Study of Cathode Materials for Lithium-Ion Batteries: Recent Progress and New Challenges
Abstract
:1. Introduction
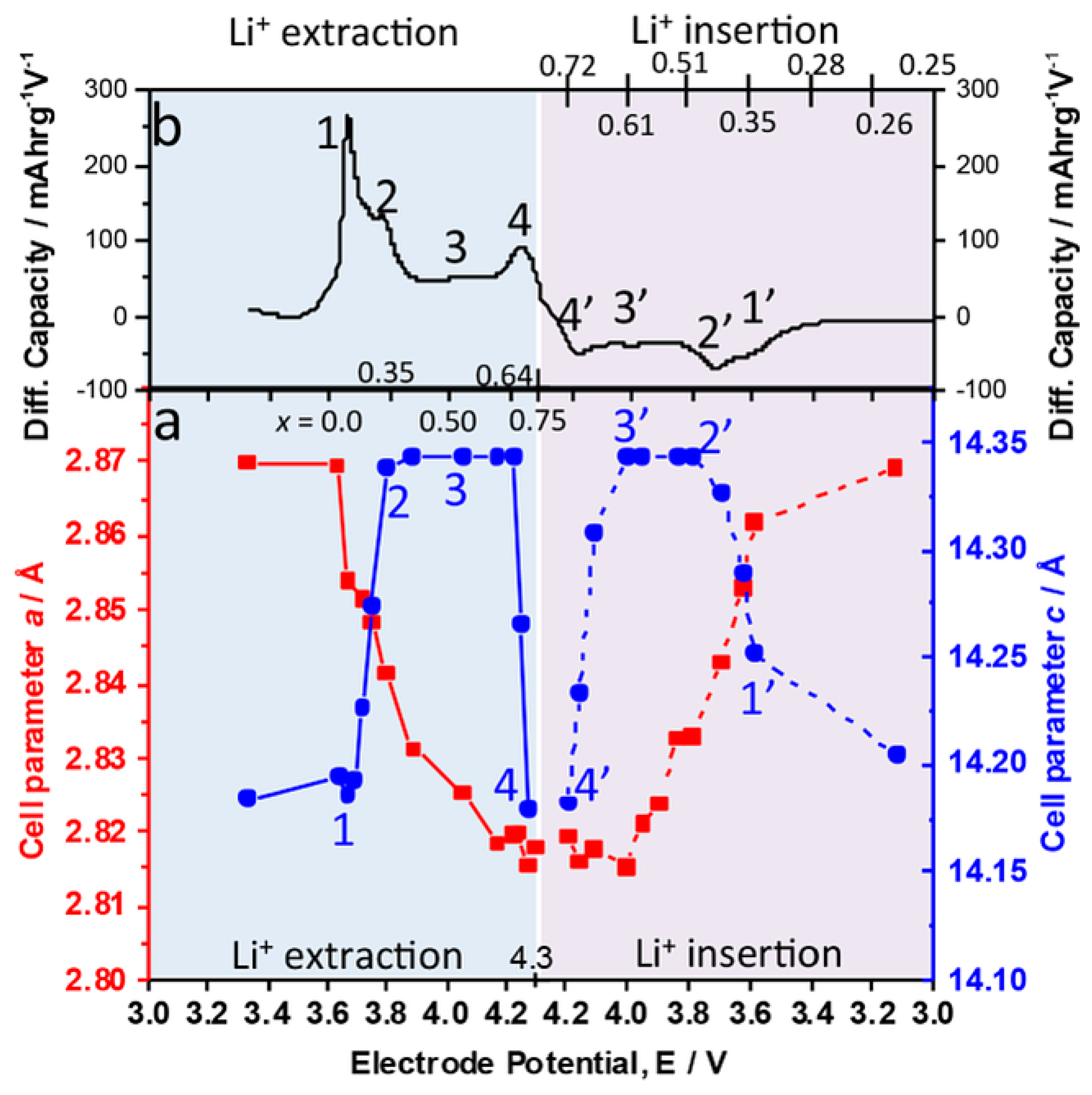
2. Lithium- and Manganese-Rich Layered Structure Materials
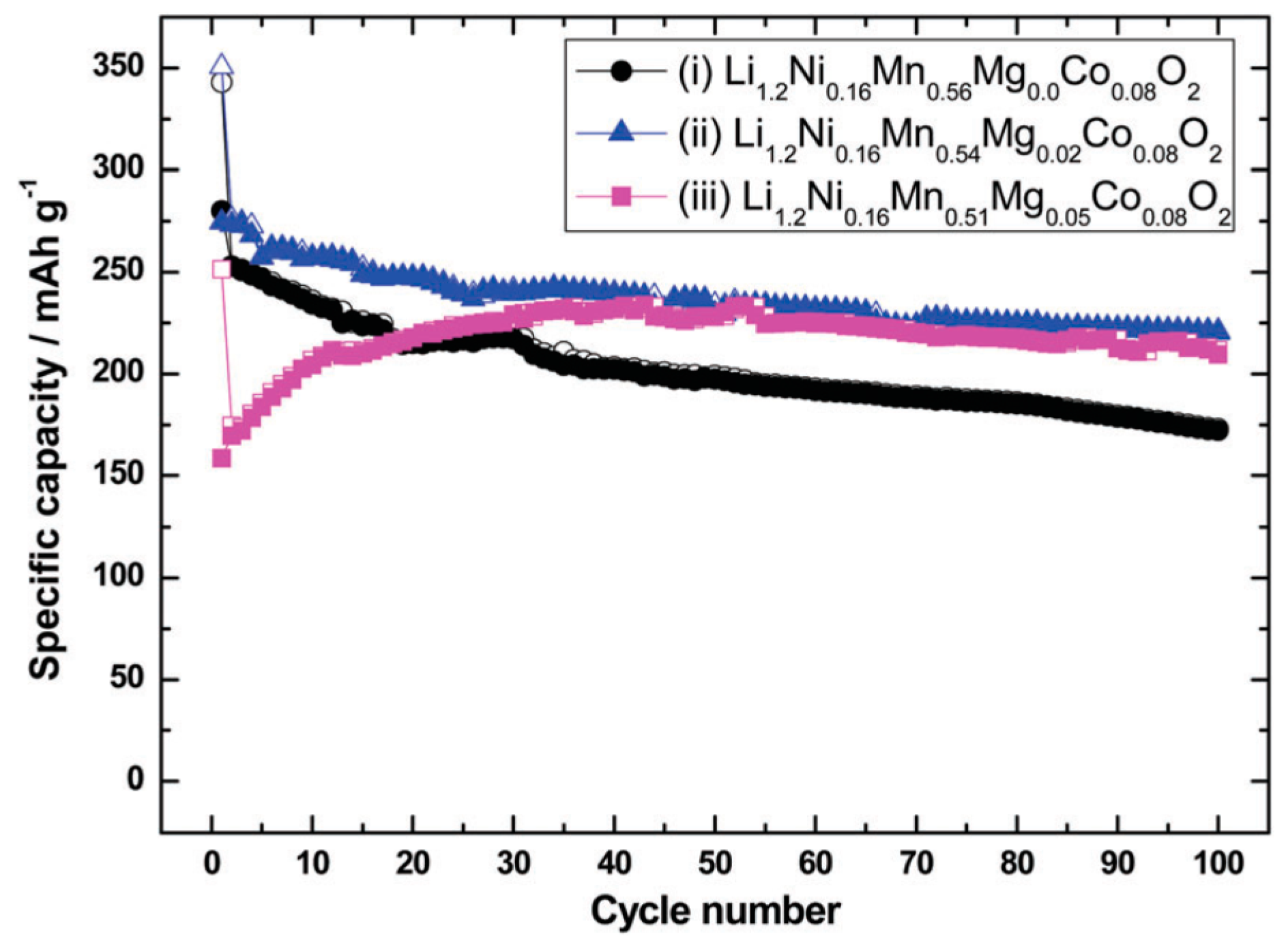
2.1. Modifications of xLi2MnO3·(1 − x)Li[NiaCobMnc]O2 Materials by Surface Coating and Lattice Doping
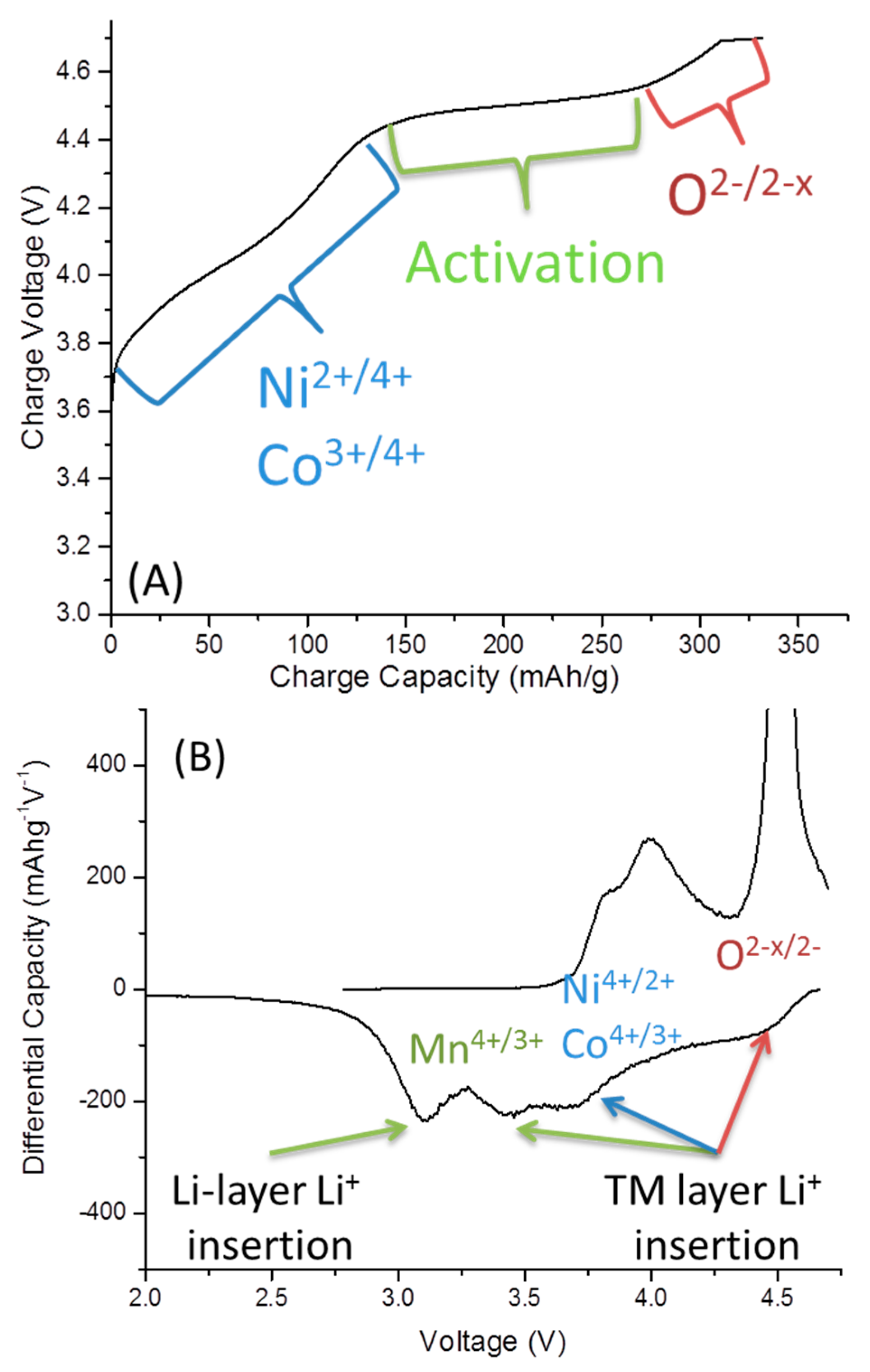
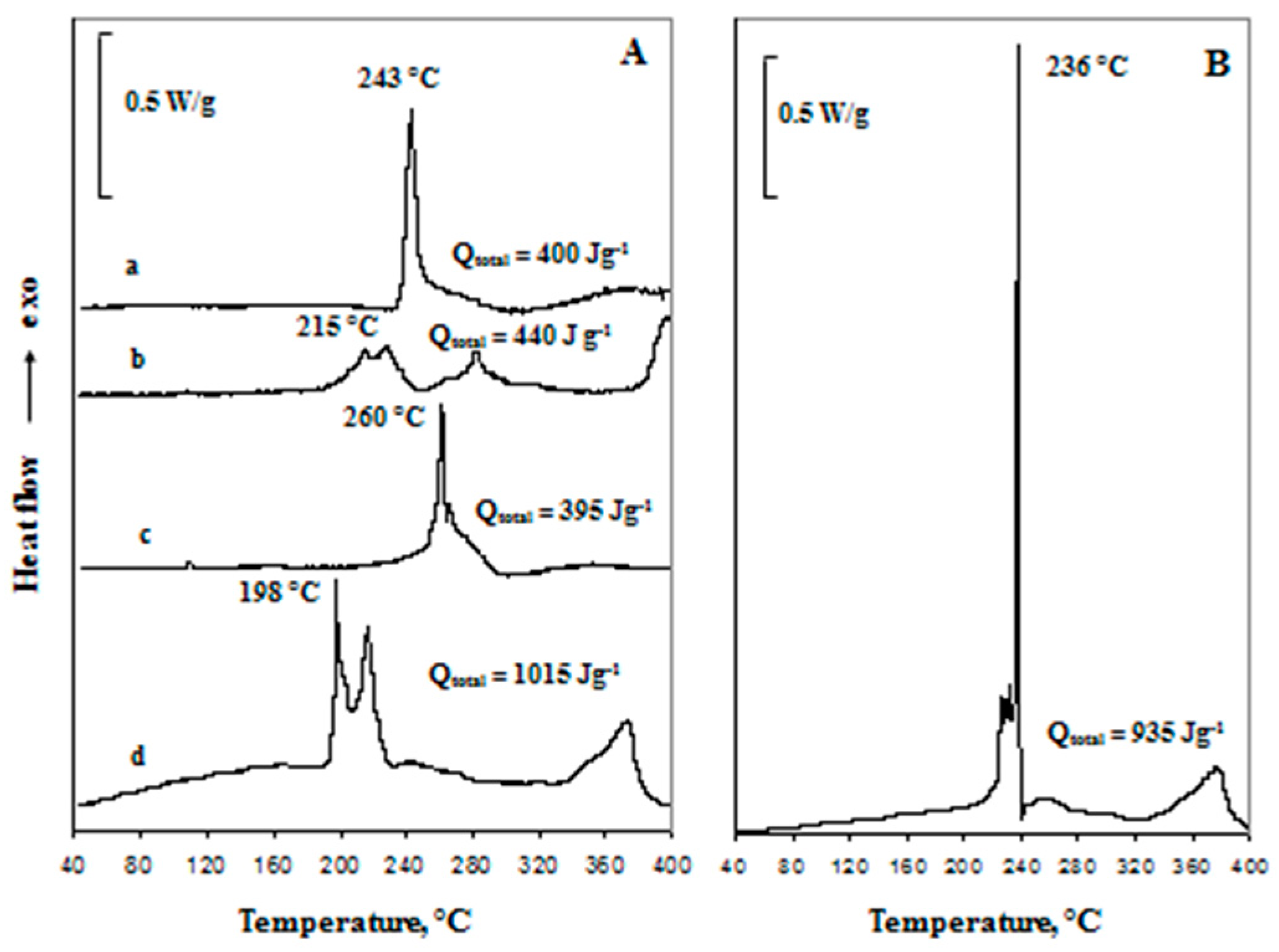
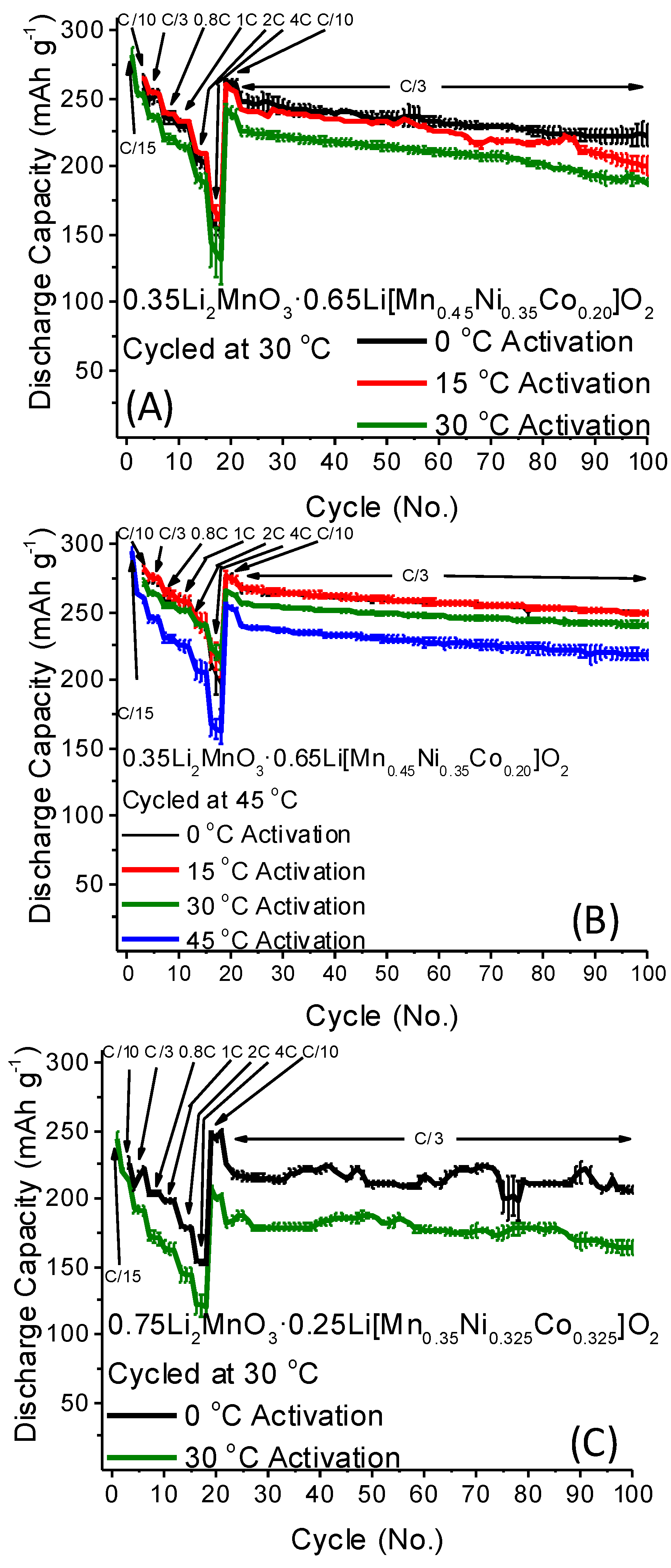
2.2. Low-Temperature Activation of xLi2MnO3·(1 − x)Li[MnyNizCow]O2 Materials
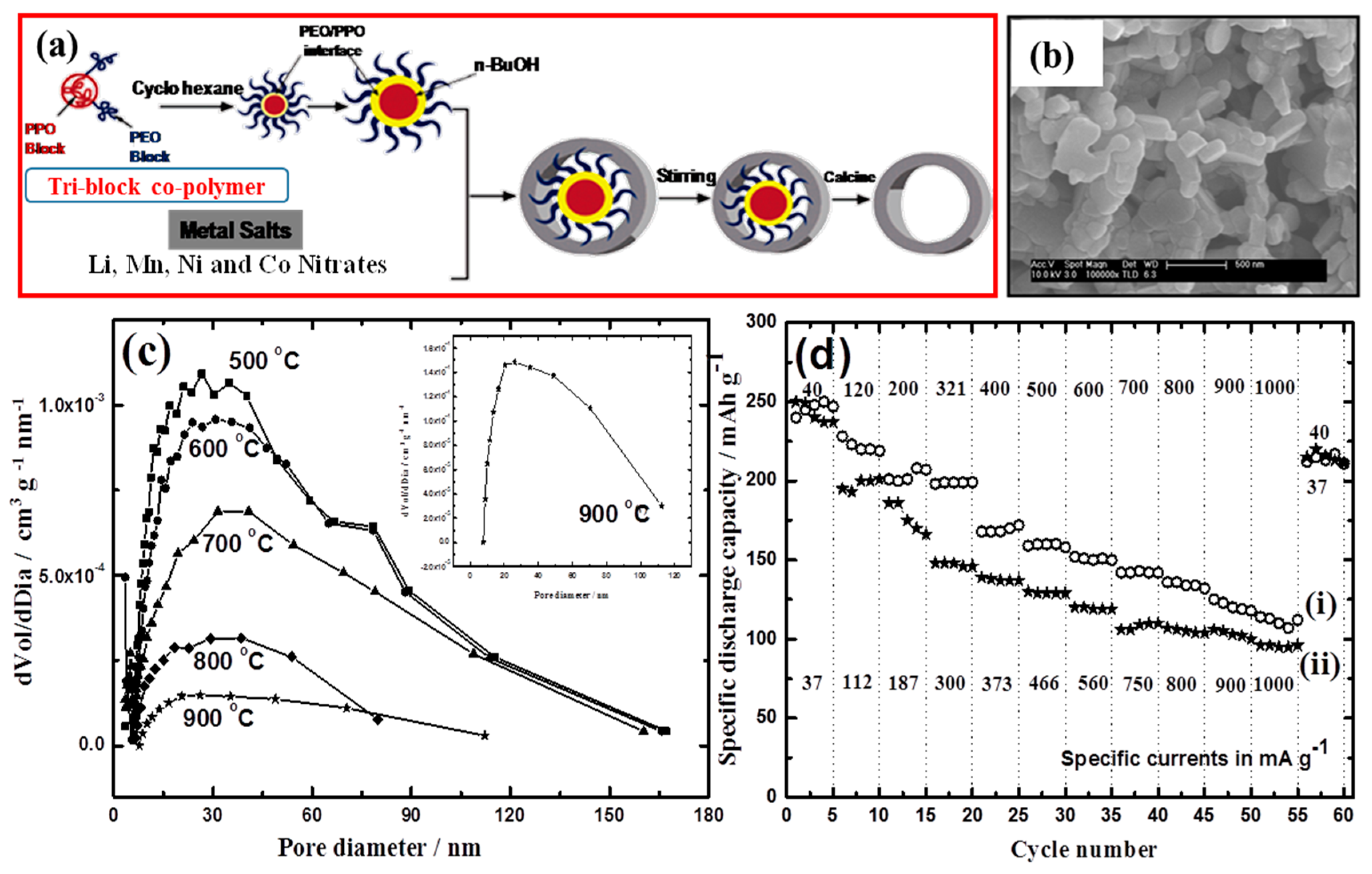
2.3. Porous Li- and Mn-Rich High-Energy-Density Cathode Materials
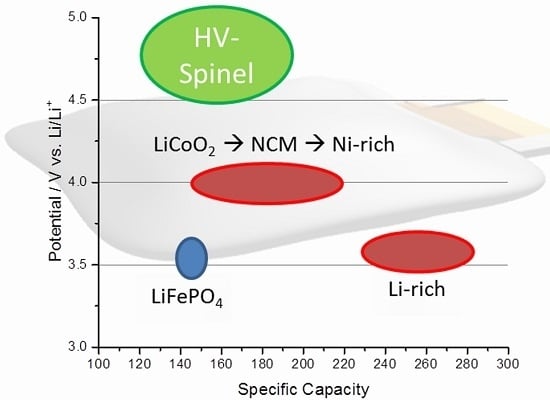
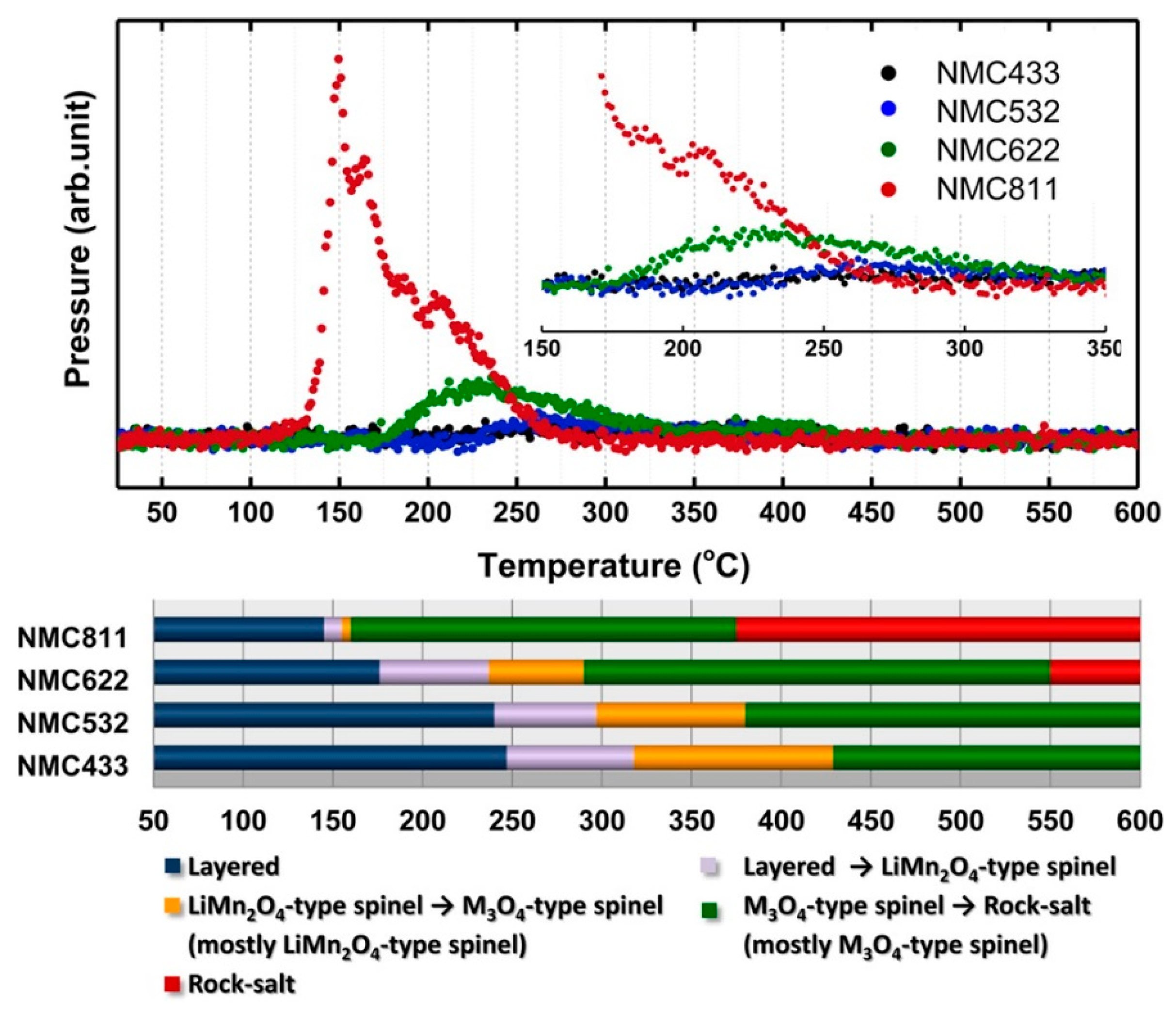
3. Nickel-Rich Li[NixCoyMnz]O2 Layered Structure Materials (NCM, x > 0.5, x + y + z = 1)
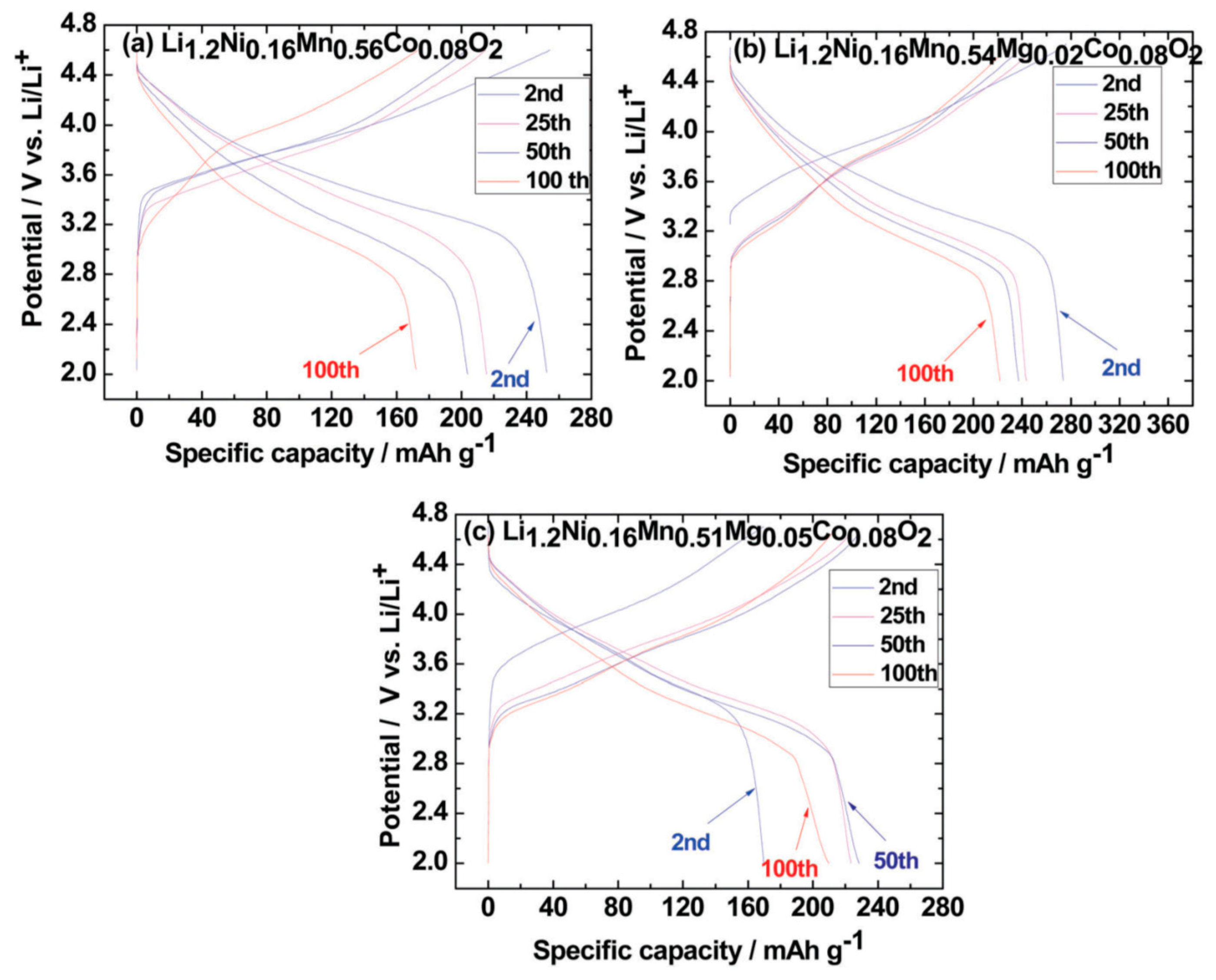
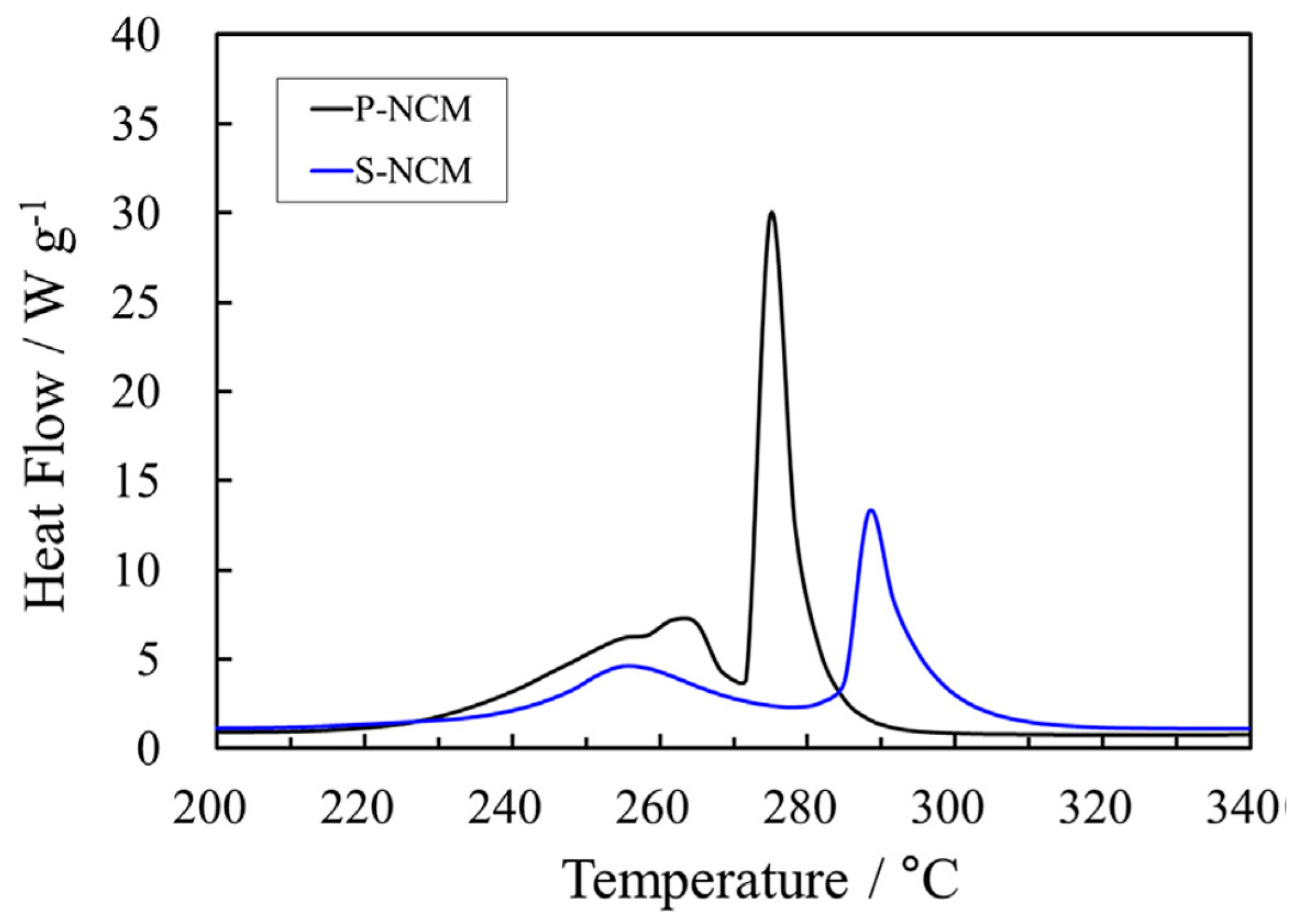
3.1. Zirconium and Aluminum Doping of Li[NixCoyMnz]O2 Materials
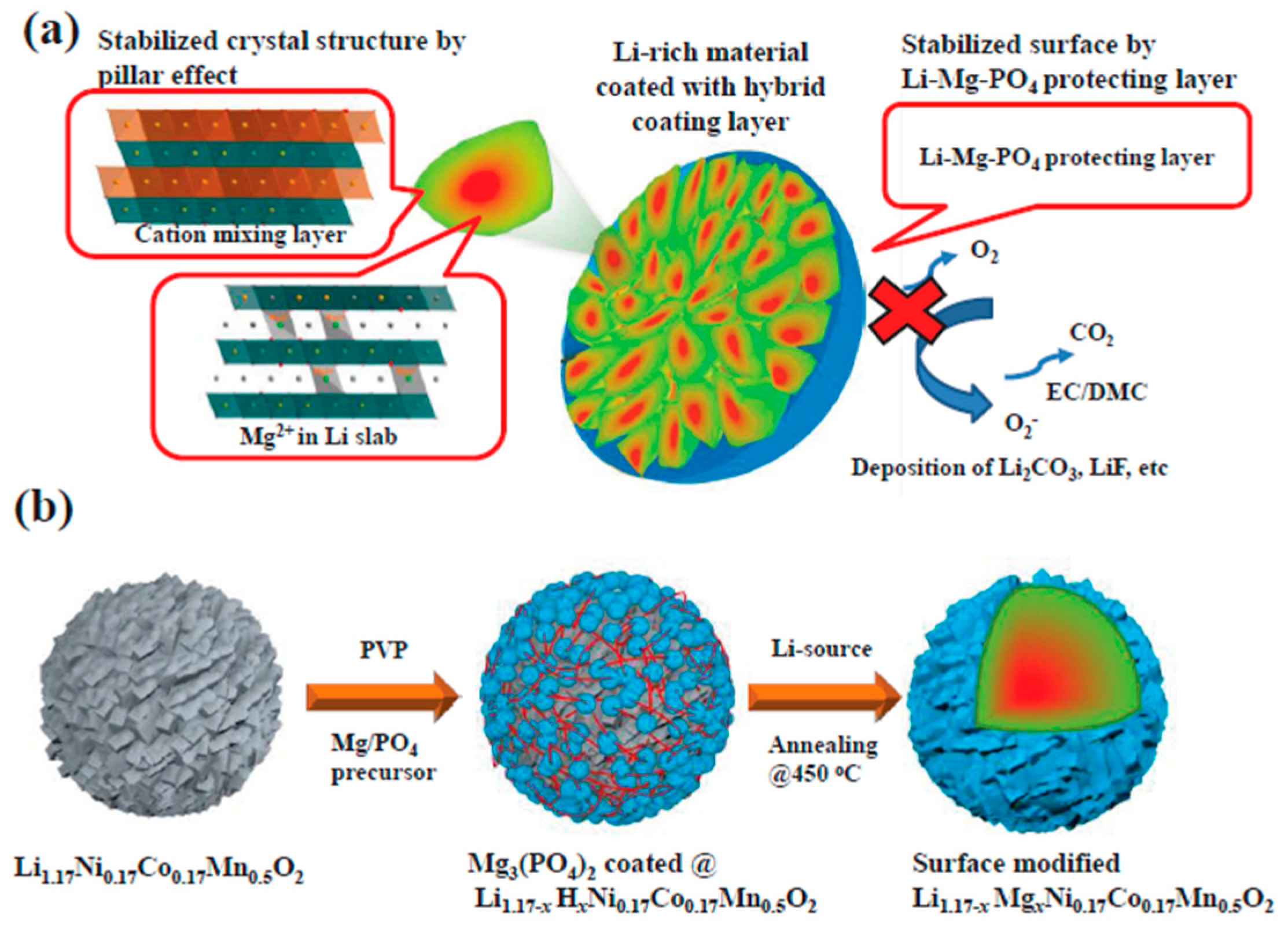
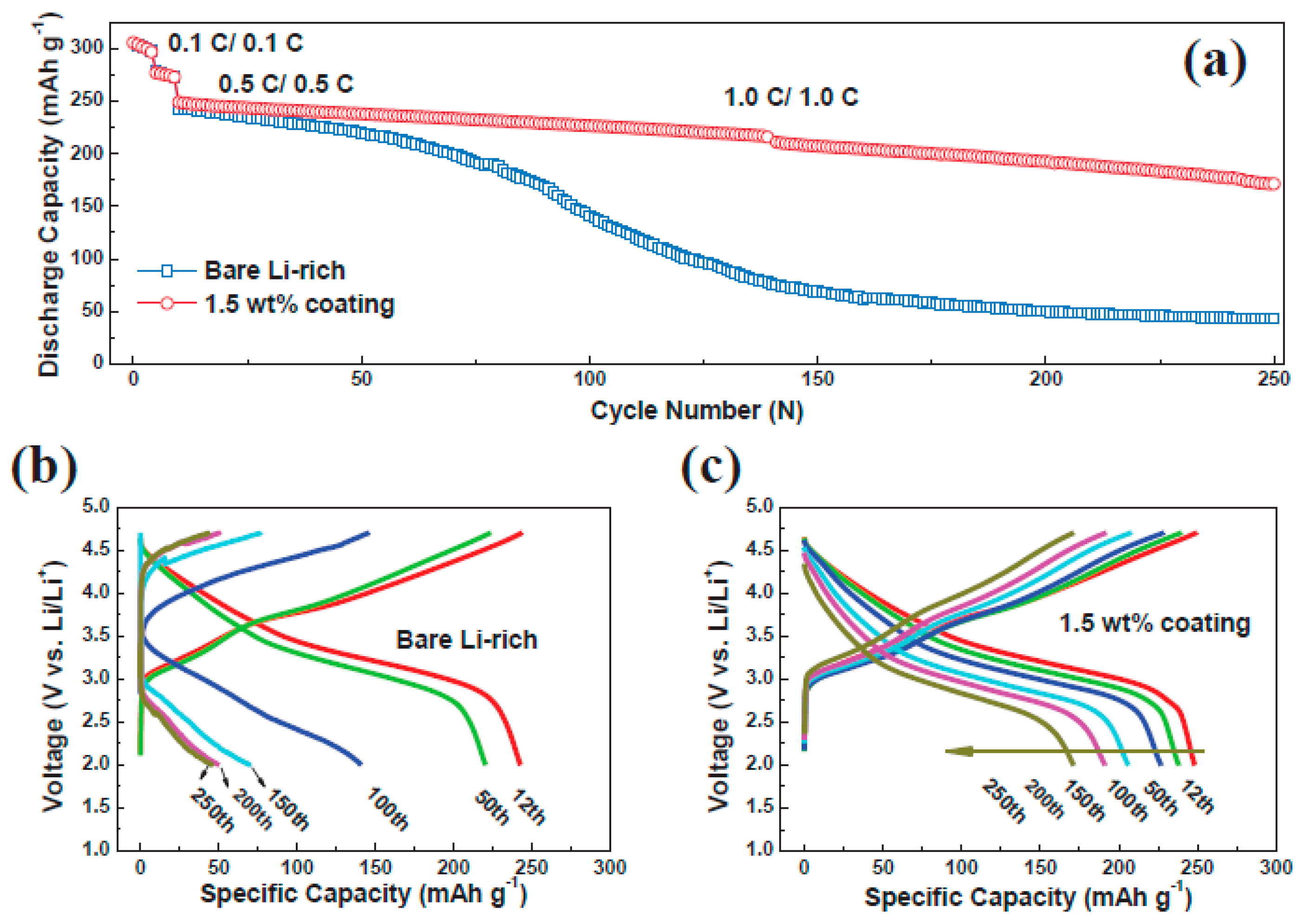
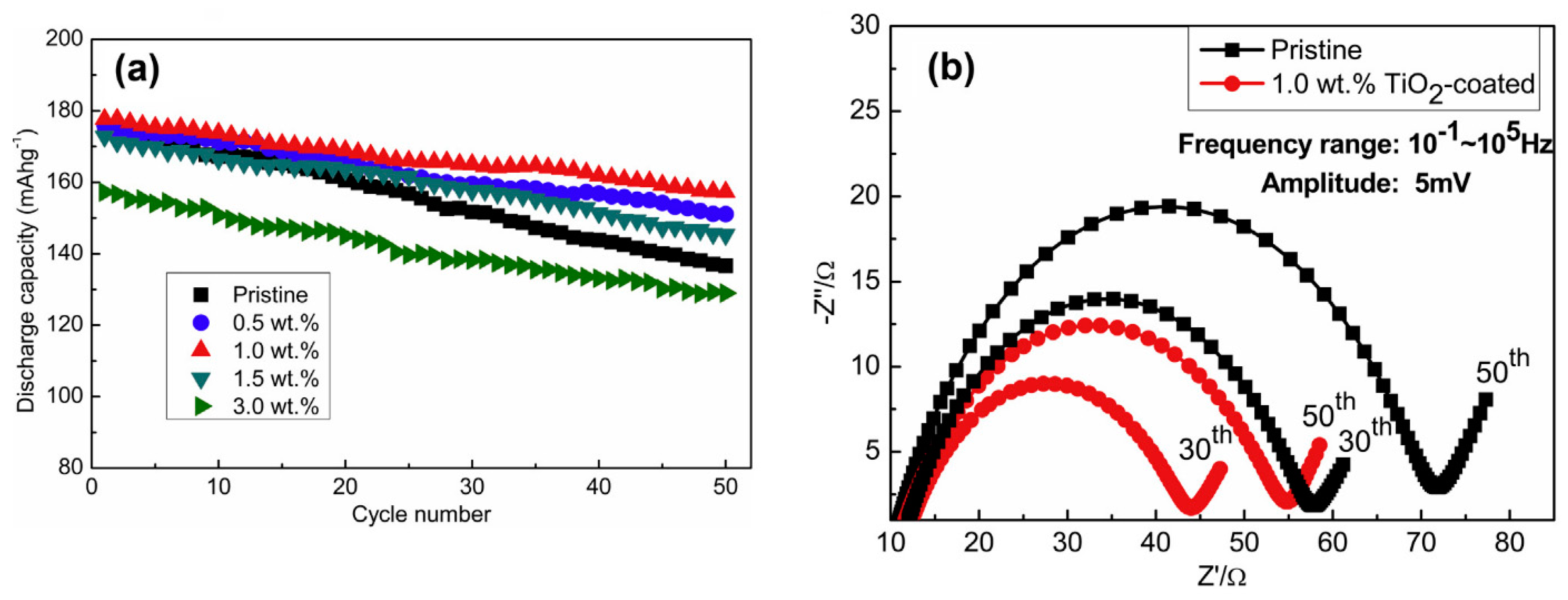
3.2. Surface Coatings of Li[NixCoyMnz]O2 Materials
4. Conclusions
Supplementary Materials
Conflicts of Interest
References
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Manthiram, A. Materials challenges and opportunities of lithium ion batteries. J. Phys. Chem. Lett. 2011, 2, 176–184. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, M.M.; Johnson, C.S.; Vaughey, J.T.; Li, N.; Hackney, S.A. Advances in manganese-oxide ‘composite’ electrodes for lithium-ion batteries. J. Mater. Chem. 2005, 15, 2257–2267. [Google Scholar] [CrossRef]
- Erickson, E.M.; Ghanty, C.; Aurbach, D. New horizons for conventional lithium ion battery technology. J. Phys. Chem. Lett. 2014, 5, 3313–3324. [Google Scholar] [CrossRef] [PubMed]
- Berg, E.J.; Villevieille, C.; Streich, D.; Trabesinger, S.; Novak, P. Rechargeable batteries: Grasping for the limits of chemistry. J. Electrochem. Soc. 2015, 162, A2468–A2475. [Google Scholar] [CrossRef]
- Yan, J.H.; Liu, X.B.; Li, B.Y. Recent progress in Li-rich layered oxides as cathode materials for Li-ion batteries. RSC Adv. 2014, 4, 63268–63284. [Google Scholar] [CrossRef]
- Amalraj, F.; Talianker, M.; Markovsky, B.; Sharon, D.; Burlaka, L.; Shafir, G.; Zinigrad, E.; Haik, O.; Aurbach, D.; Lampert, J.; et al. Study of the lithium-rich integrated compound xLi2MnO3·(1 − x)LiMO2 (x around 0.5; M = Mn, Ni, Co; 2:2:1) and its electrochemical activity as positive electrode in lithium cells. J. Electrochem. Soc. 2012, 160, A324–A337. [Google Scholar]
- Mohanty, D.; Kalnaus, S.; Meisner, R.A.; Rhodes, K.J.; Li, J.L.; Payzant, E.A.; Wood, D.L.; Daniel, C. Structural transformation of a lithium-rich Li1.2Co0.1Mn0.55Ni0.15O2 cathode during high voltage cycling resolved by in situ X-ray diffraction. J. Power Sources 2013, 229, 239–248. [Google Scholar]
- Boulineau, A.; Croguennec, L.; Delmas, C.; Weill, F. Structure of Li2MnO3 with different degrees of defects. Solid State Ion. 2010, 180, 1652–1659. [Google Scholar] [CrossRef]
- Shunmugasundaram, R.; Arumugam, R.S.; Dahn, J.R. A study of stacking faults and superlattice ordering in some Li-rich layered transition metal oxide positive electrode materials. J. Electrochem. Soc. 2016, 163, A1394–A1400. [Google Scholar] [CrossRef]
- Shukla, A.K.; Ramasse, Q.M.; Ophus, C.; Duncan, H.; Hage, F.; Chen, G. Unravelling structural ambiguities in lithium- and manganese-rich transition metal oxides. Nat Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lyu, Y.; Gu, L.; Wu, H.; Bak, S.-M.; Zhou, Y.; Amine, K.; Ehrlich, S.N.; Li, H.; Nam, K.-W.; et al. Understanding the rate capability of high-energy-density Li-rich layered Li1.2Ni0.15Co0.1Mn0.55O2 cathode materials. Adv. Energy Mater. 2014, 4, 1300950-n/a. [Google Scholar]
