The Trans Influence in Unsymmetrical Pincer Palladacycles: An Experimental and Computational Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. SCN Pincer Palladacycle Synthesis
2.2. Investigaing the Trans Influence
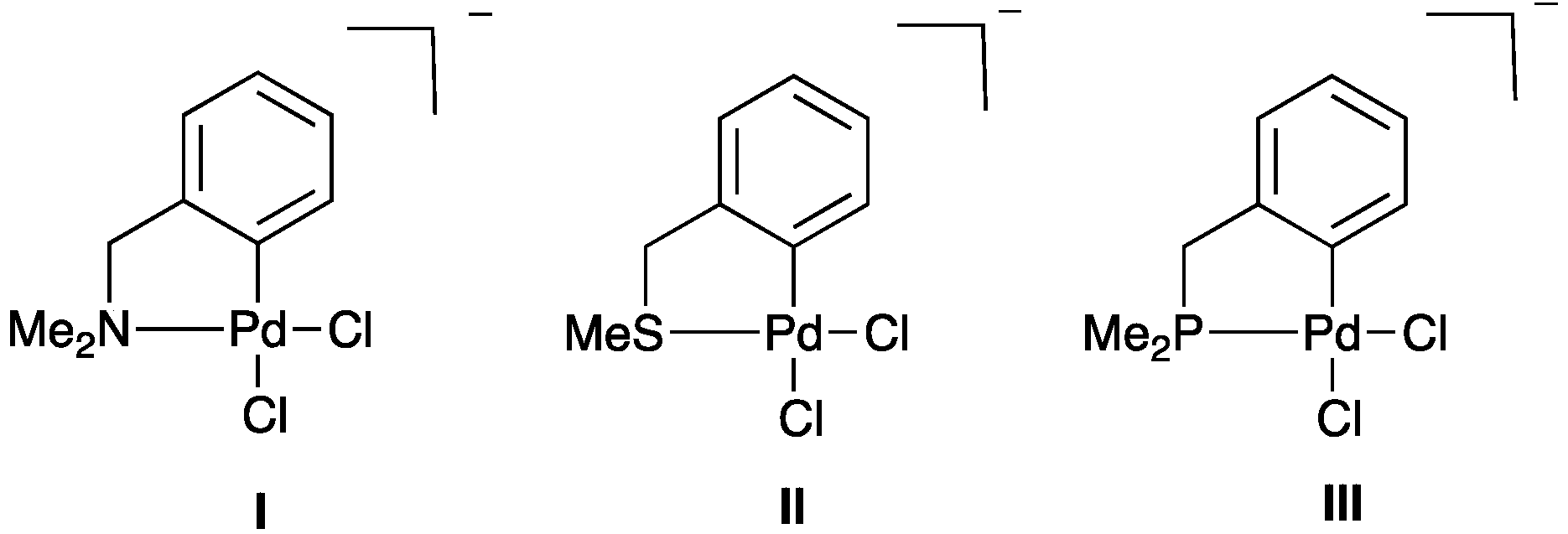
2.2.1. Trans Influence in Model Palladacycles I–III
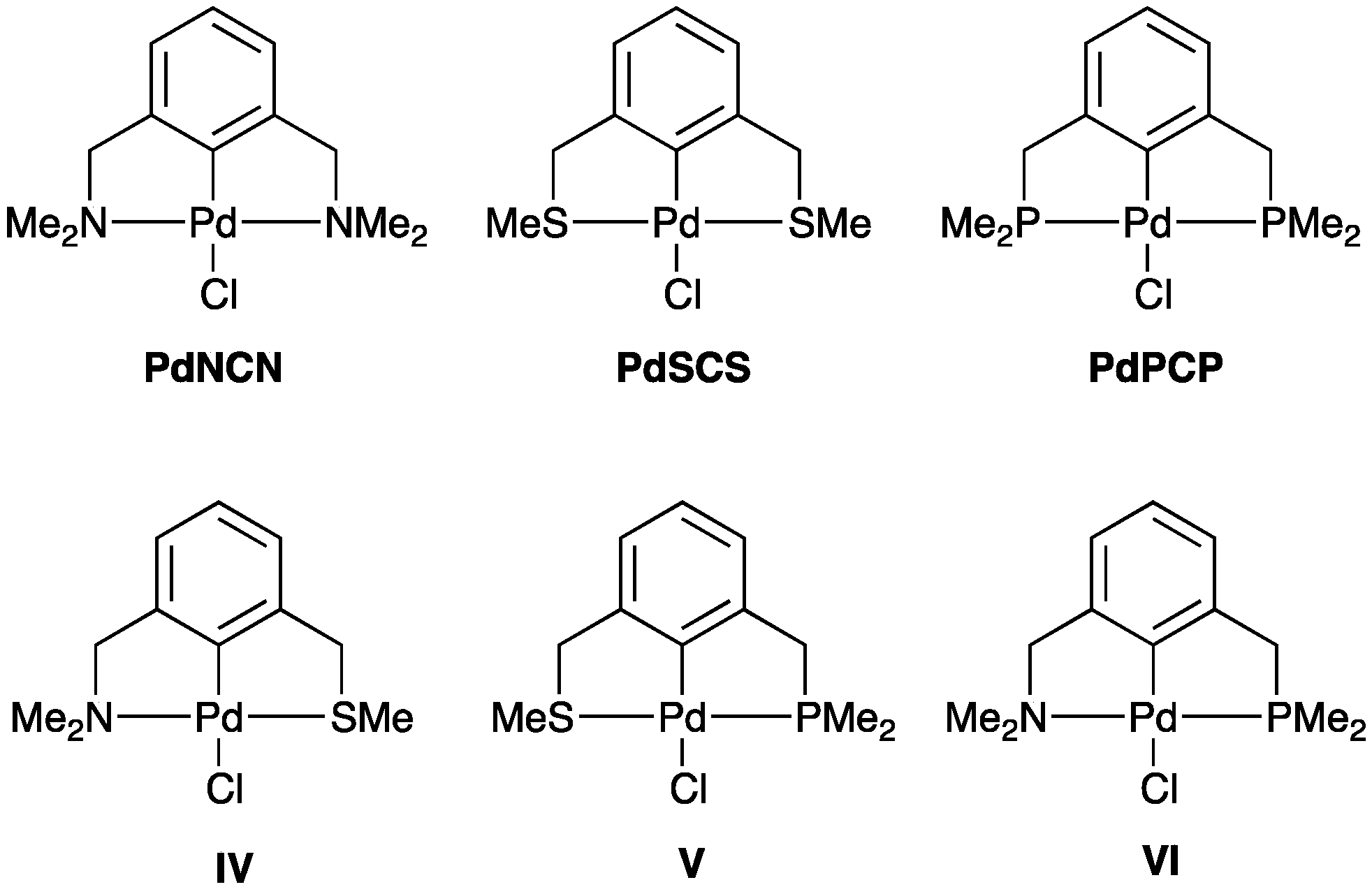
2.2.2. Trans Influence in Model Unsymmetrical YCY’ Pincer Palladacycles
2.2.3. Trans Influence in Experimentally-Characterized Unsymmetrical YCY’ Pincer Palladacycles
2.2.4. Trans Influence on Unsymmetrical Pincer Palladacycles: Donor Atom Substituent Effects
3. Experimental Section
3.1. General Details
3.2. Instrumentation
3.3. Procedure
4. Computational Section
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Pyr | Pyridine |
| DFT | Density Functional Theory |
| QTAIM | Quantum Theory of Atoms in Molecules |
| Tol | Toluene |
| HRMS | High Resolution Mass Spectrometry |
| ESI | Electrospray Ionization |
| GC | Gas Chromatography |
| DMF | Dimethylformamide |
| DCM | Dichloromethane |
| PBE | Perdew Burke Ernzerhof Exchange–Correlation Functional |
| MSE | Mean Signed Error |
| MUE | Mean Unsigned Error |
| BCP | Bond Critical Point |
| GGA | Generalized Gradient Approximation |
| SDD | Stuttgart-Dresden |
| ECP | Effective Core Potentials |
References
- Cope, A.C.; Siekman, R.W. Formation of covalent bonds from platinum or palladium to carbon by direct substitution. J. Am. Chem. Soc. 1965, 87, 3272–3273. [Google Scholar] [CrossRef]
- Dupont, J.; Consorti, C.S.; Spencer, J. The potential of palladacycles: More than just precatalysts. Chem. Rev. 2005, 105, 2527–2571. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.-L.; Hao, X.-Q.; Gong, J.-F.; Song, M.-P. Symmetrical and unsymmetrical pincer complexes with group 10 metals: Synthesis via aryl C–H activation and some catalytic applications. Dalton Trans. 2011, 40, 5135–5150. [Google Scholar] [CrossRef] [PubMed]
