Computational Structures and SAPT Interaction Energies of HXeSH···H2Y (Y=O or S) Complexes
Abstract
1. Introduction
2. Computational Details
2.1. Potential Energy Surface Analysis
2.2. Supermolecular Interaction Energy
2.3. Symmetry-Adapted Perturbation Theory (SAPT) Interaction Energy Analysis
3. Results and Discussion
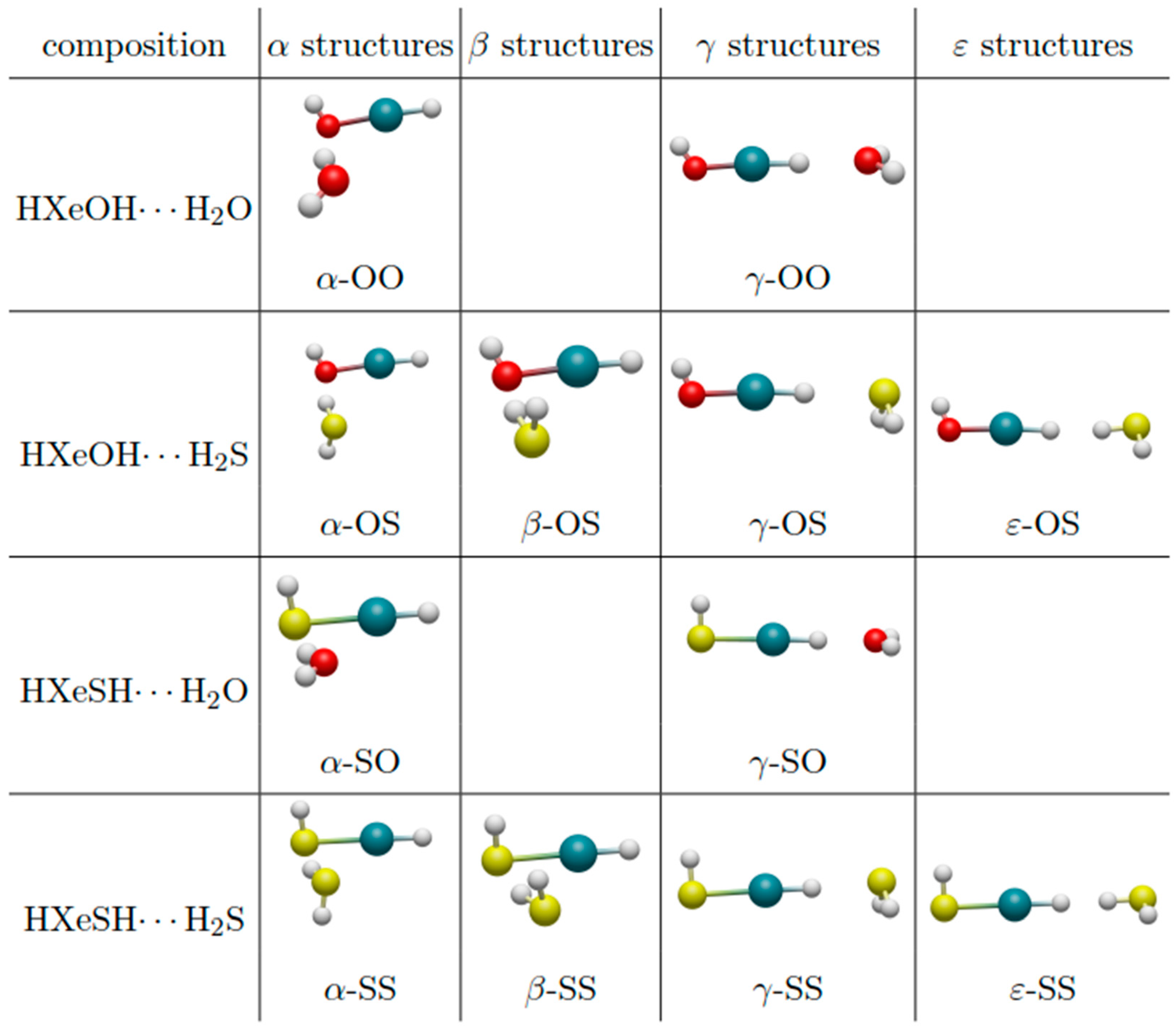
3.1. Structures of the Complexes
3.1.1. HXeOH/SH Complexes with H2O
3.1.2. HXeOH/SH Complexes with H2S
3.2. Vibrational Frequencies
3.2.1. Monomer Spectra
3.2.2. Complex Spectra
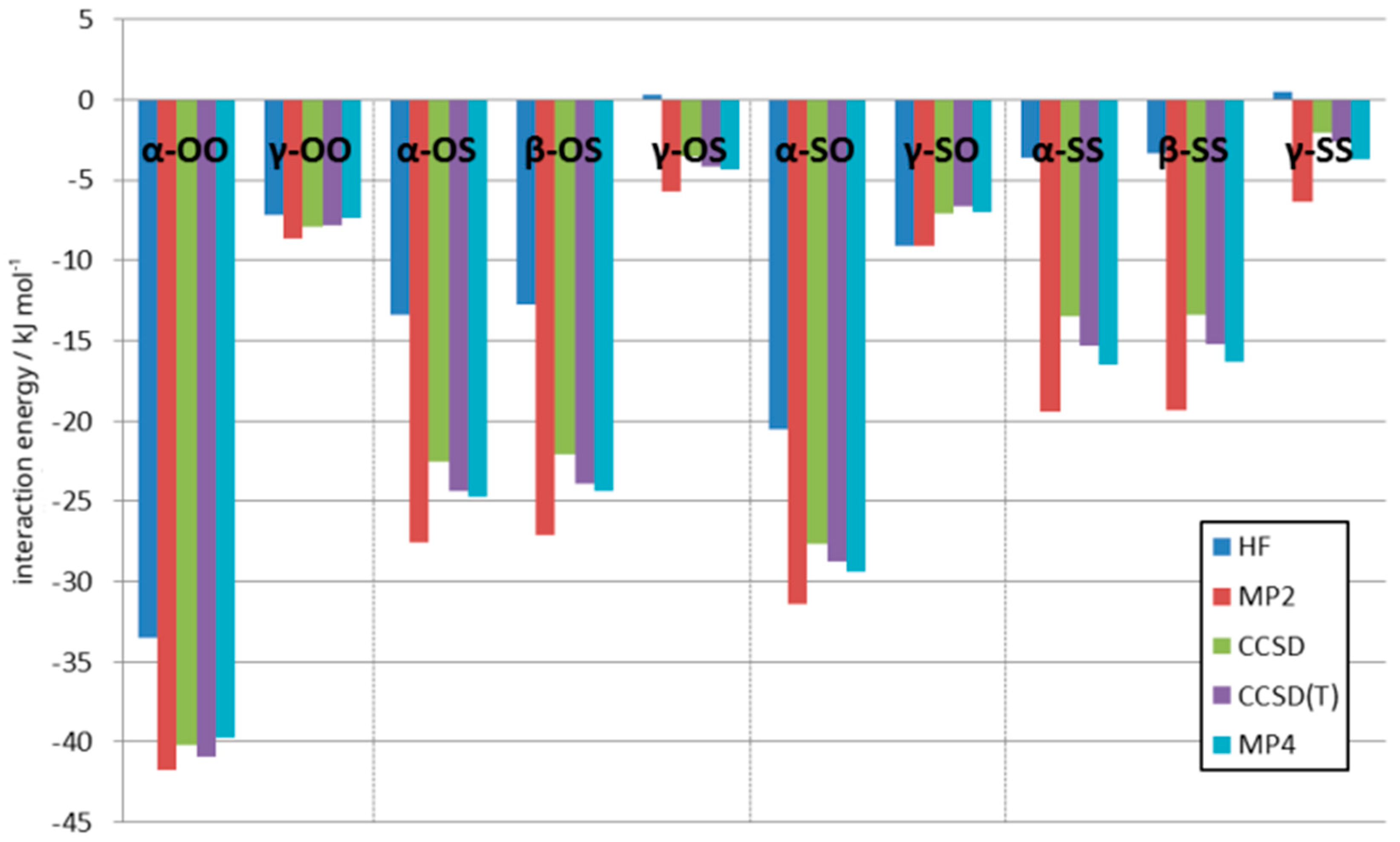
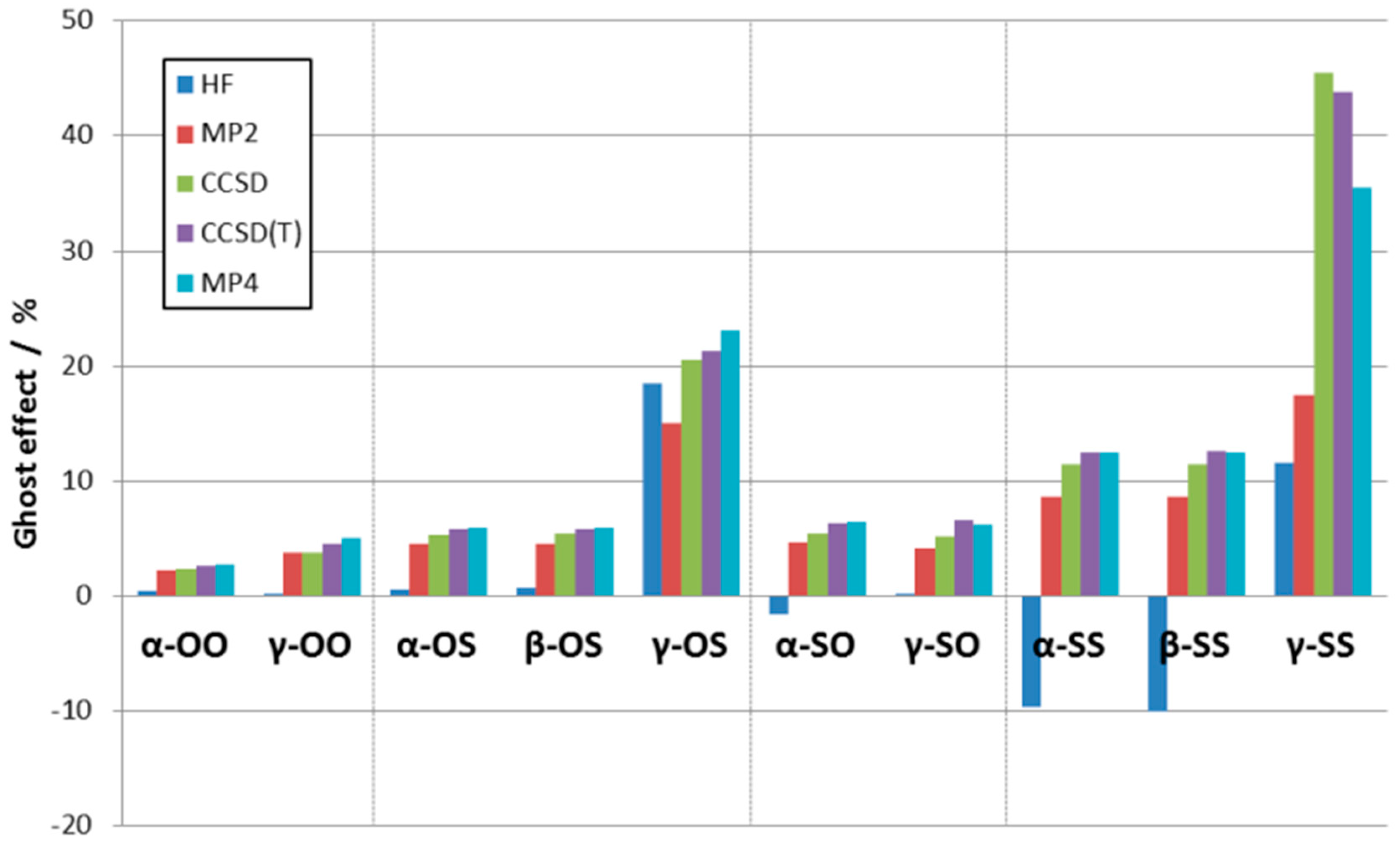
3.3. Supermolecular Interaction Analysis
3.4. Decomposition of the Interaction Energy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pimentel, G.C.; McLellan, A.L. The Hydrogen Bond; W. H. Freeman and Company: Berkeley, CA, USA, 1960. [Google Scholar]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, NY, USA, 1999; ISBN 0198502524. [Google Scholar]
- Klemperer, W.; Vaida, V. Molecular complexes in close and far away. Proc. Natl. Acad. Sci. USA 2006, 103, 10584–10588. [Google Scholar] [CrossRef] [PubMed]
- Vigasin, A.A.; Slanina, Z. Molecular Complexes in Earth’s, Planetary, Cometary and Interstellar Atmospheres; World Scientific Publising Co. Inc.: Singapore, 1998; ISBN 981023211X. [Google Scholar]
- Vaida, V. Perspective: Water cluster mediated atmospheric chemistry. J. Chem. Phys. 2011, 135, 020901. [Google Scholar] [CrossRef] [PubMed]