- Long, B.R.; Croy, J.R.; Dogan, F.; Suchomel, M.R.; Key, B.; Wen, J.; Miller, D.J.; Thackeray, M.M.; Balasubramanian, M. Effect of cooling rates on phase separation in 0.5Li2MnO3·0.5LiCoO2 electrode materials for Li-ion batteries. Chem. Mater. 2014, 26, 3565–3572. [Google Scholar]
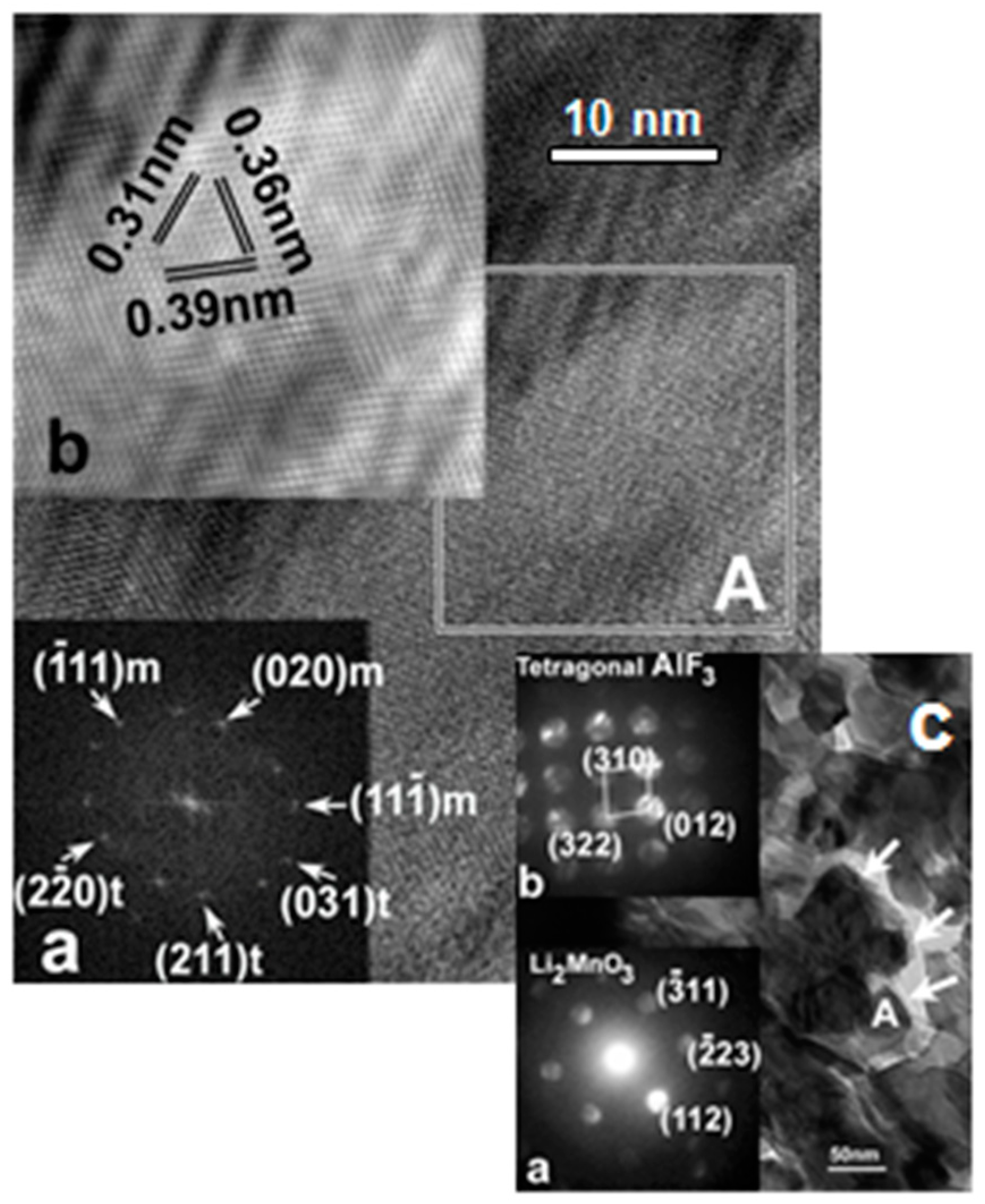
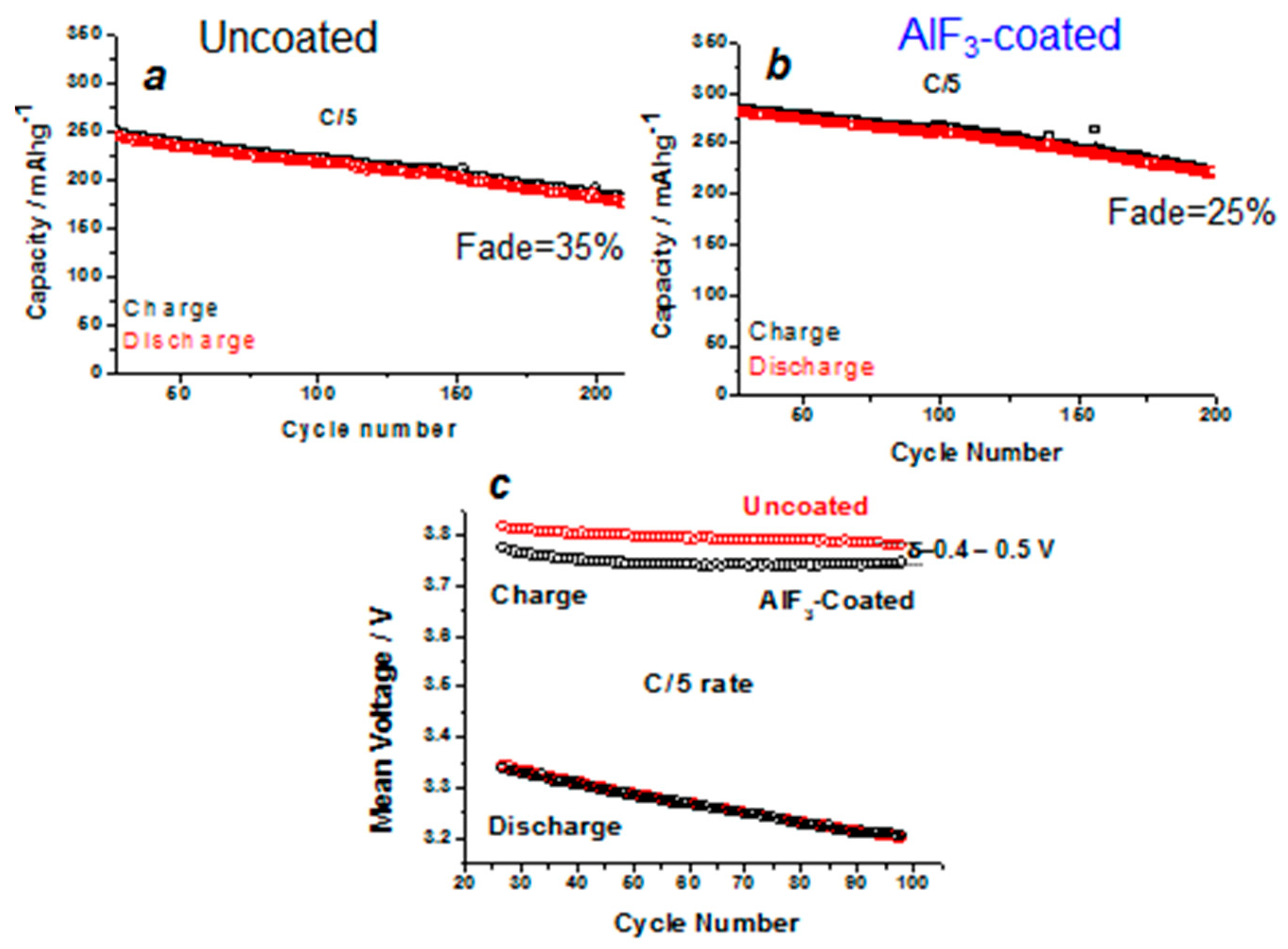
- Amalraj, F.; Talianker, M.; Markovsky, B.; Burlaka, L.; Leifer, N.; Goobes, G.; Erickson, E.M.; Haik, O.; Grinblat, J.; Zinigrad, E.; et al. Studies of Li and Mn-rich Lix[MnNiCo]O2 electrodes: Electrochemical performance, structure, and the effect of the aluminum fluoride coating. J. Electrochem. Soc. 2013, 160, A2220–A2233. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, A.; Lee, J.Y. Synthesis and characterization of LiNi1−x−yCoxMnyO2 as the cathode materials of secondary lithium batteries. J. Power Sources 1999, 81–82, 416–419. [Google Scholar]
- Yabuuchi, N.; Ohzuku, T. Novel lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries. J. Power Sources 2003, 119, 171–174. [Google Scholar] [CrossRef]
- Ohzuku, T.; Makimura, Y. Layered lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for lithium-ion batteries. Chem. Lett. 2001, 30, 642–643. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Makimura, Y.; Ohzuku, T. Solid-state chemistry and electrochemistry of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries: III. Rechargeable capacity and cycleability. J. Electrochem. Soc. 2007, 154, A314–A321. [Google Scholar] [CrossRef]
- Kang, S.H.; Johnson, C.S.; Vaughey, J.T.; Amine, K.; Thackeray, M.M. The effects of acid treatment on the electrochemical properties of 0.5 Li2MnO3·0.5 LiNi0.44Co0.25Mn0.31O2 electrodes in lithium cells. J. Electrochem. Soc. 2006, 153, A1186–A1192. [Google Scholar]
- Rossouw, M.H.; Thackeray, M.M. Lithium manganese oxides from Li2MnO3 for rechargeable lithium battery applications. Mater. Res. Bull. 1991, 26, 463–473. [Google Scholar] [CrossRef]
- Kalyani, P.; Chitra, S.; Mohan, T.; Gopukumar, S. Lithium metal rechargeable cells using Li2MnO3 as the positive electrode. J. Power Sources 1999, 80, 103–106. [Google Scholar] [CrossRef]
- Lu, Z.H.; MacNeil, D.D.; Dahn, J.R. Layered cathode materials Li(NixLi(1/3–2x/3)Mn(2/3−x/3))O2 for lithium-ion batteries. Electrochem. Solid State Lett. 2001, 4, A191–A194. [Google Scholar] [CrossRef]
- Koga, H.; Croguennec, L.; Menetrier, M.; Mannessiez, P.; Weill, F.; Delmas, C.; Belin, S. Operando X-ray absorption study of the redox processes involved upon cycling of the Li-rich layered oxide Li1.20Mn0.54Co0.13Ni0.13O2 in li ion batteries. J. Phys. Chem. C 2014, 118, 5700–5709. [Google Scholar]
- Kim, T.; Song, B.H.; Lunt, A.J.G.; Cibin, G.; Dent, A.J.; Lu, L.; Korsunsky, A.M. In operando x-ray absorption spectroscopy study of charge rate effects on the atomic environment in graphene-coated Li-rich mixed oxide cathode. Mater. Des. 2016, 98, 231–242. [Google Scholar] [CrossRef]
- Fell, C.R.; Chi, M.; Meng, Y.S.; Jones, J.L. In situ x-ray diffraction study of the lithium excess layered oxide compound Li[Li0.2Ni0.2Mn0.6]O2 during electrochemical cycling. Solid State Ion. 2012, 207, 44–49. [Google Scholar]
- Li, J.; Shunmugasundaram, R.; Doig, R.; Dahn, J.R. In situ x-ray diffraction study of layered Li–Ni–Mn–Co oxides: Effect of particle size and structural stability of core–shell materials. Chem. Mater. 2016, 28, 162–171. [Google Scholar] [CrossRef]
- Abe, M.; Matsumoto, F.; Saito, M.; Yamamura, H.; Kobayashi, G.; Ito, A.; Sanada, T.; Hatano, M.; Ohsawa, Y.; Sato, Y. Activation of a Li-rich solid-solution layered LiNi0.18Li0.20Co0.03Mn0.58O2 cathode and retention of high capacities via an electrochemical pretreatment with a low discharge voltage limit. Chem. Lett. 2012, 41, 418–419. [Google Scholar]
