Synthesis and In Vitro (Anticancer) Evaluation of η6-Arene Ruthenium Complexes Bearing Stannyl Ligands
Abstract
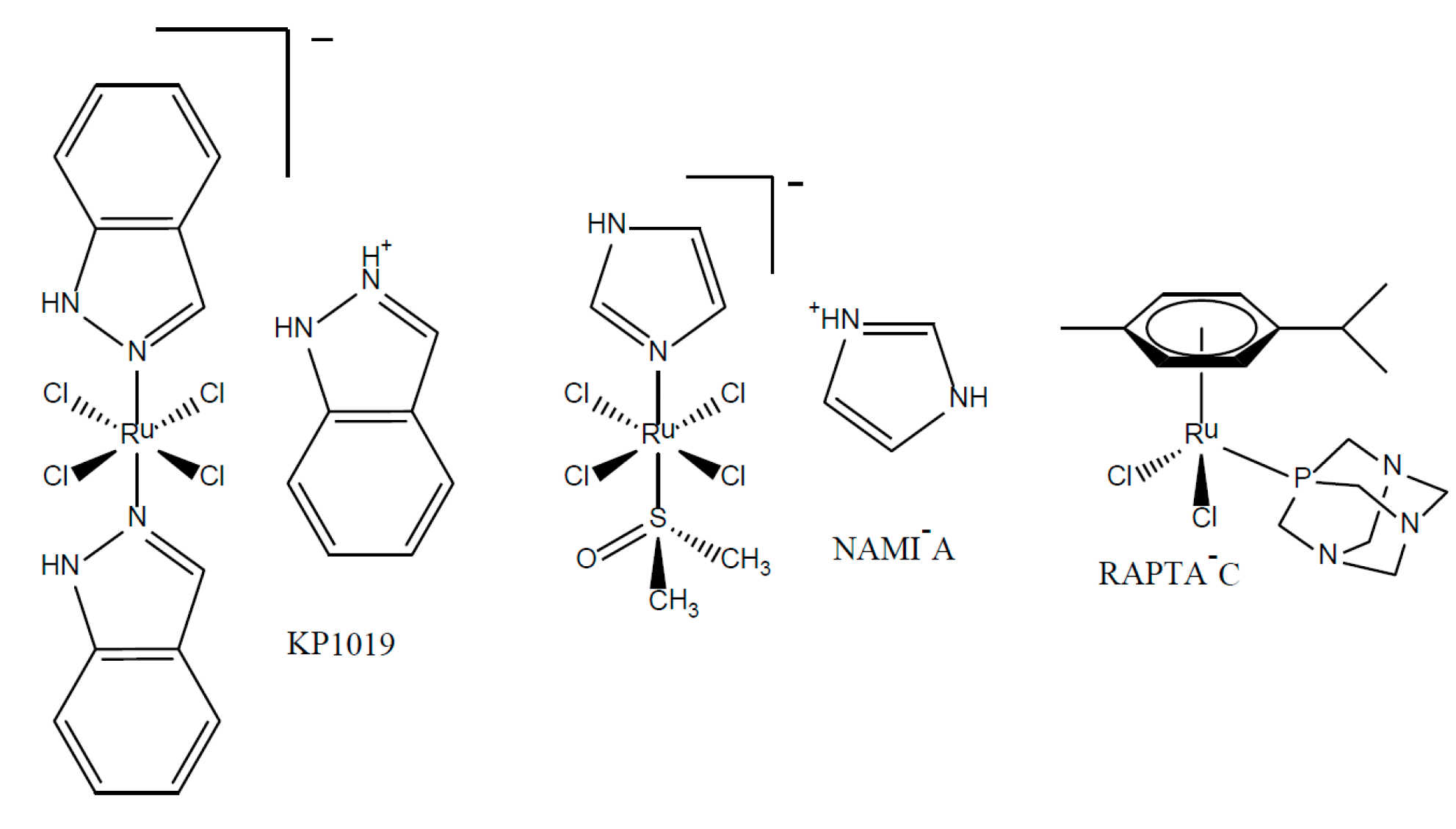
:1. Introduction
2. Results and Discussion
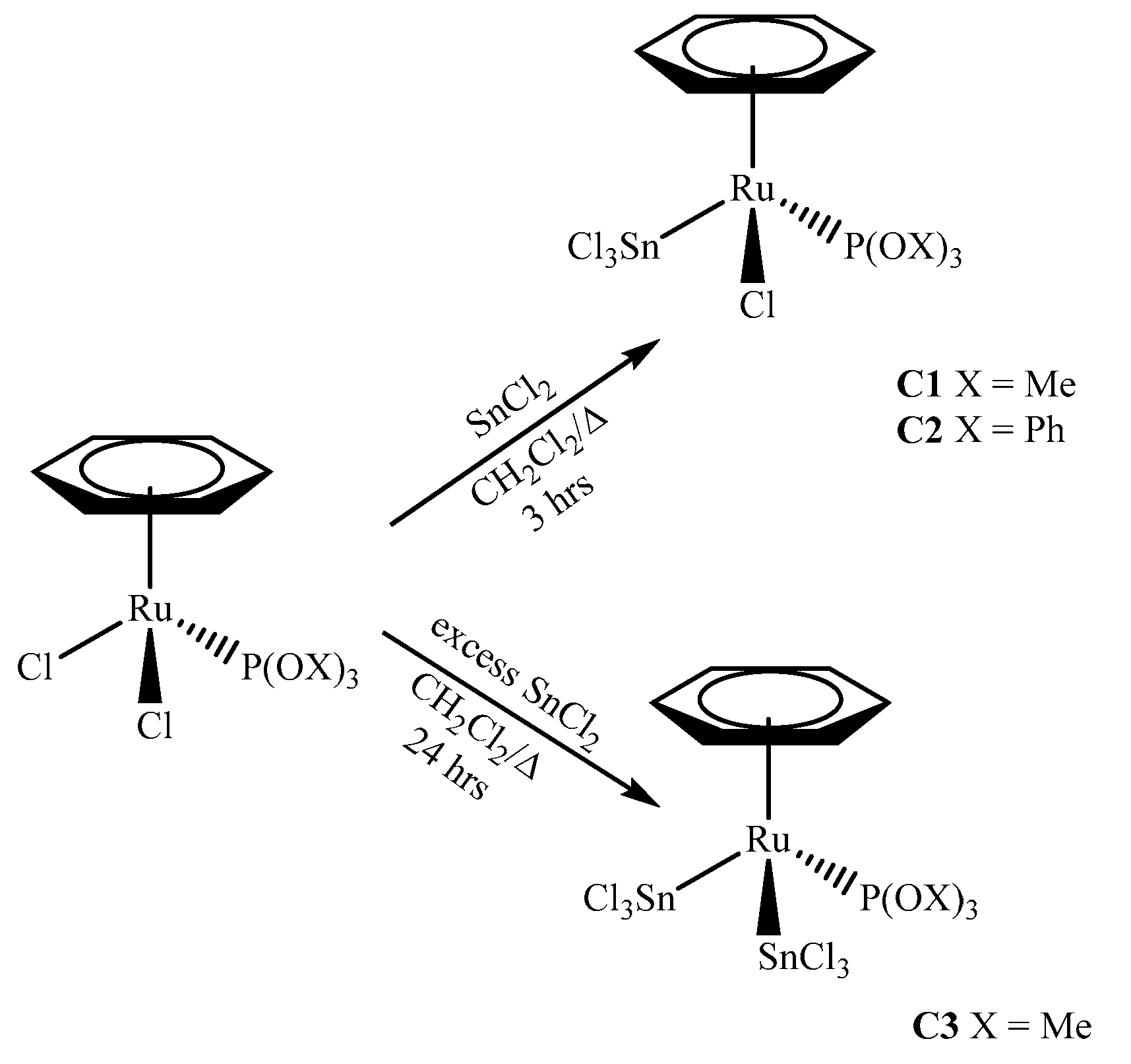
2.1. Synthesis of the Complexes
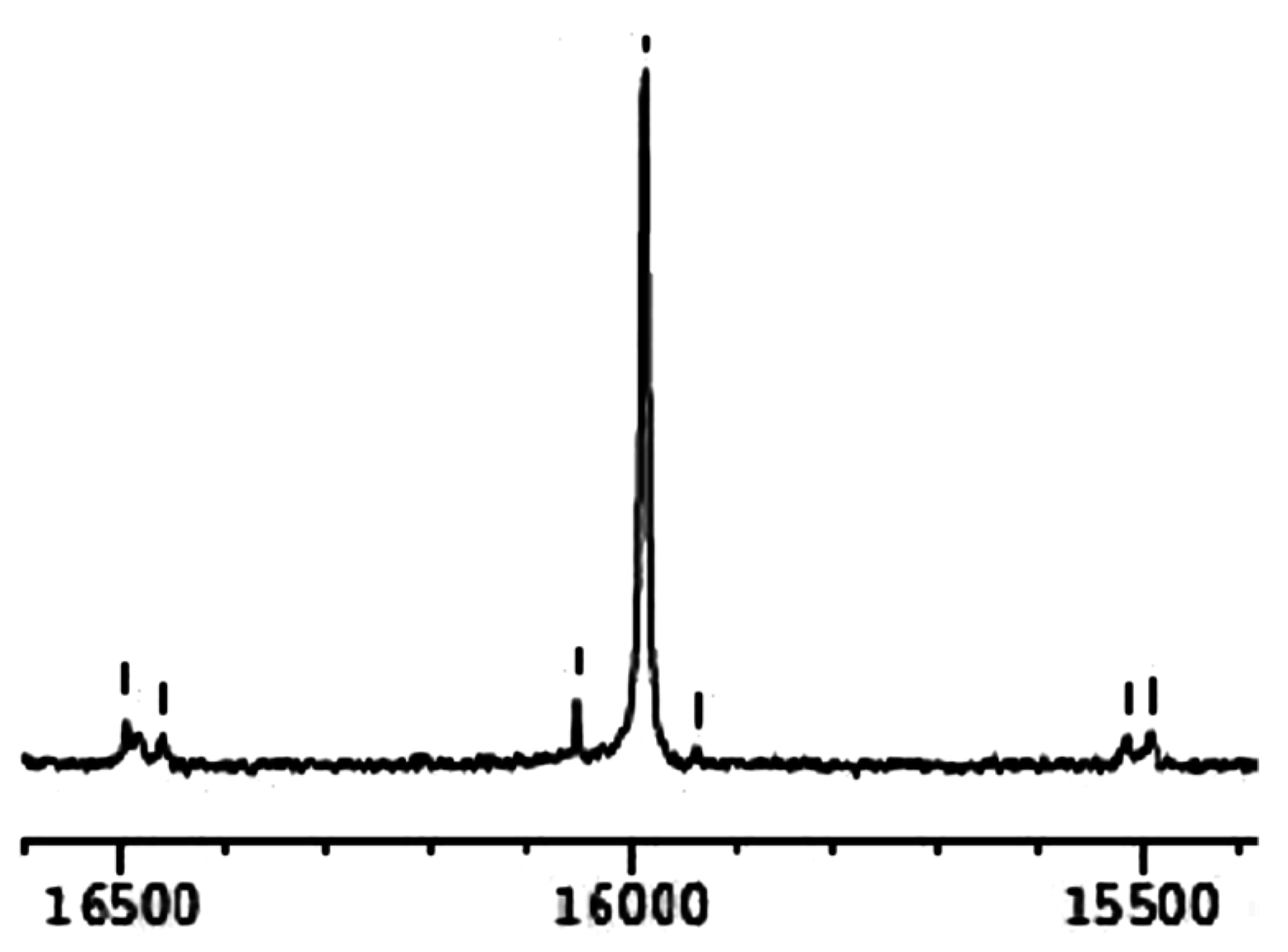
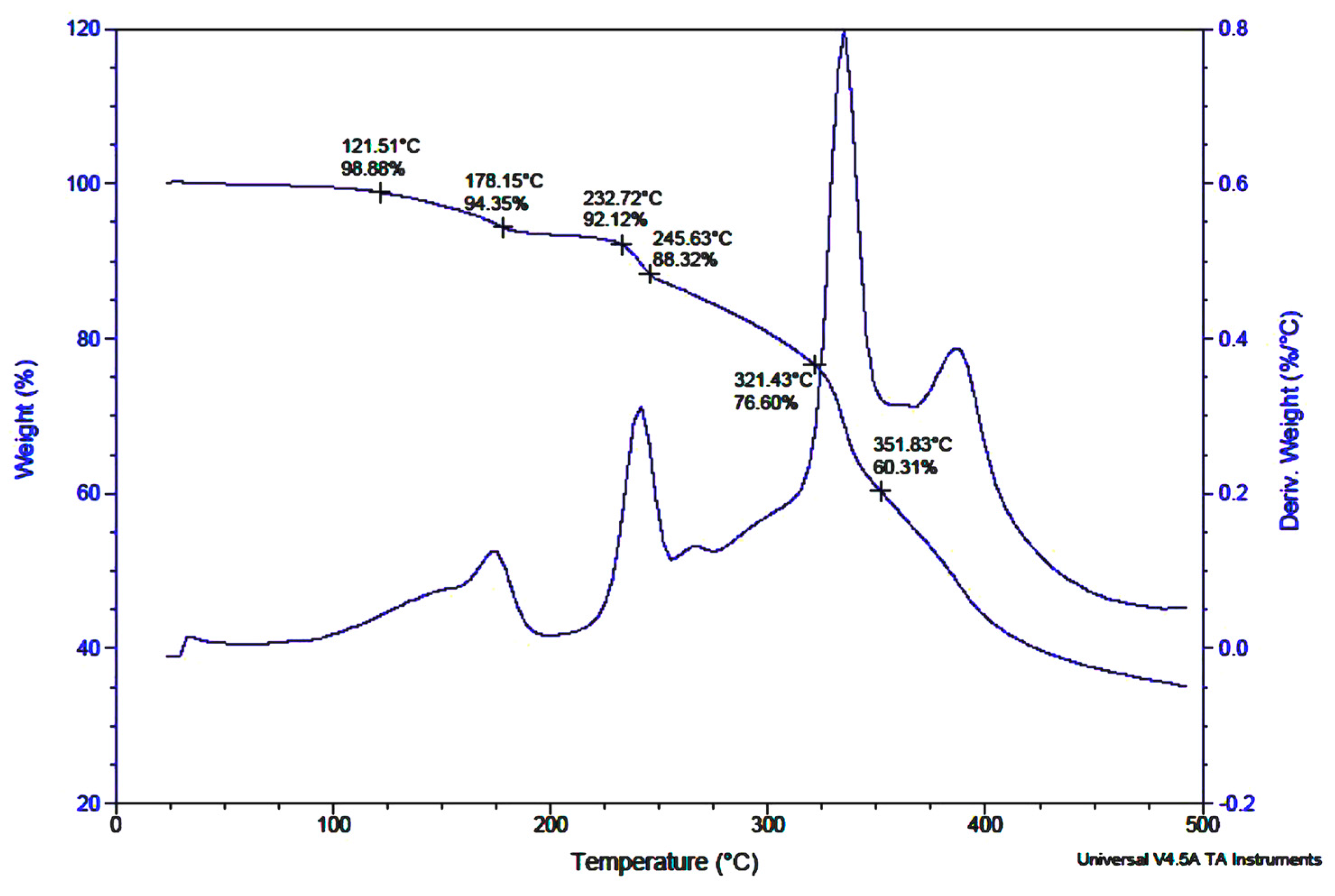
2.2. Spectroscopic Characterisation
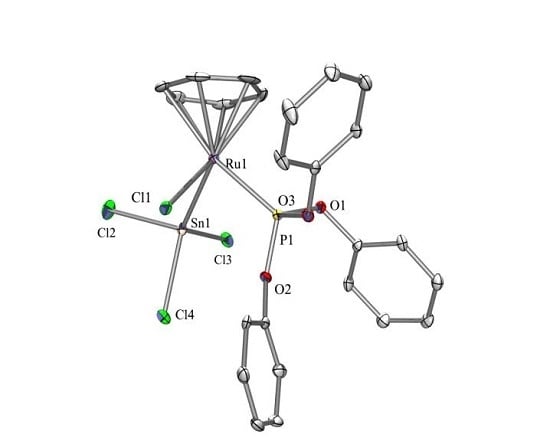
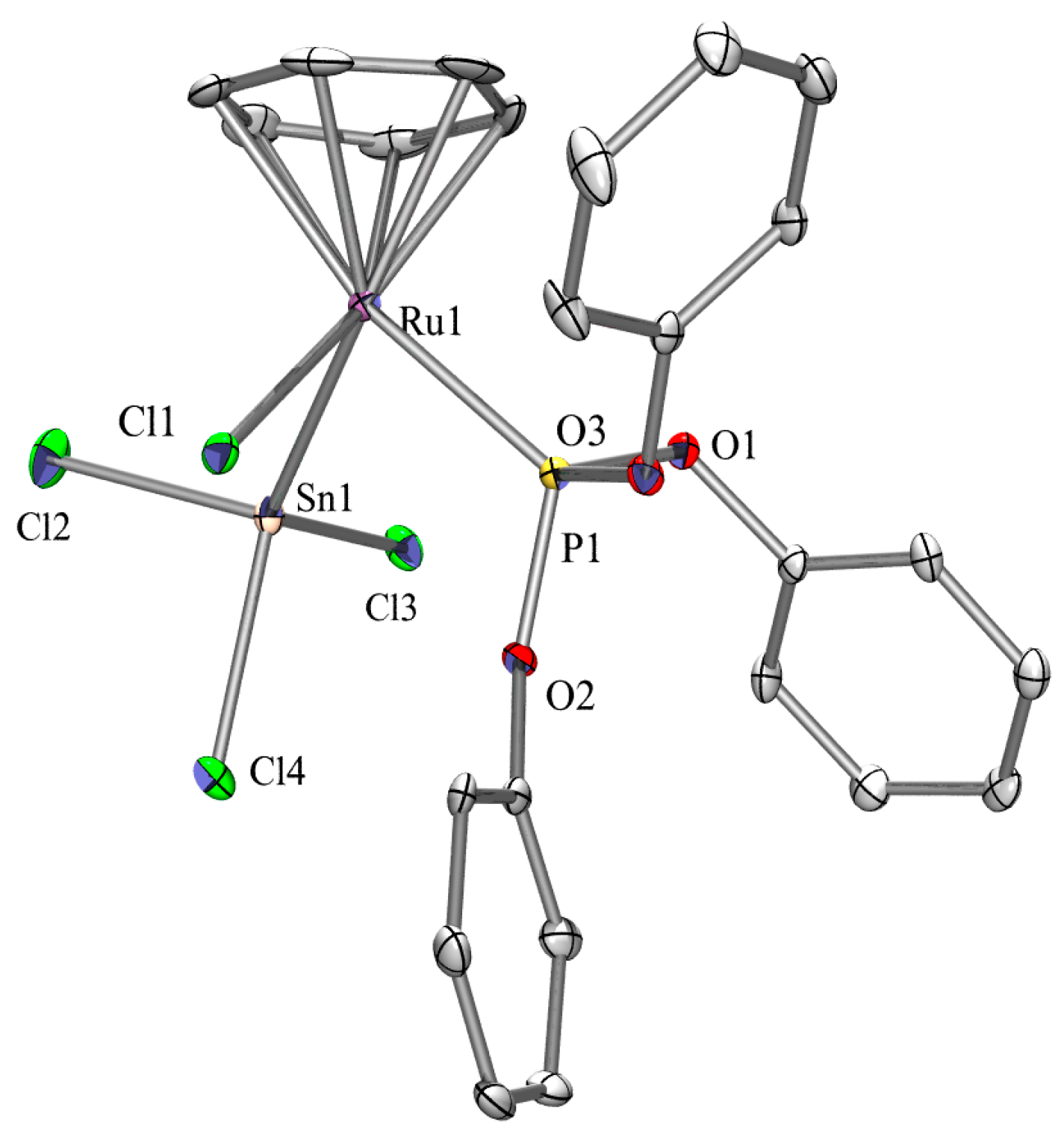
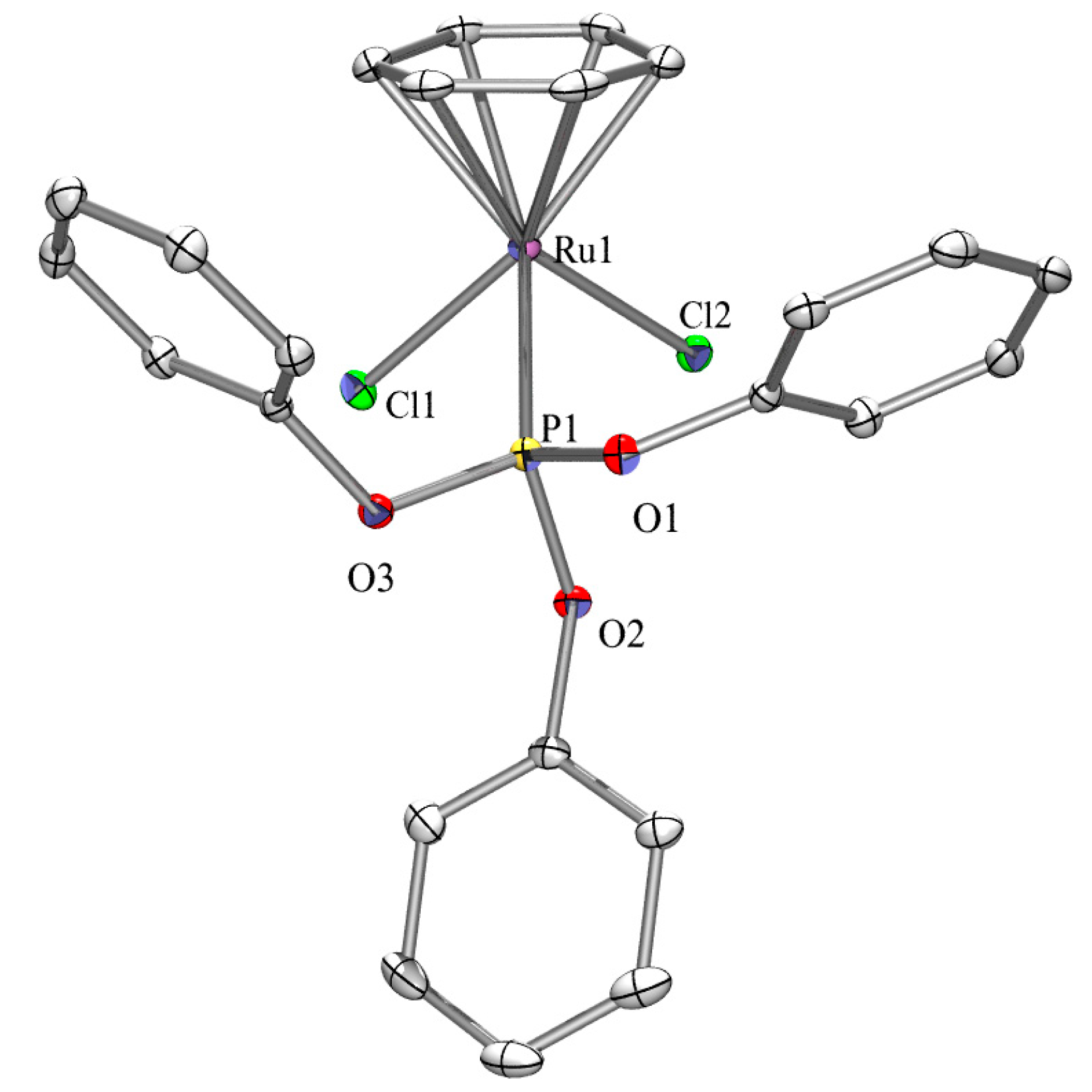
2.3. X-ray Crystallography
2.4. Cytotoxicity Studies
3. Experimental
3.1. General Procedures
3.2. Synthesis of the Complexes
3.2.1. Synthesis of [(η6-C6H6)RuCl(SnCl3)(P(OMe)3)] (C1)
3.2.2. Synthesis of [(η6-C6H6)RuCl(SnCl3)(P(OPh)3)] (C2)
3.2.3. Synthesis of [(η6-C6H6)Ru(SnCl3)2(P(OMe)3)] (C3)
3.2.4. Synthesis of [(η6-C6H6)Ru(SnCl3)(PPh3)(DMAP)]+BF4− (C4)
3.2.5. Synthesis of [(η6-C6H6)RuCl(PPh3)(DMAP)]+BF4− (C5)
3.3. Crystallographic Structure Determination
3.4. Cell Cultures and Cytotoxicity Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rosenberg, B.; Vancamp, L. Platinum compounds: A new class of potent antitumour agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Lippman, A.J.; Helson, C.; Helson, L.; Krakoff, I.H. Clinical trials of cis-diamminedichloroplatinum (nsc-119875). Cancer Chemother. Rep. 1973, 57, 191–200. [Google Scholar] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed]
- Galanski, M.; Jakupec, M.A.; Keppler, B.K. Update of the preclinical situation of anticancer platinum complexes: Novel design strategies and innovative analytical approaches. Curr. Med. Chem. 2005, 12, 2075–2094. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Shamsi, F.; Azam, A. Ruthenium complexes: An emerging ground to the development of metallopharmaceuticals for cancer therapy. Mini Rev. Med. Chem. 2016, 16, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Ott, I.; Gust, R. Non Platinum Metal Complexes as Anti-cancer Drugs. Arch. Der Pharm. 