- Selander, N.; Szabó, K.J. Catalysis by palladium pincer complexes. Chem. Rev. 2011, 111, 2048–2076. [Google Scholar] [CrossRef] [PubMed]
- Morales-Morales, D. Pincer complexes. Applications in catalysis. Rev. Soc. Quim. Mex. 2004, 48, 338–346. [Google Scholar] [CrossRef]
- Liu, B.-B.; Wang, X.-R.; Guo, Z.-F.; Lu, Z.-L. Mononuclear versus dinuclear palladacycles derived from 1,3-bis(N,N-dimethylaminomethyl)benzene: Structures and catalytic activity. Inorg. Chem. Commun. 2010, 13, 814–817. [Google Scholar] [CrossRef]
- Kruithof, C.A.; Dijkstra, H.P.; Lutz, M.; Spek, A.L.; Gebbink, R.J.M.K.; van Koten, G. X-Ray and NMR study of the structural features of SCS-pincer metal complexes of the group 10 triad. Organometallics 2008, 27, 4928–4937. [Google Scholar] [CrossRef]
- Kjellgren, J.; Aydin, J.; Wallner, O.A.; Saltanova, I.V.; Szabó, K.J. Palladium pincer complex catalyzed cross-coupling of vinyl epoxides and aziridines with organoboronic acids. Chem. Eur. J. 2005, 11, 5260–5268. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Sheets, M. A SeCSe−Pd(II) pincer complex as a highly efficient catalyst for allylation of aldehydes with allyltributyltin. J. Org. Chem. 2006, 71, 5384–5387. [Google Scholar] [CrossRef] [PubMed]
- Aydin, J.; Selander, N.; Szabó, K.J. Strategies for fine-tuning the catalytic activity of pincer-complexes. Tetrahedron Lett. 2006, 47, 8999–9001. [Google Scholar] [CrossRef]
- Gagliardo, M.; Selander, N.; Mehendale, N.C.; Van Koten, G.; Klein Gebbink, R.J.M.; Szabó, K.J. Catalytic performance of symmetrical and unsymmetrical sulfur-containing pincer complexes: Synthesis and tandem catalytic activity of the first PCS-pincer palladium complex. Chem. Eur. J. 2008, 14, 4800–4809. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; SanMartin, R.; Ines, B.; Herrero, M.T.; Domínguez, E. Recent advances in the use of unsymmetrical palladium pincer complexes. Curr. Org. Chem. 2009, 13, 878–895. [Google Scholar]
