Dye-Sensitized Photocatalytic Water Splitting and Sacrificial Hydrogen Generation: Current Status and Future Prospects
Abstract
:1. Introduction
2. Photocatalytic/Photoelectrochemical Water Splitting: Thermodynamics and Kinetics
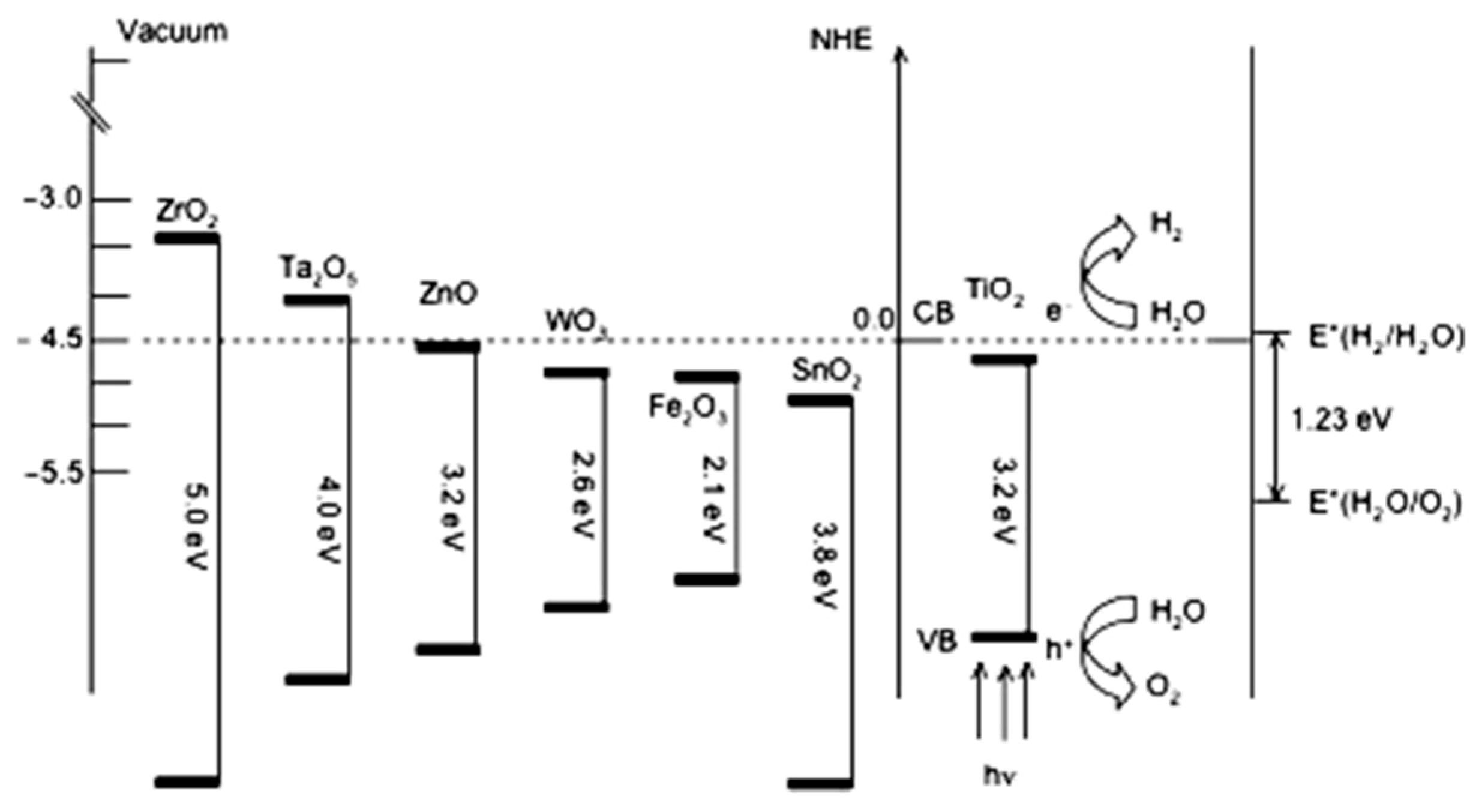
2.1. Thermodynamics of Water Splitting
2.2. Kinetic Constraint of Water Splitting
3. Visible-Light-Driven Overall Water Splitting/Sacrificial Hydrogen Generation
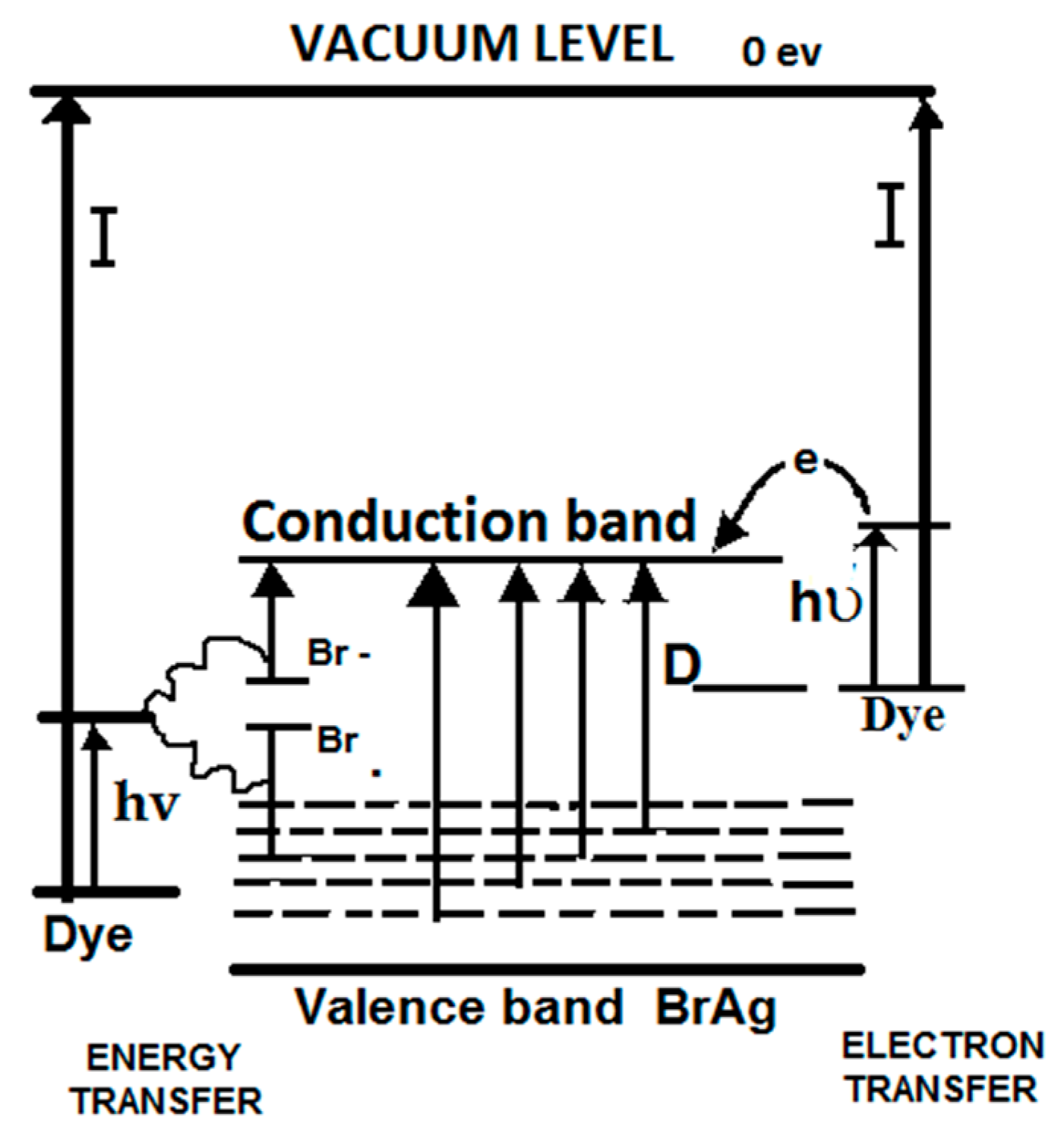
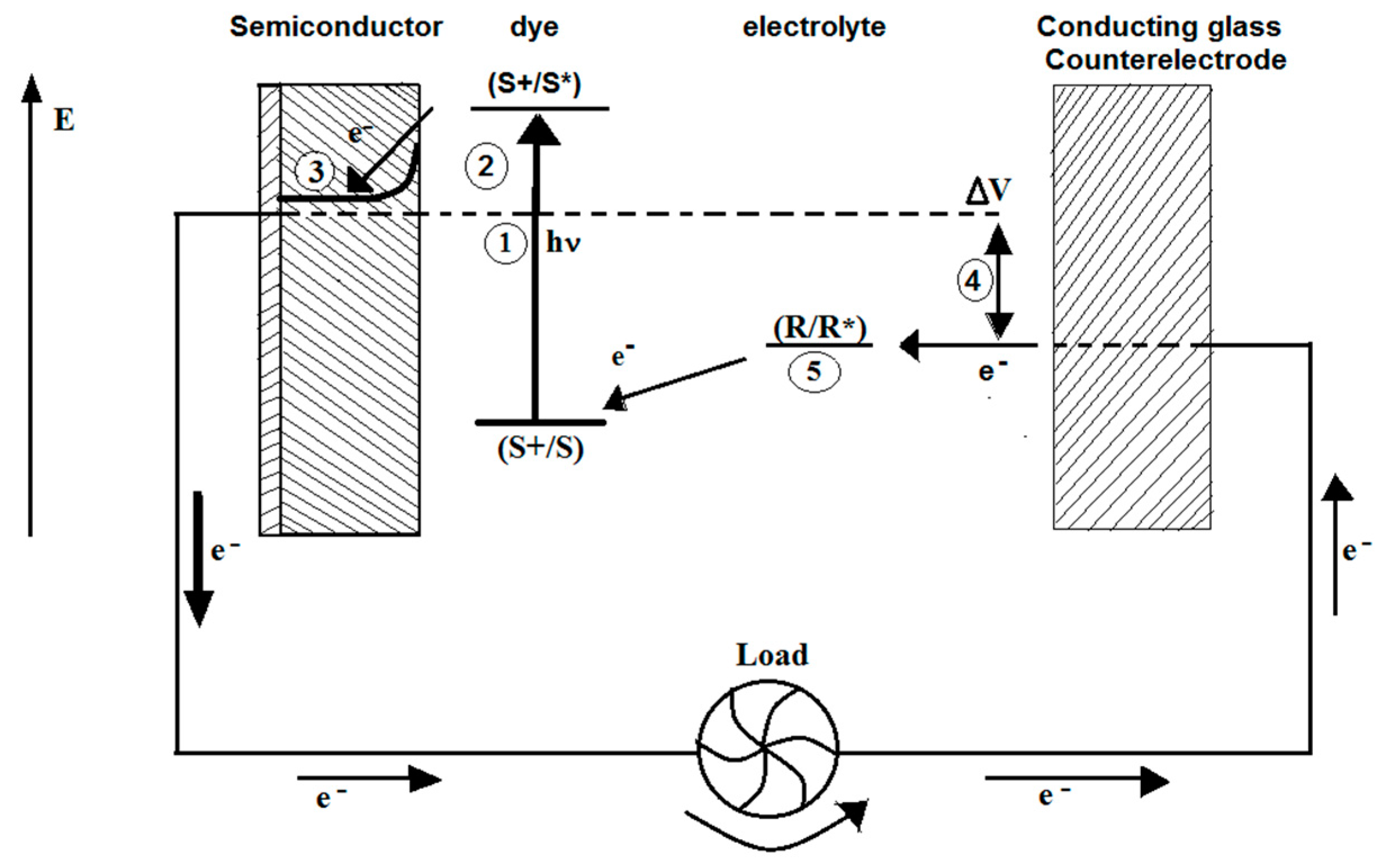
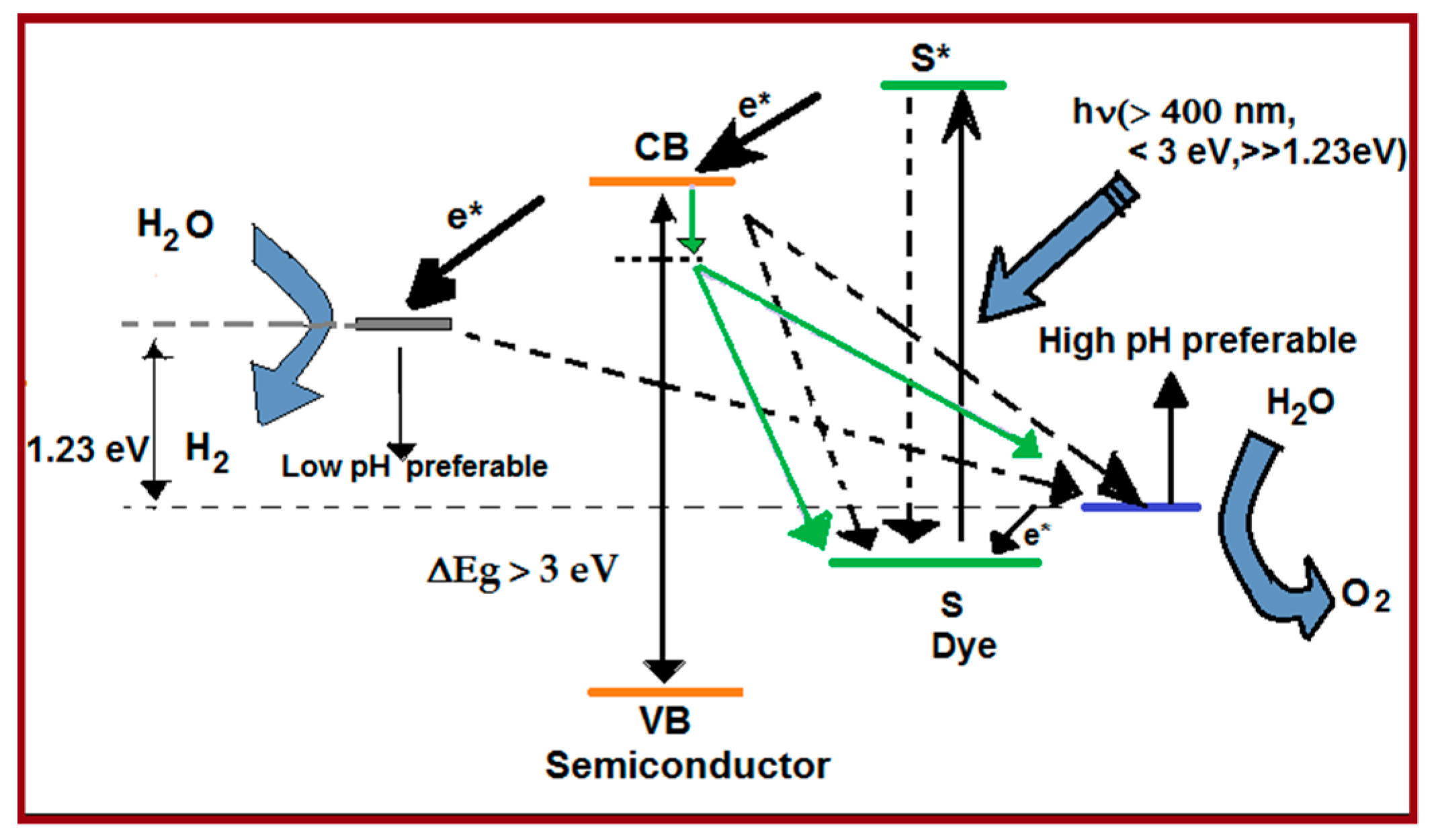
4. Dye-Sensitization Process: Visible Light Active Photocatalyst
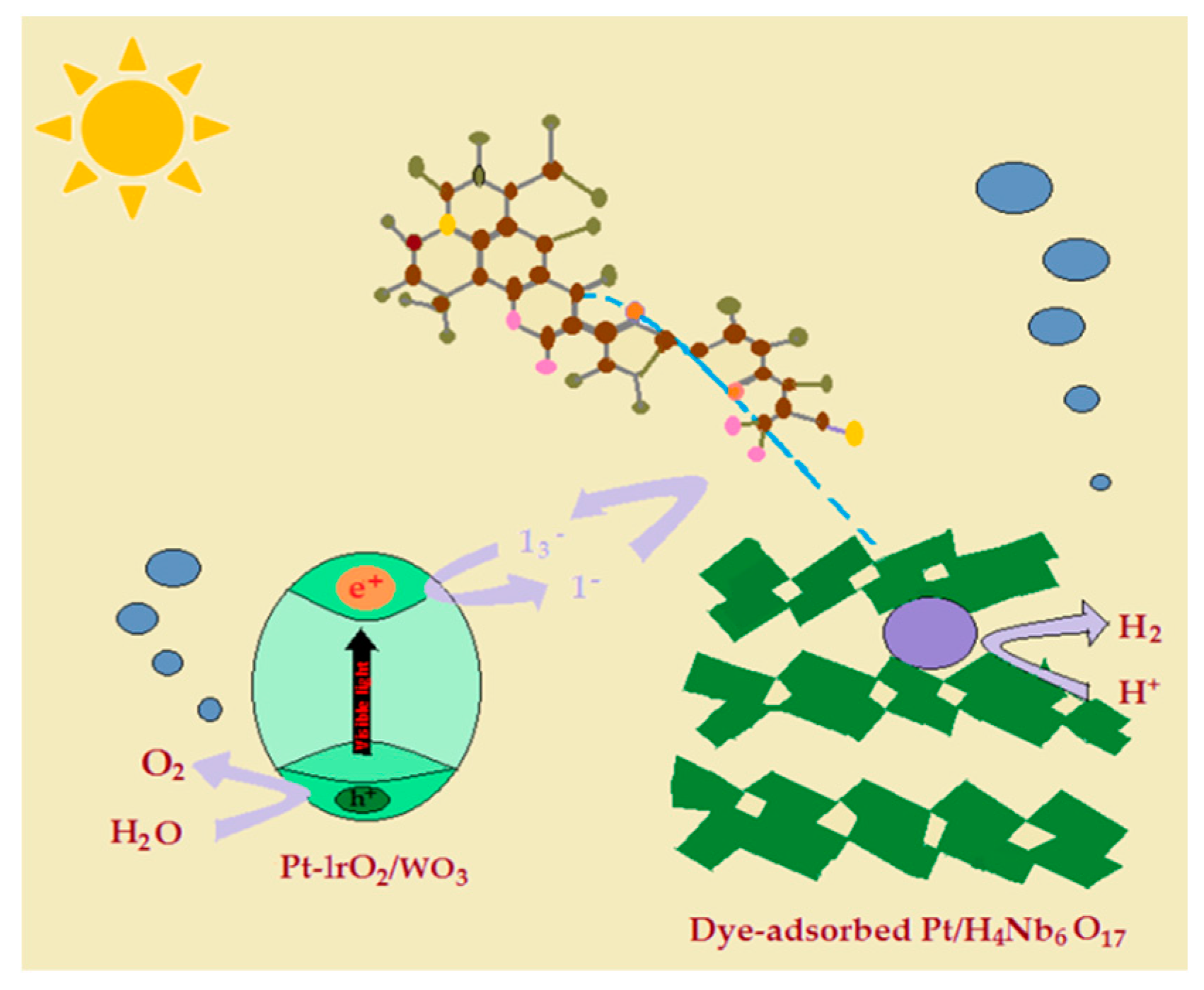
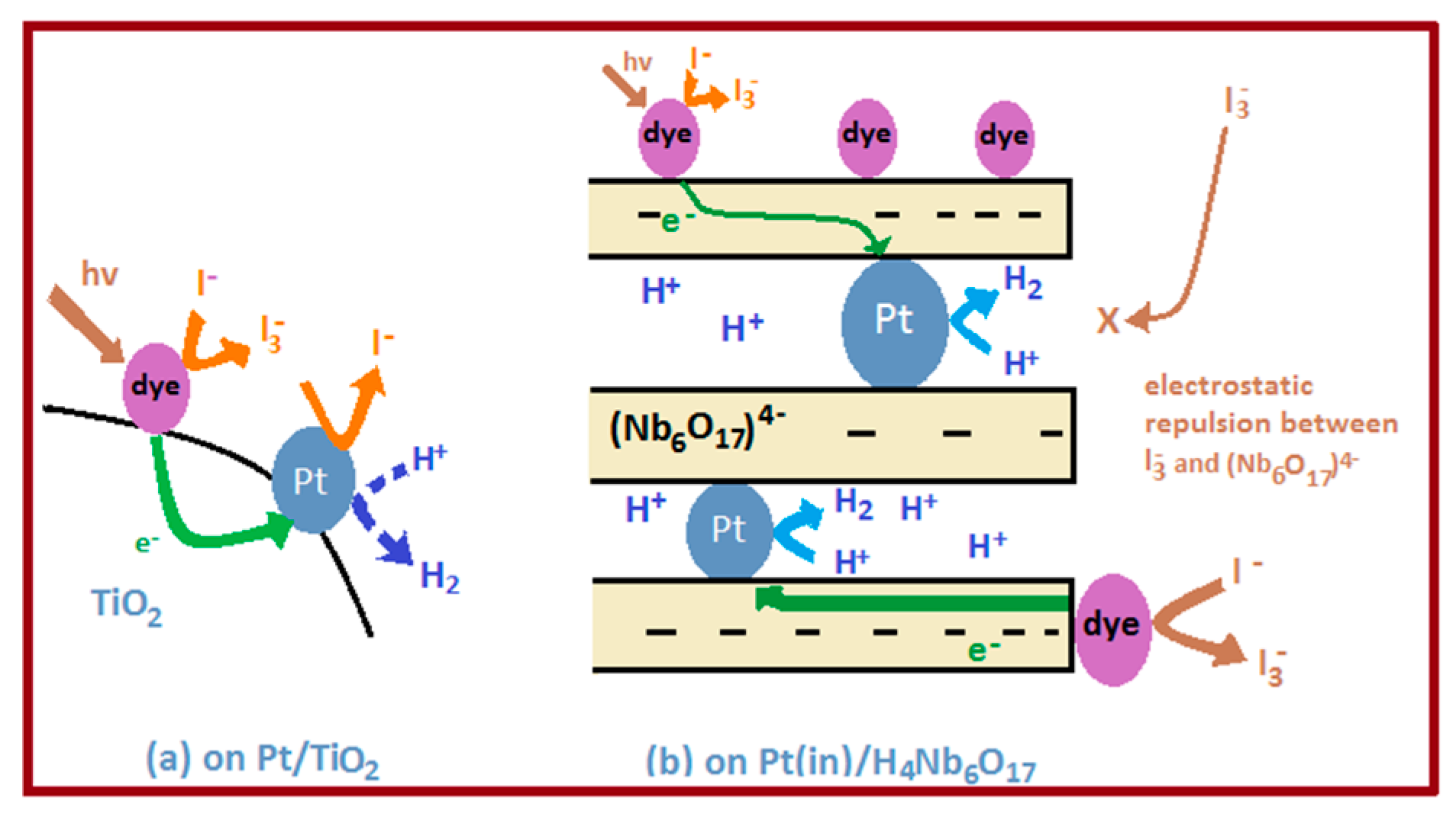
5. Review on Dye-Sensitized Overall Water Splitting
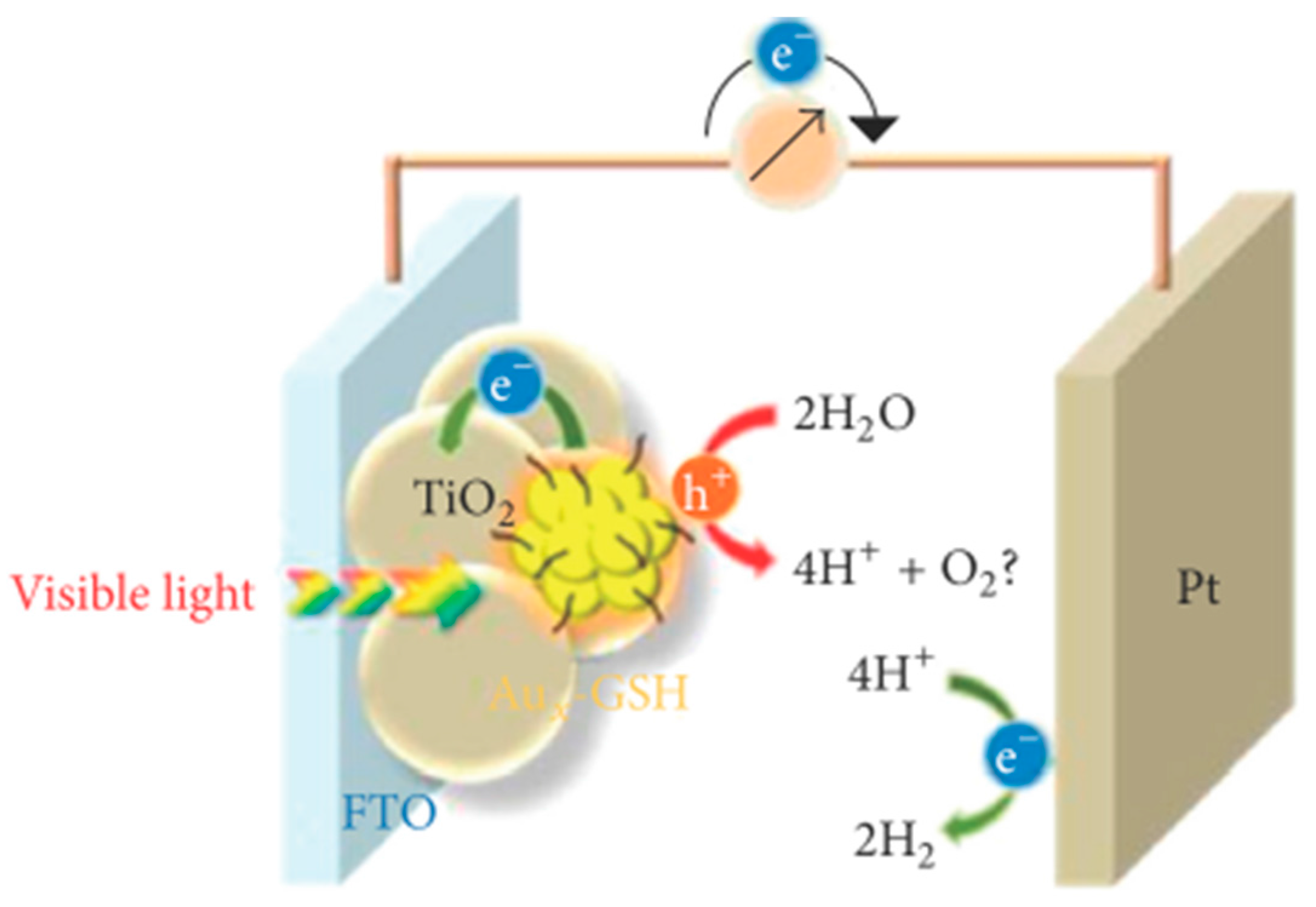