- Sanloup, C.; Bonev, S.A.; Hochlaf, M.; Maynard-Casely, H.E. Reactivity of xenon with ice at planetary conditions. Phys. Rev. Lett. 2013, 110, 265501. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, M.; Khriachtchev, L.; Lundell, J.; Räsänen, M. A chemical compound formed from water and xenon: HXeOH. J. Am. Chem. Soc. 1999, 121, 11904–11905. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Isokoski, K.; Cohen, A.; Räsänen, M.; Gerber, R.B. A small neutral molecule with two noble-gas atoms: HXeOXeH. J. Am.Chem. Soc. 2008, 130, 6114–6116. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, M.; Lundell, J.; Räsänen, M. New rare-gas-containing neutral molecules. Eur. J. Inorg. Chem. 1999, 729–737. [Google Scholar] [CrossRef]
- Gerber, R.B. Formation of novel rare-gas molecules in low temperature matrices. Ann. Rev. Phys. Chem. 2004, 55, 55–78. [Google Scholar] [CrossRef] [PubMed]
- Grochala, W.; Khriachtchev, L.; Räsänen, M. Noble Gas Chemistry. In Physics and Chemistry at Low Temperatures; Khriachtchev, L., Ed.; Pan Stanford Publishing: Singapore, 2011; Chapter 13; pp. 421–448. [Google Scholar]
- Lignell, A.; Khriachtchev, L. Intermolecular interactions involving noble-gas hydrides: Where the blue shift of vibrational frequency is a normal effect. J. Mol. Struct. 2008, 889, 1–11. [Google Scholar] [CrossRef]
- Lignell, A.; Lundell, J.; Khriachtchev, L.; Räsänen, M. Experimental and computational study of HXeY-HX complexes (X, Y = Cl and Br): An example of exceptionally large complexation effect. J. Phys. Chem. A 2008, 112, 5486–5494. [Google Scholar] [CrossRef] [PubMed]