- Roffe, G.W.; Boonseng, S.; Baltus, C.B.; Coles, S.J.; Day, I.J.; Jones, R.N.; Press, N.J.; Ruiz, M.; Tizzard, G.J.; Cox, H.; et al. A synthetic, catalytic and theoretical investigation of an unsymmetrical SCN pincer palladacycle. R. Soc. Open Sci. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Roffe, G.W.; Tizzard, G.J.; Coles, S.J.; Cox, H.; Spencer, J. Synthesis of unsymmetrical N’CN and PCN pincer palladacycles and their catalytic evaluation compared with a related SCN pincer palladacycle. Org. Chem. Front. 2016, 3, 957–965. [Google Scholar] [CrossRef]
- Braunstein, P.; Naud, F. Hemilability of hybrid ligands and the coordination chemistry of oxazoline-based systems. Angew. Chem. Int. Ed. 2001, 40, 680–699. [Google Scholar] [CrossRef]
- Khusnutdinova, J.R.; Milstein, D. Metal-ligand cooperation. Angew. Chem. Int. Ed. 2015, 54, 12236–12273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Chien, S.W.; Hor, T.S.A. Recent advances in metal catalysts with hybrid ligands. Coord. Chem. Rev. 2011, 255, 1991–2024. [Google Scholar] [CrossRef]
- Ramírez-Rave, S.; Estudiante-Negrete, F.; Toscano, R.A.; Hernández-Ortega, S.; Morales-Morales, D.; Grévy, J.M. Synthesis and characterization of new Pd(II) non-symmetrical Pincer complexes derived from thioether functionalized iminophosphoranes. Evaluation of their catalytic activity in the Suzuki–Miyaura couplings. J. Organomet. Chem. 2014, 749, 287–295. [Google Scholar] [CrossRef]
- Saha, D.; Verma, R.; Kumar, D.; Pathak, S.; Bhunya, S.; Sarkar, A. A “hemilabile” palladium–carbon bond: Characterization and its implication in catalysis. Organometallics 2014, 33, 3243–3246. [Google Scholar] [CrossRef]
