Abstract
Treatment of K[N(SiMe3)2] with N,N′-bis(2,6-difluorophenyl)formamidine (DFFormH) in toluene, resulted in the formation of [K(DFForm)]∞ (1) as a poorly soluble material. Upon dissolution in thf and layering with n-hexane, 1 was crystallised and identified as a two-dimensional polymer, in which all fluorine and nitrogen atoms, and also part of one aryl group, bridge between four symmetry equivalent potassium ions, giving rise to a completely unique μ4-(N,N′,F,F′):(N,N′):η4(Ar-C(2,3,4,5,6)):(F″,F′′′) DFForm coordination. The two-dimensional nature of the polymer could be deconstructed to one dimension by crystallisation from neat thf at −35 °C, giving [K2(DFForm)2(thf)2]∞ (2), where the thf molecules bridge the monomeric units. Complete polymer dissociation was observed when 1 was crystallised from toluene/n-hexane mixtures in the presence of 18-crown-6, giving [K(DFForm)(18-crown-6)] (3), which showed unprecedented κ(N,Cispo,F) DFForm coordination, rather than the expected κ(N,N′) coordination.
1. Introduction
With the ability to adopt numerous coordination modes, flexible N,N′-bis(aryl)formamidinates (and by extension aryl-functionalised amidinates) have earned a special place in coordination chemistry [1,2,3,4,5]. Not only does the anionic NCHN bite provide a variety of different nitrogen-based coordination modes (e.g., monodentate κ(N), bidentate κ(N,N′), or various bridging modes e.g., μ-1κ(N):2κ(N′), μ-1κ(N,N′):2κ(N,N′) to list a few) [6,7], the nitrogen-bound aromatic substituents can also provide additional coordination modes. The potential to form metal–arene interactions, such as η6 coordination, has been largely observed in group one chemistry [8,9,10,11,12,13], though some examples are known in f-block chemistry [14]. In almost all examples of this aromatic coordination, the phenyl rings contained alkyl-substituents in either the 2,6 positions (e.g., iPr, Et, Me), or in the 2,4,6 positions (e.g., Me). This is likely due to a combination of steric pressure, which starves the metal centre from coordination of additional donors, and increased electron donation from the aromatic ring caused by the alkyl substituents. Another means to engage the aromatic component in coordination is through the addition of donor functionalities (e.g., OMe, F), especially in the ortho-positions, thereby transforming the formamidinate ligand into a tri- [15,16,17], or tetra-dentate (e.g., N,N′,X or N,N′,X,X′) [17], chelate, with examples across a variety of different metal classes [18]. For s-block chemistry however, the use of such ligands has been restricted to very few examples, namely the use of N,N′-bis(2-fluorophenyl)formamidine (FForm) [19].
Nearly 15 years ago, FForm was complexed to the group one metals Li, Na, and K [19]. Akin to the transition metal complexes of Cotton and co-workers [20], the presence of the fluorine atom on the ortho-position of the aromatic rings permitted an additional coordinating site. This further led to partial, or complete, exclusion of bound donor molecules (e.g., Et2O, thf), by the formation of either binuclear, or for potassium, polymeric constructs (e.g., [Na(FForm)(Et2O)]2 or [K(FForm)]∞) [19]. This contrasts the group one complexes of the non-fluorinated N,N′-di(aryl)formamidinate ligands [21,22,23], which readily retain coordinating solvent. Since then, we have expanded the use of fluorinated formamidinate ligands to f-block chemistry [9,16,17,24,25,26,27,28], in a variety of different contexts [5]. One of the fluorinated formamidinate ligands used was N,N′-bis(2,6-difluorophenyl)formamidinate (DFForm) in both trivalent [16,17], and divalent [16] rare-earth complexes. Despite the presence of the additional fluorine atoms, the observation of any M–F interaction was rare, typically only occurred in unsolvated species, and interactions were displaced on coordination of donor solvents [16,17]. It is likely that the smaller ionic radii of the trivalent rare-earths, compared with the larger potassium ion [29], create a significant strain in the NCHN bite of the DFForm ligand when it coordinates the fluorine atoms, and therefore donor solvent coordination is preferred. Although DFForm has been used in some transition metal complexes, it has no precedent in s-block chemistry. We hypothesised that the additional two fluorine atoms over FForm could engage in further coordination chemistry, generating different coordination modes from FForm, and quite spectacular results have been obtained by way of new formamidinate binding modes.
2. Results and Discussion
Treatment of K[N(SiMe3)2] with DFFormH in toluene resulted in the formation of a colourless, poorly soluble white powder. Upon dissolution in thf, concentration, and layering with n-hexane, white crystals of targeted [K(DFForm)]∞ (1, Scheme 1i) were obtained. The structure of 1 was determined by X-ray crystallography, revealing that 1 is a two-dimensional polymer. The binding of the DFForm ligand in 1 is complex, and is discussed starting from the asymmetric unit (ASU), and then extending in both dimensions of the polymeric network.
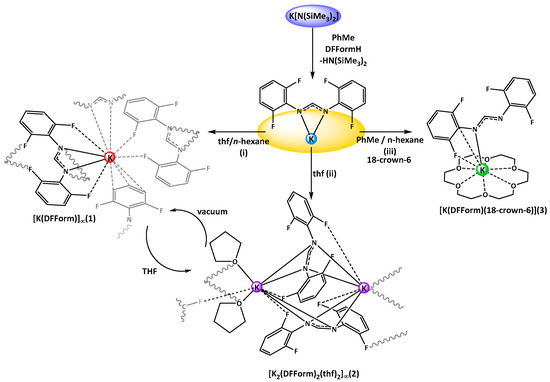
Scheme 1.
Synthesis of K(DFForm) complexes (1–3) by protonolysis and crystallisation from different solvent mixtures. (i) thf, n-hexane, at room temperature; (ii) neat thf, crystallisation at −35 °C; (iii) toluene, n-hexane, crystallisation at room temperature. The diagram further indicates the different bonding modes of the DFForm ligand in complexes, such as the (F,N,N′,F′) or arene–K interactions in 1, the twisted 1κ(F,N,N′,):2κ(N,N′,F′) DFForm coordination in 2, or the unusual (N,Cipso,F) coordination in 3.