- Zheng, J.M.; Xu, P.H.; Gu, M.; Xiao, J.; Browning, N.D.; Yan, P.F.; Wang, C.M.; Zhang, J.G. Structural and chemical evolution of Li- and Mn-rich layered cathode material. Chem. Mater. 2015, 27, 1381–1390. [Google Scholar] [CrossRef]
- Nayak, P.K.; Grinblat, J.; Levi, E.; Markovsky, B.; Aurbach, D. Effect of cycling conditions on the electrochemical performance of high capacity Li and Mn-rich cathodes for Li-ion batteries. J. Power Sources 2016, 318, 9–17. [Google Scholar] [CrossRef]
- Rana, J.; Kloepsch, R.; Li, J.; Stan, M.; Schumacher, G.; Winter, M.; Banhart, J. Structural changes in a Li-rich 0.5Li2MnO3·0.5LiMn0.4Ni0.4Co0.2O2 cathode material for Li-ion batteries: A local perspective. J. Electrochem. Soc. 2016, 163, A811–A820. [Google Scholar]
- Rozier, P.; Tarascon, J.M. Review-Li-rich layered oxide cathodes for next-generation Li-ion batteries: Chances and challenges. J. Electrochem. Soc. 2015, 162, A2490–A2499. [Google Scholar] [CrossRef]
- Sathiya, M.; Rousse, G.; Ramesha, K.; Laisa, C.P.; Vezin, H.; Sougrati, M.T.; Doublet, M.L.; Foix, D.; Gonbeau, D.; Walker, W.; et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 2013, 12, 827–835. [Google Scholar] [CrossRef] [PubMed]
- McCalla, E.; Abakumov, A.M.; Saubanere, M.; Foix, D.; Berg, E.J.; Rousse, G.; Doublet, M.-L.; Gonbeau, D.; Novak, P.; Van Tendeloo, G.; et al. Visualization of O–O peroxo-like dimers in high-capacity layered oxides for Li-ion batteries. Science 2015, 350, 1516–1521. [Google Scholar] [CrossRef] [PubMed]
- Saubanere, M.; McCalla, E.; Tarascon, J.M.; Doublet, M.L. The intriguing question of anionic redox in high-energy density cathodes for Li-ion batteries. Energy Environ. Sci. 2016, 9, 984–991. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Dupre, N.; Paterson, A.J.; Grey, C.P.; Bruce, P.G. Combined neutron diffraction, NMR, and electrochemical investigation of the layered-to-spinel transformation in LiMnO2. Chem. Mater. 2004, 16, 3106–3118. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Croy, J.R.; Balasubramanian, M.; Bettge, M.; Abraham, D.P.; Burrell, A.K.; Thackeray, M.M. Correlating hysteresis and voltage fade in lithium- and manganese-rich layered transition-metal oxide electrodes. Electrochem. Commun. 2013, 33, 96–98. [Google Scholar] [CrossRef]
- Croy, J.R.; Gallagher, K.G.; Balasubramanian, M.; Long, B.R.; Thackeray, M.M. Quantifying hysteresis and voltage fade in xLi2MnO3·(1 − x)LiMn0.5Ni0.5O2 electrodes as a function of Li2MnO3 content. J. Electrochem. Soc. 2014, 161, A318–A325. [Google Scholar]
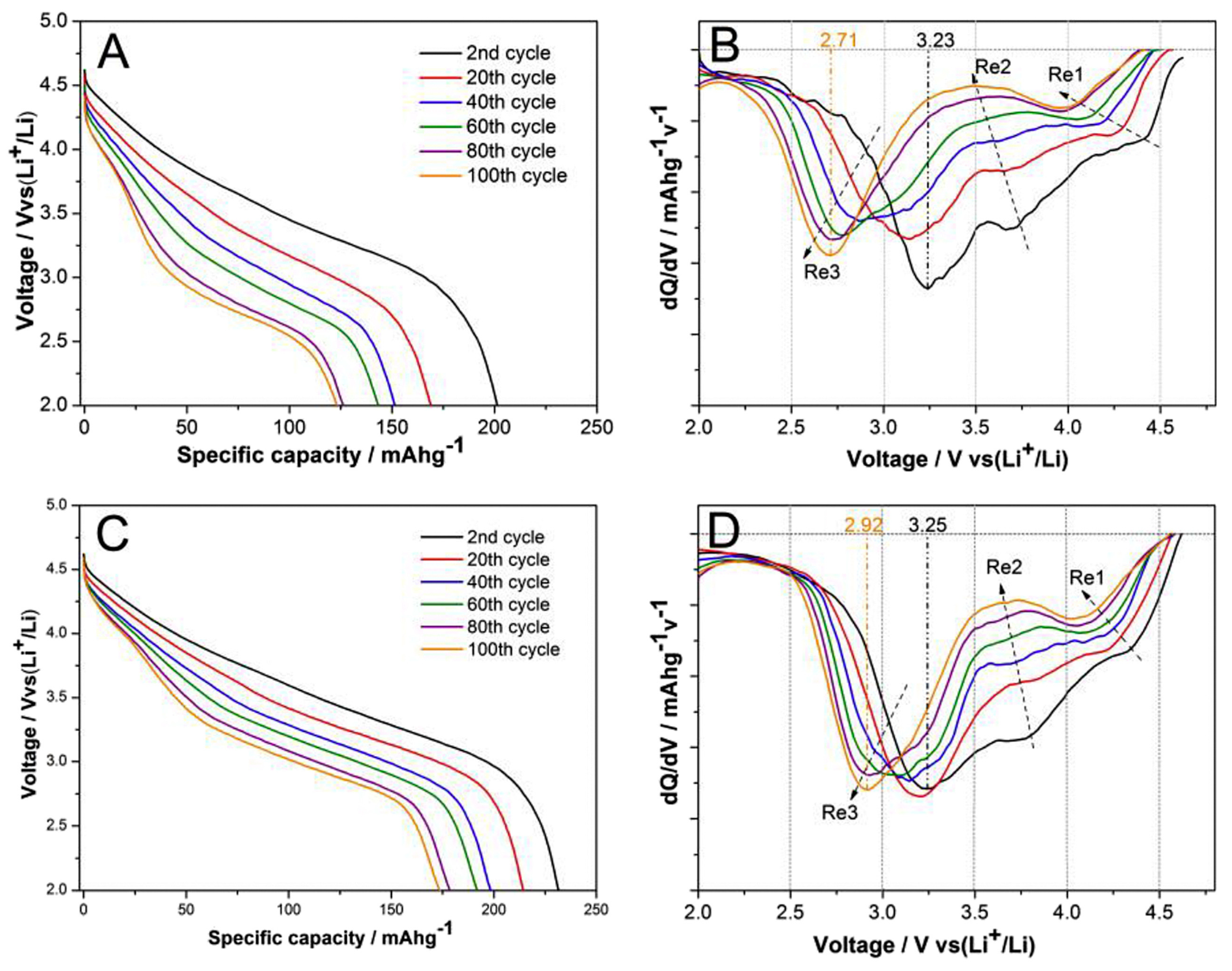
- Dogan, F.; Long, B.R.; Croy, J.R.; Gallagher, K.G.; Iddir, H.; Russell, J.T.; Balasubramanian, M.; Key, B. Re-entrant lithium local environments and defect driven electrochemistry of Li- and Mn-rich Li-ion battery cathodes. J. Am. Chem. Soc. 2015, 137, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Erickson, E.M.; Schipper, F.; Penki, T.R.; Shin, J.-Y.; Erk, C.; Chesneau, F.-F.; Markovsky, B.; Aurbach, D. Review—recent advances and remaining challenges for lithium ion battery cathodes: Li. Lithium-rich, xLi2MnO3·(1 − x)LiNiaCobMncO2. J. Electrochem. Soc. 2017, 164, A6341–A6348. [Google Scholar] [CrossRef]
- Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.-Y.; Chesneau, F.F.; Aurbach, D. Review—Recent advances and remaining challenges for lithium ion battery cathodes: I. Nickel-rich, LiNixCoyMnzO2. J. Electrochem. Soc. 2017, 164, A6220–A6228. [Google Scholar] [CrossRef]
- Freire, M.; Kosova, N.; Jordy, C.; Chateigner, D.; Lebedev, O.; Maignan, A.; Pralong, V. A new active Li-Mn–O compound for high energy density Li-ion batteries. Nat. Mater. 2016, 15, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, M.; Johnson, C.; Kim, J.-S.; Lauzze, K.; Vaughey, J.; Dietz, N.; Abraham, D.; Hackney, S.; Zeltner, W.; Anderson, M. ZrO2 and Li2ZrO3-stabilized spinel and layered electrodes for lithium batteries. Electrochem. Commun. 2003, 5, 752–758. [Google Scholar] [CrossRef]
