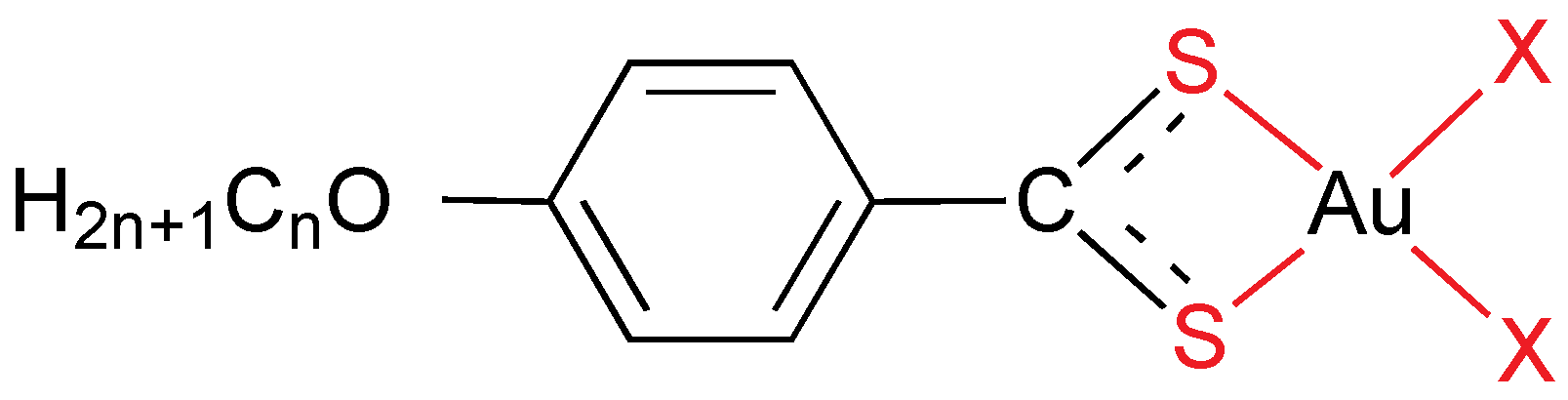
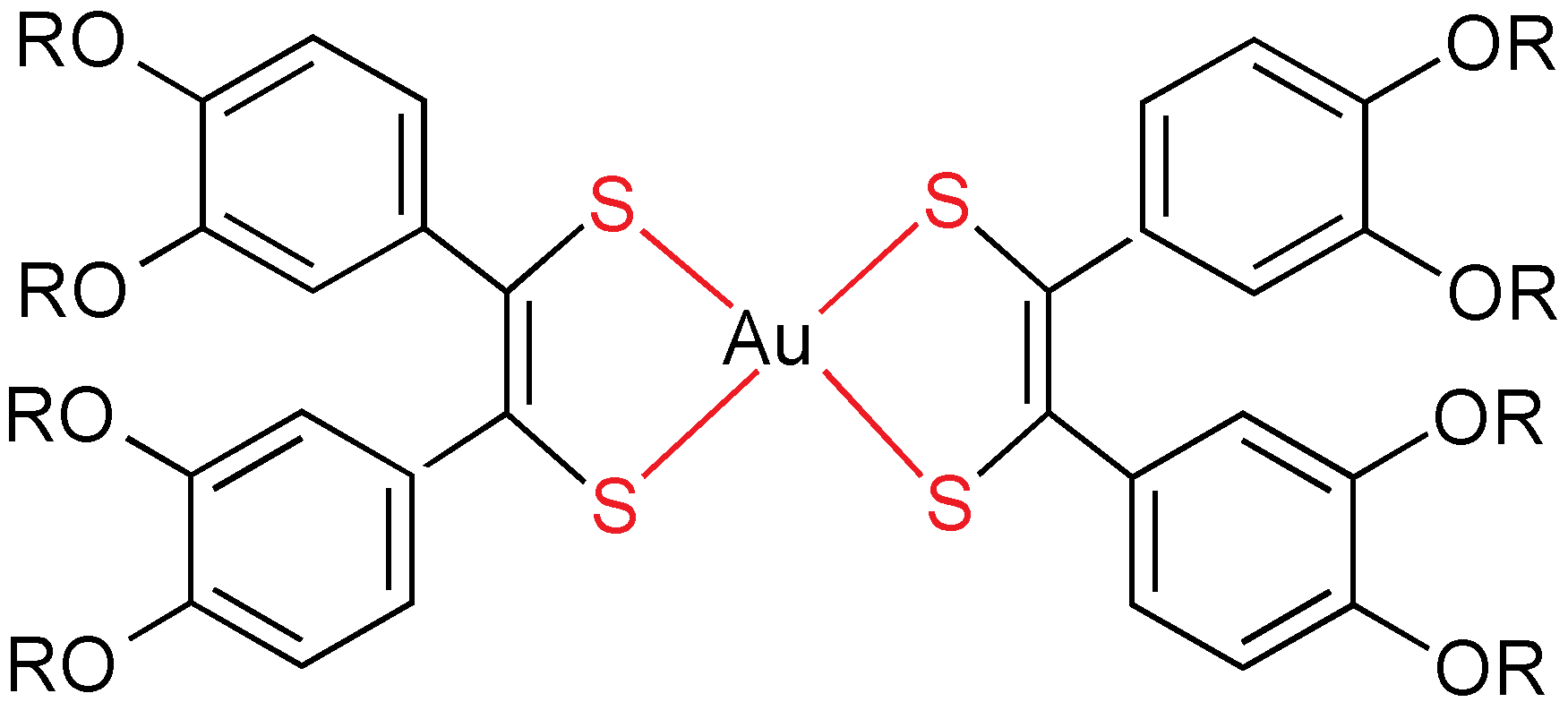
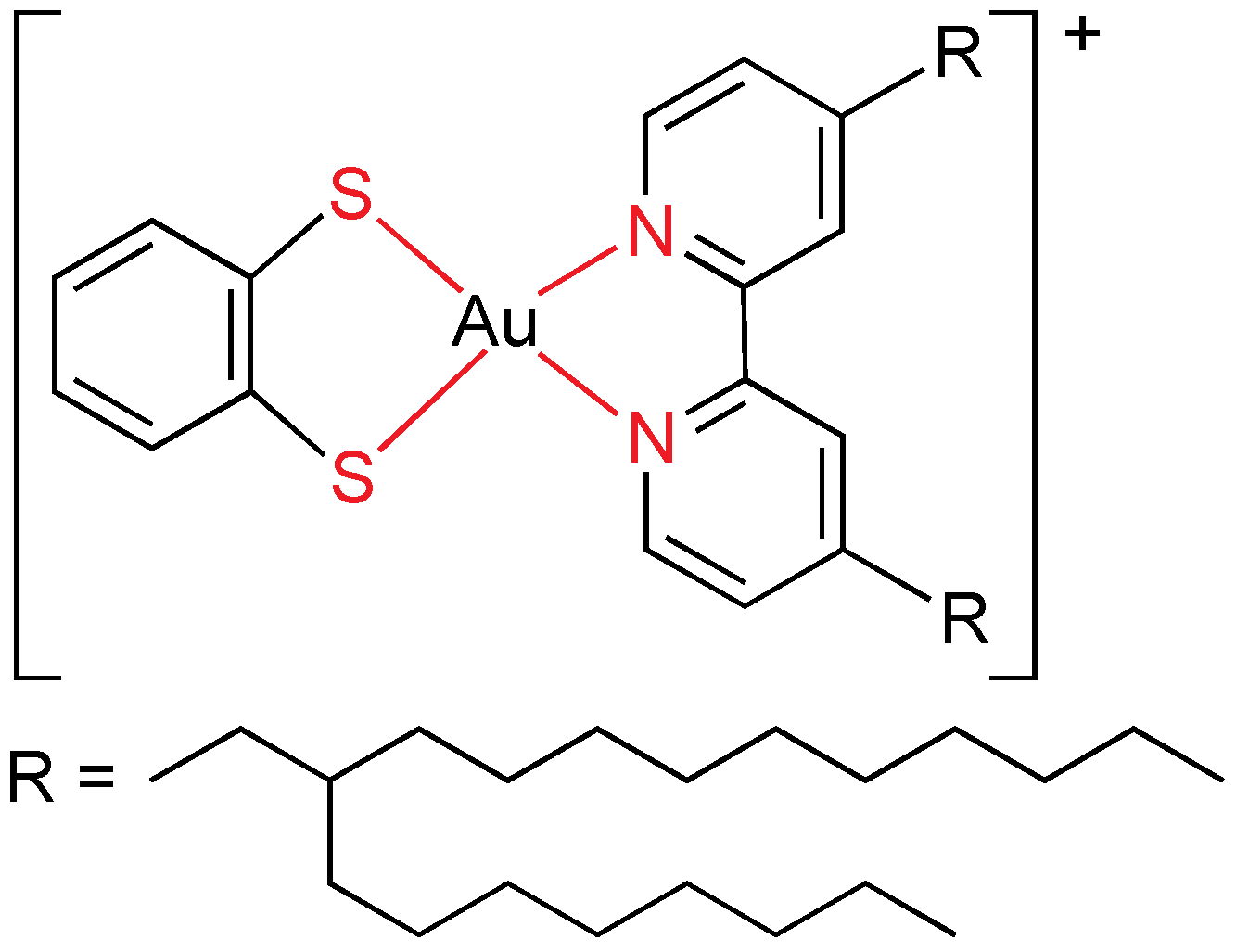
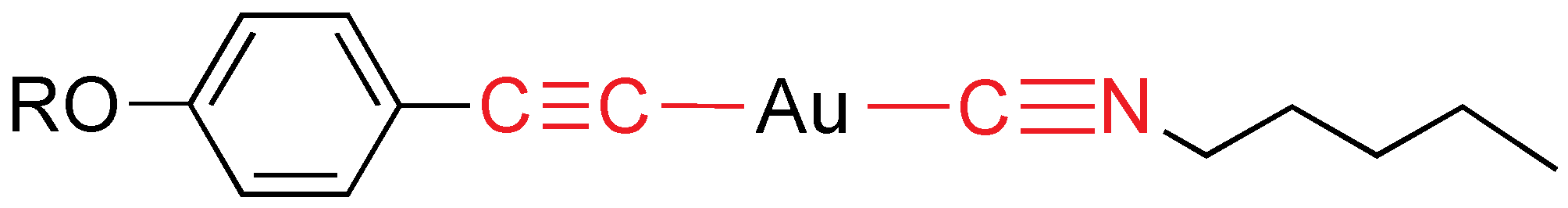As stated above, gold(I) has a strong tendency to give linear compounds, which means that a maximum of two different ligands can be used. The mesogenic ligands always possess one or more alkyl or alkoxy (additional dipole through the oxygen atom) chains, and mostly they have been designed in order to get metallomesogens by coordination chemistry. It is remarkable than non-mesomorphic ligands often become liquid crystals after bonding to a gold center. Calamitic liquid crystals are favored by rod-like molecules made by gold(I) centers. Additionally, gold dramatically increases the intermolecular interactions in condensed phases by means of the Au–L bond dipole moment, changes in dipoles associated with ligands (especially isonitrile) and gold-gold interactions. Most of gold(I) mesogens are organometallics compounds, being the Au–C bond quite stable thermally.
