Chirality Induction in Bioorganometallic Conjugates
Abstract
:1. Introduction
2. Induction of Helical Chirality into Bioorganometallic Conjugates
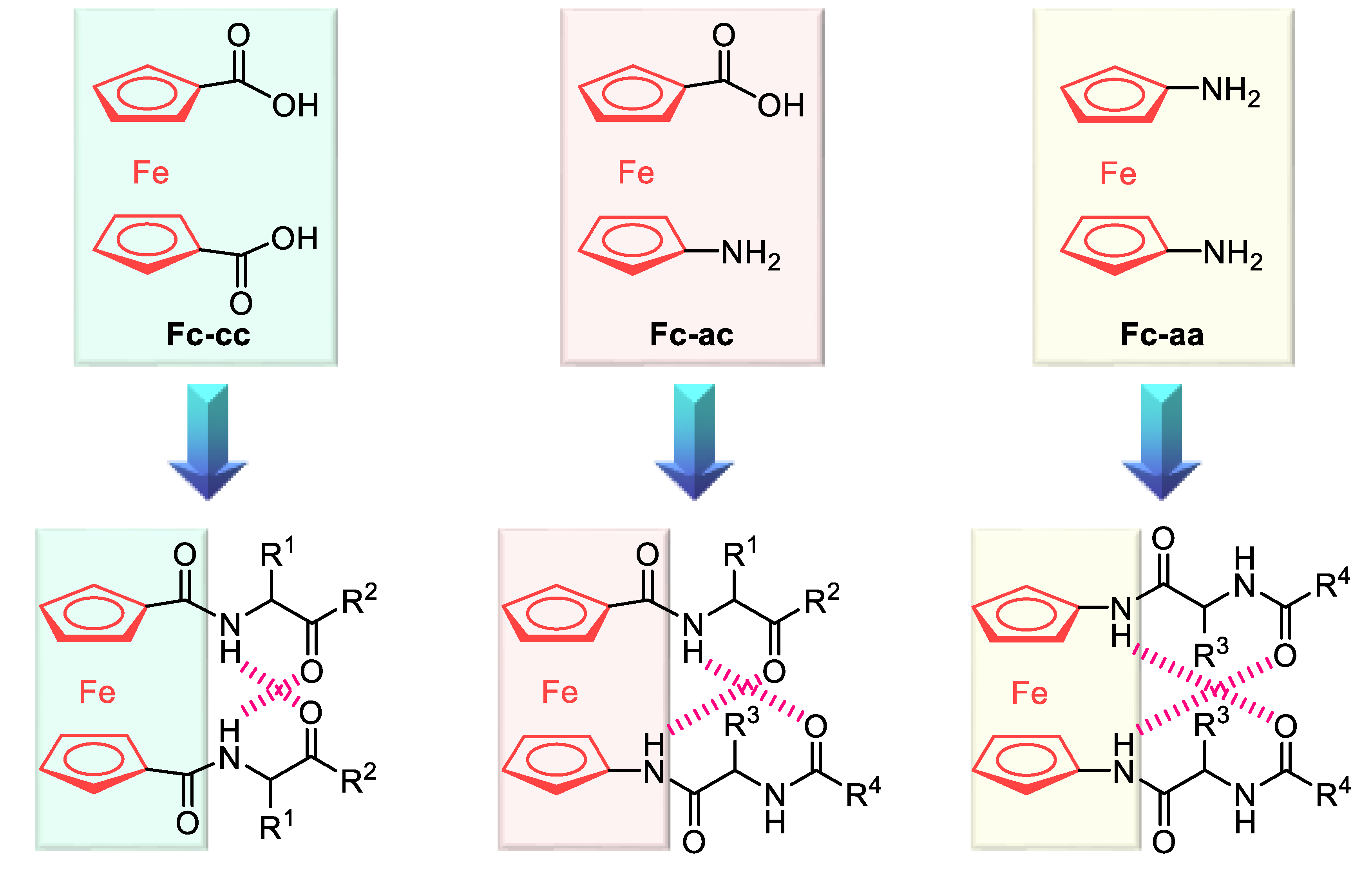
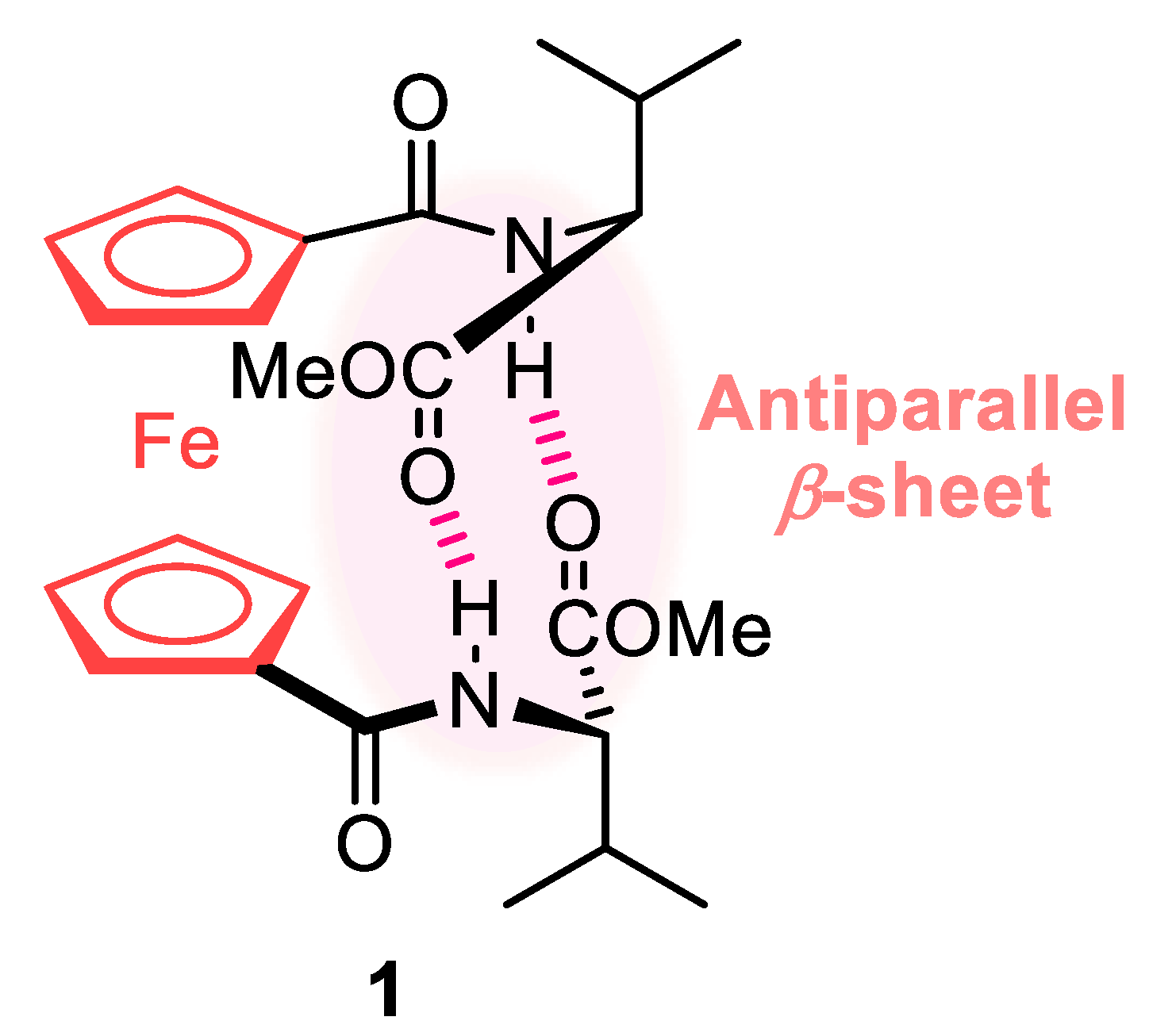
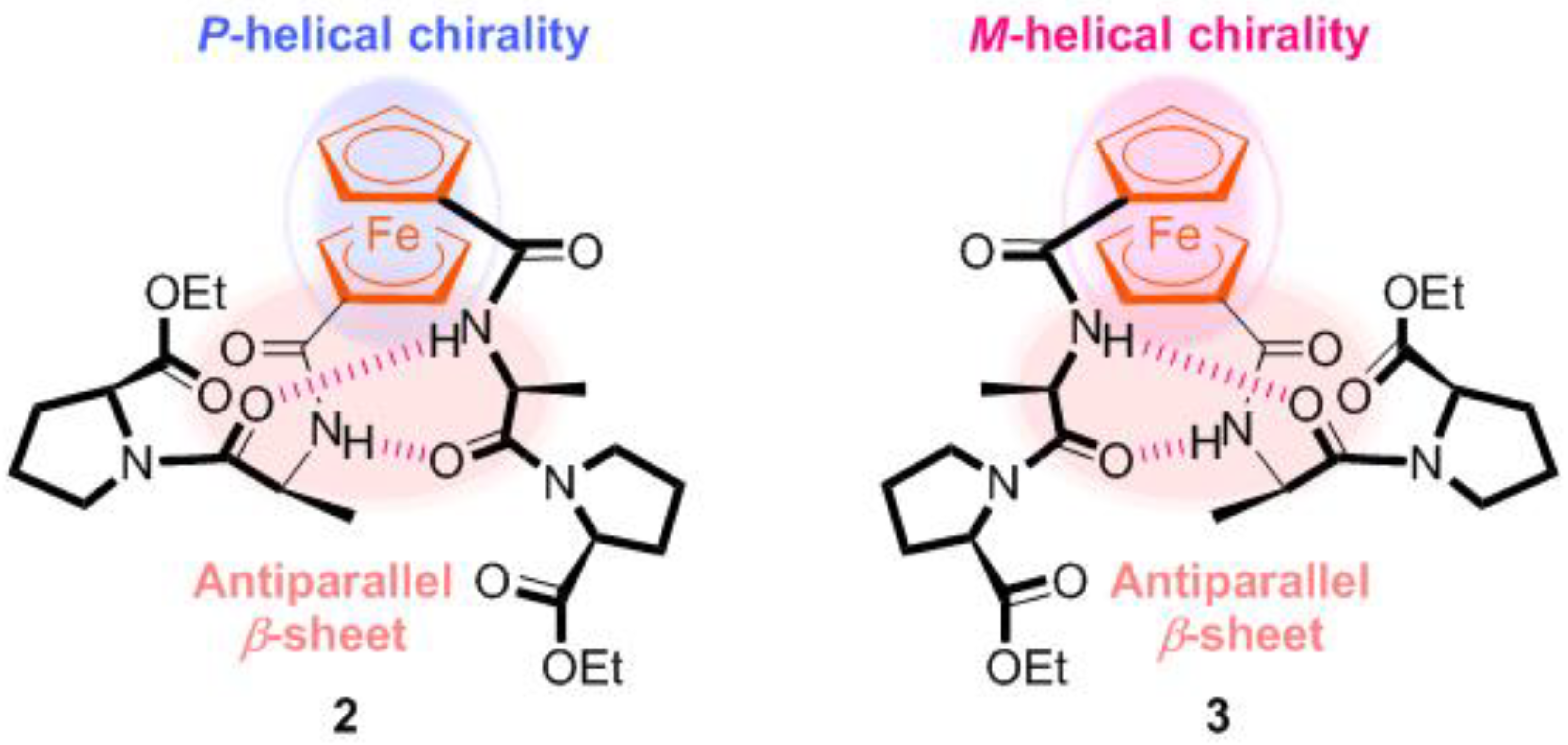
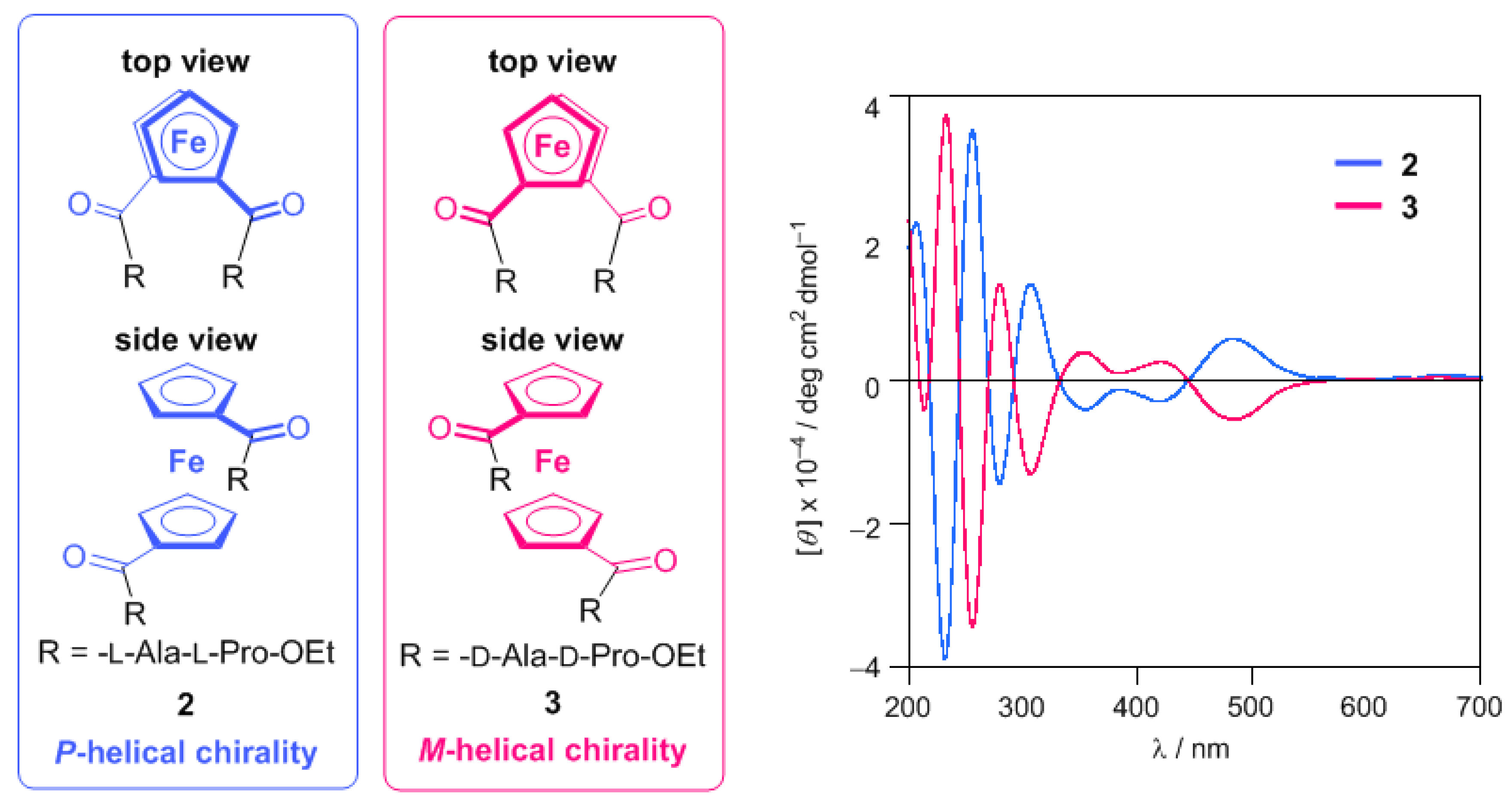
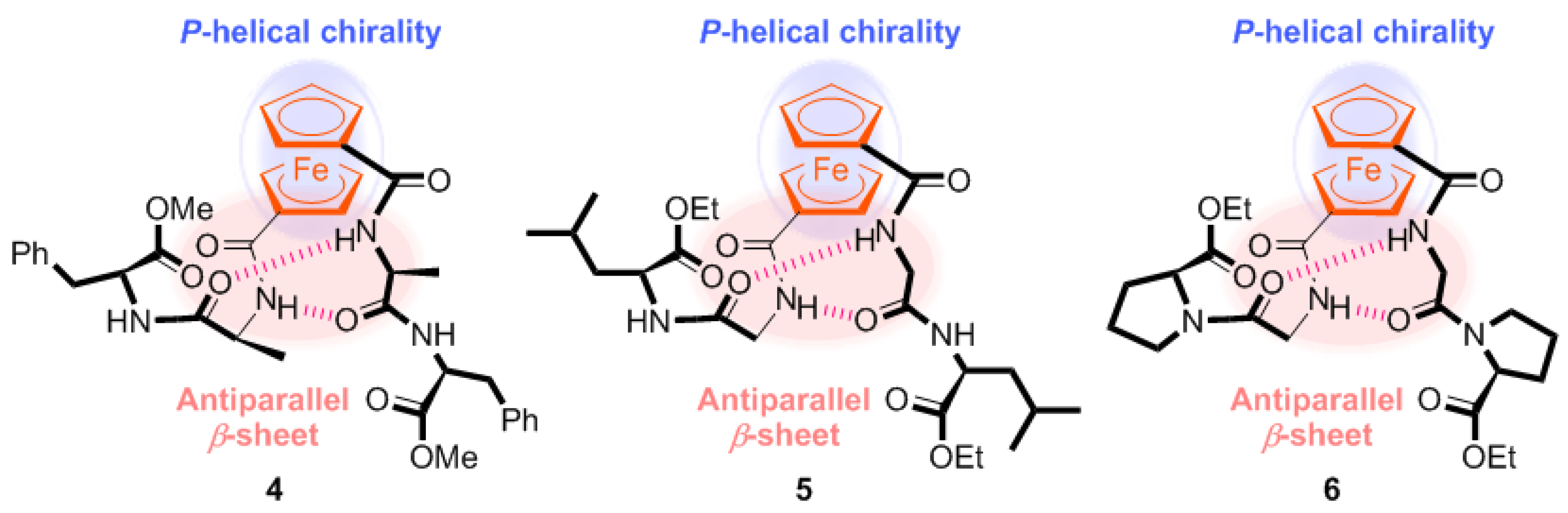
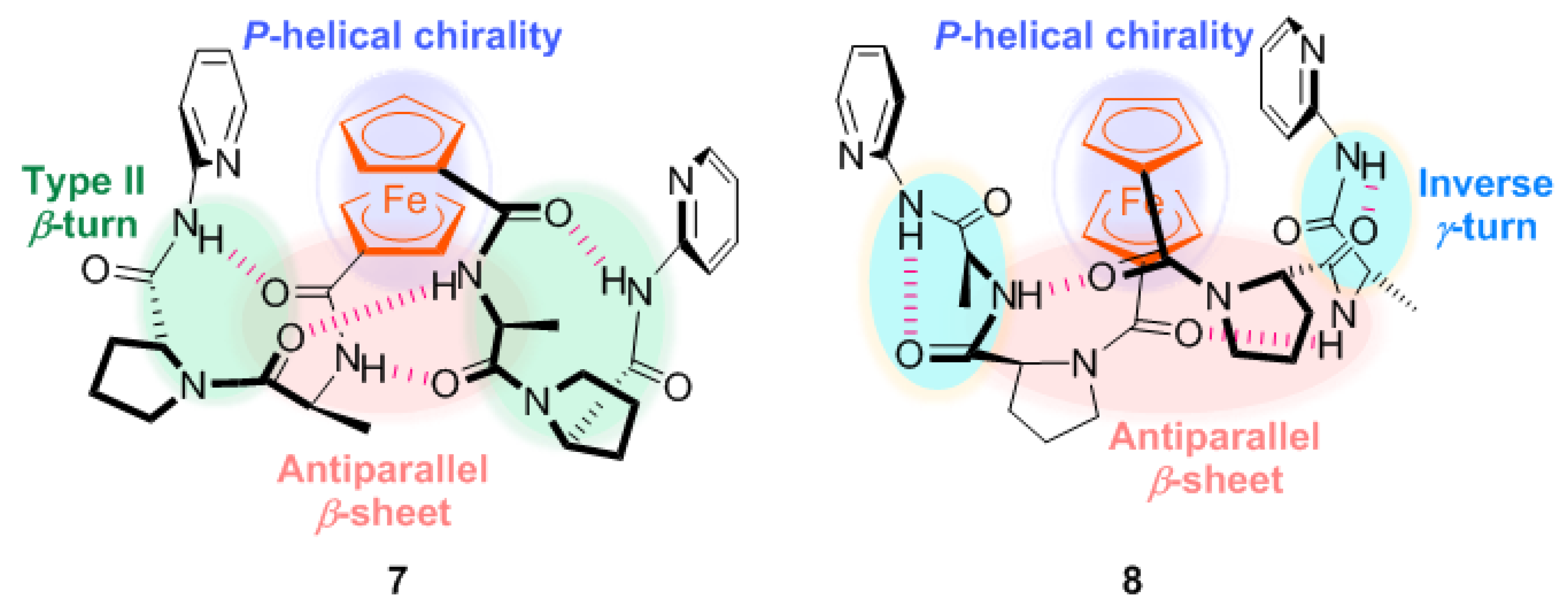
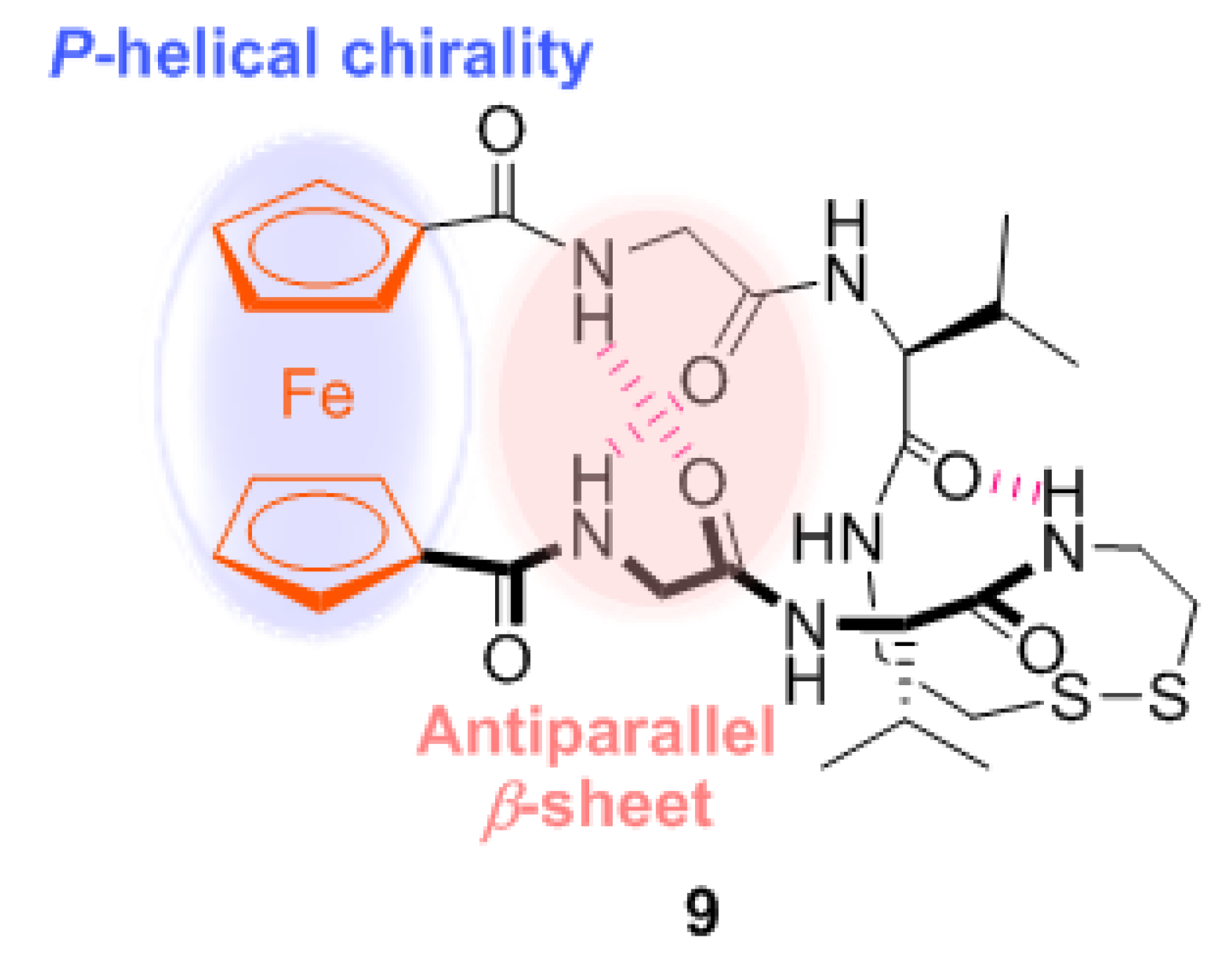
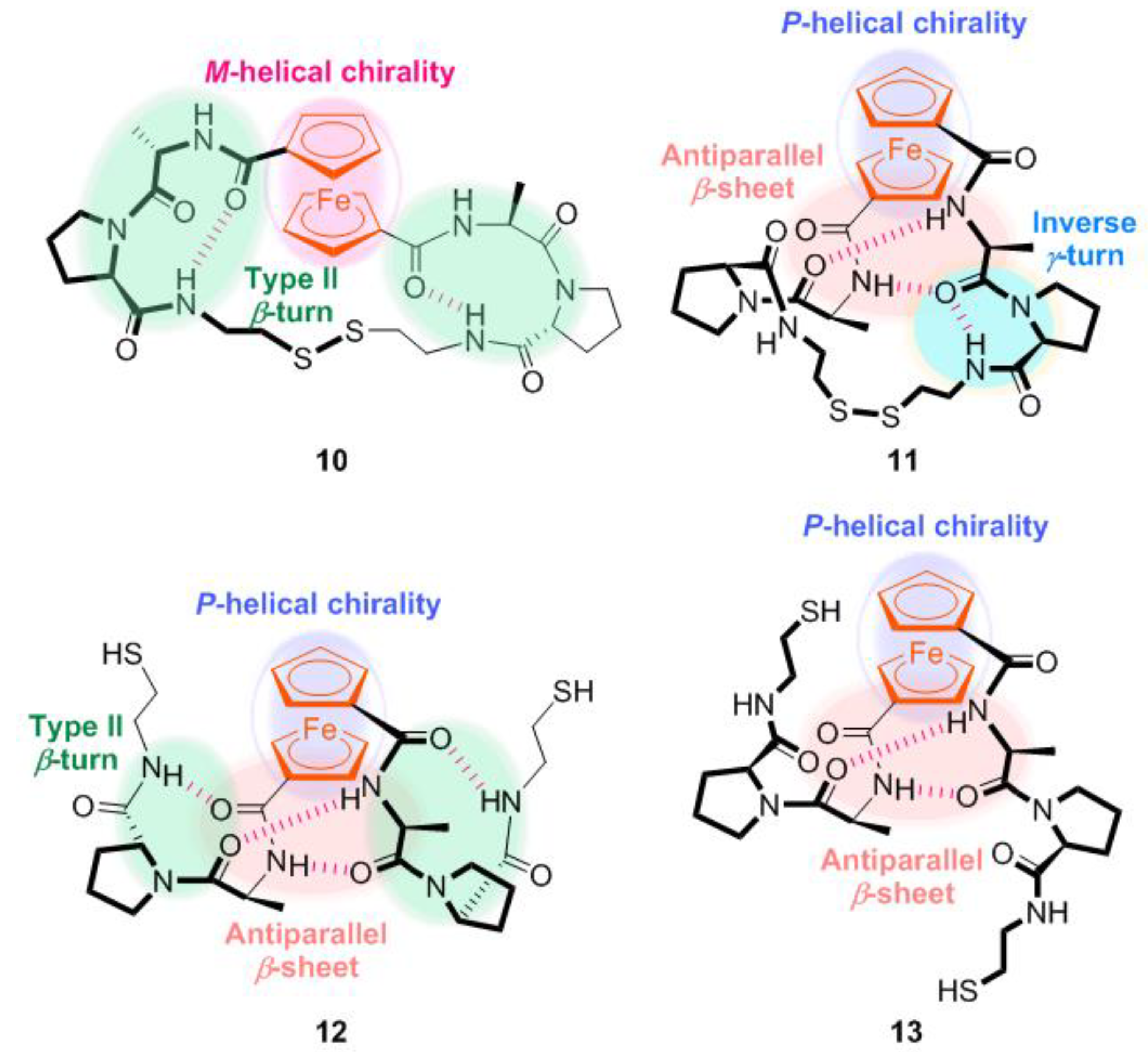
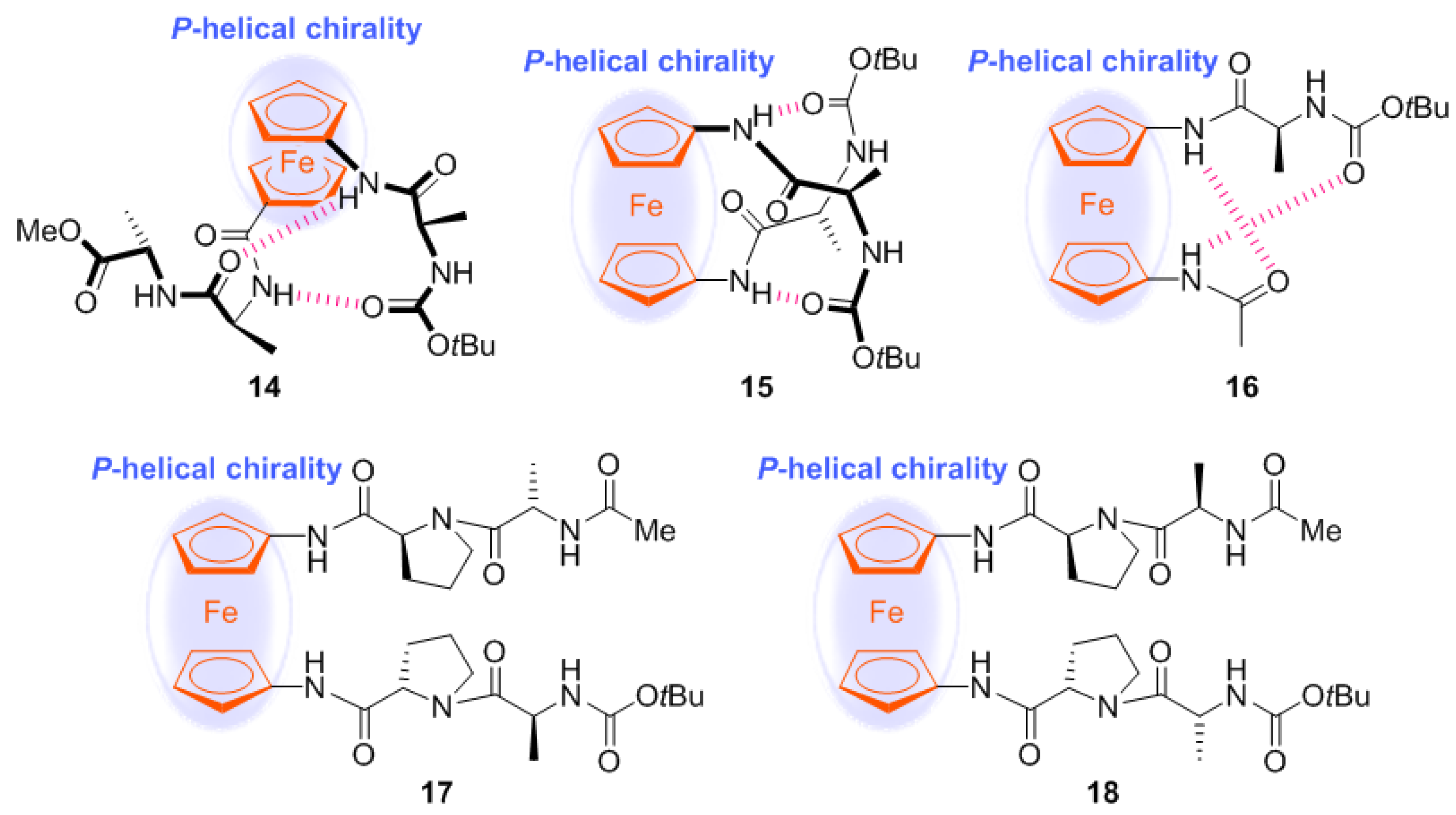
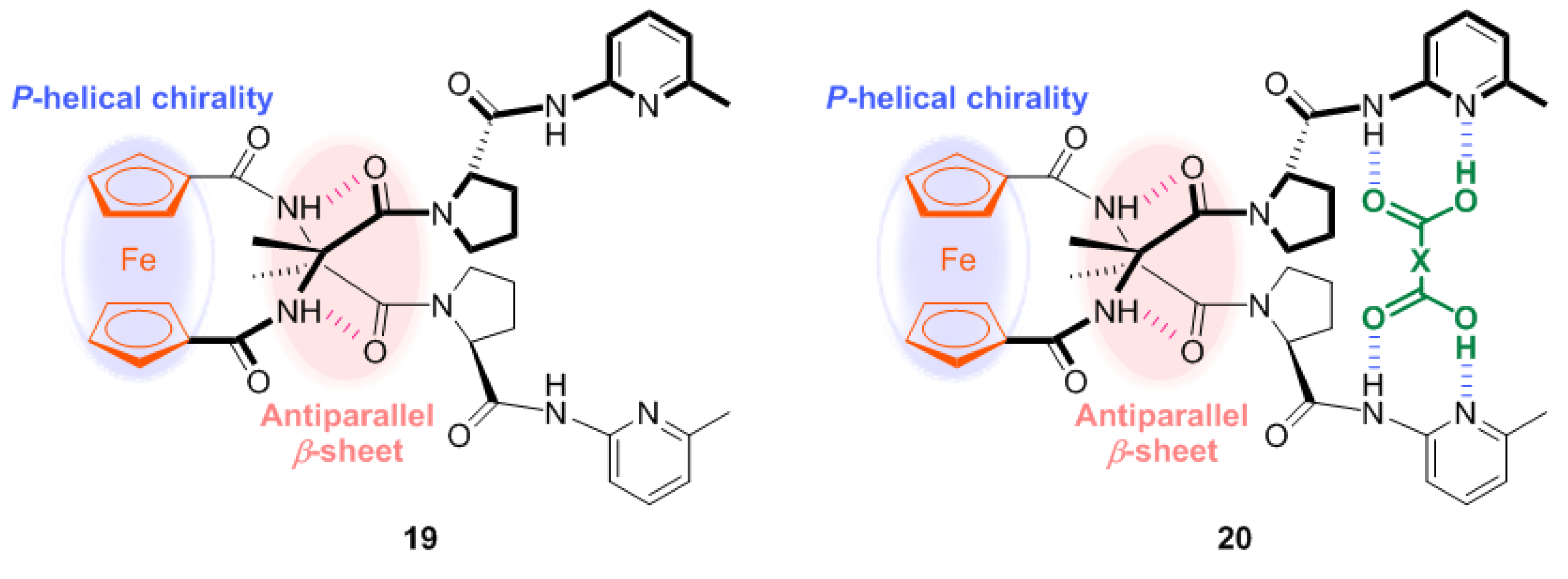
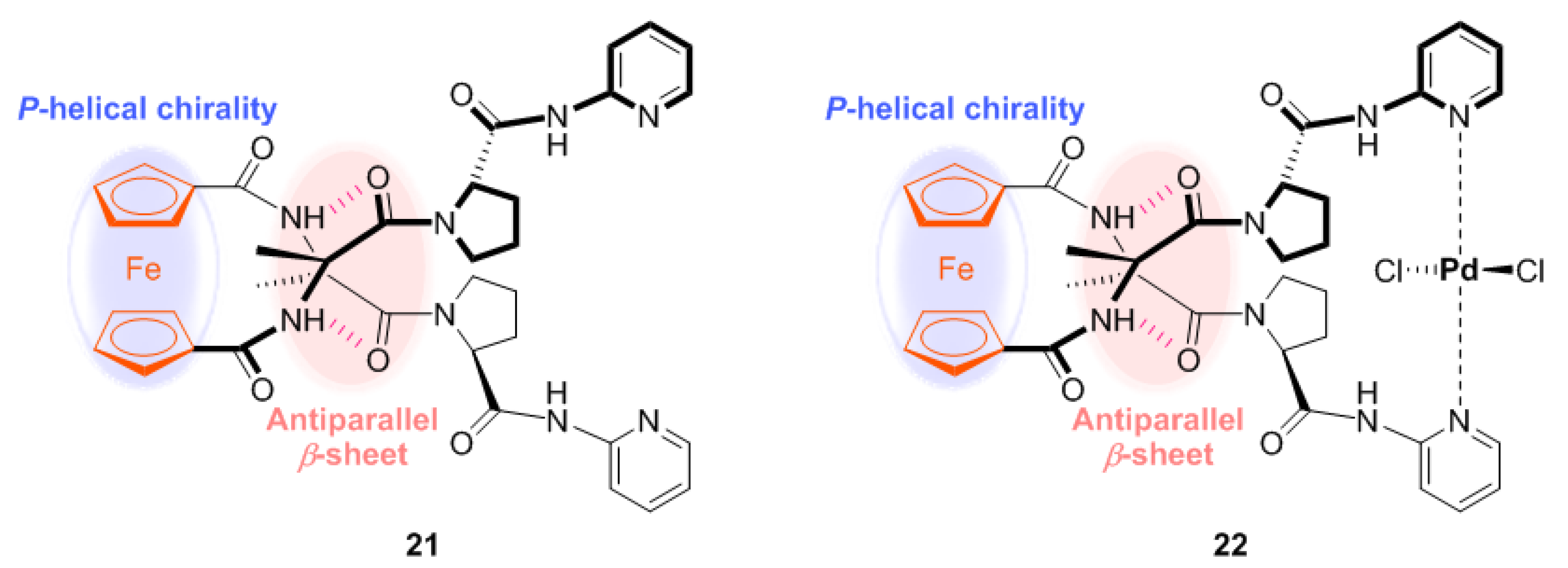
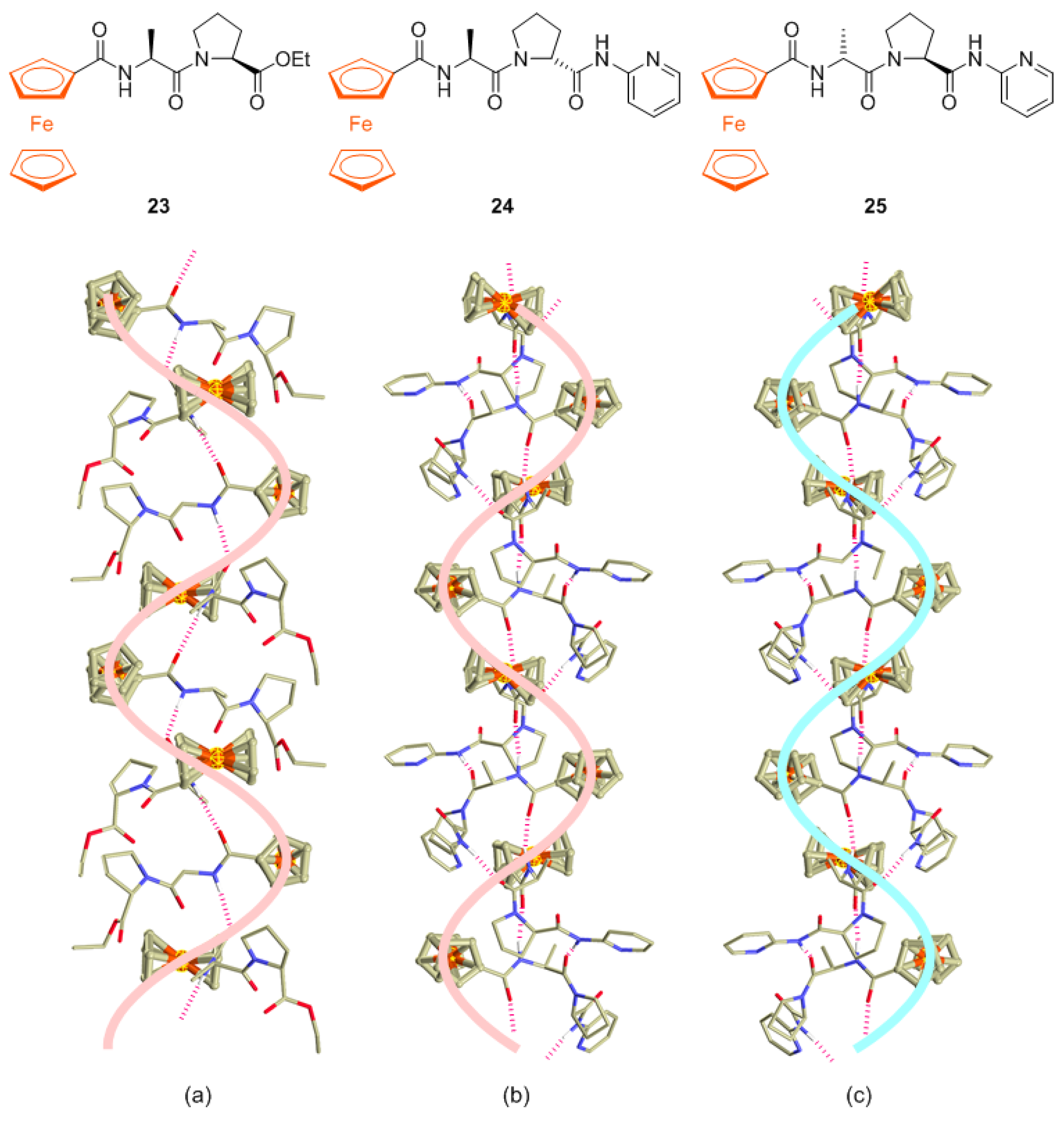
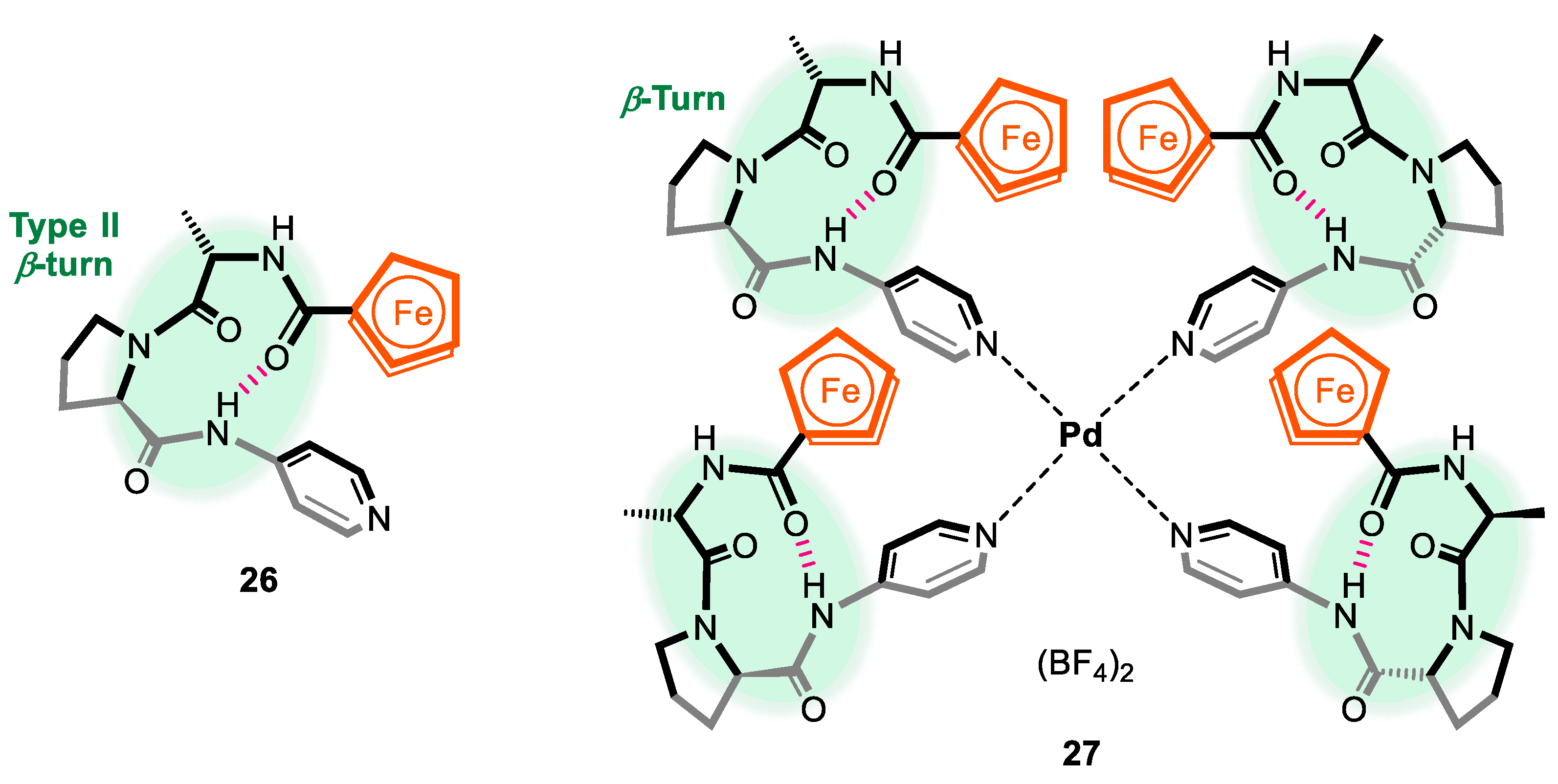
2.1. Control of Helical Chirality of Ferrocene Moieties in Ferrocene-Dipeptide Conjugates
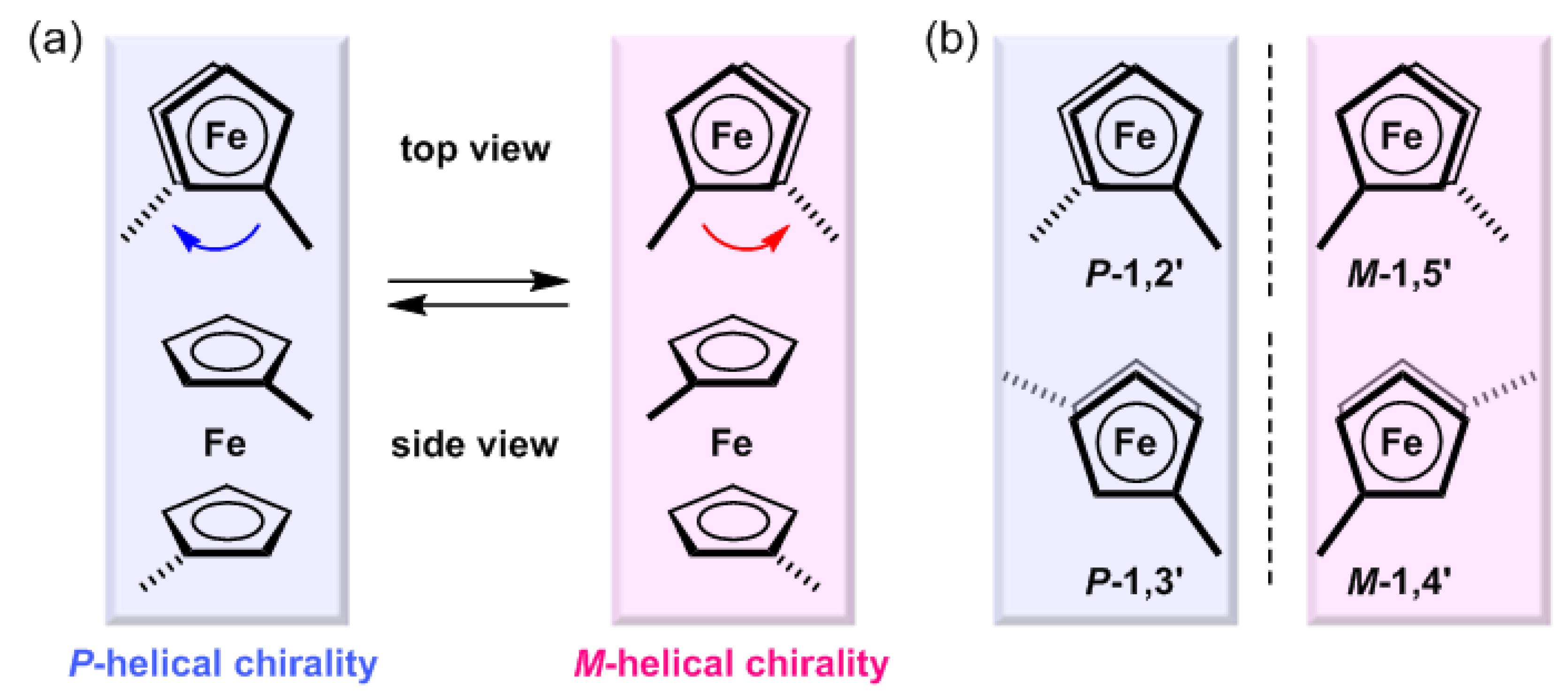
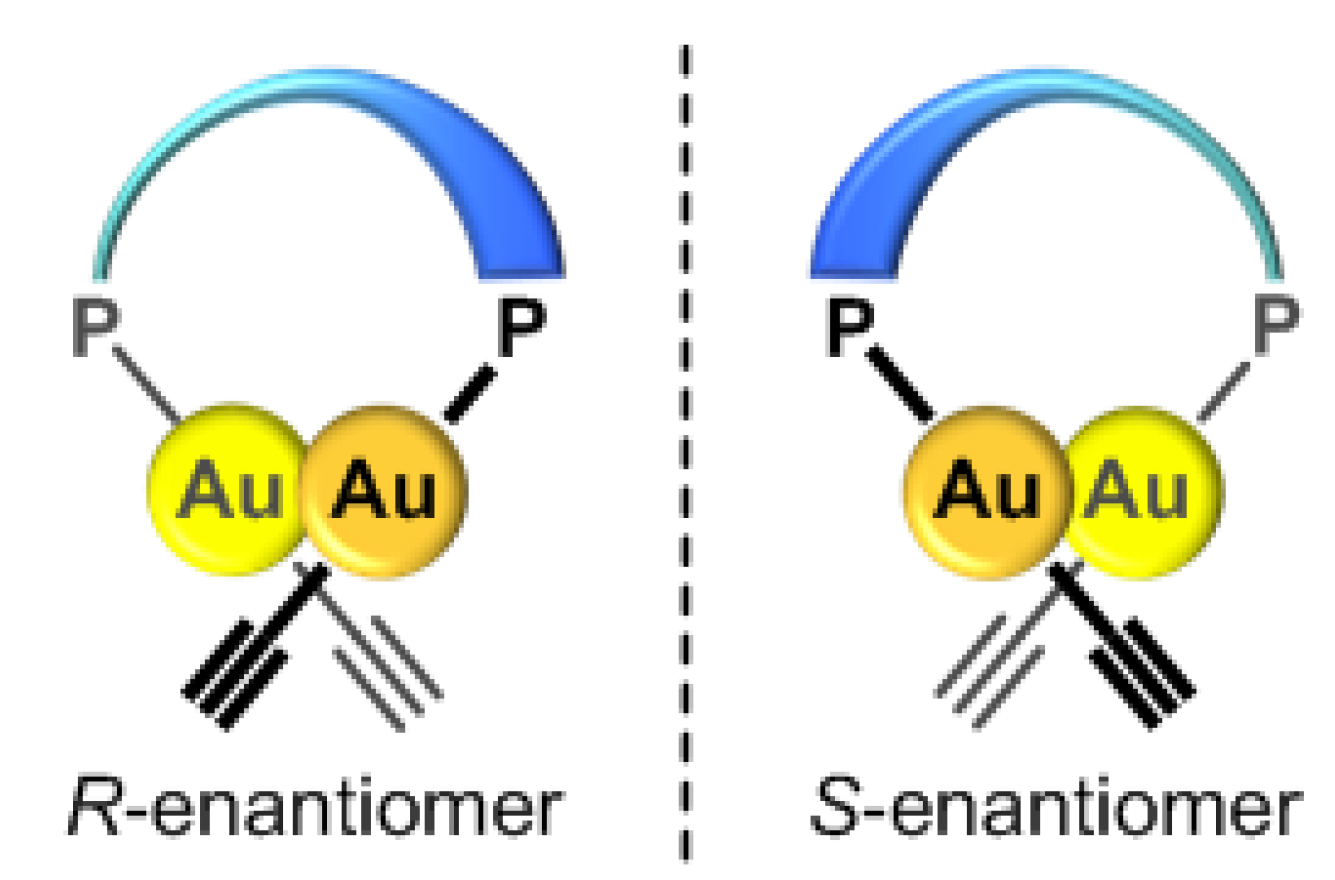
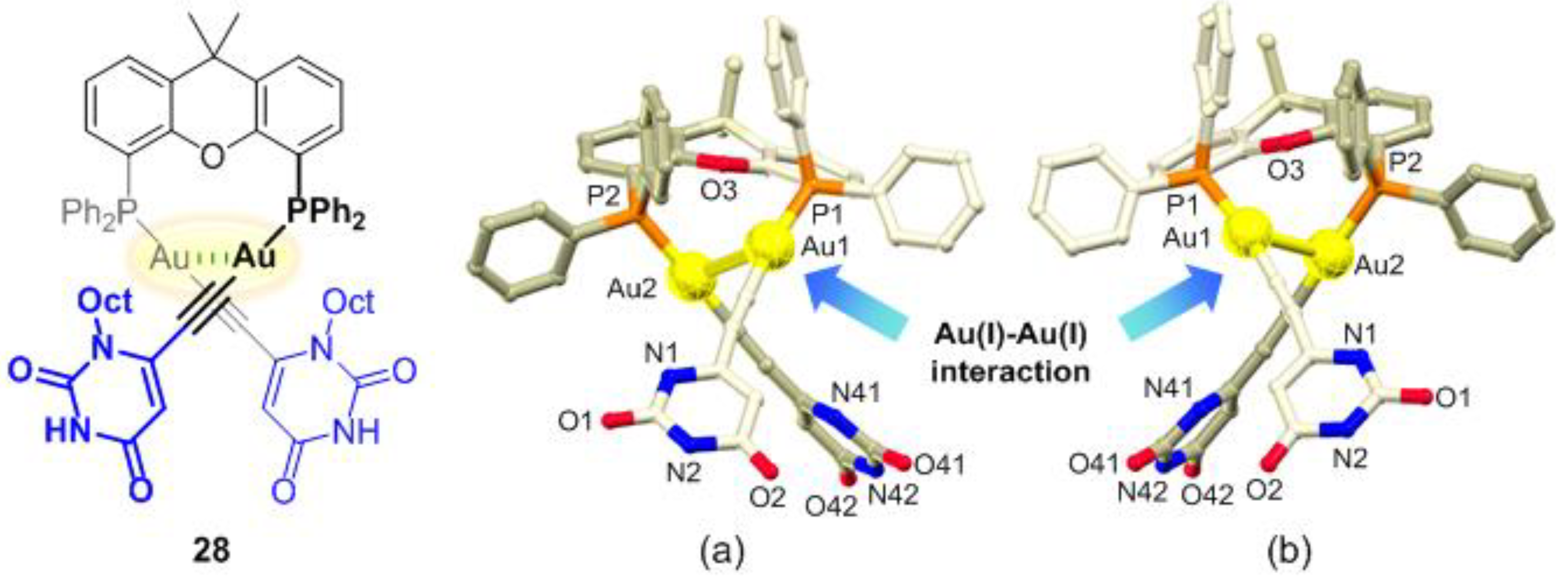
2.2. Chirality Induction of the Au(I)–Au(I) Axis in Dinuclear Organogold(I)-Uracil Conjugates
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeffrey, G.A. An Introduction to Hydrogen Bonding, 1st ed.; Oxford University Press: New York, NY, USA, 1997; ISBN 978-0-19-509549-4. [Google Scholar]
- Kyte, J. Structure in Protein Chemistry; Garland: New York, NY, USA, 1995; ISBN 978-0-81-533867-3. [Google Scholar]
- Branden, C.; Tooze, J. Introduction to Protein Structure, 2nd ed.; Garland: New York, NY, USA, 1998; ISBN 978-8-15-323050-5. [Google Scholar]
- Saenger, W. Principles of Nucleic Acid Structure; Springer: New York, NY, USA, 1984; ISBN 978-1-4612-5190-3. [Google Scholar]