Complex 1 crystallised in the triclinic space group P-1, with only one potassium ion and one DFForm ligand in the ASU (Figure 1A). The DFForm ligand of the ASU is bound (F,N,N′,F′) to the ASU potassium ion. This tetradentate binding of the DFForm ligand contrasts that of the FForm ligand in [K(FForm)]∞ [19], where the ASU contains one FForm ligand bound η4(N,(Ar-C6,5),F) to potassium. As the K ion is bound by the DFForm NCHN bite in an almost symmetrical manner, and does not favour one nitrogen donor (as observed in [K(FForm)], the K···F–C bonding is weak, and thus the C–F bonds in 1 (of either C1/F1 or C9/F3, Figure 1) are almost unchanged from those of DFFormH (C–F: 1.3596(17)–1.3625(18)) [30]. By contrast, the asymmetrical NCHN binding of FForm to K in [K(FForm)]∞ (along with the coordination across the aromatic component), brings the fluorine atom into a closer proximity to the potassium atom (K–F: 3.029(4)), and weakens the C–F bond (C–F: 1.377(6) Å) [19]. Another example of such tetradentate (F,N,N′,F′) DFForm coordination was observed in the homoleptic cerium DFForm complex, [Ce(DFForm)3] [17], where one of three DFForm ligands is tetradentate, with the other two being tri-dentate (F,N,N′). In this example, all Ce···F–C interactions were identical at 2.92 Å (range: 2.9187(13)–2.9213(13)), and consequentially each C–F bond was also strained to a similar degree (range: 1.374(1)–1.376(1) Å). However, considering that a ten-coordinate cerium(III) is smaller than a nine-coordinate potassium (difference in ionic radii: −0.3 Å) [29], the tetradentate DFForm ligand for the cerium complex had to bend the aryl-rings towards the cerium ion to bring the fluorine atoms into proximity, causing a strain on the Cipso–N–CH angle (range: 126.4(2)°–128.3(2)°). However, due to the larger ionic radii of potassium, this phenomenon is not observed in 1 (range: 120.39(9)°–120.75(9)°).
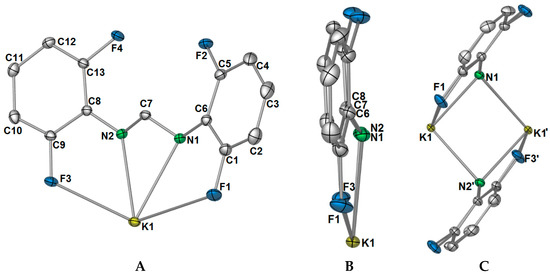
Figure 1.
(A) Asymmetric unit of [K(DFForm)]∞ (1). Selected bond lengths (Å) and angles (°): K1–F1: 3.3692(8), K1–N1: 2.8102(9), K1–N2: 2.8057(9), K1–F3: 3.3957(8), C1–F1: 1.3609(13), C9–F3: 1.3581(12), F1–K1–N1: 50.73(2), F3–K1–N2: 50.24(2). Ellipsoids were shown at the 50% probability level, and hydrogen atoms were removed for clarity; (B) side view of K(DFForm) showing that the DFForm ligand is not flat; (C) simplification of the μ-(N,N′,F,F′):(N,N′) bridging of the DFForm ligand. Selected bond lengths (Å) and angles (°): K1–N1′: 2.8102(9), K1–N2′: 2.9048(9), K1–K1′: 3.4871(4), K1–(N1/N2cent)–K1′: 84.02(1), N1/2cent–K1–N1′/2′cent: 95.98(1).
The differences in coordination between the DFForm and FForm ligands to potassium becomes considerably more apparent with expansion of the coordination mode of the ligands through bridging. Initial extension of the coordination of the DFForm ligand in 1 shows that the nitrogen atoms are further bridging to an adjacent potassium ion in a μ-(N,N′):(N,N′) manner (Figure 1C, also Figure 2A). Such formamidinate bridging is known for other s- and f-block complexes [16,22,31]. This bridging is mirrored by a symmetry equivalent DFForm ligand, generating a potassium nitrogen based cube of volume: 6.78 Å3 (Figure 1C). In stark contrast, the FForm system shows a twisted μ-(N,CipsoCortho,F):(N,N′,F′) FForm binding, where the NCHN bite is shared asymmetrically across two anent potassium atoms. It should be further noted that the aromatic group, nitrogen atoms, and backbone/ipso carbon atoms of DFForm are not flat and that the DFForm ligand is tilted (Figure 1B). The two nitrogen atoms coordinate to potassium in an almost symmetrical manner, but C7 is puckered away from the nitrogen atoms (K1–N1/2(cent)–C7: 141.10(8)°), so it is almost in line with the two ipso carbon atoms of the phenyl rings (C6–C7–C8: 177.26(6)°, c.f. (K(FForm): 168.9(3)°). This puckered nature of the DFForm ligand is typical of other formamidinate complexes which bridge in a μ-(N,N′):(N,N′) fashion (e.g., [K(p-TolForm)(dme)]∞ (K1–N1/2(cent)–C″7″: 144.8(3)°, p-TolForm = N,N′-bis(4-methylphenyl)formamidinate) [32].
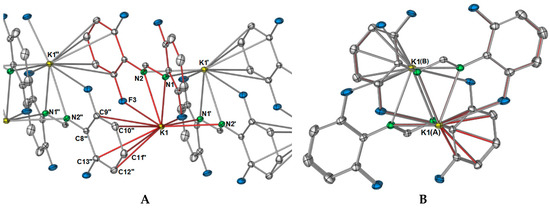
Figure 2.
Growth of one dimension of the [K(DFForm)]∞ (1) polymer network through aromatic interactions, the red bonds indicate the connectivity to the ASU potassium ion and DFForm ligand. (A) View along the side of the polymer; Selected bond lengths (Å) and angles (°): K1–C9′: 3.4980(11), K1–C10′: 3.3220(11), K1–C11″: 3.2417(11), K1–C12″: 3.3569(11), K1–C13″: 3.5066(10); (B) view down the a-axis of the polymeric network of 1.