- Poverenov, E.; Gandelman, M.; Shimon, L.J.W.; Rozenberg, H.; Ben-David, Y.; Milstein, D. Pincer “hemilabile” effect. PCN platinum(II) complexes with different amine “arm length”. Organometallics 2005, 24, 1082–1090. [Google Scholar]
- Gargir, M.; Ben-David, Y.; Leitus, G.; Diskin-Posner, Y.; Shimon, L.J.W.; Milstein, D. PNS-Type ruthenium pincer complexes. Organometallics 2012, 31, 6207–6214. [Google Scholar] [CrossRef]
- Fleckhaus, A.; Mousa, A.H.; Lawal, N.S.; Kazemifar, N.K.; Wendt, O.F. Aromatic PCN palladium pincer complexes. Probing the hemilability through reactions with nucleophiles. Organometallics 2015, 34, 1627–1634. [Google Scholar] [CrossRef]
- Pidcock, A.; Richards, R.E.; Venanzi, L.M. 195Pt–31P nuclear spin coupling constants and the nature of the trans-effect in platinum complexes. J. Chem. Soc. A Inorg. Phys. Theor. 1966, 1707–1710. [Google Scholar] [CrossRef]
- Appleton, T.G.; Clark, H.C.; Manzer, L.E. The trans-influence: Its measurement and significance. Coord. Chem. Rev. 1973, 10, 335–422. [Google Scholar] [CrossRef]
- Quagliano, J.V.; Schubert, L. The trans effect in complex inorganic compounds. Chem. Rev. 1952, 50, 201–260. [Google Scholar] [CrossRef]
- Rigamonti, L.; Rusconi, M.; Manassero, C.; Manassero, M.; Pasini, A. Quantification of cis and trans influences in [PtX(PPh3)3]+ complexes. A 31P NMR study. Inorg. Chim. Acta 2010, 363, 3498–3505. [Google Scholar] [CrossRef]
- Randaccio, L.; Bresciani-Pahor, N.; Toscano, P.J.; Marzilli, L.G. Bonding mode and trans influence of the nitromethyl ligand. Structure of trans-bis(dimethylglyoximato)(nitromethyl)(pyridine)cobalt(III). Inorg. Chem. 1981, 20, 2722–2724. [Google Scholar] [CrossRef]