2.1. Carbene Ligands
N-heterocyclic and aliphatic carbene ligands had been used to get gold(I) liquid crystals previously.
N-heterocyclic carbenes may be easily modified to tune their steric and electronic properties, a common strategy to improve catalyst activity and selectivity. Two series of
N-heterocyclic carbene (NHC) gold(I) complexes, namely [AuCl(NHC)] and [Au(NHC)
2]NO
3 have been prepared [
12]. None of them are mesomorphic although they possess two or four alkyl chains. However, the related series combining the same
N-heterocyclic carbene and imidazole [Au(NHC)(Im)]NO
3 displays a SmA mesophase, typical for ionic liquid crystals (
Figure 1). An increment in the imidazole alkyl chain length always increase the clearing point and most of the times also the melting point. In such a way, the melting and clearing temperatures can be modulated. The best results (wider intervals of liquid crystal behavior) are obtained for the C
18 carbene: the melting points are from 57.3 to 101.5 °C, and the clearing points from 96.9 to 124.1 °C. These compounds were used as precursors to obtain gold nanoparticles in organic phase by chemical reduction.
Figure 1.
Gold mesogens containing N-heterocyclic carbene and imidazole. n = 12, 18; m = 1, 6, 12, 18. n = 16, m = 10, 12, 14, 16, 18.
Figure 1.
Gold mesogens containing N-heterocyclic carbene and imidazole. n = 12, 18; m = 1, 6, 12, 18. n = 16, m = 10, 12, 14, 16, 18.
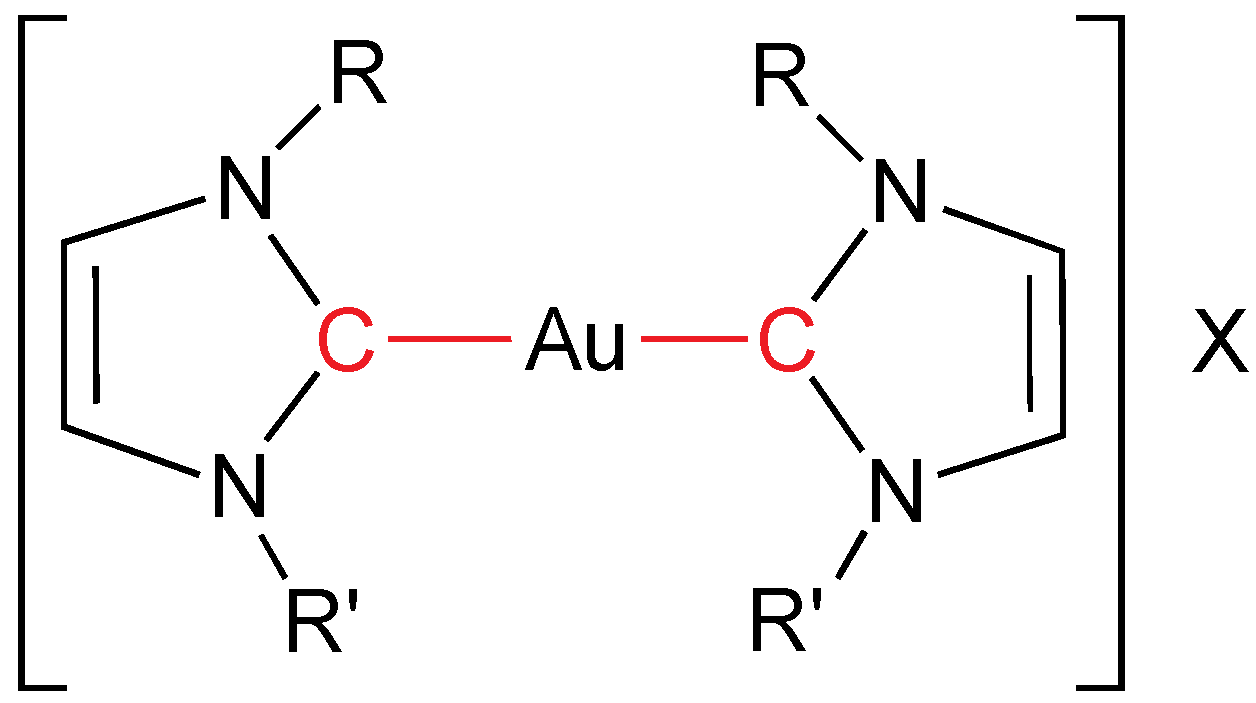
Self-assembly of doubly functionalized carbenes (now, with two different chains: amide and aliphatic;
Figure 2) gold(I) complexes leads to the formation of thermotropic liquid crystals and gels [
13]. Gold(I) compounds display SmA mesophases with melting points around 150 °C and clearing points till 226 °C (being the best with
n = 16 and bromide as anion). Gold(I) xerogels display oriental lantern-shaped bundles of belts and helical fibers when observed by scanning electron and transmission electron microscopies. These gold gels show a degree of chain motion smaller than in the mesophase, closer to found in the solid state, as studied by infrared spectroscopy.
Figure 2.
N,N-heterocyclic bis(carbene) gold mesogens. R = CnH2n+1; n = 10, 12, 14, 16. R' = CH2C(O)NH2. X = Br, NO3, BF4.
Figure 2.
N,N-heterocyclic bis(carbene) gold mesogens. R = CnH2n+1; n = 10, 12, 14, 16. R' = CH2C(O)NH2. X = Br, NO3, BF4.
2.2. Imidazole Ligand-Imidazolium Salts
Neutral and cationic imidazole gold(I) compounds, namely [AuCl(C
n-im)], [AuCl(C
n-bim)], [Au(C
n-im)
2]NO
3 and [Au(C
18-bim)
2]NO
3 (C
n-im is substituted imidazole, C
n-bim is substituted benzimidazole) have been prepared (
Figure 3) [
14]. Only the cationic imidazole compounds are liquid crystals, although they do not display any gold-gold interaction in the solid state. The melting points go from 54.4 to 79.8 °C, while the clearing points are in the range 74.5–141.5 °C. These temperatures increase with increasing chain length. The four C
18 compounds are used as precursors to make gold nanoparticles in dichloromethane by reduction with aqueous NaBH
4.
Figure 3.
Gold mesogens containing imidazole Cn-im: n = 12, 14, 16, 18.
Figure 3.
Gold mesogens containing imidazole Cn-im: n = 12, 14, 16, 18.
