst, U. Quantitative dried blood spot analysis for metallodrugs by laser ablation-inductively coupled plasma-mass spectrometry. J. Trace Elem. Med. Biol. 2019, 51, 50–56. [Google Scholar] [CrossRef]
- Chantada-Vázquez, M.P.; Moreda-Piñeiro, J.; Cantarero-Roldán, A.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Development of dried serum spot sampling techniques for the assessment of trace elements in serum samples by LA-ICP-MS. Talanta 2018, 186, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Lothian, A.; Lago, L.; Mukherjee, S.; Connor, A.R.; Fowler, C.; McLean, C.A.; Horne, M.; Masters, C.L.; Cappai, R.; Roberts, B.R. Characterization of the metal status of natively purified alpha-synuclein from human blood, brain tissue, or recombinant sources using size exclusion ICP-MS reveals no significant binding of Cu, Fe or Zn. Metallomics 2019, 11, 128–140. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Gahl, W.A. Disorders of metal metabolism. Transl. Sci. Rare Dis. 2017, 2, 101–139. [Google Scholar] [CrossRef]
- Lauer, E.; Villa, M.; Jotterand, M.; Vilarino, R.; Bollmann, M.; Michaud, K.; Grabherr, S.; Augsburger, M.; Thomas, A. Imaging mass spectrometry of elements in forensic cases by LA-ICP-MS. Int. J. Leg. Med. 2017, 131, 497–500. [Google Scholar] [CrossRef]