The polymeric network of 1 is complicated. One might expect that, as the DFForm ligand bridges in a μ-(N,N′):(N,N′) manner between potassium ions, and that this is the repeating dinuclear unit (e.g., [K2(μ-(N,N′):(N,N′)-DFForm)2]∞), but this is not the case. Instead, one dimension of the polymer is generated through aromatic interactions of one 2,6-difluorophenyl group (Figure 2A), where the aromatic ring of N2 binds to K1′, and the aromatic ring (but without the ipso carbon) of N2′ coordinates to K1, both in a η5(C2,3,4,5,6) manner. Thus, this direction of the polymeric network has an “A, B” alternating potassium ion arrangement where A = K and B = K′ and K″ (Figure 2). For the FForm system, the one and only dimension of the polymeric network is generated by additional nitrogen based bonding to two other potassium ions, namely through one (N,F) interaction, and one (N′,Cispo′) interaction, making the overall coordination of each FForm ligand shared across four potassium ions as μ4-(N,CipsoCortho,F):(N,N′,F′):(N,F):(N′,Cispo′). The DFForm ligand is also further bridging to a fourth symmetry equivalent potassium ion, and this binding is completely different from that in the FForm system.
As shown in Figure 2A, there is an apparent coordination gap in axial positions of the potassium ions, and it is in this position that the other two fluorine atoms (namely, F2 and F4) of the DFForm ligand become relevant, and expand the one-dimensional polymeric network into a two-dimensional polymer. The further fluorine atoms (F2 and F4) coordinate to an adjacent potassium ion in a μ-(F″,F′′′) manner, generating a ten-membered ring (Figure 3A). Because of this additional coordination, the DFForm ligand is nearly planar across the K and K′′′ atoms, with the bond angle of K–N1/N2cent–K′′′ being 175.66(1)°. Although the auxiliary fluorine atoms are coordinated at a considerably shorter distance than the K–F1 and K–F3 analogues, there is still only a minor shortening of the C–F bonds from those of DFFormH (C–F: 1.3596(17)–1.3625(18)) [30]).
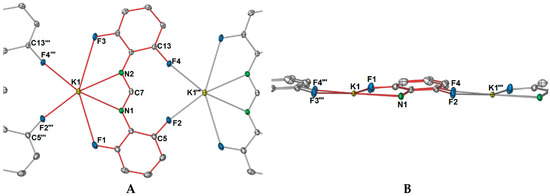
Figure 3.
Simplified diagram of the bonding of the auxiliary fluorine atoms (F2, F4) to an adjacent potassium ion, expanding the polymeric network into a second direction (across the b axis). (A) Top view of bonding showing the formation of a ten-membered ring upon fluorine coordination; (B) side view of auxiliary fluorine bonding (or side view of b-axis), highlighting the different planes within the DFForm ligand. Selected bond lengths (Å) and angles (°): K1–F2′′′: 2.7110(8), K1–F4′′′: 2.7422(7), C5–F2: 1.3641(14), C13–F4: 1.3656(11), K1–K1′′′: 7.5357(2), K1–N1/2cent–K1′′′: 175.66(1), K1–C7–K1′′′: 171.37(3), K1–F2/4cent–K′′′: 176.69(1).
In summation, each DFForm ligand binds four symmetry equivalent potassium ions in a μ4-1κ(N,N′,F,F′):2κ(N,N′):3η5(Ar-C(2,3,4,5,6)):4κ(F″,F′′′) manner, giving the potassium ion a coordination number of 11. Such an interesting binding mode exemplifies how the simple addition of other donors to a ligand system can dramatically alter the coordination network. Furthermore, it appears the 1 is the first crystallographically characterised example across all metal classes, where one N,N′-bis(aryl)formamidinate ligand generates a two-dimensional polymer network, all other examples are restricted to one dimension [18]. The complete polymeric network of 1 is displayed in Figure 4, showing both how the DFForm bridges across four potassium ions (Figure 4A) and the two dimensions of the polymer (Figure 4B,C).
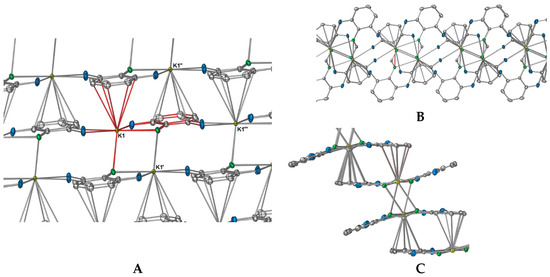
Figure 4.
Excerpt pictures from the polymeric network of 1; red-coloured bonds indicate the connectivity to the potassium atom of the ASU and the bonds of the DFForm ligand of the ASU. (A) Complete DFForm bonding network across four potassium atoms; (B,C) Simplified directions of the polymeric network showing the bridging through fluorine, nitrogen, and aromatic carbon atoms ((B) showing nitrogen-based bridging, (C) showing aryl group-based bridging).
Crystals of 1 were air- and moisture-sensitive, but under an inert atmosphere the compound appeared stable. Complex 1 was repeatedly obtained by simple exposure of thf solutions of “[K(DFForm)(thf)x]” to vacuum, giving 1 upon drying. The poor solubility in non-coordinating solvents made analysis by 1H NMR and 19F NMR spectroscopy difficult, giving only broad resonances in both spectra (NCHN at 8.88 ppm and F2,6 at −127.2 ppm).All fluorine atoms of the DFForm ligand are equivalent, but clear spectra were generated when 1 was dissolved in thf-d8. In this solvent, the NCHN resonance appeared as a pentet, owing to 5JH–F coupling with the ortho-fluorine atoms, as the pentet collapsed to a singlet with 19F decoupling. A broadening of the F resonance (corresponding to F1–F4) was also observed in the 19F NMR spectrum when it was performed without 1H decoupling. It is likely that upon dissolution in thf-d8, the polymeric network is dissociated, and a simpler DFForm coordination mode is adopted e.g., [K(DForm)(thf)x] (2 < x < 6). Attempts to crystallise a potential monomeric derivative were not successful, but upon concentration of a thf solution of 1, and storage at −35 °C, crystals of a thf-coordinated species were isolated, namely [K2(DFForm)2(thf)2]∞ (2, Scheme 1ii), identified as a one-dimensional polymer. Complex 2 is probably a transient intermediate between the putative monomeric [K(DFForm)(thf)x] solution species and polymeric 1.