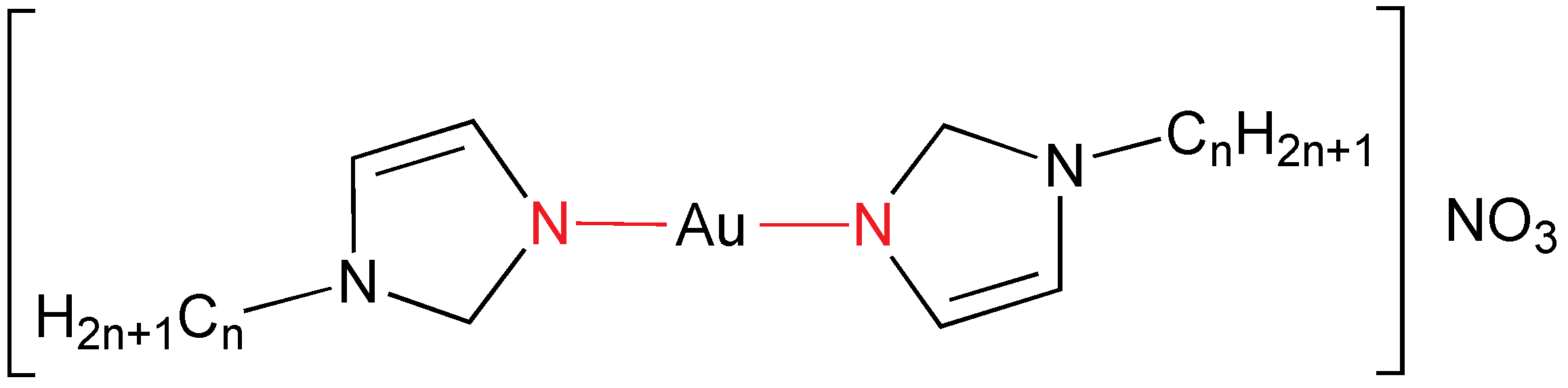
The combination of ionic liquids, salts with low melting point, and metallomesogens has been achieved by using imidazolium salts containing a gold anion as counter-ion (
Figure 4) [
15]. In fact, the dicyanoaurate(I) derivative of 3-(4-dodecyloxybenzyl)-1-methyl-1
H-imidazolium has been synthesized by metathesis from the bromide salt. The gold compound displays a SmA mesophase from 66 to 112 °C. Furthermore, the gold mesogen has been used to produce gold nanoparticles by electrodeposition. Different morphologies were obtained from the isotropic liquid state (nanodots) or from the mesophase (leaflike), demonstrating the significance of the supramolecular structure.
Figure 4.
Imidazolium dicyanoaurate(I) mesogen salt (R = CH2C6H4OC12H25).
Figure 4.
Imidazolium dicyanoaurate(I) mesogen salt (R = CH2C6H4OC12H25).
A series of dicyanoaurate(I) containing imidazolium salts has been prepared (
Figure 4 with
R = C
nH
2n+1;
n = 12, 14, 18) [
16]. They are ionic liquids and behave as liquid crystals, displaying the typical SmA characteristic for these compounds. They exhibit temperature-dependent luminescence, which depends not only on the temperature, but also on the physical state. For instance, the
n = 18 derivative emits intensely at 412 nm in the solid state and at 458 nm in the mesophase. These emissions are phosphorescence (lifetime of 9 and 11.2 μs, respectively) related to the modulation with temperature of gold-gold interactions, which is confirmed by DFT calculations. Moreover, it always displays an emission centered at 432 nm (hidden in the mesophase), which is a fluorescence coming from the imidazolium cation.
2.3. Isocyanide Ligands
Isocyanide or isonitrile ligands are strongly attached to the gold(I) centers, which leads to the suitable thermal stability of the complexes and therefore they melt without decomposition. This is critical in order to get liquid crystals and that is why these ligands are the most commonly found in gold metallomesogens. Therefore, the number of reported examples is, comparatively, quite high. Moreover, this functional group is linear, as it is the typical gold(I) coordination, which is an advantage to obtain calamitic liquid crystals. Some specific new isocyanide ligands have been designed, and in other cases the point is the right combination of the two gold’s ligands.
2.3.1. With an Alkynyl Ligand
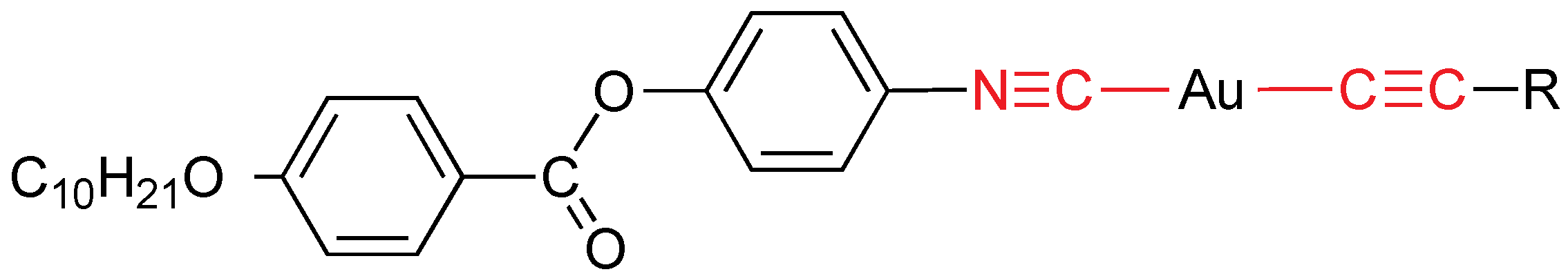
Rod-like gold(I) compounds can be prepared by combining a mesomorphic isocyanide with alkynyl ligands (
Figure 5) [
17]. They exhibit a SmA mesophase (except the non-mesogen for
R = C
6H
4CCC
5H
4N) with melting points in the range 87–144 °C and clearing points from 101 to 215 °C.
Figure 5.
Alkynyl and isocyanide gold mesogens. R = C5H4N, C6H4CN, C6H4CCC5H4N.
Figure 5.
Alkynyl and isocyanide gold mesogens. R = C5H4N, C6H4CN, C6H4CCC5H4N.
Related rod-like gold(I) compounds containing very simple ligands (
Figure 6) were prepared in order to prevent steric hindrance and facilitate a strong intermolecular aurophilic interaction [
18]. In fact, they are dimers in the crystalline state with gold-gold distances around 3.6 A. The compounds exhibit nematic or smectic A mesophases at moderate temperatures (less than 100 °C), although only on cooling and with short mesophase ranges (the best only 25 °C). The most spectacular result is about photoluminescence: blue emission with quantum yields of 8%–50% in the solid state at room temperature, with lifetime about 50 μs, typical of phosphorescence. The same emission was observed for the mesophase and the isotropic liquid, although the quantum yield is quite low; thermal deactivation of excitation states can lead to this diminution, maximized in this case by the relationship of phosphorescence and presence of gold aggregates.
Figure 6.
Alkynyl and isocyanide gold mesogens. R = CnH2n+1, n = 5–8.
Figure 6.
Alkynyl and isocyanide gold mesogens. R = CnH2n+1, n = 5–8.
