Synthesis and Catalytic Applications of Non-Metal Doped Mesoporous Titania
Abstract
:1. Introduction
2. Synthesis of Mesoporous TiO2
2.1. Titanium Precursor Chemistry
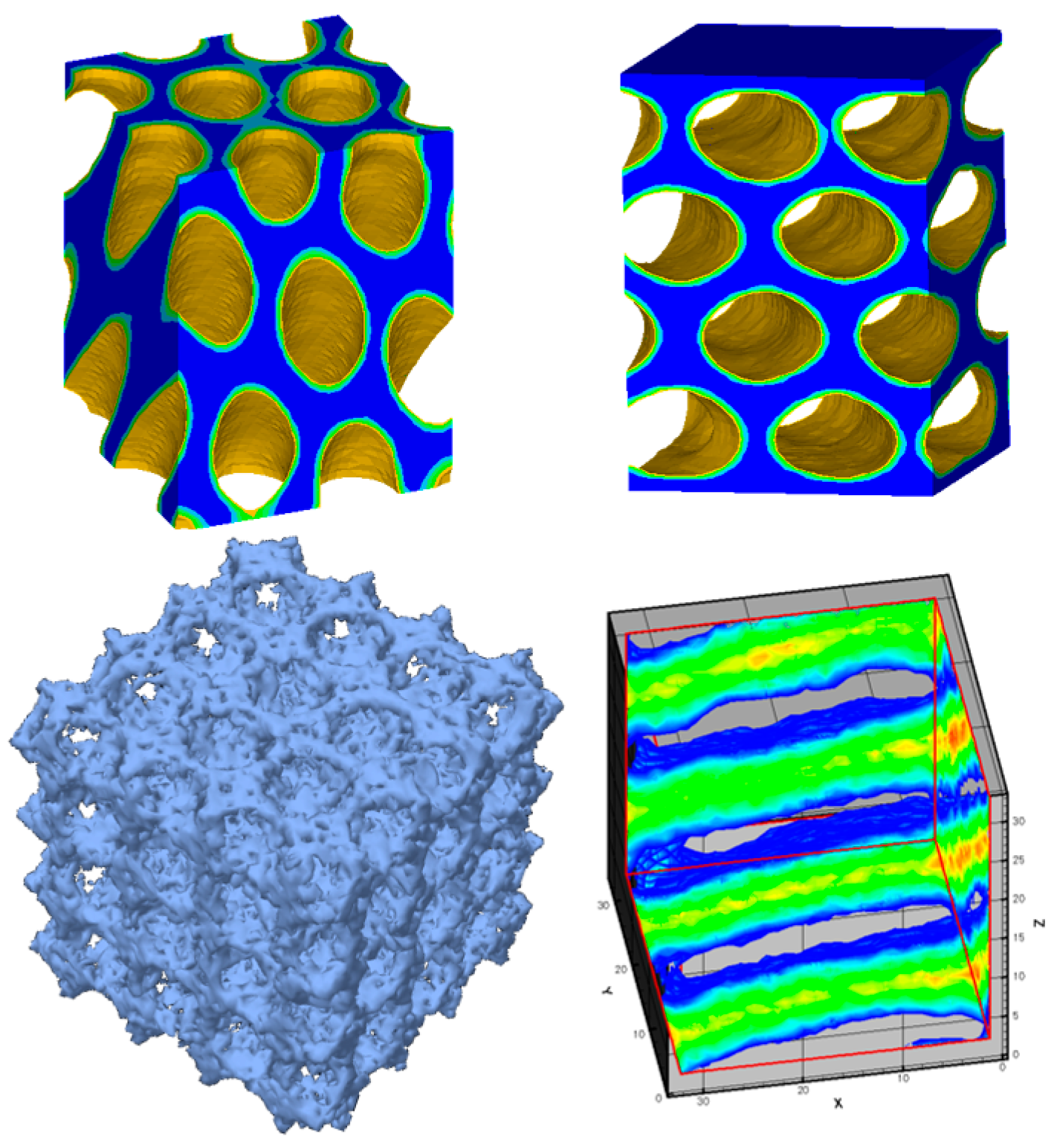
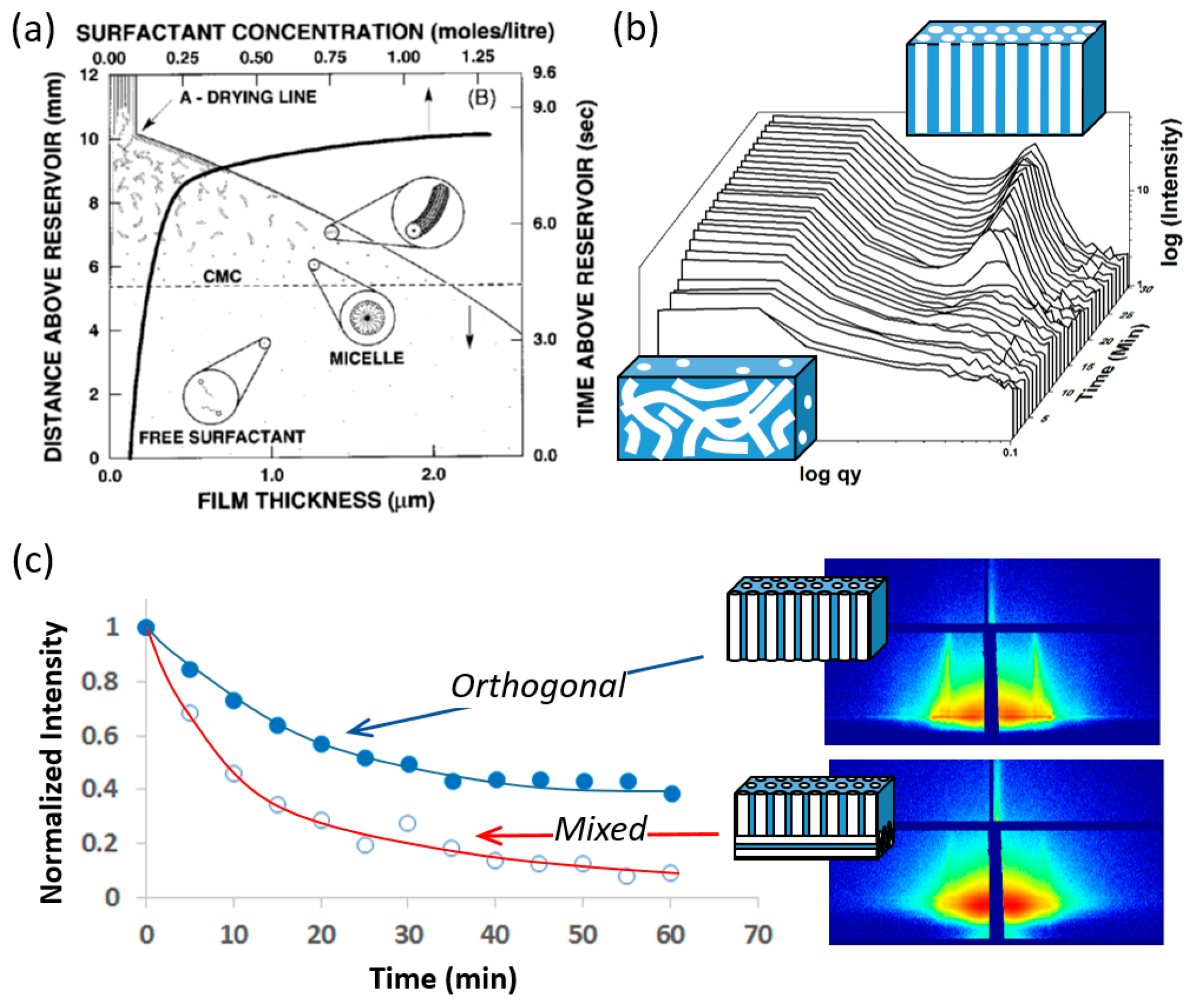
2.2. Surfactant-Templated Film Deposition
2.3. Surfactant/Titania Film Aging
2.4. Thermal Treatment
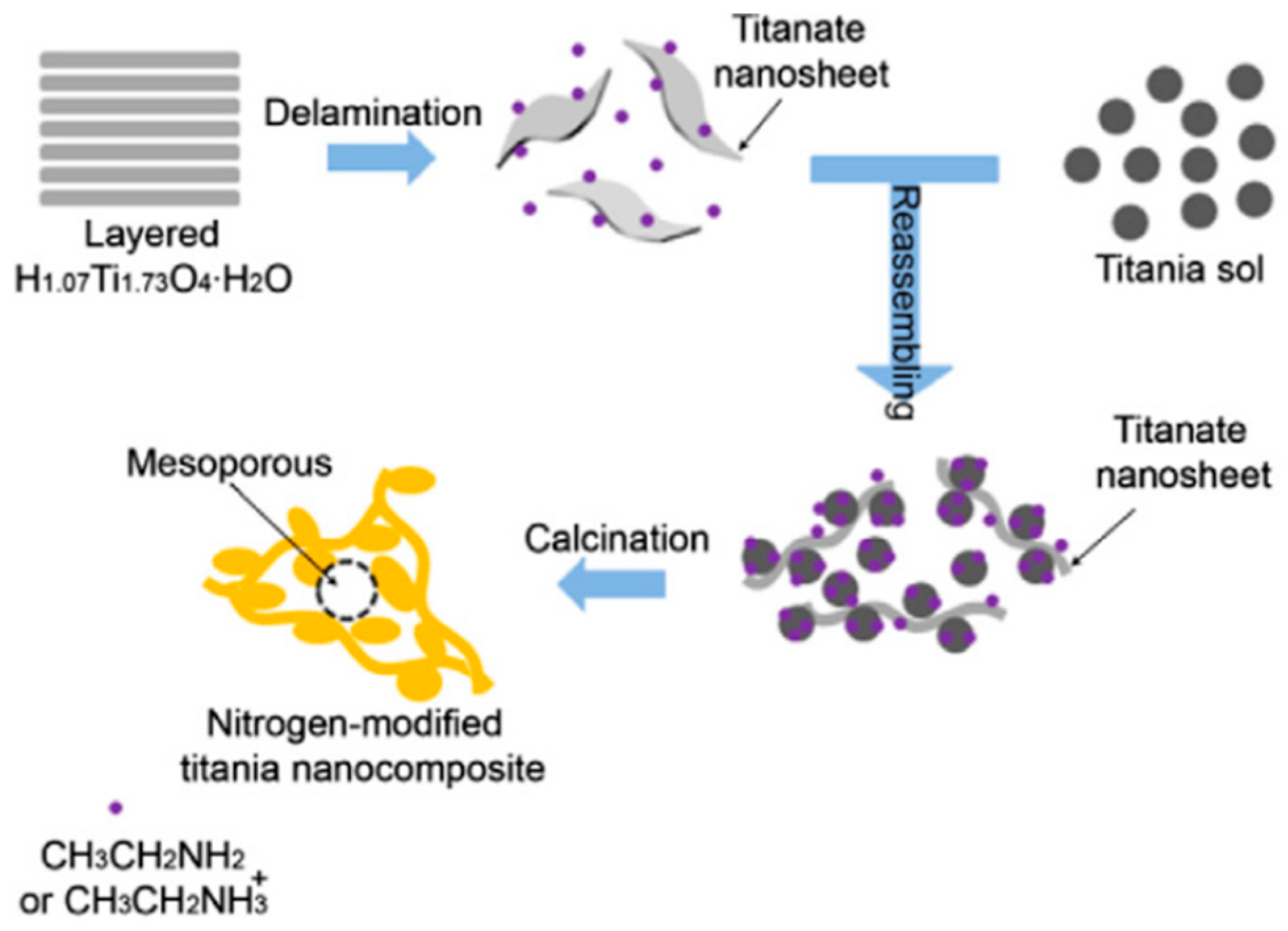
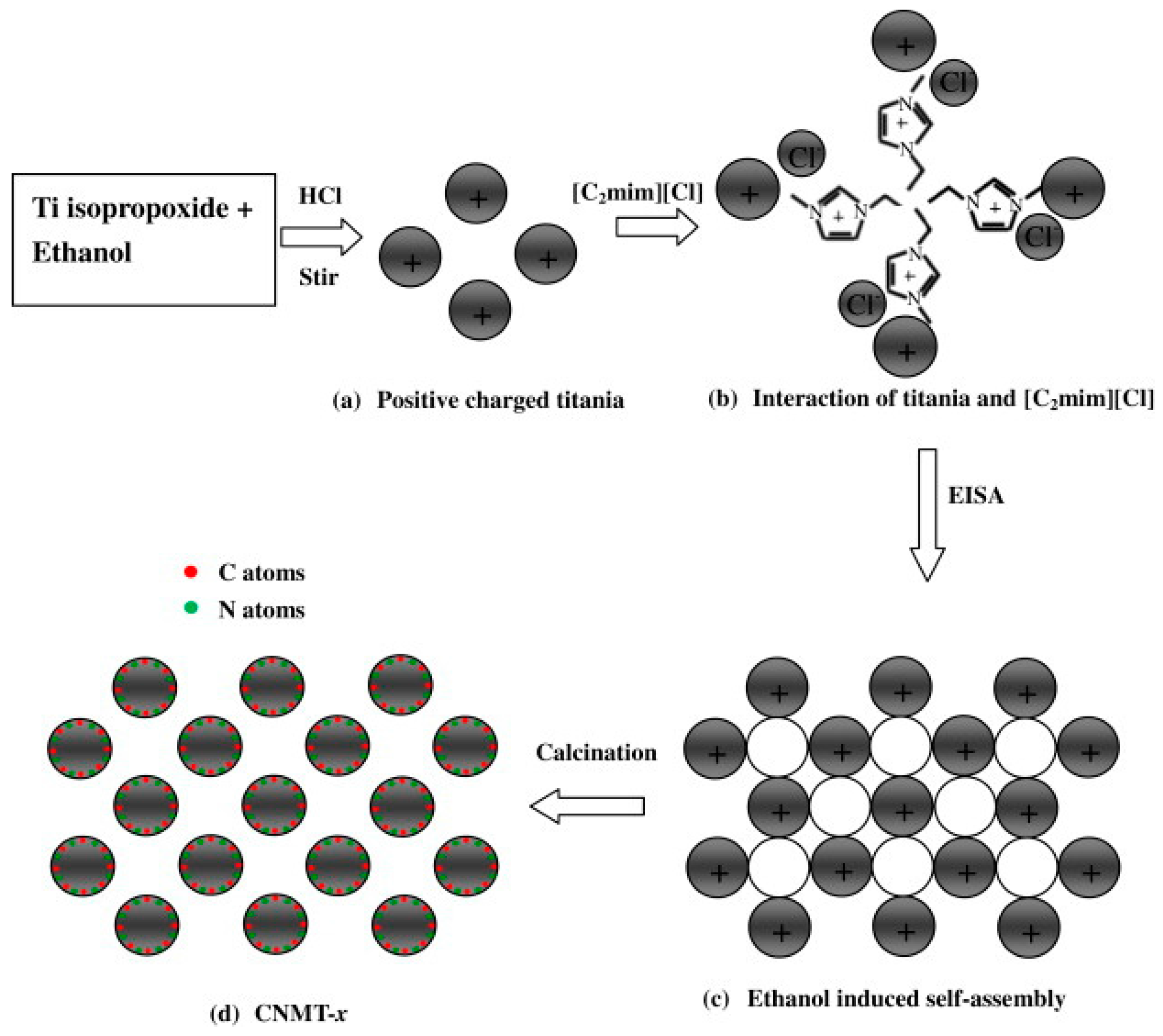
3. Nitrogen Doping
3.1. Amines
3.2. Ammonia
3.3. Hydrazine
3.4. Plasma Doping
3.5. Thiourea
3.6. Urea
3.7. Summary of Nitrogen Doped Mesoporous Titania
4. Other Non-Metal Doped Mesoporous Titania
4.1. Hydrogenation
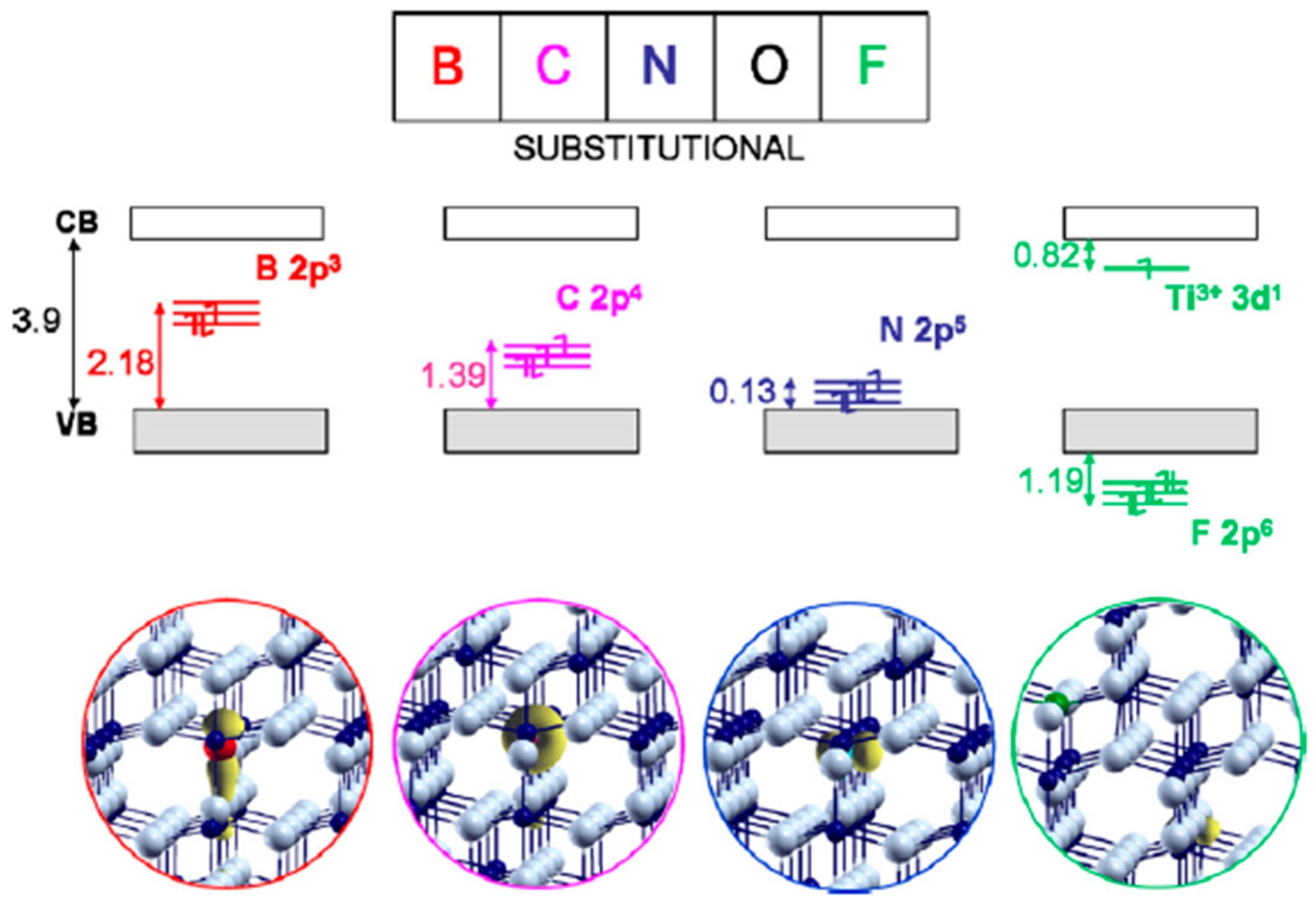
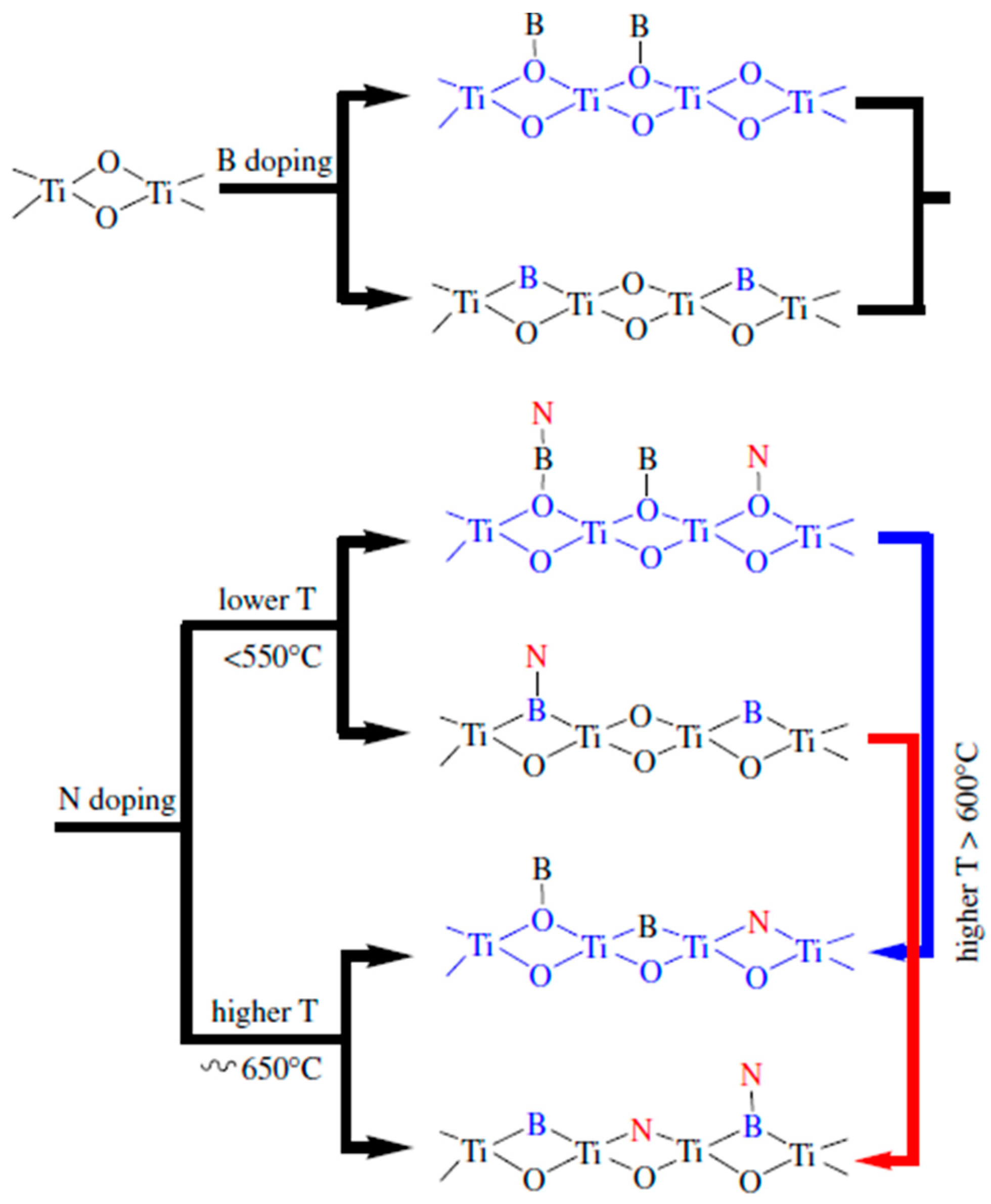
4.2. Boron Doping
4.3. Carbon Doping
4.4. Fluorine Doping
4.5. Iodine Doping
4.6. Phosphorus Doping
4.7. Summary of Nonmetal Dopants Other Than Nitrogen
5. Co-Doping of Non-Metals
6. Applications
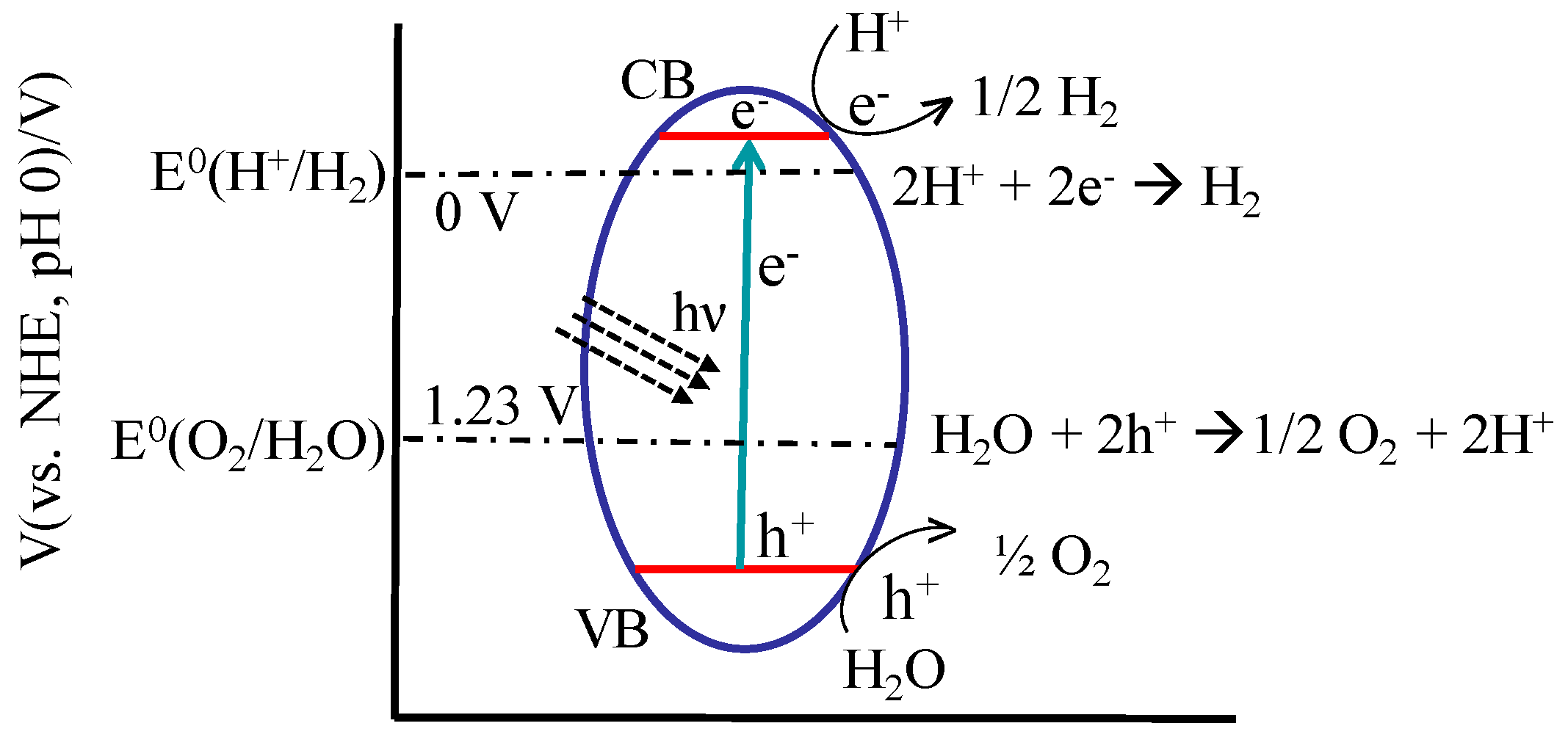
6.1. Water Splitting
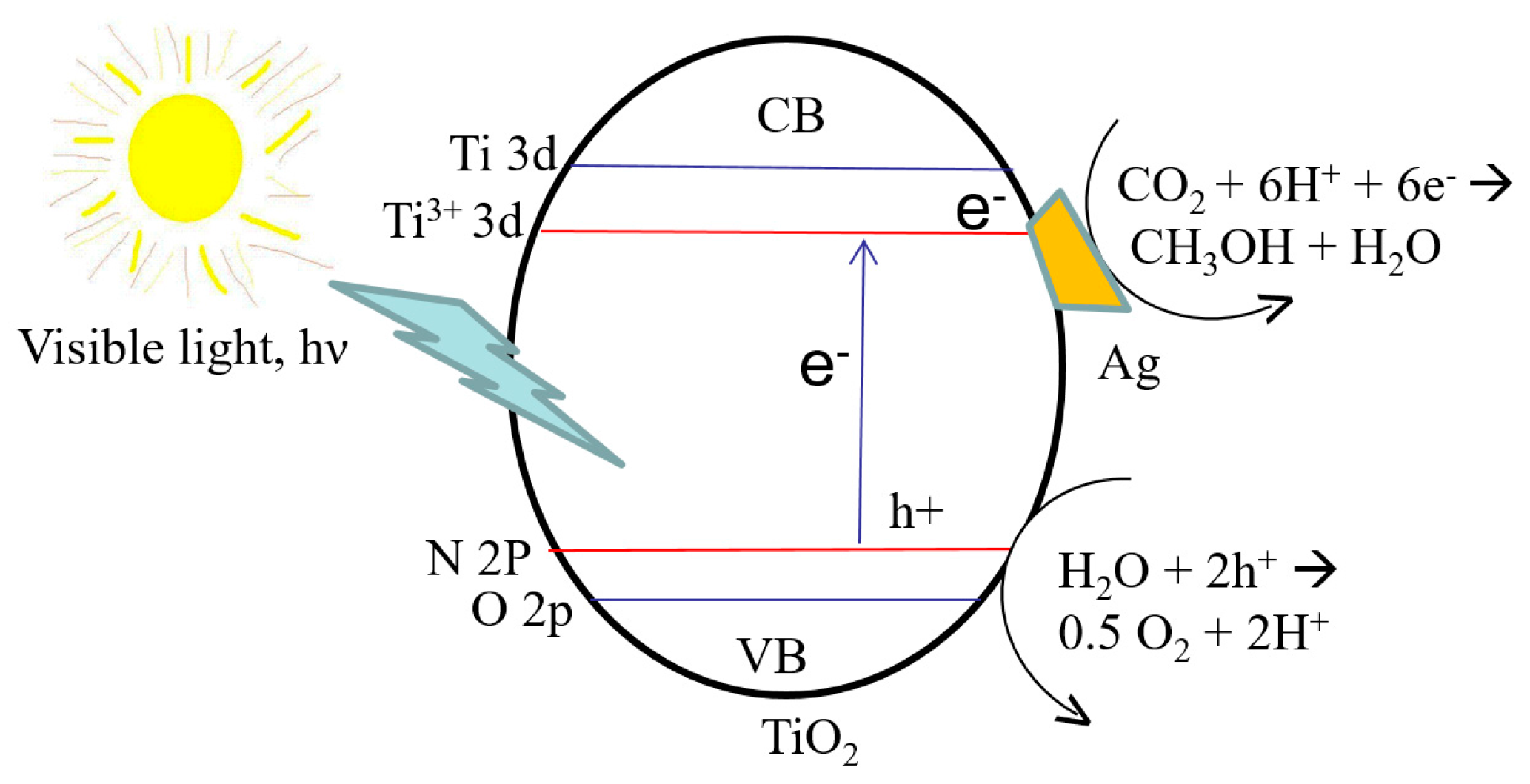
6.2. CO2 Reduction
7. Future Directions
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Leung, D.Y.C.; Fu, X.; Wang, C.; Ni, M.; Leung, M.K.H.; Wang, X.; Fu, X. Hydrogen production over titania-based photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Dholam, R.; Patel, N.; Adami, M.; Miotello, A. Hydrogen production by photocatalytic water-splitting using Cr- or Fe-doped TiO2 composite thin films photocatalyst. Int. J. Hydrogen Energy 2009, 34, 5337–5346. [Google Scholar] [CrossRef]
- Aurora, P.; Rhee, P.; Thompson, L. Titania nanotube supported gold photoanodes for photoelectrochemical cells. J. Electrochem. Soc. 2010, 157, K152–K155. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.S.; Henderson, M.J.; Bardeau, J.-F.; Gibaud, A. Visible-light photocatalysis in titania-based mesoporous thin films. Adv. Mater. 2008, 20, 1493–1498. [Google Scholar] [CrossRef]
- Shaozheng, H.; Fayun, L.; Zhiping, F.; Jianzhou, G. Improved photocatalytic hydrogen production property over Ni/NiO/N-TiO2−x heterojunction nanocomposite prepared by NH3 plasma treatment. J. Power Sources 2014, 250, 30–39. [Google Scholar]
- Lan, M.; Peng, X.; Pei-Nan, W. Experimental study on the band gap narrowings of TiO2 films calcined under N2 or NH3 atmosphere. Appl. Surf. Sci. 2008, 255, 2574–2580. [Google Scholar]
- Hidetaka, I.; Bock, J.P.; Sharma, R.; Hardcastle, F.; Kannarpady, G.K.; Mazumder, M.K.; Biris, A.S. Electrochemical synthesis of titania nanostructural arrays and their surface modification for enhanced photoelectrochemical hydrogen production. Chem. Phys. Lett. 2010, 489, 81–85. [Google Scholar]
- Lewis, N.S. Toward cost-effective solar energy use. Science 2007, 315, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.P.; Li, D.; Jing, W.H.; Xing, W.H.; Fan, Y.Q. Synthesis of visible-light responsive C, N and Ce co-doped TiO2 mesoporous membranes via weak alkaline sol–gel process. J. Mater. Chem. 2012, 22, 15309–15315. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Bahnemann, D.W. Photoelectrocatalytic materials for environmental applications. J. Mater. Chem. 2009, 19, 5089–5121. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Yang, H.G.; Cheng, H.M.; Lu, G.Q. Titania-based photocatalysts—Crystal growth, doping and heterostructuring. J. Mater. Chem. 2010, 20, 831–843. [Google Scholar] [CrossRef]
- Zhang, L.; Du, L.; Yu, X.; Tan, S.; Cai, X.; Yang, P.; Gu, Y.; Mai, W. Significantly enhanced photocatalytic activities and charge separation mechanism of Pd-decorated ZnO-graphene oxide Nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 3623–3629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, L.; Cai, X.; Yu, X.; Zhang, D.; Liang, L.; Yang, P.; Xing, X.; Mai, W.; Tan, S.; et al. Role of graphene in great enhancement of photocatalytic activity of ZnO nanoparticle–graphene hybrids. Physica E 2013, 47, 279–284. [Google Scholar] [CrossRef]
- Li, J.; Wu, N. Semiconductor-based photocatalysts and photoelectrochemical cells for solar fuel generation: A review. Catal. Sci. Technol. 2015, 5, 1360–1384. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’Shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New insights into the mechanism of visible light photocatalysis. J. Phy. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.W.; Cui, H.J.; Zong, X.; Chen, S.H.; Chen, J.S.; Xu, B.; Yang, W.Y.; Wang, L.Z.; Fu, M.L. Facile one-pot synthesis of Eu, N-codoped mesoporous titania microspheres with yolk-shell structure and high visible-light induced photocatalytic performance. Appl. Catal. A 2012, 435, 86–92. [Google Scholar] [CrossRef]
- Landmann, M.; Köhler, T.; Köppen, S.; Rauls, E.; Frauenheim, T.; Schmidt, W.G. Fingerprints of order and disorder in the electronic and optical properties of crystalline and amorphous TiO2. Phys. Rev. B 2012, 86, 064201. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O'Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Burda, C.; Lou, Y.; Chen, X.; Samia, A.C.S.; Stout, J.; Gole, J.L. Enhanced nitrogen doping in TiO2 nanoparticles. Nano Lett. 2003, 3, 1049–1051. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Mesoporous titania photocatalysts: Preparation, characterization and reaction mechanisms. J. Mater. Chem. 2011, 21, 11686–11707. [Google Scholar] [CrossRef]
- Bingham, S.; Daoud, W.A. Recent advances in making nano-sized TiO2 visible-light active through rare-earth metal doping. J. Mater. Chem. 2011, 21, 2041–2050. [Google Scholar] [CrossRef]
- Nagaveni, K.; Hegde, M.S.; Madras, G. Structure and photocatalytic activity of Ti1−xMxO2±δ (M = W, V, Ce, Zr, Fe, and Cu) synthesized by solution combustion method. J. Phys. Chem. B 2004, 108, 20204–20212. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. A review on non metal ion doped titania for the photocatalytic degradation of organic pollutants under UV/solar light: Role of photogenerated charge carrier dynamics in enhancing the activity. Appl. Catal. B 2013, 140, 559–587. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Choi, J.; Park, H.; Hoffmann, M.R. Effects of single metal-ion doping on the visible-light photoreactivity of TiO2. J. Phys. Chem. C 2010, 114, 783–792. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Huo, Y.N.; Zhang, D.Q.; Li, G.S.; Li, H.X. Ethanol supercritical route for fabricating bimodal carbon modified mesoporous TiO2 with enhanced photocatalytic capability in degrading phenol. Appl. Catal. B 2012, 115, 236–244. [Google Scholar] [CrossRef]
- Liu, S.-H.; Syu, H.-R. High visible-light photocatalytic hydrogen evolution of C,N-codoped mesoporous TiO2 nanoparticles prepared via an ionic-liquid-template approach. Int. J. Hydrogen Energy 2013, 38, 13856–13865. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M. Synthesis and characterization of nitrogen-doped TiO2 nanophotocatalyst with high visible light activity. J. Phys. Chem. C 2007, 111, 6976–6982. [Google Scholar] [CrossRef]
- Jinlong, L.; Xinxin, M.; Mingren, S.; Li, X.; Zhenlun, S. Fabrication of nitrogen-doped mesoporous TiO2 layer with higher visible photocatalytic activity by plasma-based ion implantation. Thin Solid Films 2010, 519, 101–105. [Google Scholar] [CrossRef]
