A M2L2 Redox-Active Metalla-Macrocycle Based on Electron-Rich 9-(1,3-Dithiol-2-ylidene)Fluorene
Abstract
1. Introduction
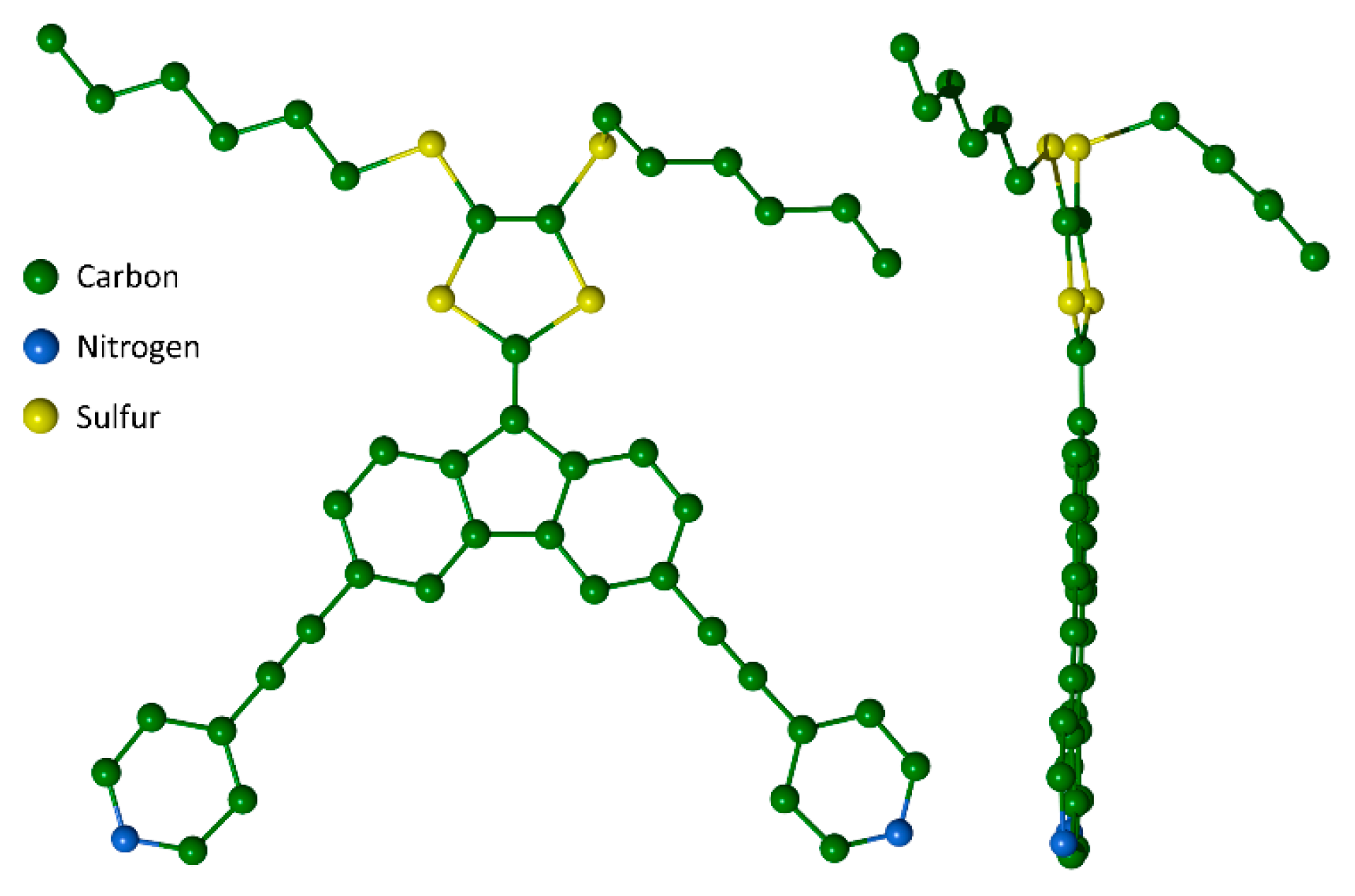
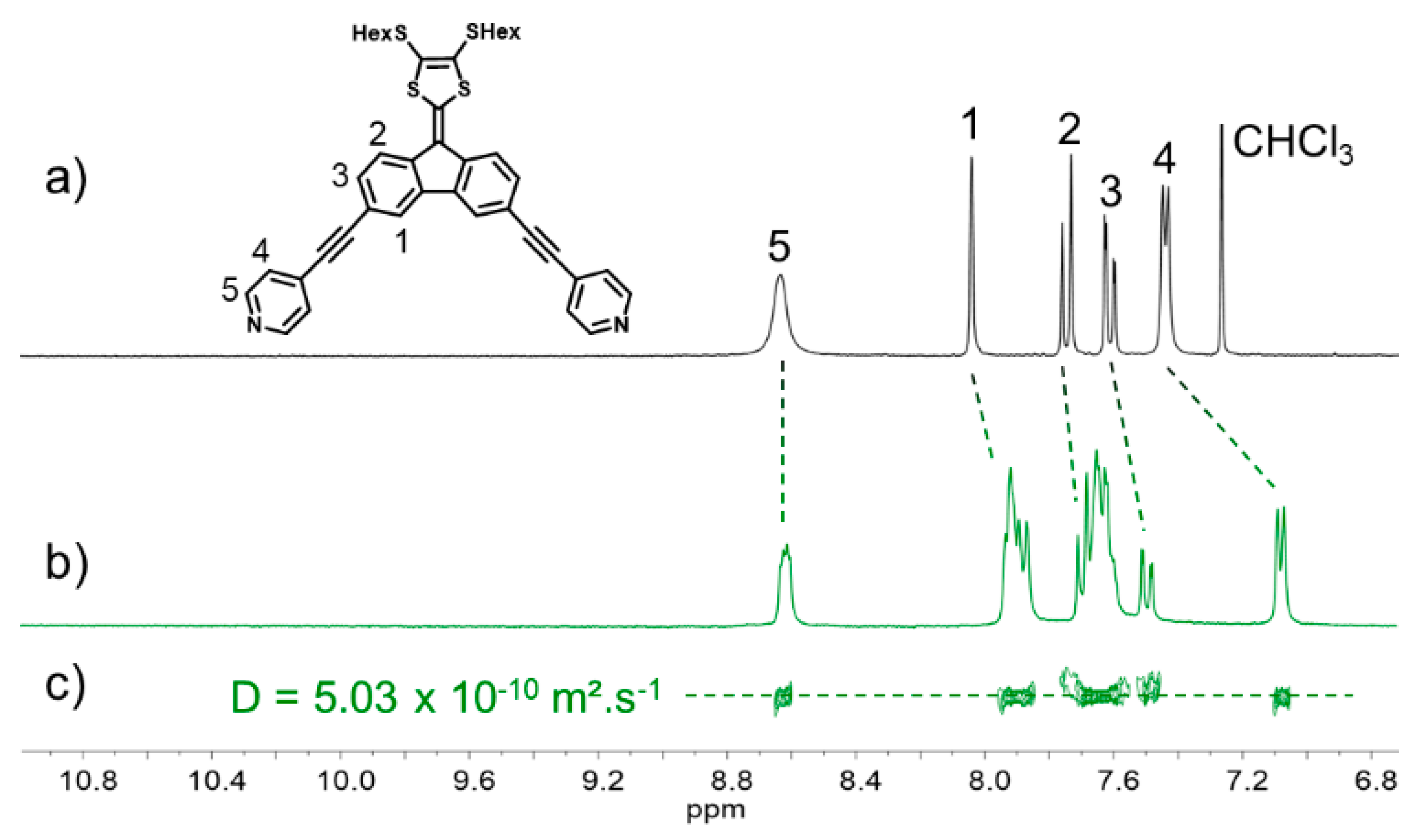
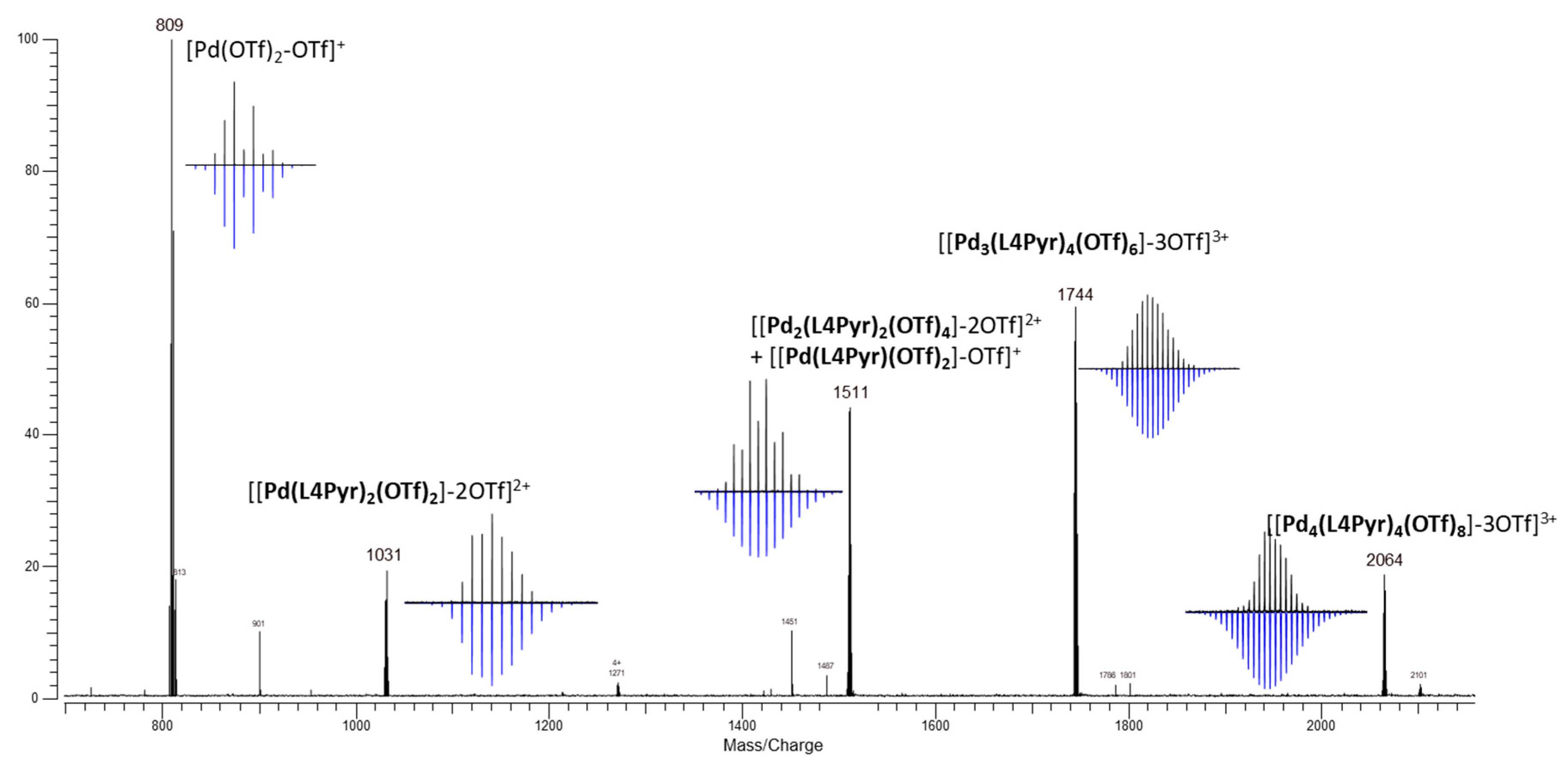
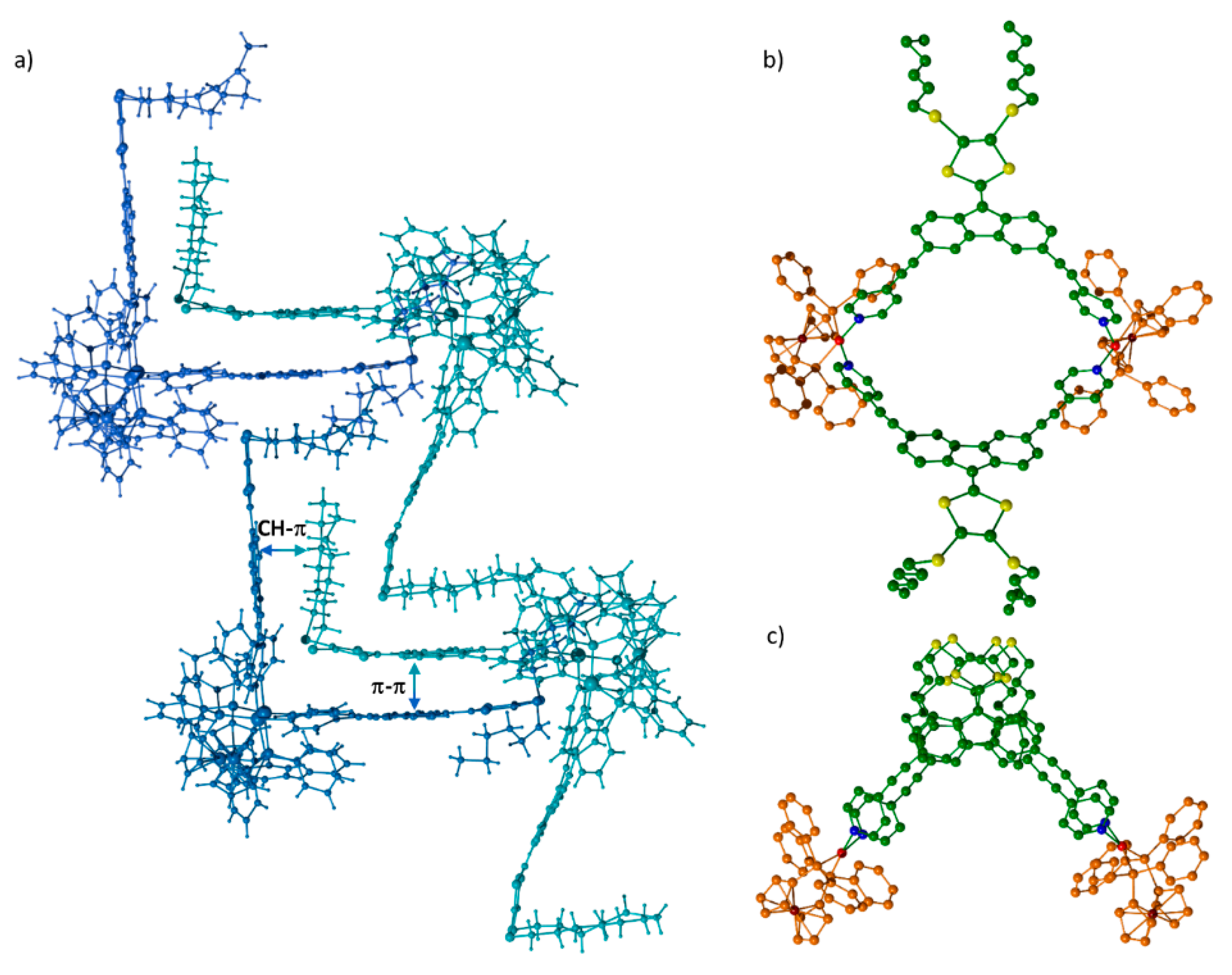
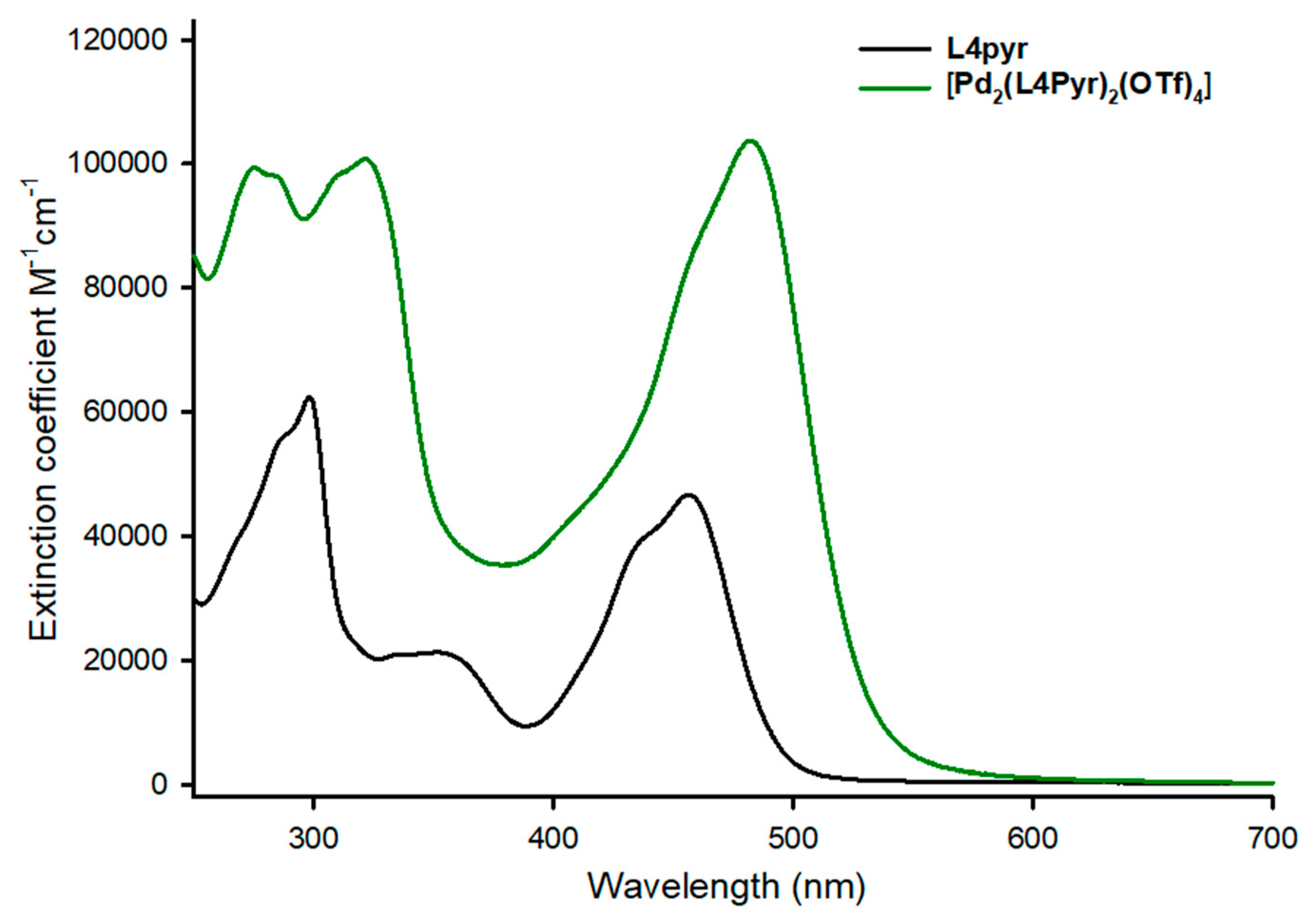
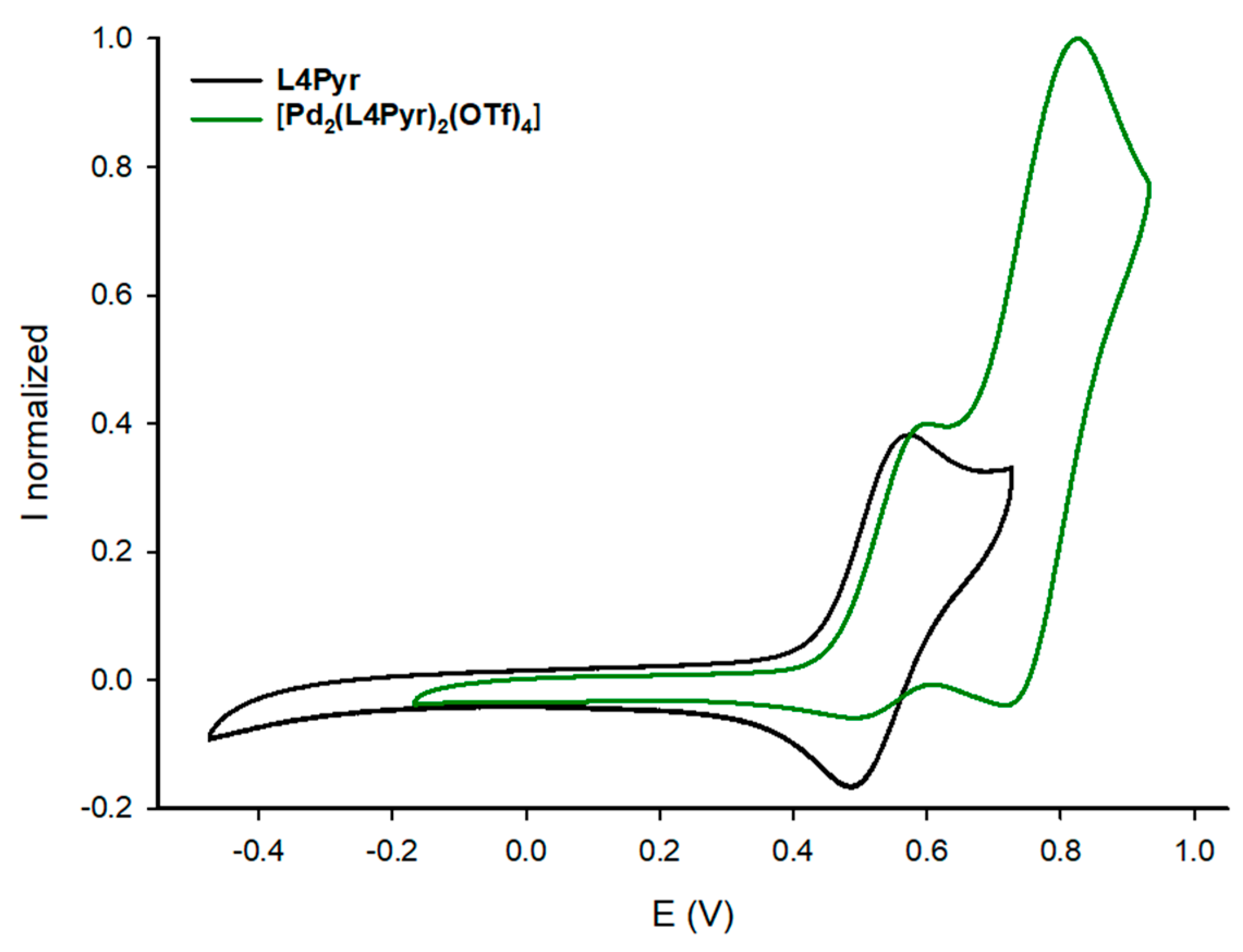
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Instrumentation
3.3. Experimental Procedure and Characterization Data
3.3.1. Synthesis of 3,6-bis(Pyridin-4-ylethynyl)-9H-fluoren-9-one (2)
3.3.2. Synthesis of Ligand L4Pyr
3.3.3. Synthesis of Metalla-Macrocycle [Pd2(L4Pyr)2(OTf)4]
3.4. X-ray Crystallographic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fujita, M.; Yazaki, J.; Ogura, K. Preparation of a macrocyclic polynuclear complex, [(en)Pd(4,4’-bpy)]4(NO3)8 (en = ethylenediamine, bpy = bipyridine), which recognizes an organic molecule in aqueous media. J. Am. Chem. Soc. 1990, 112, 5645–5647. [Google Scholar] [CrossRef]
- Cook, T.R.; Stang, P.J. Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Kang, S.C.; Chi, K.-W. Coordination-Driven Self-Assembly of Arene–Ruthenium Compounds. Eur. J. Inorg. Chem. 2013, 5222–5232. [Google Scholar] [CrossRef]