- Weiskirchen, S.; Kim, P.; Weiskirchen, R. Determination of copper poisoning in Wilson’s disease using laser ablation inductively coupled plasma spectrometry. Ann. Transl. Med. 2018. [Google Scholar] [CrossRef]
- Uerlings, R.; Matusch, A.; Weiskirchen, R. Reconstruction of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) spatial distribution images in Microsoft Excel 2007. Int. J. Mass Spectrom. 2016, 395, 27–35. [Google Scholar] [CrossRef]

| Specimens | Major Findings | Refs |
|---|---|---|
| Liver sections of Atp7b−/− and wild type mouse (each n = 5) | The average hepatic concentrations of Cu, Mn, Fe, and Zn were 4, 0.7, 41, and 18 µg/g tissue in control samples, while they were 143, 0.6, 80, and 32 µg/g tissue in livers of Atp7b−/− mouse | [37] |
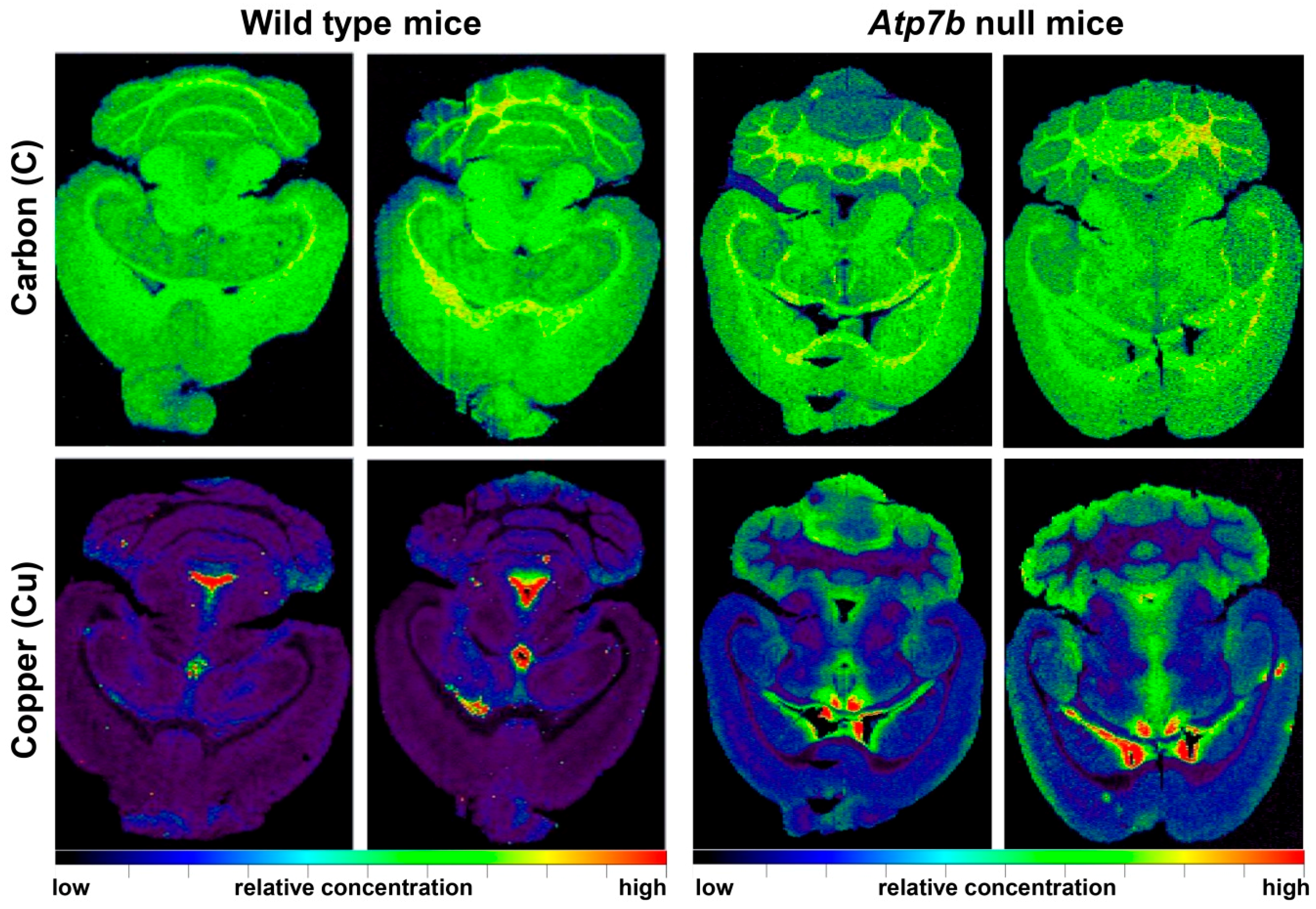
| Brain sections of wild type (n = 8) and Atp7b−/− (n = 9) mice in the age range of 11-24 months | Cu was increased proportionally during ageing throughout all cerebral regions; Atp7b null mice showed ~2-fold stable increase of Cu throughout brain parenchyma; Cu was ~3.5-fold decreased in periventricular regions | [38] |
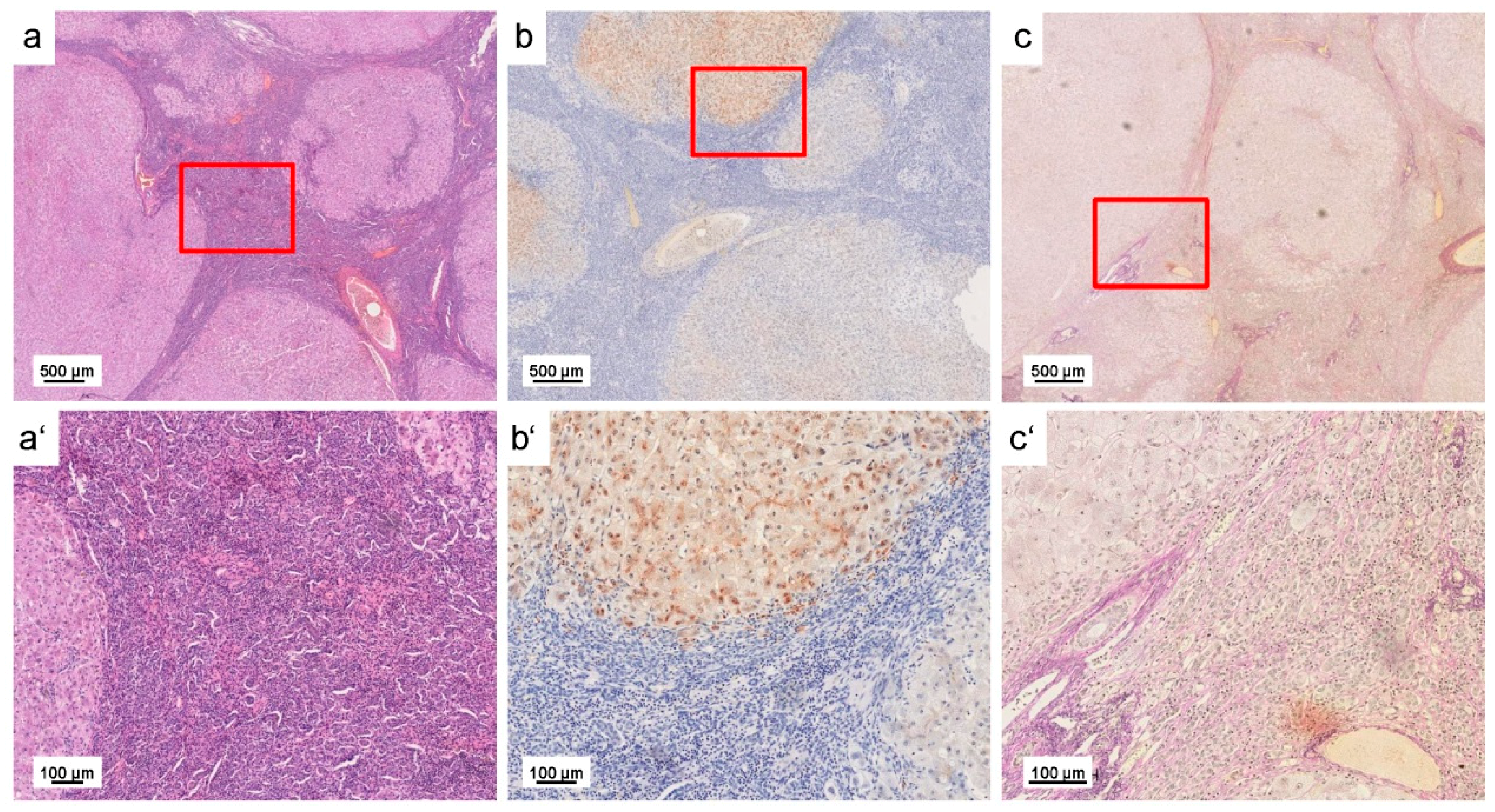
| Liver sections of wild type and Atp7b−/− mice and human WD liver samples | Liver sections of 10 months old Atp7b null mice and patients with WD showed irregular (patchy) Cu distribution with high regional concentrations; age-dependent accumulation of hepatic Cu was strictly associated with a simultaneous increase in iron (Fe) and Zn; human liver samples confirmed accumulation of hepatic Fe and Zn in WD patients; tumorigenic regions are highly enriched in Cu | [39] |
