Dynamic Helicity Control of Oligo(salamo)-Based Metal Helicates
Abstract
1. Introduction
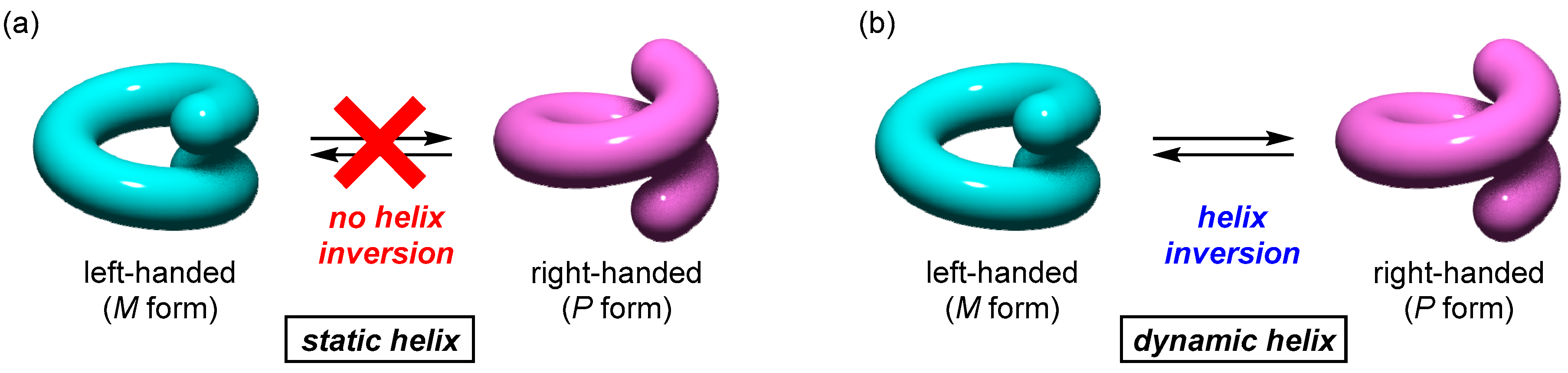
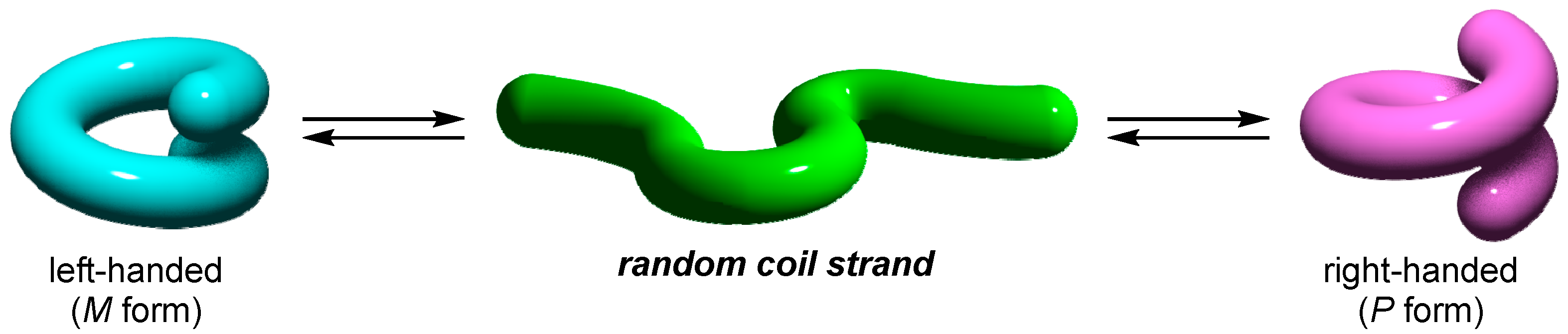
1.1. Dynamic Helical Structures
1.2. Helicity Control of Dynamic Helical Structures
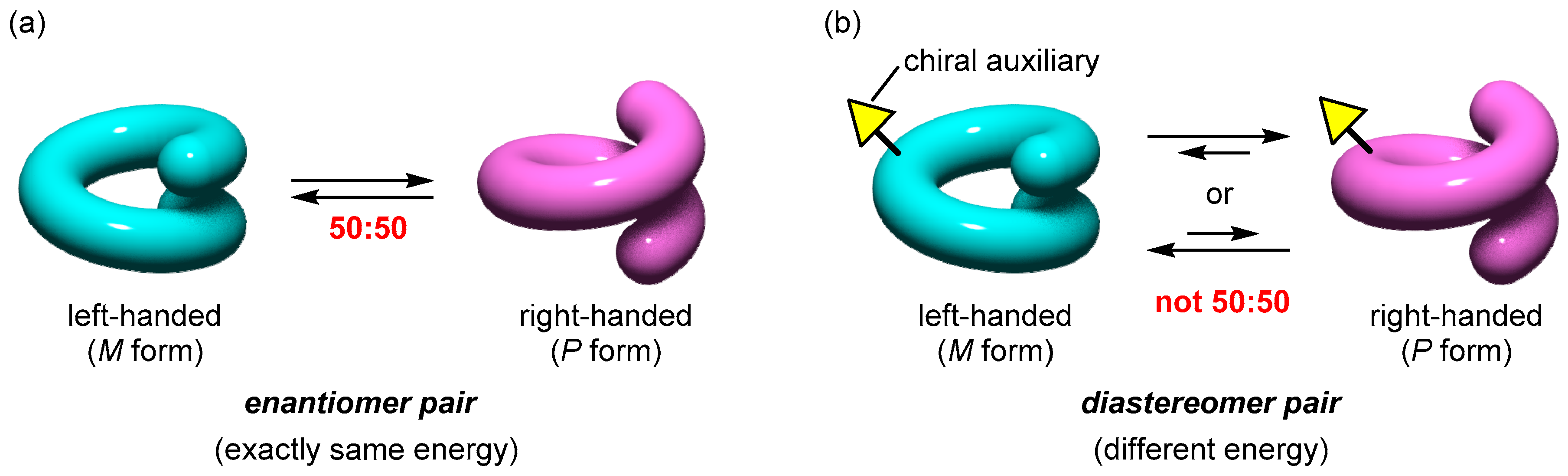
1.3. Classification of Helicity Control and Helicity Inversion of Dynamic Helical Metal Complexes
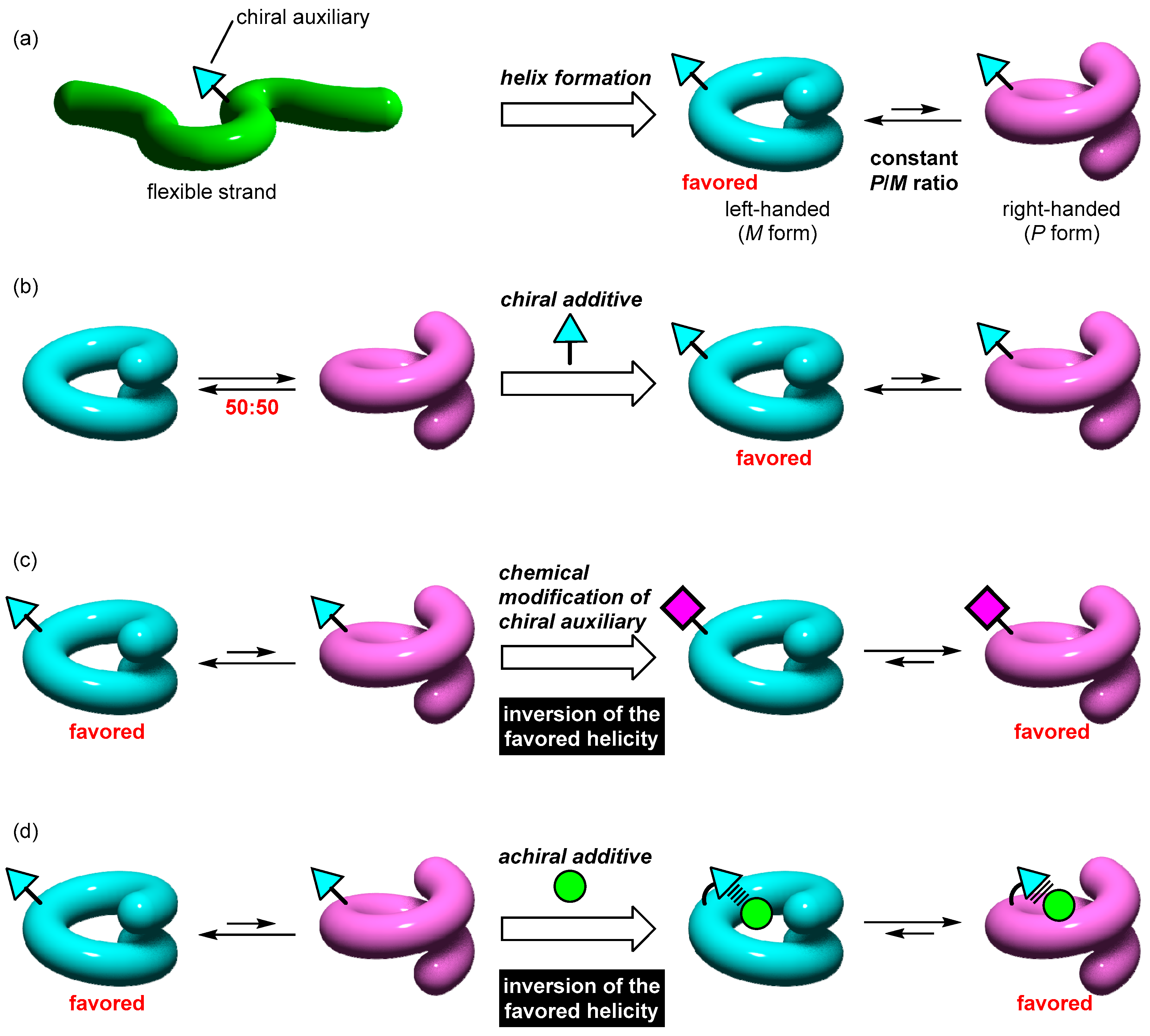
- Category A. Formation of helical structures with a biased P/M ratio.In this case, helical structures with a biased P/M ratio are obtained upon the helix formation by the metal complexation of an acyclic ligand having a chiral auxiliary (Figure 4a). The chiral auxiliary that is pre-installed into the helix scaffold causes the deviation of the P/M ratio from 50:50 as a result of the thermodynamic equilibration, because the introduction of the chiral auxiliary converts the P/M enantiomer pair into a diastereomer pair.
- Category B. Shift of the 50:50 P/M ratios of racemic helical structures.In this case, stimuli-responsiveness is introduced so that the exactly 50:50 P/M ratio of a racemic helical structure is biased upon the addition of a chiral auxiliary as an additive (Figure 4b). A chiral stimulus converts the enantiomer pair into a diastereomer pair, which causes the dynamic shift of the P/M equilibrium. This change is regarded as the helicity induction triggered by a chiral additive, which can be detected by the induced CD signal.
- Category C. Increase, decrease, or inversion of P/M ratios by chemical modification.In this case, stimuli-responsiveness is used for helicity changes such as helicity inversion. For example, the P/M ratio is changed by replacing or modifying the chiral auxiliary (Figure 4c). If the pre-installed chiral auxiliary becomes more effective after the modification, the helical biases should be increased. If the chiral auxiliary after the modification stabilizes the opposite helicity from the original form, a responsive helicity inversion is expected. The helicity change can also be achieved without altering the chiral auxiliary, by using chemical stimuli such as achiral ions (Figure 4d). If the structures of a helix framework are chemically modified, the P/M preference is also changed even if the chiral auxiliary is unchanged. Therefore, for a helix having a pre-installed chiral auxiliary, the P/M equilibrium ratio can be increased, decreased, or inverted upon stimulation with the suitable achiral additive.
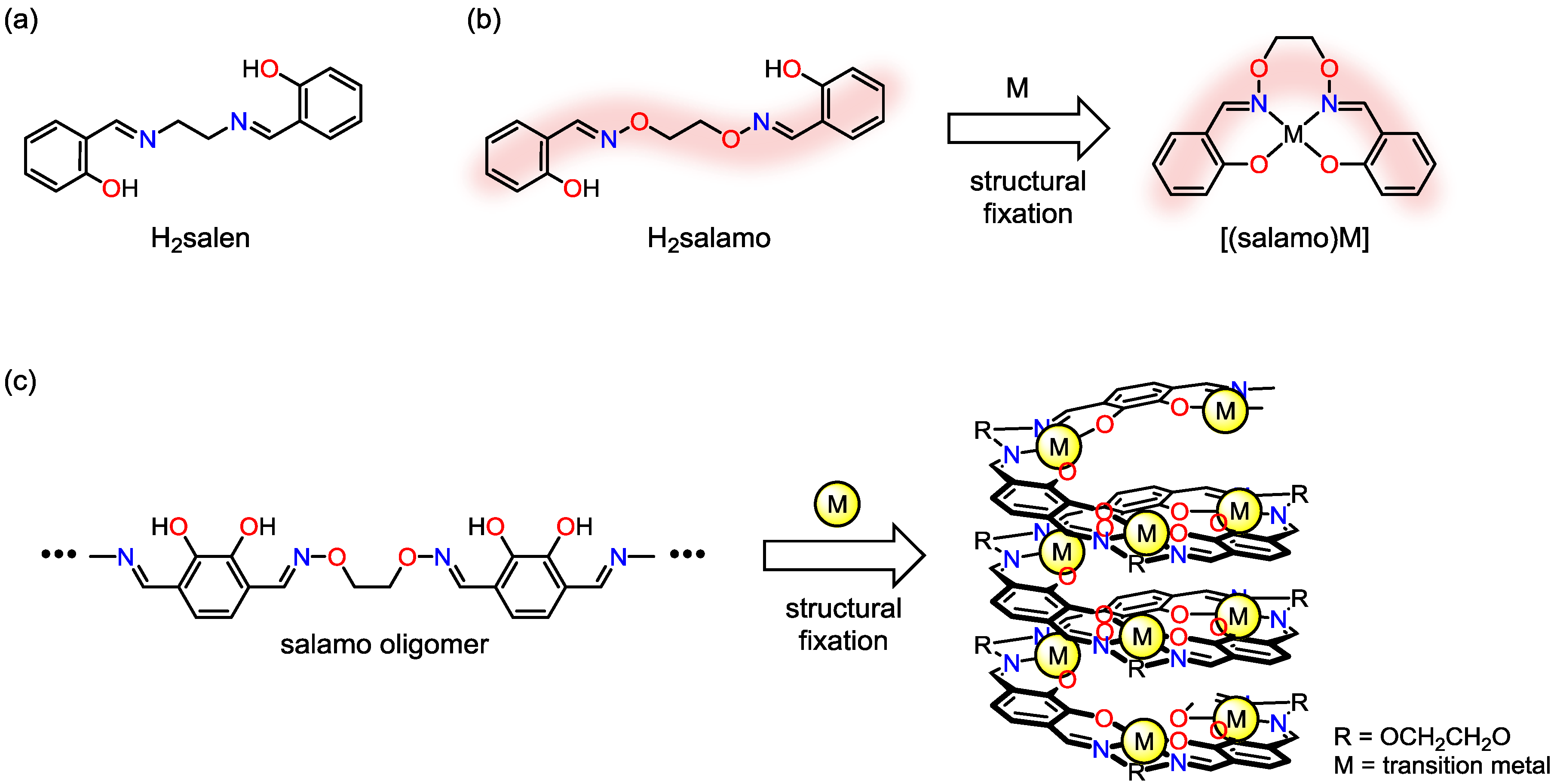
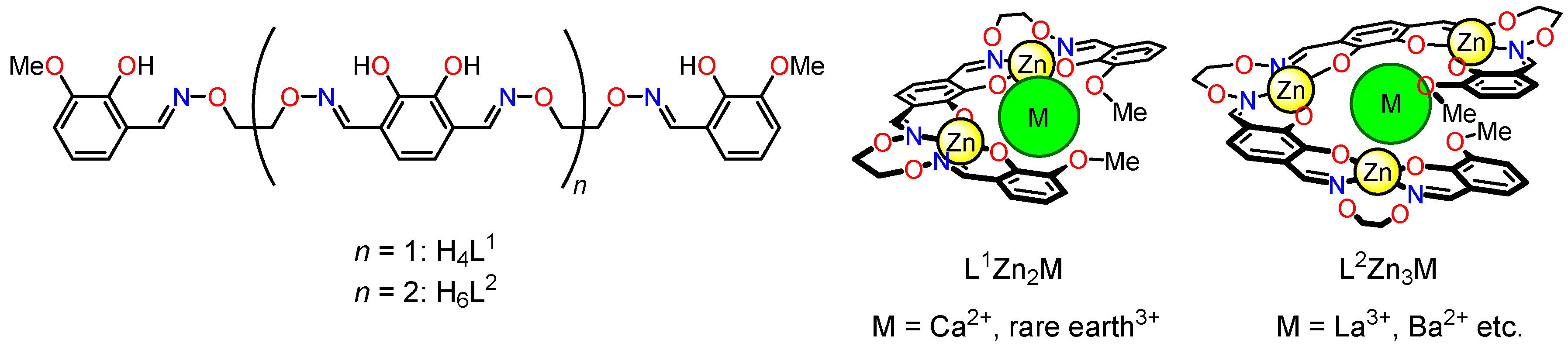
2. Molecular Design of Oligo(salen)-Type Helical Structures
2.1. Molecular Design
2.2. Construction of Helical Structures and Their Dynamic Helix Inversion Behavior
3. Dynamic Helicity Control by Chiral Counteranions
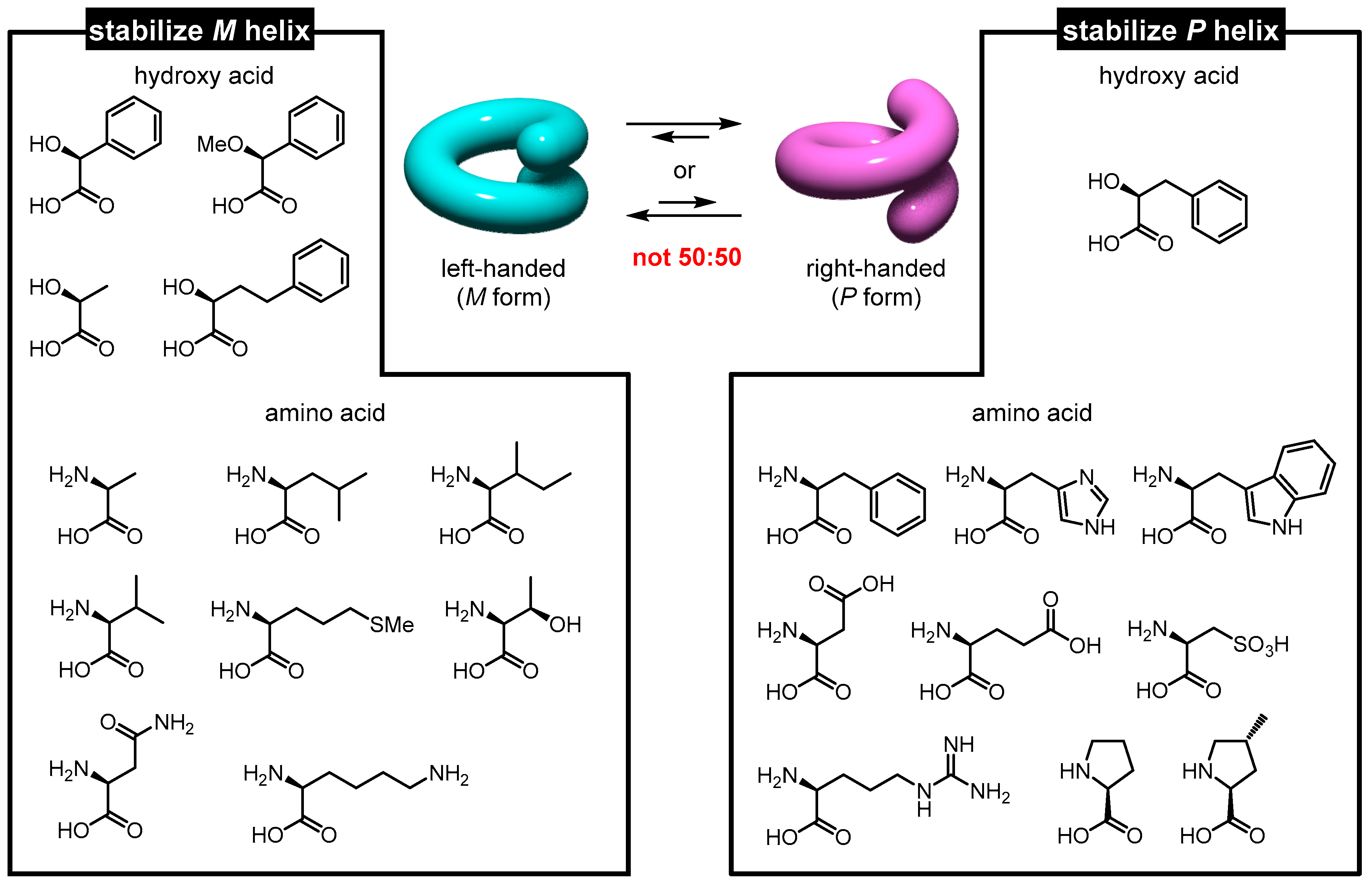
3.1. Strategy
3.2. Helicity Control Using Hydroxy Acids
3.3. Helicity Control Using Amino Acids
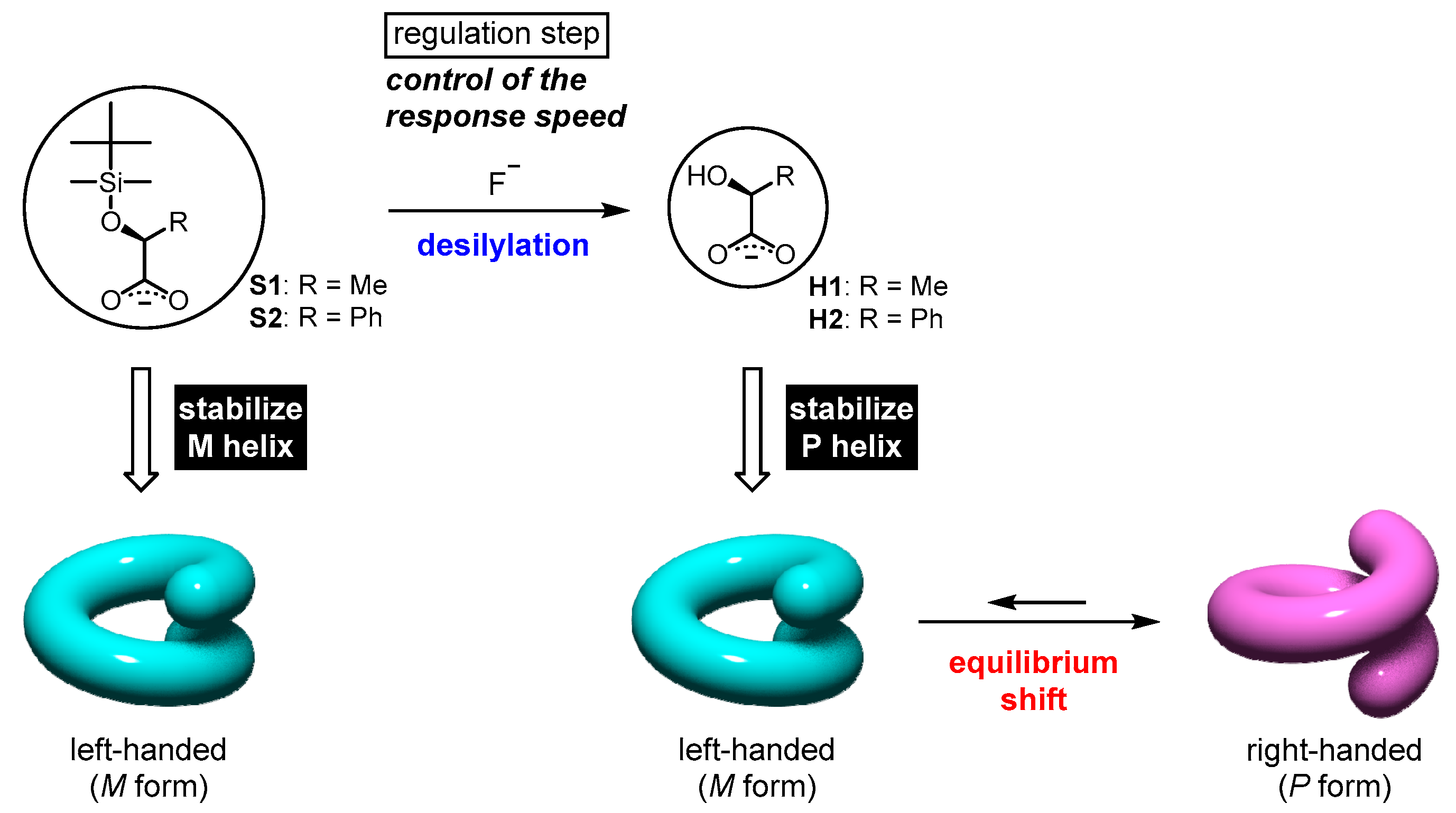
3.4. Dynamic Helicity Inversion by Chemical Modifications
4. Dynamic Helicity Control by Chiral Tethers
4.1. Strategy
4.2. Helicity Control of Helical Complexes
4.3. Helicity Inversion by Metal Exchange
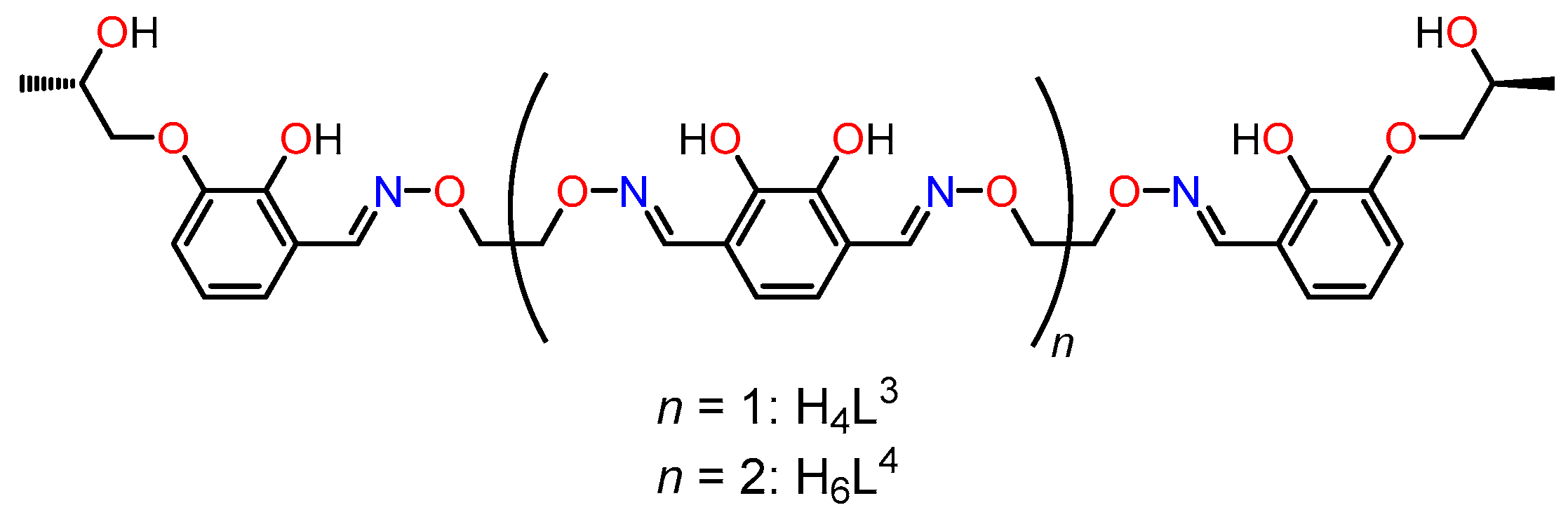
