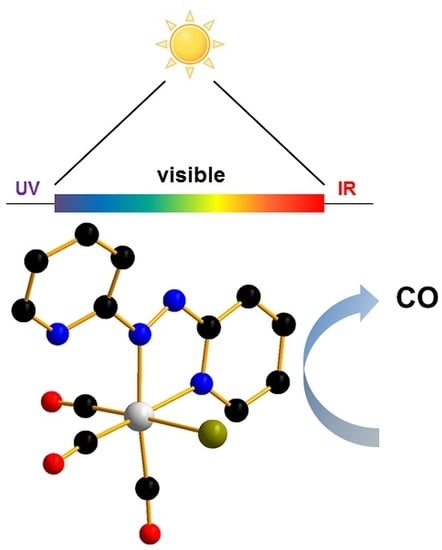
Visible Light-Activated PhotoCORMs
Abstract
:1. Introduction
2. PhotoCORMs: Identification and Design
3. UV Light Photoactivated CORMs
4. Visible Light and Near Infrared-Light (NIR) Photoactivated CORMs
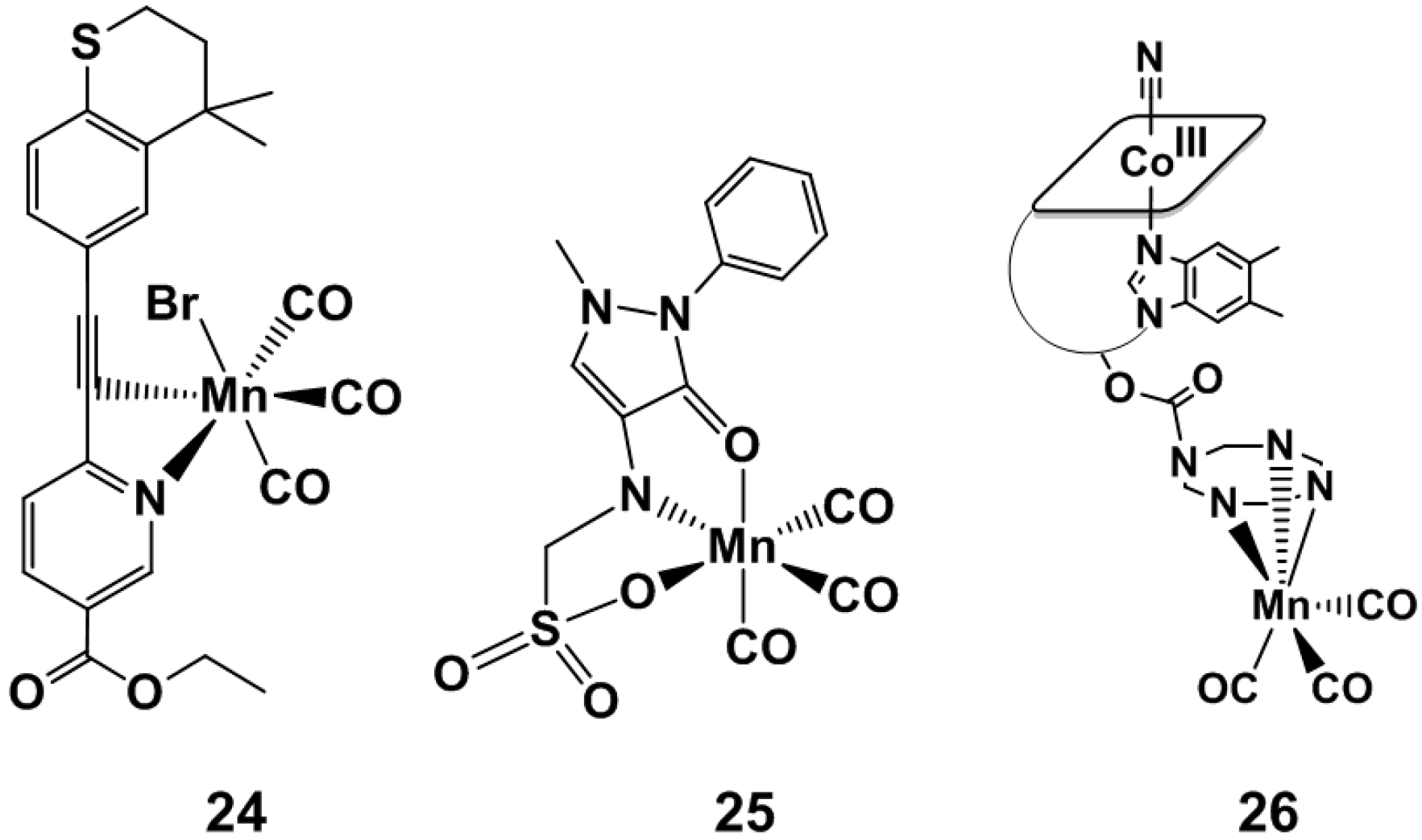
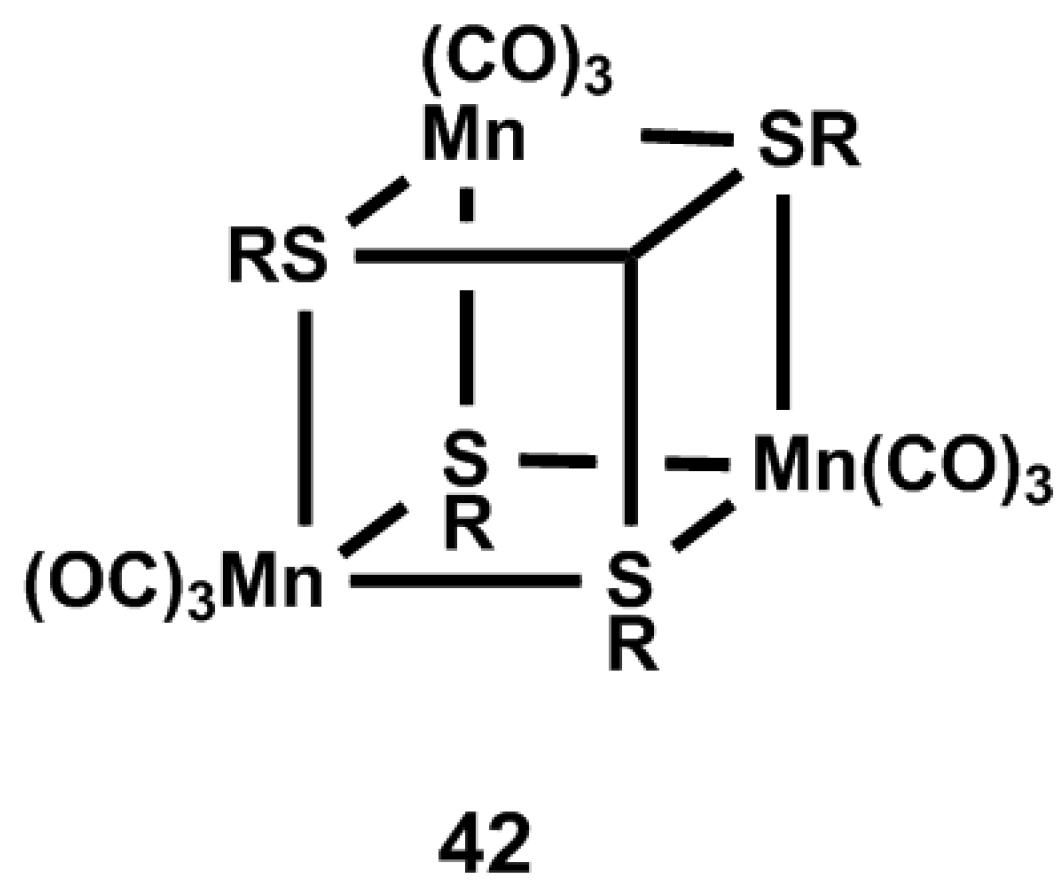
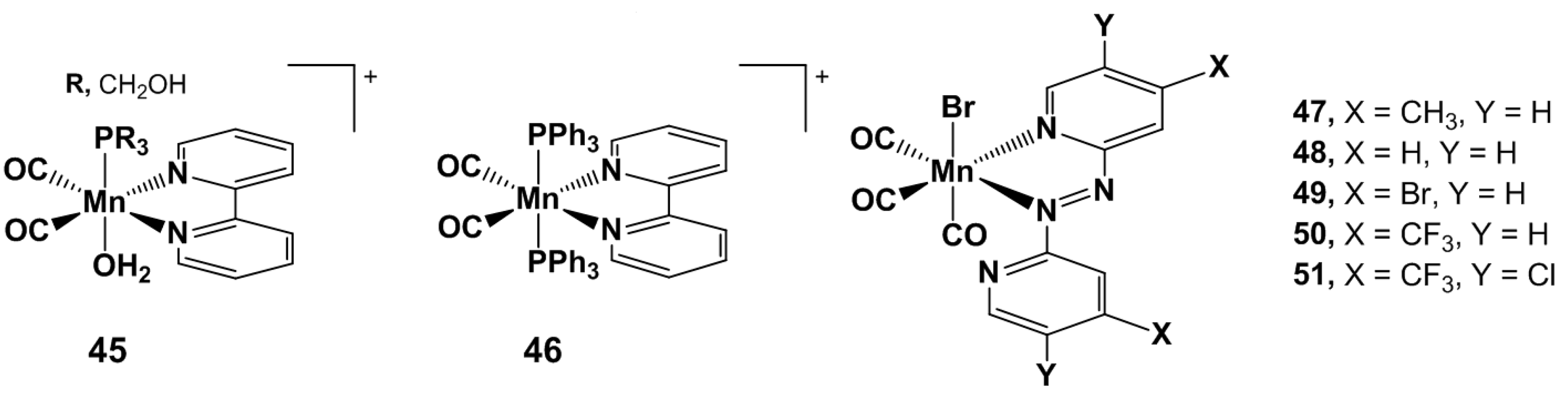
4.1. Inorganic and Organometallic PhotoCORMs
4.2. Organic PhotoCORMs
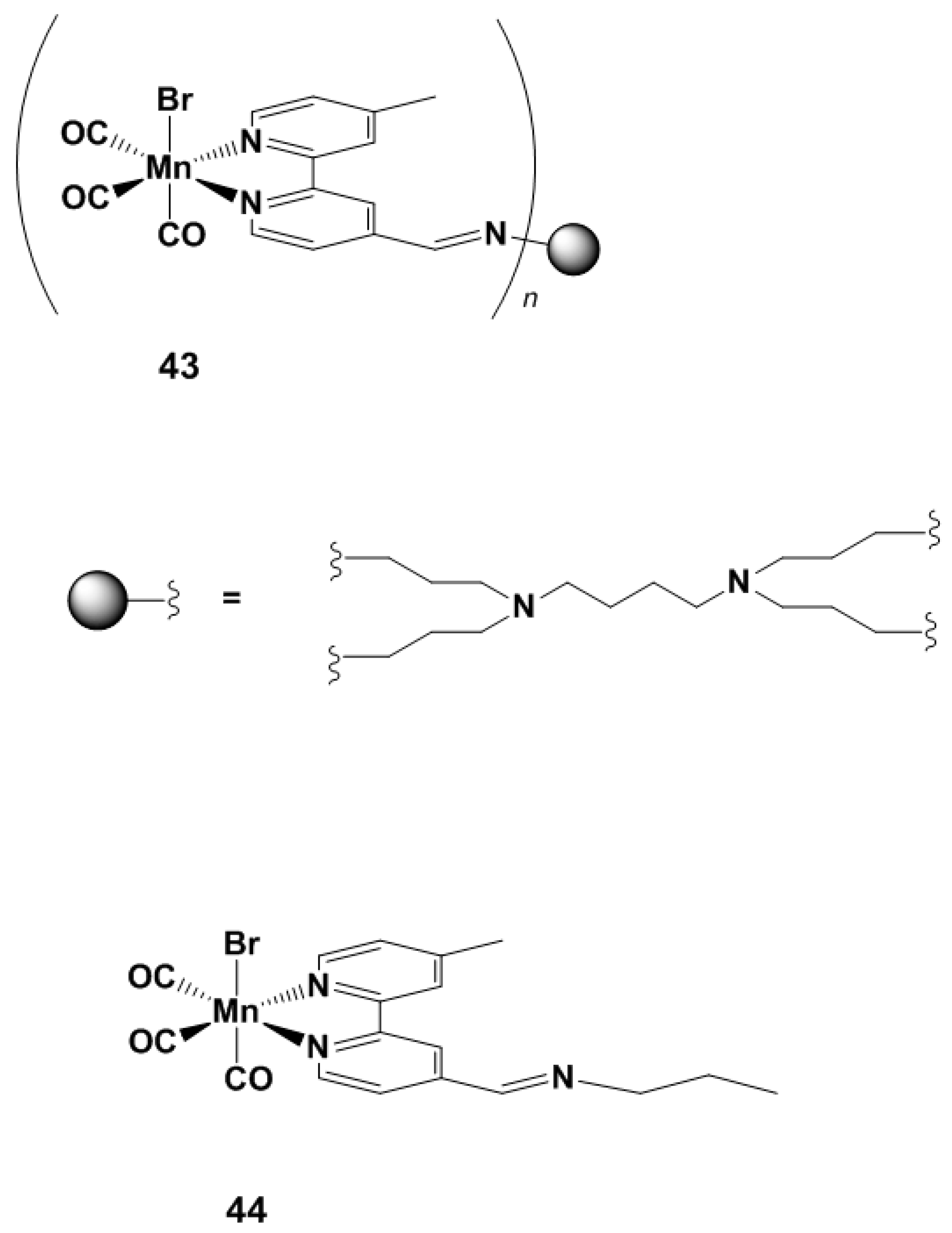
4.3. PhotoCORM Materials
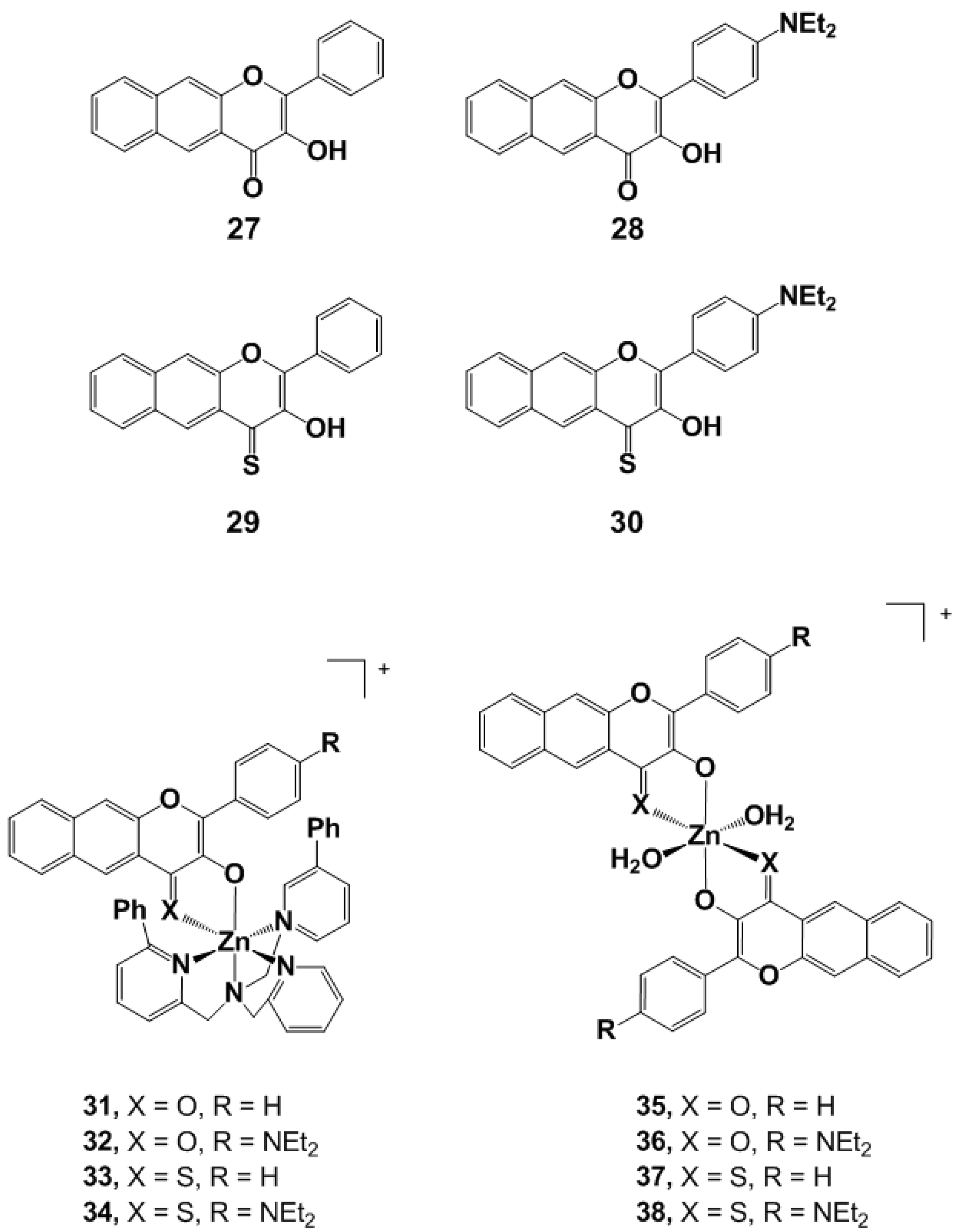
4.4. NIR PhotoCORMs
5. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Widdop, B. Analysis of Carbon Monoxide. Ann. Clin. Biochem. 2002, 39, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Romao, C.C.; Blättler, W.A.; Seixas, J.D.; Bernardes, G.J. Developing Drug Molecules for Therapy with Carbon Monoxide. Chem. Soc. Rev. 2012, 41, 3571–3583. [Google Scholar] [CrossRef] [PubMed]
- Fukuto, J.M.; Carrington, S.J.; Tantillo, D.J.; Harrison, J.G.; Ignarro, L.J.; Freeman, B.A.; Chen, A.; Wink, D.A. Small Molecule Signaling Agents: The Integrated Chemistry Biochemistry of Nitrogen Oxides, Oxides of Carbon, Dioxygen, Hydrogen Sulfide, and Their Derived Species. Chem. Res. Toxicol. 2012, 25, 769–793. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Otterbein, L.E. The Therapeutic Potential of Carbon Monoxide. Nat. Rev. Drug. Discov. 2010, 9, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Halilovic, A.; Patil, K.A.; Bellner, L.; Marrazzo, G.; Castellano, K.; Cullaro, G.; Dunn, M.W.; Schwartzman, M.L. Knockdown of Heme Oxygenase-2 Impairs Corneal Epithelial Cell Wound Healing. J. Cell. Physiol. 2011, 226, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Gonzales, A.; Foresti, R.; Clark, J.E.; Green, C.J.; Winslow, R.M. Heme Oxygenase-1-Derived Carbon Monoxide Contributes to the Suppression of Acute Hypertensive Responses in Vivo. Circ. Res. 1998, 83, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E. Carbon Monoxide: Innovative Anti-Inflammatory Properties of an Age-Old Gas Molecule. Antioxid. Redox Signal. 2002, 4, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Kaczorowski, D.J.; Sugimoto, R.; Billiar, T.R.; McCurry, K.R. Serial Review Application of Heme Oxygenase-1, Carbon Monoxide and Biliverdin for the Prevention of Intestinal Ischemia/Reperfusion Injury. J. Clin. Biochem. Nutr. 2008, 42, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Lu, H.T.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M. Carbon Monoxide Has Anti-Inflammatory Effects Involving the Mitogen-Activated Protein Kinase Pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [PubMed]