X-ray data for 2 were solved and refined in the monoclinic space group P21, with two potassium ions, two DFForm ligands, and two coordinating thf molecules occupying the asymmetric unit (Figure 5A). For the ASU component, the two DFForm ligands bridge between both potassium centres in a μ-1κ(N,N′,F):2κ(N,N′,F′) manner, and N1 and N3 coordinate closer to K1, and N2 and N4 coordinate closer to K2. The K···F–C coordination in this arrangement is overall shorter than those observed for the tetradentate (N,N′,F,F′) DFForm coordination in 1, but longer than the auxiliary fluorine K···F–C coordination in 1. All the C–F bond lengths exhibit only a slight elongation, with the exception of the C13–F4 bond, which is notably longer than the others. An explanation behind the elongation of only C13–F4 is due to the involvement of this fluorine atom in additional bridging to an adjacent potassium atom. This, in conjunction with the two bridging thf ligands, leads to a one-dimensional polymer (Figure 5B).
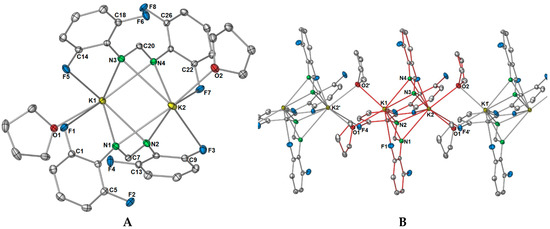
Figure 5.
Molecular structure of [K2(DFForm)2(thf)2]∞ (2). Ellipsoids are shown at 50% probability; hydrogen atoms and lattice solvent were removed for clarity. (A) Asymmetric unit. (B) Growth of the one-dimensional polymer chain; red bonding indicates the ASU. Selected bond lengths (Å) and angles (°): K1–N1: 2.7921(13), K1–N2: 2.9118(13), K1–N3: 2.7504(13), K1–N4: 3.0472(12), K1–F1: 3.3090(9), K1–F5: 3.1141(10), K1–O1: 2.7830(11), K1–O2′: 2.9351(13), K2–N1: 3.2913(12), K2–N2: 2.7581(13), K2–N3: 3.2957(13), K2–N4: 2.8039(13), K2–F3: 3.3678(10), K2–F4′: 3.2276(11), K2–F7: 2.9353(9), K2–O2: 2.8039(12), K2–O1′: 2.8172(11): C1–F1: 1.3575(19), C5–F2: 1.3644(16), C9–F3: 1.3610(17), C13–F4: 1.3724(14), C14–F5: 1.3608(18), C18–F6: 1.3675(16), C22–F7: 1.3578(16), C26–F8: 1.3640(15), K1–C7–K2: 70.41(3), K1–C20–K2: 68.86(3), O1–K1–K2: 147.97(3), O2–K2–K1: 136.59(3). K1–O1–K2′: 98.05(4), K1–O2′–K2′: 101.41(3).
The thf ligands in 2 bridge in an almost symmetrical manner between the two potassium atoms, though O2′ coordinates closer to K2′ than K1. This type of μ-1κ(O):2κ(O) bridging of two thf molecules is no stranger to group one chemistry [18], for example in the polymeric sodium diphenyloxidomethanide (Ph2CO)2− polymer [Na2(Ph2CO)(thf)2] [33], or the potassium 2,4,6-tris(trifluoromethyl)phenolate (OArCF3) complex [K2(OArCF3)2(thf)4(μ-O-thf)2] [34]. However, examples where the polymeric structure is generated by two thf ligands connecting the dinuclear units is restricted to only one other example in group one chemistry, namely [K4(COT)2(thf)6]∞ [35]. One difference between the COT (cyclooctatetraenyl) system and 2 is that asymmetric bridging of the thf ligands is more apparent, as both thf ligands favour one metal centre over the other (e.g., K1–O1: 2.839(3), K1–O2: 2.846(5), K2–O1:2.781(3), K2–O2: 2.783(4)). It should also be noted that there are examples where three thf ligands, not two, bridge the monomeric units to create a polymeric network [32,36]. Exposure of crystalline 2 to vacuum immediately causes fracturing of the crystals, giving 1. Furthermore, when crystals of 2 are isolated and allowed to stand at room temperature, some degree of thf liberation is apparent as the elemental analysis performed on these crystals gave a lower than expected carbon value. The best fit was obtained when the composition was calculated with loss of 0.4 thf molecules from 2. By examining the structure of 2, it seems that upon the liberation of bound thf, the DFForm ligand changes from the asymmetric μ-1κ(N,N′,F):2κ(N,N′,F′) coordination to a μ-(N,N′,F,F′):(N,N′) binding mode, and the auxiliary fluorine and aromatic carbon atoms become free to engage with adjacent potassium ions, building the complex polymeric network of 1. Owing to the rapid loss of thf from 2, additional characterisation was difficult. Dissolution in C6D6 gave rapid formation of a powder, presumably 1, as a large excess of thf was observed in the 1H NMR spectrum.
Although no monomeric [K(DFForm)(thf)x] species could be obtained from thf, we exploited the well-known affinity of 18-crown-6 for the potassium ion. Treatment of 1 with 18-crown-6 and crystallisation from a n-hexane/toluene solution, gave monomeric [K(DFForm)(18-crown-6)] (3). The structure was determined by X-ray crystallography, where the data were solved and refined in the monoclinic space group P21/n, with two molecules occupying the asymmetric unit (only one is depicted in Figure 6). The most surprising feature of this structure is the κ(N,C,F) coordination of the DFForm ligand to the potassium centre, as opposed to the expected κ(N,N′) coordination that is observed in [K(p-TolForm)(18-crown-6)] [32], and in several other C{NCXN}−C based ligand systems [18], such as [K(pyr)(18-crown-6)] (pyr = 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidide) [37]. The ipso carbon–potassium bond length (Figure 6) lies in the expected range for such interactions. For example, the ipso-carbon potassium interactions observed in the bimetallic 2,6-diphenylphenolate complex [KCa(OArPh)3], has a K–Cipso bond lengths of 3.391(6) Å [38] and the K–Cipso bond length in the phenylthiolato complex [K2Fe(SPh)4] is 3.477 (5) Å [39]. Despite the non-binding of N2, there is still charge delocalisation across the NCN bite, as there is only a slight shortening of the free C7–N2 bond, making it far too long for a formal double bond (e.g., DFForm(CPh3): C=N: 1.2762(12) Å) [17].