- Myung, S.-T.; Izumi, K.; Komaba, S.; Yashiro, H.; Bang, H.J.; Sun, Y.-K.; Kumagai, N. Functionality of oxide coating for Li[Li0.05Ni0.4Co0.15Mn0.4]O2 as positive electrode materials for lithium-ion secondary batteries. J. Phys. Chem. C 2007, 111, 4061–4067. [Google Scholar]
- Liu, J.; Manthiram, A. Functional surface modifications of a high capacity layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode. J. Mater. Chem. 2010, 20, 3961–3967. [Google Scholar]
- Myung, S.-T.; Izumi, K.; Komaba, S.; Sun, Y.-K.; Yashiro, H.; Kumagai, N. Role of alumina coating on Li–Ni–Co–Mn–O particles as positive electrode material for lithium-ion batteries. Chem. Mater. 2005, 17, 3695–3704. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Z.; Wu, X.; Dong, Z.; Zhu, Z.; Yang, Y. The effects of ALF3 coating on the performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 positive electrode material for lithium-ion battery. J. Electrochem. Soc. 2008, 155, A775–A782. [Google Scholar]
- Yun, S.H.; Park, K.-S.; Park, Y.J. The electrochemical property of ZrFx-coated Li[Ni1/3Co1/3Mn1/3]O2 cathode material. J. Power Sources 2010, 195, 6108–6115. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.; Hong, Y.-S.; Kim, Y.; Kim, M.G.; Shin, N.-S.; Cho, J. Structural characterization of the surface-modified LixNi0.9Co0.1O2 cathode materials by MPO4 coating (M = Al, Ce, Sr, and Fe) for Li-ion cells. J. Electrochem. Soc. 2006, 153, A781–A786. [Google Scholar]
- Sun, Y.K.; Lee, M.J.; Yoon, C.S.; Hassoun, J.; Amine, K.; Scrosati, B. The role of AlF3 coatings in improving electrochemical cycling of Li-enriched nickel-manganese oxide electrodes for Li-ion batteries. Adv. Mater. 2012, 24, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- König, R.; Scholz, G.; Scheurell, K.; Heidemann, D.; Buchem, I.; Unger, W.E.S.; Kemnitz, E. Spectroscopic characterization of crystalline AlF3 phases. J. Fluor. Chem. 2010, 131, 91–97. [Google Scholar] [CrossRef]
- Amalraj, S.F.; Markovsky, B.; Sharon, D.; Talianker, M.; Zinigrad, E.; Persky, R.; Haik, O.; Grinblat, J.; Lampert, J.; Schulz-Dobrick, M. Study of the electrochemical behavior of the “inactive” Li2MnO3. Electrochim. Acta 2012, 78, 32–39. [Google Scholar] [CrossRef]
- Stechert, T.; Rushton, M.; Grimes, R.; Dillon, A. Predicted structure, thermo-mechanical properties and Li ion transport in LiAlF4 glass. J. Non Cryst. Solids 2012, 358, 1917–1923. [Google Scholar] [CrossRef]
- Amalraj, F.; Kovacheva, D.; Talianker, M.; Zeiri, L.; Grinblat, J.; Leifer, N.; Goobes, G.; Markovsky, B.; Aurbach, D. Synthesis of integrated cathode materials xLi2MnO3·(1 − x)LiMn1/3Ni1/3Co1/3O2 (x = 0.3, 0.5, 0.7) and studies of their electrochemical behavior. J. Electrochem. Soc. 2010, 157, A1121–A1130. [Google Scholar]
- Armstrong, A.; Robertson, A.; Bruce, P. Structural transformation on cycling layered Li(Mn1−yCoy)O2 cathode materials. Electrochim. Acta 1999, 45, 285–294. [Google Scholar] [CrossRef]
- Ito, A.; Shoda, K.; Sato, Y.; Hatano, M.; Horie, H.; Ohsawa, Y. Direct observation of the partial formation of a framework structure for Li-rich layered cathode material Li[Ni0.17Li0.2Co0.07Mn0.56]O2 upon the first charge and discharge. J. Power Sources 2011, 196, 4785–4790. [Google Scholar]
- Myung, S.-T.; Lee, K.-S.; Yoon, C.S.; Sun, Y.-K.; Amine, K.; Yashiro, H. Effect of ALF3 coating on thermal behavior of chemically delithiated Li0.35[Ni1/3Co1/3Mn1/3]O2. J. Phys. Chem. C 2010, 114, 4710–4718. [Google Scholar]
- Kim, J.-H.; Park, M.-S.; Song, J.-H.; Byun, D.-J.; Kim, Y.-J.; Kim, J.-S. Effect of aluminum fluoride coating on the electrochemical and thermal properties of 0.5 Li2MnO3·0.5LiNi0.5Co0.2Mn0.3O2 composite material. J. Alloy. Compd. 2012, 517, 20–25. [Google Scholar]