- Wu, M.L.; Ho, Y.C.; Yet, S.F. A Central Role of Heme Oxygenase-1 in Cardiovascular Protection. Antioxid. Redox Signal. 2011, 15, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.S.; Nakao, A.; Kimizuka, K.; Romanosky, A.J.; Stolz, D.B.; Uchiyama, T.; Nalesnik, M.A.; Otterbein, L.E.; Murase, N. Protection of Transplant-Induced Renal Ischemia-Reperfusion Injury with Carbon Monoxide. Am. J. Physiol. Renal 2004, 287, F979–F989. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Zuckerbraun, B.S.; Haga, M.; Liu, F.; Song, R.; Usheva, A.; Stachulak, C.; Bodyak, N.; Smith, R.N.; Csizmadia, E. Carbon Monoxide Suppresses Arteriosclerotic Lesions Associated with Chronic Graft Rejection and with Balloon Injury. Nat. Med. 2003, 9, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Katori, M.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Heme Oxygenase-1 System in Organ Transplantation1. Transplantation 2002, 74, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.H.; Hoshi, T.; Westerhausen, M.; Schiller, A. Carbon Monoxide–Physiology, Detection and Controlled Release. Chem. Commun. 2014, 50, 3644–3660. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, R.D.; Pierri, A.E.; Ford, P.C. Photochemically Activated Carbon Monoxide Release for Biological Targets. Toward Developing Air-Stable Photocorms Labilized by Visible Light. Coord. Chem. Rev. 2012, 256, 1509–1519. [Google Scholar] [CrossRef]
- Schatzschneider, U. Photocorms: Light-Triggered Release of Carbon Monoxide from the Coordination Sphere of Transition Metal Complexes for Biological Applications. Inorg. Chim. Acta 2011, 374, 19–23. [Google Scholar] [CrossRef]
- Schatzschneider, U. Novel Lead Structures Activation Mechanisms for CO-releasing molecules (CORMs). Br. J. Pharmacol. 2015, 172, 1638–1650. [Google Scholar] [CrossRef] [PubMed]
- Zobi, F. CO and CO-Releasing Molecules in Medicinal Chemistry. Future Med. Chem. 2013, 5, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Clark, J.E.; Foresti, R.; Sarathchandra, P.; Mann, B.E.; Green, C.J. Carbon Monoxide-Releasing Molecules Characterization of Biochemical and Vascular Activities. Circ. Res. 2002, 90, e17–e24. [Google Scholar] [CrossRef] [PubMed]
- Hewison, L.; Crook, S.H.; Mann, B.E.; Meijer, A.J.; Adams, H.; Sawle, P.; Motterlini, R.A. New Types of CO-Releasing Molecules (CO-RMs), Based on Iron Dithiocarbamate Complexes and [Fe(CO)3I(S2COEt)]. Organometallics 2012, 31, 5823–5834. [Google Scholar] [CrossRef]
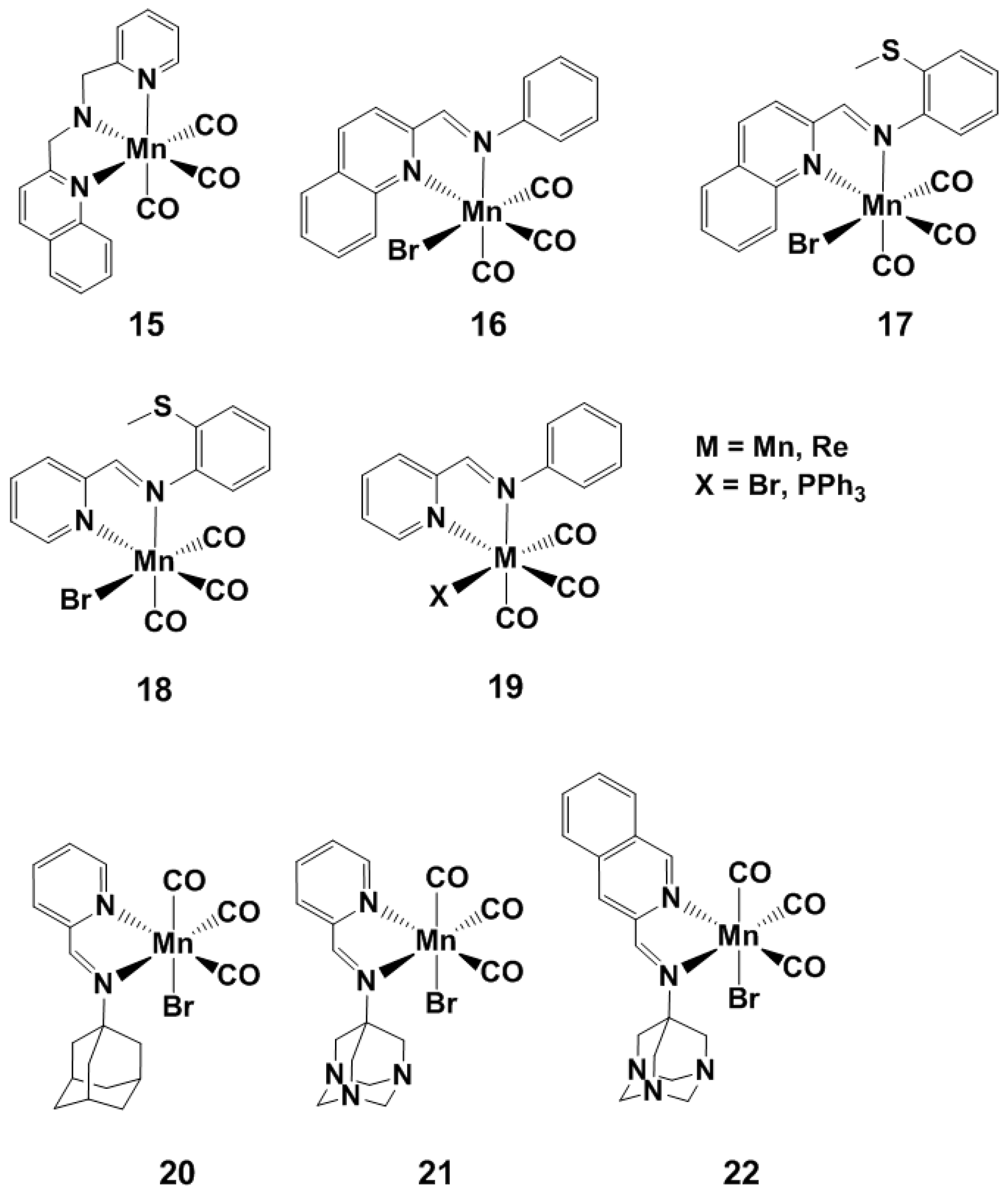
- Kottelat, E.; Ruggi, A.; Zobi, F. Red-Light Activated Photocorms of Mn(I) Species Bearing Electron Deficient 2,2′-Light Activated. Dalton Trans. 2016, 45, 6920–6927. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Carrington, S.J.; Mascharak, P.K. Design Strategies to Improve the Sensitivity of Photoactive Metal Carbonyl Complexes (PhotoCORMs) to Visible Light and Their Potential as CO-Donors to Biological Targets. Accounts Chem. Res. 2014, 47, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Romanski, S.; Kraus, B.; Schatzschneider, U.; Neudörfl, J.M.; Amslinger, S.; Schmalz, H.G. Acyloxybutadiene Iron Tricarbonyl Complexes as Enzyme-Triggered CO-Releasing Molecules (ET-CORMs). Angew. Chem. Int. Ed. 2011, 50, 2392–2396. [Google Scholar] [CrossRef] [PubMed]
- Romanski, S.; Rücker, H.; Stamellou, E.; Guttentag, M.; Neudörfl, J.M.; Alberto, R.; Amslinger, S.; Yard, B.; Schmalz, H.G. Iron Dienylphosphate Tricarbonyl Complexes as Water-Soluble Enzyme-Triggered CO-Releasing Molecules (ET-CORMs). Organometallics 2012, 31, 5800–5809. [Google Scholar] [CrossRef]