| Paraffin-embedded human liver needle biopsy (n = 2) | WD patients showed inhomogeneous Cu distribution and high Cu concentrations of up to 1200 μg/g; inverse correlation of regions with elevated Cu and region with high Fe concentrations | [40] |
| Liver of D-Penicillamine (DPA)-treated PINA/Atp7b−/− (LPP−/−)** rats and a liver samples from a patient before and after DPA treatment | DPA-treatment resulted in a significant decrease in hepatic Cu by more than a factor two; Cu distribution maps after DPA treatment were highly inhomogeneous and lowest Cu concentrations were found in direct proximity to blood vessels | [41] |
| Human stained and unstained liver needle biopsies (n = 8) | When comparing unstained and rhodanine-stained sections of each WD liver sample, unstained sections showed distinct structures of Cu distribution, while rhodanine-stained sections revealed blurred Cu distribution with 20–90% decreased concentrations | [42] |
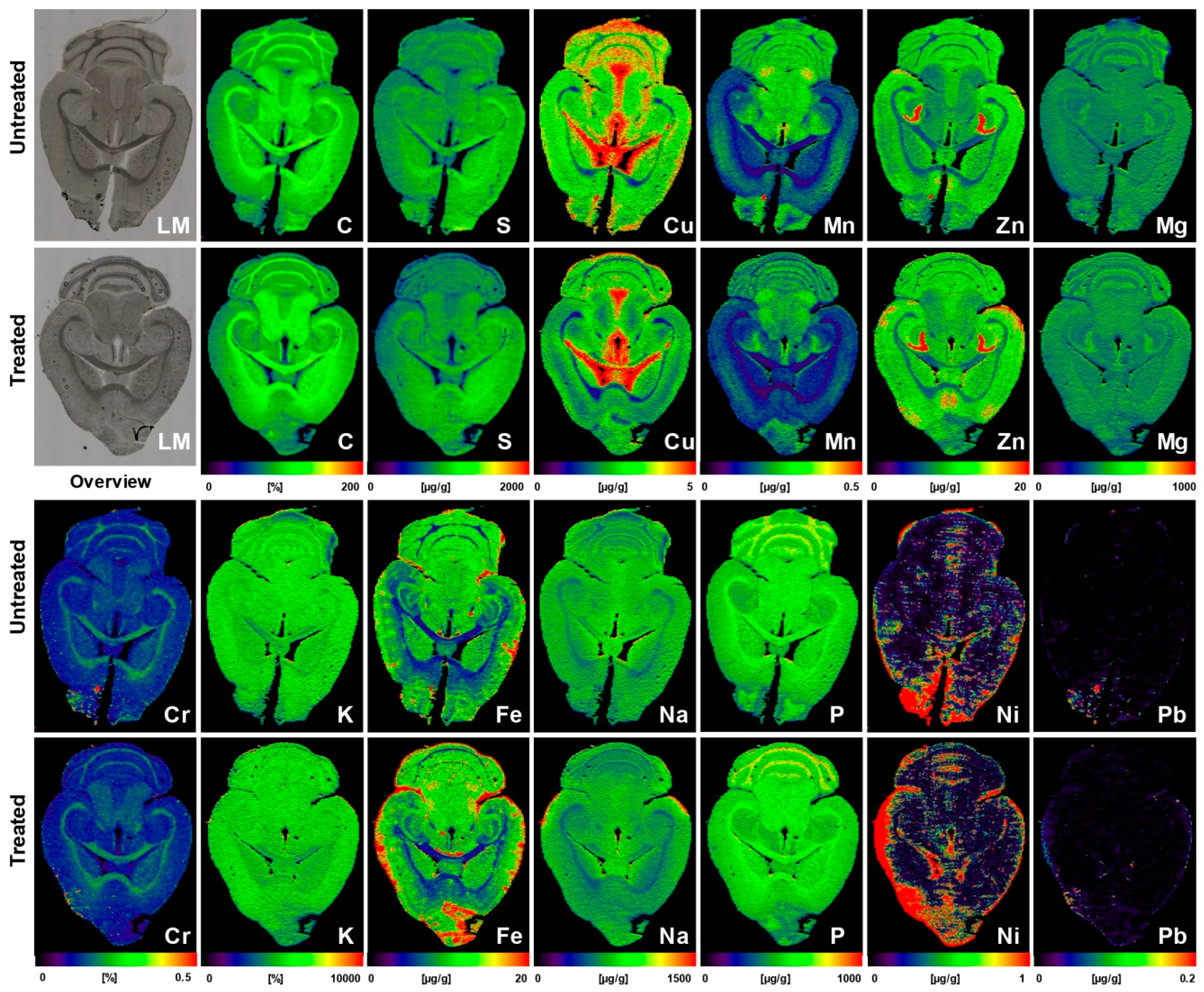
| Liver sections of untreated or AAV-AAT-co-miATP7B-treated Atp7b−/− mice (n = 5) | While the mean of hepatic Cu was 112.7 ± 13.3 µg/g liver tissue in the untreated group, the delivery of the transgene reduced Cu content to a mean of 43.3 ± 3.6 µg/g liver tissue; removal of Cu provoked a simultaneous decrease in hepatic Fe (314 ± 38 vs. 150.2 ± 25.2 µg/g liver tissue) and a slight reduction in hepatic Zn (43.1 ± 3.5 vs. 32.4 ± 4.3 µg/g liver tissue) | [43] |