- Smulders, M.M.J.; Riddell, I.A.; Browne, C.; Nitschke, J.R. Building on architectural principles for three-dimensional metallosupramolecular construction. Chem. Soc. Rev. 2013, 42, 1728–1754. [Google Scholar] [CrossRef] [PubMed]
- Young, N.J.; Hay, B.P. Structural design principles for self-assembled coordination polygons and polyhedra. Chem. Commun. 2013, 49, 1354–1379. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal–organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis, and functionality of metal–organic materials. Chem. Rev. 2012, 113, 734–777. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular coordination: Self-assembly of finite two-and three-dimensional ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef] [PubMed]
- Zarra, S.; Wood, D.M.; Roberts, D.A.; Nitschke, J.R. Molecular containers in complex chemical systems. Chem. Soc. Rev. 2015, 44, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Amouri, H.; Desmarets, C.; Moussa, J. Confined nanospaces in metallocages: guest molecules, weakly encapsulated anions, and catalyst sequestration. Chem. Rev. 2012, 112, 2015–2041. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Woods, B.; Wenzel, M. The Promise of Self-Assembled 3D Supramolecular Coordination Complexes for Biomedical Applications. Inorg. Chem. 2017, 56, 14715–14729. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Younus, H.A.; Chughtai, A.H.; Verpoort, F. Metal–organic molecular cages: Applications of biochemical implications. Chem. Soc. Rev. 2015, 44, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Vajpayee, V.; Lee, M.H.; Stang, P.J.; Chi, K.-W. Biomedical and biochemical applications of self-assembled metallacycles and metallacages. Acc. Chem. Res. 2013, 46, 2464–2474. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Moscoso, A.; Ballester, P. Light-responsive molecular containers. Chem. Commun. 2017, 53, 4635–4652. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.-X.; Yang, H.-B. Supramolecular transformations within discrete coordination-driven supramolecular architectures. Chem. Soc. Rev. 2016, 45, 2656–2693. [Google Scholar] [CrossRef] [PubMed]
- McConnell, A.J.; Wood, C.S.; Neelakandan, P.P.; Nitschke, J.R. Stimuli-responsive metal–ligand assemblies. Chem. Rev. 2015, 115, 7729–7793. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.-H.; Wang, Q.-C.; Zhang, Q.-W.; Ma, X.; Tian, H. Photoresponsive host–guest functional systems. Chem. Rev. 2015, 115, 7543–7588. [Google Scholar] [CrossRef] [PubMed]