- Varley, J.B.; Janotti, A.; van de Walle, C.G. Mechanism of visible-light photocatalysis in nitrogen-doped TiO2. Adv. Mater. 2011, 23, 2343–2347. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N. Is the band gap of pristine TiO2 narrowed by anion- and cation-doping of titanium dioxide in second-generation photocatalysts. J. Phys. Chem. B 2006, 110, 24287–24293. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhang, Y.W.; Shenoy, V.B.; Gao, H. Effects of H-, N-, and (H, N)-doping on the photocatalytic activity of TiO2. J. Phys. Chem. C 2011, 115, 12224–12231. [Google Scholar] [CrossRef]
- Lynch, J.; Giannini, C.; Cooper, J.K.; Loiudice, A.; Sharp, I.D.; Buonsanti, R. Substitutional or interstitial site-selective nitrogen doping in TiO2 nanostructures. J. Phys. Chem. C 2015, 119, 7443–7452. [Google Scholar] [CrossRef]
- Wang, G.; Xiao, X.; Li, W.; Lin, Z.; Zhao, Z.; Chen, C.; Wang, C.; Li, Y.; Huang, X.; Miao, L.; et al. Significantly enhanced visible light photoelectrochemical activity in TiO2 nanowire arrays by nitrogen implantation. Nano Lett. 2015, 15, 4692–4698. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Lou, Y.B.; Samia, A.C.S.; Burda, C.; Gole, J.L. Formation of oxynitride as the photocatalytic enhancing site in nitrogen-doped titania nanocatalysts: Comparison to a commercial nanopowder. Adv. Funct. Mater. 2005, 15, 41–49. [Google Scholar] [CrossRef]
- Wang, J.; Mao, B.; Gole, J.L.; Burda, C. Visible-light-driven reversible and switchable hydrophobic to hydrophilic nitrogen-doped titania surfaces: Correlation with photocatalysis. Nanoscale 2010, 2, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Di Valentin, C.; Pacchioni, G. Trends in non-metal doping of anatase TiO2: B, C, N and F. Catal. Today 2013, 206, 12–18. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, S.M.; Zhang, G.S.; El-Hosainy, H.M.; Ismail, A.A.; O’Shea, K.E.; Falaras, P.; Kontos, A.G.; Dionysiou, D.D. High performance sulfur, nitrogen and carbon doped mesoporous anatase-brookite TiO2 photocatalyst for the removal of microcystin-LR under visible light irradiation. J. Hazard. Mater. 2014, 280, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhao, Y.; Sun, C.; Li, F.; Lu, G.Q.; Cheng, H.M. Synergistic effects of B/N doping on the visible-light photocatalytic activity of mesoporous TiO2. Angew. Chem. Int. Ed. 2008, 47, 5277. [Google Scholar] [CrossRef]
- Xu, J.-H.; Li, J.; Dai, W.-L.; Cao, Y.; Li, H.; Fan, K. Simple fabrication of twist-like helix N,S-codoped titania photocatalyst with visible-light response. Appl. Catal. B 2008, 79, 72–80. [Google Scholar] [CrossRef]
- Liu, G.; Han, C.; Pelaez, M.; Zhu, D.; Liao, S.; Likodimos, V.; Kontos, A.G.; Falaras, P.; Dionysiou, D.D. Enhanced visible light photocatalytic activity of CN-codoped TiO2 films for the degradation of microcystin-LR. J. Mol. Catal. A Chem. 2013, 372, 58–65. [Google Scholar] [CrossRef]
- Katsanaki, A.V.; Kontos, A.G.; Maggos, T.; Pelaez, M.; Likodimos, V.; Pavlatou, E.A.; Dionysiou, D.D.; Falaras, P. Photocatalytic oxidation of nitrogen oxides on N–F-doped titania thin films. Appl. Catal. B 2013, 140–141, 619–625. [Google Scholar] [CrossRef]
- Periyat, P.; McCormack, D.E.; Hinder, S.J.; Pillai, S.C. One-pot synthesis of anionic (nitrogen) and cationic (sulfur) codoped high-temperature stable, visible light active, anatase photocatalysts. J. Phys. Chem. C 2009, 113, 3246–3253. [Google Scholar] [CrossRef]
- Pelaez, M.; Falaras, P.; Kontos, A.G.; de la Cruz, A.A.; O’Shea, K.; Dunlop, P.S.M.; Byrne, J.A.; Dionysiou, D.D. A comparative study on the removal of cylindrospermopsin and microcystins from water with NF-TiO2-P25 composite films with visible and UV–vis light photocatalytic activity. Appl. Catal. B 2012, 121–122, 30–39. [Google Scholar] [CrossRef]
- Kontos, A.G.; Pelaez, M.; Likodimos, V.; Vaenas, N.; Dionysiou, D.D.; Falaras, P. Visible light induced wetting of nanostructured N–F co-doped titania films. Photochem. Photobiol. Sci. 2011, 10, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; de la Cruz, A.A.; Stathatos, E.; Falaras, P.; Dionysiou, D.D. Visible light-activated N–F-codoped TiO2 nanoparticles for the photocatalytic degradation of microcystin-LR in water. Catal. Today 2009, 144, 19–25. [Google Scholar] [CrossRef]
- Zhao, C.; Pelaez, M.; Dionysiou, D.D.; Pillai, S.C.; Byrne, J.A.; O’Shea, K.E. UV and visible light activated TiO2 photocatalysis of 6-hydroxymethyl uracil, a model compound for the potent cyanotoxin cylindrospermopsin. Catal. Today 2014, 224, 70–76. [Google Scholar] [CrossRef]
- Shao, G.S.; Wang, F.Y.; Ren, T.Z.; Liu, Y.P.; Yuan, Z.Y. Hierarchical mesoporous phosphorus and nitrogen doped titania materials: Synthesis, characterization and visible-light photocatalytic activity. Appl. Catal. B 2009, 92, 61–67. [Google Scholar] [CrossRef]
- Cavalcante, R.P.; Dantas, R.F.; Bayarri, B.; González, O.; Giménez, J.; Esplugas, S.; Machulek Junior, A. Synthesis and characterization of B-doped TiO2 and their performance for the degradation of metoprolol. Catal. Today 2015, 252, 27–34. [Google Scholar] [CrossRef]
- Crepaldi, E.L.; Soler-lllia, G.J.D.A.A.; Grosso, D.; Cagnol, G.; Ribot, F.; Sanchez, C. Controlled formation of highly organized mesoporous titania thin films: From mesostructured hybrids to mesoporous nanoanatase TiO2. J. Am. Chem. Soc. 2003, 125, 9770–9786. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Luo, W.; Zhang, J.; Xu, M.; Zhang, R.; Wu, H.; Lv, Y.; Asiri, A.M.; Khan, S.B.; Rahman, M.M.; et al. Multi-layered mesoporous TiO2 thin films with large pores and highly crystalline frameworks for efficient photoelectrochemical conversion. J. Mater. Chem. A 2013, 1, 1591–1599. [Google Scholar] [CrossRef]
- Martinez-Ferrero, E.; Sakatani, Y.; Boissiere, C.; Grosso, D.; Fuertes, A.; Fraxedas, J.; Sanchez, C. Nanostructured titanium oxynitride porous thin films as efficient visible-active photocatalysts. Adv. Funct. Mater. 2007, 17, 3348–3354. [Google Scholar] [CrossRef]
- Liao, Y.T.; Huang, C.W.; Liao, C.H.; Wu, J.C.S.; Wu, K.C.W. Synthesis of mesoporous titania thin films (MTTFs) with two different structures as photocatalysts for generating hydrogen from water splitting. Appl. Energy 2012, 100, 75–80. [Google Scholar] [CrossRef]
- Li, L.; Yan, J.; Wang, T.; Zhao, Z.J.; Zhang, J.; Gong, J.; Guan, N. Sub-10 nm rutile titanium dioxide nanoparticles for efficient visible-light-driven photocatalytic hydrogen production. Nat. Commun. 2015, 6, 5881. [Google Scholar] [CrossRef] [PubMed]
- Salari, M.; Aboutalebi, S.H.; Aghassi, A.; Wagner, P.; Mozera, A.J.; Wallace, G.G. Disorder engineering of undoped TiO2 nanotube arrays for highly efficient solar-driven oxygen evolution. Phys. Chem. Chem. Phys. 2015, 17, 5642–5649. [Google Scholar] [CrossRef] [PubMed]
- Varghese, O.K.; Paulose, M.; LaTempa, T.J.; Grimes, C.A. High-rate solar photocatalytic conversion of CO2 and water vapor to hydrocarbon fuels. Nano Lett. 2009, 9, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.Z.; Reed, A.; Kim, D.Y.; Rankin, S.E. N2/Ar plasma induced doping of ordered mesoporous TiO2 thin films for visible light active photocatalysis. Microporous Mesoporous Mater. 2016, 220, 120–128. [Google Scholar] [CrossRef]
- Bagheri, S.; Mohd Hir, Z.A.; Yousefi, A.T.; Abdul Hamid, S.B. Progress on mesoporous titanium dioxide: Synthesis, modification and applications. Microporous Mesoporous Mater. 2015, 218, 206–222. [Google Scholar] [CrossRef]
- Mahoney, L.; Koodali, R. Versatility of evaporation-induced self-assembly (EISA) method for preparation of mesoporous TiO2 for energy and environmental applications. Materials 2014, 7, 2697. [Google Scholar] [CrossRef]