- Otto, S.; Johansson, M.H. Quantifying the trans influence of triphenylarsine. Crystal and molecular structures of cis-[PtCl2(SMe2)(AsPh3)] and cis-[PtCl2(AsPh3)2]·CHCl3. Inorg. Chim. Acta 2002, 329, 135–140. [Google Scholar] [CrossRef]
- Kaltsoyannis, N.; Mountford, P. Theoretical study of the geometric and electronic structures of pseudo-octahedral d0 imido compounds of titanium: The trans influence in mer-[Ti(NR)Cl2(NH3)3] (R = But, C6H5 or C6H4NO2-4). Dalt. Trans. 1999, 781–790. [Google Scholar] [CrossRef]
- Lyne, P.D.; P. Mingos, D.M. The effects of back-bonding to phosphines on the trans influence in [Mo(NH)Cl3(PR3)2]0,±1 (R = H, Me and F). J. Organomet. Chem. 1994, 478, 141–151. [Google Scholar] [CrossRef]
- Jacobsen, H.; Berke, H. Tuning of the transition metal hydrogen bond: How do trans ligands influence bond strength and hydridicity? Dalt. Trans. 2002, 3117–3122. [Google Scholar] [CrossRef]
- Deeth, R.J. The trans influence in [RH(Ph3)3Cl]: A density functional theory study. Dalt. Trans. 1993, 3711–3713. [Google Scholar] [CrossRef]
- Burdett, J.K.; Albright, T.A. Trans influence and mutual influence of ligands coordinated to a central atom. Inorg. Chem. 1979, 18, 2112–2120. [Google Scholar] [CrossRef]
- Loeb, S.J.; Shimizu, G.K.H.; Wisner, J.A. Mono- versus dipalladation of the durene-based tetrathioether Ligand 1,2,4,5-(tBuSCH2)4C6H2. Structures of [PdCl((tBuSCH2)4C6H)] and [Pd2((tBuSCH2)4C6)(MeCN)2][BF4]2. Organometallics 1998, 17, 2324–2327. [Google Scholar] [CrossRef]
- Bader, R.F.W. A bond path: A universal indicator of bonded interactions. J. Phys. Chem. A 1998, 5639, 7314–7323. [Google Scholar] [CrossRef]
- Boonseng, S.; Roffe, G.W.; Spencer, J.; Cox, H. The nature of the bonding in symmetrical pincer palladacycles. Dalt. Trans. 2015, 44, 7570–7577. [Google Scholar] [CrossRef] [PubMed]
- Hartley, F.R. The cis- and trans-effects of ligands. Chem. Soc. Rev. 1973, 2. [Google Scholar] [CrossRef]