6. Review on Dye-Sensitized Sacrificial Hydrogen Generation
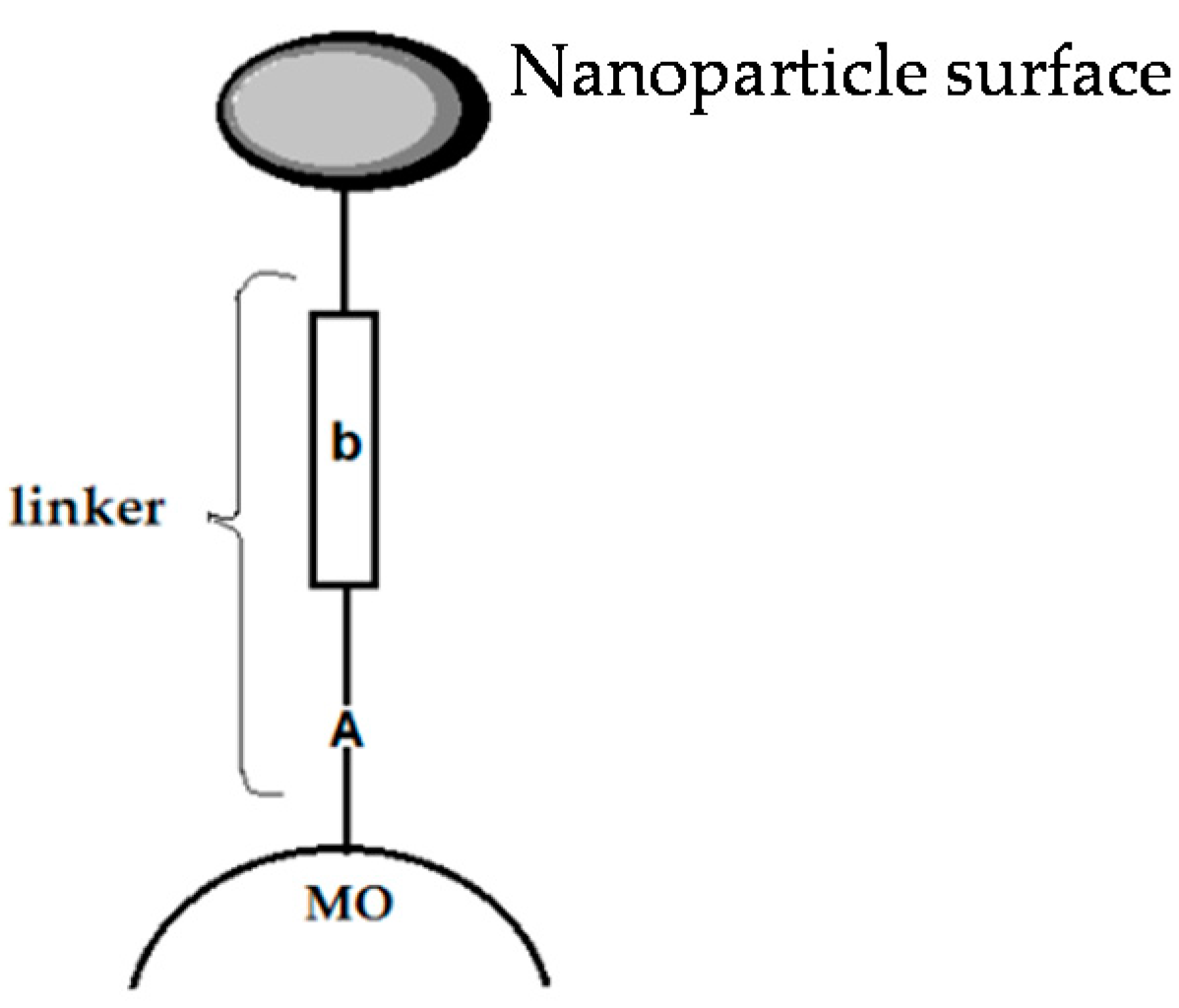
6.1. An Overview of Dyes as Photosensitizers
6.2. An Overview of Sacrificial Agents
6.3. An Overview of Photocatalyst Modification
6.3.1. Role of Co-Catalyst
Noble-Metal Based Co-Catalysts
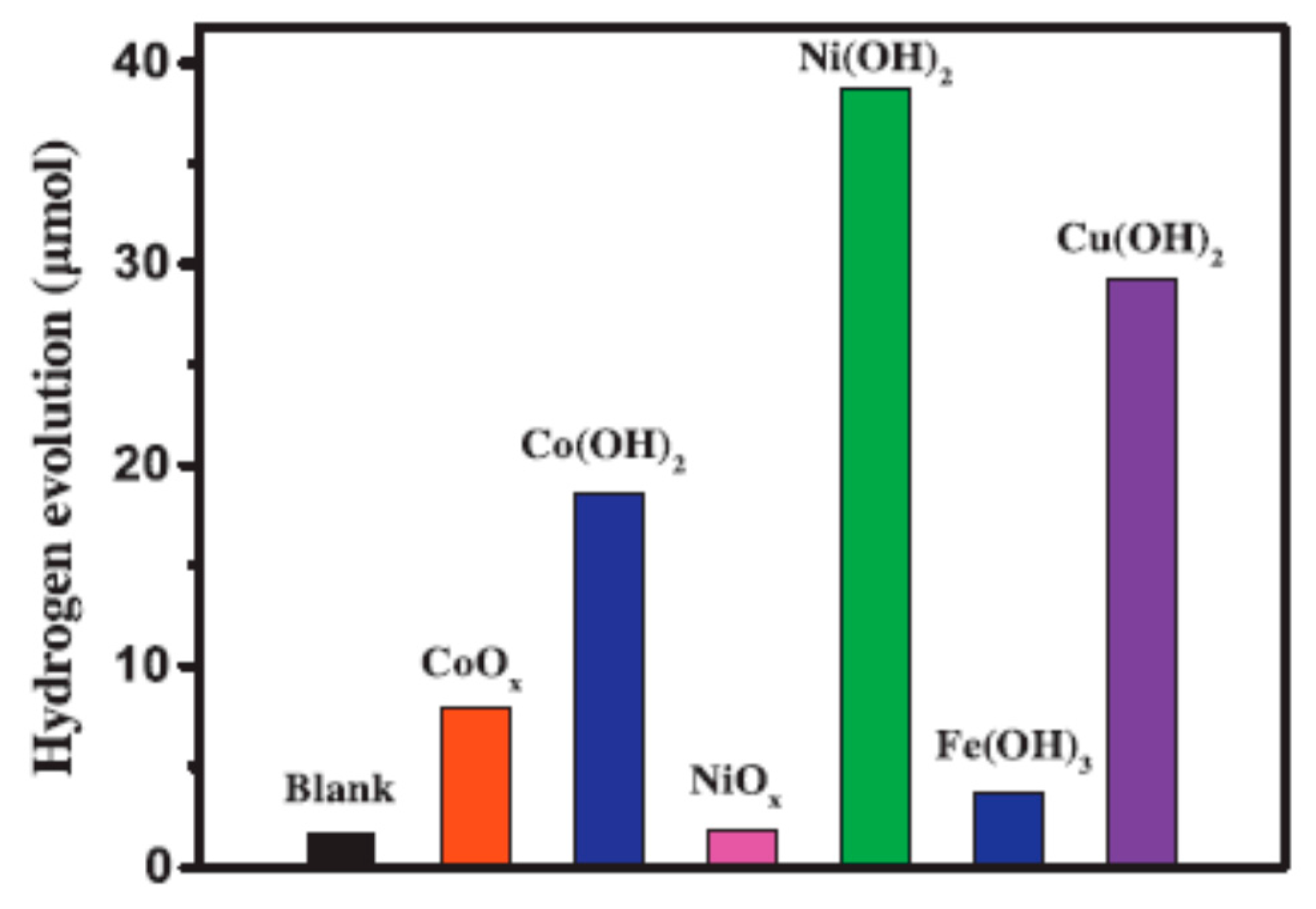
Noble-Metal Free Co-Catalysts
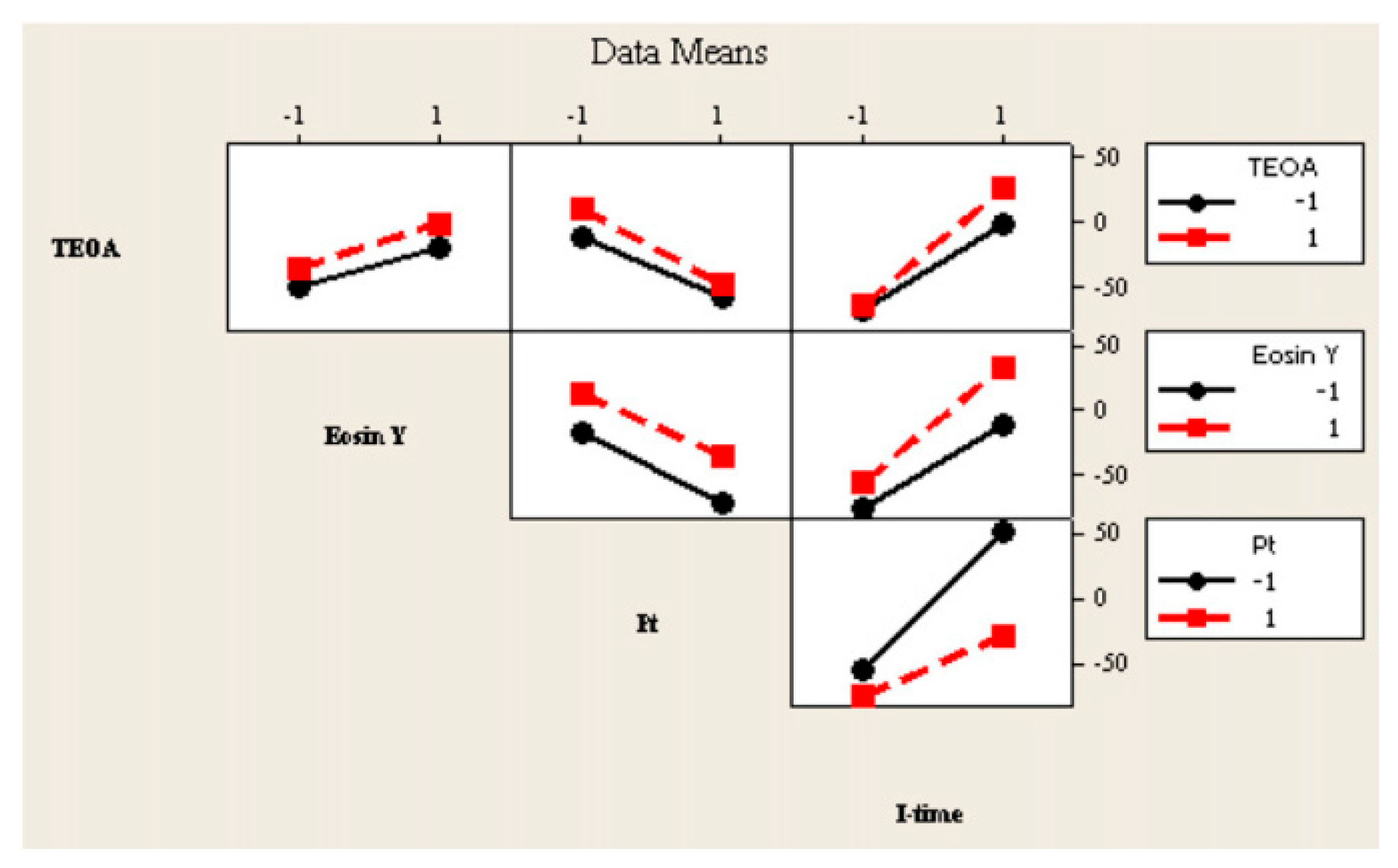
7. Application of Design of Experiment in Photocatalytic Hydrogen Generation
8. Photoreactor Configurations for the Improvement of Water Splitting/Hydrogen Generation
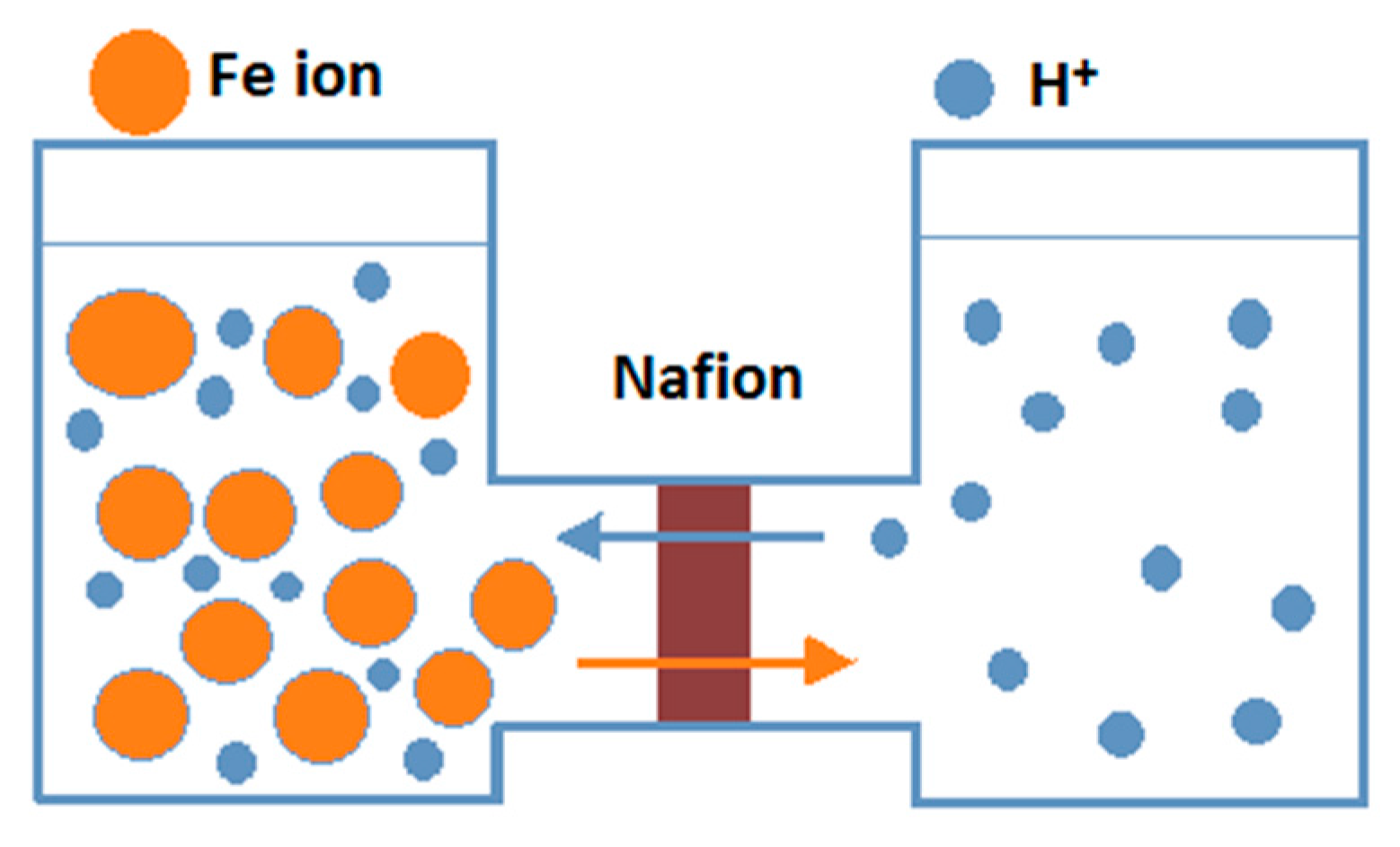
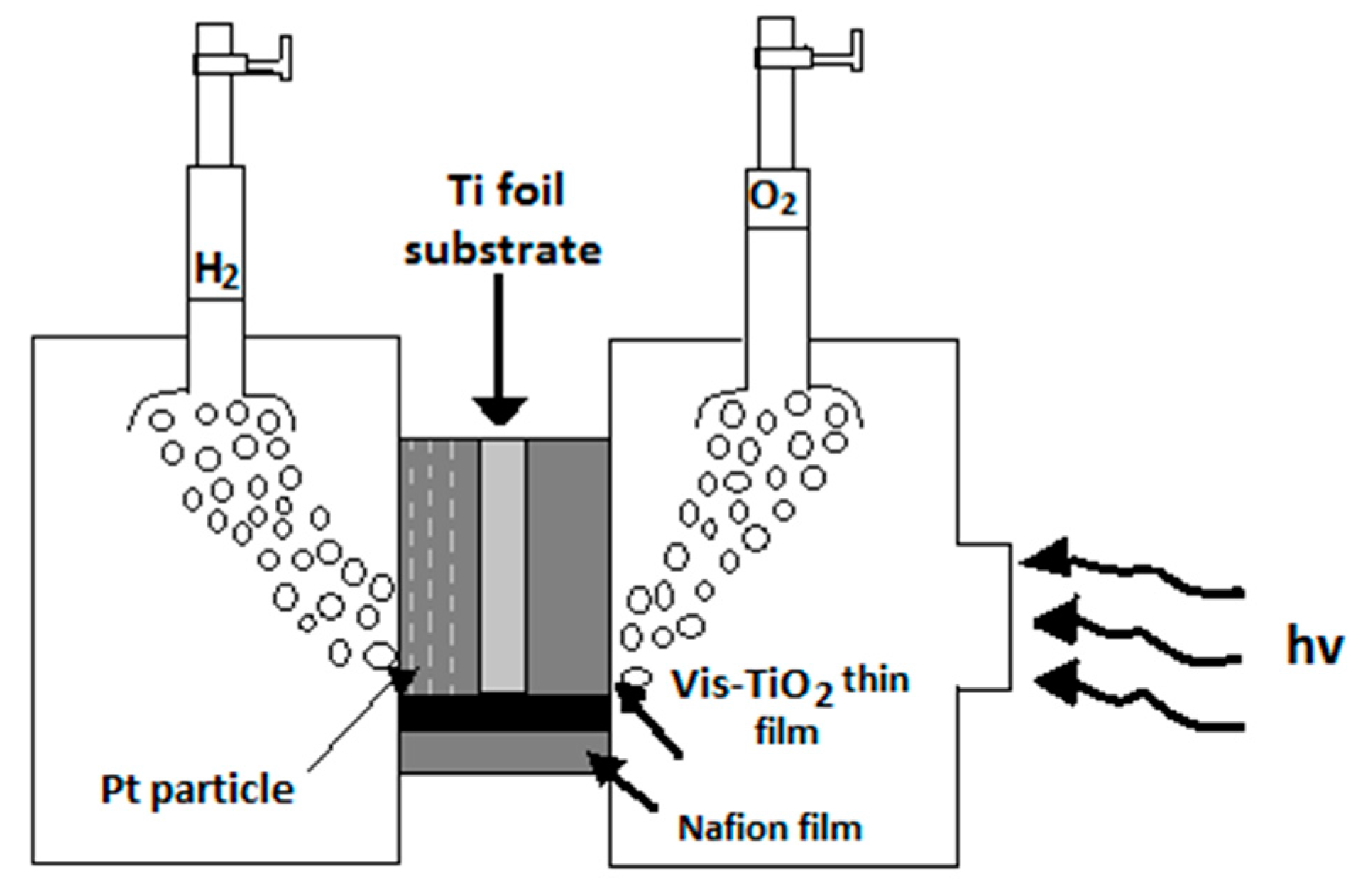
8.1. Photocatalytic Membrane Reactor for Separating Hydrogen and Oxygen
8.2. Dual Bed Photoreactor for Water Splitting
8.3. Self-Mixing Photoreactor for Large-Scale Application