- Romanski, S.; Kraus, B.; Guttentag, M.; Schlundt, W.; Rücker, H.; Adler, A.; Neudörfl, J.M.; Alberto, R.; Amslinger, S.; Schmalz, H.G. Acyloxybutadiene Tricarbonyl Iron Complexes as Enzyme-Triggered CO-Releasing Molecules (ET-CORMs): A Structure–Activity Relationship Study. Dalton Trans. 2012, 41, 13862–13875. [Google Scholar] [CrossRef] [PubMed]
- Botov, S.; Stamellou, E.; Romanski, S.; Guttentag, M.; Alberto, R.; Neudörfl, J M.; Yard, B.; Schmalz, H.G. Synthesis and Performance of Acyloxy-Diene-Fe (CO)3 Complexes with Variable Chain Lengths as Enzyme-Triggered Carbon Monoxide-Releasing Molecules. Organometallics 2013, 32, 3587–3594. [Google Scholar] [CrossRef]
- Kunz, P.C.; Meyer, H.; Barthel, J.; Sollazzo, S.; Schmidt, A.M.; Janiak, C. Metal Carbonyls Supported on Iron Oxide Nanoparticles to Trigger the CO-Gasotransmitter Release by Magnetic Heating. Chem. Commun. 2013, 49, 4896–4898. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.A.; Mascharak, P.K. Photoactive Metal Carbonyl Complexes as Potential Agents for Targeted CO Delivery. J. Inorg. Biochem. 2014, 133, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.A.; Wright, J.A. PhotoCORMs: CO Release Moves into the Visible. Dalton Trans. 2016, 45, 6801–6811. [Google Scholar] [CrossRef] [PubMed]
- Dördelmann, G.; Pfeiffer, H.; Birkner, A.; Schatzschneider, U. Silicium Dioxide Nanoparticles as Carriers for Photoactivatable CO-Releasing Molecules (PhotoCORMs). Inorg. Chem. 2011, 50, 4362–4367. [Google Scholar] [CrossRef] [PubMed]
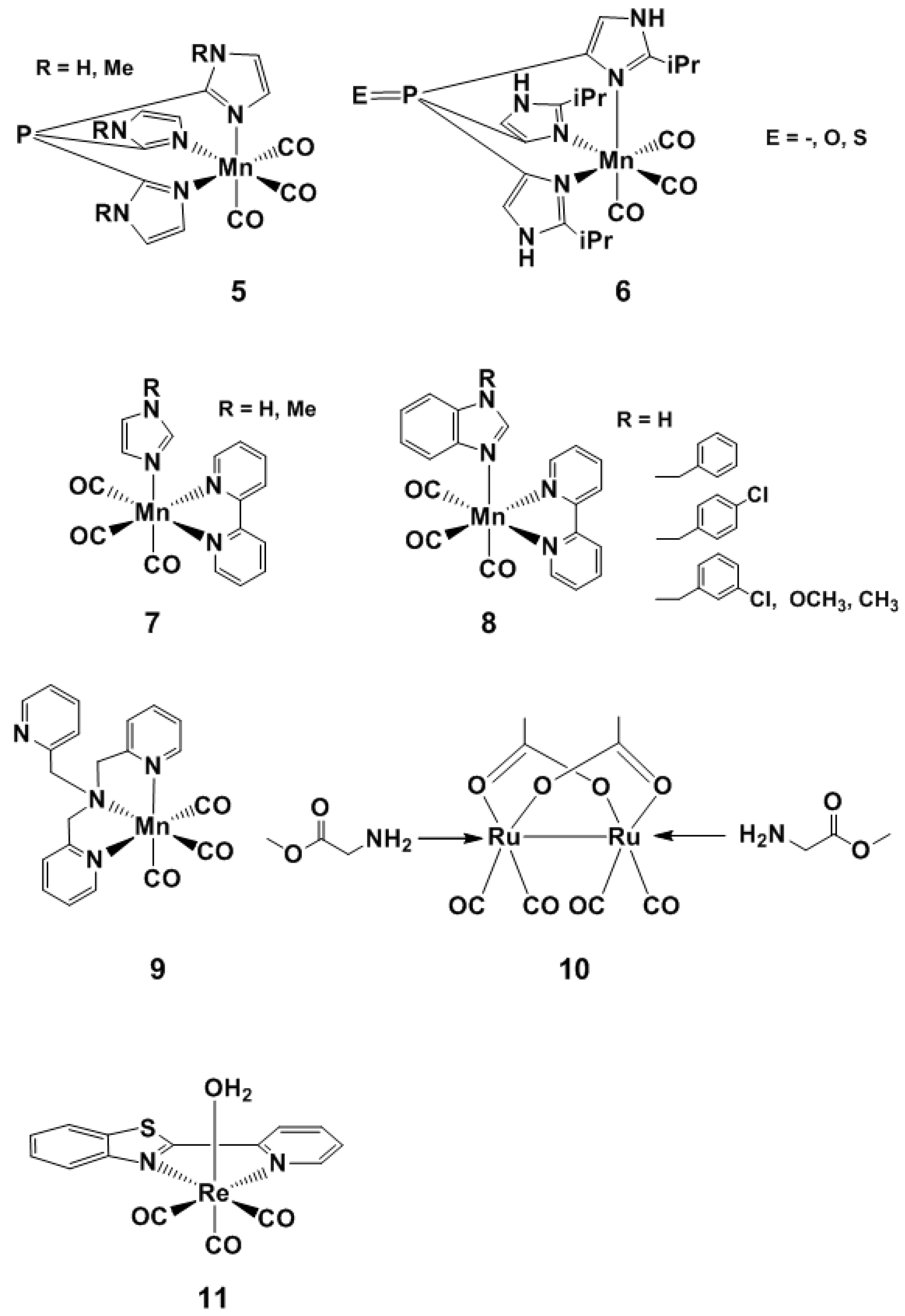
- Niesel, J.; Pinto, A.; NDongo, H.W.P.; Merz, K.; Ott, I.; Gust, R.; Schatzschneider, U. Photoinduced CO Release, Cellular Uptake and Cytotoxicity of a Tris(Pyrazolyl)Methane (tpm) Manganese Tricarbonyl Complex. Chem. Commun. 2008, 1798–1800. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, P.; Kanal, F.; Knorr, J.; Nagel, C.; Niesel, J.; Brixner, T.; Schatzschneider, U.; Nuernberger, P. Ultrafast Photochemistry of a Manganese-Tricarbonyl CO-Releasing Molecule (CORM) in Aqueous Solution. J. Phys. Chem. Lett. 2013, 4, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, H.; Rojas, A.; Niesel, J.; Schatzschneider, U. Sonogashira “Click” Reactions for the N-Terminal and Side-Chain Functionalization of Peptides with [Mn(CO)3(tpm)]+-Based CO Releasing Molecules (tpm = Tris(Pyrazolyl)Methane). Dalton Trans. 2009, 4292–4298. [Google Scholar] [CrossRef] [PubMed]
- Meister, K.; Niesel, J.; Schatzschneider, U.; Metzler-Nolte, N.; Schmidt, D.A.; Havenith, M. Label-Free Imaging of Metal–Carbonyl Complexes in Live Cells by Raman Microspectroscopy. Angew. Chem. Int. Ed. 2010, 49, 3310–3312. [Google Scholar] [CrossRef] [PubMed]
- Pierri, A.E.; Huang, P.J.; Garcia, J.V.; Stanfill, J.G.; Chui, M.; Wu, G.; Zheng, N.; Ford, P.C. A PhotoCORM Nanocarrier for CO Release Using NIR Light. Chem. Commun. 2015, 51, 2072–2075. [Google Scholar] [CrossRef] [PubMed]
- Crespy, D.; Landfester, K.; Schubert, U.S.; Schiller, A. Potential Photoactivated Metallopharmaceuticals: From Active Molecules to Supported Drugs. Chem. Commun. 2010, 46, 6651–6662. [Google Scholar] [CrossRef] [PubMed]