- Sajith, P.K.; Suresh, C.H. Bond dissociation energies of ligands in square planar Pd(II) and Pt(II) complexes: An assessment using trans influence. J. Organomet. Chem. 2011, 696, 2086–2092. [Google Scholar] [CrossRef]
- Manojlovic-Muir, L.J.; Muir, K.W. The trans-influence of ligands in platinum(II) complexes. The significance of the bond length data. Inorg. Chim. Acta 1974, 10, 47–49. [Google Scholar] [CrossRef]
- Kapoor, P.N.; Kakkar, R. Trans and cis influence in square planar Pt(II) complexes: A density functional study of [PtClX(dms)2] and related complexes. J. Mol. Struct. Theochem. 2004, 679, 149–156. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Sajith, P.K.; Suresh, C.H. Quantification of mutual trans influence of ligands in Pd(II) complexes: A combined approach using isodesmic reactions and AIM analysis. Dalton Trans. 2010, 39, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Coles, S.J.; Gale, P.A. Changing and challenging times for service crystallography. Chem. Sci. 2012, 3, 683–689. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01. Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple . Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple [Phys. Rev. Lett. 77, 3865 (1996)]. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-D.; Head-Gordon, M. Long-Range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Sajith, P.K.; Suresh, C.H. Mechanisms of reductive eliminations in square planar Pd(II) complexes: Nature of eliminated bonds and role of trans influence. Inorg. Chem. 2011, 50, 8085–8093. [Google Scholar] [CrossRef] [PubMed]
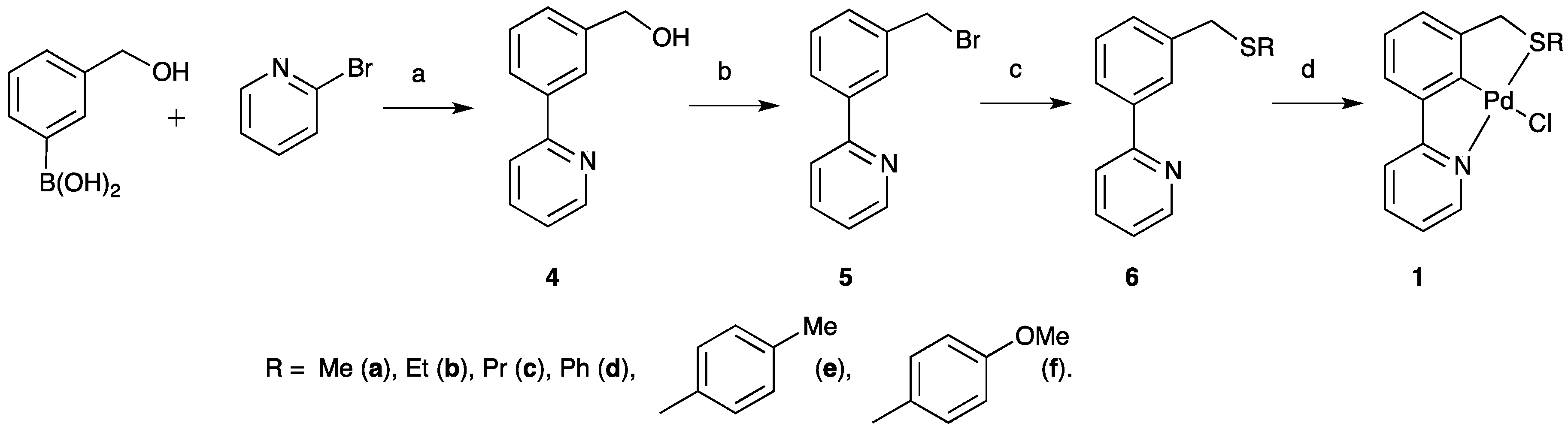
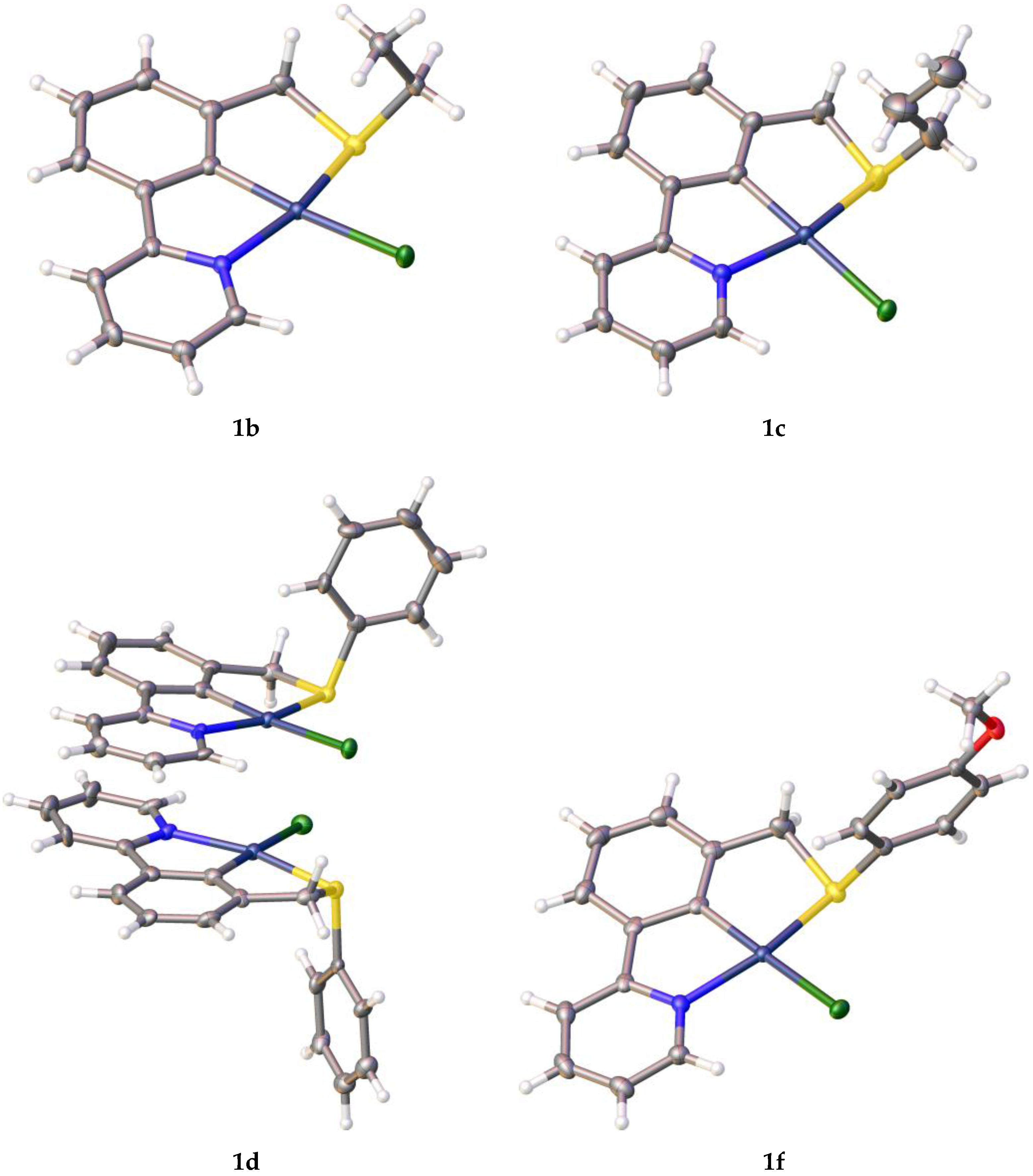
| Entry | Palladacycle | Ligand Synthesis Conditions | Ligand Synthesis Isolated Yield/% | Palladacycle Synthesis Yield/% |