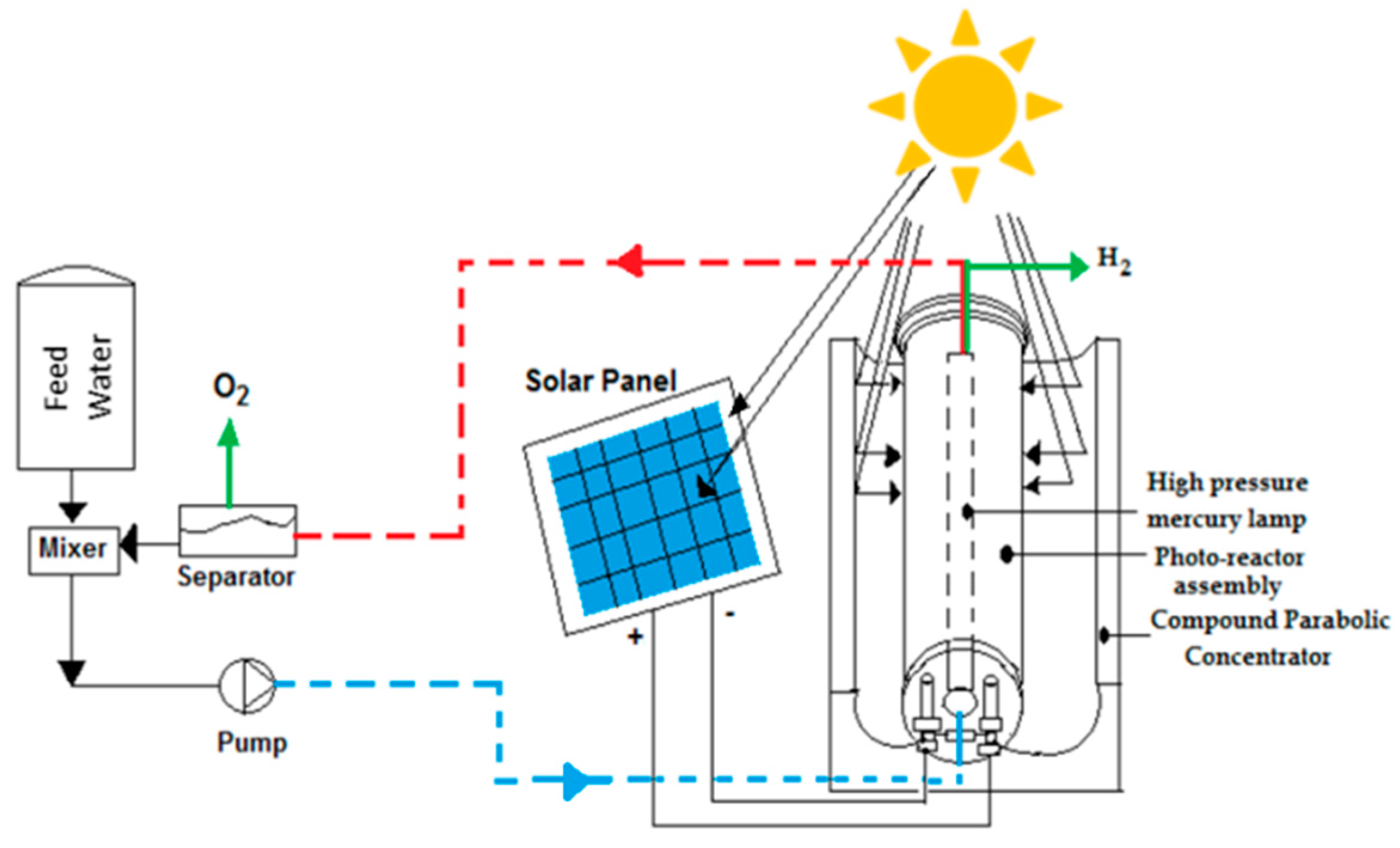
8.4. Solar Photocatalytic Reactor for Water Splitting
9. Conclusions and Future Research Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dunn, S. Hydrogen futures: Toward a sustainable energy system. Int. J. Hydrog. Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- Chowdhury, P. Solar and Visible Light Driven Photocatalysis for Sacrificial Hydrogen Generation and Water Detoxification with Chemically Modified TiO2; Western University: London, ON, Canada, 2012. [Google Scholar]
- Grimes, C.A.; Varghese, O.K.; Ranjan, S. Hydrogen Generation by Water Splitting; Springer: New York, NY, USA, 2008. [Google Scholar]
- The Kelling Curve. Available online: https://scripps.ucsd.edu/programs/keelingcurve/ (accessed on 12 March 2017).