- Zhang, R.; Elzatahry, A.A.; Al-Deyab, S.S.; Zhao, D. Mesoporous titania: From synthesis to application. Nano Today 2012, 7, 344–366. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.X.; Wang, J.X.; Elzatahry, A.A.; Zhao, D.Y. A perspective on mesoporous TiO2 materials. Chem. Mater. 2014, 26, 287–298. [Google Scholar] [CrossRef]
- Zhou, W.; Fu, H. Mesoporous TiO2: Preparation, doping, and as a composite for photocatalysis. ChemCatChem 2013, 5, 885–894. [Google Scholar] [CrossRef]
- Antonelli, D.M.; Ying, J.Y. Synthesis of hexagonally packed mesoporous TiO2 by a modified sol–gel method. Angew. Chem. Int. Ed. 1995, 34, 2014–2017. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L.; Cheng, B.; Su, Y. Hydrothermal preparation and photocatalytic activity of hierarchically sponge-like macro-/mesoporous titania. J. Phys. Chem. C 2007, 111, 10582–10589. [Google Scholar] [CrossRef]
- Wang, H.; Miao, J.J.; Zhu, J.M.; Ma, H.M.; Zhu, J.J.; Chen, H.Y. Mesoporous spherical aggregates of anatase nanocrystals with wormhole-like framework structures: Their chemical fabrication, characterization, and photocatalytic performance. Langmuir 2004, 20, 11738–11747. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, Y.; Cao, C.; Luo, H.; Wang, W. Highly crystalline spindle-shaped mesoporous anatase titania particles: Solution-phase synthesis, characterization, and photocatalytic properties. Langmuir 2010, 26, 7671–7674. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.B.; Zhang, Q.; Lee, I.; Dahl, M.; Zaera, F.; Yin, Y. Mesoporous anatase titania hollow nanostructures though silica-protected calcination. Adv. Funct. Mater. 2012, 22, 166–174. [Google Scholar] [CrossRef]
- Da Silva, R.O.; Goncalves, R.H.; Stroppa, D.G.; Ramirez, A.J.; Leite, E.R. Synthesis of recrystallized anatase TiO2 mesocrystals with Wulff shape assisted by oriented attachment. Nanoscale 2011, 3, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Bleta, R.; Alphonse, P.; Lorenzato, L. Nanoparticle route for the preparation in aqueous medium of mesoporous TiO2 with controlled porosity and crystalline framework. J. Phys. Chem. C 2010, 114, 2039–2048. [Google Scholar] [CrossRef]
- Moonoosawmy, K.R.; Katzke, H.; Es-Souni, M.; Dietze, M.; Es-Souni, M. Mesoporous and macroporous brookite thin films having a large thermal stability range. Langmuir 2012, 28, 6706–6713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.; Li, H.; Zuo, C.; Wang, H. Thermal stability and optimal photoinduced hydrophilicity of mesoporous TiO2 thin films. J. Phys. Chem. C 2012, 116, 9517–9525. [Google Scholar] [CrossRef]
- Yin, J.B.; Zhao, X.P. Enhanced electrorheological activity of mesoporous Cr-doped TiO2 from activated pore wall and high surface area. J. Phys. Chem. B 2006, 110, 12916–12925. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Xu, J.; Zhang, Y.; He, Y. Hierarchical mesoporous TiO2 microspheres for the enhanced photocatalytic oxidation of sulfonamides and their mechanism. RSC Adv. 2012, 2, 4720–4727. [Google Scholar] [CrossRef]
- Nagpure, S.; Das, S.; Garlapalli, R.K.; Strzalka, J.; Rankin, S.E. In situ GISAXS investigation of low-temperature aging in oriented surfactant-mesostructured titania thin films. J. Phys. Chem. C 2015, 119, 22970–22984. [Google Scholar] [CrossRef]
- Das, S.; Wu, Q.; Garlapalli, R.K.; Nagpure, S.; Strzalka, J.; Jiang, Z.; Rankin, S.E. In-situ GISAXS investigation of pore orientation effects on the thermal transformation mechanism in mesoporous titania thin films. J. Phys. Chem. C 2014, 118, 968–976. [Google Scholar] [CrossRef]
- Das, S.; Nagpure, S.; Garlapalli, R.K.; Wu, Q.; Islam, S.Z.; Strzalka, J.; Rankin, S.E. Pore orientation effects on the kinetics of mesostructure loss in surfactant templated titania thin films. Phys. Chem. Chem. Phys. 2016, 18, 2896–2905. [Google Scholar] [CrossRef] [PubMed]
- Han, O.H.; Paik, Y.; Moon, Y.S.; Lee, S.K.; Kim, T.Y.; Lee, Y.H.; Lee, W.I. Selective synthesis of lamellar titania with carboxylate precursor and characterization by solid-state NMR. Chem. Mater. 2007, 19, 3615–3623. [Google Scholar] [CrossRef]
- Livage, J.; Henry, M.; Sanchez, C. Sol–gel chemistry of transition metal oxides. Prog. Solid State Chem. 1988, 18, 259–341. [Google Scholar] [CrossRef]
- Schubert, U. Chemical modification of titanium alkoxides for sol–gel processing. J. Mater. Chem. 2005, 15, 3701–3715. [Google Scholar] [CrossRef]
- Kallala, M.; Sanchez, C.; Cabane, B. Structures of inorganic polymers in sol–gel processes based on titanium oxide. Phys. Rev. E 1993, 48, 3692–3704. [Google Scholar] [CrossRef]
- Park, J.K.; Myoung, J.J.; Kyong, J.B.; Kim, H.K. Reaction mechanism for the hydrolysis of titanium alkoxides. Bull. Korean Chem. Soc. 2003, 24, 671–673. [Google Scholar]
- Weymann-Schildknetch, S.; Henry, M. Mechanistic aspects of the hydrolysis and condensation of titanium alkoxides complexed by tripodal ligands. J. Chem. Soc. Dalton Trans. 2001, 2425–2428. [Google Scholar] [CrossRef]
- Soler-Illia, G.J.D.A.A.; Sanchez, C. Interactions between poly(ethylene oxide)-based surfactants and transition metal alkoxides: Their role in the templated construction of mesostructured hybrid organic-inorganic composites. New J. Chem. 2000, 24, 493–499. [Google Scholar] [CrossRef]
- Antonelli, D.M. Synthesis of phosphorus-free mesoporous titania via templating with amine surfactants. Microporous Mesoporous Mater. 1999, 30, 315–319. [Google Scholar] [CrossRef]
- Brinker, C.J.; Lu, Y.; Sellinger, A.; Fan, H. Evaporation-induced self-assembly: Nanostructures made easy. Adv. Mater. 1999, 11, 579–585. [Google Scholar] [CrossRef]
- Alberius, P.C.A.; Frindell, K.L.; Hayward, R.C.; Kramer, E.J.; Stucky, G.D.; Chmelka, B.F. General predictive syntheses of cubic, hexagonal, and lamellar silica and titania mesostructured thin films. Chem. Mater. 2002, 14, 3284–3294. [Google Scholar] [CrossRef]
- Kirsch, B.L.; Richman, E.K.; Riley, A.E.; Tolbert, S.H. In-situ X-ray diffraction study of the crystallization kinetics of mesoporous titania films. J. Phys. Chem. B 2004, 108, 12698–12706. [Google Scholar] [CrossRef]
- Choi, S.Y.; Mamak, M.; Coombs, N.; Chopra, N.; Ozin, G.A. Thermally stable two-dimensional hexagonal mesoporous nanocrystalline anatase, meso-nc-TiO2: Bulk and crack-free thin film morphologies. Adv. Funct. Mater. 2004, 14, 335–344. [Google Scholar] [CrossRef]
- Haseloh, S.; Choi, S.Y.; Mamak, M.; Coombs, N.; Petrov, S.; Chopra, N.; Ozin, G.A. Towards flexible inorganic “mesomaterials”: One-pot low temperature synthesis of mesostructured nanocrystalline titania. Chem. Commun. 2004, 13, 1460–1461. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Boissière, C.; Grosso, D.; Laberty, C.; Nicole, L. Design, synthesis, and properties of inorganic and hybrid thin films having periodically organized nanoporosity. Chem. Mater. 2008, 20, 682–737. [Google Scholar] [CrossRef]
- Huo, Q.; Margolese, D.I.; Stucky, G.D. Surfactant control of phases in the synthesis of mesoporous silica-based materials. Chem. Mater. 1996, 8, 1147–1160. [Google Scholar] [CrossRef]
- Bosc, F.; Ayral, A.; Albouy, P.A.; Datas, L.; Guizard, C. Mesostructure of anatase thin films prepared by mesophase templating. Chem. Mater. 2004, 16, 2208–2214. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 2nd ed.; Academic Press: New York, NY, USA, 1992; pp. 366–394. [Google Scholar]