- Jaouen, G.; Vessiéres, A.; Butler, I.S. Bioorganometallic Chemistry: A Future Direction for Transition Metal Organometallic Chemistry? Acc. Chem. Res. 1993, 26, 361–369. [Google Scholar] [CrossRef]
- Severin, R.; Bergs, R.; Beck, W. Bioorganometallic Chemistry—Transition Metal Complexes with α-Amino Acids and Peptides. Angew. Chem. Int. Ed. 1998, 37, 1634–1654. [Google Scholar] [CrossRef]
- Fish, R.H.; Jaouen, G. Bioorganometallic Chemistry: Structural Diversity of Organometallic Complexes with Bioligands and Molecular Recognition Studies of Several Supramolecular Hosts with Biomolecules, Alkali-Metal Ions, and Organometallic Pharmaceuticals. Organometallics 2003, 22, 2166–2177. [Google Scholar] [CrossRef]
- Jaouen, G. Bioorganometallics: Biomolecules, Labeling, Medicine; Wiley-VCH: Weinheim, Germany, 2006; ISBN 978-3-527-30990-0. [Google Scholar]
- Hartinger, C.G.; Dyson, P.J. Bioorganometallic Chemistry—From Teaching Paradigms to Medicinal Applications. Chem. Soc. Rev. 2009, 38, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Hirao, T.; Moriuchi, T.; Groß, A. Bioconjugates to induce chirality organization. In Functionalized Redox Systems; Hirao, T., Ed.; Springer Japan: Tokyo, Japan, 2015; pp. 111–150. ISBN 978-4-431-55305-2. [Google Scholar]
- Kowalski, K. Ferrocenyl-Nucleobase Complexes: Synthesis, Chemistry and Applications. Coord. Chem. Rev. 2016, 317, 132–156. [Google Scholar] [CrossRef]
- Merlino, A. Interactions between Proteins and Ru Compounds of Medicinal Interest: A Structural Perspective. Coord. Chem. Rev. 2016, 326, 111–134. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G. The Medicinal Chemistry of Ferrocene and Its Derivatives. Nat. Rev. Chem. 2017, 1, 0066. [Google Scholar] [CrossRef]