- Sun, Y.-K.; Cho, S.-W.; Lee, S.-W.; Yoon, C.; Amine, K. ALF3-coating to improve high voltage cycling performance of Li[Ni1/3Co1/3Mn1/3]O2 cathode materials for lithium secondary batteries. J. Electrochem. Soc. 2007, 154, A168–A172. [Google Scholar] [CrossRef]
- Lee, K.-S.; Myung, S.-T.; Kim, D.-W.; Sun, Y.-K. AlF3-coated LiCoO2 and Li[Ni1/3Co1/3Mn1/3]O2 blend composite cathode for lithium ion batteries. J. Power Sources 2011, 196, 6974–6977. [Google Scholar] [CrossRef]
- Liu, W.; Oh, P.; Liu, X.; Myeong, S.; Cho, W.; Cho, J. Countering voltage decay and capacity fading of lithium-rich cathode material at 60 °C by hybrid surface protection layers. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Guan, C.; Wang, J. Recent development of advanced electrode materials by atomic layer deposition for electrochemical energy storage. Adv. Sci. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Ham, G.; Jin, B.-S.; Lee, S.-M.; Lee, Y.M.; Wang, G.; Kim, H.-S. Ultra-thin Al2O3 coating on the acid-treated 0.3Li2MnO3·0.7LiMn0.60Ni0.25Co0.15O2 electrode for Li-ion batteries. J. Alloys Compd. 2014, 608, 110–117. [Google Scholar]
- Oh, P.; Ko, M.; Myeong, S.; Kim, Y.; Cho, J. A novel surface treatment method and new insight into discharge voltage deterioration for high-performance 0.4Li2MnO3–0.6LiNi1/3Co1/3Mn1/3O2 cathode materials. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Nayak, P.K.; Grinblat, J.; Levi, M.; Markovsky, B.; Aurbach, D. Structural and electrochemical evidence of layered to spinel phase transformation of Li and Mn rich layered cathode materials of the formulae xLi[Li1/3Mn2/3]O2·(1 − x)LiMn1/3Ni1/3Co1/3O2 (x = 0.2, 0.4, 0.6) upon cycling. J. Electrochem. Soc. 2014, 161, A1534–A1547. [Google Scholar]
- Qiao, Q.-Q.; Qin, L.; Li, G.-R.; Wang, Y.-L.; Gao, X.-P. Sn-stabilized Li-rich layered Li(Li0.17Ni0.25Mn0.58)O2 oxide as a cathode for advanced lithium-ion batteries. J. Mater. Chem. A 2015, 3, 17627–17634. [Google Scholar]
- Wang, D.; Huang, Y.; Huo, Z.; Chen, L. Synthesize and electrochemical characterization of Mg-doped Li-rich layered Li[Li0.2Ni0.2Mn0.6]O2 cathode material. Electrochim. Acta 2013, 107, 461–466. [Google Scholar]
- Nayak, P.K.; Grinblat, J.; Levi, M.; Levi, E.; Kim, S.; Choi, J.W.; Aurbach, D. Al doping for mitigating the capacity fading and voltage decay of layered Li and Mn-rich cathodes for Li-ion batteries. Adv. Energy Mater. 2016. [Google Scholar] [CrossRef]
- Nayak, P.K.; Grinblat, J.; Levi, E.; Levi, M.; Markovsky, B.; Aurbach, D. Understanding the influence of Mg doping for the stabilization of capacity and higher discharge voltage of Li- and Mn-rich cathodes for Li-ion batteries. Phys. Chem. Chem. Phys. 2017, 19, 6142–6152. [Google Scholar] [CrossRef] [PubMed]
- Erickson, E.M.; Schipper, F.; Tian, R.; Shin, J.-Y.; Erk, C.; Chesneau, F.F.; Lampert, J.K.; Markovsky, B.; Aurbach, D. Enhanced capacity and lower mean charge voltage of Li-rich cathodes for lithium ion batteries resulting from low-temperature electrochemical activation. RSC Adv. 2017, 7, 7116–7121. [Google Scholar] [CrossRef]
- Vu, A.; Qian, Y.; Stein, A. Porous electrode materials for lithium-ion batteries—How to prepare them and what makes them special. Adv. Energy Mater. 2012, 2, 1056–1085. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Li, Y.; Lemaire, A.; Yu, J.-G.; Su, B.-L. Hierarchically structured functional materials: Synthesis strategies for multimodal porous networks. Pure Appl. Chem. 2009, 81, 2265–2307. [Google Scholar] [CrossRef]
- Zhang, L.; Borong, W.; Ning, L.; Feng, W. Hierarchically porous micro-rod lithium-rich cathode material Li1.2Ni0.13Mn0.54Co0.13O2 for high performance lithium-ion batteries. Electrochim. Acta 2014, 118, 67–74. [Google Scholar]