- García-Gallego, S.; Bernardes, G. Carbon-Monoxide-Releasing Molecules for the Delivery of Therapeutic CO In Vivo. Angew. Chem. Int. Ed. 2014, 53, 9712–9721. [Google Scholar] [CrossRef] [PubMed]
- Mann, B.E. Carbon monoxide: An essential signalling molecule. In Medicinal Organometallic Chemistry; Gerard, J., Metzler-Note, N., Eds.; Springer: New York, NY, USA, 2010; Volume 32, pp. 247–285. [Google Scholar]
- Marhenke, J.; Trevino, K.; Works, C. The Chemistry, Biology and Design of Photochemical CO Releasing Molecules and the Efforts to Detect CO for Biological Applications. Coord. Chem. Rev. 2016, 306, 533–543. [Google Scholar] [CrossRef]
- Ji, X.; Damera, K.; Zheng, Y.; Yu, B.; Otterbein, L.E.; Wang, B. Toward Carbon Monoxide-Based Therapeutics: Critical Drug Delivery and Developability Issues. J. Pharm. Sci. 2016, 105, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, R.D.; Richter, H.; Ford, P.C. A Photochemical Precursor for Carbon Monoxide Release in Aerated Aqueous Media. Inorg. Chem. 2009, 49, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Zobi, F.; Degonda, A.; Schaub, M.C.; Bogdanova, A.Y. CO Releasing Properties and Cytoprotective Effect of cis-trans-[ReII(CO)2Br2L2]n Complexes. Inorg. Chem. 2010, 49, 7313–7322. [Google Scholar] [CrossRef] [PubMed]
- McLean, S.; Mann, B.E.; Poole, R.K. Sulfite Species Enhance Carbon Monoxide Release from CO-Releasing Molecules: Implications for the Deoxymyoglobin Assay of Activity. Anal. Biochem. 2012, 427, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Gläser, S.; Mede, R.; Görls, H.; Seupel, S.; Bohlender, C.; Wyrwa, R.; Schirmer, S.; Dochow, S.; Reddy, G.U.; Popp, J. Remote-Controlled Delivery of CO Via Photoactive CO-Releasing Materials on a Fiber Optical Device. Dalton Trans. 2016, 45, 13222–13233. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Neugebauer, U.; Gheisari, A.; Malassa, A.; Jazzazi, T.M.; Froehlich, F.; Westerhausen, M.; Schmitt, M.; Popp, J.R. IR Spectroscopic Methods for the Investigation of the CO Release from CORMs. J. Phys. Chem. A 2014, 118, 5381–5390. [Google Scholar] [CrossRef] [PubMed]
- Michel, B.W.; Lippert, A.R.; Chang, C.J. A Reaction-Based Fluorescent Probe for Selective Imaging of Carbon Monoxide in Living Cells Using a Palladium-Mediated Carbonylation. J. Am. Chem. Soc. 2012, 134, 15668–15671. [Google Scholar] [CrossRef] [PubMed]
- Chapman, O.; Wojtkowski, P.; Adam, W.; Rodriguez, O.; Rucktäschel, R. Photochemical transformations. XLIV. Cyclic peroxides. Synthesis and chemistry of.alpha.-lactones. J. Am. Chem. Soc. 1972, 94, 1365–1367. [Google Scholar] [CrossRef]
- Kuzmanich, G.; Garcia, P.; Adam, W.; Rodriguez, O.; Rucktäschel, R. Ring strain release as a strategy to enable the singlet state photodecarbonylation of crystalline 1,4-cyclobutanediones. J. Phys. Org. Chem. 2011, 24, 883–888. [Google Scholar] [CrossRef]