- Wenzel, M.; Casini, A. Mass Spectrometry as a Powerful Tool to Study Therapeutic Metallodrugs Speciation Mechanisms: Current Frontiers and Perspectives. Coord. Chem. Rev. 2017, 352, 432–460. [Google Scholar] [CrossRef]
- Thota, S.; Rodrigues, D.A.; Crans, D.C.; Barreiro, E.J. Ru(II) Compounds: Next-Generation Anticancer Metallotherapeutics? J. Med. Chem. 2018, 61, 5805–5821. [Google Scholar] [CrossRef] [PubMed]
- Togni, A.; Hayashi, T. Ferrocenes: Homogeneous Catalysis/Organic Synthesis/Materials Science; Wiley-VCH: Weinheim, Germany, 1995; ISBN (print: 978-3-527-29048-2; online: 978-3-527-61559-9). [Google Scholar]
- Kirin, S.I.; Kraatz, H.-B.; Metzler-Nolte, N. Systematizing Structural Motifs and Nomenclature in 1,n′-Disubstituted Ferrocene Peptides. Chem. Soc. Rev. 2006, 35, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Yam, V.W.-W.; Cheng, E.C.-C. Highlights on the Recent Advances in Gold Chemistry—A Photophysical Perspective. Chem. Soc. Rev. 2008, 37, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.J.; Sakai, K.; Leznoff, D.B. The Use of Aurophilic and Other Metal–Metal Interactions as Crystal Engineering Design Elements to Increase Structural Dimensionality. Chem. Soc. Rev. 2008, 37, 1884–1895. [Google Scholar] [CrossRef] [PubMed]
- Schmidbaur, H.; Schier, A. Aurophilic Interactions as a Subject of Current Research: An Up-Date. Chem. Soc. Rev. 2012, 41, 370–412. [Google Scholar] [CrossRef] [PubMed]
- Schlögl, K. Über Ferrocen-Aminosäuren und verwandte Verbindungen. Monatsh. Chem. 1957, 88, 601–621. [Google Scholar] [CrossRef]