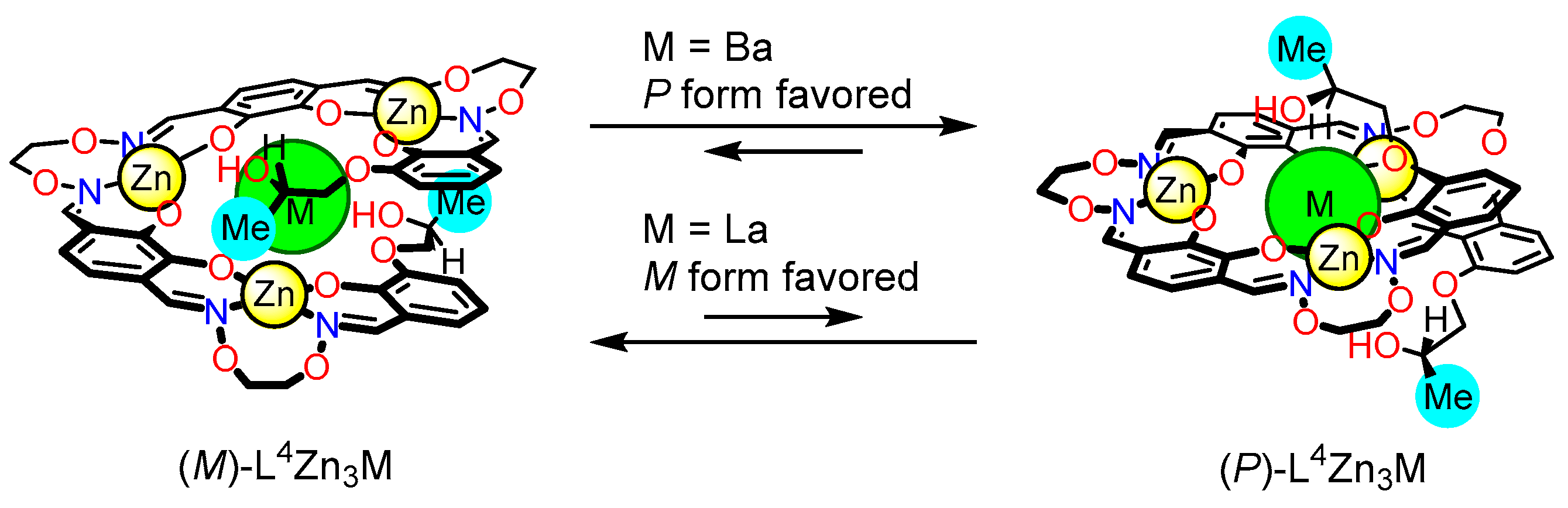
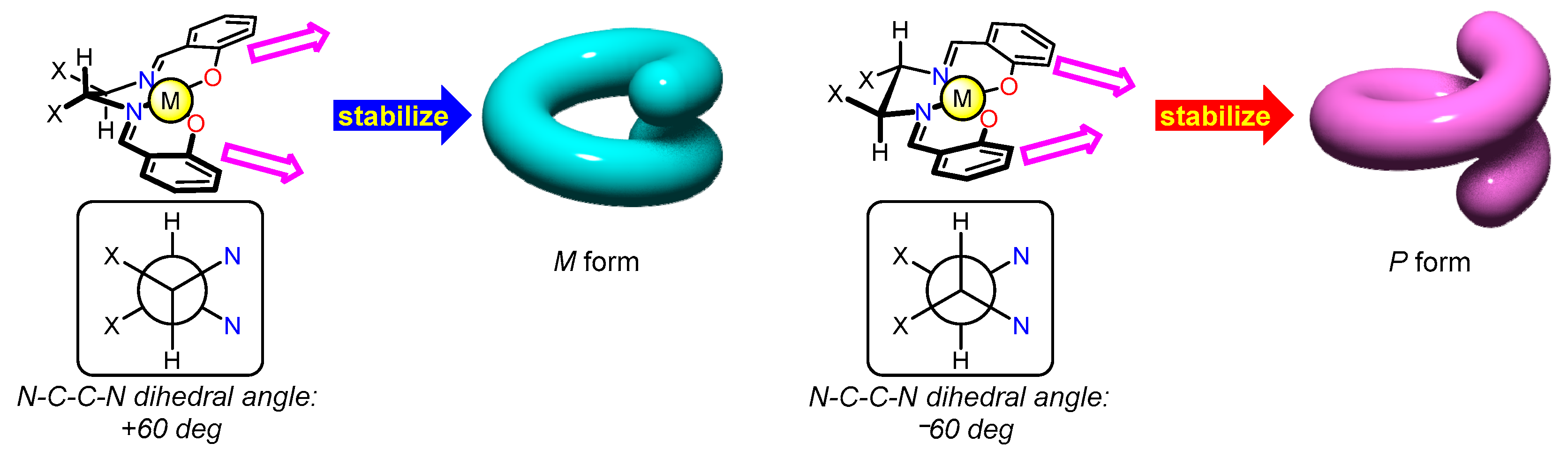
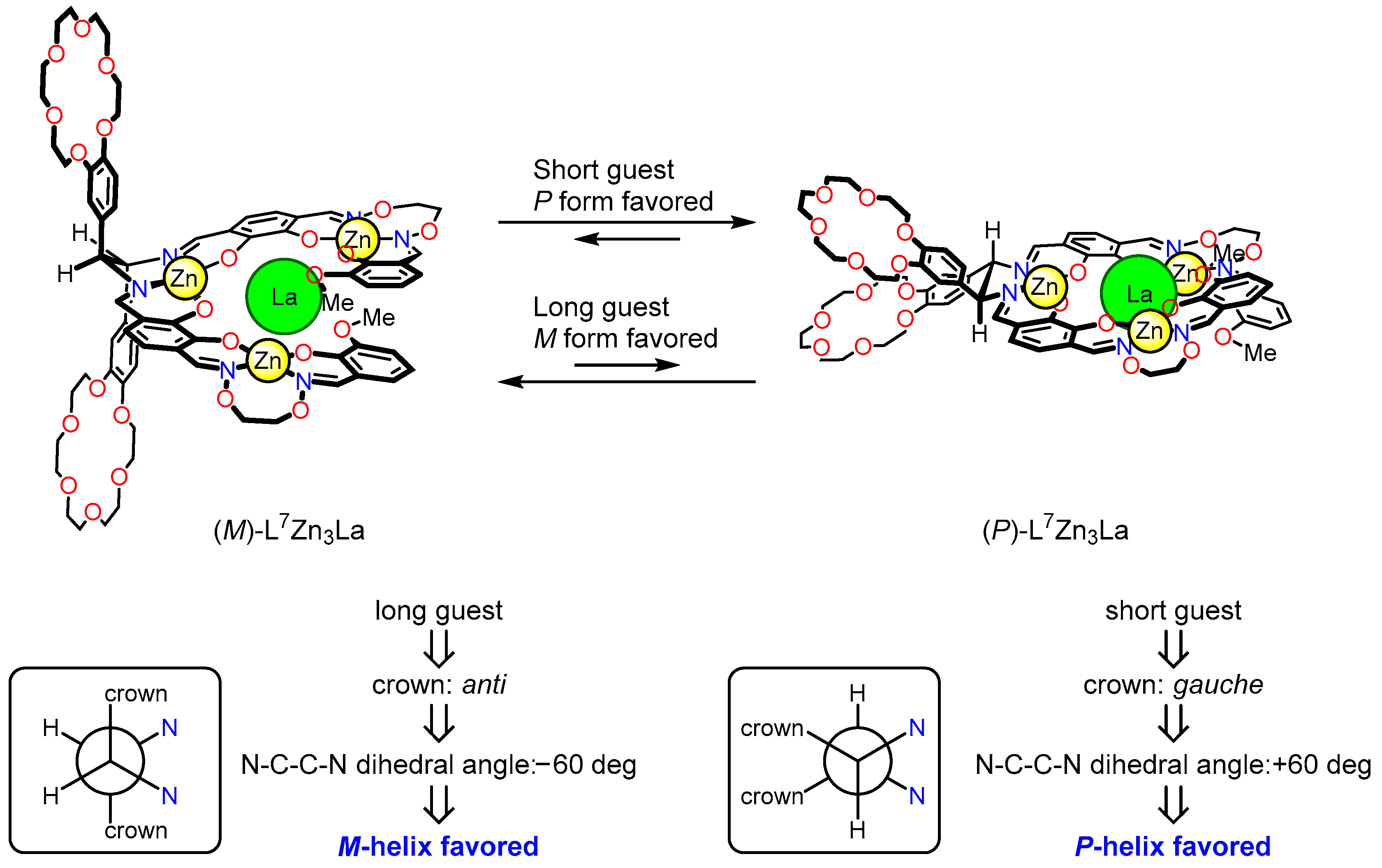
5. Dynamic Helicity Control Using Chiral Salen Units
5.1. Strategy
5.2. Dynamic Helicity Control of Chiral Salen Units
5.3. Helicity Inverison by Leverage Mechanism
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lehn, J.-M. Supramolecular Chemistry Concepts and Perspectives; VCH: Weinheim, Germany, 1995; ISBN 3-527-29311-6. [Google Scholar]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; Wiley: Chichester, UK, 2000; ISBN 0-471-98791-3. [Google Scholar]
- Fujita, M.; Tominaga, M.; Hori, A.; Therrien, B. Coordination Assemblies from a Pd(II)-Cornered Square Complex. Acc. Chem. Res. 2005, 38, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef] [PubMed]
- McConnell, A.J.; Wood, C.S.; Neelakandan, P.P.; Nitschke, J.R. Stimuli-Responsive Metal–Ligand Assemblies. Chem. Rev. 2015, 115, 7729–7793. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, C.-F. Helicenes: Synthesis and Applications. Chem. Rev. 2012, 112, 1463–1535. [Google Scholar] [CrossRef] [PubMed]
- Gingras, M. One hundred years of helicene chemistry. Part 1: Non-stereoselective syntheses of carbohelicenes. Chem. Soc. Rev. 2013, 42, 968–1006. [Google Scholar] [CrossRef] [PubMed]
- Gingras, M.; Félix, G.; Peresutti, R. One hundred years of helicene chemistry. Part 2: Stereoselective syntheses and chiral separations of carbohelicenes. Chem. Soc. Rev. 2013, 42, 1007–1050. [Google Scholar] [CrossRef] [PubMed]
- Gingras, M. One hundred years of helicene chemistry. Part 3: Applications and properties of carbohelicenes. Chem. Soc. Rev. 2013, 42, 1051–1095. [Google Scholar] [CrossRef] [PubMed]
- Starý, I.; Stará, I.G.; Alexandrová, Z.; Sehnal, P.; Teplý, F.; Šaman, D.; Rulíšek, L. Helicity control in the synthesis of helicenes and related compounds. Pure Appl. Chem. 2006, 78, 495–499. [Google Scholar] [CrossRef]
- Hill, D.J.; Mio, M.J.; Prince, R.B.; Hughes, T.S.; Moore, J.S. A Field Guide to Foldamers. Chem. Rev. 2001, 101, 3893–4011. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.-M.; Rigault, A.; Siegel, J.; Harrowfield, J.; Chevrier, B.; Moras, D. Spontaneous assembly of double-stranded helicates from oligobipyridine ligands and copper(I) cations: Structure of an inorganic double helix. Proc. Natl. Acad. Sci. USA 1987, 84, 2565–2569. [Google Scholar] [CrossRef] [PubMed]
- Piguet, C.; Bernardinelli, G.; Hopfgartner, G. Helicates as Versatile Supramolecular Complexes. Chem. Rev. 1997, 97, 2005–2062. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M. “Let’s Twist Again”—Double-Stranded, Triple-Stranded, and Circular Helicates. Chem. Rev. 2001, 101, 3457–3497. [Google Scholar] [CrossRef] [PubMed]
- Porter, W.H. Resolution of chiral drugs. Pure Appl. Chem. 1991, 63, 1119–1122. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Nagai, K. Helical Polymers: Synthesis, Structures, and Functions. Chem. Rev. 2009, 109, 6102–6211. [Google Scholar] [CrossRef] [PubMed]
- Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K. Supramolecular Helical Systems: Helical Assemblies of Small Molecules, Foldamers, and Polymers with Chiral Amplification and Their Functions. Chem. Rev. 2016, 116, 13752–13990. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H.; Tsukube, H. Helix Architecture and Helicity Switching via Dynamic Metal Coordination Chemistry. Supramol. Chem. 2005, 17, 53–59. [Google Scholar] [CrossRef]
- Miyake, H.; Tsukube, H. Coordination chemistry strategies for dynamic helicates: Time-programmable chirality switching with labile and inert metal helicates. Chem. Soc. Rev. 2012, 41, 6977–6991. [Google Scholar] [CrossRef] [PubMed]
- Crassous, J. Transfer of chirality from ligands to metal centers: Recent examples. Chem. Commun. 2012, 48, 9684–9692. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Yashima, E. Helical Polyacetylenes Induced via Noncovalent Chiral Interactions and Their Applications as Chiral Materials. Top. Curr. Chem. 2017, 375, 72. [Google Scholar] [CrossRef] [PubMed]
- Bisoyi, H.K.; Li, Q. Light-Directed Dynamic Chirality Inversion in Functional Self-Organized Helical Superstructures. Angew. Chem. Int. Ed. 2016, 55, 2994–3010. [Google Scholar] [CrossRef] [PubMed]
- Pijper, D.; Feringa, B.L. Control of dynamic helicity at the macro- and supramolecular level. Soft Matter 2008, 4, 1349–1372. [Google Scholar] [CrossRef]
- Feringa, B.L.; van Delden, R.A.; Koumura, N.; Geertsema, E.M. Chiroptical Molecular Switches. Chem. Rev. 2000, 100, 1789–1816. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Nabeshima, T. Cyclic and acyclic oligo(N2O2) ligands for cooperative multi-metal complexation. Dalton Trans. 2009, 10395–10408. [Google Scholar] [CrossRef] [PubMed]
- Akine, S. Novel Ion Recognition Systems Based on Cyclic and Acyclic Oligo(salen)-type Ligands. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 25–54. [Google Scholar] [CrossRef]
- Akine, S. Metal Complexes with Oligo(salen)-Type Ligands. In The Chemistry of Metal Phenolates, Volume 2, Patai’s Chemistry of Functional Groups; Zabicky, J., Ed.; John Wiley and Sons, Ltd.: Chichester, UK, 2018; pp. 153–194. ISBN 978-1-119-08328-3. [Google Scholar]
- Wezenberg, S.J.; Kleij, A.W. Material Applications for Salen Frameworks. Angew. Chem. Int. Ed. 2008, 47, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Kleij, A.W. New Templating Strategies with Salen Scaffolds (Salen = N,N’-Bis(salicylidene)ethylenediamine Dianion). Chem. Eur. J. 2008, 14, 10520–10529. [Google Scholar] [CrossRef] [PubMed]
- Feltham, H.L.C.; Brooker, S. Ligands and polymetallic complexes derived from 1,4-diformyl-2,3-dihydroxybenzene and two close analogues. Coord. Chem. Rev. 2009, 253, 1458–1475. [Google Scholar] [CrossRef]
- Wiznycia, A.V.; Desper, J.; Levy, C.J. Iron(II) and zinc(II) monohelical binaphthyl salen complexes. Chem. Commun. 2005, 4693–4695. [Google Scholar] [CrossRef] [PubMed]
- Wiznycia, A.V.; Desper, J.; Levy, C.J. Monohelical Iron(II) and Zinc(II) Complexes of a (1R,2R)-Cyclohexyl Salen Ligand with Benz[a]anthryl Sidearms. Inorg. Chem. 2006, 45, 10034–10036. [Google Scholar] [CrossRef] [PubMed]
- Wiznycia, A.V.; Desper, J.; Levy, C.J. Iron(II) and zinc(II) monohelical binaphthyl-salen complexes with overlapping benz[a]anthryl sidearms. Dalton Trans. 2007, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Patil, S.; Desper, J.; Aikens, C.M.; Levy, C.J. Helical Oxidovanadium(IV) Salen-Type Complexes: Synthesis, Characterisation and Catalytic Behaviour. Eur. J. Inorg. Chem. 2013, 5708–5717. [Google Scholar] [CrossRef]