2007, 340, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Momekov, G.; Bakalova, A.; Karaivanova, M. Novel approaches towards development of non-classical platinum-based antineoplastic agents: Design of platinum complexes characterized by an alternative DNA-binding pattern and/or tumor-targeted cytotoxicity. Curr. Med. Chem. 2005, 12, 2177–2191. [Google Scholar] [CrossRef] [PubMed]
- Xin Zhang, C.; Lippard, S. New metal complexes as potential therapeutics. Curr. Opin. Chem. Biol. 2003, 7, 481–489. [Google Scholar]
- Wu, Q.; Fan, C.; Chen, T.; Liu, C.; Mei, W.; Chen, S.; Wang, B.; Chen, Y.; Zheng, W. Microwave-assisted synthesis of arene ruthenium(II) complexes that induce s-phase arrest in cancer cells by DNA damage-mediated p53 phosphorylation. Eur. J. Med. Chem. 2013, 63, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Sava, G.; Bergamo, A. Ruthenium-based compounds and tumour growth control. Int. J. Oncol. 2000, 17, 353–418. [Google Scholar] [CrossRef] [PubMed]
- Sava, G.; Bergamo, A. Ruthenium Drugs for Cancer Chemotherapy: An Ongoing Challenge to Treat Solid Tumours. In Platinum and Other Heavy Metal Compounds in Cancer Chemotherapy: Molecular Mechanisms and Clinical Applications; Bonetti, A., Leone, R., Muggia, F.M., Howell, S.B., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 57–66. [Google Scholar]
- Barry, N.P.; Sadler, P.J. 100 Years of metal coordination chemistry: From Alfred Werner to anticancer metallodrugs. Pure Appl. Chem. 2014, 86, 1897–1910. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Esteban Leon, I.; Fernando Cadavid-Vargas, J.; Laura Di Virgilio, A.; Beatriz Etcheverry, S. Vanadium, ruthenium and copper compounds: A new class of nonplatinum metallodrugs with anticancer activity. Curr. Med. Chem. 2017, 24, 112–148. [Google Scholar] [CrossRef] [PubMed]