- Chowdhury, P.; Gomaa, H.; Ray, A.K. Factorial design analysis for dye-sensitized hydrogen generation from water. Int. J. Hydrog. Energy 2011, 36, 13442–13451. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Domen, K.; Kudo, A.; Onishi, T. Mechanism of photocatalytic decomposition of water into H2 and O2 over NiO–SrTiO3. J. Catal. 1986, 102, 92–98. [Google Scholar] [CrossRef]
- Polo, A.S.; Itokazu, M.K.; Iha, N.Y.M. Metal complex sensitizers in dye-sensitized solar cells. Coord. Chem. Rev. 2004, 248, 1343–1361. [Google Scholar] [CrossRef]
- Chowdhury, P.; Gomaa, H.; Ray, A.K. Dye-sensitized photocatalyst: A breakthrough in green energy and environmental detoxification. In Sustainable Nanotechnology and the Environment: Advances and Achievements; Shamim, N., Sharma, V.K., Eds.; American Chemical Society: Washington, DC, USA, 2013; Volume 1124, pp. 231–266. [Google Scholar]
- Pei, D.; Luan, J. Development of visible light-responsive sensitized photocatalysts. Int. J. Photoenergy 2012, 2012, 262831. [Google Scholar] [CrossRef]
- Chowdhury, P.; Gomaa, H.; Ray, A.K. Sacrificial hydrogen generation from aqueous triethanolamine with Eosin Y-sensitized Pt/TiO2 photocatalyst in UV, visible and solar light irradiation. Chemosphere 2015, 121, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Salvador, P. Thermodynamic and kinetic considerations about water splitting and competitive reactions in a photoelectrochemical cell. New J. Chem. 1988, 12, 35–43. [Google Scholar]
- Chen, Y.-S.; Kamat, P.V. Glutathione-capped gold nanoclusters as photosensitizers. Visible light-induced hydrogen generation in neutral water. J. Am. Chem. Soc. 2014, 136, 6075–6082. [Google Scholar]
- Lee, J.S. Photocatalytic water splitting under visible light with particulate semiconductor catalysts. Catal. Surv. Asia 2005, 9, 217–227. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. TiO2 photoelectrochemistry and photocatalysis. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Kato, H. Photocatalytic decomposition of water into H2 and O2 over novel photocatalyst K3Ta3Si2O13 with pillared structure consisting of three TaO6 chains. Chem. Lett. 1997, 26, 867–868. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Sakthivel, S.; Shankar, M.V.; Palanichamy, M.; Arabindoo, B.; Bahnemann, D.W.; Murugesan, V. Enhancement of photocatalytic activity by metal deposition: Characterization and photonic efficiency of Pt, Au and Pd deposited on TiO2 catalyst. Water Res. 2004, 38, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Catalysis with TiO2/gold nanocomposites. Effect of metal particle size on the Fermi level equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Green emission to probe photoinduced charging events in ZnO–Au nanoparticles. Charge distribution and Fermi-level equilibration. J. Phys. Chem. B 2003, 107, 7479–7485. [Google Scholar] [CrossRef]
- Jakob, M.; Levanon, H.; Kamat, P.V. Charge distribution between UV-irradiated TiO2 and gold nanoparticles: Determination of shift in the Fermi level. Nano Lett. 2003, 3, 353–358. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Therefore, W.-W.; Kim, K.-J.; Moon, S.-J. Photo-production of hydrogen over the CdS–TiO2 nano-composite particulate films treated with TiCl4. Int. J. Hydrog. Energy 2004, 29, 229–234. [Google Scholar]
- Gurunathan, K.; Maruthamuthu, P.; Sastri, M.V.C. Photocatalytic hydrogen production by dye-sensitized Pt/SnO2 and Pt/SnO2/RuO2 in aqueous methyl viologen solution. Int. J. Hydrog. Energy 1997, 22, 57–62. [Google Scholar] [CrossRef]
- Kudo, A.; Ueda, K.; Kato, H.; Mikami, I. Photocatalytic O2 evolution under visible light irradiation on BiVO4 in aqueous AgNO3 solution. Catal. Lett. 1998, 53, 229–230. [Google Scholar] [CrossRef]
- Kato, H.; Kobayashi, H.; Kudo, A. Role of Ag+ in the band structures and photocatalytic properties of AgMO3 (M: Ta and Nb) with the perovskite structure. J. Phys. Chem. B 2002, 106, 12441–12447. [Google Scholar] [CrossRef]
- Hosogi, Y.; Tanabe, K.; Kato, H.; Kobayashi, H.; Kudo, A. Energy structure and photocatalytic activity of niobates and tantalates containing Sn(II) with a 5s2 electron configuration. Chem. Lett. 2003, 33, 28–29. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Gratzel, M. Encyclopedia of Electrochemistry. In Semiconductor Electrodes and Photoelectronchemistry; Licht, S., Ed.; Wiley-VCH: Weinheim, Germany, 2002; Volume 6, pp. 407–431. [Google Scholar]
- Hagfeldt, A.; Gratzel, M. Molecular Photovoltaics. Acc. Chem. Res. 2000, 33, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, J. Spectral Sensitization of Chemical Effects in Solids. J. Phys. Chem. 1965, 69, 705–713. [Google Scholar] [CrossRef]
- Oregan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Youngblood, W.J.; Lee, S.-H.A.; Maeda, K.; Mallouk, T.E. Visible light water splitting using dye-sensitized oxide semiconductors. Acc. Chem. Res. 2009, 42, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Tsubomura, H.; Matsumura, M.; Nomura, Y.; Amamiya, T. Dye sensitised zinc oxide: Aqueous electrolyte: Platinum photocell. Nature 1976, 261, 402–403. [Google Scholar] [CrossRef]
- Galoppini, E. Linkers for anchoring sensitizers to semiconductor nanoparticles. Coord. Chem. Rev. 2004, 248, 1283–1297. [Google Scholar] [CrossRef]
- Gratzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Abe, R.; Shinmei, K.; Hara, K.; Ohtani, B. Robust dye-sensitized overall water splitting system with two-step photoexcitation of coumarin dyes and metal oxide semiconductors. Chem. Commun. 2009, 24, 3577–3579. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Shinmei, K.; Koumura, N.; Hara, K.; Ohtani, B. Visible-light-induced water splitting based on two-step photoexcitation between dye-sensitized layered niobate and tungsten oxide photocatalysts in the presence of a triiodide/iodide shuttle redox mediator. J. Am. Chem. Soc. 2013, 135, 16872–16884. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Akhtar, M.S.; Park, D.M.; Lee, J.C.; Yang, O.B. Water splitting on Rhodamine-B dye sensitized Co-doped TiO2 catalyst under visible light. Appl. Catal. B 2012, 111–112, 397–401. [Google Scholar] [CrossRef]
- Alibabaei, L.; Sherman, B.D.; Norris, M.R.; Brennaman, M.K.; Meyer, T.J. Visible photoelectrochemical water splitting into H2 and O2 in a dye-sensitized photoelectrosynthesis cell. Proc. Natl. Acad. Sci. USA 2015, 112, 5899–5902. [Google Scholar] [CrossRef] [PubMed]