- Khriachtchev, L.; Tapio, S.; Räsänen, M.; Domanskaya, A.; Lignell, A. HY···N2 and HXeY···N2 complexes in solid xenon (Y = Cl and Br): Unexpected suppression of the complex formation for deposition at higher temperature. J. Chem. Phys. 2010, 133, 084309. [Google Scholar] [CrossRef] [PubMed]
- Domanskaya, A.; Kobzarenko, A.V.; Tsivion, E.; Khriachtchev, L.; Feldman, V.I.; Gerber, R.B.; Räsänen, M. Matrix-isolation and ab initio study of HXeCCH complexed with acetylene. Chem. Phys. Lett. 2009, 481, 83–87. [Google Scholar] [CrossRef]
- Yen, S.-Y.; Mou, C.-H.; Hu, W.-P. Strong hydrogen bonding between neutral noble-gas molecules (HNgF, Ng = Ar, Kr, and Xe) and hydrogen fluoride: A theoretical study. Chem. Phys. Lett. 2004, 383, 606–611. [Google Scholar] [CrossRef]
- Jankowska, J.; Sadlej, J. Spectroscopic parameters in noble gas molecule: HXeF and its complex with HF. Chem. Phys. Lett. 2011, 517, 155–161. [Google Scholar] [CrossRef]
- Esrafili, M.D. A theoretical investigation of the characteristics of hydrogen/halogen bonding interaction in dibromonitroaniline. J. Mol. Mod. 2013, 19, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Huang, Z.; Niu, X.; Shen, T.; Guo, L. A theoretical study of the hydrogen bonding interactions in HXeCCH···Y (Y = H2O and HF) complexes. Comp. Theor. Chem. 2013, 1017, 14–21. [Google Scholar] [CrossRef]
- Mondal, S.; Chandra Singh, P. Noble gas indiced surprisingly higher stability of π hydrogen bonded complex: Comparative study of hydrogen bonded complexes of HKrCCH and HCCH with H2O, NH3, CH3OH and CH3NH2. RSC Adv. 2014, 4, 20752–20760. [Google Scholar] [CrossRef]