- Croué, V.; Goeb, S.; Sallé, M. Metal-driven self-assembly: The case of redox-active discrete architectures. Chem. Commun. 2015, 51, 7275–7289. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.-X.; Chen, L.-J.; Yang, H.-B. Construction of multiferrocenyl metallacycles and metallacages via coordination-driven self-assembly: From structure to functions. Chem. Soc. Rev. 2015, 44, 2148–2167. [Google Scholar] [CrossRef] [PubMed]
- Szalóki, G.; Croué, V.; Carré, V.; Aubriet, F.; Aleveque, O.; Levillain, E.; Allain, M.; Arago, J.; Orti, E.; Goeb, S.; et al. Controlling the Host–Guest Interaction Mode through a Redox Stimulus. Angew. Chem. Int. Ed. 2017, 56, 16272–16276. [Google Scholar] [CrossRef] [PubMed]
- Croué, V.; Goeb, S.; Szalóki, G.; Allain, M.; Sallé, M. Reversible Guest Uptake/Release by Redox-Controlled Assembly/Disassembly of a Coordination Cage. Angew. Chem. Int. Ed. 2016, 55, 1746–1750. [Google Scholar] [CrossRef] [PubMed]
- Szalóki, G.; Croué, V.; Allain, M.; Goeb, S.; Sallé, M. Neutral versus polycationic coordination cages: A comparison regarding neutral guest inclusion. Chem. Commun. 2016, 52, 10012–10015. [Google Scholar] [CrossRef] [PubMed]
- Bivaud, S.; Goeb, S.; Croué, V.; Allain, M.; Pop, F.; Sallé, M. Tuning the size of a redox-active tetrathiafulvalene-based self-assembled ring. Beilstein J. Org. Chem. 2015, 11, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Vajpayee, V.; Bivaud, S.; Goeb, S.; Croué, V.; Allain, M.; Popp, B.V.; Garci, A.; Therrien, B.; Sallé, M. Electron-Rich Arene–Ruthenium Metalla-architectures Incorporating Tetrapyridyl–Tetrathiafulvene Donor Moieties. Organometallics 2014, 33, 1651–1658. [Google Scholar] [CrossRef]
- Bivaud, S.; Goeb, S.; Croué, V.; Dron, P.I.; Allain, M.; Sallé, M. Self-assembled containers based on extended tetrathiafulvalene. J. Am. Chem. Soc. 2013, 135, 10018–10021. [Google Scholar] [CrossRef] [PubMed]
- Bivaud, S.; Balandier, J.Y.; Chas, M.; Allain, M.; Goeb, S.; Sallé, M. A metal-directed self-assembled electroactive cage with bis(pyrrolo) tetrathiafulvalene (BPTTF) side walls. J. Am. Chem. Soc. 2012, 134, 11968–11970. [Google Scholar] [CrossRef] [PubMed]
- Goeb, S.; Bivaud, S.; Dron, P.I.; Balandier, J.-Y.; Chas, M.; Sallé, M. A BPTTF-based self-assembled electron-donating triangle capable of C60 binding. Chem. Commun. 2012, 48, 3106–3108. [Google Scholar] [CrossRef] [PubMed]
- Perepichka, D.F.; Perepichka, I.F.; Ivasenko, O.; Moore, A.J.; Bryce, M.R.; Kuz’mina, L.G.; Batsanov, A.S.; Sokolov, N.I. Combining High Electron Affinity and Intramolecular Charge Transfer in 1,3-Dithiole–Nitrofluorene Push–Pull Diads. Chem. Eur. J. 2008, 14, 2757–2770. [Google Scholar] [CrossRef] [PubMed]