- Hauser, C.R.; Lindsay, J.K. Certain Alkylations with the Methiodide of N,N-Dimethylaminomethylferrocene. Synthesis of an α-Amino Acid Having the Ferrocene Group. J. Org. Chem. 1957, 22, 1246–1247. [Google Scholar] [CrossRef]
- Osgerby, J.M.; Pauson, P.L. Ferrocene Derivatives. Part VI. dl-Ferrocenylalanine. J. Chem. Soc. 1958, 656–660. [Google Scholar] [CrossRef]
- Moriuchi, T.; Hirao, T. Highly Ordered Structures of Peptides by Using Molecular Scaffolds. Chem. Soc. Rev. 2004, 33, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Van Staveren, D.R.; Metzler-Nolte, N. Bioorganometallic Chemistry of Ferrocene. Chem. Rev. 2004, 104, 5931–5986. [Google Scholar] [CrossRef] [PubMed]
- Moriuchi, T.; Hirao, T. Ferrocene-Peptide Bioconjugates. In Bioorganometallic Chemistry; Simonneaux, G., Ed.; Springer: Berlin, Germany, 2006; Volume 17, pp. 143–175, ISBN (print: 978-3-540-33047-9; online: 978-3-540-33049-3). [Google Scholar]
- Salmain, M.; Metzler-Nolte, N. Bioorganometallic Chemistry of Ferrocene. In Ferrocenes; Stepnicka, P., Ed.; John Wiley & Sons: Chichester, UK, 2008; pp. 499–639, ISBN (print: 9780470035856; online: 9780470985663). [Google Scholar]
- Lataifeh, A.; Beheshti, S.; Kraatz, H.-B. Designer Peptides: Attempt to Control Peptide Structure by Exploiting Ferrocene as a Scaffold. Eur. J. Inorg. Chem. 2009, 3205–3218. [Google Scholar] [CrossRef]
- Moriuchi, T.; Hirao, T. Design of Ferrocene-Dipeptide Bioorganometallic Conjugates to Induce Chirality-Organized Structures. Acc. Chem. Res. 2010, 43, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Moriuchi, T.; Hirao, T. Dipeptide-Induced Chirality Organization. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 23–40. [Google Scholar] [CrossRef]