- Cohen, A.; Tsuge, M.; Khriachtchev, L.; Räsänen, M.; Gerber, R.B. Modeling of HXeBr in CO2 and Xe environments: Structure, energetics and vibrational spectra. Chem. Phys. Lett. 2014, 594, 18–22. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Juyban, P.; Solimannejad, M. Exploring lithium bonding interactions between noble-gas hydrides HXeY and LiX molecules (Y = H, CN, NC and X = H, CN, NC, OH, NH2, CH3): A theoretical study. Comp. Theor. Chem. 2014, 1027, 84–90. [Google Scholar] [CrossRef]
- Ryazantsev, S.V.; Lundell, J.; Feldman, V.I.; Khriachtchev, L. Photochemistry of the H2O/CO system revisited: The HXeOH···CO complex in a xenon matrix. J. Phys. Chem. A 2018, 122, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lundell, J.; Berski, S.; Latajka, Z. Dihydrogen-bonded complexes of xenon dihydride with water: Ab initio calculations and topological analysis of electron localisation function (ELF). Phys. Chem. Chem. Phys. 2000, 2, 5521–5527. [Google Scholar] [CrossRef]
- Nemukhin, A.V.; Grigorenko, B.L.; Khriachtchev, L.; Tanskanen, H.; Pettersson, M.; Räsänen, M. Intermolecular complexes of HXeOH with water: Stabilization and destabilization effects. J. Am. Chem. Soc. 2002, 124, 10706–10711. [Google Scholar] [CrossRef] [PubMed]
- Lundell, J.; Berski, S.; Lignell, A.; Latajka, Z. Quantum chemical study of the hydrogen-bonded HXeOH-H2O complex. J. Mol. Struct. 2006, 790, 31–39. [Google Scholar] [CrossRef]
- Cukras, J.; Sadlej, J. Theoretical predictions of the spectroscopic parameters in noble-gas molecules: HXeOH and its complex with water. Phys. Chem. Chem. Phys. 2011, 13, 15455–15467. [Google Scholar] [CrossRef] [PubMed]