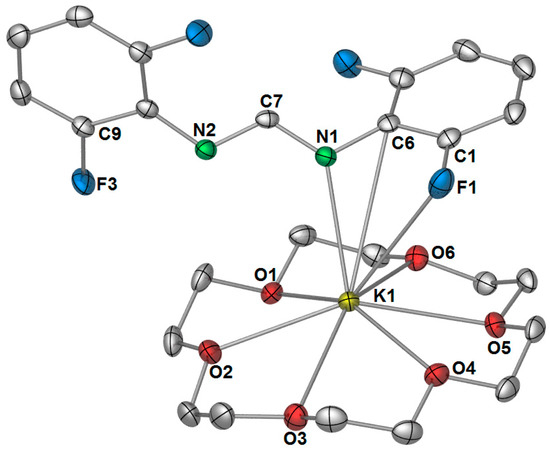
Figure 6.
Molecular structure of [K(DFForm)(18-crown-6)] (3). Ellipsoids shown at 50% probability with hydrogen atoms omitted for clarity. Selected bond lengths (Å): K1–N1: 2.7994(12), K1–C6: 3.4836(14), K1–C1(non-bonding): 3.6886(15), K1–F1: 3.2809(11), K1–N2(non-bonding): 3.9843(13), K1–O(crown): range: 2.8255(11)–2.9525(11), average: 2.89. C1–F1: 1.3584(18), C1–C6:1.401(2), C9–F3: 1.3576(16), C7–N1: 1.3224(18), C7–N2: 1.3161(18).
3. Materials and Methods
3.1. General Experimental Details
All reactions were undertaken using Schlenk line and glove box techniques. Solvents (thf, toluene, hexane, C6D6, thf-d8) were purified by distillation over sodium or sodium benzophenone, and were degassed prior to use. NMR experiments were recorded on a Bruker Avance 300 spectrometer or a Bruker AVII+400 machine (Billerica, MA, USA). 1H NMR resonances were referenced to tetramethylsilane by way of the residual 1H resonance of C6D6 (and 19F coupled unless specified otherwise). 19F-NMR data were 1H decoupled (unless specified otherwise) and referenced to external CFCl3. Microanalyses were performed by the elemental analysis service of London Metropolitan University or by an Elementar Vario Micro cube (Elementar, Langenselbold, Germany) by Wolfgang Bock of Tübingen University. IR spectra were recorded on a Perkin–Elmer 1600 Fourier transform infrared spectrometer (ῦ = 4000–500 cm−1), as either mulls in sodium-dried Nujol, or a Nicolet 6700 FTIR spectrometer (Thermo Nicolet, Madison, WI, USA) or using a DRIFT chamber with dry KBr/sample mixtures and KBr windows. K[(NSiMe3)2] was purchased from Sigma-Aldrich (St Louis, MO, USA) and used as received. N,N′-bis(2,6-difluorophenyl)formamidine (DFFormH) was synthesised by a published procedure [40], 18-crown-6 was purchased from Sigma-Aldrich and used as received.
[K(DFForm)]∞ (1): K[(NSiMe3)2] (0.25 g, 1.3 mmol) and DFFormH (0.33 g, 1.2 mmol) were each dissolved in toluene and combined with stirring, immediately forming a white, poorly soluble powder. The supernatant solution was decanted and the resulting powder was dried in vacuo. After the addition of thf, the powder dissolved, and then the solution was concentrated and layered with n-hexane. Colourless white block crystals grew overnight and were suitable for X-ray diffraction, revealing the composition [K(DFForm)]∞ (1, Yield = 0.30 g, 80%).1H NMR (C6D6, 400 MHz, 25 °C): δ 8.88 (br s, 1H, NCHN), 6.68 (m, 4 H, Ar-H(3,5)), 6.63 (m, 2 H, Ar-H(4)).19F NMR (C6D6, 25 °C): δ −127.2 (br s). 1H NMR (thf-d8, 400 MHz, 25 °C): δ 8.92 (p 5JH–F: 3.09 Hz, 1 H, NCHN), 6.68 (m, 4 H, Ar-H(3,5)), 6.46 (m, 2 H, Ar-H(4)). 1H NMR (thf-d8, 400 MHz, 25 °C, 19F decoupled): δ 8.89 (s, 1 H, NCHN), 6.68 (m, 4 H, Ar-H(3,5)), 6.46 (m, 2 H, Ar-H(4)).19F NMR (thf-d8, 25 °C): δ −127.8 (s). 19F NMR (thf-d8, 25 °C, F–H coupled) −127.8 (br s). IR (DRIFT): ν 1612 (m), 1562 (vs), 1513 (s), 1477 (s), 1464 (s), 1395 (w), 1326 (m), 1287 (w), 1254 (m), 1231 (m), 1199 (s), 1062 (w), 1005 (m), 984 (s), 954 (w), 922 (w), 828 (w), 779 (m), 766 (m). Elemental analysis (C13H7F4KN2, 306.31 g·mol−1): calcd.: C 50.97, H 2.30, N 9.15, found: C 50.81, H 2.34, N 9.07.
[K2(DFForm)2(thf)2]∞ (2): 1 (0.10 g, 0.32 mmol) was dissolved in minimal thf and concentrated in vacuo, giving colourless crystals that were not suitable for X-ray diffraction. The concentrated solution was stored at −35 °C, where large colourless block crystals grew of [K2(DFForm)2(thf)2] (2), suitable for X-ray diffraction. Upon exposure to vacuum, the crystals fractured and a white powder was obtained, likely consisting of a mixture of 1 and 2. (Yield = 0.11 g, 89%). 1H NMR (C6D6, 400 MHz, 25 °C, formation of insoluble white powder upon solvent addition, giving a large excess of thf in solution): δ 1.42 (m, 232 H, thf-β-CH2), 3.57 (m, 232 H, thf-α-CH2), 6.36 (m, 2 H, Ar-H4) 6.67 (m, 4 H, Ar-H(3,5)), 8.88 (br s, 1 H, NCHN). 19F NMR (C6D6, 25 °C): δ −127.1 (br s). IR (DRIFT): ν 1613 (m), 1564 (vs), 1551 (vs), 1514 (m), 1477 (vs), 1464 (vs), 1395 (w), 1325 (s), 1254 (m), 1231 (w), 1200 (s), 1062 (w), 1005 (w), 984.6 (s), 955 (w), 922 (w), 827 (w), 799 (w), 766 (m), 742 (w), 716 (m). Elemental analysis calcd (%) (for C34H30F8K2N4O2, 756.81 g·mol−1, pre-dried powder under vacuum). C 53.95, H 4.00, N 7.40, found: C 49.64, H 2.74, N 8.44. When the crystals were dried by slow evaporation in a glove box, a composition of [K2(DFForm)2(thf)1.6] was supported, calcd. (C58.4H40.8F8K2N4O1.6, 727.96 g·mol−1): C 53.46, H 3.71, N 7.69 found: C 53.08, H 3.66, N 7.35.