- Amriou, S.; Wang, C.; Batsanov, A.S.; Bryce, M.R.; Perepichka, D.F.; Ortí, E.; Viruela, R.; Vidal-Gancedo, J.; Rovira, C. The Interplay of Inverted Redox Potentials and Aromaticity in the Oxidized States of New π-Electron Donors: 9-(1,3-Dithiol-2-ylidene) fluorene and 9-(1,3-Dithiol-2-ylidene) thioxanthene Derivatives. Chem. Eur. J. 2006, 12, 3389–3400. [Google Scholar] [CrossRef] [PubMed]
- Croué, V.; Krykun, S.; Allain, M.; Morille, Y.; Aubriet, F.; Carré, V.; Voitenko, Z.; Goeb, S.; Sallé, M. A self-assembled M2L4 cage incorporating electron-rich 9-(1,3-dithiol-2-ylidene) fluorene units. New J. Chem. 2017, 41, 3238–3241. [Google Scholar] [CrossRef]
- Cohen, Y.; Avram, L.; Frish, L. Diffusion NMR spectroscopy in supramolecular and combinatorial chemistry: An old parameter—New insights. Angew. Chem. Int. Ed. 2005, 44, 520–554. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraju, S.; Vajpayee, V.; Lee, S.; Chi, K.-W.; Stang, P.J.; Mukherjee, P.S. Coordination-driven self-assembly of 2d-metallamacrocycles using a new carbazole-based dipyridyl donor: Synthesis, characterization, and C60 binding study. Inorg. Chem. 2012, 51, 4817–4823. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Ravikumar, S.; Hong, S.H.; Kim, H.; Vajpayee, V.; Lee, H.; Ahn, B.; Wang, M.; Stang, P.J.; Chi, K.