- Tsivion, E.; Gerber, R.B. Lifetimes of compounds made of noble-gas atoms with water. Chem. Phys. Lett. 2009, 482, 30–33. [Google Scholar] [CrossRef]
- Tsivion, E.; Räsänen, M.; Gerber, R.B. Destabilization of noble-gas hydrides by a water environment: Calculations for HXeOH@(H2O)n, HXeOXeH@(H2O)n, HXeBr@(H2O)n, HXeCCH@(H2O)n. Phys. Chem. Chem. Phys. 2013, 15, 12610–12616. [Google Scholar] [CrossRef] [PubMed]
- Barone, V. Vibrational zero-point energies and theormodynamic functions beyond the harmonic approximation. J. Chem. Phys. 2004, 120, 3059–3065. [Google Scholar] [CrossRef] [PubMed]
- Barone, V. Anharmonic vibrational properties by a fully automated second-order perturbative approach. J. Chem. Phys. 2005, 122, 014108. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian09; revision E.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Jeziorski, B.; Moszyński, R.; Szalewicz, K. Perturbation theory approach to intermolecular potential energy surfaces of van der Waals complexes. Chem. Rev. 1994, 94, 1887–1930. [Google Scholar] [CrossRef]
- Jeziorska, M.; Jeziorski, B.; Čížek, J. Direct calculation of the Hartree-Fockinteraction energy via exchange-perturbation expansion. The He···He interaction. Int. J. Quantum Chem. 1987, 32, 149–164. [Google Scholar] [CrossRef]
- Werner, H.-J.; Knowles, P.J.; Lindh, R.; Manby, F.R.; Schütz, M.; Celani, P.; Korona, T.; Mitrushenkov, A.; Rauhut, G.; Adler, T.B.; et al. MOLPRO, version 2009.1; A Package of ab Initio Programs. Available online: http://140.123.79.88/~silvercy/Data/Reference/quickstart.pdf (accessed on 13 September 2018).