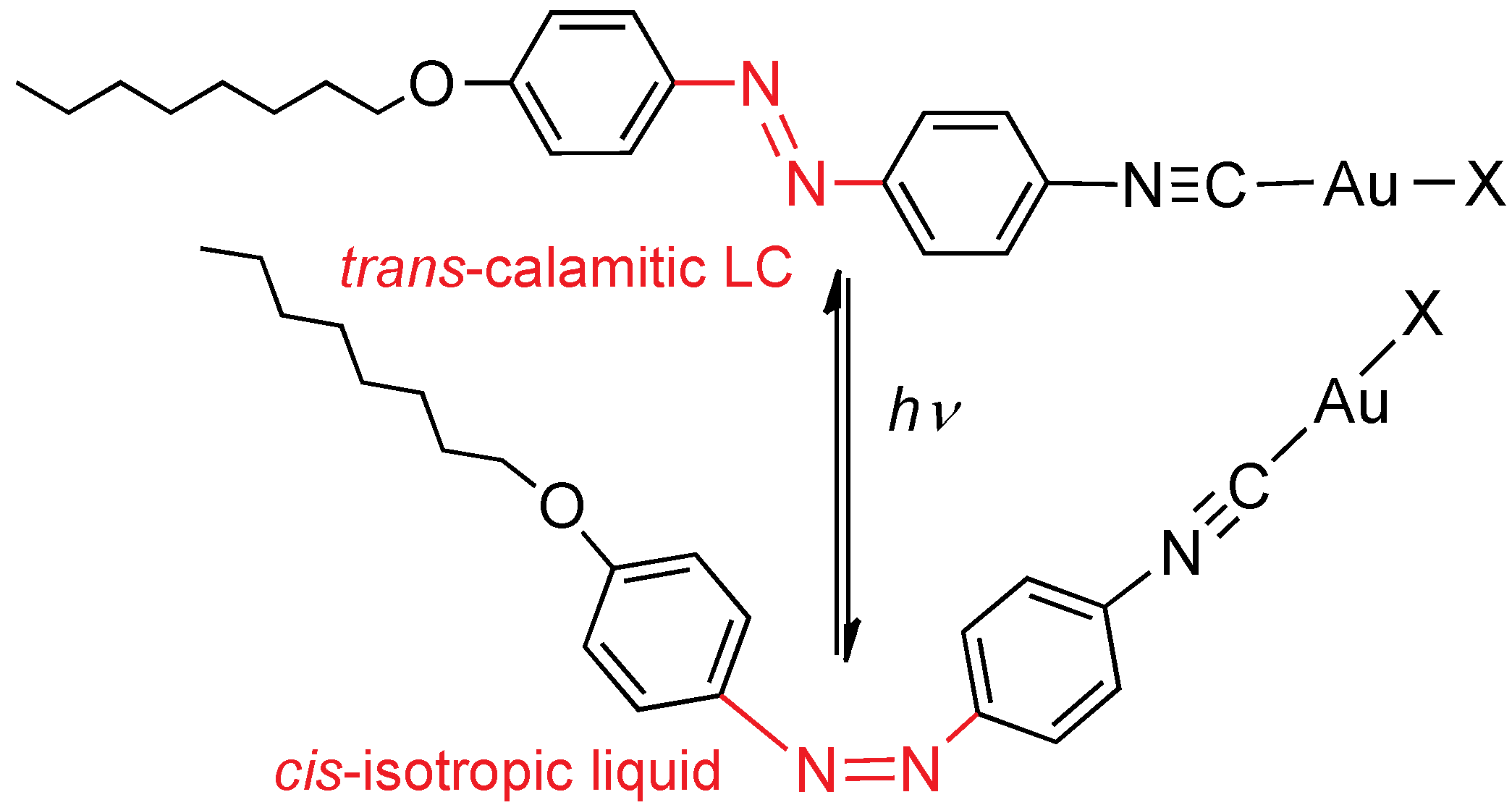
2.3.2. With an Azo-Isocyanide Ligand
4,4'-Disubstituted azobenzene compounds with appropriate substituents are liquid crystals that have been used to prepare orthometalated metallomesogens. In the cyclometalated mesogens, the azo fragment is obviously blocked in its
trans conformation, and the photosensitivity is suppressed. New azo isocyanide ligands were designed, containing: an azo, an isocyanide, and an alkoxy chain (
Figure 7) [
19]. The ligands display nematic and SmA mesophases (for
n > 4), and are able to coordinate through the isocyanide functional group, leaving a free azo functional group. Their gold(I) compounds [Au
X(CN
R)] (
X = Cl, C
6F
5;
R = C
6H
4N=NC
6H
4OC
nH
2n+1,
n = 4, 8, 12), also exhibit nematic and SmA mesophases, with higher transition temperatures and wider mesophase ranges. All of the derivatives are photosensitive in solution because of
trans to
cis isomerization of the azo group under UV light (365 nm lamp), which reverts photochemically or thermally to the
trans isomer. Irradiation in the mesophase with a very intense He–Cd laser also induces isomerization, with consequent destabilization of the mesophase to an isotropic liquid; the mesophase is recovered as soon as illumination stops (
Figure 7). These azo mesogens show high birefringence values, higher for the linear gold complexes (Δ
n from 0.51 to 0.59) than for the free azo ligand (Δ
n are 0.32), which confirms that the presence of gold plays an important role in increasing the molecular anisotropy. Therefore, these colored azo gold-containing liquid crystals are photosensitive, not only in solution but also in the mesophase. It is important to note that the photoisomerization has been demonstrated in a mesophase displayed by a pure metallomesogen.
Figure 7.
Changes in azo isocyanide gold mesogens after irradiation with a laser. X = Cl, C6F5; alkoxy chain with n = 4, 8, 12.
Figure 7.
Changes in azo isocyanide gold mesogens after irradiation with a laser. X = Cl, C6F5; alkoxy chain with n = 4, 8, 12.
2.3.3. Cationic Bis(Isocyanide) Compounds
A structure-mesomorphism study was carried out in cationic gold(I) derivatives containing two isocyanides [
20] by taking into account the number of phenyl rings (one or two), the length and number of alkoxy chains (one or three) and the counter-ions (BF
4, PF
6, NO
3) (
Figure 8 and
Figure 9). All of the new compounds are liquid crystals (except PF
6 complexes with
n = 4). These cationic gold mesogens exhibit calamitic SmA and SmC mesophases for one alkoxy chain, and hexagonal columnar mesophases for trialkoxyphenyl isocyanide. All of the gold nitrate derivatives are non-mesomorphic and undergo extensive decomposition at relatively low temperatures, which is likely to be related to the easy decomposition of the anion in the presence of many heavy metal cations. Some of these compounds can be described as ionic liquids because of their low melting point (below 100 °C).
Figure 8.
One alkoxy chain cationic mesogens. X = PF6, BF4; m = 1, 2; n = 4, 8, 12.
Figure 8.
One alkoxy chain cationic mesogens. X = PF6, BF4; m = 1, 2; n = 4, 8, 12.
Figure 9.
Three alkoxy chains cationic mesogens. X = PF6, BF4; n = 4, 8, 12.
Figure 9.
Three alkoxy chains cationic mesogens. X = PF6, BF4; n = 4, 8, 12.
2.3.4. Chiral Isocyanide
Pioneering work about chiral gold mesogens was reported in 1999 [
21]. [AuCl(CNR)] was prepared by means of complexation to an enantiomerically pure chiral isocyanide (non-mesomorphic). It exhibits a SmC* mesophase. The influence of introducing one or two chiral centers in gold(I) perfluoroaryl isocyanide complexes has been studied (
Figure 10) [
22]. They exhibit cholesteric phases (N*), as well as rare twist-grain boundary (TGBA*) and blue phases (BP), two types of frustrated phases, which are found exclusively in optically active systems. The complexes equipped with one chiral substituent show an enantiotropic cholesteric and a monotropic SmA phase for shorter alkoxy chains. A TGBA* phase is observed for the compound containing the chiral isocyanide combined with the diethyloxy, when the SmA to cholesteric transition is studied. The complexes with two chiral ligands display a monotropic chiral nematic transition. When this compound is cooled very slowly from the isotropic liquid, it exhibits blue phases BP-III, BP-II, and BP-I.
Figure 10.
Chiral gold mesogens. R' = (R)-2-butyl, R'' = CnH2n+1 (n = 2, 10); R' = CmH2m+1 (m = 2, 10), R'' = (R)-2-butyl; R' = R'' = (R)-2-butyl.
Figure 10.
Chiral gold mesogens. R' = (R)-2-butyl, R'' = CnH2n+1 (n = 2, 10); R' = CmH2m+1 (m = 2, 10), R'' = (R)-2-butyl; R' = R'' = (R)-2-butyl.
2.3.5. Isocyanide Dendrimers
Dendrimers, arborols or cascade molecules are highly branched and functionalized molecules formed by an iterative sequence of reactions. They are repetitive molecules and maintain fractal symmetry from the center. The size, shape, topology and surface of dendrimers can be varied, which allows building stoichiometric and predictable structures. Metallodendrimers are dendrimer containing metals, which combine the metallic fragment properties with the dendrimeric structure. The metallic fragment can be on the core, on the branches or as terminal group. The design of liquid crystal metallodendrimers has attracted much interest during the last years [
23]. We will describe the results obtained for gold.
First, nitro-dendrons were designed, in such a way that nitro was always in the focal point, then, the nitro functional group was transformed in an isocyanide through the amine and amide. By this approach, two generation of isocyanide dendrimers were reported, and, also, the corresponding Au, Cu, Pd and Pt organometallic complexes (
Figure 11) [
24]. The free ligands and the first-generation complexes are no mesomorphic, but the second-generation complexes exhibit a thermotropic micellar cubic mesophase, over a large temperature range, some of them at room temperature. In particular the coordination to Au–Cl fragment leads to a mesophase in a wide temperature range of 92–260 °C.
Figure 11.
First and second-generation isocyanide dendrons.
Figure 11.
First and second-generation isocyanide dendrons.