- Zhang, F.; Bai, S.; Yap, G.P.A.; Tarwade, V.; Fox, J.M. Abiotic Metallofoldamers as Electrochemically Responsive Molecules. J. Am. Chem. Soc. 2005, 127, 10590–10599. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Karpowicz, R.J., Jr.; Bai, S.; Yap, G.P.A.; Fox, J.M. Control of Absolute Helicity in Single-Stranded Abiotic Metallofoldamers. J. Am. Chem. Soc. 2006, 128, 14242–14243. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Yap, G.P.A.; Fox, J.M. trans-Cyclohexane-1,2-diamine is a Weak Director of Absolute Helicity in Chiral Nickel–Salen Complexes. J. Am. Chem. Soc. 2007, 129, 11850–11853. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.A.; Zhang, F.; Yap, G.P.A.; Fox, J.M. Weak absolute helicity direction in Ni–salen by trans-cyclohexane-(1R,2R)-diamine. Inorg. Chim. Acta 2010, 364, 259–260. [Google Scholar] [CrossRef]
- Dong, Z.; Plampin, J.N., III; Yap, G.P.A.; Fox, J.M. Minimalist End Groups for Control of Absolute Helicity in Salen- and Salophen-Based Metallofoldamers. Org. Lett. 2010, 12, 4002–4005. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Bai, S.; Yap, G.P.A.; Fox, J.M. Interplay between the diamine structure and absolute helicity in Ni–salen metallofoldamers. Chem. Commun. 2011, 47, 3781–3783. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, S.; Houjou, H.; Nagawa, Y.; Kanesato, M. First helicat zinc(II) complex with a salen ligand. Chem. Commun. 2003, 1148–1149. [Google Scholar] [CrossRef]
- Wezenberg, S.J.; Salassa, G.; Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Effective Chirogenesis in a Bis(metallosalphen) Complex through Host–Guest Binding with Carboxylic Acids. Angew. Chem. Int. Ed. 2011, 50, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Achira, H.; Ito, M.; Mutai, T.; Yoshikawa, I.; Araki, K.; Houjou, H. Spontaneous helical folding of bis(Ni-salphen) complexes in solution and in the solid state: Spectroscopic tracking of the unfolding process induced by Na+ ions. Dalton Trans. 2014, 43, 5899–5907. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Katz, T.J.; Nichols, D.A. Synthesis of a Helical Conjugated Ladder Polymer. Angew. Chem. Int. Ed. Engl. 1996, 35, 2109–2111. [Google Scholar] [CrossRef]
- Zhang, H.-C.; Huang, W.-S.; Pu, L. Biaryl-Based Macrocyclic and Polymeric Chiral (Salophen)Ni(II) Complexes: Synthesis and Spectroscopic Study. J. Org. Chem. 2001, 66, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Furusho, Y.; Maeda, T.; Takeuchi, T.; Makino, N.; Takata, T. A Rational Design of Helix: Absolute Helix Synthesis by Binaphthyl-Salen Fusion. Chem. Lett. 2001, 1020–1021. [Google Scholar] [CrossRef]
- Akine, S.; Taniguchi, T.; Nabeshima, T. Synthesis and Characterization of Novel Ligands 1,2-Bis(salicylideneaminooxy)ethanes. Chem. Lett. 2001, 682–683. [Google Scholar] [CrossRef]