| Brain sections of untreated (n = 5) or AAV-AAT-co-miATP7B*-treated Atp7b−/− mice (n = 6) | Brains of animals receiving the transgene had overall lower concentrations of total cerebral Cu (3.8 ± 0.2 vs. 3.05 ± 0.17 µg/g brain tissue), most prominently noticeable in the cerebellum, cerebellar white matter, corpus callosum, 3rd and 4th ventricles, and surrounding tissue, and a slight decrease in the basal ganglia; the content in the Atp7b+/− control mice that showed no alterations in Cu metabolism was 2.34 ± 0.09 μg/g. Concentrations of Fe, Zn, Mn, Na, Mg, K, Ca, P, Cr, Ni, and Pb were unaffected by the therapeutic approach | [44] |
| Liver samples from PINA/Atp7b−/− (LPP−/−) rats treated with Methanobactin OB3b*** | Hepatic Cu hotspots were effectively removed by treatment with Methanobactin OB3b; Cu re-accumulation was observed after interruption of therapy | [45] |
| Parameter | Experimental Setting |
|---|---|
| ICP mass spectrometer | ICP-QMS (e.g., Thermo XSeries II*) |
| ICP RF power | 1450 W |
| Cooling gas flow rate | 16.0 L·min−1 |
| Auxiliary gas flow rate | 0.7 L·min−1 |
| Carrier gas flow rate | 1.0 L·min−1 |
| Dwell time | 20 ms |
| Extraction lens potential | 3400 V |