[K(DFForm)(18-crown-6)] (3): If 1 (~0.10 g, 0.32 mmol) was crystallised from toluene/hexane solutions in the presence of one equivalent of 18-crown-6 (~0.09 g, 0.34 mmol), pale yellow block crystals of [K(DFForm)(18-crown-6)] (3) developed. (Yield = ~0.07 g, 34%). 1H NMR (C6D6, 300 MHz 303.2 K): δ 9.15 (s, 1 H, NCHN), 6.84 (m, 4 H, Ar-H(3,5)), 6.42 (m, 2 H, Ar-H(4)), 3.22 (br s, 24 H, 18-crown-6). 19F NMR (C6D6, 303.2 K): δ = −125.81 (br s). IR (Nujol): ῦ = 1588 (vs), 1540 (vs), 1259 (vs), 1193 (m), 1096 (s), 1004 (s), 956 (m), 818 (m). Elemental analysis returned poor C H N values. (C25H31F4KN2O6, 570.62 g·mol−1): calcd. C 52.62, H 5.47, N 4.91, found: C 46.87, H 4.93, N 5.21.
3.2. X-ray Crystallography
All compounds were examined on a “Bruker APEX-II CCD” diffractometer at 100.15 or 150.15 K, mounted on a fibre loop in Paratone-N. Absorption corrections were completed using Apex II program suite [41]. Structural solutions were obtained by charge flipping (1, 2, 3) [42] methods, and refined using full matrix least squares methods against F2 using SHELX2013 [43], within the OLEX 2 graphical interface [43]. CCDC numbers: 1 (1540263), 2 (1540264), 3 (1540265).
[K(DFForm)]∞ (1): C13H7F4KN2 (M = 306.31 g/mol): triclinic, space group P-1 (no. 2), a = 7.4437(2) Å, b = 7.5357(2) Å, c = 11.8891(3) Å, α = 100.6590(10)°, β = 101.9020(10)°, γ = 101.1070(10)°, V = 622.56(3) Å3, Z = 2, T = 100(2) K, μ(MoKα) = 0.466 mm−1, Dcalc = 1.634 g/cm3, 10877 reflections measured (5.66° ≤ 2Θ ≤ 60.48°), 3657 unique (Rint = 0.0152, Rsigma = 0.0174) which were used in all calculations. The final R1 was 0.0279 (>2σ(I)) and wR2 was 0.0734 (all data). Note: NCHN hydrogen atom manually assigned from identified Q peak.
[K2(DFForm)2(thf)2]∞ (2): C34H30F8K2N4O2 (M = 756.82 g/mol): monoclinic, space group P21 (no. 4), a = 7.55040(10) Å, b = 19.8885(3) Å, c = 11.6408(2) Å, β = 105.4479(6)°, V = 1684.90(4) Å3, Z = 4, T = 100.1 K, μ(MoKα) = 0.364 mm−1, Dcalc = 1.492 g/cm3, 17983 reflections measured (3.62° ≤ 2Θ ≤ 60.66°), 8299 unique (Rint = 0.0136, Rsigma = 0.0201), which were used in all calculations. The final R1 was 0.0262 (>2σ(I)) and wR2 was 0.0652 (all data).
2[K(DFForm)(18-crown-6)] (3): C50H62F8K2N4O12 (M = 1141.23): note: two molecules present in the asymmetric unit. monoclinic, space group P21/n (no. 14), a = 10.9773(4) Å, b = 15.3047(5) Å, c = 31.3860(10) Å, β = 93.674(2)°, V = 5262.1(3) Å3, Z = 4, T = 123.15 K, μ(MoKα) = 0.273 mm−1, Dcalc = 1.441 g/mm3, 78411 reflections measured (2.6 ≤ 2Θ ≤ 56.76), 13119 unique (Rint = 0.0369, Rsigma = 0.0266) which were used in all calculations. The final R1 was 0.0321 (I > 2σ(I)) and wR2 was 0.1146 (all data).
4. Conclusions
Complexation of N,N′-bis(2,6-difluorophenyl)formamidinate to potassium generates a species which rapidly liberated coordinated thf, giving a two-dimensional polymeric network [K(DFForm)]∞ (1), based on a complex and unprecedented formamidinate binding mode. This binding was shown to be completely different from the analogous [K(FForm)]∞ (FForm: N,N′-bis(2-fluorophenyl)formamidinate) mono-directional polymer. With access to two additional auxiliary o-fluorine atoms (namely F2 and F4), a new dimension for the polymeric network could be generated, which was further reinforced by potassium–arene interactions and nitrogen-based bridging of the DFForm ligand. The formation of this network was so favourable that it could be generated by simple n-hexane layering of thf solutions, or the evaporation of thf solutions to dryness, and is the first example of a two-dimensional polymeric N,N′-bis(aryl)formamidinate network. A likely transient species between a monomeric thf solution derivative [K(DFForm)(thf)x] and 1 was also obtained and identified as a one-dimensional polymer with two bridging thf ligands, namely [K2(DFForm)2(thf)2]∞ (2). Complex 2 lost thf in the solid state, slowly forming 1 upon storage at room temperature. A monomeric derivative of 1 was obtained through use of 18-crown-6, giving [K(DFForm)(18-crown-6)] (3), which showed a highly unexpected κ(N,Cispo,F) coordination. Such examples as these highlight the strong affinity of potassium for donor atoms, especially fluorine, and how the simple addition of more fluorine atoms to a ligand system can expand the coordination network of the ligand, and can generate unexpected structural consequences.
Supplementary Materials
The following are available online at www.mdpi.com/2304-6740/5/2/26/s1, CIFs and CIF checked files.