- Ma, S.; Hou, X.; Li, Y.; Ru, Q.; Hu, S.; Lam, K.-H. Performance and mechanism research of hierarchically structured Li-rich cathode materials for advanced lithium-ion batteries. J. Mater. Sci.. Mater. Electron. 2017, 28, 2705–2715. [Google Scholar] [CrossRef]
- Duraisamy, S.; Penki, T.R.; Nookala, M. Hierarchically porous Li1.2Mn0.6Ni0.2O2 as a high capacity and high rate capability positive electrode material. New J. Chem. 2016, 40, 1312–1322. [Google Scholar]
- Penki, T.R.; Shanmughasundaram, D.; Jeyaseelan, A.; Subramani, A.; Munichandraiah, N. Polymer template assisted synthesis of porous Li1.2Mn0.53Ni0.13Co0.13O2 as a high capacity and high rate capability positive electrode material. J. Electrochem. Soc. 2014, 161, A33–A39. [Google Scholar]
- Penki, T.R.; Shanmughasundaram, D.; Kishore, B.; Jeyaseelan, A.; Subramani, A.; Munichandraiah, N. Composite of Li-rich Mn, Ni and Fe oxides as positive electrode materials for Li-ion battery. J. Electrochem. Soc. 2016, 163, A1493–A1502. [Google Scholar] [CrossRef]
- Penki, T.R.; Shanmughasundaram, D.; Munichandraiah, N. Porous lithium rich Li1.2Mn0.54Ni0.22Fe0.04O2 prepared by microemulsion route as a high capacity and high rate capability positive electrode material. Electrochim. Acta 2014, 143, 152–160. [Google Scholar]
- Boutonnet, M.; Kizling, J.; Stenius, P.; Maire, G. The preparation of monodisperse colloidal metal particles from microemulsions. Coll. Surf. 1982, 5, 209–225. [Google Scholar] [CrossRef]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic ia 3D large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 2003, 17, 2136–2137. [Google Scholar] [CrossRef]
- Chen, M.; Xiang, X.; Chen, D.; Liao, Y.; Huang, Q.; Li, W. Polyethylene glycol-assisted synthesis of hierarchically porous layered lithium-rich oxide as cathode of lithium ion battery. J. Power Sources 2015, 279, 197–204. [Google Scholar] [CrossRef]
- Li, Q.; Li, G.; Fu, C.; Luo, D.; Fan, J.; Li, L. K+-doped Li1.2Mn0.54Co0.13Ni0.13O2: A novel cathode material with an enhanced cycling stability for lithium-ion batteries. ACS Appl. Mater. Interf. 2014, 6, 10330–10341. [Google Scholar]
- Song, B.; Liu, H.; Liu, Z.; Xiao, P.; Lai, M.O.; Lu, L. High rate capability caused by surface cubic spinels in Li-rich layer-structured cathodes for Li-ion batteries. Sci. Rep. 2013, 3, 3094. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Belharouak, I.; Zheng, J.; Wu, H.; Xiao, J.; Genc, A.; Amine, K.; Thevuthasan, S.; Baer, D.R.; Zhang, J.-G. Formation of the spinel phase in the layered composite cathode used in Li-ion batteries. ACS Nano 2012, 7, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Fell, C.R.; Chi, M.; Meng, Y.S. Identifying surface structural changes in layered Li-excess nickel manganese oxides in high voltage lithium ion batteries: A joint experimental and theoretical study. Energy Environ. Sci. 2011, 4, 2223–2233. [Google Scholar] [CrossRef]
- Zheng, J.; Gu, M.; Genc, A.; Xiao, J.; Xu, P.; Chen, X.; Zhu, Z.; Zhao, W.; Pullan, L.; Wang, C. Mitigating voltage fade in cathode materials by improving the atomic level uniformity of elemental distribution. Nano Lett. 2014, 14, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Gu, M.; Xiao, J.; Zuo, P.; Wang, C.; Zhang, J.-G. Corrosion/fragmentation of layered composite cathode and related capacity/voltage fading during cycling process. Nano Lett. 2013, 13, 3824–3830. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhuang, H.; Ma, Q.; Jiao, Z.; Zhang, H.; Liu, R.; Chu, Y.; Zhao, B. Synthesis of porous Li2MnO3-LiNi1/3Co1/3Mn1/3O2 nanoplates via colloidal crystal template. J. Mater. Res. 2013, 28, 1505–1511. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, J.; Zhang, C.; Wu, B.; Wu, F. High-rate layered lithium-rich cathode nanomaterials for lithium-ion batteries synthesized with the assist of carbon spheres templates. J. Power Sources 2016, 331, 247–257. [Google Scholar] [CrossRef]
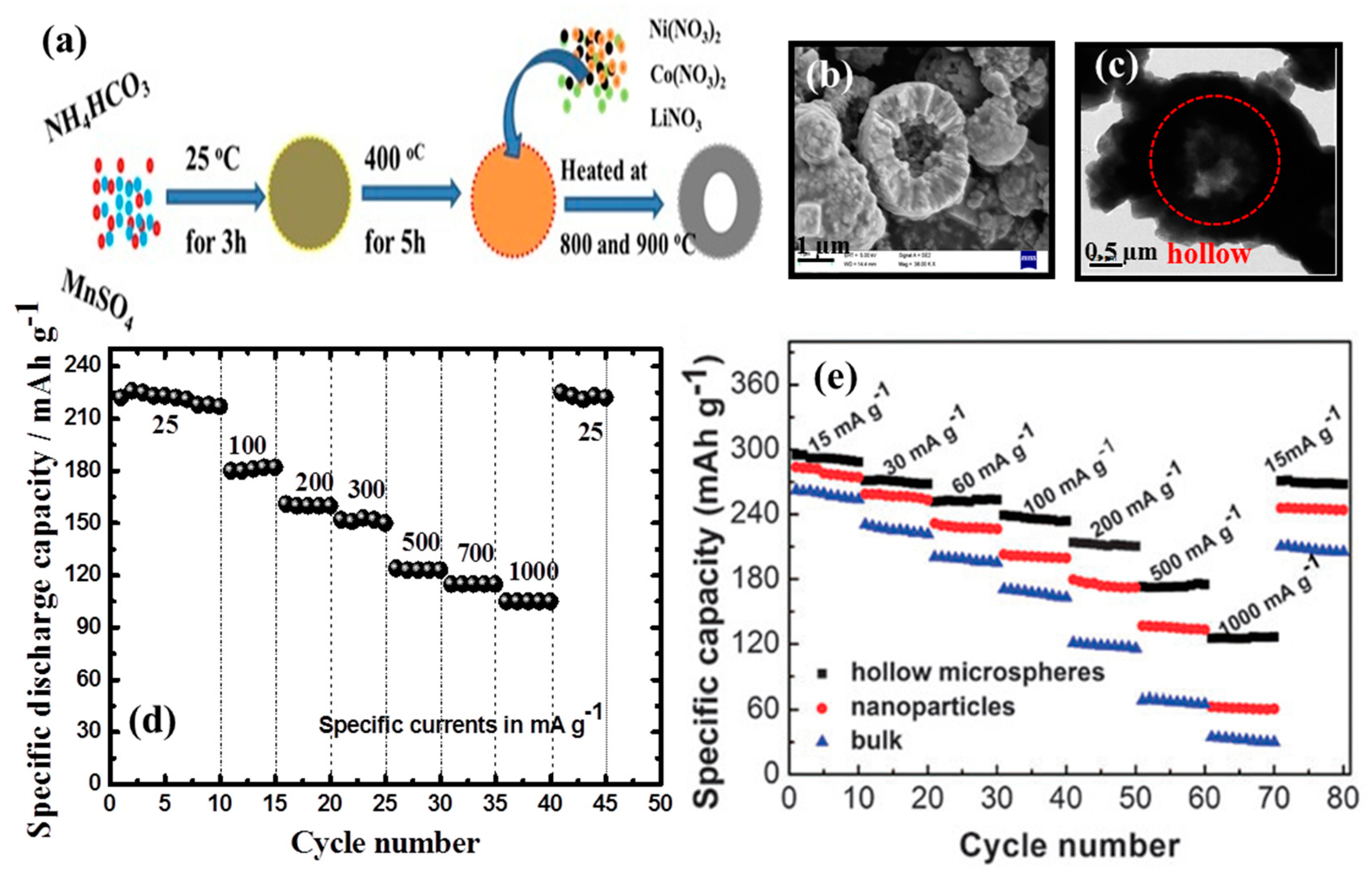