- Brabec, V.; Hrabina, O.; Kasparkova, J. Cytotoxic platinum coordination compounds. DNA binding agents. Coord. Chem. Rev. In Press. [CrossRef]
- Hartinger, C.G.; Jakupec, M.A.; Zorbas-Seifried, S.; Groessl, M.; Egger, A.; Berger, W.; Zorbas, H.; Dyson, P.J.; Keppler, B.K. Kp1019, a new redox-active anticancer agent-preclinical development and results of a clinical phase I study in tumor patients. Chem. Biodivers. 2008, 5, 2140–2155. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, C.G.; Zorbas-Seifried, S.; Jakupec, M.A.; Kynast, B.; Zorbas, H.; Keppler, B.K. From bench to bedside—Preclinical and early clinical development of the anticancer agent indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(III)] (kp1019 or ffc14a). J. Inorg. Biochem. 2005, 100, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, R.; Petrini, A.; Marchetti, F.; Pettinari, C.; Riedel, T.; Therrien, B.; Dyson, P.J. Arene-ruthenium(II) complexes with bioactive ortho-hydroxydibenzoylmethane ligands: Synthesis, structure, and cytotoxicity. Eur. J. Inorg. Chem. 2017, 12, 1800–1806. [Google Scholar] [CrossRef]
- Scolaro, C.; Geldbach, T.J.; Rochat, S.; Dorcier, A.; Gossens, C.; Bergamo, A.; Cocchietto, M.; Tavernelli, I.; Sava, G.; Rothlisberger, U.; et al. Influence of Hydrogen-Bonding Substituents on the Cytotoxicity of RAPTA Compounds. Organometallics 2006, 25, 756–765. [Google Scholar] [CrossRef]
- Scolaro, C.; Chaplin, A.B.; Hartinger, C.G.; Bergamo, A.; Cocchietto, M.; Keppler, B.K.; Sava, G.; Dyson, P.J. Tuning the hydrophobicity of ruthenium(II)-arene (RAPTA) drugs to modify uptake, biomolecular interactions and efficacy. Dalton Trans. 2007, 43, 5065–5072. [Google Scholar] [CrossRef] [PubMed]