Acknowledgments
We thank the ARC (DP16010640) for funding, and Professor Reiner Anwander for use of the elemental analysis and IR services of Tuebingen University.
Author Contributions
The project concept was devised equally by all authors. Daniel Werner performed the experiments and analyzed the resulting data. The materials were provided by Glen B. Deacon and Peter C. Junk. The manuscript was written by Daniel Werner and edited by Glen B. Deacon and Peter C. Junk.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Edelmann, F.T. Chapter two—Recent progress in the chemistry of metal amidinates and guanidinates: Syntheses, catalysis and materials. In Advances in Organometallic Chemistry; Anthony, F.H., Mark, J.F., Eds.; Academic Press: London, UK, 2013; Volume 61, pp. 55–374. [Google Scholar]
- Edelmann, F.T. Chapter 3 advances in the coordination chemistry of amidinate and guanidinate ligands. In Advances in Organometallic Chemistry; Anthony, F.H., Mark, J.F., Eds.; Academic Press: London, UK, 2008; Volume 57, pp. 183–352. [Google Scholar]
- Junk, P.C.; Cole, M.L. Alkali-metal bis(aryl)formamidinates: A study of coordinative versatility. Chem. Commun. 2007, 16, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Cotton, F.A.; Daniels, L.M.; Murillo, C.A. A systematic approach in the preparation of compounds with σ2-π4 vanadium-to-vanadium triple bonds: Synthesis, reactivity, and structural characterization. Inorg. Chem. 1993, 32, 2881–2885. [Google Scholar] [CrossRef]
- Deacon, G.B.; Hossain, M.E.; Junk, P.C.; Salehisaki, M. Rare-earth N,N′-diarylformamidinate complexes. Coord. Chem. Rev. 2017. [Google Scholar] [CrossRef]
- Cotton, F.A.; Daniels, L.M.; Murillo, C.A. The first complex with a σ2π4 triple bond between vanadium atoms in a ligand framework of fourfold symmetry—[V2{(p-CH3C6H4)NC(H)N(p-C6H4CH3)}4]. Angew. Chem. Int. Ed. 1992, 31, 737–738. [Google Scholar] [CrossRef]
- Cotton, F.A.; Daniels, L.M.; Maloney, D.J.; Matonic, J.H.; Murillo, C.A. Divalent metal chloride formamidine complexes, M11 = Fe, Co and Pt. Syntheses and structural characterization. Polyhedron 1994, 13, 815–823. [Google Scholar] [CrossRef]
- Lyhs, B.; Bläser, D.; Wölper, C.; Schulz, S. Syntheses and X-ray crystal structures of organoantimony diazides. Chem. Eur. J. 2011, 17, 4914–4920. [Google Scholar] [CrossRef] [PubMed]
- Deacon, G.B.; Junk, P.C.; Wang, J.; Werner, D. Reactivity of bulky formamidinatosamarium(II or III) complexes with C=O and C=S bonds. Inorg. Chem. 2014, 53, 12553–12563. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Mills, D.P.; Rivard, E.; Stasch, A.; Woodul, W.D. Synthesis and crystal structures of anionic gallium(II) and gallium(III) heterocyclic compounds derived from a gallium(I) n-heterocyclic carbene analogue. J. Chem. Crystallogr. 2010, 40, 965–969. [Google Scholar] [CrossRef]
- Cole, M.L.; Davies, A.J.; Jones, C.; Junk, P.C. Persistent π-arene interactions in bulky formamidinate complexes of potassium. J. Organomet. Chem. 2007, 692, 2508–2518. [Google Scholar] [CrossRef]
- Cole, M.L.; Junk, P.C. Potassium complexes of the ‘super’ formamidine (2,6-pr2iC6H3)NC(H)NH(2,6-pr2iC6H3), Hdippform.: Synthesis and molecular structure of [{K(DippForm)2K(thf)2}n]·nthf and [K(DippForm)(thf)3]·HDippForm. J. Organomet. Chem. 2003, 666, 55–62. [Google Scholar] [CrossRef]
- Baldamus, J.; Berghof, C.; Cole, M.L.; Evans, D.J.; Hey-Hawkins, E.; Junk, P.C. Attenuation of reactivity by product solvation: Synthesis and molecular structure of [K{(η6-Mes)NC(H)N(Mes)}{(η6-Mes)NHC(H)N(Mes)], the first formamidinate complex of potassium. J. Chem. Soc. Dalton Trans. 2002, 14, 2802–2804. [Google Scholar] [CrossRef]
- Hamidi, S.; Jende, L.N.; Martin Dietrich, H.; Maichle-Mössmer, C.; Törnroos, K.W.; Deacon, G.B.; Junk, P.C.; Anwander, R. C–H bond activation and isoprene polymerization by rare-earth-metal tetramethylaluminate complexes bearing formamidinato n-ancillary ligands. Organometallics 2013, 32, 1209–1223. [Google Scholar] [CrossRef]
- Kulkarni, N.V.; Elkin, T.; Tumaniskii, B.; Botoshansky, M.; Shimon, L.J.W.; Eisen, M.S. Asymmetric bis(formamidinate) group 4 complexes: Synthesis, structure and their reactivity in the polymerization of α-olefins. Organometallics 2014, 33, 3119–3136. [Google Scholar] [CrossRef]
- Deacon, G.B.; Junk, P.C.; Werner, D. Enhancing the value of free metals in the synthesis of lanthanoid formamidinates: Is a co-oxidant needed? Chem. Eur. J. 2016, 22, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Werner, D.; Deacon, G.B.; Junk, P.C.; Anwander, R. Cerium(III/IV)formamidinate chemistry, and a stable cerium(IV) diolate. Chem. Eur. J. 2014, 20, 4426–4438. [Google Scholar] [CrossRef] [PubMed]
- Allen, F.H. The cambridge structural database: A quarter of a million crystal structures and rising. Acta Crystallogr. Sec. B 2002, 58, 380–388. [Google Scholar] [CrossRef]