- Jiang, Y.; Yang, Z.; Luo, W.; Hu, X.; Huang, Y. Hollow 0.3Li2MnO3·0.7LiNi0.5Mn0.5O2 microspheres as a high-performance cathode material for lithium–ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 2954–2960. [Google Scholar]
- Wei, C.; Deng, J.; Xi, L.; Zhou, H.; Wang, Z.; Chung, C.Y.; Yao, Q.; Rao, G. High power LiMn2O4 hollow microsphere cathode materials for lithium ion batteries. Int. J. Electrochem. Sci. 2013, 8, 6775–6783. [Google Scholar]
- Duraisamy, S.; Penki, T.R.; Kishore, B.; Barpanda, P.; Nayak, P.K.; Aurbach, D.; Munichandraiah, N. Porous, hollow Li1.2Mn0.53Ni0.13Co0.13O2 microspheres as a positive electrode material for Li-ion batteries. J. Solid State Electrochem. 2017, 21, 437–445. [Google Scholar]
- Hu, Y.S.; Adelhelm, P.; Smarsly, B.M.; Hore, S.; Antonietti, M.; Maier, J. Synthesis of hierarchically porous carbon monoliths with highly ordered microstructure and their application in rechargeable lithium batteries with high-rate capability. Adv. Funct. Mater. 2007, 17, 1873–1878. [Google Scholar] [CrossRef]
- Sinha, N.N.; Shivakumara, C.; Munichandraiah, N. High rate capability of a dual-porosity LiFePO4/C composite. ACS Appl. Mater. Interf. 2010, 2, 2031–2038. [Google Scholar] [CrossRef]
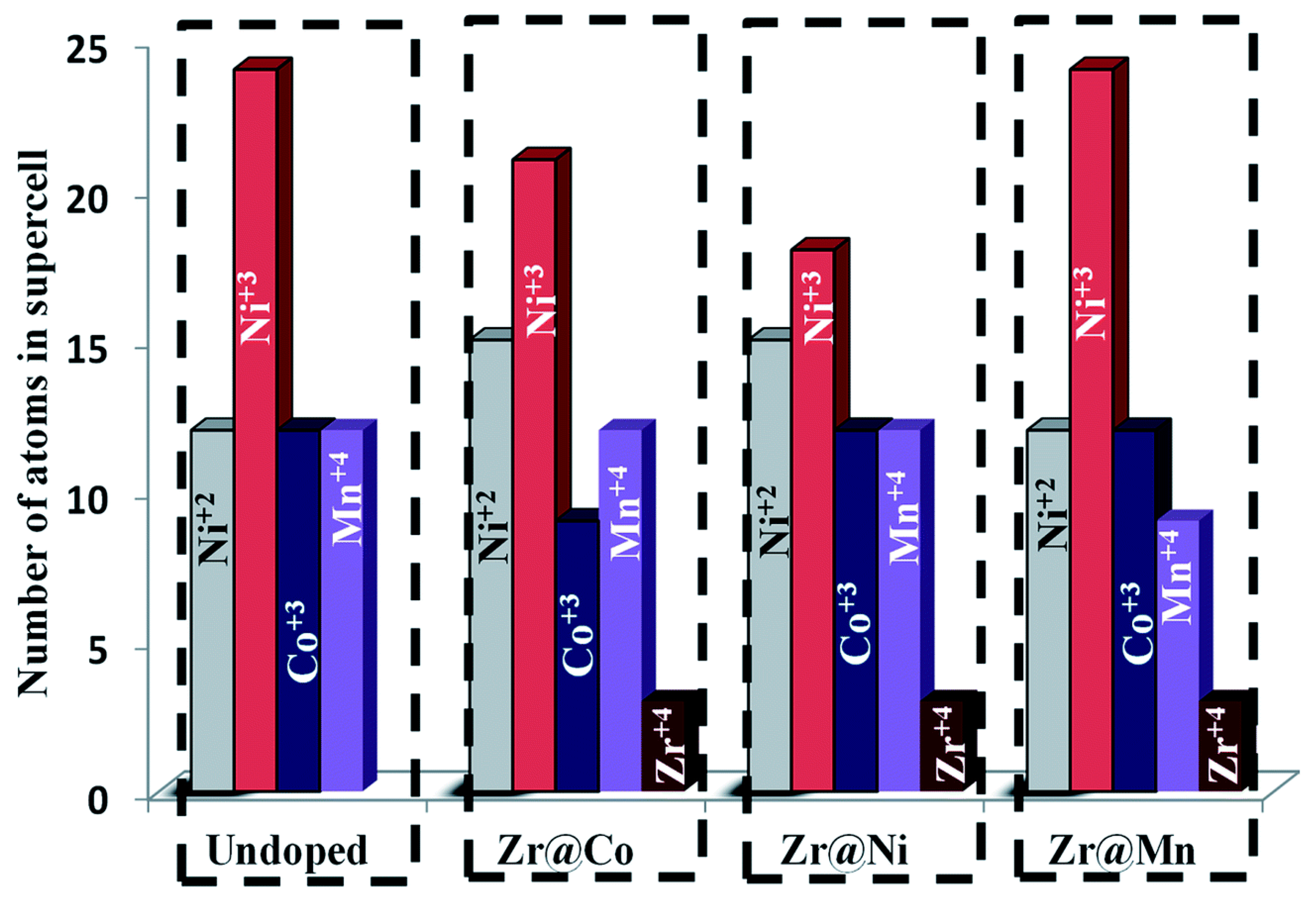
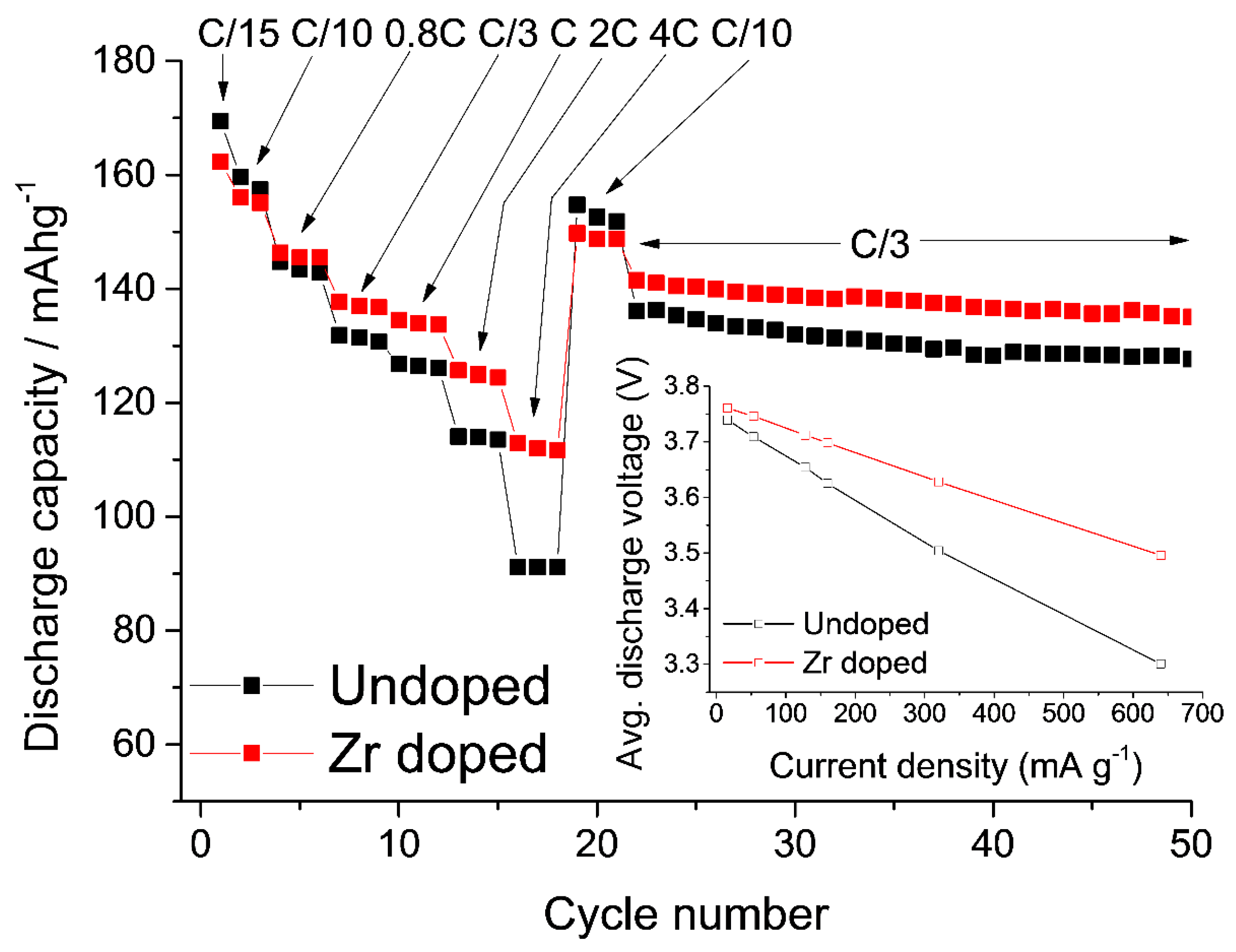
- Schipper, F.; Dixit, M.; Kovacheva, D.; Talianker, M.; Haik, O.; Grinblat, J.; Erickson, E.M.; Ghanty, C.; Major, D.T.; Markovsky, B.; et al. Stabilizing nickel-rich layered cathode materials by a high-charge cation doping strategy: Zirconium-doped LiNi0.6Co0.2Mn0.2O2. J. Mater. Chem. A 2016, 4, 16073–16084. [Google Scholar] [CrossRef]
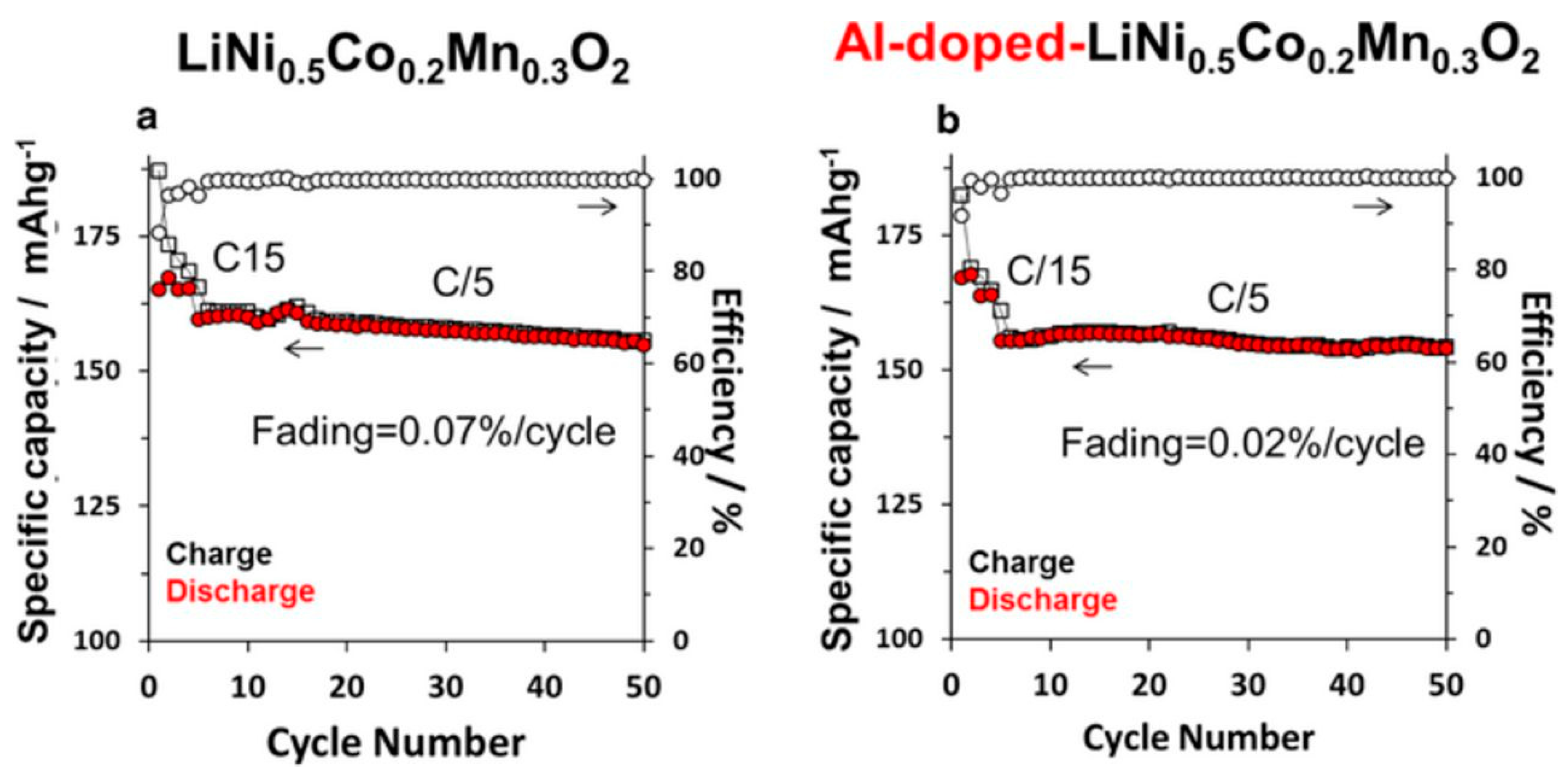
- Aurbach, D.; Srur-Lavi, O.; Ghanty, C.; Dixit, M.; Haik, O.; Talianker, M.; Grinblat, Y.; Leifer, N.; Lavi, R.; Major, D.T.; et al. Studies of aluminum-doped LiNi0.5Co0.2Mn0.3O2: Electrochemical behavior, aging, structural transformations, and thermal characteristics. J. Electrochem. Soc. 2015, 162, A1014–A1027. [Google Scholar]
- Jin, X.; Xu, Q.; Liu, H.; Yuan, X.; Xia, Y. Excellent rate capability of Mg doped Li[Li0.2Ni0.13Co0.13Mn0.54]O2 cathode material for lithium-ion battery. Electrochim. Acta 2014, 136, 19–26. [Google Scholar]
- Liu, L.; Sun, K.N.; Zhang, N.Q.; Yang, T.Y. Improvement of high-voltage cycling behavior of Li(Ni1/3Co1/3Mn1/3)O2 cathodes by Mg, Cr, and Al substitution. J. Solid State Electrochem. 2009, 13, 1381–1386. [Google Scholar] [CrossRef]
- Liu, D.T.; Wang, Z.X.; Chen, L.Q. Comparison of structure and electrochemistry of Al- and Fe-doped LiNi1/3Co1/3Mn1/3O2. Electrochim. Acta 2006, 51, 4199–4203. [Google Scholar] [CrossRef]
- Chen, C.H.; Liu, J.; Stoll, M.E.; Henriksen, G.; Vissers, D.R.; Amine, K. Aluminum-doped lithium nickel cobalt oxide electrodes for high-power lithium-ion batteries. J. Power Sources 2004, 128, 278–285. [Google Scholar] [CrossRef]
- Jung, S.-K.; Gwon, H.; Hong, J.; Park, K.-Y.; Seo, D.-H.; Kim, H.; Hyun, J.; Yang, W.; Kang, K. Understanding the degradation mechanisms of LiNi0.5Co0.2Mn0.3O2 cathode material in lithium ion batteries. Adv. Energy Mater. 2014, 4, 1300787-n/a. [Google Scholar]
- Shin, D.W.; Bridges, C.A.; Huq, A.; Paranthaman, M.P.; Manthiram, A. Role of cation ordering and surface segregation in high-voltage spinel LiMn1.5Ni0.5–xMxO4 (M = Cr, Fe, and Ga) cathodes for lithium-ion batteries. Chem. Mater. 2012, 24, 3720–3731. [Google Scholar]
- Park, B.C.; Kim, H.B.; Myung, S.T.; Amine, K.; Belharouak, I.; Lee, S.M.; Sun, Y.K. Improvement of structural and electrochemical properties of AlF3-coated Li[Ni1/3Co1/3Mn1/3]O2 cathode materials on high voltage region. J. Power Sources 2008, 178, 826–831. [Google Scholar] [CrossRef]