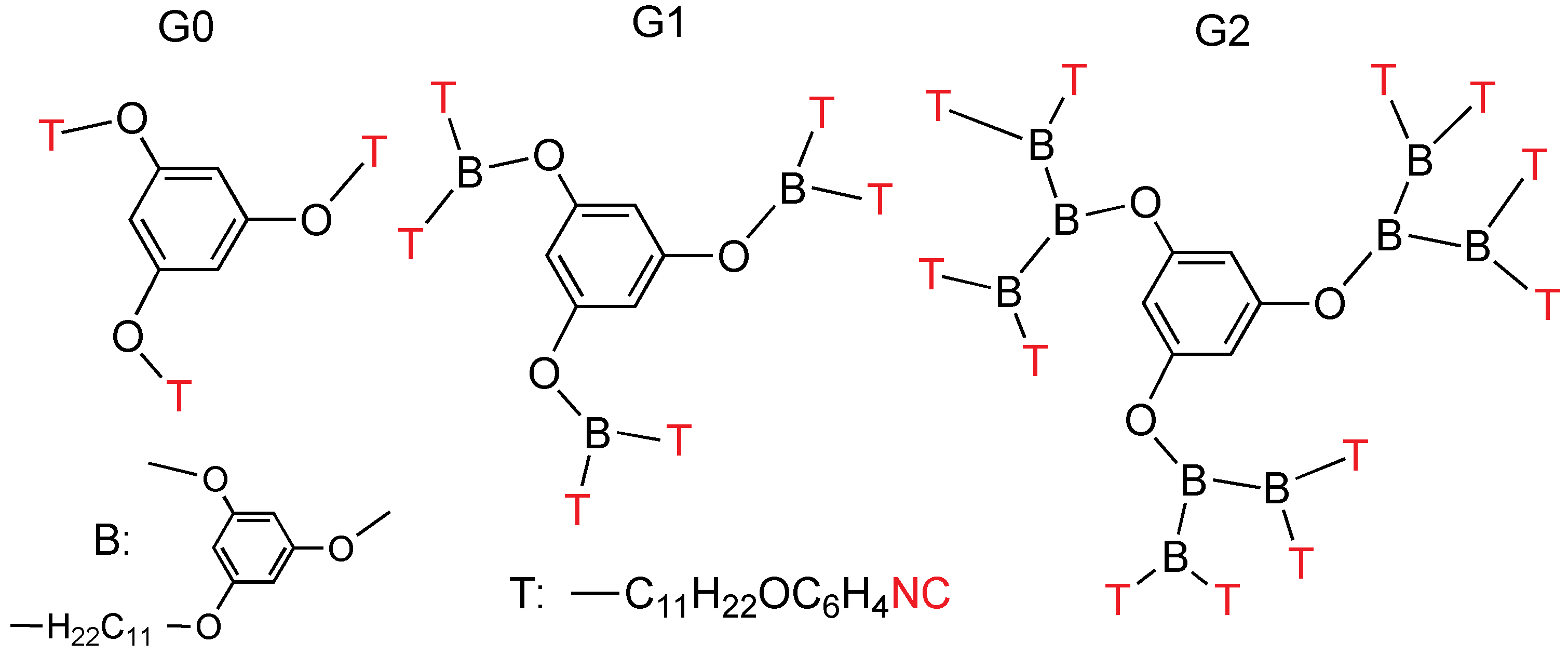
More recently dendrimers containing isocyanide as terminal functional groups were designed and synthesized as non-mesomorphic oils (
Figure 12) [
25]. Dendritic polyisocyanides can be considered as promising polytopic ligands to generate a great diversity of metallodendrimers due to the ability of the isocyanide moiety to bind to various transition metals. Moreover, they can be used to construct novel liquid-crystalline organometallic dendrimers. Generation zero, one, and two, containing 3, 6, or 12 nitro terminal groups were prepared, and then transformed in the corresponding polyisocyanides. Three types of gold(I) fragments were connected at the periphery of the dendrimers, namely AuCl, AuCCC
6H
4OC
12H
25 and AuCCC
6H
2(OC
12H
25)
3.
Figure 12.
Polyisocyanides G0, G1 and G2 containing 3, 6, and 12 terminal isocyanide groups.
Figure 12.
Polyisocyanides G0, G1 and G2 containing 3, 6, and 12 terminal isocyanide groups.
Their mesomorphic properties are largely influenced by the nature of the peripheral gold fragments, while the increase of the dendritic generation contributes to a strong stabilization of the mesophases. Smectic C mesophases are observed after binding of the dendrimers to gold(I) chloride or to rod-like gold(I) acetylide fragments: G0-AuCl, G1-AuR (R = Cl, AuCCC6H4OC12H25), with melting points from 36 °C and clearing points till 178 °C. The mesophase temperature ranges are about 85 °C for Au–Cl, but only 15 °C for Au-acetylide. A columnar hexagonal mesophase is displayed by the three generations of dendritic gold complexes bearing hemipolycatenars. The clearing temperatures decrease with the generation from 116 °C down to 73 and 36 °C from the G0 to the G2 derivatives, respectively. The mesophase is built by supramolecular aggregation of molecular dendrimers into one-dimensional cylindrical assemblies, which are further organized to give a hexagonal network.
2.3.6. Partially Fluorinated Hydrocarbon Chain
During the last years, fluorous or mixed fluorous-hydrogen alkyl chains have been used instead of the standard fully hydrocarbon chains. Incorporation of perfluorinated alkyl chains into classical organic molecules produces a microsegregation at the molecular level that results in an enhancement of highly ordered lamellar mesophases. This effect results from the differences in the cohesive energy between fluorocarbon and hydrocarbon groups, and has been named as the fluorophobic effect. It was prepared a new isocyanide ligand containing a partially fluorinated hydrocarbon alkoxy chain, which is already is mesomorphic, as opposed to its hydrocarbon analogue. Ag, Au, Cu, Pd, and Pt liquid crystals were prepared [
26]. Gold(I) compound AuCl(CNAr) [Ar = 4-CF
3(CF
2)
7(CH
2)
4OC
6H
4] shows a SmA calamitic mesophase from 191 to 274 °C. Melting and clearing points are higher than for the H-analog (too high for practical application), being the mesogenic range larger too; this was found for all of the metallomesogens reported.
2.3.7. With Halides or Pseudohalides
Gold(I) derivatives containing alkylisocyanides have been reported (
Figure 13) [
27]. These compounds exhibit some intermediate phases, described as crystalline but mechanically soft and easy to deform. The transition temperatures are in the range 45–52 °C for the melting points, and 51–59 °C for the clearing points. These phases could be described as rotator phases induced by aurophilic bonding, similarly to those induced by hydrogen bonding for primary alcohols. The gold derivatives are geometrically similar to the alcohols and form aurophilic bonds of similar energy to the hydrogen bonds.
Figure 13.
Isocyanide and halide gold mesogens. R = CnH2n+1, n = 7–11.
Figure 13.
Isocyanide and halide gold mesogens. R = CnH2n+1, n = 7–11.
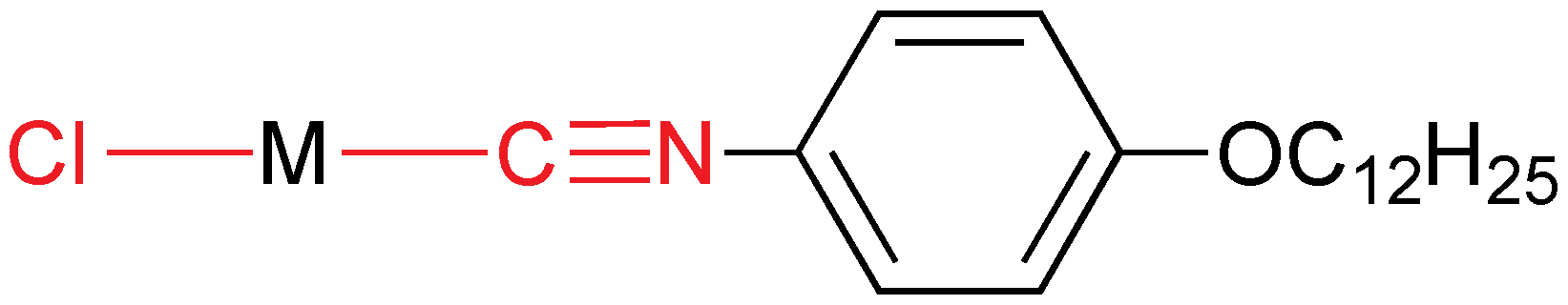
2.3.8. Binary Mixtures of Isocyanide Derivatives
Applications for liquid crystals are very demanding in temperature range, adequate response to an electric field, stability, viscosity, and that is why all the industrial devices use mixtures. In the field of metallomesogens, these studies are usually limited to binary mixtures. The thermotropic behavior and phase diagrams of binary mixtures [
MX(CN
R)] (
M = Au, Cu,
X = anionic ligand,
R = p-alkoxyaryl chain) have been studied [
28] and the main results are:
(a) The mixture of two compounds where the only difference is the alkoxy length, [AuCl(CN–C6H4–C6H4–OCnH2n+1–p)] (n = 4 and 12), gives rise to a wider range of SmA mesophases with lower melting points and to SmC mesophases for mixtures richer in the dodecyloxy compound.