- Swierk, J.R.; Mallouk, T.E. Design and development of photoanodes for water-splitting dye-sensitized photoelectrochemical cells. Chem. Soc. Rev. 2013, 42, 2357–2387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Swierk, J.R.; Megiatto, J.D.; Sherman, B.; Youngblood, W.J.; Qin, D.; Lentz, D.M.; Moore, A.L.; Moore, T.A.; Gust, D. Improving the efficiency of water splitting in dye-sensitized solar cells by using a biomimetic electron transfer mediator. Proc. Natl. Acad. Sci. USA 2012, 109, 15612–15616. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Nagatomo, M.; Ida, S.; Ishihara, T. Photocatalytic Splitting of Water into Hydrogen and Oxygen on Organic Dye Modified KTa(Zr)O3 Catalyst. Energy Procedia 2012, 22, 53–60. [Google Scholar] [CrossRef]
- Park, J.H.; Bard, A.J. Unassisted Water Splitting from Bipolar Pt/Dye-Sensitized TiO2 Photoelectrode Arrays. Electrochem. Solid State Lett. 2005, 8, G371–G375. [Google Scholar] [CrossRef]
- Li, L.; Duan, L.; Xu, Y.; Gorlov, M.; Hagfeldt, A.; Sun, L. A photoelectrochemical device for visible light driven water splitting by a molecular ruthenium catalyst assembled on dye-sensitized nanostructured TiO2. Chem. Commun. 2010, 46, 7307–7309. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fan, K.; Xu, B.; Gabrielsson, E.; Daniel, Q.; Li, L.; Sun, L. Organic dye-sensitized tandem photoelectrochemical cell for light driven total water splitting. J. Am. Chem. Soc. 2015, 137, 9153–9159. [Google Scholar] [CrossRef] [PubMed]
- Graetzel, M. Artificial photosynthesis: Water cleavage into hydrogen and oxygen by visible light. Acc. Chem. Res. 1981, 14, 376–384. [Google Scholar] [CrossRef]
- Houlding, V.H.; Gratzel, M. Photochemical H2 generation by visible light: Sensitization of TiO2 particles by surface complexation with 8-hydroxyquinoline. J. Am. Chem. Soc. 1983, 105, 5695–5696. [Google Scholar] [CrossRef]
- Furlong, D.N.; Wells, D.; Sasse, W.H.F. Colloidal semiconductors in systems for the sacrificial photolysis of water: Sensitization of TiO2 by adsorption of ruthenium complexes. J. Phys. Chem. 1986, 90, 1107–1115. [Google Scholar] [CrossRef]
- Kim, Y.I.; Keller, S.W.; Krueger, J.S.; Yonemoto, E.H.; Saupe, G.B.; Mallouk, T.E. Photochemical charge transfer and hydrogen evolution mediated by oxide semiconductor particles in zeolite-based molecular assemblies. J. Phys. Chem. B 1997, 101, 2491–2500. [Google Scholar] [CrossRef]
- Lakshminarasimhan, N.; Bae, E.; Choi, W. Enhanced photocatalytic production of H2 on mesoporous TiO2 prepared by template-free method: Role of interparticle charge transfer. J. Phys. Chem. C 2007, 111, 15244–15250. [Google Scholar] [CrossRef]
- Chen, Y.; Mou, Z.; Yin, S.; Huang, H.; Yang, P.; Wang, X.; Du, Y. Graphene enhanced photocatalytic hydrogen evolution performance of dye-sensitized TiO2 under visible light irradiation. Mater. Lett. 2013, 107, 31–34. [Google Scholar] [CrossRef]
- Vinodgopal, K.; Hua, X.; Dahlgren, R.L.; Lappin, A.G.; Patterson, L.K.; Kamat, P.V. Photochemistry of Ru(bpy)2(dcbpy)2+ on Al2O3 and TiO2 surfaces. An insight into the mechanism of photosensitization. J. Phys. Chem. 1995, 99, 10883–10889. [Google Scholar] [CrossRef]
- Lakadamyali, F.; Reisner, E. Photocatalytic H2 evolution from neutral water with a molecular cobalt catalyst on a dye-sensitised TiO2 nanoparticle. Chem. Commun. 2011, 47, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Lakadamyali, F.; Reynal, A.; Kato, M.; Durrant, J.R.; Reisner, E. Electron transfer in dye-sensitized semiconductors modified with molecular cobalt catalysts: Photoreduction of aqueous protons. Chem. Eur. J. 2012, 18, 15464–15475. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.; Choi, W.; Park, J.; Shin, H.S.; Kim, S.B.; Lee, J.S. Effects of surface anchoring groups (carboxylate vs phosphonate) in ruthenium-complex-sensitized TiO2 on visible light reactivity in aqueous suspensions. J. Phys. Chem. B 2004, 108, 14093–14101. [Google Scholar] [CrossRef]
- Bae, E.; Choi, W. Effect of the anchoring group (carboxylate vs phosphonate) in Ru-complex-sensitized TiO2 on hydrogen production under visible light. J. Phys. Chem. B 2006, 110, 14792. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.A.; Hamed, H.A.; Barakat, M.H.; Mohamed, N.R.; Veziroglu, T.N. Enhancement of photocatalytic hydrogen production rate using photosensitized TiO2/RuO2–MV2+. Int. J. Hydrog. Energy 2008, 33, 3264–3269. [Google Scholar] [CrossRef]
- Maitani, M.M.; Zhan, C.; Mochizuki, D.; Suzuki, E.; Wada, Y. Influence of co-existing species on charge transfer in dye-sensitized nanocrystalline oxide semiconductors in aqueous suspension for H2 evolution under visible light. Appl. Catal. B 2014, 147, 770–778. [Google Scholar] [CrossRef]
- Hagiwara, H.; Ono, N.; Inoue, T.; Matsumoto, H.; Ishihara, T. Dye-Sensitizer Effects on a Pt/KTa(Zr)O3 Catalyst for the Photocatalytic Splitting of Water. Angew. Chem. Int. Ed. 2006, 45, 1420–1422. [Google Scholar] [CrossRef] [PubMed]
- Astuti, Y.; Palomares, E.; Haque, S.A.; Durrant, J.R. Triplet state photosensitization of nanocrystalline metal oxide electrodes by zinc-substituted cytochrome c: Application to hydrogen evolution. J. Am. Chem. Soc 2005, 127, 15120–15126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, L.; Zhuang, C.; Peng, T.; Li, R.; Li, X. Highly asymmetric phthalocyanine as a sensitizer of graphitic carbon nitride for extremely efficient photocatalytic H2 production under near-infrared light. ACS Catal. 2014, 4, 162–170. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, T.; Song, S. Recent advances in dye-sensitized semiconductor systems for photocatalytic hydrogen production. J. Mater. Chem. A 2016, 4, 2365–2402. [Google Scholar] [CrossRef]
- Kong, C.; Min, S.; Lu, G. Dye-sensitized NiSx catalyst decorated on graphene for highly efficient reduction of water to hydrogen under visible light irradiation. ACS Catal. 2014, 4, 2763–2769. [Google Scholar] [CrossRef]
- Watanabe, M.; Hagiwara, H.; Iribe, A.; Ogata, Y.; Shiomi, K.; Staykov, A.; Ida, S.; Tanaka, K.; Ishihara, T. Spacer effects in metal-free organic dyes for visible-light-driven dye-sensitized photocatalytic hydrogen production. J. Mater. Chem. A 2014, 2, 12952–12961. [Google Scholar] [CrossRef]
- Latorre-Sanchez, M.; Lavorato, C.; Puche, M.; Fornes, V.; Molinari, R.; Garcia, H. Visible-light photocatalytic hydrogen generation by using dye-sensitized graphene oxide as a photocatalyst. Chem. Eur. J. 2012, 18, 16774–16783. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.; Dong, Y.; Li, S.; Du, Y.; Wang, X.; Yang, P.; Wang, S. Eosin Y functionalized graphene for photocatalytic hydrogen production from water. Int. J. Hydrog. Energy 2011, 36, 8885–8893. [Google Scholar] [CrossRef]