- Bak, S.-M.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.D.; Cho, S.-J.; Kim, K.-B.; Chung, K.Y.; Yang, X.-Q.; Nam, K.-W. Structural changes and thermal stability of charged LiNixMnyCozO2 cathode materials studied by combined in situ time-resolved XRD and mass spectroscopy. ACS Appl. Mater. Interf. 2014, 6, 22594–22601. [Google Scholar] [CrossRef] [PubMed]
- Ghanty, C.; Markovsky, B.; Erickson, E.M.; Talianker, M.; Haik, O.; Tal-Yossef, Y.; Mor, A.; Aurbach, D.; Lampert, J.; Volkov, A.; et al. Li+-ion extraction/insertion of Ni-rich Li1+x(NiyCozMnz)wO2 (0.005 <x <0.03; y:z=8:1, w ≈ 1) electrodes: Insitu XRD and raman spectroscopy study. ChemElectroChem 2015, 2, 1479–1486. [Google Scholar]
- Amalraj, S.F.; Sharon, D.; Talianker, M.; Julien, C.M.; Burlaka, L.; Lavi, R.; Zhecheva, E.; Markovsky, B.; Zinigrad, E.; Kovacheva, D. Study of the nanosized Li2MnO3: Electrochemical behavior, structure, magnetic properties, and vibrational modes. Electrochim. Acta 2013, 97, 259–270. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, Y.; Amine, K.; Sun, Y.K. Role of surface coating on cathode materials for lithium-ion batteries. J. Mater. Chem. 2010, 20, 7606–7612. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Meng, X.; Tang, Y.; Banis, M.N.; Yang, J.; Hu, Y.; Li, R.; Cai, M.; Sun, X. Significant impact on cathode performance of lithium-ion batteries by precisely controlled metal oxide nanocoatings via atomic layer deposition. J. Power Sources 2014, 247, 57–69. [Google Scholar] [CrossRef]
- Ghanty, C.; Dahiya, P.P.; Basu, R.N.; Chang, J.-K.; Majumder, S.B. Improvement of the electrochemical characteristics of lithium and manganese rich layered cathode materials: Effect of surface coating. J. Electrochem. Soc. 2015, 162, A1957–A1965. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Chen, B.; Wang, Z.; Lu, C. An approach to application for LiNi0.6Co0.2Mn0.2O2 cathode material at high cutoff voltage by TiO2 coating. J. Power Sources 2014, 256, 20–27. [Google Scholar]
- Qin, C.; Cao, J.; Chen, J.; Dai, G.; Wu, T.; Chen, Y.; Tang, Y.; Li, A.; Chen, Y. Improvement of electrochemical performance of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode active material by ultrathin TiO2 coating. Dalton Trans. 2016, 45, 9669–9675. [Google Scholar] [PubMed]
- Cho, W.; Kim, S.-M.; Song, J.H.; Yim, T.; Woo, S.-G.; Lee, K.-W.; Kim, J.-S.; Kim, Y.-J. Improved electrochemical and thermal properties of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode materials by SiO2 coating. J. Power Sources 2015, 282, 45–50. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schipper, F.; Nayak, P.K.; Erickson, E.M.; Amalraj, S.F.; Srur-Lavi, O.; Penki, T.R.; Talianker, M.; Grinblat, J.; Sclar, H.; Breuer, O.; et al. Study of Cathode Materials for Lithium-Ion Batteries: Recent Progress and New Challenges. Inorganics 2017, 5, 32. https://doi.org/10.3390/inorganics5020032
Schipper F, Nayak PK, Erickson EM, Amalraj SF, Srur-Lavi O, Penki TR, Talianker M, Grinblat J, Sclar H, Breuer O, et al. Study of Cathode Materials for Lithium-Ion Batteries: Recent Progress and New Challenges. Inorganics. 2017; 5(2):32. https://doi.org/10.3390/inorganics5020032
Chicago/Turabian StyleSchipper, Florian, Prasant Kumar Nayak, Evan M. Erickson, S. Francis Amalraj, Onit Srur-Lavi, Tirupathi Rao Penki, Michael Talianker, Judith Grinblat, Hadar Sclar, Ortal Breuer, and et al. 2017. "Study of Cathode Materials for Lithium-Ion Batteries: Recent Progress and New Challenges" Inorganics 5, no. 2: 32. https://doi.org/10.3390/inorganics5020032
APA StyleSchipper, F., Nayak, P. K., Erickson, E. M., Amalraj, S. F., Srur-Lavi, O., Penki, T. R., Talianker, M., Grinblat, J., Sclar, H., Breuer, O., Julien, C. M., Munichandraiah, N., Kovacheva, D., Dixit, M., Major, D. T., Markovsky, B., & Aurbach, D. (2017). Study of Cathode Materials for Lithium-Ion Batteries: Recent Progress and New Challenges. Inorganics, 5(2), 32. https://doi.org/10.3390/inorganics5020032