(b) The mixture of the phenyl and biphenylisocyanide gold(I) complexes [AuCl(CN–(C6H4)x–OC12H25−p)] (x = 1, 2) produces an enhancement of the range of the SmA mesophase mainly at the eutectic composition, by comparison to the pure components.
(c) The mixture of two derivatives (
Figure 14), being the only difference where the alkoxy chain is bonded, displays melting temperatures lower than those of pure complexes, leading to room-temperature SmC mesophases, and to a range of mesophases higher than for the pure components. Actually, by tuning the mixture, liquid crystal behavior can be obtained from room temperature to about 200 °C. This is the only mixture in these studies that gives a solid solution, whereas the others separated in the components after crystallization.
Figure 14.
Isocyanide and perfluoroaryl gold mesogen. n = 10 and m = 6; n = 6 and m = 10.
Figure 14.
Isocyanide and perfluoroaryl gold mesogen. n = 10 and m = 6; n = 6 and m = 10.
(d) The mixture of two compounds which only differ in the metal (
Figure 15), shows liquid crystal behavior for a large range of concentrations (at least 30% mol of gold complex), although the starting copper compound is not a liquid crystal. In fact, a single fluid material with an ordered homogeneous distribution of the two metal complexes is obtained.
Figure 15.
Binary mixture of copper and gold compounds. M = Au, Cu.
Figure 15.
Binary mixture of copper and gold compounds. M = Au, Cu.
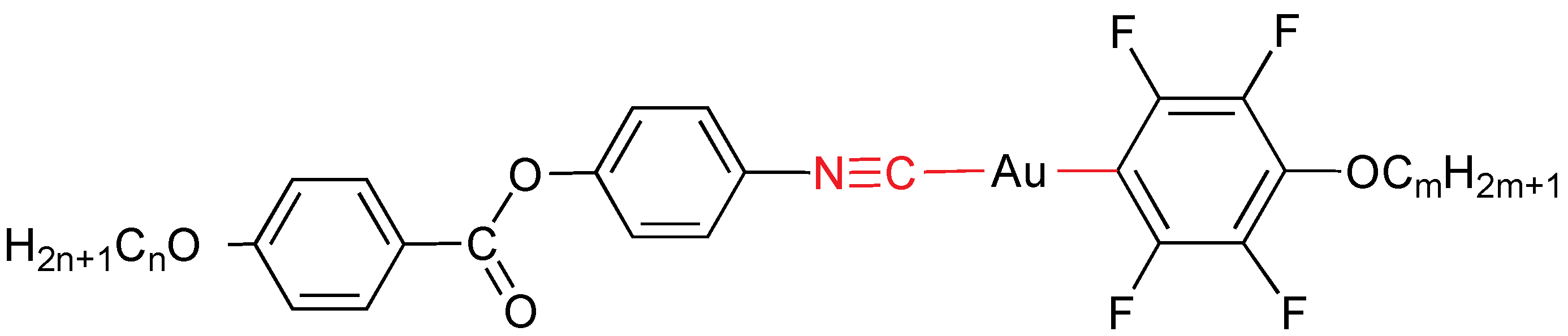
2.4. Perhaloaryl Ligands
Most of gold mesogens with perhaloaryl ligands as mesogenic unit contain isocyanide as mesogenic fragment too. They are all described in this section. Mononuclear gold(I) mesogens have been obtained by combining two mesogenic ligands: an isocyanide and a fluoroaryl ligand with an alkoxy chain (
Figure 16) [
29]. All of the derivatives are liquid crystals and their thermal stability is high, even in the isotropic state. Compounds exhibit nematic, SmA and SmC phases depending on
n and
m values.
Figure 16.
Isocyanide and perfluoroaryl gold mesogens. m = 4, 8; n = 4, 6, 8, 10.
Figure 16.
Isocyanide and perfluoroaryl gold mesogens. m = 4, 8; n = 4, 6, 8, 10.
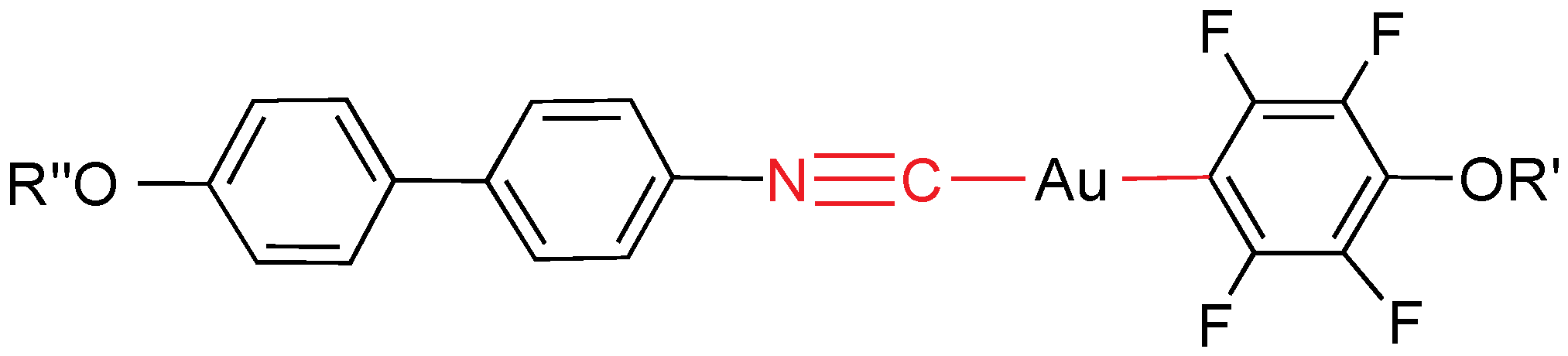
Similar compounds have been prepared with a fluoroaryl ligand without alkoxy chain (
Figure 17) [
17]. All of them are mesogens and exhibit short-range SmA mesophases, except the perfluorophenylpyridine derivative, which show a SmA phase in the range 87–215 °C. This much longer range seems to be related to the non-planar structure, because as is well known, the perfluorophenyl ring is significantly twisted with respect to the plane of the pyridine ring.
Figure 17.
Isocyanide and perfluoroaryl gold mesogens. Y = N, C–C5H4N, CF.
Figure 17.
Isocyanide and perfluoroaryl gold mesogens. Y = N, C–C5H4N, CF.
The analogous compounds with biphenylisocyanides (also mesogens themselves) instead of the two aromatic ring isocyanides exhibit N, SmA and SmC mesophases, too (
Figure 14 with
m = 2,
n = 4, 10;
m = 6,
n = 10;
m = 10,
n = 6, 10) [
30]. In addition, they show photoluminescence in the mesophase, as well as in the solid state and in solution. Therefore, both properties are displayed at the same time. It was reported in 2005. The emission spectra show maxima at around 390, 485, and 520 nm in the solid and liquid crystal states, while only the maxima at
ca. 390 nm is kept in solution (although all the emissions are recovered in solution at 77 K). X-ray diffraction studies confirm its rod-like structure, the absence of gold-gold interactions and the presence of short F–F intermolecular distances.
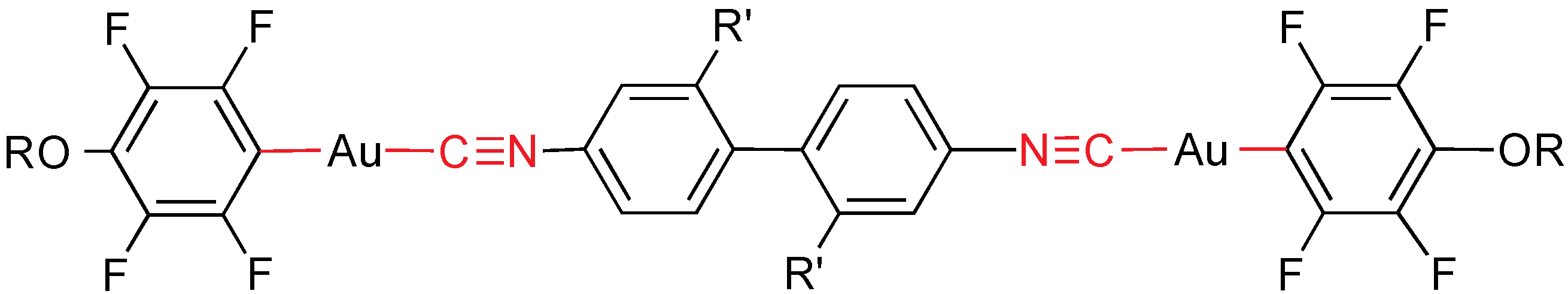
Dinuclear gold(I) complexes containing two equal mesogenic alkoxytetrafluoroaryl fragments bridged by a non-mesogenic biphenyl diisocyanide have been reported (
Figure 18) [
31]. All of them exhibit N mesophases, whose transition temperatures decrease in the order 4,4'-biphenylene > 2,2'-dichloro-4,4'-biphenylene > 2,2'-dimethyl-4,4'-biphenylene. This has been related to the planarity of the molecule: the twist angle of the biphenyl follows the trend Me > Cl >> H and more planar means stronger interactions and therefore higher transition temperatures. All compounds emit at room temperature in the solid state with emission maxima from 480 to 532 nm, and also in solution, from 452 to 524 nm.