- Li, Y.; Guo, M.; Peng, S.; Lu, G.; Li, S. Formation of multilayer-Eosin Y-sensitized TiO2 via Fe3+ coupling for efficient visible-light photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2009, 34, 5629–5636. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Wang, C. Visible-light-driven photocatalytic H2 evolution from aqueous suspensions of perylenediimide dye-sensitized Pt/TiO2 catalysts. RSC Adv. 2015, 5, 15880–15885. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Zeng, X.; Peng, S. Synergetic effect of metal nickel and graphene as a cocatalyst for enhanced photocatalytic hydrogen evolution via dye sensitization. Sci. Rep. 2015, 5, 10589. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.; Gratzel, M. Photosensitized electron injection in colloidal semiconductors. J. Am. Chem. Soc. 1984, 106, 6557–6564. [Google Scholar] [CrossRef]
- Kamegawa, T.; Matsuura, S.; Seto, H.; Yamashita, H. A Visible-light-harvesting assembly with a sulfocalixarene linker between Dyes and a Pt–TiO2 Photocatalyst. Angew. Chem. Int. Ed. 2013, 52, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Maia, D.L.S.; Pepe, I.; da Silva, A.F.; Silva, L.A. Visible-light-driven photocatalytic hydrogen production over dye-sensitized β-BiTaO4. J. Photochem. Photobiol. A Chem. 2012, 243, 61–64. [Google Scholar] [CrossRef]
- Min, S.; Lu, G. Dye-cosensitized graphene/Pt photocatalyst for high efficient visible light hydrogen evolution. Int. J. Hydrog. Energy 2012, 37, 10564–10574. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, T.; Yu, L.; Li, R.; Li, Q.; Li, Z. Visible/near-infrared-light-induced H2 production over g-C3N4 co-sensitized by organic dye and zinc phthalocyanine derivative. ACS Catal. 2015, 5, 504–510. [Google Scholar] [CrossRef]
- Abe, R.; Sayama, K.; Sugihara, H. Effect of water/acetonitrile ratio on dye-sensitized photocatalytic H2 evolution under visible light irradiation. J. Sol. Energy. Eng. 2005, 127, 413–416. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, X.; Huang, H.; Zhu, M.; Yang, P.; Wang, Y.; Du, Y. Improved superiority by covalently binding dye to graphene for hydrogen evolution from water under visible-light irradiation. J. Phys. Chem. C 2013, 117, 21303–21311. [Google Scholar] [CrossRef]
- Peng, T.; Ke, D.; Cai, P.; Dai, K.; Ma, L.; Zan, L. Influence of different ruthenium(II) bipyridyl complex on the photocatalytic H2 evolution over TiO2 nanoparticles with mesostructures. J. Power Sources 2008, 180, 498–505. [Google Scholar] [CrossRef]
- George, L.; Sappati, S.; Ghosh, P.; Devi, R.N. Surface Site Modulations by Conjugated Organic Molecules To Enhance Visible Light Activity of ZnO Nanostructures in Photocatalytic Water Splitting. J. Phys. Chem. C 2015, 119, 3060–3067. [Google Scholar] [CrossRef]
- He, K.; Li, M.; Guo, L. Preparation and photocatalytic activity of PANI–CdS composites for hydrogen evolution. Int. J. Hydrog. Energy 2012, 37, 755–759. [Google Scholar] [CrossRef]
- Yan, H.; Huang, Y. Polymer composites of carbon nitride and poly(3-hexylthiophene) to achieve enhanced hydrogen production from water under visible light. Chem. Commun. 2011, 47, 4168–4170. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Suzuki, E.; Ishikawa, A.; Moroi, T.; Shiroishi, H.; Kaneko, M. Sensitization of TiO2 particles by dyes to achieve H2 evolution by visible light. J. Photochem. Photobiol. A Chem. 2000, 136, 157–161. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, B.; Zhang, S.; Peng, T. Robust wide visible-light-responsive photoactivity for H2 production over a polymer/polymer heterojunction photocatalyst: The significance of sacrificial reagent. ACS Sustain. Chem. Eng. 2015, 3, 1501–1509. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Lu, G. Visible-light-induced photocatalytic hydrogen generation on dye-sensitized multiwalled carbon nanotube/Pt catalyst. J. Phys. Chem. C 2007, 111, 11494–11499. [Google Scholar] [CrossRef]
- Min, S.; Lu, G. Dye-sensitized reduced graphene oxide photocatalysts for highly efficient visible-light-driven water reduction. J. Phys. Chem. C 2011, 115, 13938–13945. [Google Scholar] [CrossRef]
- Min, S.; Lu, G. Promoted photoinduced charge separation and directional electron transfer over dispersible xanthene dyes sensitized graphene sheets for efficient solar H2 evolution. Int. J. Hydrog. Energy 2013, 38, 2106–2116. [Google Scholar] [CrossRef]
- Wang, D.; Huang, J.; Li, X.; Yang, P.; Du, Y.; Goh, C.M.; Lu, C. Photocatalytic H2 production under visible-light irradiation based on covalent attachment of manganese phthalocyanine to graphene. J. Mater. Chem. A 2015, 3, 4195–4202. [Google Scholar] [CrossRef]
- Malekshoar, G.; Pal, K.; He, Q.; Yu, A.; Ray, A.K. Enhanced solar photocatalytic degradation of phenol with coupled graphene-based titanium dioxide and zinc oxide. Ind. Eng. Chem. Res. 2014, 53, 18824–18832. [Google Scholar] [CrossRef]
- Potje-Kamloth, K. Semiconductor junction gas sensors. Chem. Rev. 2008, 108, 367–399. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-P.; Ruan, L.-W.; Barber, J.; Loo, S.C.J.; Xue, C. Hetero-nanostructured suspended photocatalysts for solar-to-fuel conversion. Energy Environ. Sci. 2014, 7, 3934–3951. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Sayama, K.; Arakawa, H. Dye-sensitized photocatalysts for efficient hydrogen production from aqueous I− solution under visible light irradiation. J. Photochem. Photobiol. A Chem. 2004, 166, 115–122. [Google Scholar] [CrossRef]
- Abe, R.; Sayama, K.; Arakawa, H. Efficient hydrogen evolution from aqueous mixture of I− and acetonitrile using a merocyanine dye-sensitized Pt/TiO2 photocatalyst under visible light irradiation. Chem. Phys. Lett. 2002, 362, 441–444. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, X.; Lu, G.; Li, S. Improved quantum yield for photocatalytic hydrogen generation under visible light irradiation over eosin sensitized TiO2—Investigation of different noble metal loading. J. Mol. Catal. A Chem. 2006, 259, 275–280. [Google Scholar] [CrossRef]
- Yan, Z.; Yu, X.; Zhang, Y.; Jia, H.; Sun, Z.; Du, P. Enhanced visible light-driven hydrogen production from water by a noble-metal-free system containing organic dye-sensitized titanium dioxide loaded with nickel hydroxide as the cocatalyst. Appl. Catal. B 2014, 160, 173–178. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, X.; Li, Y.; Li, S.; Lu, G. 5.1% Apparent quantum efficiency for stable hydrogen generation over eosin-sensitized CuO/TiO2 photocatalyst under visible light irradiation. Catal. Commun. 2007, 8, 1267–1273. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Kang, S.; Li, G.; Mu, J. Visible light photocatalytic activity of CuO/Cr2O3 co-loaded multiwalled carbon nanotubes sensitized with eosin Y for hydrogen evolution from water. Ceram. Int. 2014, 40, 10171–10176. [Google Scholar] [CrossRef]