- Herrick, R.S.; Jarret, R.M.; Curran, T.P.; Dragoli, D.R.; Flaherty, M.B.; Lindyberg, S.E.; Slate, R.A.; Thornton, L.C. Ordered Conformations in Bis(amino acid) Derivatives of 1,1′-Ferrocenedicarboxylic Acid. Tetrahedron Lett. 1996, 37, 5289–5292. [Google Scholar] [CrossRef]
- Nomoto, A.; Moriuchi, T.; Yamazaki, S.; Ogawa, A.; Hirao, T. A Highly Ordered Ferrocene System Regulated by Podand Peptide Chains. Chem. Commun. 1998, 1963–1964. [Google Scholar] [CrossRef]
- Moriuchi, T.; Nomoto, A.; Yoshida, K.; Hirao, T. Characterization of Ferrocene Derivatives Bearing Podand Dipeptide Chains (-l-Ala-l-Pro-OR). J. Organomet. Chem. 1999, 589, 50–58. [Google Scholar] [CrossRef]
- Moriuchi, T.; Nomoto, A.; Yoshida, K.; Ogawa, A.; Hirao, T. Chirality Organization of Ferrocenes Bearing Podand Dipeptide Chains: Synthesis and Structural Characterization. J. Am. Chem. Soc. 2001, 123, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Moriuchi, T.; Wu, H.; Tayano, Y.; Hirao, T. Structural Characterization of Chirality-Organized Ferrocene-Dipeptide Conjugates that Contain Pyridine N-Oxide Moieties. Asian. J. Org. Chem. 2017, 6, 1250–1256. [Google Scholar] [CrossRef]
- Van Staveren, D.R.; Weyhermüller, T.; Metzler-Nolte, N. Organometallic β-Turn Mimetics. A Structural and Spectroscopic Study of Inter-Strand Hydrogen Bonding in Ferrocene and Cobaltocenium Conjugates of Amino Acids and Dipeptides. Dalton. Trans. 2003, 210–220. [Google Scholar] [CrossRef]