- Akine, S.; Taniguchi, T.; Dong, W.; Masubuchi, S.; Nabeshima, T. Oxime-Based Salen-Type Tetradentate Ligands with High Stability against Imine Metathesis Reaction. J. Org. Chem. 2005, 70, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Taniguchi, T.; Nabeshima, T. Cooperative Formation of Trinuclear Zinc(II) Complexes via Complexation of a Tetradentate Oxime Chelate Ligand, Salamo, and Zinc(II) Acetate. Inorg. Chem. 2004, 43, 6142–6144. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Nabeshima, T. Novel Thiosalamo Ligand as a Remarkably Stable N2S2 Salen-type Chelate and Synthesis of a Nickel(II) Complex. Inorg. Chem. 2005, 44, 1205–1207. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Taniguchi, T.; Nabeshima, T. Heterometallic Zn2La and ZnLu Complexes Formed by Site-selective Transmetalation of a Dimeric Homotrinuclear Zinc(II) Complex. Chem. Lett. 2006, 35, 604–605. [Google Scholar] [CrossRef]
- Akine, S.; Dong, W.; Nabeshima, T. Octanuclear Zinc(II) and Cobalt(II) Clusters Produced by Cooperative Tetrameric Assembling of Oxime Chelate Ligands. Inorg. Chem. 2006, 45, 4677–4684. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Akimoto, A.; Shiga, T.; Oshio, H.; Nabeshima, T. Synthesis, Stability, and Complexation Behavior of Isolable Salen-Type N2S2 and N2SO Ligands Based on Thiol and Oxime Functionalities. Inorg. Chem. 2008, 47, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Utsuno, F.; Taniguchi, T.; Nabeshima, T. Dinuclear Complexes of the N2O2 Oxime Chelate Ligand with Zinc(II)–Lanthanide(III) as a Selective Sensitization System for Sm3+. Eur. J. Inorg. Chem. 2010, 3143–3152. [Google Scholar] [CrossRef]
- Akine, S.; Nabeshima, T. Increased Nuclearity of Salen-Type Transition Metal Complexes by Incorporation of O-Alkyloxime Functionality. Heteroat. Chem. 2014, 25, 410–421. [Google Scholar] [CrossRef]
- Akine, S.; Taniguchi, T.; Nabeshima, T. Novel Synthetic Approach to Trinuclear 3d–4f Complexes: Specific Exchange of the Central Metal of a Trinuclear Zinc(II) Complex of a Tetraoxime Ligand with a Lanthanide(III) Ion. Angew. Chem. Int. Ed. 2002, 41, 4670–4673. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Taniguchi, T.; Nabeshima, T. Helical Metallohost–Guest Complexes via Site-Selective Transmetalation of Homotrinuclear Complexes. J. Am. Chem. Soc. 2006, 128, 15765–15774. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Taniguchi, T.; Saiki, T.; Nabeshima, T. Ca2+- and Ba2+-Selective Receptors Based on Site-Selective Transmetalation of Multinuclear Polyoxime–Zinc(II) Complexes. J. Am. Chem. Soc. 2005, 127, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Matsumoto, T.; Sairenji, S.; Nabeshima, T. Synthesis of Acyclic Tetrakis- and Pentakis(N2O2) Ligands for Single-helical Heterometallic Complexes with a Greater Number of Winding Turns. Supramol. Chem. 2011, 23, 106–112. [Google Scholar] [CrossRef]
- Akine, S.; Taniguchi, T.; Matsumoto, T.; Nabeshima, T. Guest-dependent inversion rate of a tetranuclear single metallohelicate. Chem. Commun. 2006, 4961–4963. [Google Scholar] [CrossRef] [PubMed]
- Sairenji, S.; Akine, S.; Nabeshima, T. Dynamic helicity control of single-helical oligooxime metal complexes by coordination of chiral carboxylate ions. Tetrahedron Lett. 2014, 55, 1987–1990. [Google Scholar] [CrossRef]