Figure 18.
Isocyanide and perfluoroaryl gold mesogens. R = CnH2n+1, n = 4, 6, 8, 10, R' = H, Cl, Me.
Figure 18.
Isocyanide and perfluoroaryl gold mesogens. R = CnH2n+1, n = 4, 6, 8, 10, R' = H, Cl, Me.
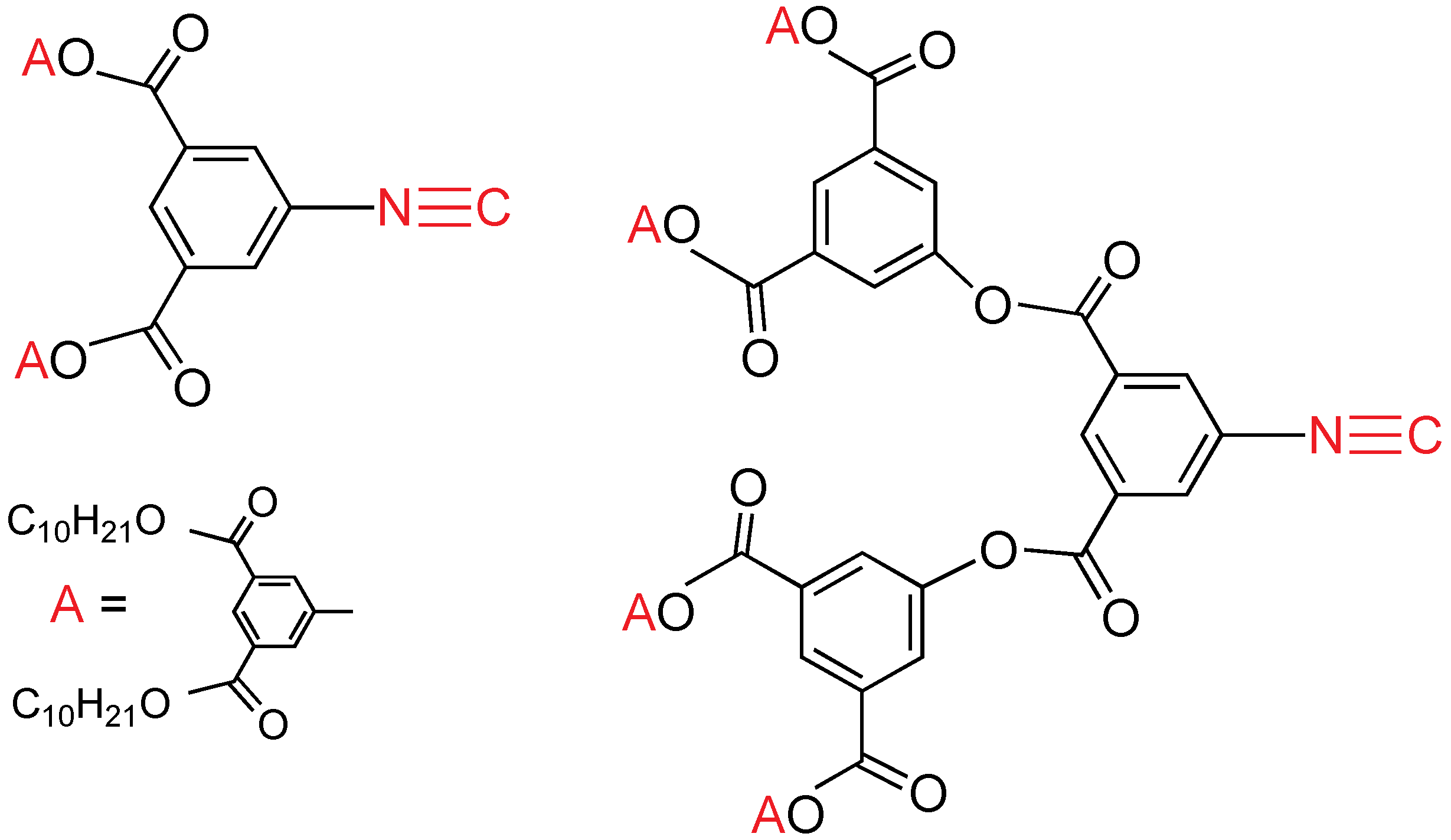
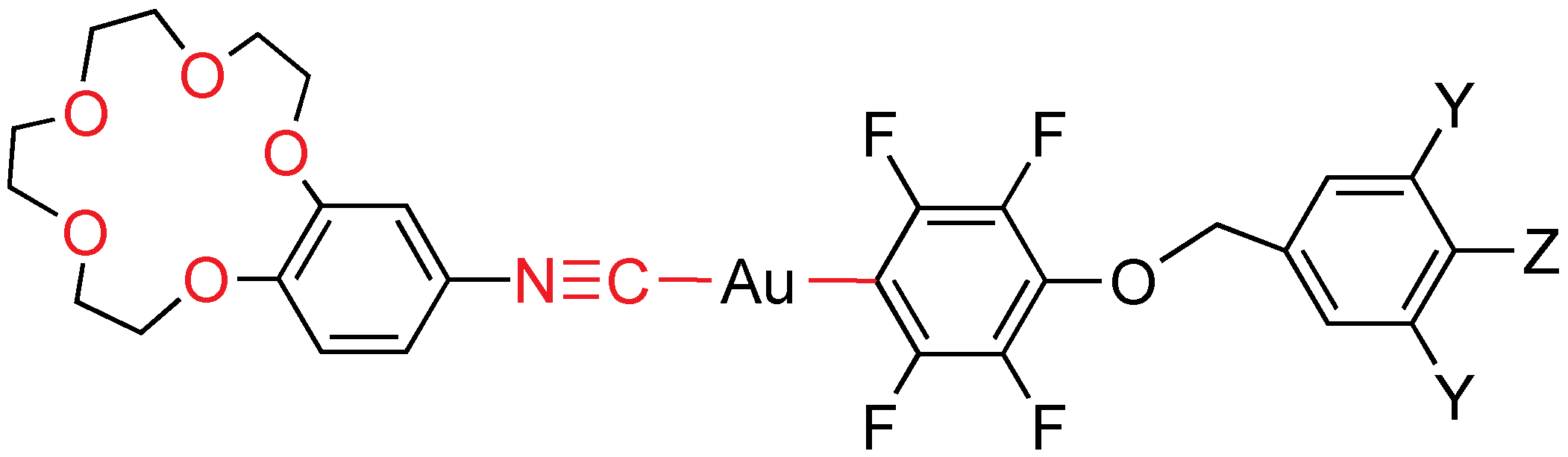
A family of mononuclear gold(I) compounds combining one or three alkoxy chain perfluoro mesogenic unit, and a specifically made crown ether isocyanide has been synthesized (
Figure 19) [
32]. One alkoxy chain compounds are monotropic liquid crystals, displaying a smectic C mesophase at temperatures close to room temperature. The trialkoxy-chain compounds display unidentified or smectic C mesophases, which are more clearly seen on cooling. In all the cases, the increase of the alkoxy chain length reduces the melting temperature. The use of the fairly bulky and flexible crown ether fragment in the ligand results in a reduction of intermolecular attractions, producing lower melting points, less ordered mesophases, and shorter mesophase ranges than found for the related derivatives [AuCl(CNR)] (
R = C
6H
4OC
nH
2n+1−p). The 15-crown ether moiety in these complexes is able to coordinate sodium from NaClO
4, solubilizing it in chlorinated solvents, affording the corresponding bimetallic complexes (
Figure 20), although the mesomorphic behavior is lost. All of the derivatives are luminescent at room temperature in the solid state with emission maxima in the range 457–511 nm; they emit at 77 K from 450 to 492 nm. They also emit in solution at room temperature at 426–445 nm, and at 77 K from 425 to 497 nm. Very interestingly, these mesogens are luminescent not only in the solid, and in solution, but also in the mesophase, and even in the isotropic liquid at mild temperatures.
Figure 19.
Crown ether isocyanide and perfluoroaryl gold mesogens. Y = H, Z = OC4H9, OC8H17, OC10H21, OC12H25; Y = Z = OC4H9, OC8H17, OC12H25.
Figure 19.
Crown ether isocyanide and perfluoroaryl gold mesogens. Y = H, Z = OC4H9, OC8H17, OC10H21, OC12H25; Y = Z = OC4H9, OC8H17, OC12H25.
Figure 20.
Sodium adduct of crown ether isocyanide gold compound. Y = H, Z = OC8H17; Y = Z = OC8H17.
Figure 20.