- Scolaro, C.; Bergamo, A.; Brescacin, L.; Delfino, R.; Cocchietto, M.; Laurenczy, G.; Sava, G.; Dyson, P.J. In Vitro and In Vivo Evaluation of Ruthenium(II)-Arene PTA Complexes. J. Med. Chem. 2005, 48, 4161–4171. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Ding, X.; van Beijnum, J.R.; Wong, I.; Wong, T.J.; Berndsen, R.H.; Dormond, O.; Dallinga, M.; Shen, L.; Schingemann, R.O.; et al. Rapid optimization of drug combinations for the optimal angiostatic treatment of cancer. Angiogenesis 2015, 18, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Allardyce, C.S.; Dyson, P.J.; Ellis, D.J.; Heath, S.L. [Ru(η-cymene)Cl(PTA)] (PTA = 1,3,5-triaza-7-phosphatricyclo-[3.3.1.1]decane): A water soluble compound that exhibits pH dependent DNA binding providing selectivity for diseased cells. Chem. Commun. 2001, 15, 1396–1397. [Google Scholar] [CrossRef]
- Pettinari, R.; Marchetti, F.; Petrini, A.; Pettinari, C.; Lupidi, G.; Fernández, B.; Diéguez, A.R.; Santoni, G.; Nabissi, M. Ruthenium(II)-arene complexes with dibenzoylmethane induce apoptotic cell death in multiple myeloma cell lines. Inorg. Chim. Acta 2017, 454, 139–148. [Google Scholar] [CrossRef]
- Kurzwernhart, A.; Kandioller, W.; Enyedy, É.A.; Novak, M.; Jakupec, M.A.; Keppler, B.K.; Hartinger, C.G. 3-hydroxyflavones vs. 3-hydroxyquinolinones: Structure–activity relationships and stability studies on Ru(II) (arene) anticancer complexes with biologically active ligands. Dalton Trans. 2013, 42, 6193–6202. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Pandey, D.S.; Xu, Q.; Braunstein, P. Recent advances in supramolecular and biological aspects of arene ruthenium (II) complexes. Coord. Chem. Rev. 2014, 270, 31–56. [Google Scholar] [CrossRef]
- Betanzos-Lara, S.; Liu, Z.; Habtemariam, A.; Pizarro, A.M.; Qamar, B.; Sadler, P.J. Organometallic ruthenium and iridium transfer-hydrogenation catalysts using coenzyme NADH as a cofactor. Angew. Chem. Int. Ed. 2012, 51, 3897–3900. [Google Scholar] [CrossRef] [PubMed]