- Poloukhtine, A.; Popik, V.V. Mechanism of the Cyclopropenone Decarbonylation Reaction.A Density Functional Theory and Transient Spectroscopy Study. J. Phys. Chem. A 2006, 110, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Poloukhtine, A.; Popik, V.V. Highly Efficient Photochemical Generation of a Triple Bond: Synthesis, Properties, and Photodecarbonylation of Cyclopropenones. J. Org. Chem. 2003, 68, 7833–7840. [Google Scholar] [CrossRef] [PubMed]
- Poloukhtine, A.A.; Mbua, N.E.; Wolfert, M.A.; Boons, G.J.; Popik, V.V. Selective Labeling of Living Cells by a Photo-Triggered Click Reaction. J. Am. Chem. Soc. 2009, 131, 15769–15776. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanich, G.; Gard, M.N.; Garcia-Garibay, M.A. Photonic Amplification by a Singlet-State Quantum Chain Reaction in the Photodecarbonylation of Crystalline Diarylcyclopropenones. J. Am. Chem. Soc. 2009, 131, 11606–11614. [Google Scholar] [CrossRef] [PubMed]
- Berends, H.M.; Kurz, P. Investigation of Light-Triggered Carbon Monoxide Release from Two Manganese PhotoCORMs by IR, UV-vis EPR Spectroscopy. Inorg. Chim. Acta 2012, 380, 141–147. [Google Scholar] [CrossRef]
- Üstün, E.; Ayvaz, M.Ç.; Çelebi, M.S.; Aşcı, G.; Demir, S.; Özdemir, İ. Structure, CO-Releasing Property, Electrochemistry, DFT Calculation, and Antioxidant Activity of Benzimidazole Derivative Substituted [Mn(CO)3(bpy)L]PF6 Type Novel Manganese Complexes. Inorg. Chim. Acta 2016, 450, 182–189. [Google Scholar] [CrossRef]
- Üstün, E.; Özgür, A.; Coşkun, K.A.; Demir, S.; Özdemir, İ; Tutar, Y. CO-Releasing Properties and Anticancer Activities of Manganese Complexes with Imidazole/Benzimidazole Ligands. J. Coord. Chem. 2016, 69, 3384–3394. [Google Scholar]
- Tinajero-Trejo, M.; Rana, N.; Nagel, C.; Jesse, H.E.; Smith, T.W.; Wareham, L.K.; Hippler, M.; Schatzschneider, U.; Poole, R.K. Antimicrobial Activity of the Manganese Photoactivated Carbon Monoxide-Releasing Molecule [Mn(CO)3(tpa-κ3N)]+ against a Pathogenic Escherichia coli That Causes Urinary Infections. Antioxid. Redox Signal. 2016, 24, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, M.; Zhou, L.; Zhang, G.; Gao, Z.; Zhang, W. Photo-Activated Co-Releasing Molecules (Photocorms) of Robust Sawhorse Scaffolds [μ2-OOCR1, η 1-NH2CHR2(C=O]OCH3, Ru(I)2CO4. Dalton Trans. 2016, 45, 3727–3733. [Google Scholar] [CrossRef] [PubMed]
- Carrington, S.J.; Chakraborty, I.; Bernard, J.M.; Mascharak, P.K. A Theranostic Two-Tone Luminescent Photocorm Derived from Re (I) and (2-Pyridyl)-Benzothiazole: Trackable CO Delivery to Malignant Cells. Inorg. Chem. 2016, 55, 7852–7858. [Google Scholar] [CrossRef] [PubMed]
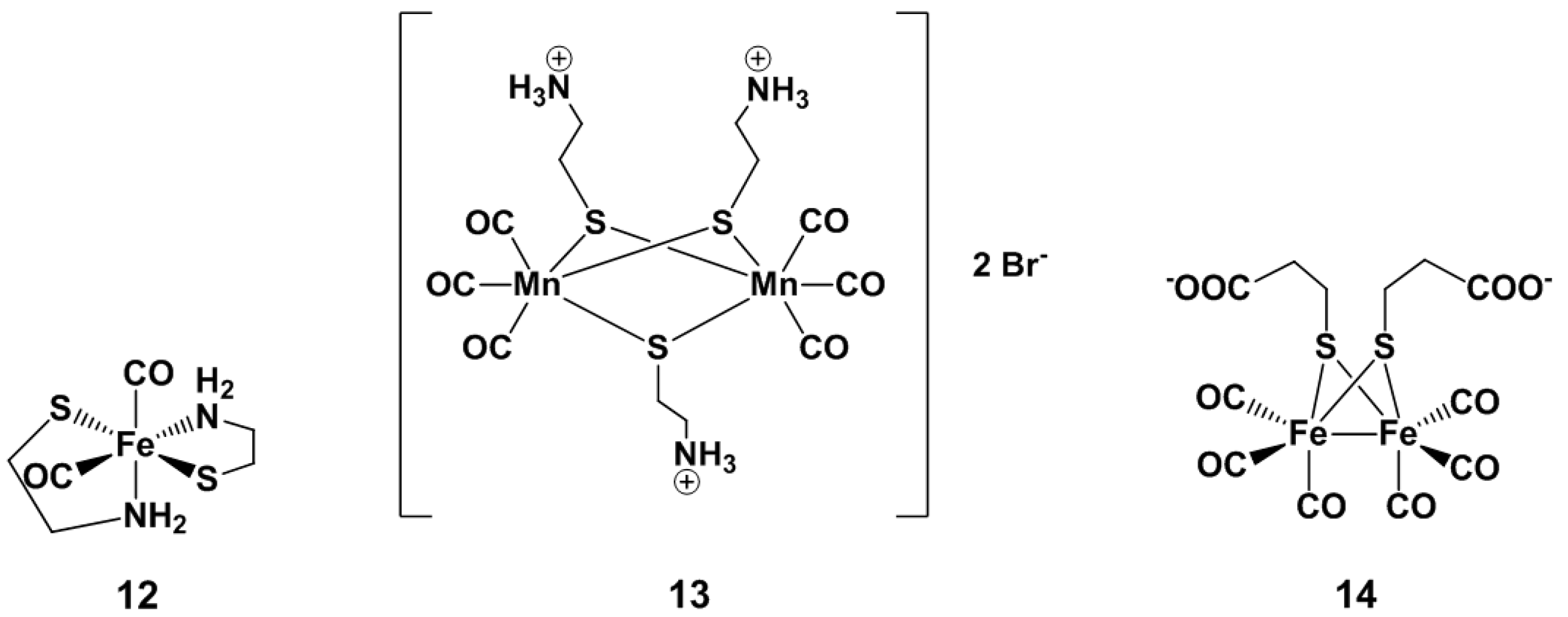
- Kretschmer, R.; Gessner, G.; Görls, H.; Heinemann, S.H.; Westerhausen, M. Dicarbonyl-Bis(Cysteamine) Iron (II): A Light Induced Carbon Monoxide Releasing Molecule Based on Iron (CORM-S1). J. Inorg. Biochem. 2011, 105, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Hewison, L.; Johnson, T.R.; Mann, B.E.; Meijer, A.J.; Sawle, P.; Motterlini, R. A Re-Investigation of [Fe(l-Cysteinate)2(CO)2]2−: An Example of Non-Heme CO Coordination of Possible Relevance to CO Binding to Ion Channel Receptors. Dalton Trans. 2011, 40, 8328–8334. [Google Scholar] [CrossRef] [PubMed]