- Moriuchi, T.; Nomoto, A.; Yoshida, K.; Hirao, T. Intramolecular Conformational Control in Ferrocenes Bearing Podand Dipeptide Chains. Organometallics 2001, 20, 1008–1013. [Google Scholar] [CrossRef]
- Moriuchi, T.; Nagai, T.; Hirao, T. Chirality Organization of Ferrocenes Bearing Dipeptide Chains of Heterochiral Sequence. Org. Lett. 2005, 7, 5265–5268. [Google Scholar] [CrossRef] [PubMed]
- Kirin, S.I.; Wissenbach, D.; Metzler-Nolte, N. Unsymmetrical 1,n′-Disubstituted Ferrocenoyl Peptides: Convenient One Pot Synthesis and Solution Structures by CD and NMR Spectroscopy. New J. Chem. 2005, 29, 1168–1173. [Google Scholar] [CrossRef]
- Heinze, K.; Beckmann, M. Conformational Analysis of Chiral Ferrocene-Peptides. Eur. J. Inorg. Chem. 2005, 3450–3457. [Google Scholar] [CrossRef]
- Moriuchi, T.; Nagai, T.; Hirao, T. Induction of γ-Turn-Like Structure in Ferrocene Bearing Dipeptide Chains via Conformational Control. Org. Lett. 2006, 8, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Sanders, D.A.R.; Schatte, G.; Kraatz, H.-B. Discovery of a Pseudo β Barrel: Synthesis and Formation by Tiling of Ferrocene Cyclopeptides. Angew. Chem. Int. Ed. 2006, 45, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Moriuchi, T.; Nishiyama, T.; Nobu, M.; Hirao, T. Control of Helical Chirality of Ferrocene-Dipeptide Conjugates by the Secondary Structure of Dipeptide Chains. Chem. Eur. J. 2017, 23, 12704–12708. [Google Scholar] [CrossRef] [PubMed]
- Barišić, L.; Dropučić, M.; Rapić, V.; Pritzkow, H.; Kirin, S.I.; Metzler-Nolte, N. The First Oligopeptide Derivative of 1′-Aminoferrocene-1-Carboxylic Acid Shows Helical Chirality with Antiparallel Strands. Chem. Commun. 2004, 2004–2005. [Google Scholar] [CrossRef]