| Mass resolution (m/Δm) | 300 |
| Scanning mode | peak hopping |
| Typical analysis time per brain or liver sample (10 mm × 10 mm) | 4 h |
| Laser ablation system | New Wave (NRW213) |
| Wavelength of Nd:YAG** laser | 213 nm |
| Laser fluence | 0.24 J·cm−2 |
| Repetition frequency | 20 Hz |
| Laser spot size | 60–80 µm |
| Scan speed | 60 µm·s−1 |
| Ablation mode | line scan |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiskirchen, S.; Kim, P.; Weiskirchen, R. Laser Ablation Inductively Coupled Plasma Spectrometry: Metal Imaging in Experimental and Clinical Wilson Disease. Inorganics 2019, 7, 54. https://doi.org/10.3390/inorganics7040054
Weiskirchen S, Kim P, Weiskirchen R. Laser Ablation Inductively Coupled Plasma Spectrometry: Metal Imaging in Experimental and Clinical Wilson Disease. Inorganics. 2019; 7(4):54. https://doi.org/10.3390/inorganics7040054
Chicago/Turabian StyleWeiskirchen, Sabine, Philipp Kim, and Ralf Weiskirchen. 2019. "Laser Ablation Inductively Coupled Plasma Spectrometry: Metal Imaging in Experimental and Clinical Wilson Disease" Inorganics 7, no. 4: 54. https://doi.org/10.3390/inorganics7040054
APA StyleWeiskirchen, S., Kim, P., & Weiskirchen, R. (2019). Laser Ablation Inductively Coupled Plasma Spectrometry: Metal Imaging in Experimental and Clinical Wilson Disease. Inorganics, 7(4), 54. https://doi.org/10.3390/inorganics7040054