- Mede, R.; Klein, M.; Claus, R.A.; Krieck, S.; Quickert, S.; Görls, H.; Neugebauer, U.; Schmitt, M.; Gessner, G.; Heinemann, S.H. CORM-EDE1: A Highly Water-Soluble and Nontoxic Manganese-Based PhotoCORM with a Biogenic Ligand Sphere. Inorg. Chem. 2015, 55, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Poh, H.T.; Sim, B.T.; Chwee, T.S.; Leong, W.K.; and Fan, W.Y. The Dithiolate-Bridged Diiron Hexacarbonyl Complex Na2[(M-SCH2CH2COO)Fe(CO)3]2 as a Water-Soluble PhotoCORM. Organometallics 2014, 33, 959–963. [Google Scholar] [CrossRef]
- Gonzalez, M.A.; Yim, M.A.; Cheng, S.; Moyes, A.; Hobbs, A.J.; Mascharak, P.K. Manganese Carbonyls Bearing Tripodal Polypyridine Ligands as Photoactive Carbon Monoxide-Releasing Molecules. Inorg. Chem. 2011, 51, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.A.; Carrington, S.J.; Fry, N.L.; Martinez, J.L.; Mascharak, P.K. Syntheses, Structures, and Properties of New Manganese Carbonyls as Photoactive CO-Releasing Molecules: Design Strategies That Lead to CO Photolability in the Visible Region. Inorg. Chem. 2012, 51, 11930–11940. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Carrington, S.J.; Mascharak, P.K. Photodelivery of CO by Designed Photocorms: Correlation between Absorption in the Visible Region and Metal–CO Bond Labilization in Carbonyl Complexes. ChemMedChem 2014, 9, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Vlček, A., Jr.; Farrell, I.R.; Liard, D.J.; Matousek, P.; Towrie, M.; Parker, A.W.; Grills, D.C.; George, M.W. Early Photochemical Dynamics of Organometallic Compounds Studied by Ultrafast Time-Resolved Spectroscopic Techniques. Dalton Trans. 2002, 701–712. [Google Scholar]
- Jimenez, J.; Chakraborty, I.; Carrington, S.J.; Mascharak, P.K. Light-Triggered CO Delivery by a Water-Soluble and Biocompatible Manganese PhotoCORM. Dalton Trans. 2016, 45, 13204–13213. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Chakraborty, I.; Mascharak, P.K. Synthesis and Assessment of Coered Cls, D.C.; George, M.W.; Early Photochemical Dynamics of α-imenez, J.; Chakof Varied Complexity. Eur. J. Inorg. Chem. 2015, 2015, 5021–5026. [Google Scholar] [CrossRef] [PubMed]
- Santoro, G.; Beltrami, R.; Kottelat, E.; Blacque, O.; Bogdanova, A.Y.; Zobi, F. N-Nitrosamine-{cis-Re[CO]2}2+ Cobalamin Conjugates as Mixed Co/No-Releasing Molecules. Dalton Trans. 2016, 45, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Blanářová, O.V.; Jelínková, I.; Szöőr, Á.; Skender, B.; Souček, K.; Horváth, V.; Vaculová, A.; Sova, P.; Szöllősi, J. Cisplatin and a Potent Platinum (IV) Complex-Mediated Enhancement of Trail-Induced Cancer Cells Killing Is Associated with Modulation of Upstream Events in the Extrinsic Apoptotic Pathway. Carcinogenesis 2011, 32, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Horváth, V.; Blanářová, O.; Švihálková-Šindlerová, L.; Souček, K.; Hofmanová, J.; Sova, P.; Kroutil, A.; Fedoročko, P.; Kozubík, A. Platinum (IV) Complex with Adamantylamine Overcomes Intrinsic Resistance to Cisplatin in Ovarian Cancer Cells. Gynecol. Oncol. 2006, 102, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Yempally, V.; Kyran, S.J.; Raju, R.K.; Fan, W.Y.; Brothers, E.N.; Darensbourg, D.J.; Bengali, A.A. Thermal and Photochemical Reactivity of Manganese Tricarbonyl and Tetracarbonyl Complexes with a Bulky Diazabutadiene Ligand. Inorg. Chem. 2014, 53, 4081–4088. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.M.; Shehab, O.R. Experimental and Quantum Chemical Calculations of Novel Photoactivatable Manganese (I) Tricarbonyl Complexes. J. Organomet. Chem. 2016, 822, 91–99. [Google Scholar] [CrossRef]