- Cole, M.L.; Evans, D.J.; Junk, P.C.; Smith, M.K. Structural studies of N,N′-di(ortho-fluorophenyl)formamidine group 1 metallation. Chem. Eur. J. 2003, 9, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Cotton, F.A.; Murillo, C.A.; Pascual, I. Quadruply bonded dichromium complexes with variously fluorinated formamidinate ligands. Inorg. Chem. 1999, 38, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.L.; Davies, A.J.; Jones, C.; Junk, P.C. Lithium and sodium N,N′-di(2,6-dialkylphenyl)formamidinate complexes. J. Organomet. Chem. 2004, 689, 3093–3107. [Google Scholar] [CrossRef]
- Cole, M.L.; Junk, P.C.; Louis, L.M. Synthesis and structural characterisation of some novel lithium and sodium N,N′-di(para-tolyl)formamidinate complexes. J. Chem. Soc. Dalton Trans. 2002, 3906–3914. [Google Scholar] [CrossRef]
- Cole, M.L.; Davies, A.J.; Jones, C.; Junk, P.C. Mononuclear formamidinate complexes of lithium and sodium. Z. Anorg. Allg. Chem. 2011, 637, 50–55. [Google Scholar] [CrossRef]
- Cole, M.L.; Deacon, G.B.; Forsyth, C.M.; Junk, P.C.; Konstas, K.; Wang, J.; Bittig, H.; Werner, D. Synthesis, structures and reactivity of lanthanoid(II)formamidinates of varying steric bulk. Chem. Eur. J. 2013, 19, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Werner, D.; Zhao, X.; Best, S.P.; Maron, L.; Junk, P.C.; Deacon, G.B. Bulky ytterbium formamidinates stabilise complexes with radical ligands, and related samarium “tetracyclone” chemistry. Chem. Eur. J. 2017, 23, 2084–2102. [Google Scholar] [CrossRef] [PubMed]
- Deacon, G.B.; Junk, P.C.; Werner, D. The synthesis and structures of rare earth 2-fluorophenyl- and 2,3,4,5-tetrafluorophenyl-N,N′-bis(aryl)formamidinate complexes. Polyhedron 2016, A103, 178–186. [Google Scholar] [CrossRef]
- Cole, M.L.; Deacon, G.B.; Forsyth, C.M.; Junk, P.C.; Konstas, K.; Wang, J. Steric modulation of coordination number and reactivity in the synthesis of lanthanoid(III)formamidinates. Chem. Eur. J. 2007, 13, 8092–8110. [Google Scholar] [CrossRef] [PubMed]
- Deacon, G.B.; Junk, P.C.; Werner, D. Lanthanoid induced C–F activation of all fluorine atoms of one CF3 group. Eur. J. Inorg. Chem. 2015, 9, 1484–1489. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 155–169. [Google Scholar] [CrossRef]
- Krackl, S.; Inoue, S.; Driess, M.; Enthaler, S. Intermolecular hydrogen–fluorine interaction in dimolybdenum triply bonded complexes modified by fluorinated formamidine ligands for the construction of 2D- and 3D-networks. Eur. J. Inorg. Chem. 2011, 13, 2103–2111. [Google Scholar] [CrossRef]
- Deacon, G.B.; Junk, P.C.; Macreadie, L.K.; Werner, D. Structural and reactivity consequences of reducing steric bulk of N,N′-diarylformamidinates coordinated to lanthanoid ions. Eur. J. Inorg. Chem. 2014, 5240–5250. [Google Scholar] [CrossRef]
- Baldamus, J.; Berghof, C.; Cole, M.L.; Evans, D.J.; Hey-Hawkins, E.; Junk, P.C. N,N′-di(tolyl)formamidinate complexes of potassium: Studies of ancillary donor imposed molecular and supramolecular structure. J. Chem. Soc. Dalton Trans. 2002, 22, 4185–4192. [Google Scholar] [CrossRef]
- Geier, J.; Ruegger, H.; Grutzmacher, H. Sodium compounds of the benzophenone dianion (diphenyloxidomethanide). Dalton Trans. 2006, 1, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Brooker, S.; Edelmann, F.T.; Kottke, T.; Roesky, H.W.; Sheldrick, G.M.; Stalke, D.; Whitmire, K.H. Comparison of the X-ray crystal structures of the sodium and potassium 2,4,6-tris(trifluoromethyl)phenoxides (RO-) and 2,4,6-tris(trifluoromethyl)benzenethiolates (RS-); [Na(OR)(thf)2]2, [k(OR)(thf)2(µ-thf)]2, [Na(SR)(thf)2∙0.25thf] and [K(SR)(thf)](thf = tetrahydrofuran). J. Chem. Soc. Chem. Commun. 1991, 3, 144–146. [Google Scholar]
- Hu, N.; Gong, L.; Jin, Z.; Chen, W. Crystal structure of cyclooctatetraenylpotassium, C8H8K2·(OC4H8)3. J. Organomet. Chem. 1988, 352, 61–66. [Google Scholar] [CrossRef]
- Antolini, F.; Hitchcock, P.B.; Khvostov, A.V.; Lappert, M.F. Synthesis and structures of alkali metal amides derived from the ligands [N(SiMe2Ph)(SiMe3)]−, [N(tbu)(SiMe3)]−, [N(Ph)(2-C5H4N)]−, and [N(2-C5H4N)2]−. Eur. J. Inorg. Chem. 2003, 18, 3391–3400. [Google Scholar] [CrossRef]
- Coles, M.P.; Hitchcock, P.B. Bicyclic guanidinates in mono- and di-valent metal complexes, including group 1/2 and group 1/12 heterometallic systems. Aus. J. Chem. 2013, 66, 1124–1130. [Google Scholar] [CrossRef]
- Zuniga, M.F.; Deacon, G.B.; Ruhlandt-Senge, K. Developments in heterobimetallic s-block systems: Synthesis and structural survey of molecular M/AE (M = Li, Na, K, Cs; AE = Ca, Sr) aryloxo complexes. Inorg. Chem. 2008, 47, 4669–4681. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-Y.; Jin, G.-X.; Weng, L.-H. Phenylthiolate as a σ and π donor ligand: Synthesis of a 3-D organometallic coordination polymer [K2Fe(SPh)4]n. Chem. Commun. 2004, 13, 1542–1543. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.M. Acid Catalyzed Reaction of Diarylformamidines with Ethyl Orthoformate. J. Am. Chem. Soc. 1949, 71, 3848–3849. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS; program for empirical absorption correction; University of Gottingen: Gottingen, Germany, 1996. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta. Crystallogr. 2008, A64, 112. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).