- Wu, Q.; Rankin, S.E. Tuning the wall thickness and pore orientation in mesoporous titania films prepared with low-temperature aging. J. Solgel Sci. Technol. 2011, 60, 81–90. [Google Scholar] [CrossRef]
- Jang, K.S.; Song, M.G.; Cho, S.H.; Kim, J.D. Using the effects of pH and moisture to synthesize highly organized mesoporous titania thin films. Chem. Commun. 2004, 13, 1514–1515. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, G.; Walton, D.; Mayes, A.; Lambooy, P.; Russell, T.; Gallagher, P.; Satija, S. Observed surface energy effects in confined diblock copolymers. Phys. Rev. Lett. 1996, 76, 2503. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Pruzinsky, S.; Russell, T.; Mays, J.; Hawker, C. Neutrality conditions for block copolymer systems on random copolymer brush surfaces. Macromolecules 1999, 32, 5299–5303. [Google Scholar] [CrossRef]
- Huang, E.; Russell, T.; Harrison, C.; Chaikin, P.; Register, R.; Hawker, C.; Mays, J. Using surface active random copolymers to control the domain orientation in diblock copolymer thin films. Macromolecules 1998, 31, 7641–7650. [Google Scholar] [CrossRef]
- Thurn-Albrecht, T.; Steiner, R.; DeRouchey, J.; Stafford, C.M.; Huang, E.; Bal, M.; Tuominen, M.; Hawker, C.J.; Russell, T.P. Nanoscopic templates from oriented block copolymer films. Adv. Mater. 2000, 12, 787–791. [Google Scholar] [CrossRef]
- Huinink, H.; Brokken-Zijp, J.; van Dijk, M.; Sevink, G. Asymmetric block copolymers confined in a thin film. J. Chem. Phys. 2000, 112, 2452–2462. [Google Scholar] [CrossRef]
- Pickett, G.T.; Balazs, A.C. Equilibrium orientation of confined diblock copolymer films. Macromolecules 1997, 30, 3097–3103. [Google Scholar] [CrossRef]
- Koganti, V.R.; Dunphy, D.; Gowrishankar, V.; McGehee, M.D.; Li, X.; Wang, J.; Rankin, S.E. Generalized coating route to silica and titania films with orthogonally tilted cylindrical nanopore arrays. Nano Lett. 2006, 6, 2567–2570. [Google Scholar] [CrossRef] [PubMed]
- Tasinkevych, M.; Ciach, A. Structural transformations in confined lamellar phases in oil–water–surfactant mixtures. J. Chem. Phys. 2001, 115, 8705–8713. [Google Scholar] [CrossRef]
- Rankin, S.E.; Malanoski, A.P.; van Swol, F. Monte Carlo simulation of amphiphile self-assembly during dip coating. In Proceedings of the Nonlithigraphic and Lithographic Methods of Nanofabrication—From Ultralarge-Scale Integration to Photonics to Molecular Electronics, Boston, MA, USA, 26 November–1 December 2000; Materials Research Society: Boston, MA, USA, 2001; pp. D121–D126. [Google Scholar]
- Richman, E.K.; Brezesinski, T.; Tolbert, S.H. Vertically oriented hexagonal mesoporous films formed through nanometre-scale epitaxy. Nat. Mater. 2008, 7, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Grosso, D.; Babonneau, F.; Sanchez, C.; Soler-Illia, G.J.D.A.A.; Crepaldi, E.L.; Albouy, P.A.; Amenitsch, H.; Balkenende, A.R.; Brunet-Bruneau, A. A first insight in the mechanisms involved in the self-assembly of 2D-hexagonal templated SiO2 and TiO2 mesostructured films during dip-coating. J. Solgel Sci. Technol. 2003, 26, 561–565. [Google Scholar] [CrossRef]
- Grosso, D.; Babonneau, F.; Albouy, P.A.; Amenitsch, H.; Balkenende, A.R.; Brunet-Bruneau, A.; Rivory, J. An in situ study of mesostructured CTAB–silica film formation during dip coating using time-resolved SAXS and interferometry measurements. Chem. Mater. 2002, 14, 931–939. [Google Scholar] [CrossRef]
- Grosso, D.; Balkenende, A.R.; Albouy, P.A.; Ayral, A.; Amenitsch, H.; Babonneau, F. Two-dimensional hexagonal mesoporous silica thin films prepared from block copolymers: Detailed characterization and formation mechanism. Chem. Mater. 2001, 13, 1848–1856. [Google Scholar] [CrossRef]
- Grosso, D.; Babonneau, F.; Soler-Illia, G.J.D.A.A.; Albouy, P.A.; Amenitsch, H. Phase transformation during cubic mesostructured silica film formation. Chem. Commun. 2002, 748–749. [Google Scholar] [CrossRef]
- Grosso, D.; Cagnol, F.; Soler-Illia, G.J.D.A.A.; Crepaldi, E.L.; Amenitsch, H.; Brunet-Bruneau, A.; Bourgeois, A.; Sanchez, C. Fundamentals of mesostructuring through evaporation-induced self-assembly. Adv. Funct. Mater. 2004, 14, 309–322. [Google Scholar] [CrossRef]
- Faustini, M.; Boissière, C.; Nicole, L.; Grosso, D. From chemical solutions to inorganic nanostructured materials: A journey into evaporation-driven processes. Chem. Mater. 2014, 26, 709–723. [Google Scholar] [CrossRef]
- Koganti, V.R.; Das, S.; Rankin, S.E. In situ FTIR investigation of the kinetics of silica polycondensation in surfactant templated, mesostructured thin films. J. Phys. Chem. C 2014, 118, 19450–19461. [Google Scholar] [CrossRef]
- Ko, Y.-S.; Koh, C.-W.; Lee, U.-H.; Kwon, Y.-U. Synthesis of mesoporous titania thin films with vertical pore channels and thick and crystalline walls. Microporous Mesoporous Mater. 2011, 145, 141–145. [Google Scholar] [CrossRef]
- Koh, C.-W.; Lee, U.-H.; Song, J.-K.; Lee, H.-R.; Kim, M.-H.; Suh, M.; Kwon, Y.-U. Mesoporous titania thin film with highly ordered and fully accessible vertical pores and crystalline walls. Chem. Asian J. 2008, 3, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Grosso, D.; Soler-Illia, G.J.D.A.A.; Crepaldi, E.L.; Cagnol, F.; Sinturel, C.; Bourgeois, A.; Brunet-Bruneau, A.; Amenitsch, H.; Albouy, P.A.; Sanchez, C. Highly porous TiO2 anatase optical thin films with cubic mesostructure stabilized at 700 °C. Chem. Mater. 2003, 15, 4562–4570. [Google Scholar] [CrossRef]
- Wanka, G.; Hoffmann, H.; Ulbricht, W. Phase diagrams and aggregation behavior of poly(oxyethylene)-poly(oxypropylene)-poly(oxyethylene) triblock copolymers in aqueous solutions. Macromolecules 1994, 27, 4145–4159. [Google Scholar] [CrossRef]
- Lee, U.H.; Lee, H.; Wen, S.; Mho, S.-I.; Kwon, Y.-U. Mesoporous titania thin films with pseudo-cubic structure: Synthetic studies and applications to nanomembranes and nanotemplates. Microporous Mesoporous Mater. 2006, 88, 48–55. [Google Scholar] [CrossRef]
- Liu, K.; Fu, H.; Shi, K.; Xiao, F.; Jing, L.; Xin, B. Preparation of large-pore mesoporous nanocrystalline TiO2 thin films with tailored pore diameters. J. Phys. Chem. B 2005, 109, 18719–18722. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Fryxell, G.E.; Birnbaum, J.C.; Wang, C. Effects of template and precursor chemistry on structure and properties of mesoporous TiO2 thin films. Langmuir 2004, 20, 9095–9102. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Park, S.J.; Ihm, S.K.; Lee, K.H. Synthesis of bimodal mesoporous titania with high thermal stability via replication of citric acid-templated mesoporous silica. Chem. Mater. 2007, 19, 937–941. [Google Scholar] [CrossRef]
- Yue, W.; Xu, X.; Irvine, J.T.S.; Attidekou, P.S.; Liu, C.; He, H.; Zhao, D.; Zhou, W. Mesoporous monocrystalline TiO2 and its solid-state electrochemical properties. Chem. Mater. 2009, 21, 2540–2546. [Google Scholar] [CrossRef]
- Zhang, Z.; Zuo, F.; Feng, P. Hard template synthesis of crystalline mesoporous anatase TiO2 for photocatalytic hydrogen evolution. J. Mater. Chem. 2010, 20, 2206–2212. [Google Scholar] [CrossRef]
- McDonnell, K.A.; English, N.J.; Rahman, M.; Dowling, D.P. Influence of doping on the photoactive properties of magnetron-sputtered titania coatings: Experimental and theoretical study. Phys. Rev. B Condens. Matter 2012, 86, 115306. [Google Scholar] [CrossRef]