- Green, M.M.; Peterson, N.C.; Sato, T.; Teramoto, A.; Cook, R.; Lifson, S. A Helical Polymer with a Cooperative Response to Chiral Information. Science 1995, 268, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Palmans, A.R.A.; Meijer, E.W. Amplification of Chirality in Dynamic Supramolecular Aggregates. Angew. Chem. Int. Ed. 2007, 46, 8948–8968. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Yamada, T.; Adachi, T.; Akai, Y.; Yamamoto, T.; Suginome, M. Solvent-Dependent Switch of Helical Main-Chain Chirality in Sergeants-and-Soldiers-Type Poly(quinoxaline-2,3-diyl)s: Effect of the Position and Structures of the “Sergeant” Chiral Units on the Screw-Sense Induction. J. Am. Chem. Soc. 2013, 135, 10104–10113. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Yagi, S.; Honmaru, A.; Murakami, S.; Furusyo, M.; Takagishi, T.; Ogoshi, H. Helical Chirality Induction in Zinc Bilindiones by Amino Acid Esters and Amines. J. Org. Chem. 1998, 63, 8769–8784. [Google Scholar] [CrossRef]
- Mizutani, T.; Sakai, N.; Yagi, S.; Takagishi, T.; Kitagawa, S.; Ogoshi, H. Allosteric Chirality Amplification in Zinc Bilinone Dimer. J. Am. Chem. Soc. 2000, 122, 748–749. [Google Scholar] [CrossRef]
- Nonokawa, R.; Yashima, E. Detection and Amplification of a Small Enantiomeric Imbalance in α-Amino Acids by a Helical Poly(phenylacetylene) with Crown Ether Pendants. J. Am. Chem. Soc. 2003, 125, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Sakai, R.; Otsuka, I.; Satoh, T.; Kakuchi, R.; Kaga, H.; Kakuchi, T. Thermoresponsive On–Off Switching of Chiroptical Property Induced in Poly(4′-ethynylbenzo-15-crown-5)/α-Amino Acid System. Macromolecules 2006, 39, 4032–4037. [Google Scholar] [CrossRef]
- Misaki, H.; Miyake, H.; Shinoda, S.; Tsukube, H. Asymmetric Twisting and Chirality Probing Properties of Quadruple-Stranded Helicates: Coordination Versatility and Chirality Response of Na+, Ca2+, and La3+ Complexes with Octadentate Cyclen Ligand. Inorg. Chem. 2009, 48, 11921–11928. [Google Scholar] [CrossRef] [PubMed]
- Sairenji, S.; Akine, S.; Nabeshima, T. Dynamic Helicity Control of a Single-Helical Oligooxime Complex and Acid–Base-triggered Repeated Helicity Inversion Mediated by Amino Acids. Chem. Lett. 2014, 43, 1107–1109. [Google Scholar] [CrossRef]
- Sanji, T.; Kato, N.; Tanaka, M. Switching of Optical Activity in Oligosailane through pH-Responsive Chiral Wrapping with Amylose. Macromolecules 2006, 39, 7508–7512. [Google Scholar] [CrossRef]
- Maeda, T.; Furusho, Y.; Sakurai, S.-I.; Kumaki, J.; Okoshi, K.; Yashima, E. Double-Stranded Helical Polymers Consisting of Complementary Homopolymers. J. Am. Chem. Soc. 2008, 130, 7938–7945. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.G.A.; Ruiz-Carretero, A.; González-Rodríguez, D.; Meijer, E.W.; Schenning, A.P.H.J. pH-Switchable Helicity of DNA-Templated Assemblies. Angew. Chem. Int. Ed. 2009, 48, 8103–8106. [Google Scholar] [CrossRef] [PubMed]
- Sairenji, S.; Akine, S.; Nabeshima, T. Response speed control of helicity inversion based on a “regulatory enzyme”-like strategy. Sci. Rep. 2018, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Traut, T.W. Allosteric Regulatory Enzymes; Springer: New York, NY, USA, 2008; ISBN 0-387-72888-0. [Google Scholar]