- Carrión, M.C.; Sepúlveda, F.; Jalón, F.A.; Manzano, B.R.; Rodríguez, A.M. Arene-ruthenium (II) complexes containing bispyrazolylmethane ligands: Effect of the ligand substituents on the formation of an isomer and on the fluxional behaviour. Eur. J. Inorg. Chem. 2013, 217–227. [Google Scholar] [CrossRef]
- Singh, S.K.; Pandey, D.S. Multifaceted half-sandwich arene-ruthenium complexes: Interactions with biomolecules, photoactivation, and multinuclearity approach. RSC Adv. 2014, 4, 1819–1840. [Google Scholar] [CrossRef]
- Therrien, B. Functionalised η6-arene ruthenium complexes. Coord. Chem. Rev. 2009, 253, 493–519. [Google Scholar] [CrossRef]
- Robertson, D.R.; Stephenson, T.; Arthur, T. Cationic, neutral and anionic complexes of ruthenium(II) containing η6-arene ligands. J. Organomet. Chem. 1978, 162, 121–136. [Google Scholar] [CrossRef]
- Zelonka, R.A.; Baird, M.C. Benzene complexes of ruthenium(II). Can. J. Chem. 1972, 50, 3063–3072. [Google Scholar] [CrossRef]
- Zelonka, R.; Baird, M. Reactions of π-benzeneruthenium(II) complexes with alkylating reagents. J. Organomet. Chem. 1972, 44, 383–389. [Google Scholar] [CrossRef]
- Bennett, M.A.; Smith, A.K. Arene ruthenium(II) complexes formed by dehydrogenation of cyclohexadienes with ruthenium (III) trichloride. J. Chem. Soc. Dalton Trans. 1974, 2, 233–241. [Google Scholar] [CrossRef]
- Therrien, B.; Thai, T.-T.; Freudenreich, J.; Süss-Fink, G.; Shapovalov, S.S.; Pasynskii, A.A.; Plasseraud, L. Bimetallic ruthenium–tin chemistry: Synthesis and molecular structure of arene ruthenium complexes containing trichlorostannyl ligands. J. Organomet. Chem. 2010, 695, 409–414. [Google Scholar] [CrossRef]