- Kong, C.; Min, S.; Lu, G. Dye-sensitized cobalt catalysts for high efficient visible light hydrogen evolution. Int. J. Hydrog. Energy 2014, 39, 4836–4844. [Google Scholar] [CrossRef]
- Yin, M.; Ma, S.; Wu, C.; Fan, Y. A noble-metal-free photocatalytic hydrogen production system based on cobalt(III) complex and eosin Y-sensitized TiO2. RSC Adv. 2015, 5, 1852–1858. [Google Scholar] [CrossRef]
- Malekshoar, G.; Ray, A.K. In situ grown molybdenum sulfide on TiO2 for dye-sensitized solar photocatalytic hydrogen generation. Chem. Eng. Sci. 2016, 152, 35–44. [Google Scholar] [CrossRef]
- Min, S.; Lu, G. Sites for high efficient photocatalytic hydrogen evolution on a limited-layered MoS2cocatalyst confined on graphene sheets—The role of graphene. J. Phys. Chem. C 2012, 116, 25415–25424. [Google Scholar] [CrossRef]
- Feilizadeh, M.; Mul, G.; Vossoughi, M. E. coli inactivation by visible light irradiation using a Fe–Cd/TiO2 photocatalyst: Statistical analysis and optimization of operating parameters. Appl. Catal. B 2015, 168, 441–447. [Google Scholar] [CrossRef]
- Chen, J.; Luo, H.; Shi, H.; Li, G.; An, T. Anatase TiO2 nanoparticles–carbon nanotubes composite: Optimization synthesis and the relationship of photocatalytic degradation activity of acyclovir in water. Appl. Catal. A 2014, 485, 188–195. [Google Scholar] [CrossRef]
- Antony, J. Design of Experiments for Engineers and Scientists; Elsevier: New York, NY, USA, 2014. [Google Scholar]
- Ray, A.K. Photocatalytic reactor configurations for water purification: Experimentation and modeling. Adv. Chem. Eng. 2009, 36, 145–184. [Google Scholar]
- Chen, D.; Ray, A.K. Photodegradation kinetics of 4-nitrophenol in TiO2 suspension. Water Res. 1998, 32, 3223–3234. [Google Scholar] [CrossRef]
- Assink, J.W.; Koster, T.P.M.; Slaager, J.M. Fotokatalytische Oxydatie Voor Afvalwaterbehandeling, Internal Report Ref. No. 93-137; Instituut voor Milieu en Energie-technologie (TNO): Apeldoorn, The Netherlands, June 2014.
- Ray, A.K.; Beenackers, A.A.C.M. Development of a new photocatalytic reactor for water purification. Catal. Today 1998, 40, 73–83. [Google Scholar] [CrossRef]
- Ray, A.K. Design, modelling and experimentation of a new large-scale photocatalytic reactor for water treatment. Chem. Eng. Sci. 1999, 54, 3113–3125. [Google Scholar] [CrossRef]
- Xing, Z.; Zong, X.; Pan, J.; Wang, L. On the engineering part of solar hydrogen production from water splitting: Photoreactor design. Chem. Eng. Sci. 2013, 104, 125–146. [Google Scholar] [CrossRef]
- Molinari, R.; Marino, T.; Argurio, P. Photocatalytic membrane reactors for hydrogen production from water. Int. J. Hydrog. Energy 2014, 39, 7247–7261. [Google Scholar] [CrossRef]
- Yu, S.-C.; Huang, C.-W.; Liao, C.-H.; Wu, J.C.S.; Chang, S.-T.; Chen, K.-H. A novel membrane reactor for separating hydrogen and oxygen in photocatalytic water splitting. J. Membr. Sci. 2011, 382, 291–299. [Google Scholar] [CrossRef]
- Lo, C.-C.; Huang, C.-W.; Liao, C.-H.; Wu, J.C.S. Novel twin reactor for separate evolution of hydrogen and oxygen in photocatalytic water splitting. Int. J. Hydrog. Energy 2010, 35, 1523–1529. [Google Scholar] [CrossRef]
- Kitano, M.; Tsujimaru, K.; Anpo, M. Decomposition of water in the separate evolution of hydrogen and oxygen using visible light-responsive TiO2 thin film photocatalysts: Effect of the work function of the substrates on the yield of the reaction. Appl. Catal. A 2006, 314, 179–183. [Google Scholar] [CrossRef]
- Sasaki, Y.; Kato, H.; Kudo, A. [Co(bpy)3]3+/2+ and [Co(phen)3]3+/2+ electron mediators for overall water splitting under sunlight irradiation using Z-scheme photocatalyst system. J. Am. Chem. Soc. 2013, 135, 5441–5449. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zheng, C.L.; Liu, Y.L.; Guo, L.J. A novel dual-bed photocatalytic water splitting system for hydrogen production. Int. J. Hydrog. Energy 2011, 36, 7405–7409. [Google Scholar] [CrossRef]
- Bae, S.W.; Ji, S.M.; Hong, S.J.; Jang, J.W.; Lee, J.S. Photocatalytic overall water splitting with dual-bed system under visible light irradiation. Int. J. Hydrog. Energy 2009, 34, 3243–3249. [Google Scholar] [CrossRef]
- Huang, C.; Yao, W.; Ali, T.; Muradov, N. Development of efficient photoreactors for solar hydrogen production. Solar Energy 2011, 85, 19–27. [Google Scholar] [CrossRef]
- Jing, D.; Liu, H.; Zhang, X.; Zhao, L.; Guo, L. Photocatalytic hydrogen production under direct solar light in a CPC based solar reactor: Reactor design and preliminary results. Energy Convers. Manag. 2009, 50, 2919–2926. [Google Scholar] [CrossRef]
- Jing, D.; Guo, L.; Zhao, L.; Zhang, X.; Liu, H.; Li, M.; Shen, S.; Liu, G.; Hu, X.; Zhang, X. Efficient solar hydrogen production by photocatalytic water splitting: From fundamental study to pilot demonstration. Int. J. Hydrog. Energy 2010, 35, 7087–7097. [Google Scholar] [CrossRef]
- Priya, R.; Kanmani, S. Design of pilot-scale solar photocatalytic reactor for the generation of hydrogen from alkaline sulfide wastewater of sewage treatment plant. Environ. Technol. 2013, 34, 2817–2823. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, E.; Dincer, I.; Naterer, G.F. Exergy and environmental impact assessment of solar photoreactors for catalytic hydrogen production. Chem. Eng. J. 2012, 213, 330–337. [Google Scholar] [CrossRef]


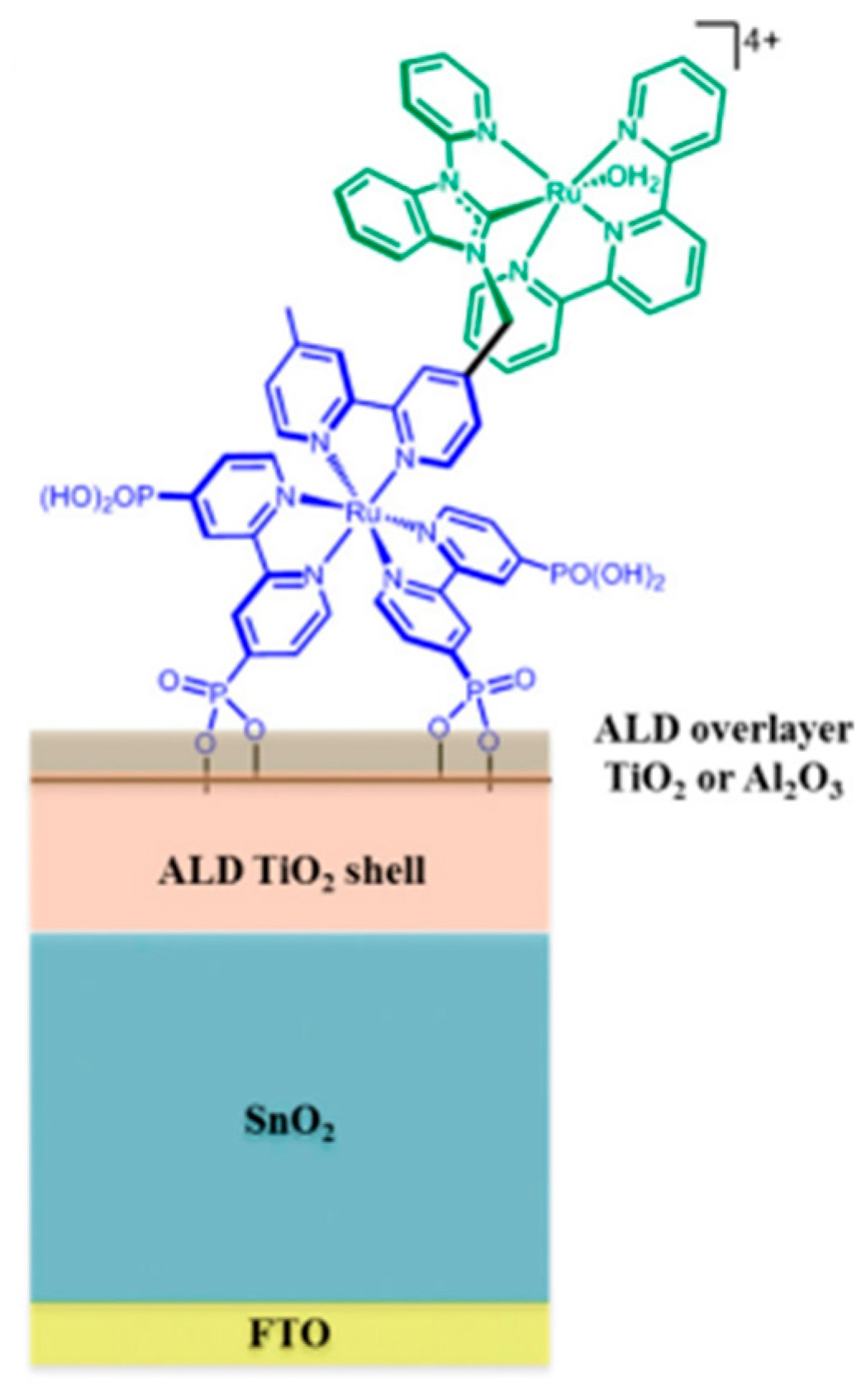
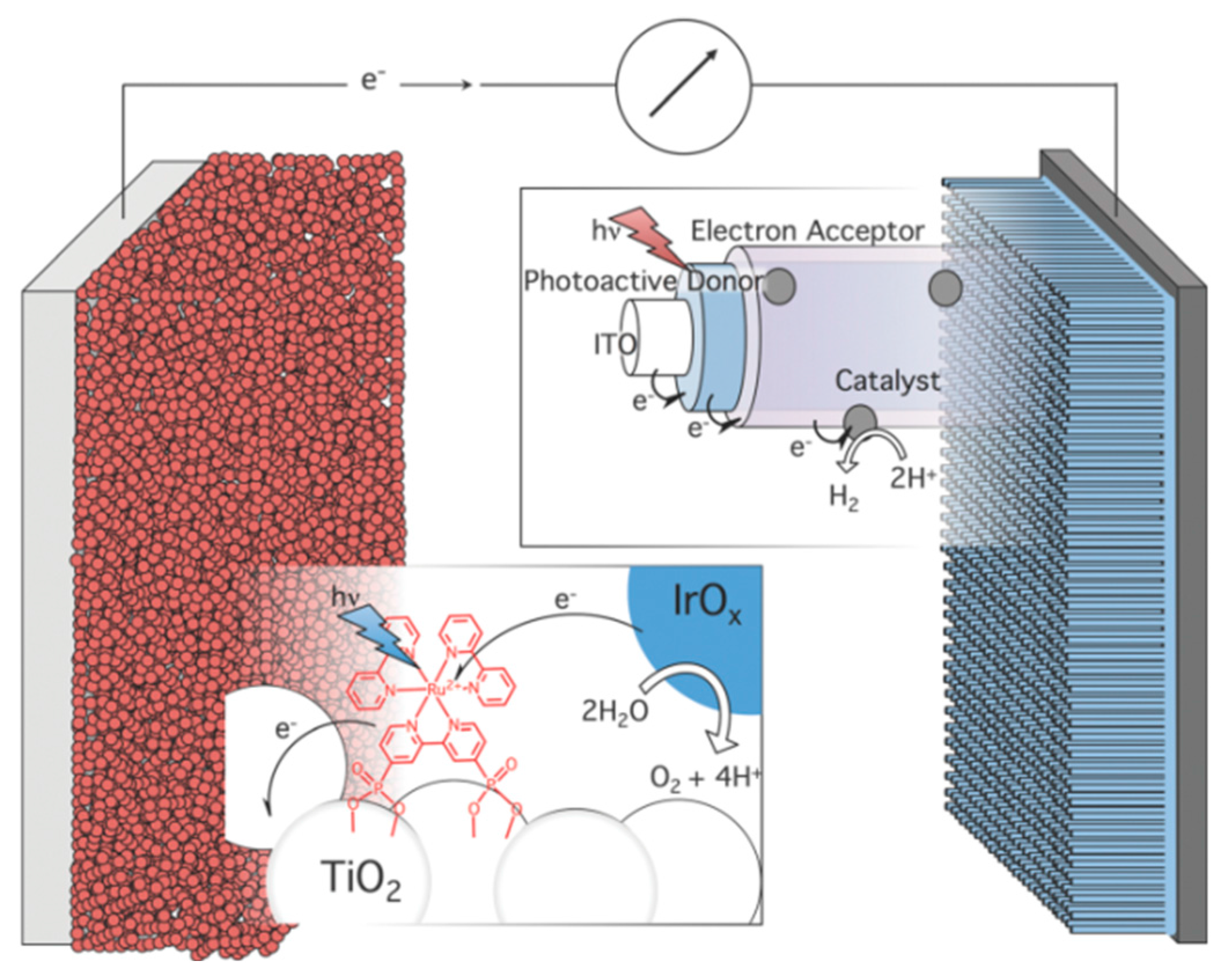
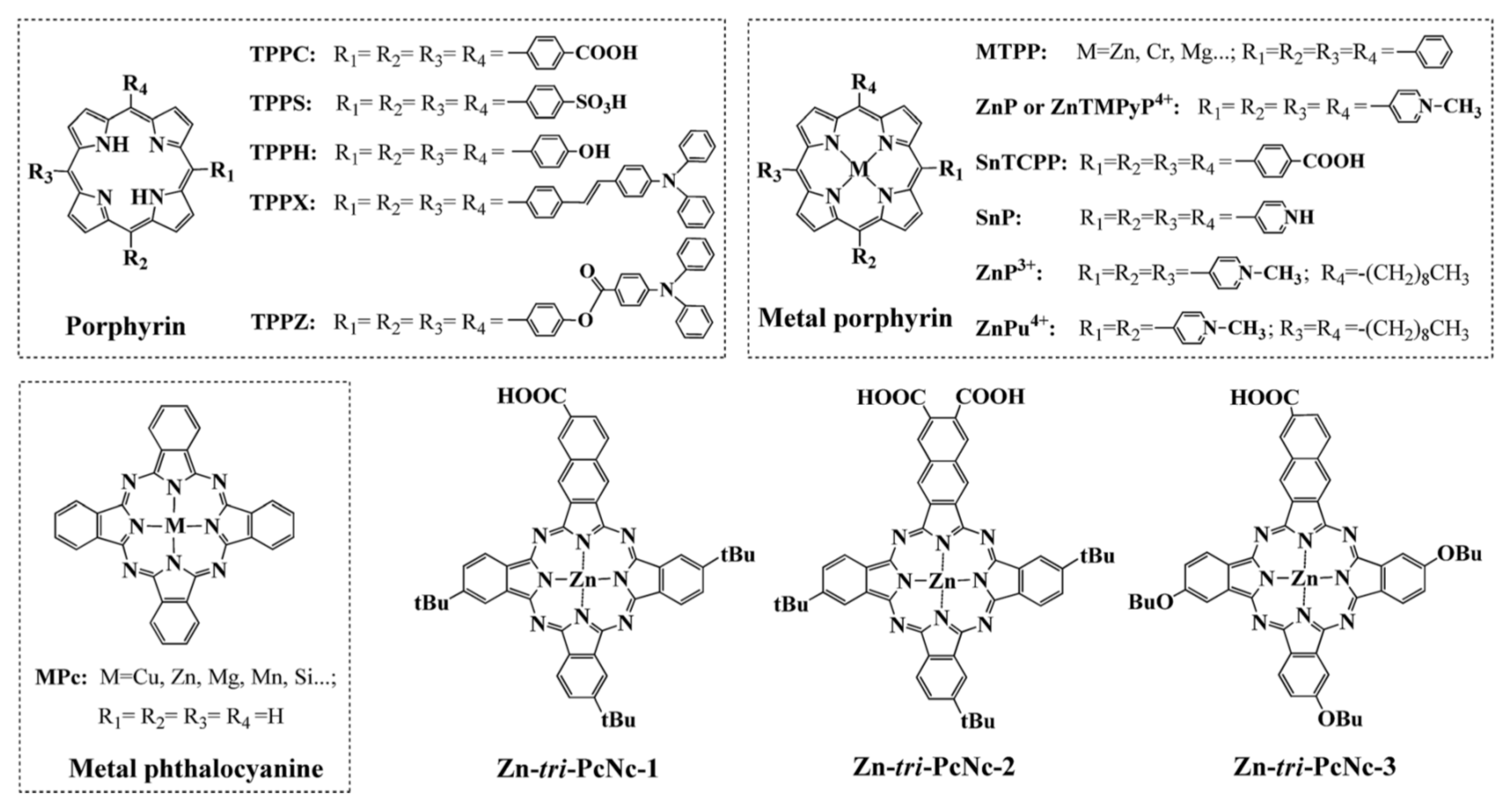
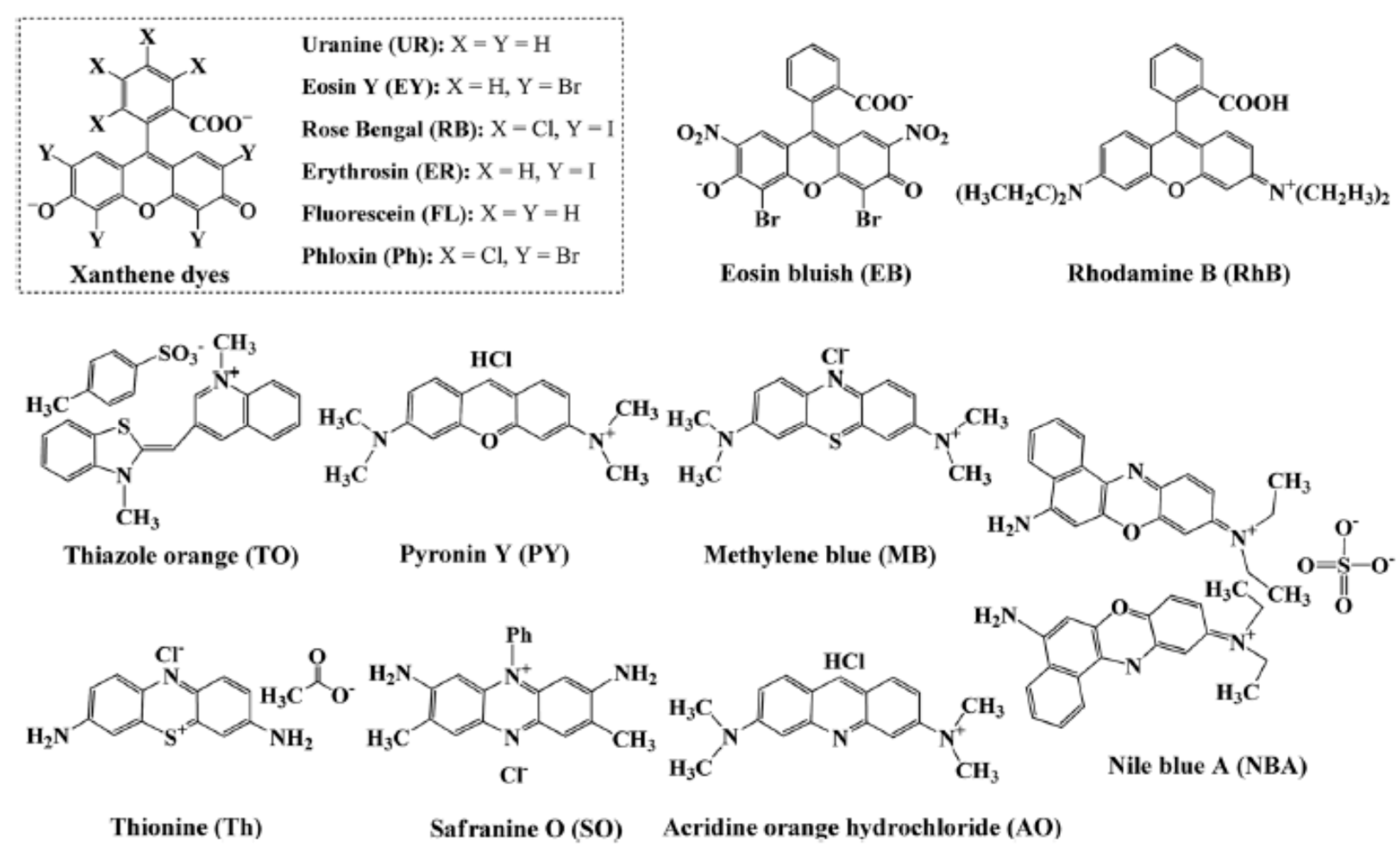

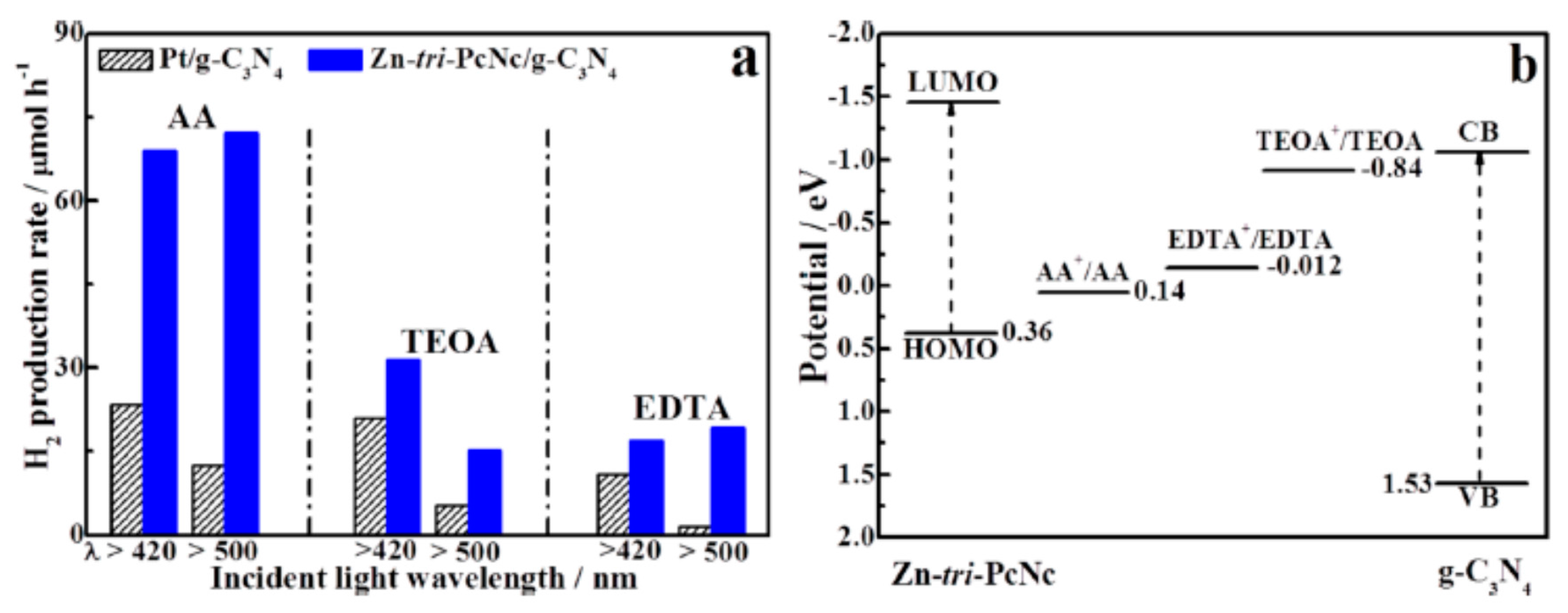
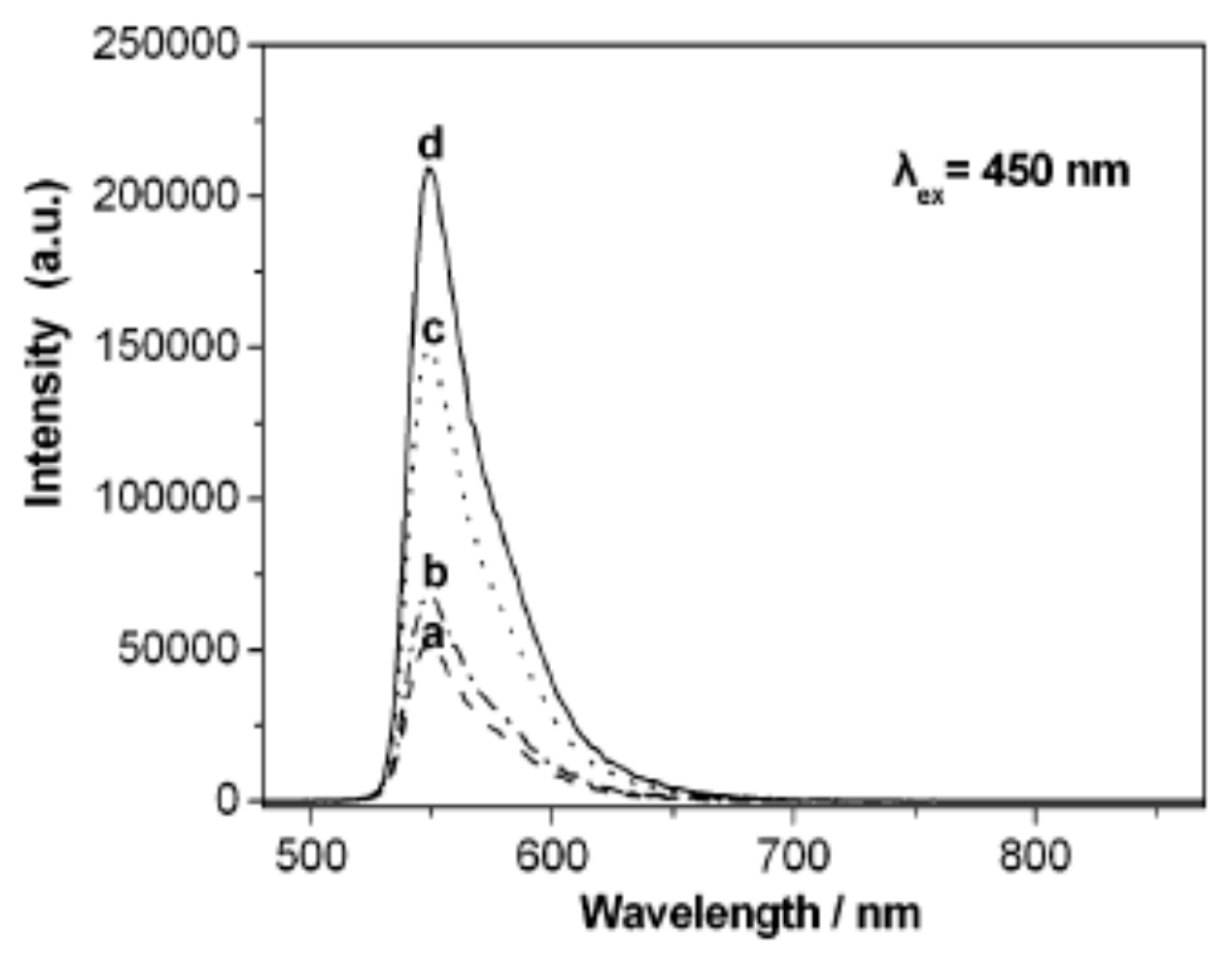
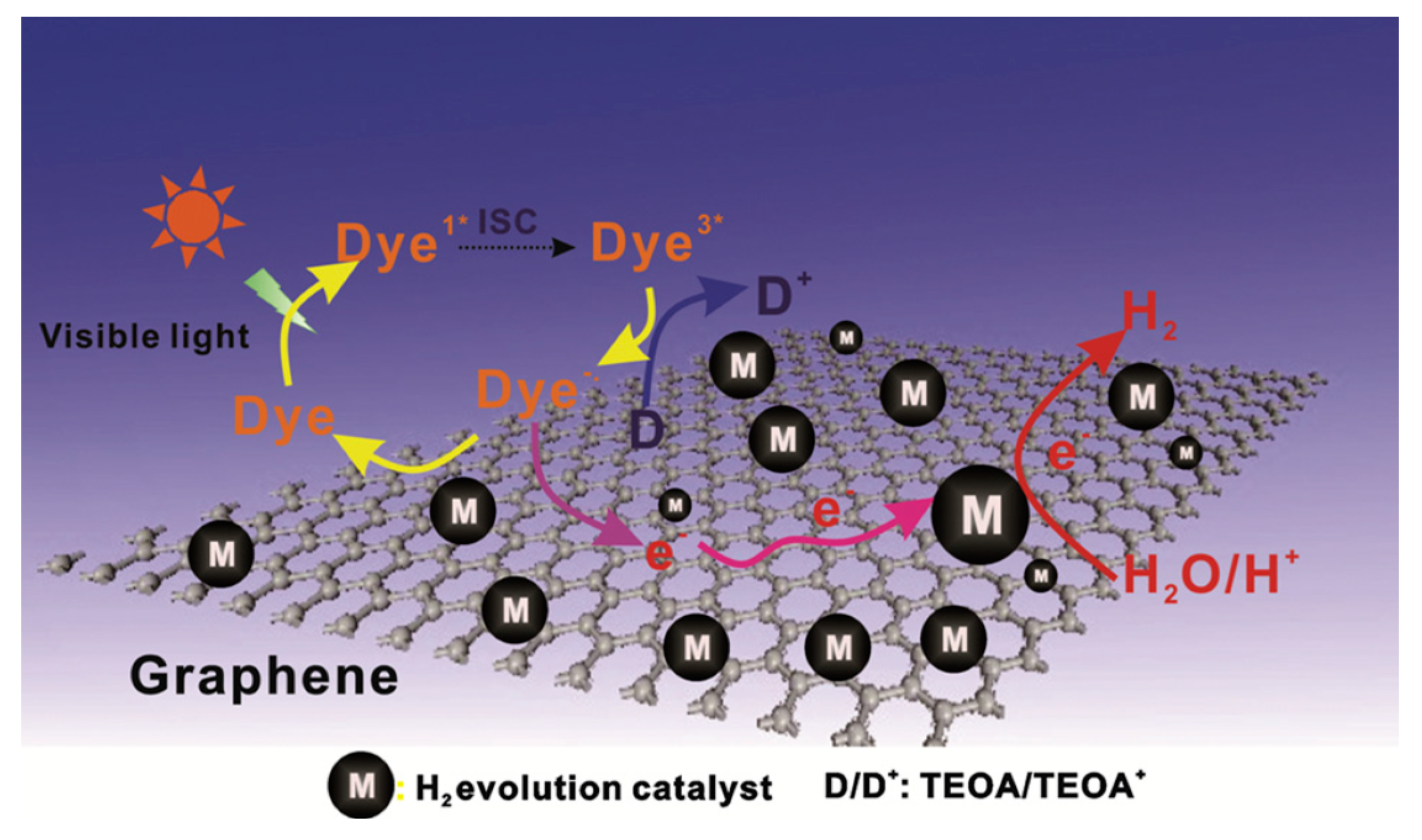
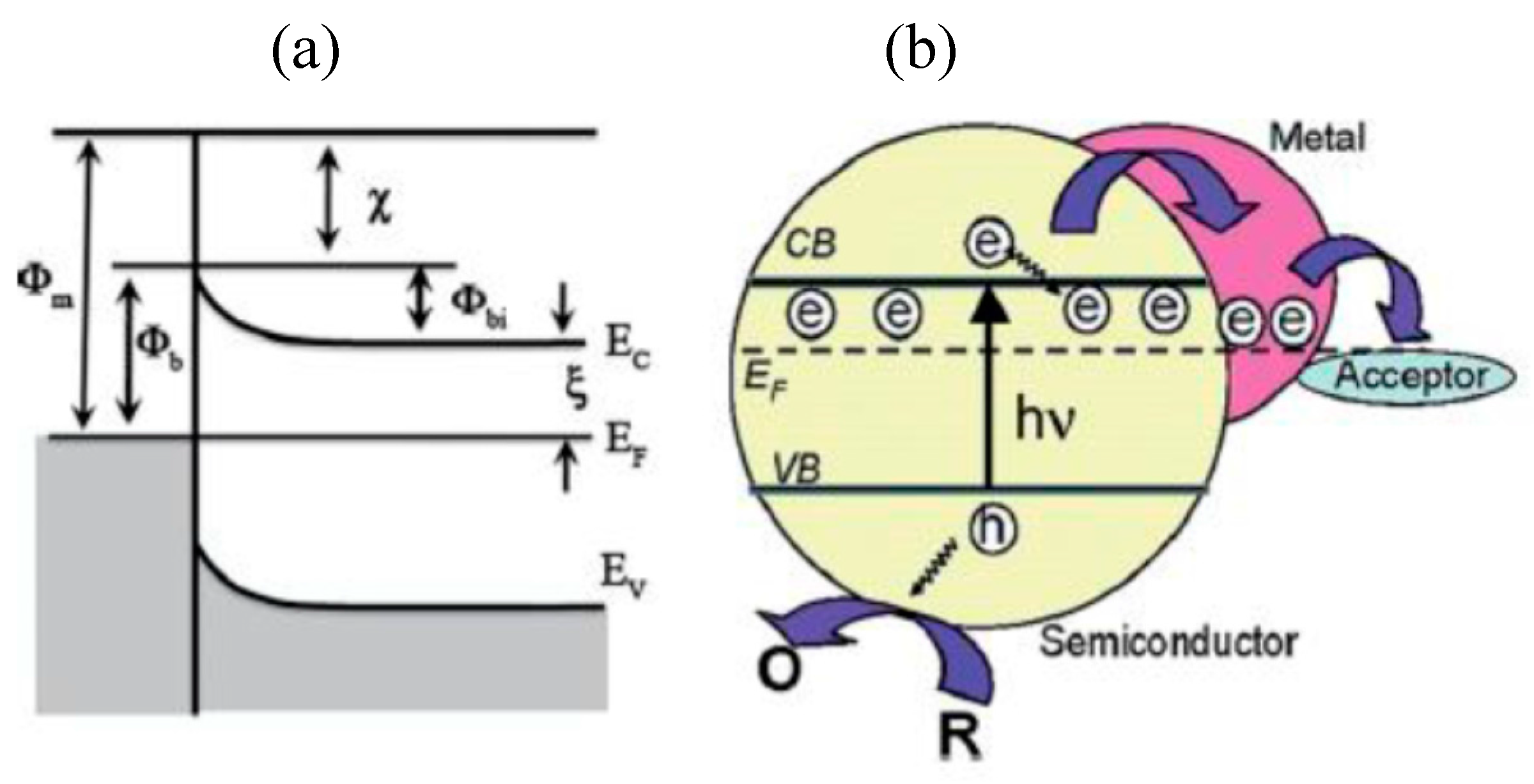

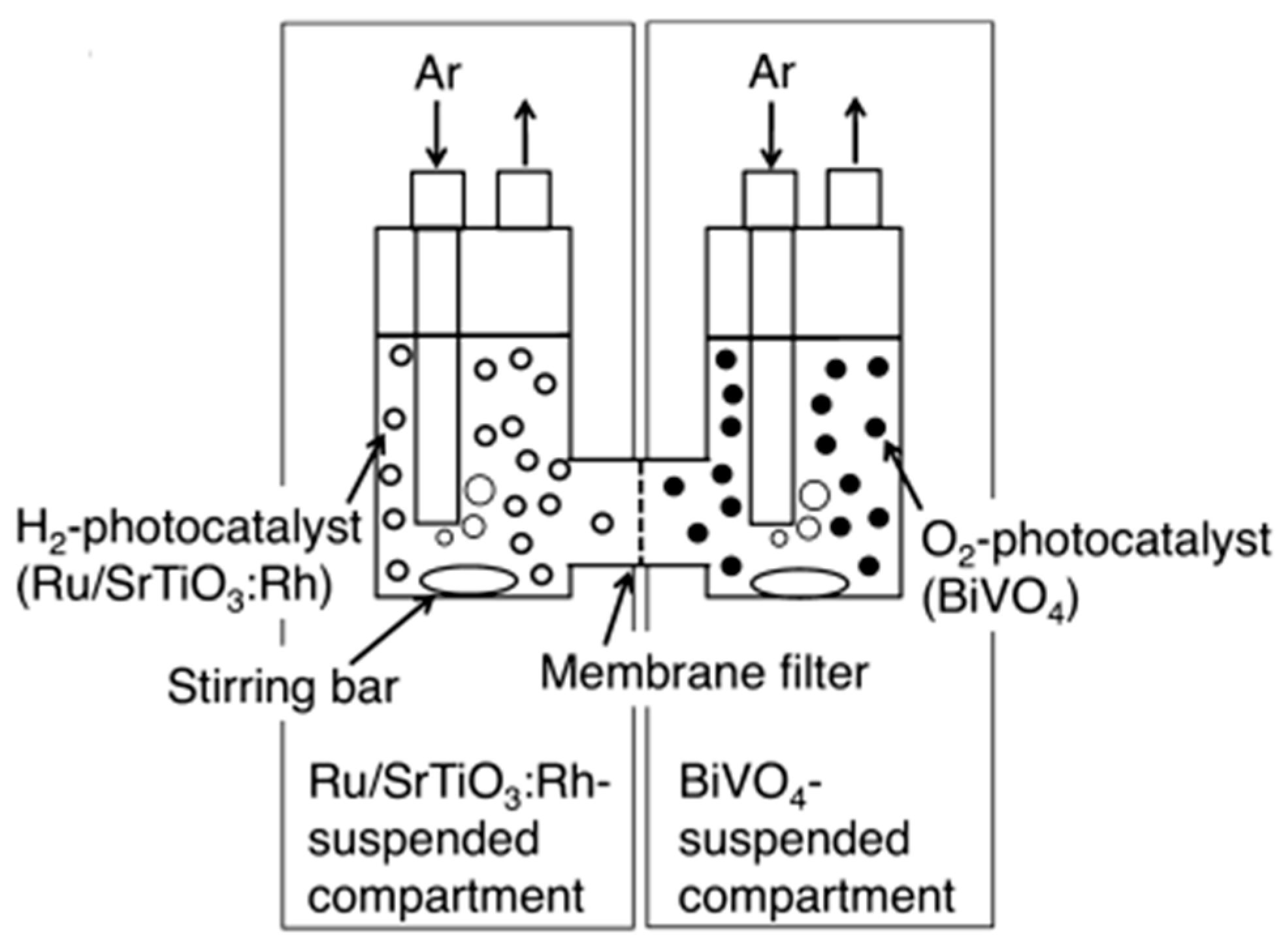

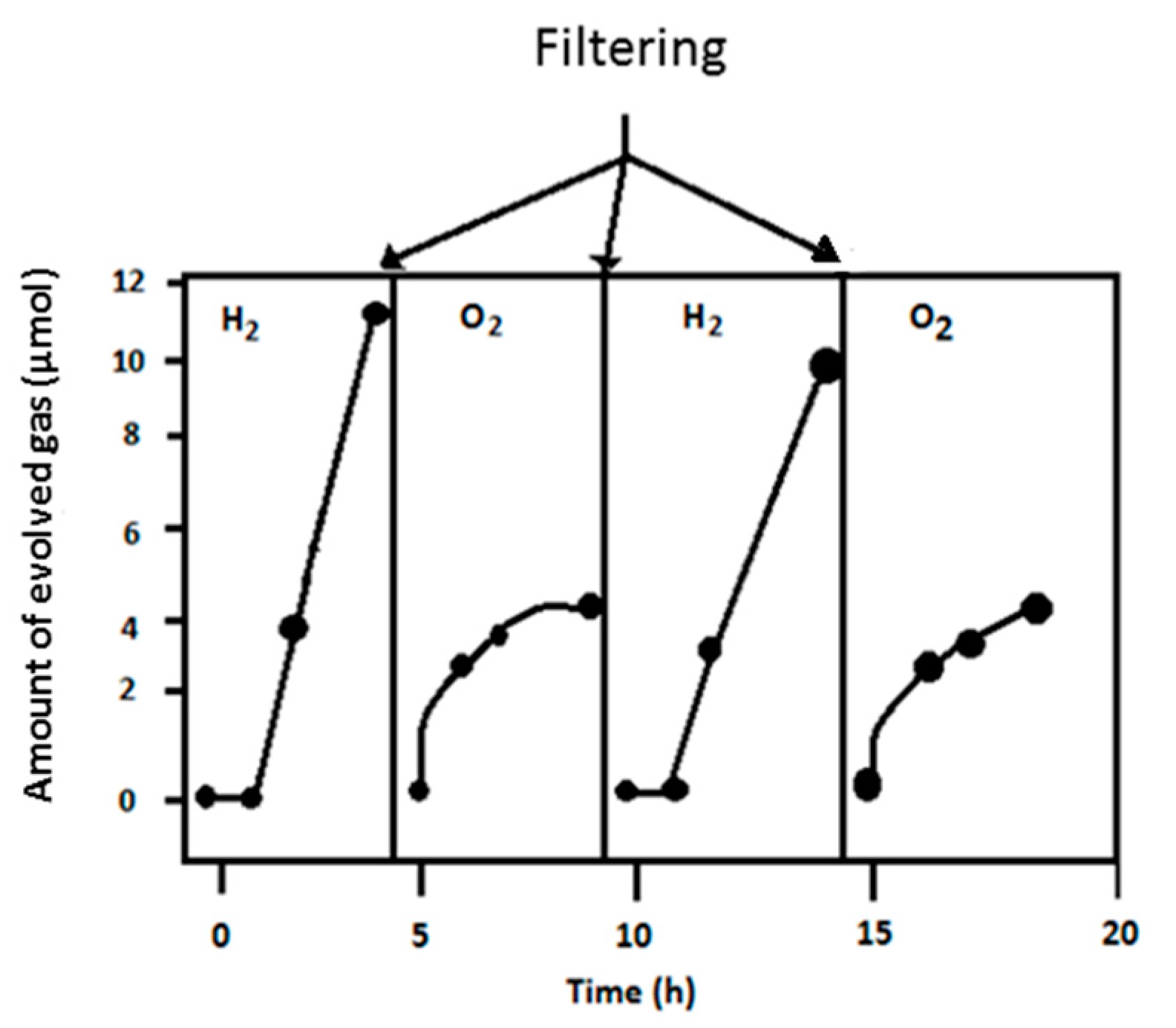
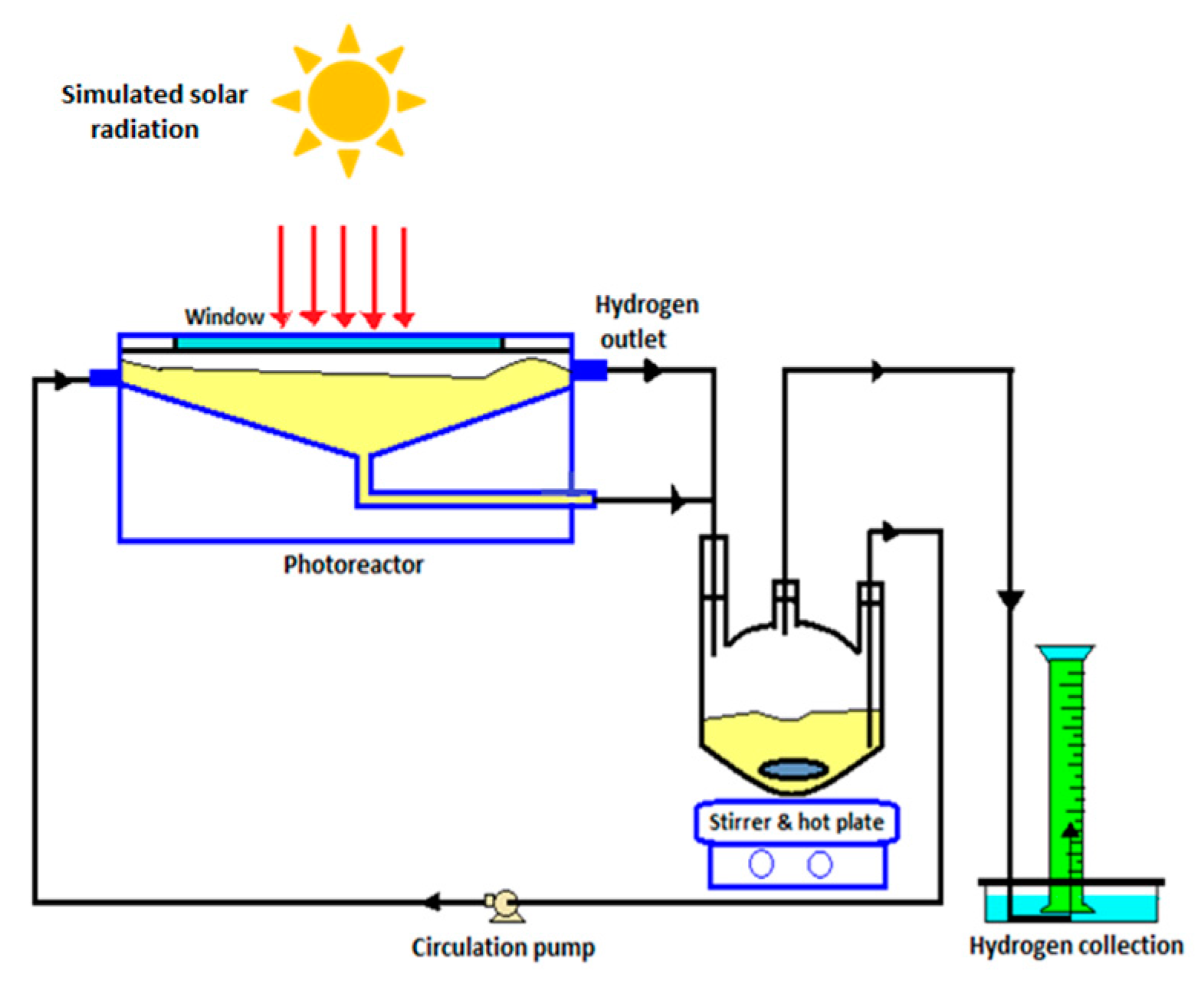
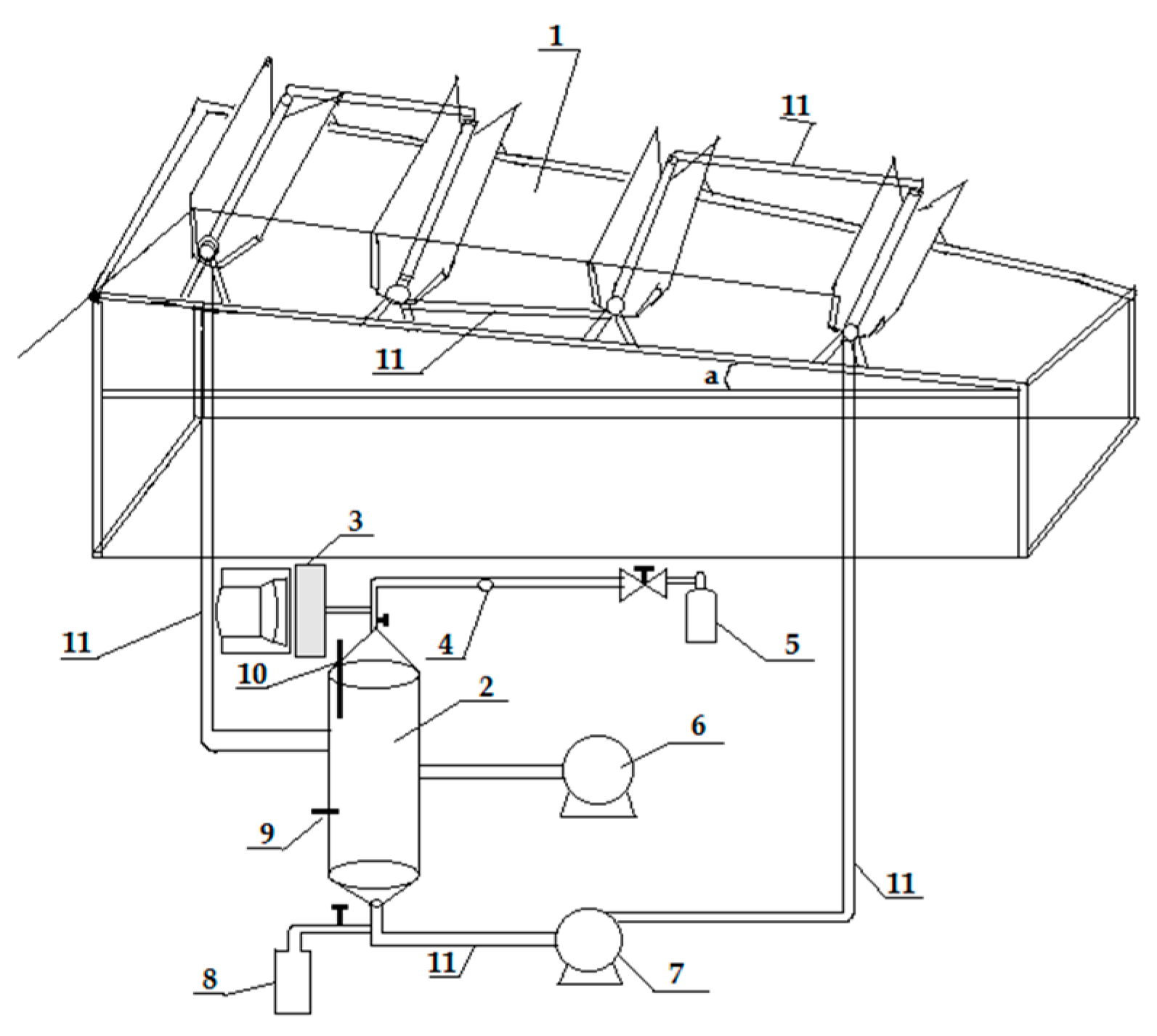

| No. | Photocatalyst Modification Technique | Mechanism of the Process | Advantage/Disadvantage | Results/Comments | References |
|---|---|---|---|---|---|
| 1. | Noble metal (Au, Ag, Cu, Pt, Ru, etc.) loading | Fermi levels of the noble metals are less than the semiconductor, and thus the conduction band electron transfer occurs more easily to the metal particles deposited on the surface of the semiconductor | (i) suppress the e−/h+ recombination (ii) help electron transfer in water-splitting (iii) noble metals are expensive (iv) much higher metal deposition results lower efficiency | platinum and gold loading is beneficial than palladium loading due to their opposite work function and electron affinity | [17,18,19,20,21] |