- Ghicov, A.; Macak, J.M.; Tsuchiya, H.; Kunze, J.; Haeublein, V.; Kleber, S.; Schmuki, P. TiO2 nanotube layers: Dose effects during nitrogen doping by ion implantation. Chem. Phys. Lett. 2006, 419, 426–429. [Google Scholar] [CrossRef]
- Ghicov, A.; Macak, J.M.; Tsuchiya, H.; Kunze, J.; Haeublein, V.; Frey, L.; Schmuki, P. Ion implantation and annealing for an efficient N-doping of TiO2 nanotubes. Nano Lett. 2006, 6, 1080–1082. [Google Scholar] [CrossRef]
- Aman, N.; Mishra, T.; Sahu, R.K.; Tiwari, J.P. Facile synthesis of mesoporous N doped zirconium titanium mixed oxide nanomaterial with enhanced photocatalytic activity under visible light. J. Mater. Chem. 2010, 20, 10876–10882. [Google Scholar] [CrossRef]
- Mao, C.; Zuo, F.; Hou, Y.; Bu, X.; Feng, P. In situ preparation of a Ti3+ self-doped TiO2 film with enhanced activity as photoanode by N2H4 reduction. Angew. Chem. Int. Ed. 2014, 53, 10485–10489. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.Z.; Rankin, S.E. Hydrazine-based synergistic Ti(III)/N doping of surfactant-templated TiO2 thin films for enhanced visible light photocatalysis. Mater. Chem. Phys. 2016, 182, 382–393. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Chu, W.; Li, Z.; Ge, C. Characteristics of doped TiO2 photocatalysts for the degradation of methylene blue waste water under visible light. J. Alloys Compd. 2010, 501, 54–59. [Google Scholar] [CrossRef]
- Gohin, M.; Maurin, I.; Gacoin, T.; Boilot, J.P. Photocatalytic activity of mesoporous films based on N-doped TiO2 nanoparticles. J. Mater. Chem. 2010, 20, 8070–8077. [Google Scholar] [CrossRef]
- Sivaranjani, K.; Gopinath, C.S. Porosity driven photocatalytic activity of wormhole mesoporous TiO2−xNx in direct sunlight. J. Mater. Chem. 2011, 21, 2639–2647. [Google Scholar] [CrossRef]
- Ananpattarachai, J.; Kajitvichyanukul, P.; Seraphin, S. Visible light absorption ability and photocatalytic oxidation activity of various interstitial N-doped TiO2 prepared from different nitrogen dopants. J. Hazard. Mater. 2009, 168, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, G.; Shi, X. N/Zr-codoped TiO2 nanotube arrays: Fabrication, characterization, and enhanced photocatalytic cctivity. Colloids Surf. A 2010, 363, 35–40. [Google Scholar] [CrossRef]
- Kafizas, A.; Parkin, I.P. The combinatorial atmospheric pressure chemical vapour deposition (cAPCVD) of a gradating N-doped mixed phase titania thin film. J. Mater. Chem. 2010, 20, 2157–2169. [Google Scholar] [CrossRef]
- Miao, L.; Tanemura, S.; Watanabe, H.; Toh, S.; Kaneko, K. Structural and compositional characterization of N2–H2 plasma surface-treated TiO2 thin films. Appl. Surf. Sci. 2005, 244, 412–417. [Google Scholar] [CrossRef]
- Sharma, R.; Das, P.P.; Misra, M.; Mahajan, V.; Bock, J.P.; Trigwell, S.; Biris, A.S.; Mazumder, M.K. Enhancement of the photoelectrochemical conversion efficiency of nanotubular TiO2 photoanodes using nitrogen plasma assisted surface modification. Nanotechnology 2009, 20, 075704. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Z.; Zheng, J.; Yan, X.; Li, D.; Chen, S.; Chu, W. Characteristics of N-Doped TiO2 nanotube arrays by N2-plasma for visible light-driven photocatalysis. J. Alloys Compd. 2011, 509, 9970–9976. [Google Scholar] [CrossRef]
- Lee, H.U.; Lee, Y.; Lee, S.C.; Park, S.Y.; Son, B.; Lee, J.W.; Lim, C.; Choi, C.; Choi, M.; Lee, S.Y.; et al. Visible-light-responsive bicrystalline (anatase/brookite) nanoporous nitrogen-doped TiO2 photocatalysts by plasma treatment. Chem. Eng. J. 2014, 254, 268–275. [Google Scholar] [CrossRef]
- Pulsipher, D.J.; Martin, I.T.; Fisher, E.R. Controlled nitrogen doping and film colorimetrics in porous TiO2 materials using plasma processing. ACS Appl. Mater. Interfaces 2010, 2, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.M.; Chen, L.C.; Cheng, K.W.; Pan, G.T. Effect of nitrogen-plasma surface treatment to the enhancement of TiO2 photocatalytic activity under visible light irradiation. J. Mol. Catal. A Chem. 2007, 261, 218–224. [Google Scholar] [CrossRef]
- Chen, C.; Bai, H.; Chang, C. Effect of plasma processing gas composition on the nitrogen-doping status and visible light photocatalysis of TiO2. J. Phys. Chem. C 2007, 111, 15228–15235. [Google Scholar] [CrossRef]
- Napoli, F.; Chiesa, M.; Livraghi, S.; Giamello, E.; Agnoli, S.; Granozzi, G.; Pacchioni, G.; Di Valentin, C. The Nitrogen photoactive centre in N-doped titanium dioxide formed via interaction of N atoms with the solid. nature and energy level of the species. Chem. Phys. Lett. 2009, 477, 135–138. [Google Scholar] [CrossRef]
- Li, G.S.; Yu, J.C.; Zhang, D.Q.; Hu, X.L.; Lau, W.M. A mesoporous TiO2−xNx photocatalyst prepared by sonication pretreatment and in situ pyrolysis. Sep. Purif. Technol. 2009, 67, 152–157. [Google Scholar] [CrossRef]
- Liu, G.; Wang, X.; Wang, L.; Chen, Z.; Li, F.; Lu, G.Q.; Cheng, H.M. Drastically enhanced photocatalytic activity in nitrogen doped mesoporous TiO2 with abundant surface states. J. Colloid Interface Sci. 2009, 334, 171–175. [Google Scholar] [CrossRef]
- Nassoko, D.; Li, Y.-F.; Wang, H.; Li, J.-L.; Li, Y.-Z.; Yu, Y. Nitrogen-doped TiO2 nanoparticles by using EDTA as nitrogen source and soft template: Simple preparation, mesoporous structure, and photocatalytic activity under visible light. J. Alloys Compd. 2012, 540, 228–235. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Subramaniam, V.P.; Gong, D.G.; Tang, Y.X.; Highfield, J.; Pehkonen, S.O.; Pichat, P.; Schreyer, M.K.; Chen, Z. Nitrogen-sensitized dual phase titanate/titania for visible-light driven phenol degradation. J. Solid State Chem. 2012, 196, 518–527. [Google Scholar] [CrossRef]
- Qiao, M.; Chen, Q.A.; Wu, S.S.; Shen, J.A. Novel sol–gel synthesis of N-doped TiO2 hollow spheres with high photocatalytic activity under visible light. J. Solgel Sci. Technol. 2010, 55, 377–384. [Google Scholar] [CrossRef]
- Fu, J.; Tian, Y.L.; Chang, B.B.; Xi, F.N.; Dong, X.P. Soft-chemical synthesis of mesoporous nitrogen-modified titania with superior photocatalytic performance under visible light irradiation. Chem. Eng. J. 2013, 219, 155–161. [Google Scholar] [CrossRef]
- Elghniji, K.; Ksibi, M.; Elaloui, E. Sol–gel reverse micelle preparation and characterization of N-doped TiO2: Efficient photocatalytic degradation of methylene blue in water under visible light. J. Ind. Eng. Chem. 2012, 18, 178–182. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, C.; Fan, Q.; Wei, L.; Chen, J.; Yu, J.C. Ultrasonic fabrication of N-doped TiO2 nanocrystals with mesoporous structure and enhanced visible light photocatalytic activity. Chin. J. Catal. 2013, 34, 1250–1255. [Google Scholar] [CrossRef]
- Nolan, N.T.; Synnott, D.W.; Seery, M.K.; Hinder, S.J.; Wassenhoven, A.V.; Pillai, S.C. Effect of N-doping on the photocatalytic activity of sol–gel TiO2. J. Hazard. Mater. 2012, 211–212, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Xu, L.; Chen, J. Ordered arrays of N-doped mesoporous titania spheres with high visible light photocatalytic activity. Mater. Lett. 2013, 106, 421–424. [Google Scholar] [CrossRef]
- Li, X.; Liu, P.W.; Mao, Y.; Xing, M.Y.; Zhang, J.L. Preparation of homogeneous nitrogen-doped mesoporous TiO2 spheres with enhanced visible-Light photocatalysis. Appl. Catal. B 2015, 164, 352–359. [Google Scholar] [CrossRef]