- Novák, M.; Bouška, M.; Dostál, L.; Lutter, M.; Jurkschat, K.; Turek, J.; De Proft, F.; Růžičková, Z.; Jambor, R. Role of the trichlorostannyl ligand in tin–ruthenium arene complexes: Experimental and computational studies. Eur. J. Inorg. Chem. 2017, 2017, 1292–1300. [Google Scholar] [CrossRef]
- Thoonen, S.H.; Lutz, M.; Spek, A.L.; Deelman, B.-J.; van Koten, G. Synthesis of novel trialkyl (trichlorostannyl) platinum(IV) complexes through SnCl2 insertion into the Pt–Cl bond. Organometallics 2003, 22, 1156–1159. [Google Scholar] [CrossRef]
- Blom, B. Reactivity of Ylenes at Late Transition Metal Centres. Ph.D. Thesis, Bonn University, Bonn, Germany, 2011. [Google Scholar]
- Gras, M.; Therrien, B.; Süss-Fink, G.; Casini, A.; Edafe, F.; Dyson, P.J. Anticancer activity of new organo-ruthenium, rhodium and iridium complexes containing the 2-(pyridine-2-yl) thiazole N,N-chelating ligand. J. Organomet. Chem. 2010, 695, 1119–1125. [Google Scholar] [CrossRef]
- Joslin, E.E.; McMullin, C.L.; Gunnoe, T.B.; Cundari, T.R.; Sabat, M.; Myers, W.H. Coordination chemistry of 4-methyl-2,6,7-trioxa-1-phosphabicyclo[2,2,1]heptane: Preparation and characterization of Ru(II) complexes. Inorg. Chem. 2012, 51, 4791–4801. [Google Scholar] [CrossRef] [PubMed]
- Meijboom, R.; Muller, A.; Roodt, A. (η6-Benzene)dichloro[tris(2-isopropylphenyl) phosphite]ruthenium(II) dichloromethane solvate. Acta Crystallogr. Sect. E 2006, 62, m1866–m1868. [Google Scholar] [CrossRef]
- Fernandez-Zumel, M.A.; Lastra-Barreira, B.; Scheele, M.; Diez, J.; Crochet, P.; Gimeno, J. Chiral phosphonite, phosphite and phosphoramidite η6-arene-ruthenium(II) complexes: Application to the kinetic resolution of allylic alcohols. Dalton Trans. 2010, 39, 7780–7785. [Google Scholar] [CrossRef] [PubMed]
- SAINTPLUS: Software Reference Manual; Version 6.45; Bruker-AXS: Madison, WI, USA, 1997–2003.