| 2. | Doping with transition metal ions such as Cr, Co, Ni, Mn, and Fe | Upon incorporation of metal ion into semiconductor photocatalyst, an impurity energy level is formed in the band gap of semiconductor | (i) doping with transition metal ions expand the photo-response of semiconductor photocatalyst into the visible region (ii) deep doping create recombination centers | Metal ions doped near the semiconductor photocatalyst surface for efficient charge transfer | [17,22] |
| 3. | Doping with anions such as C, P, S, N, O, and F | Upon doping band gap narrowing occurs in the semiconductor photocatalyst | (i) doping with anions in TiO2 shift its photo-response to the visible region (ii) anions hardly form any recombination centers like cations | TiO2 band gap narrow down occurs due to the overlapping of 2p orbitals of O with p orbitals of N | [17,23] |
| 4. | Composite semiconductor; small band gap CdS (2.4 eV) and large band gap semiconductors such as TiO2 (3.2 eV), SnO2 (3.5 eV) | A large band gap semiconductor is attached to a small band gap semiconductor; conduction band electrons are injected from the small band gap semiconductor to the large band gap semiconductor | (i) combination of small and large band gap semiconductors provide improved photocatalytic performance due to adequate charge separation | CdS–SnO2 system produces hydrogen under visible light irradiation in the presence of EDTA; CdS–TiO2 system produces hydrogen under visible light in the presence of Na2S. | [17,24,25] |
| 5. | Valence band controlled photocatalyst | Metals such as bismuth (6s orbital), tin (5s orbital) and silver (4d orbital) contribute to the formation of valence band of these photocatalysts above the 2p orbital of oxygen | (i) this process makes the photocatalysts visible light active | BiVO4 and AgNbO3 are suitable for O2 evolution, and Pt/SnNb2O6 is suitable for H2 evolution | [17,26,27,28] |
| 6. | Metal ion implantation | In this method, high energy ions are injected into the semiconductor which modifies the electronic structure | (i) overlapping of the d-orbital of Titanium with implant metal d-orbital occurs and that leads to a reduction of the band gap | Metal ion implantation is very efficient for red shift, and no electron mediator is required | [17] |
| 7. | Dye-sensitization | Upon visible light illumination, the excited dye molecule introduce electron into the conduction band of semiconductor to begin the photocatalytic reaction; the conduction band electron is transmitted to noble metal at the semiconductor surface to undertake water reduction | (i) hydrogen generation rate is improved by dye absorption onto photocatalyst surface (ii) careful design of dye and semiconductor couple is necessary to prevent charge trapping and recombination which lowers the efficiency of the dye-sensitized semiconductor | Transition metal-based dyes are the best dyes regarding intense charge transfer in the visible spectral range | [8,17] |
| No. | Process Details | Light Source and Accessories | Other Experimental Details | Results/Comments | References |
|---|---|---|---|---|---|
| 1. | Photocatalytic water splitting on organic dye-sensitized KTa(Zr)O3 photocatalyst | Xenon lamp (300 or 500 W) | A total of 17 sensitizers (dye) were used to modify the photocatalytic activity of PtO x/KTa(Zr)O3 for water splitting | Modification with cyanocobalamin (B12) was the most promising with energy conversion efficiency of about 0.013% | [43] |
| 2. | Ruthenium polypyridyl-sensitized solar cells for water splitting in the presence of a biometric electron transfer mediator; | Filtered white light illumination (λ = 450 nm light) at 4.5 mW·cm−2 intensity | Iridium oxide nanoparticles are bonded to benzimidazole-phenol mediator and 2-carboxyethylphosphonic acid anchoring molecule | QY (2.3%) is more than doubled with the use of an electron transfer mediator | [42] |
| 3. | Heteroleptic ruthenium dye-sensitized photoelectrochemical cell for water splitting | 450 nm light at 7.8 mW·cm−2 | Modified ruthenium dye adsorbed on IrO2·nH2O nanoparticles in aqueous solution (1 M) of Na2S2O8; this system undergoes rapid electron transfer into anatase TiO2 | QY of approximately 0.9% | [33] |
| 4. | Water splitting from bipolar Pt/dye-sensitized TiO2 photoanode arrays | Xenon lamp (2500 W) with IR filter, light intensity 100 mW·cm−2 | Anode: Ru-dye Z-907 coated TiO2, Electrolyte: iodide/iodine, Cathode: Pt | QY 3.7% | [44] |
| 5. | Water splitting in a photoelectrochemical device with a molecular Ru catalyst assembled on dye-sensitized nano-TiO2 | Xenon lamp (500 W) with 400 nm cut-off filter | Sensitizer: [Ru(bpy)2(4,4′-(PO3H2)2bpy)]2+; Nafion membrane was used to immobilize the hydrophobic catalyst; Pt cathode | Strong acidic pH of commercial Nafion membrane led to a fast decay of the photocurrent | [45] |
| 6. | Organic dye-sensitized Tandem photoelectrochemical cell for water splitting | White LED light (λ > 400 nm), light intensity 100 mW·cm−2 | Photocathode: organic dye P1 as photo absorber and a complex molecular Co1 as H2 generation catalyst on nano-NiO; Photoanode: organic dye L0 as photosensitizer and a molecular complex Ru1 as O2 generation catalyst on mesoporous TiO2 | IPCE of 25% at 380 nm under neutral condition without bias | [46] |
| 7. | Water splitting on Rhodamine-B dye-sensitized Co-doped TiO2 catalyst | Ozone-free Xenon arc lamp (300 W) with UV cut-off filter | Photocatalytic reaction was carried out in an outer irradiated Pyrex cell with Rh–B–Co/TiO2 photocatalyst powder (50 mg) dispersed in pure water (70 mL) | Dye-sensitized photocatalyst achieved stoichiometric evolution of H2 and O2 gas; the H2 generation was six times higher than sensitized photocatalyst | [39] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, P.; Malekshoar, G.; Ray, A.K. Dye-Sensitized Photocatalytic Water Splitting and Sacrificial Hydrogen Generation: Current Status and Future Prospects. Inorganics 2017, 5, 34. https://doi.org/10.3390/inorganics5020034
Chowdhury P, Malekshoar G, Ray AK. Dye-Sensitized Photocatalytic Water Splitting and Sacrificial Hydrogen Generation: Current Status and Future Prospects. Inorganics. 2017; 5(2):34. https://doi.org/10.3390/inorganics5020034
Chicago/Turabian StyleChowdhury, Pankaj, Ghodsieh Malekshoar, and Ajay K. Ray. 2017. "Dye-Sensitized Photocatalytic Water Splitting and Sacrificial Hydrogen Generation: Current Status and Future Prospects" Inorganics 5, no. 2: 34. https://doi.org/10.3390/inorganics5020034
APA StyleChowdhury, P., Malekshoar, G., & Ray, A. K. (2017). Dye-Sensitized Photocatalytic Water Splitting and Sacrificial Hydrogen Generation: Current Status and Future Prospects. Inorganics, 5(2), 34. https://doi.org/10.3390/inorganics5020034