- Chowdhury, S.; Schatte, G.; Mahmoud, K.A.; Kraatz, H.-B. Amino Acid Conjugates of 1,1′-Diaminoferrocene. Synthesis and Chiral Organization. Org. Biomol. Chem. 2005, 3, 3018–3023. [Google Scholar] [CrossRef] [PubMed]
- Djaković, S.; Siebler, D.; Semenčić, M.C.; Heinze, K.; Rapić, V. Spectroscopic and Theoretical Study of Asymmetric 1,1′-Diaminoferrocene Conjugates of α-Amino Acids. Organometallics 2008, 27, 1447–1453. [Google Scholar] [CrossRef]
- Kovačević, M.; Kodrin, I.; Roca, S.; Molčanov, K.; Shen, Y.; Adhikari, B.; Kraatz, H.-B.; Barišić, L. Helically Chiral Peptides That Contain Ferrocene-1,1′-Diamine Scaffolds as a Turn Inducer. Chem. Eur. J. 2017, 23, 10372–10395. [Google Scholar] [CrossRef] [PubMed]
- Moriuchi, T.; Yoshida, K.; Hirao, T. Chirality-Organized Ferrocene Receptor Bearing Podand Dipeptide Chains (-l-Ala-l-Pro-NHPyMe) for the Selective Recognition of Dicarboxylic Acids. Org. Lett. 2003, 5, 4285–4288. [Google Scholar] [CrossRef] [PubMed]
- Moriuchi, T.; Yoshida, K.; Hirao, T. Complexation Stabilized Conformational Regulation of Ferrocene Bearing Podand Dipeptide Chains (-l-Ala-l-Pro-NHPy). Organometallics 2001, 20, 3101–3105. [Google Scholar] [CrossRef]
- Moriuchi, T.; Fujiwara, T.; Hirao, T. β-Turn-Structure-Assembled Palladium Complexes by Complexation-Induced Self-Organization of Ferrocene-Dipeptide Conjugates. Dalton Trans. 2009, 4286–4288. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Moriuchi, T.; Kawahata, M.; Yamaguchi, K.; Hirao, T. A G-Octamer Scaffold via Self-Assembly of a Guanosine-Based Au(I) Isonitrile Complex for Au(I)–Au(I) Interaction. Chem. Commun. 2011, 47, 4682–4684. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Moriuchi, T.; Sakamoto, Y.; Kawahata, M.; Yamaguchi, K.; Hirao, T. La(OTf)3-Mediated Self-Organization of the Guanosine with an Alkynyl-Au(I)PPh3 Moiety to Induce Au(I)–Au(I) Interaction. RSC Adv. 2012, 2, 4359–4363. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Moriuchi, T.; Hirao, T. Organogold(I)-Uracil Conjugates: Synthesis and Structural Characterization. J. Organomet. Chem. 2015, 782, 77–81. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Moriuchi, T.; Hirao, T. Dinuclear Organogold(I) Complexes Bearing Uracil Moieties: Chirality of Au(I)–Au(I) Axis and Self-Assembling. CrystEngComm 2015, 17, 3460–3467. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriuchi, T.; Ohmura, S.D.; Moriuchi-Kawakami, T. Chirality Induction in Bioorganometallic Conjugates. Inorganics 2018, 6, 111. https://doi.org/10.3390/inorganics6040111
Moriuchi T, Ohmura SD, Moriuchi-Kawakami T. Chirality Induction in Bioorganometallic Conjugates. Inorganics. 2018; 6(4):111. https://doi.org/10.3390/inorganics6040111
Chicago/Turabian StyleMoriuchi, Toshiyuki, Satoshi D. Ohmura, and Takayo Moriuchi-Kawakami. 2018. "Chirality Induction in Bioorganometallic Conjugates" Inorganics 6, no. 4: 111. https://doi.org/10.3390/inorganics6040111
APA StyleMoriuchi, T., Ohmura, S. D., & Moriuchi-Kawakami, T. (2018). Chirality Induction in Bioorganometallic Conjugates. Inorganics, 6(4), 111. https://doi.org/10.3390/inorganics6040111