- Blessing, R. An empirical correction for absorption anisotropy. Acta Crystallogr. Sect. A 1995, 51, 33–38. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS; Version 2.10; Bruker-AXS Inc.: Madison, WI, USA, 2003. [Google Scholar]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- POVRAY 3.6. Persistence of Vision Pty. Ltd.: Williamstown, Victoria, Australia, 2004. Available online: http://www.povray.org/download/ (accessed on 9 July 2008).
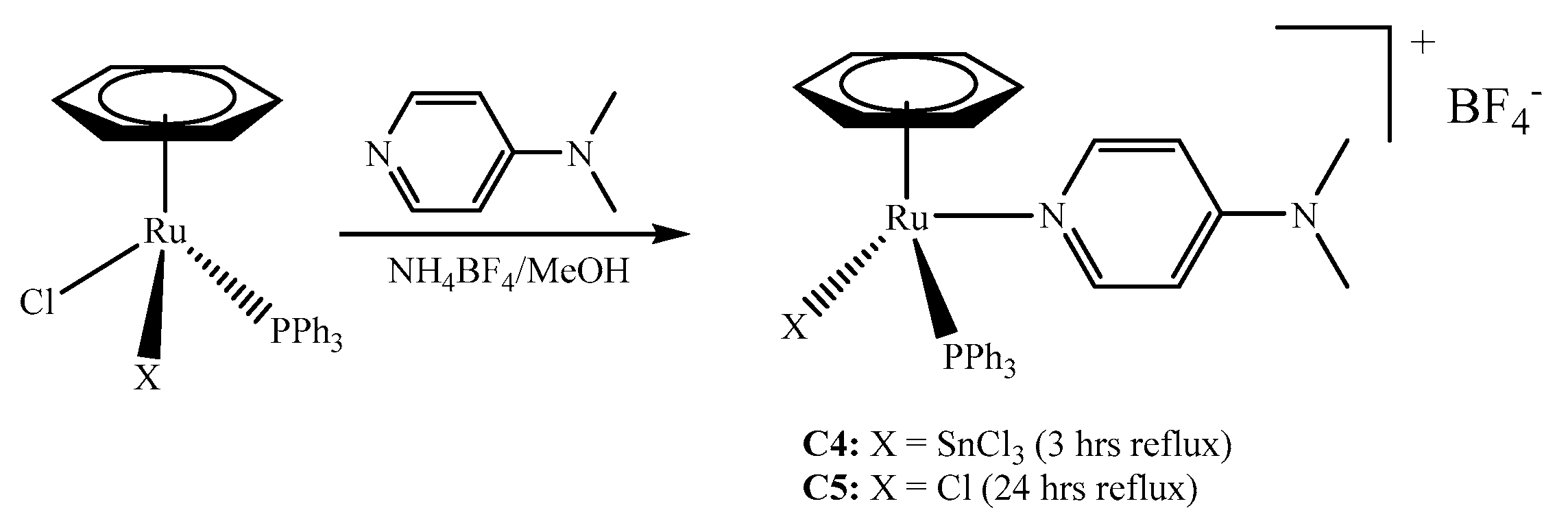
| Compound | IC50 (µM) a | ||
|---|---|---|---|
| A2780 | A2780cisR | HEK293 | |
| C1 | >500 | >500 | >500 |
| C2 | 51.5 ± 0.1 | 51.5 ± 0.1 | 25.0 ± 6.2 |
| C3 | >200 | >200 | >200 |
| C4 | 3.4 ± 0.4 | 4.1 ± 0.9 | 14.2 ± 4.9 |
| C5 | 6.5 ± 0.9 | 7.1 ± 1.6 | 3.2 ± 0.3 |
| [(η6-C6H6)RuCl2(PPh3)] | 30.5 ± 0.7 | 27.0 ± 5.6 | 27.8 ± 6.8 |
| [(η6-C6H6)RuCl2(P(OPh)3)] | 2.3 ± 0.02 | 3.2 ± 0.4 | 1.0 ± 0.5 |
| [(η6-C6H6)RuCl(SnCl3)(PPh3)] | 12.4 ± 0.4 | 12.5 ± 3.0 | 4.9 ± 0.1 |
| cis-platin | 1.1 ± 0.4 | 14.4 ± 2.1 | 10.6 ± 1.4 |
| Complex | C2 | [(η6-C6H6)RuCl2{P(OPh)3}] |
|---|---|---|
| Empirical formula | C24H21Cl4PRuSn | C24H21Cl2O3PRu |
| Formula weight | 749.94 | 560.35 |
| T/K | 100(2) K | 446(2) K |
| λ/Å Crystal system | 0.71073 Orthorhombic | 0.71073 Orthorhombic |
| Space group | P2(1)2(1)2(1) | Pbca |
| a/Å | 8.7164(17) | 17.371(3) |
| b/Å | 16.420(3) | 14.997(3) |
| c/Å | 18.514(4) | 17.551(3) |
| α(deg.) | 90 | 90 |
| β(deg.) | 90 | 90 |
| γ(deg.) | 90 | 90 |
| V(Å3) | 2649.9(9) | 4572.6(14) |
| Z | 4 | 8 |
| Densitycalc(mg·m−3) | 1.880 | 1.628 |
| Absorption coefficient (mm−1) | 2.001 | 1.013 |
| F(000) | 1464 | 2256 |
| Crystal size (mm) | 0.42 × 0.08 × 0.06 | 0.38 × 0.36 × 0.16 |
| Theta range for data collection (deg.) | 1.66–26.33 | 2.14–26.34 |
| Limiting indices | −10 ≤ h ≤ 10, −16 ≤ k ≤ 20, −23 ≤ l ≤23 | −21 ≤h ≤21, −18 ≤ k ≤18, −21 ≤ l ≤ 21 |