- Perez, F.; Granger, B.E. IPython: A system for interactive scientific computing. Comput. Sci. Eng. 2007, 9, 21–29. [Google Scholar] [CrossRef]
- Jones, E.; Oliphant, T.; Peterson, P. SciPy: Open Source Scientific Tools for Python. 2001. Available online: www.scipy.org (accessed on 15 January 2018).
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Cukras, J.; Sadlej, J. The influence of the dispersion corrections on the performance of DFT method in modeling HNgY noble gas molecules and their complexes. Chem. Phys. Lett. 2018, 691, 319–324. [Google Scholar] [CrossRef]
- Lundell, J.; Pettersson, M. The dihydrogen-bonded complex HXeH-H2O. Phys. Chem. Chem. Phys. 1999, 1, 1691–1697. [Google Scholar] [CrossRef]
- Crabtree, R.H.; Siegbahn, P.E.M.; Eisenstein, O.; Rheingold, A.L.; Koetzle, T.F. A new intermolecular interaction: Unconventional hydrogen bonds with element-hydride bonds as proton acceptor. Acc. Chem. Res. 1996, 29, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Klooster, W.T.; Koetzle, T.F.; Siegbahn, P.E.M.; Richardson, T.B.; Crabtree, R.H. Study of the N–H···H–B dihydrogen bond including the crystal structure of BH3NH3 by neutron diffraction. J. Am. Chem. Soc. 1999, 121, 6337–6343. [Google Scholar] [CrossRef]
- Bakhmutov, V.I. Dihydrogen Bond: Principles, Experiments, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-18096-9. [Google Scholar]
- Shimanouchi, T. Tables of Molecular Vibrational Frequencies. Consolidated Volume I; National Bureau of Standards: Gaithersburg, MD, USA, 1971; pp. 1–160. Available online: https://nvlpubs.nist.gov/nistpubs/Legacy/NSRDS/nbsnsrds6.pdf (accessed on 20 March 2018).
- Lundell, J.; Chaban, G.M.; Gerber, R.B. Anharmonic vibrational spectroscopy calculations for novel rare-gas-containing compounds: HXeH, HXeCl, HXeBr, and HXeOH. J. Phys. Chem. A 2000, 104, 7944–7949. [Google Scholar] [CrossRef]
- Patkowski, K.; Szalewicz, K.; Jeziorski, B. Orbital relaxation and the third-order induction energy in symmetry-adapted perturbation theory. Theor. Chem. Acc. 2010, 127, 211–221. [Google Scholar] [CrossRef]
| Atom | HXeOH | ε-OS | HXeSH | ε-SS |
|---|---|---|---|---|
| H | 0.032 | 0.084 | −0.117 | 0.044 |
| O/S | −0.622 | −0.785 | −0.519 | −0.601 |
| Xe | 0.622 | 0.874 | 0.611 | 0.932 |
| H(bonded to Xe) | −0.032 | −0.198 | −0.025 | −0.360 |
| H(DHB) | - | −0.021 | - | −0.046 |
| S | - | −0.045 | - | −0.054 |
| H | - | 0.091 | - | 0.085 |
| νharm | νanh | I | νharm | νanh | Δνanh 1 | I | νharm | νanh | Δνanh 1 | I | νharm | νanh | Δνanh 1 | I |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2O | α-OO | α-OS | β-OS | |||||||||||
| 3938 | 3747 | 67 | 3894 | 3708 | −39 | 55 | - | - | - | - | - | - | - | - |