- Hou, Y.D.; Wang, X.C.; Wu, L.; Chen, X.F.; Ding, Z.X.; Wang, X.X.; Fu, X.Z. N-doped SiO2/TiO2 mesoporous nanoparticles with enhanced photocatalytic activity under visible-light irradiation. Chemosphere 2008, 72, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.M.; Labhsetwar, N.K.; Mangrulkar, P.A.; Tijare, S.N.; Kamble, S.P.; Rayalu, S.S. Visible light induced photoreduction of methyl orange by N-doped mesoporous titania. Appl. Catal. A 2009, 357, 26–33. [Google Scholar] [CrossRef]
- Zuo, F.; Wang, L.; Wu, T.; Zhang, Z.; Borchardt, D.; Feng, P. Self-doped Ti3+ enhanced photocatalyst for hydrogen production under visible light. J. Am. Chem. Soc. 2010, 132, 11856–11857. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Bozhilov, K.; Dillon, R.J.; Wang, L.; Smith, P.; Zhao, X.; Bardeen, C.; Feng, P. Active facets on titanium(III)-doped TiO2: An effective strategy to improve the visible-light photocatalytic activity. Angew. Chem. Int. Ed. 2012, 51, 6223–6226. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Bai, Y.; Jin, W.; Xu, N. Visible-light-driven TiO2 catalysts doped with low-concentration nitrogen species. Sol. Energy Mater. Sol. Cells 2008, 92, 76–83. [Google Scholar] [CrossRef]
- Xu, J.; Ao, Y.; Chen, M.; Fu, D. Photoelectrochemical property and photocatalytic activity of N-doped TiO2 nanotube arrays. Appl. Surf. Sci. 2010, 256, 4397–4401. [Google Scholar] [CrossRef]
- Ao, Y.; Xu, J.; Fu, D.; Yuan, C. Visible-light responsive C,N-codoped titania hollow spheres for X-3B dye photodegradation. Microporous Mesoporous Mater. 2009, 118, 382–386. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Su, J.; Zou, X.; Chen, J.S. Self-modification of titanium dioxide materials by Ti3+ and/or oxygen vacancies: New insights into defect chemistry of metal oxides. RSC Adv. 2014, 4, 13979–13988. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Li, X.; Zhu, J.; Li, H. Synchronical pollutant degradation and H2 production on a Ti3+-doped TiO2 visible photocatalyst with dominant (001) facets. Appl. Catal. B 2013, 134–135, 198–204. [Google Scholar] [CrossRef]
- Selvam, K.; Balachandran, S.; Velmurugan, R.; Swaminathan, M. Mesoporous nitrogen doped nano titania—A green photocatalyst for the effective reductive cleavage of azoxybenzenes to amines or 2-phenyl indazoles in methanol. Appl. Catal. A 2012, 413–414, 213–222. [Google Scholar] [CrossRef]
- Islam, S.Z.; Reed, A.; Wanninayake, N.; Kim, D.Y.; Rankin, S.E. Remarkable enhancement of photocatalytic water oxidation in N2/Ar plasma treated, mesoporous TiO2 films. J. Phys. Chem. C 2016, 120, 14069–14081. [Google Scholar] [CrossRef]
- Mei, P.; Henderson, M.; Kassiba, A.; Gibaud, A. EPR study of nitrogen-doped mesoporous TiO2 powders. J. Phys. Chem. Solids 2010, 71, 1–6. [Google Scholar] [CrossRef]
- Soni, S.S.; Dave, G.S.; Henderson, M.J.; Gibaud, A. Visible light induced cell damage of Gram positive bacteria by N-doped TiO2 mesoporous thin films. Thin Solid Films 2013, 531, 559–565. [Google Scholar] [CrossRef]
- Yao, N.; Wu, C.; Jia, L.; Han, S.; Chi, B.; Pu, J.; Jian, L. Simple synthesis and characterization of mesoporous (N, S)-codoped TiO2 with enhanced visible-light photocatalytic activity. Ceram. Int. 2012, 38, 1671–1675. [Google Scholar] [CrossRef]
- Xiao, Q.; Ouyang, L.; Gao, L.; Yao, C. Preparation and visible light photocatalytic activity of mesoporous N, S-codoped TiO2(B) nanobelts. Appl. Surf. Sci. 2011, 257, 3652–3656. [Google Scholar] [CrossRef]
- Ao, Y.; Xu, J.; Fu, D.; Yuan, C. Synthesis of C,N,S-tridoped mesoporous titania with enhanced visible light-induced photocatalytic activity. Microporous Mesoporous Mater. 2009, 122, 1–6. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Li, H.; Zou, C. Mesoporous TiO2−xAy (A = N, S) as a visible-light-response photocatalyst. Solid State Sci. 2010, 12, 490–497. [Google Scholar] [CrossRef]
- Zalas, M. Synthesis of N-doped template-free mesoporous titania for visible light photocatalytic applications. Catal. Today 2014, 230, 91–96. [Google Scholar] [CrossRef]
- Liu, S.H.; Syu, H.R. One-step fabrication of N-doped mesoporous TiO2 nanoparticles by self-assembly for photocatalytic water splitting under visible light. Appl. Energy 2012, 100, 148–154. [Google Scholar] [CrossRef]
- Gandhe, A.R.; Naik, S.P.; Fernandes, J.B. Selective synthesis of N-doped mesoporous TiO2 phases having enhanced photocatalytic activity. Microporous Mesoporous Mater. 2005, 87, 103–109. [Google Scholar] [CrossRef]
- Chi, B.; Zhao, L.; Jin, T. One-step template-free route for synthesis of mesoporous N-doped titania spheres. J. Phys. Chem. C 2007, 111, 6189–6193. [Google Scholar] [CrossRef]
- Horikawa, T.; Katoh, M.; Tomida, T. Preparation and characterization of nitrogen-doped mesoporous titania with high specific surface area. Microporous Mesoporous Mater. 2008, 110, 397–404. [Google Scholar] [CrossRef]
- Shao, G.S.; Zhang, X.J.; Yuan, Z.Y. Preparation and photocatalytic activity of hierarchically mesoporous-macroporous TiO2−xNx. Appl. Catal. B 2008, 82, 208–218. [Google Scholar] [CrossRef]
- Parida, K.M.; Naik, B. Synthesis of mesoporous TiO2−xNx spheres by template free homogeneous co-precipitation method and their photo-catalytic activity under visible light illumination. J. Colloid Interface Sci. 2009, 333, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Xing, M.Y.; Tian, B.Z.; Zhang, J.L.; Chen, F. Preparation of nitrogen and fluorine co-doped mesoporous TiO2 microsphere and photodegradation of acid orange 7 under visible light. Chem. Eng. J. 2010, 162, 710–717. [Google Scholar] [CrossRef]
- Liu, E.; Guo, X.; Qin, L.; Shen, G.; Wang, X. Fabrication and photocatalytic activity of highly crystalline nitrogen doped mesoporous TiO2. Chin. J. Catal. 2012, 33, 1665–1671. [Google Scholar] [CrossRef]
- Jia, T.; Fu, F.; Zhao, J.; Chen, J.; Wang, X.; Fan, Z.; Cui, L.; Meng, F. Sonochemical synthesis, characterization, and photocatalytic activity of N-doped TiO2 nanocrystals with mesoporous structure. Int. J. Photoenergy 2014, 2014, 516806. [Google Scholar]
- Sreethawong, T.; Laehsalee, S.; Chavadej, S. Comparative investigation of mesoporous- and non-mesoporous-assembled TiO2 nanocrystals for photocatalytic H2 production over N-doped TiO2 under visible light irradiation. Int. J. Hydrogen Energy 2008, 33, 5947–5957. [Google Scholar] [CrossRef]
- Sreethawong, T.; Laehsalee, S.; Chavadej, S. Use of Pt/N-doped mesoporous-assembled nanocrystalline TiO2 for photocatalytic H2 production under visible light irradiation. Catal. Commun. 2009, 10, 538–543. [Google Scholar] [CrossRef]
- Lu, X.H.; Wang, G.M.; Zhai, T.; Yu, M.H.; Gan, J.Y.; Tong, Y.X.; Li, Y. Hydrogenated TiO2 nanotube arrays for supercapacitors. Nano Lett. 2012, 12, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.H. A highly efficient photocatalyst—Hydrogenated black TiO2 for the photocatalytic splitting of water. Angew. Chem. Int. Ed. 2012, 51, 12410–12412. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, C.Y.; Lin, T.Q.; Yin, H.; Chen, P.; Wan, D.Y.; Xu, F.F.; Huang, F.Q.; Lin, J.H.; Xie, X.M.; et al. H-doped black titania with very high solar absorption and excellent photocatalysis enhanced by localized surface plasmon resonance. Adv. Funct. Mater. 2013, 23, 5444–5450. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Lin, T.; Yin, H.; Lu, X.; Wan, D.; Xu, T.; Zheng, C.; Lin, J.; Huang, F.; et al. Core-shell nanostructured “black” rutile titania as excellent catalyst for hydrogen production enhanced by sulfur doping. J. Am. Chem. Soc. 2013, 135, 17831–17838. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhong, Y.; He, Z.; Zhang, L.; Wang, J.; Zhang, J.; Cao, C.-N. Three-dimensional nanoporous TiO2 network films with excellent electrochemical capacitance performance. J. Alloys Compd. 2014, 597, 1–7. [Google Scholar] [CrossRef]