-W. DNA binding and unwinding by self-assembled supramolecular heterobimetallacycles. Organometallics 2011, 30, 6343–6346. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Gutiérrez, A.; Rodríguez, L.; Rossell, O.; Ruiz, E.; Engeser, M.; Lorenz, Y.; Schilling, R.; Gómez-Sal, P.; Martín, A. Self-assembly of heterometallic metallomacrocycles via ditopic fluoroaryl gold (I) organometallic metalloligands. Organometallics 2012, 31, 1533–1545. [Google Scholar] [CrossRef]
- Niu, Z.; Li, D.; Liu, D.; Xia, D.; Zou, Y.; Sun, W.; Li, G. Syntheses, electrochemical behaviors, spectral properties and DFT calculations of two 1,3-dithiole derivatives. Chem. Res. Chin. Univ. 2014, 30, 425–430. [Google Scholar] [CrossRef]
- Zhao, Y.-P.; Wu, L.-Z.; Si, G.; Liu, Y.; Xue, H.; Zhang, L.-P.; Tung, C.-H. Synthesis, spectroscopic, electrochemical and Pb2+-binding studies of tetrathiafulvalene acetylene derivatives. J. Org. Chem. 2007, 72, 3632–3639. [Google Scholar] [CrossRef] [PubMed]
- Estrada, L.A.; Neckers, D.C. Synthesis and photophysics of ambipolar fluoren-9-ylidene malononitrile derivatives. J. Org. Chem. 2009, 74, 8484–8487. [Google Scholar] [CrossRef] [PubMed]