| 3803 | 3622 | 4 | 3401 | 3239 | −383 | 679 | - | - | - | - | - | - | - | - |
| 1622 | 1575 | 67 | 1663 | 1605 | 30 | 57 | - | - | - | - | - | - | - | - |
| HXeOH | ||||||||||||||
| 3766 | 3610 | 55 | 3778 | 3600 | −10 | 55 | 3767 | 3591 | −19 | 60 | 3770 | 3595 | −15 | 62 |
| 1809 | 1722 | 1087 | 1917 | 1812 | 90 | 826 | 1888 | 1782 | 60 | 894 | 1888 | 1781 | 59 | 891 |
| 824 | 818 | 6 | 779 | 732 | −86 | 33 | 789 | 773 | −45 | 1 | 787 | 768 | −50 | 1 |
| 633 | 630 | 0 | 617 | 599 | −31 | 1 | 630 | 581 | −49 | 26 | 626 | 593 | −37 | 13 |
| 586 | 597 | 3 | 564 | 554 | −43 | 5 | 610 | 563 | −34 | 73 | 597 | 546 | −51 | 9 |
| - | - | - | - | - | - | - | 547 | 518 | −79 | 62 | 541 | 494 | −103 | 101 |
| 447 | 437 | 130 | 415 | 408 | −29 | 132 | 420 | 413 | −24 | 129 | 420 | 417 | −20 | 103 |
| H2S | ||||||||||||||
| 2780 | 2682 | 0 | - | - | - | - | 2765 | 2666 | −16 | 1 | 2763 | 2665 | −17 | 2 |
| 2755 | 2658 | 0 | - | - | - | - | 2425 | 2276 | −382 | 440 | 2443 | 2292 | −366 | 349 |
| 1193 | 1166 | 0 | - | - | - | - | 1215 | 1172 | 6 | 3 | 1220 | 1172 | 6 | 5 |
| H2O | α-SO | α-SS | β-SS | |||||||||||
| 3938 | 3747 | 67 | 3887 | 3691 | −56 | 75 | - | - | - | - | - | - | - | - |
| 3803 | 3622 | 4 | 3585 | 3387 | −235 | 367 | - | - | - | - | - | - | - | - |
| 1622 | 1575 | 67 | 1642 | 1584 | 9 | 1868 | - | - | - | - | - | - | - | - |
| HXeSH | ||||||||||||||
| 2724 | 2634 | 7 | 2724 | 2628 | −6 | 5 | 2721 | 2626 | −8 | 5 | 2721 | 2626 | −8 | 5 |
| 1450 | 1375 | 2800 | 1621 | 1488 | 113 | 3043 | 1573 | 1436 | 61 | 2317 | 1573 | 1436 | 61 | 2322 |
| 640 | 818 | 6 | 629 | 601 | −25 | 12 | 632 | 601 | −25 | 3 | 632 | 605 | −21 | 2 |
| 538 | 630 | 0 | 530 | 512 | −12 | 2 | 535 | 509 | −15 | 0 | 535 | 508 | −16 | 0 |
| 459 | 597 | 3 | 446 | 442 | −18 | 2 | 447 | 443 | −17 | 1 | 448 | 443 | −17 | 1 |
| 253 | 248 | 43 | 247 | 242 | −6 | 49 | 248 | 243 | −5 | 53 | 248 | 240 | −8 | 255 |
| H2S | ||||||||||||||
| 2780 | 2682 | 0 | - | - | - | - | 2764 | 2664 | −18 | 1 | 2764 | 2664 | −18 | 1 |
| 2755 | 2658 | 0 | - | - | - | - | 2580 | 2482 | −176 | 267 | 2580 | 2482 | −176 | 267 |
| 1193 | 1166 | 0 | - | - | - | - | 1203 | 1167 | 1 | 4 | 1204 | 1169 | 3 | 4 |
| Energy | α-OO | γ-OO | α-OS | β-OS | γ-OS | ε-OS | α-SO | γ-SO | α-SS | β-SS | γ-SS | ε-SS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −33.47 | −7.16 | −13.38 | −12.80 | 0.27 | 8.55 | −20.55 | −9.06 | −3.64 | −3.38 | 0.52 | 11.99 | |
| −41.70 | −8.68 | −27.58 | −27.13 | −5.68 | −0.37 | −31.37 | −9.10 | −19.45 | −19.30 | −6.34 | −1.52 | |
| −40.20 | −7.95 | −22.06 | −22.06 | −3.50 | 1.78 | −27.69 | −7.11 | −13.52 | −13.36 | −2.02 | 2.43 | |
| −40.96 | −7.85 | −23.91 | −23.91 | −4.19 | 0.03 | −28.73 | −6.59 | −15.36 | 15.21 | −2.60 | −0.13 | |