- Shahid, M.; Choi, S.-Y.; Liu, J.; Kwon, Y.-U. Reduced titania films with ordered nanopores and their application to visible light water splitting. Bull. Korean Chem. Soc. 2013, 34, 2271–2275. [Google Scholar] [CrossRef]
- Lin, X.; Rong, F.; Ji, X.; Fu, D. Carbon-doped mesoporous TiO2 film and its photocatalytic activity. Microporous Mesoporous Mater. 2011, 142, 276–281. [Google Scholar] [CrossRef]
- Chang, P.-Y.; Huang, C.-H.; Doong, R.-A. Ordered mesoporous carbon–TiO2 materials for improved electrochemical performance of lithium ion battery. Carbon 2012, 50, 4259–4268. [Google Scholar] [CrossRef]
- Wang, T.; He, J.; Zhou, J.; Tang, J.; Guo, Y.; Ding, X.; Wu, S.; Zhao, J. Microwave absorption properties and infrared emissivities of ordered mesoporous C–TiO2 nanocomposites with crystalline framework. J. Solid State Chem. 2010, 183, 2797–2804. [Google Scholar] [CrossRef]
- Ren, W.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B 2007, 69, 138–144. [Google Scholar] [CrossRef]
- Lin, X.X.; Fu, D.G. Synthesis of nitrogen-doped mesoporous TiO2 film and its visible light photocatalytic activity. In Sustainable Development of Urban Environment and Building Material, Pts 1–4; Li, H., Liu, Y.F., Guo, M., Zhang, R., Du, J., Eds.; Trans Tech Publications Ltd.: Zurich, Switzerland, 2012; Volume 374–377, pp. 895–898. [Google Scholar]
- Huang, Y.; Ho, W.; Lee, S.; Zhang, L.; Li, G.; Yu, J.C. Effect of carbon doping on the mesoporous structure of nanocrystalline titanium dioxide and its solar-light-driven photocatalytic degradation of NOx. Langmuir 2008, 24, 3510–3516. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yu, J.C.; Chan, M. Sonochemical fabrication of fluorinated mesoporous titanium dioxide microspheres. J. Solid State Chem. 2009, 182, 1061–1069. [Google Scholar] [CrossRef]
- Pan, J.H.; Zhang, X.; Du, A.J.; Sun, D.D.; Leckie, J.O. Self-etching reconstruction of hierarchically mesoporous F-TiO2 hollow microspherical photocatalyst for concurrent membrane water Purifications. J. Am. Chem. Soc. 2008, 130, 11256–11257. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Ho, W.K.; Yu, J.G.; Hark, S.K.; Iu, K. Effects of trifluoroacetic acid modification on the surface microstructures and photocatalytic activity of mesoporous TiO2 thin films. Langmuir 2003, 19, 3889–3896. [Google Scholar] [CrossRef]
- Yu, J.; Wang, W.; Cheng, B.; Su, B.-L. Enhancement of photocatalytic activity of mesporous TiO2 powders by hydrothermal surface fluorination treatment. J. Phys. Chem. C 2009, 113, 6743–6750. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Ho, W.; Jiang, Z.; Zhang, L. Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Park, H.; Choi, W. Effects of TiO2 surface fluorination on photocatalytic reactions and photoelectrochemical behaviors. J. Phys. Chem. B 2004, 108, 4086–4093. [Google Scholar] [CrossRef]
- Shi, Q.; Jiang, C.; Wang, Y.; Yang, W.; Yang, C. Preparation and characterization of PVA-I complex doped mesoporous TiO2 by hydrothermal method. Appl. Surf. Sci. 2013, 273, 769–775. [Google Scholar] [CrossRef]
- Liu, G.; Chen, Z.G.; Dong, C.L.; Zhao, Y.N.; Li, F.; Lu, G.Q.; Cheng, H.M. Visible light photocatalyst: Iodine-doped mesoporous titania with a bicrystalline framework. J. Phys. Chem. B 2006, 110, 20823–20828. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.Y.; Han, S.; Mao, H.F.; Zeng, C.H.; Sun, Y.; Chi, B.; Pu, J.; Li, J. Synthesis of phosphorus-doped titania with mesoporous structure and excellent photocatalytic activity. Mater. Res. Bull. 2013, 48, 3032–3036. [Google Scholar] [CrossRef]
- Yu, J.C.; Zhang, L.; Zheng, Z.; Zhao, J. Synthesis and characterization of phosphated mesoporous titanium dioxide with high photocatalytic activity. Chem. Mater. 2003, 15, 2280–2286. [Google Scholar] [CrossRef]
- Fan, X.; Yu, T.; Wang, Y.; Zheng, J.; Gao, L.; Li, Z.; Ye, J.; Zou, Z. Role of phosphorus in synthesis of phosphated mesoporous TiO2 photocatalytic materials by EISA method. Appl. Surf. Sci. 2008, 254, 5191–5198. [Google Scholar] [CrossRef]
- Feng, H.J.; Zhang, M.H.; Yu, L.E. Phosphorus-Doped TiO2 Catalysts with Stable Anatase-Brookite Biphase Structure: Synthesis and Photocatalytic Performance. J. Nanosci. Nanotechnol. 2013, 13, 4981–4989. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Yu, J.C.; Zhou, W.Q.; Yang, K. WO3 coupled P-TiO2 photocatalysts with mesoporous structure. Catal. Lett. 2010, 140, 172–183. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.D.; He, T.N.; Guo, X.L.; Feng, Y.N. Preparation and photocatalytic activity of B–N co-doped mesoporous TiO2. Powder Technol. 2014, 253, 608–613. [Google Scholar] [CrossRef]
- He, T.N.; Guo, X.L.; Zhang, K.; Feng, Y.M.; Wang, X.D. Synthesis and characterization of B–N co-doped mesoporous TiO2 with enhanced photocatalytic activity. Rsc Adv. 2014, 4, 5880–5886. [Google Scholar] [CrossRef]
- Lee, J.H.; Youn, I.; Kim, Y.J.; Kim, I.K.; Jang, K.W.; Oh, H.J. Photocatalytic characteristics of boron and nitrogen doped titania film synthesized by micro-arc oxidation. Ceram. Int. 2015, 41, 11899–11907. [Google Scholar] [CrossRef]
- Zhou, X.; Peng, F.; Wang, H.; Yu, H.; Yang, J. Effect of nitrogen-doping temperature on the structure and photocatalytic activity of the B,N-doped TiO2. J. Solid State Chem. 2011, 184, 134–140. [Google Scholar] [CrossRef]
- Abdollahi Kakroudi, M.; Kazemi, F.; Kaboudin, B. Highly efficient photodeoximation under green and blue LEDs catalyzed by mesoporous CN codoped nano TiO2. J. Mol. Catal. A Chem. 2014, 392, 112–119. [Google Scholar] [CrossRef]
- Pan, L.; Zou, J.-J.; Wang, S.; Huang, Z.-F.; Zhang, X.; Wang, L. Enhancement of visible-light-induced photodegradation over hierarchical porous TiO2 by nonmetal doping and water-mediated dye sensitization. Appl. Surf. Sci. 2013, 268, 252–258. [Google Scholar] [CrossRef]
- Xu, J.; Yang, B.; Wu, M.; Fu, Z.; Lv, Y.; Zhao, Y. Novel N–F-codoped TiO2 inverse opal with a hierarchical meso-/macroporous structure: Synthesis, characterization, and photocatalysis. J. Phys. Chem. C 2010, 114, 15251–15259. [Google Scholar] [CrossRef]
- Li, Y.; Jia, L.; Wu, C.; Han, S.; Gong, Y.; Chi, B.; Pu, J.; Jian, L. Mesoporous (N,S)-codoped TiO2 nanoparticles as effective photoanode for dye-sensitized solar cells. J. Alloys Compd. 2012, 512, 23–26. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Li, H.; Yin, S.; Sato, T. Photocatalytic activity of (sulfur, nitrogen)-codoped mesoporous TiO2 thin films. Res. Chem. Intermed. 2010, 36, 27–37. [Google Scholar] [CrossRef]
- Yu, C.; Cai, D.; Yang, K.; Yu, J.C.; Zhou, Y.; Fan, C. Sol–gel derived S,I-codoped mesoporous TiO2 photocatalyst with high visible-light photocatalytic activity. J. Phys. Chem. Solids 2010, 71, 1337–1343. [Google Scholar] [CrossRef]
- Islam, S.Z.; Deshmane, V.G.; Ilias, S. Thermal stability study of Pd-composite membrane fabricated by surfactant induced electroless plating (SIEP). Sep. Sci. Technol. 2016, 51, 1176–1188. [Google Scholar] [CrossRef]