| Reflections Collected/Unique | 17168/5368 [R(int) = 0.0409] | 34691/4657 [R(int) = 0.0339] |
| Completeness of theta max. | 26.33 (99.6%) | 26.34 (99.9%) |
| Absorption correction | SADABS | SADABS |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/Restraints/Parameters | 5368/0/308 | 4657/0/280 |
| Goodness-of-fit on F2 (GOF) | 1.042 | 1.117 |
| Largest diff. peak and hole (e·Å−3) | 0.805 and −0.471 | 0.566 and −0.404 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renier, O.; Deacon-Price, C.; Peters, J.E.B.; Nurekeyeva, K.; Russon, C.; Dyson, S.; Ngubane, S.; Baumgartner, J.; Dyson, P.J.; Riedel, T.; et al. Synthesis and In Vitro (Anticancer) Evaluation of η6-Arene Ruthenium Complexes Bearing Stannyl Ligands. Inorganics 2017, 5, 44. https://doi.org/10.3390/inorganics5030044
Renier O, Deacon-Price C, Peters JEB, Nurekeyeva K, Russon C, Dyson S, Ngubane S, Baumgartner J, Dyson PJ, Riedel T, et al. Synthesis and In Vitro (Anticancer) Evaluation of η6-Arene Ruthenium Complexes Bearing Stannyl Ligands. Inorganics. 2017; 5(3):44. https://doi.org/10.3390/inorganics5030044
Chicago/Turabian StyleRenier, Olivier, Connor Deacon-Price, Joannes E. B. Peters, Kunsulu Nurekeyeva, Catherine Russon, Simba Dyson, Siyabonga Ngubane, Judith Baumgartner, Paul J. Dyson, Tina Riedel, and et al. 2017. "Synthesis and In Vitro (Anticancer) Evaluation of η6-Arene Ruthenium Complexes Bearing Stannyl Ligands" Inorganics 5, no. 3: 44. https://doi.org/10.3390/inorganics5030044
APA StyleRenier, O., Deacon-Price, C., Peters, J. E. B., Nurekeyeva, K., Russon, C., Dyson, S., Ngubane, S., Baumgartner, J., Dyson, P. J., Riedel, T., Chiririwa, H., & Blom, B. (2017). Synthesis and In Vitro (Anticancer) Evaluation of η6-Arene Ruthenium Complexes Bearing Stannyl Ligands. Inorganics, 5(3), 44. https://doi.org/10.3390/inorganics5030044