| −39.69 | −7.38 | −2477 | −24.40 | −4.38 | −0.90 | −29.43 | −7.04 | −16.47 | −16.35 | −3.75 | 1.41 | |
| 9.66 | 2.99 | 6.08 | 5.99 | 2.76 | 1.86 | 8.45 | 3.41 | 5.30 | 5.38 | 3.13 | 2.78 |
| Energy | α-OO | γ-OO | α-OS | β-OS | γ-OS | ε-OS | α-SO | γ-SO | α-SS | β-SS | γ-SS | ε-SS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −33.47 | −7.16 | −13.38 | −12.80 | 0.27 | 8.55 | −20.55 | −9.06 | −3.64 | −3.38 | 0.52 | 11.99 | |
| −82.44 | −17.98 | −59.17 | −57.11 | −11.91 | 2.20 | −54.07 | −23.54 | −35.75 | −35.46 | −17.98 | 0.43 | |
| 77.27 | 17.30 | 73.22 | 70.84 | 18.21 | 10.53 | 50.92 | 24.70 | 46.22 | 46.11 | 30.09 | 20.41 | |
| −42.14 | −8.39 | −41.36 | −40.56 | −9.48 | −4.29 | −32.94 | −14.06 | −32.91 | −33.02 | −19.75 | −9.80 | |
| 23.87 | 5.10 | 25.55 | 24.99 | 7.00 | 2.37 | 21.89 | 9.41 | 24.16 | 24.26 | 15.60 | 6.35 | |
| −27.13 | −9.18 | −28.11 | −27.66 | −10.85 | −8.20 | −22.48 | −11.68 | −23.20 | −23.24 | −15.31 | −12.53 | |
| 5.76 | 1.72 | 6.13 | 5.99 | 1.85 | 0.90 | 4.44 | 2.50 | 4.43 | 4.44 | 2.97 | 1.67 | |
| −5.17 | −0.68 | 14.04 | 13.73 | 6.30 | 12.73 | −3.15 | 1.16 | 10.47 | 10.65 | 12.11 | 20.84 | |
| −39.63 | −10.75 | −37.78 | −37.24 | −11.49 | −9.22 | −29.10 | −13.83 | −27.51 | −27.55 | −16.49 | −14.31 | |
| −10.04 | −3.20 | −11.61 | −10.96 | −3.55 | −2.27 | −6.34 | −5.57 | −5.36 | −5.27 | −7.44 | −5.40 | |
| −44.80 | −11.43 | −23.74 | −23.51 | −5.19 | 3.52 | −32.25 | −12.68 | −17.05 | −16.90 | −4.38 | 6.53 | |
| 1.84 | 1.57 | 2.49 | 2.43 | 2.29 | 0.62 | 1.68 | 1.86 | 2.10 | 2.10 | 4.11 | 0.07 | |
| −1.72 | −1.51 | −3.08 | −3.01 | −3.51 | 2.99 | −1.58 | −1.95 | −2.71 | −2.73 | −6.87 | 3.13 | |
| 0.41 | 0.29 | 0.67 | 0.66 | 0.48 | −0.55 | 0.34 | 0.37 | 0.51 | 0.52 | 0.95 | −0.53 | |
| 0.48 | 0.65 | 0.93 | 0.92 | 1.73 | −2.07 | 0.56 | 0.72 | 1.10 | 1.11 | 2.82 | −1.66 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cukras, J.; Skóra, G.; Jankowska, J.; Lundell, J. Computational Structures and SAPT Interaction Energies of HXeSH···H2Y (Y=O or S) Complexes. Inorganics 2018, 6, 100. https://doi.org/10.3390/inorganics6030100
Cukras J, Skóra G, Jankowska J, Lundell J. Computational Structures and SAPT Interaction Energies of HXeSH···H2Y (Y=O or S) Complexes. Inorganics. 2018; 6(3):100. https://doi.org/10.3390/inorganics6030100
Chicago/Turabian StyleCukras, Janusz, Grzegorz Skóra, Joanna Jankowska, and Jan Lundell. 2018. "Computational Structures and SAPT Interaction Energies of HXeSH···H2Y (Y=O or S) Complexes" Inorganics 6, no. 3: 100. https://doi.org/10.3390/inorganics6030100
APA StyleCukras, J., Skóra, G., Jankowska, J., & Lundell, J. (2018). Computational Structures and SAPT Interaction Energies of HXeSH···H2Y (Y=O or S) Complexes. Inorganics, 6(3), 100. https://doi.org/10.3390/inorganics6030100