- Akine, S.; Taniguchi, T.; Nabeshima, T. Chiral single-stranded metallohelix: Metal-mediated folding of linear oligooxime ligand. Tetrahedron Lett. 2006, 47, 8419–8422. [Google Scholar] [CrossRef]
- Akine, S.; Sairenji, S.; Taniguchi, T.; Nabeshima, T. Stepwise Helicity Inversions by Multisequential Metal Exchange. J. Am. Chem. Soc. 2013, 135, 12948–12951. [Google Scholar] [CrossRef] [PubMed]
- Sairenji, S.; Akine, S.; Nabeshima, T. Lanthanide contraction for helicity fine-tuning and helix-winding control of single-helical metal complexes. Dalton Trans. 2016, 45, 14902–14906. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Kagiyama, S.; Nabeshima, T. Modulation of Multimetal Complexation Behavior of Tetraoxime Ligand by Covalent Transformation of Olefinic Functionalities. Inorg. Chem. 2010, 49, 2141–2152. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Morita, Y.; Utsuno, F.; Nabeshima, T. Multiple Folding Structures Mediated by Metal Coordination of Acyclic Multidentate Ligand. Inorg. Chem. 2009, 48, 10670–10678. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Tadokoro, T.; Nabeshima, T. Oligometallic Template Strategy for Synthesis of a Macrocyclic Dimer-Type Octaoxime Ligand for Its Cooperative Complexation. Inorg. Chem. 2012, 51, 11478–11486. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Sunaga, S.; Nabeshima, T. Multistep Oligometal Complexation of the Macrocyclic Tris(N2O2) Hexaoxime Ligand. Chem. Eur. J. 2011, 17, 6853–6861. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Sunaga, S.; Taniguchi, T.; Miyazaki, H.; Nabeshima, T. Core/Shell Oligometallic Template Synthesis of Macrocyclic Hexaoxime. Inorg. Chem. 2007, 46, 2959–2961. [Google Scholar] [CrossRef] [PubMed]
- Canali, L.; Sherrington, D.C. Utilisation of homogeneous and supported chiral metal(salen) complexes in asymmetric catalysis. Chem. Soc. Rev. 1999, 28, 85–93. [Google Scholar] [CrossRef]
- Ito, Y.N.; Katsuki, T. Asymmetric Catalysis of New Generation Chiral Metallosalen Complexes. Bull. Chem. Soc. Jpn. 1999, 72, 603–619. [Google Scholar] [CrossRef]
- Jacobsen, E.N. Asymmetric Catalysis of Epoxide Ring-Opening Reactions. Acc. Chem. Res. 2000, 33, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Baleizão, C.; Garcia, H. Chiral Salen Complexes: An Overview to Recoverable and Reusable Homogeneous and Heterogeneous Catalysts. Chem. Rev. 2006, 106, 3987–4043. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Matsumoto, T.; Nabeshima, T. Spontaneous formation of a chiral supramolecular superhelix in the crystalline state using a single-stranded tetranuclear metallohelicate. Chem. Commun. 2008, 4604–4606. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Hotate, S.; Matsumoto, T.; Nabeshima, T. Spontaneous enrichment of one-handed helices by dissolution of quasiracemic crystals of a tetranuclear single helical complex. Chem. Commun. 2011, 47, 2925–2927. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Nagumo, H.; Nabeshima, T. Hierarchical Helix of Helix in the Crystal: Formation of Variable-Pitch Helical π-Stacked Array of Single-Helical Dinuclear Metal Complexes. Inorg. Chem. 2012, 51, 5506–5508. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Matsumoto, T.; Nabeshima, T. Overcoming Statistical Complexity: Selective Coordination of Three Different Metal Ions to a Ligand with Three Different Coordination Sites. Angew. Chem. Int. Ed. 2016, 55, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Hotate, S.; Nabeshima, T. A Molecular Leverage for Helicity Control and Helix Inversion. J. Am. Chem. Soc. 2011, 133, 13868–13871. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Miyashita, M.; Piao, S.; Nabeshima, T. Perfect encapsulation of a guanidinium ion in a helical trinickel(II) metallocryptand for efficient regulation of the helix inversion rate. Inorg. Chem. Front. 2014, 1, 53–57. [Google Scholar] [CrossRef]
- Akine, S.; Miyashita, M.; Nabeshima, T. A Metallo-molecular Cage That Can Close the Apertures with Coordination Bonds. J. Am. Chem. Soc. 2017, 139, 4631–4634. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Shimada, T.; Nagumo, H.; Nabeshima, T. Highly cooperative double metalation of a bis(N2O2) ligand based on bipyridine-phenol framework driven by intramolecular π-stacking of square planar nickel(II) complex moieties. Dalton Trans. 2011, 40, 8507–8509. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Nagumo, H.; Nabeshima, T. Programmed multiple complexation for the creation of helical structures from acyclic phenol–bipyridine oligomer ligands. Dalton Trans. 2013, 42, 15974–15986. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Akimoto, A.; Nabeshima, T. Synthesis of Ag+-Selective Dipalladium(II) Metallohost Based on O-Alkyloxime Bis(N2SO) Ligands. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 1000–1007. [Google Scholar] [CrossRef]
- Akine, S.; Matsumoto, T.; Taniguchi, T.; Nabeshima, T. Synthesis, Structures, and Magnetic Properties of Tri- and Dinuclear Copper(II)–Gadolinium(III) Complexes of Linear Oligooxime Ligands. Inorg. Chem. 2005, 44, 3270–3274. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Kagiyama, S.; Nabeshima, T. Oligometallic Template Strategy for Ring-Closing Olefin Metathesis: Highly Cis- and Trans-Selective Synthesis of a 32-Membered Macrocyclic Tetraoxime. Inorg. Chem. 2007, 46, 9525–9527. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Taniguchi, T.; Nabeshima, T. Acyclic Bis(N2O2 chelate) Ligand for Trinuclear d-Block Homo- and Heterometal Complexes. Inorg. Chem. 2008, 47, 3255–3264. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Utsuno, F.; Nabeshima, T. Visible and Near-infrared Luminescence of Helical Zinc(II)–Lanthanide(III) Trinuclear Complexes Having Acyclic Bis(N2O2) Oxime Ligand. IOP Conf. Ser. Mater. Sci. Eng. 2009, 1, 012009. [Google Scholar] [CrossRef]





© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akine, S. Dynamic Helicity Control of Oligo(salamo)-Based Metal Helicates. Inorganics 2018, 6, 80. https://doi.org/10.3390/inorganics6030080
Akine S. Dynamic Helicity Control of Oligo(salamo)-Based Metal Helicates. Inorganics. 2018; 6(3):80. https://doi.org/10.3390/inorganics6030080
Chicago/Turabian StyleAkine, Shigehisa. 2018. "Dynamic Helicity Control of Oligo(salamo)-Based Metal Helicates" Inorganics 6, no. 3: 80. https://doi.org/10.3390/inorganics6030080
APA StyleAkine, S. (2018). Dynamic Helicity Control of Oligo(salamo)-Based Metal Helicates. Inorganics, 6(3), 80. https://doi.org/10.3390/inorganics6030080