- Mansour, A.M. Crystal Structure, DFT, Spectroscopic and Biological Activity Evaluation of Analgin Complexes with Co (II), Ni (II) and Cu (II). Dalton Trans. 2014, 43, 15950–15957. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.M. Tazarotene Copper Complexes: Synthesis, Crystal Structure, DFT and Biological Activity Evaluation. Polyhedron 2016, 109, 99–106. [Google Scholar] [CrossRef]
- Anderson, S.N.; Larson, M.T.; Berreau, L.M. Solution or Solid—It Doesn’t Matter: Visible Light-Induced CO Release Reactivity of Zinc Flavonolato Complexes. Dalton Trans. 2016, 45, 14570–14580. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.N.; Richards, J.M.; Esquer, H.J.; Benninghoff, A.D.; Arif, A.M.; Berreau, L.M. A Structurally-Tunable 3-Hydroxyflavone Motif for Visible Light-Induced Carbon Monoxide-Releasing Molecules (CORMs). ChemistryOpen 2015, 4, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Viennois, E.; Ji, K.; Damera, K.; Draganov, A.; Zheng, Y.; Dai, C.; Merlin, D.; Wang, B. A Click-and-Release Approach to CO Prodrugs. Chem. Commun. 2014, 50, 15890–15893. [Google Scholar] [CrossRef] [PubMed]
- Palao Utiel, E.; Slanina, T.; Muchova, L.; Šolomek, T.; Vitek, L.; Klán, P. Transition-Metal-Free CO-Releasing Bodipy Derivatives Activatable by Visible to NIR Light as Promising Bioactive Molecules. J. Am. Chem. Soc. 2016, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Pap, J.S.; Kaizer, J.; Speier, G. Model Systems for the CO-Releasing Flavonol 2,4-Dioxygenase Enzyme. Coord. Chem. Rev. 2010, 254, 781–793. [Google Scholar] [CrossRef]
- Fetzner, S. Ring-Cleaving Dioxygenases with a Cupin Fold. Appl. Environ. Microbiol. 2012, 78, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Wang, C.; Shi, Z.; Johns, V.K.; Ma, L.; Oyer, J.; Copik, A.; Igarashi, R.; Liao, Y. Visible-Light Activatable Organic CO-Releasing Molecules (PhotoCORMs) That Simultaneously Generate Fluorophores. Org. Biomol. Chem. 2013, 11, 6671–6674. [Google Scholar] [CrossRef] [PubMed]
- Michael, E.; Abeyrathna, N.; Patel, A.V.; Liao, Y.; Bashur, C.A. Incorporation of Photo-Carbon Monoxide Releasing Materials into Electrospun Scaffolds for Vascular Tissue Engineering. Biomed. Mater. 2016, 11, 025009. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.; Pannen, B.H. Bench-to-Bedside Review: Carbon Monoxide—from Mitochondrial Poisoning to Therapeutic Use. Crit. Care 2009, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Bohlender, C.; Gläser, S.; Klein, M.; Weisser, J.; Thein, S.; Neugebauer, U.; Popp, J.; Wyrwa, R.; Schiller, A. Light-Triggered CO Release from Nanoporous Non-Wovens. J. Mater. Chem. B 2014, 2, 1454–1463. [Google Scholar] [CrossRef]