|---|---|---|---|---|
| 1 | 1b | A | 72 | 83 |
| 2 | 1c | B | >99 | 85 |
| 3 | 1d | B | 99 | 71 |
| 4 | 1e | C | 51 | 89 |
| 5 | 1f | B | 60 | 54 |
| Compound | ρ(r) of Pd–Cl (a.u.) | Pd–Cl Bond Length (Å) |
|---|---|---|
| I | 0.080 | 2.334 |
| II | 0.077 | 2.352 |
| III | 0.070 | 2.395 |
| PdYCY’ | Y | Y’ | ρ(r) of Pd–Y | ρ(r) of Pd–Y’ |
|---|---|---|---|---|
| PdNCN | Me2NCH2 | Me2NCH2 | 0.086 | 0.086 |
| PdSCS | MeSCH2 | MeSCH2 | 0.091 | 0.091 |
| PdPCP | Me2PCH2 | Me2PCH2 | 0.101 | 0.101 |
| IV | MeSCH2 | Me2NCH2 | 0.097 | 0.083 |
| V | Me2PCH2 | MeSCH2 | 0.110 | 0.082 |
| VI | Me2PCH2 | Me2NCH2 | 0.114 | 0.075 |
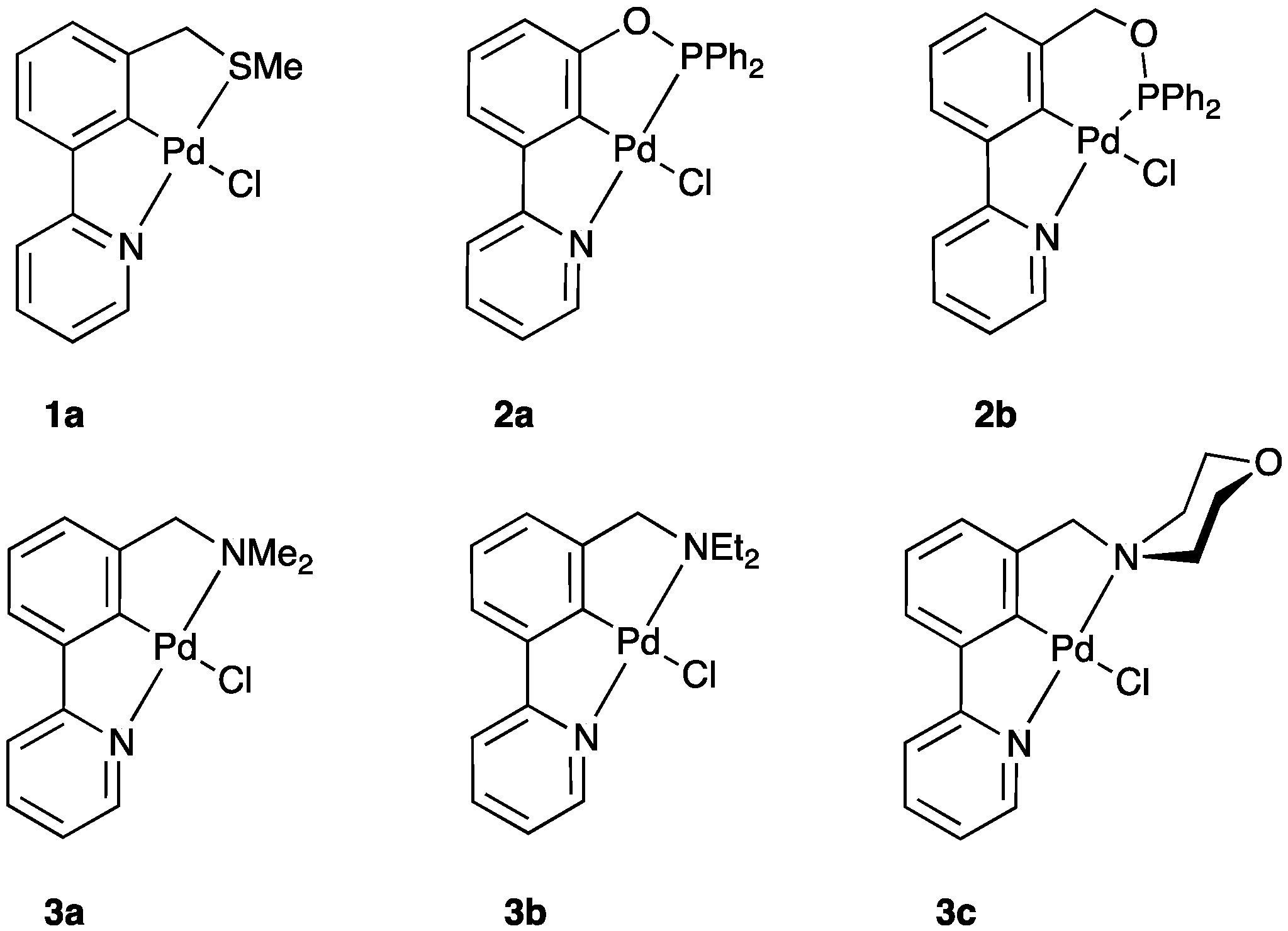
| 1a | MeSCH2 | 2-NC5H4 | 0.096 | 0.098 |
| 1b | EtSCH2 | 2-NC5H4 | 0.095 | 0.098 |
| 1c | PrSCH2 | 2-NC5H4 | 0.095 | 0.098 |
| 1d | PhSCH2 | 2-NC5H4 | 0.092 | 0.098 |
| 1e | (p-MeC6H4)SCH2 | 2-NC5H4 | 0.092 | 0.098 |
| 1f | (p-MeOC6H4)SCH2 | 2-NC5H4 | 0.092 | 0.098 |
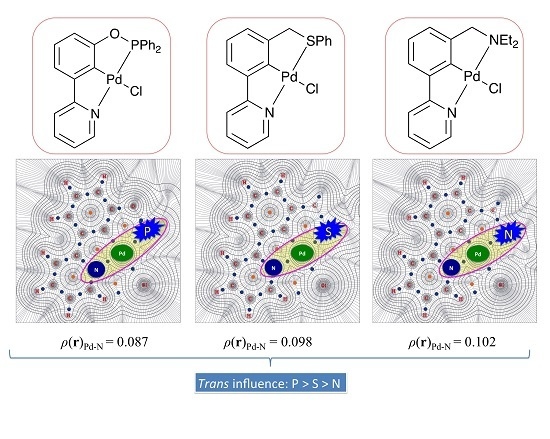
| 2a | Ph2PO | 2-NC5H4 | 0.114 | 0.087 |
| 2b | Ph2POCH2 | 2-NC5H4 | 0.113 | 0.089 |
| 3a | Me2NCH2 | 2-NC5H4 | 0.086 | 0.102 |
| 3b | Et2NCH2 | 2-NC5H4 | 0.085 | 0.102 |
| 3c | (C4H8O)NCH2 | 2-NC5H4 | 0.084 | 0.102 |
| PdYCY’ | Y | Y’ | Calculation | X-ray | Ref. X-ray | ||
|---|---|---|---|---|---|---|---|
| Pd–Y | Pd–Y’ | Pd–Y | Pd–Y’ | ||||
| PdNCN | Me2NCH2 | Me2NCH2 | 2.140 | 2.140 | 2.103(3) | 2.102(3) | [6] |
| PdSCS | MeSCH2 | MeSCH2 | 2.313 | 2.313 | 2.2831(11) | 2.2911(11) | [7] |
| PdPCP | Me2PCH2 | Me2PCH2 | 2.287 | 2.287 | n/a | n/a | n/a |
| IV | MeSCH2 | Me2NCH2 | 2.285 | 2.156 | n/a | n/a | n/a |
| V | Me2PCH2 | MeSCH2 | 2.240 | 2.364 | n/a | n/a | n/a |
| VI | Me2PCH2 | Me2NCH2 | 2.222 | 2.203 | n/a | n/a | n/a |
| 1a | MeSCH2 | 2-NC5H4 | 2.288 | 2.074 | 2.291(8) | 2.09(3) | [13] |