- Broman, S.L.; Andersen, C.L.; Jousselin-Oba, T.; Manso, M.; Hammerich, O.; Frigoli, M.; Nielsen, M.B. Tetraceno [2,1,12,11-opqra] tetracene-extended tetrathiafulvalene–redox-controlled generation of a large PAH core. Org. Biomol. Chem. 2017, 15, 807–811. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krykun, S.; Allain, M.; Carré, V.; Aubriet, F.; Voitenko, Z.; Goeb, S.; Sallé, M. A M2L2 Redox-Active Metalla-Macrocycle Based on Electron-Rich 9-(1,3-Dithiol-2-ylidene)Fluorene. Inorganics 2018, 6, 44. https://doi.org/10.3390/inorganics6020044
Krykun S, Allain M, Carré V, Aubriet F, Voitenko Z, Goeb S, Sallé M. A M2L2 Redox-Active Metalla-Macrocycle Based on Electron-Rich 9-(1,3-Dithiol-2-ylidene)Fluorene. Inorganics. 2018; 6(2):44. https://doi.org/10.3390/inorganics6020044
Chicago/Turabian StyleKrykun, Serhii, Magali Allain, Vincent Carré, Frédéric Aubriet, Zoia Voitenko, Sébastien Goeb, and Marc Sallé. 2018. "A M2L2 Redox-Active Metalla-Macrocycle Based on Electron-Rich 9-(1,3-Dithiol-2-ylidene)Fluorene" Inorganics 6, no. 2: 44. https://doi.org/10.3390/inorganics6020044
APA StyleKrykun, S., Allain, M., Carré, V., Aubriet, F., Voitenko, Z., Goeb, S., & Sallé, M. (2018). A M2L2 Redox-Active Metalla-Macrocycle Based on Electron-Rich 9-(1,3-Dithiol-2-ylidene)Fluorene. Inorganics, 6(2), 44. https://doi.org/10.3390/inorganics6020044