- Tabe, H.; Shimoi, T.; Boudes, M.; Abe, S.; Coulibaly, F.; Kitagawa, S.; Mori, H.; Ueno, T. Photoactivatable CO Release from Engineered Protein Crystals to Modulate NF-κB Activation. Chem. Commun. 2016, 52, 4545–4548. [Google Scholar] [CrossRef] [PubMed]
- Tabe, H.; Fujita, K.; Abe, S.; Tsujimoto, M.; Kuchimaru, T.; Kizaka-Kondoh, S.; Takano, M.; Kitagawa, S.; Ueno, T. Preparation of a Cross-Linked Porous Protein Crystal Containing Ru Carbonyl Complexes as a CO-Releasing Extracellular Scaffold. Inorg. Chem. 2014, 54, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Tabe, H.; Shimoi, T.; Fujita, K.; Abe, S.; Ijiri, H.; Tsujimoto, M.; Kuchimaru, T.; Kizaka-Kondo, S.; Mori, H.; Kitagawa, S. Design of a CO-Releasing Extracellular Scaffold Using in Vivo Protein Crystals. Chem. Lett. 2015, 44, 342–344. [Google Scholar] [CrossRef]
- Chlopicki, S.; Lomnicka, M.; Fedorowicz, A.; Grochal, E.; Kramkowski, K.; Mogielnicki, A.; Buczko, W.; Motterlini, R. Inhibition of Platelet Aggregation by Carbon Monoxide-Releasing Molecules (CO-RMs): Comparison with NO Donors. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Brasier, A.; Tate, J.; Habener, J. Optimized Use of the Firefly Luciferase Assay as a Reporter Gene in Mammalian Cell Lines. Biotechniques 1988, 7, 1116–1122. [Google Scholar]
- Govender, P.; Pai, S.; Schatzschneider, U.; Smith, G.S. Next Generation PhotoCORMs: Polynuclear Tricarbonylmanganese (I)-Functionalized Polypyridyl Metallodendrimers. Inorg. Chem. 2013, 52, 5470–5478. [Google Scholar] [CrossRef] [PubMed]
- Pierri, A.E.; Pallaoro, A.; Wu, G.; Ford, P.C. A Luminescent Biocompatible PhotoCORM. J. Am. Chem. Soc. 2012, 134, 18197–18200. [Google Scholar] [CrossRef] [PubMed]
- Carling, C.J.; Nourmohammadian, F.; Boyer, J.C.; Branda, N.R. Remote-Control Photorelease of Caged Compounds Using Near-Infrared Light and Upconverting Nanoparticles. Angew. Chem. Int. Ed. 2010, 122, 3870–3873. [Google Scholar] [CrossRef]
- Zheng, Q.; Bonoiu, A.; Ohulchanskyy, T.Y.; He, G.S.; Prasad, P.N. Water-Soluble Two-Photon Absorbing Nitrosyl Complex for Light-Activated Therapy through Nitric Oxide Release. Mol. Pharm. 2008, 5, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.V.; Yang, J.; Shen, D.; Yao, C.; Li, X.; Wang, R.; Stucky, G.D.; Zhao, D.; Ford, P.C.; Zhang, F. Nanostructured Materials. Small 2012, 8, 3800–3805. [Google Scholar] [CrossRef] [PubMed]
- Wecksler, S.; Mikhailovsky, A.; Ford, P.C. Photochemical Production of Nitric Oxide Via Two-Photon Excitation with NIR Light. J. Am. Chem. Soc. 2004, 126, 13566–13567. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kottelat, E.; Fabio, Z. Visible Light-Activated PhotoCORMs. Inorganics 2017, 5, 24. https://doi.org/10.3390/inorganics5020024
Kottelat E, Fabio Z. Visible Light-Activated PhotoCORMs. Inorganics. 2017; 5(2):24. https://doi.org/10.3390/inorganics5020024
Chicago/Turabian StyleKottelat, Emmanuel, and Zobi Fabio. 2017. "Visible Light-Activated PhotoCORMs" Inorganics 5, no. 2: 24. https://doi.org/10.3390/inorganics5020024
APA StyleKottelat, E., & Fabio, Z. (2017). Visible Light-Activated PhotoCORMs. Inorganics, 5(2), 24. https://doi.org/10.3390/inorganics5020024