| 1b | EtSCH2 | 2-NC5H4 | 2.290 | 2.076 | 2.2638(4) | 2.0672(13) | * |
| 1c | PrSCH2 | 2-NC5H4 | 2.291 | 2.076 | 2.2705(7) | 2.066(2) | * |
| 1d | PhSCH2 | 2-NC5H4 | 2.303 | 2.078 | 2.2846(17) | 2.069(5) | * |
| 1e | (p-MeC6H4)SCH2 | 2-NC5H4 | 2.302 | 2.078 | n/a | n/a | n/a |
| 1f | (p-MeOC6H4)SCH2 | 2-NC5H4 | 2.303 | 2.078 | 2.2674(5) | 2.0708(15) | * |
| 2a | Ph2PO | 2-NC5H4 | 2.219 | 2.129 | 2.2028(6) | 2.1216(18) | [14] |
| 2b | Ph2POCH2 | 2-NC5H4 | 2.232 | 2.114 | 2.2159(7) | 2.103(2) | [14] |
| 3a | Me2NCH2 | 2-NC5H4 | 2.145 | 2.060 | 2.105(6) | 2.062(5) | [14] |
| 3b | Et2NCH2 | 2-NC5H4 | 2.149 | 2.063 | 2.1145(16) | 2.0639(16) | [14] |
| 3c | (C4H8O)NCH2 | 2-NC5H4 | 2.154 | 2.060 | 2.1239(19) | 2.0521(19) | [14] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonseng, S.; Roffe, G.W.; Jones, R.N.; Tizzard, G.J.; Coles, S.J.; Spencer, J.; Cox, H. The Trans Influence in Unsymmetrical Pincer Palladacycles: An Experimental and Computational Study. Inorganics 2016, 4, 25. https://doi.org/10.3390/inorganics4030025
Boonseng S, Roffe GW, Jones RN, Tizzard GJ, Coles SJ, Spencer J, Cox H. The Trans Influence in Unsymmetrical Pincer Palladacycles: An Experimental and Computational Study. Inorganics. 2016; 4(3):25. https://doi.org/10.3390/inorganics4030025
Chicago/Turabian StyleBoonseng, Sarote, Gavin W. Roffe, Rhiannon N. Jones, Graham J. Tizzard, Simon J. Coles, John Spencer, and Hazel Cox. 2016. "The Trans Influence in Unsymmetrical Pincer Palladacycles: An Experimental and Computational Study" Inorganics 4, no. 3: 25. https://doi.org/10.3390/inorganics4030025
APA StyleBoonseng, S., Roffe, G. W., Jones, R. N., Tizzard, G. J., Coles, S. J., Spencer, J., & Cox, H. (2016). The Trans Influence in Unsymmetrical Pincer Palladacycles: An Experimental and Computational Study. Inorganics, 4(3), 25. https://doi.org/10.3390/inorganics4030025