- Hartmann, P.; Lee, D.-K.; Smarsly, B.M.; Janek, J. Mesoporous TiO2: Comparison of classical sol–gel and nanoparticle based photoelectrodes for the water splitting reaction. ACS Nano 2010, 4, 3147–3154. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Shi, F.; Bu, J.; Ding, J.; Xu, S.; Bao, J.; Ma, Y.; Jiang, Z.; Zhang, W.; Gao, C.; et al. One-step synthesis of bifunctional TiO2 catalysts and their photocatalytic activity. J. Phys. Chem. C 2010, 114, 7940–7948. [Google Scholar] [CrossRef]
- Atabaev, T.S.; Hossain, M.A.; Lee, D.; Kim, H.-K.; Hwang, Y.-H. Pt-coated TiO2 nanorods for photoelectrochemical water splitting applications. Results Phys. 2016, 6, 373–376. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Corma, A.; Garcia, H. Photocatalytic CO2 reduction by TiO2 and related titanium containing solids. Energy Environ. Sci. 2012, 5, 9217–9233. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon-dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Gao, M.Y.; Jiang, D.; Sun, D.K.; Hou, B.; Li, D.B. Synthesis of Ag/N-TiO2/SBA-15 photocatalysts and photocatalytic reduction of CO2 under visible light irradiation. Acta Chim. Sin. 2014, 72, 1092–1098. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, S.-J.; Choi, W. Visible light active platinum-ion-doped TiO2 photocatalyst. J. Phys. Chem. B 2005, 109, 24260–24267. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Chen, F.; Zhang, J.; Anpo, M. A novel deposition precipitation method for preparation of Ag-loaded titanium dioxide. Catal. Lett. 2005, 102, 247–250. [Google Scholar] [CrossRef]
- Tian, B.; Zhang, J.; Tong, T.; Chen, F. Preparation of Au/TiO2 catalysts from Au(I)–thiosulfate complex and study of their photocatalytic activity for the degradation of methyl orange. Appl. Catal. B 2008, 79, 394–401. [Google Scholar] [CrossRef]
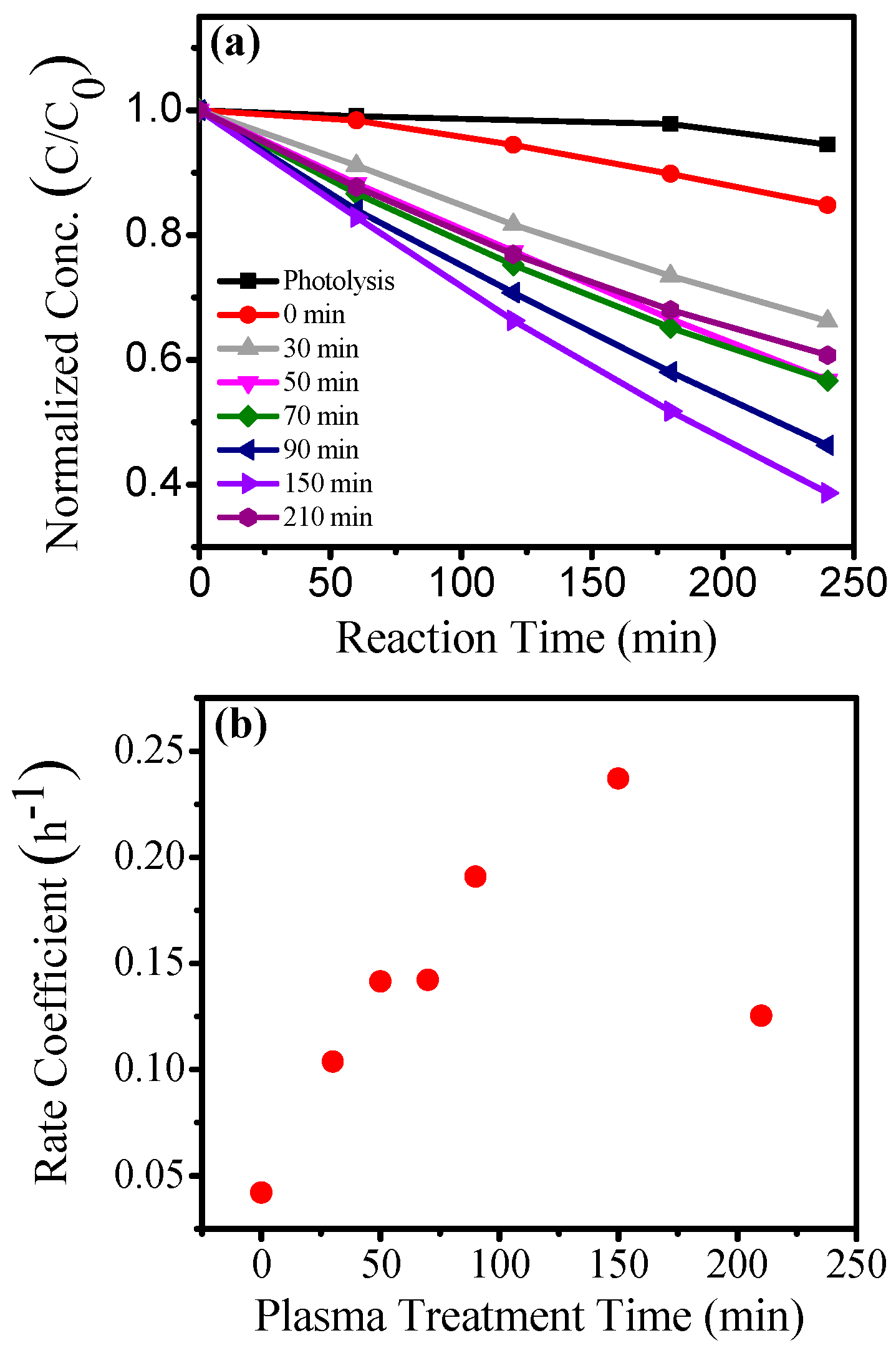
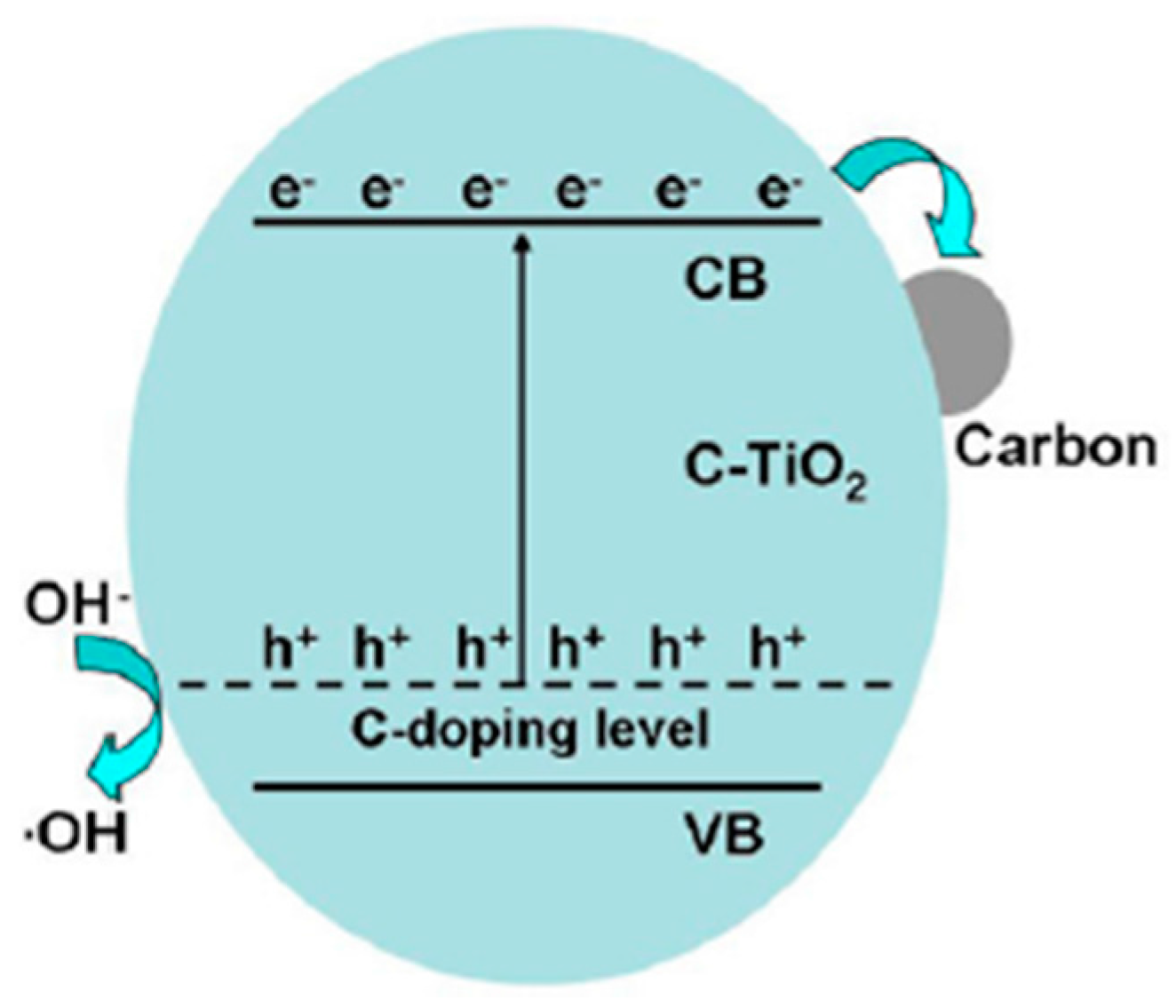
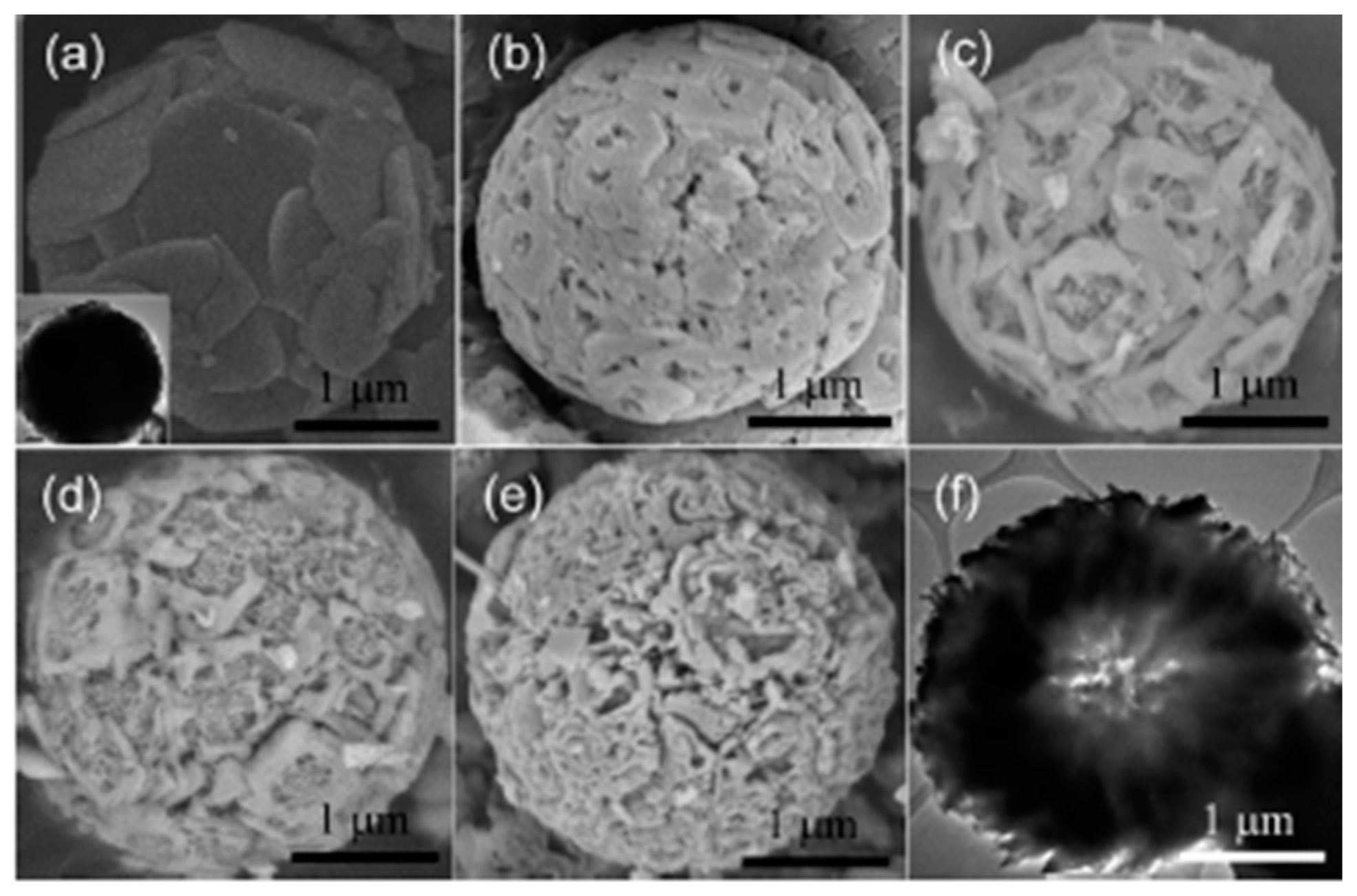
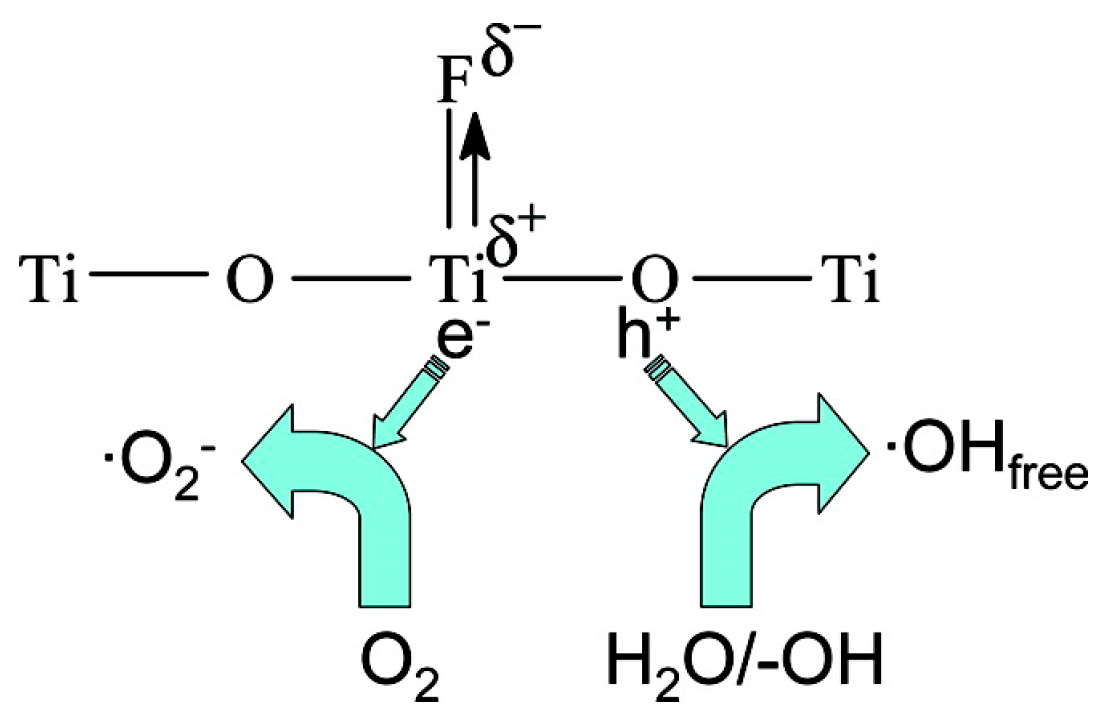
| Method | Template | N Source | N Comp (atom %) | Binding Energy (eV) | Initial BG (eV) | Final BG (eV) | SBET (m2/g) | Test Compound | Enhance-Ment | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Solvothermal | Free | Urea | 1.43 | 400 | 3.14 | 3.09 | 121 | Rh B | 1.43 | [185] |
| Solvothermal | Free | Urea | - | 399.5 | 3.16 | 3.02 | 23.6 | Acetic Acid | 1.54 | [180] |
| EISA | F127 | Urea | 3.46 | 398.8 | - | - | 67 | Water Splitting | 2.37 | [181] |
| Solvothermal | Urea | Urea | 0.8 | 399.5 | - | - | 154 | MB | 3.1 | [183] |
| Supercritical CO2 drying | - | Urea | - | 396 | 3.1 | 1.92 | 116 | - | - | [184] |
| Homogeneous precipitation | - | Urea | 0.91 | 395.4, 401.5 | - | - | 89 | MB | 3 | [186] |
| Sol–gel | PAM + PEG | Urea | 0.6 | 397, 398.8, 402.4 | 2.9 | 2.75 | 110 | MO | 3 | [188] |
| Sol–gel | Laurylamine hydrochloride | Urea | 26.2 wt % | 400.8 | 3.2 | 3.1 | 110.3 | Water Splitting | 1.44 | [191] |
| Sol–gel | F127 | N2 plasma | 3.2 | 396 | 3.5 | 3.02 | 143 | MB | 6 | [65] |
| Exfoliation-reassembly | - | Ethylamine | - | 399.8 | - | - | 217 | MO | 9 | [156] |
| Solvothermal | F127 | Ethylenediamine | 4.1 wt % | 398.6 | 3.1 | 2.2 | 180.2 | MB | - | [151] |
| Solvothermal | F127 | Ethylenediamine | 0.13 (N/Ti) | 399.5, 401.4 | 3.32 | 2.98 | 185.4 | Dimethyl Phthalate | - | [158] |
| Sol–gel | Triethylamine | Triethylamine | 4 | 401.6 | - | - | 180 | Phenol | 7.5 | [154] |
| Sol–gel | - | Triethanolamine | 1.09 | 397, 399.9 | - | - | 70.5 | MB | [155] | |
| Hydrothermal | P123 | Triethylamine | - | 399 | - | - | 150 | Rh B | 3.3 | [152] |
| Sol–gel reverse micelle | TritonX100 | Na2EDTA | 6 wt % | - | 3.13 | 3.06 | 58 | MB | 4 | [157] |
| Sol–gel | EDTA | EDTA | - | - | 3.1 | 2.29 | 72.46 | Rh B | 1.5 | [153] |
| Hydrothermal | CTAB | Ammonia solution | 1.31 | 399.5, 400.6, 401.5 | - | - | 83.1 | Rh B | 5 | [161] |
| Sol–gel | P123 | Tetramethylammonium hydroxide | - | 400 | - | - | 105 | Rh B | 4 | [160] |
| Sol–gel | Chitosan | Chitosan and NH4OH | 0.72 wt % | 394.2, 400.9 | - | 2.65 | 132.26 | MO | 2 | [163] |
| EISA | F127 | NH3 gas | - | 395.9 | - | - | 173 m2/cm3 | MB | 1.47 | [60] |
| Wet Impregnation | Hydrazine Hydrate | Hydrazine Hydrate | - | 398.1 | - | - | 74 | Azoxybenzene | - | [172] |
| Method | Template | Dopant Source | Dopant Composition (atom %) | XPS Binding Energy (eV) | Initial BG (eV) | Final BG (eV) | SBET (m2/g) | Test Compounds | Enhancement | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Sol–gel plus hydrothermal | Glucose | Glucose | C | 282.4, 284.8, 286.2, 288.6 | - | - | 190 | Reactive Brilliant X-3B | 3.6 | [199] |
| Solvothermal | Free | Rice | C | 284.9, 285.8, 288.2, 288.6 | 3.1 | 2.04 | 138 | MO | 25.8 | [32] |
| Hydrothermal | Free | TiF4 | 0.16 (atomic ratio, F/Ti) | 684.3 | - | - | 21.6 | MB | 1.06 | [206] |
| Hydrothermal | - | NH4HF2-H2O-C2H5OH | 0.5 (atomic ratio, F/Ti) | 684 | - | - | 196.6 | Acetone | 3 | [208] |
| Hydrothermal | P123 | Iodic acid | 5.2 (I) | 624.5 | - | - | 157 | MB | 5.6 | [212] |
| Hydrothermal | Polyvinyl alcohol | KI and I2 | 0.62 (I) | - | - | 1.39 | 190.22 (doped) | MB | 9 | [211] |
| Sol–gel | - | Boric acid | 17.8 (B) | 192 | 3.05 | 3.04 | 68.11 | Metoprolol | 1.45 | [57] |
| Sol–gel | P123 | Phosphoric acid | 14 (atomic ratio, P/Ti) | - | 3.1 | 3.17 | - | Pentane | 1.42 | [214] |
| Hydrothermal and sol–gel | - | Phosphoric acid | - | 133.4, 133.5 | - | - | 106.86 | MB | 2 | [213] |
| Method | Template | Dopant Source | Dopant Composition (atom %) | XPS Binding Energy (eV) | Initial BG (eV) | Final BG (eV) | SBET (m2/g) | Test Compounds | Enhancement | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Sol–gel | P123 | Thiourea | 1.41 (N), 2.33 (S), C | 399.7, 401.8 | 3.1 | 2.9 | 85.1 | Cyanotoxin microcystin-LR | 11.5 | [46] |
| Sol–gel | CTAB | Thiourea | - | 396, 399.2, 284.6, 288.2, 165, 169 | - | - | 123.8 | Reactive Brilliant Red X-3B | 5 | [178] |
| EISA | F127 | Thiourea | 0.044 (N/O), 0.048 (S/O) | 400.2, 396, 168.5 | - | 2.38 | 105 | MO | 18 | [179] |
| Solvothermal | Thiourea | Thiourea | 0.62 (N), 0.35 (S) | 168.6, 169.7, 395.9, 399.7 | - | - | 29.11 | MB | 1.23 | [176] |
| Hydrothermal | - | Thiourea | 1.1 (N), 1.1 (S) | 395.2, 396.9, 400.1, 168.9, 170.3 | - | - | 22.8 (doped) | Potassium ethyl xanthate | - | [177] |
| Solvothermal | - | Urea, Ammonium Fluoride | 0.57 (N), 1.9 (F) | 683.7–684.6, 688–688.6, 399.1, 400.1 | 3.02 | 2.74 | 36 | Acid Orange 7 | 5.5 | [187] |
| Sol–gel | PAM and PEG | Urea (N), Boric acid (B) | 1.78 (N), 1.23 (B) | 400.5, 192.1 | 3.18 | 2.78 | 121.6 | MB | 3 | [219] |
| EISA | [C2mim][Cl] doped/F127 undoped | [C2mim][Cl] | 1.75 (N), C | 398.2, 400.2 | 3.1 | 2.98 | 54 | Water splitting | 10.5 | [33] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, S.Z.; Nagpure, S.; Kim, D.Y.; Rankin, S.E. Synthesis and Catalytic Applications of Non-Metal Doped Mesoporous Titania. Inorganics 2017, 5, 15. https://doi.org/10.3390/inorganics5010015
Islam SZ, Nagpure S, Kim DY, Rankin SE. Synthesis and Catalytic Applications of Non-Metal Doped Mesoporous Titania. Inorganics. 2017; 5(1):15. https://doi.org/10.3390/inorganics5010015
Chicago/Turabian StyleIslam, Syed Z., Suraj Nagpure, Doo Young Kim, and Stephen E. Rankin. 2017. "Synthesis and Catalytic Applications of Non-Metal Doped Mesoporous Titania" Inorganics 5, no. 1: 15. https://doi.org/10.3390/inorganics5010015
APA StyleIslam, S. Z., Nagpure, S., Kim, D. Y., & Rankin, S. E. (2017). Synthesis and Catalytic Applications of Non-Metal Doped Mesoporous Titania. Inorganics, 5(1), 15. https://doi.org/10.3390/inorganics5010015