Sodium adduct of crown ether isocyanide gold compound. Y = H, Z = OC8H17; Y = Z = OC8H17.
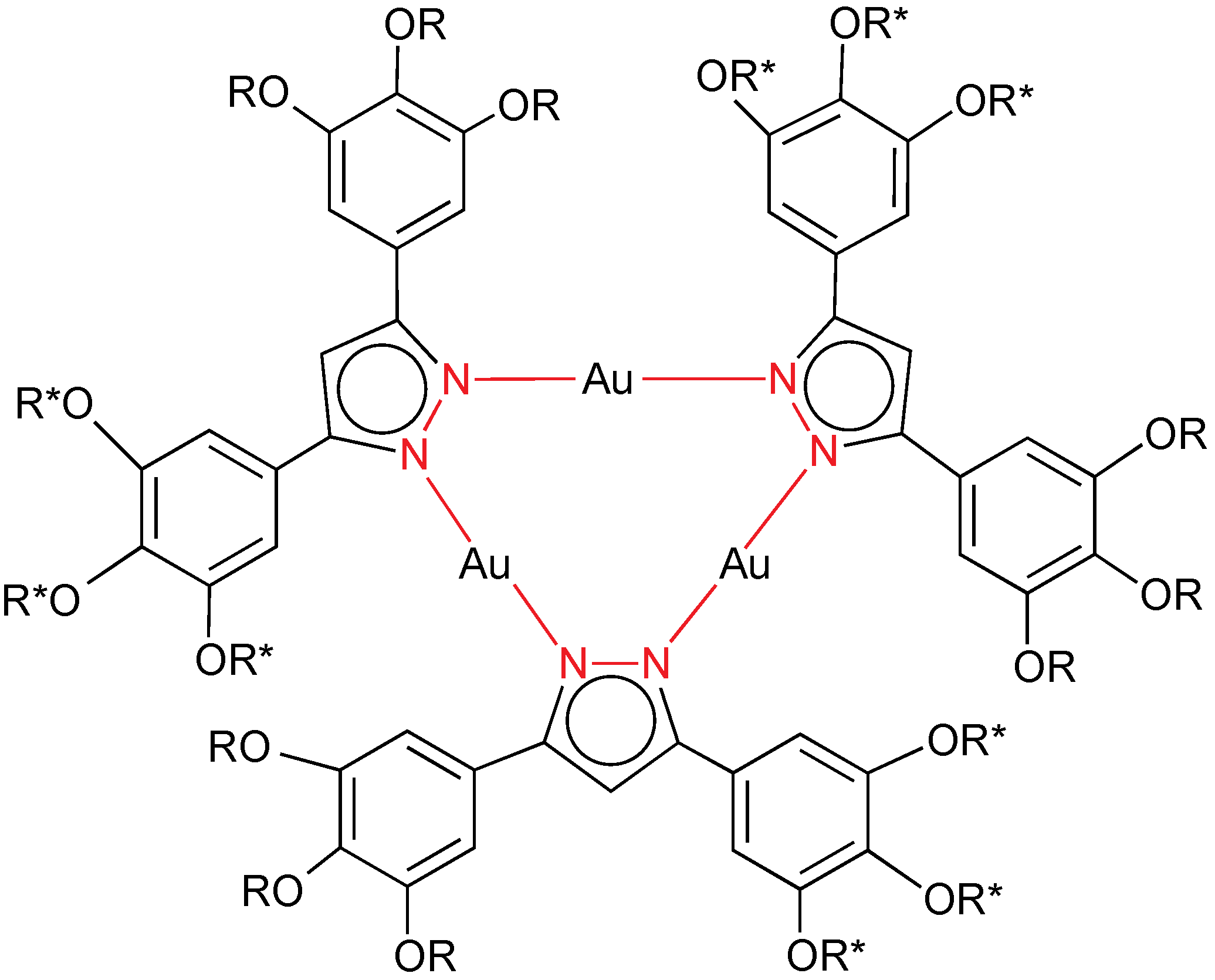
2.5. Pyrazolate Ligands
The first discotic gold mesogens reported in 1996 were homoleptic trinuclear gold(I) compounds with pyrazolate ligands as bridges as seen in
Figure 20, but with different alkoxy chain; they were named as metallocrowns [
33,
34]. Now, helical columnar mesophases by using chiral substituents in the pyrazolate have been reported (
Figure 21) [
35]. The compounds combine
n-decyloxy- and/or (
S)-3,7-dimethyloctyloxy terminal groups, and behave as room temperature columnar liquid crystals (hexagonal or rectangular) in the case of fifteen (nine non-chiral and six chiral) and eighteen chains (nine non-chiral and nine chiral; 18 chiral). The eighteen-chain molecules stack at regular distances and mutually rotated by 40° to form a helical columnar phase, which displays a well-formed hexagonal two-dimensional lattice. Moreover, all of the complexes are luminescent at room temperature in solution (only in UV, related to the ligand) and in thin films (UV + blue emission related to the gold centers that has been assigned as a ligand-to-metal charge-transfer transition).
The optical properties of these trinuclear derivatives have been attracted much attention. For instance, the compound drawn in
Figure 22 containing 6 alkoxy chains (
R = H,
R' = C
18H
37) forms a gel in hexane and exhibits an intense RGB (red-green-blue) phosphorescence [
36]. Initially, it is observed the red emission, after doping with Ag
+ the blue, and heating to get a sol leads to the green emission. Actually, the liquid crystal behavior is not studied.
Figure 21.
Compound shows a helical columnar mesophase. R = C10H21, R* = (CH2)2CH(Me)(CH2)3CHMe2.
Figure 21.
Compound shows a helical columnar mesophase. R = C10H21, R* = (CH2)2CH(Me)(CH2)3CHMe2.
A close trinuclear compound equipped with 9 alkoxy chains (half chain hydrophobic and the other half hydrophilic,
R =
R' = (CH
2)
10(OCH
2CH
2)
3CH
3,
Figure 22) displays a discotic lamellar mesophase from −3 to 44 °C [
37].
Figure 22.
Trinuclear gold(I) compounds. R = H, R' = C18H37. R = R' = (CH2)10(OCH2CH2)3CH3.
Figure 22.
Trinuclear gold(I) compounds. R = H, R' = C18H37. R = R' = (CH2)10(OCH2CH2)3CH3.
This compound can be confined into nanoscopic channels of mesoporous silica by sol-gel synthesis, and exhibits self-repairable luminescence properties because of a nanoscopic template effect. The emission is coming from the supramolecular cylindrical assembly formed by metallophilic interactions, and it is lost after heating. However, this temperature dependency is smaller in the silica. Additionaly, the phosphorescence is fully recovered upon cooling because of complete reconstruction of the supramolecular structure in the silica.
The related mono and disubstituted pyrazole ligands have been synthesized. The free disubstituted pyrazole ligands are calamitic mesogens, while the monosubstituted are non-mesomorphic materials (
Figure 23). Surprisingly, only the metal derivative [{Au(pz)}
3] containing the asymmetric monosubstituted pyrazolate behaves as liquid crystal [
38]. In fact, only the dodecyloxy chain does, and the compound exhibits a columnar mesophase, which is only observed on cooling very slowly in a narrow temperature range (76.4–83.5 °C). The corresponding pyrazole mononuclear derivatives [AuCl(pzH)] have been prepared with the same ligands [
39]. Again, only the monosubstituted non-mesomorphic pyrazole leads to a liquid crystal, but not the disubstituted mesomorphic ligand. The complex displays a SmA mesophase on cooling at 69.2 °C, although mixed with some crystallization. Moreover, this derivative is luminescent at 77 K in the solid state. These facts illustrate that the mesomorphism in these compounds is related to the disc-like shape of the molecular core and its supramolecular rearrangement, which is affected by the length, number, and position of the chains. Therefore, very subtle changes can inhibit the mesomorphism.
Figure 23.
Mono and disubstituted pyrazole ligands.
Figure 23.
Mono and disubstituted pyrazole ligands.
A series of ionic gold(I) complexes [Au(Hpz)
2][
A] (
A = BF
4−, PF
6−, NO
3−) has been prepared by coordination of the mesomorphic 3,5-bis(4-alkyloxyphenyl)pyrazole or non-mesomorphic 3-(4-alkyloxyphenyl)pyrazole (
Figure 24) [
40]. The complexes show smectic A mesophases, independently of the anion. The melting points go from 56 to 120 °C, and the clearing points in the short-range 127–135 °C. The choice of the ligands allows the achievement of H or U molecular shapes (see
Figure 22), which appear to be responsible for the liquid crystal mesophases. These metallomesogens are emissive both in the solid state and in solution at room temperature, and also in the mesophase (the same emission as observed in solid but less intense).
Figure 24.
H and U shape structures [Au(Hpz)2]+. R = C12H25, C14H29.
Figure 24.
H and U shape structures [Au(Hpz)2]+. R = C12H25, C14H29.
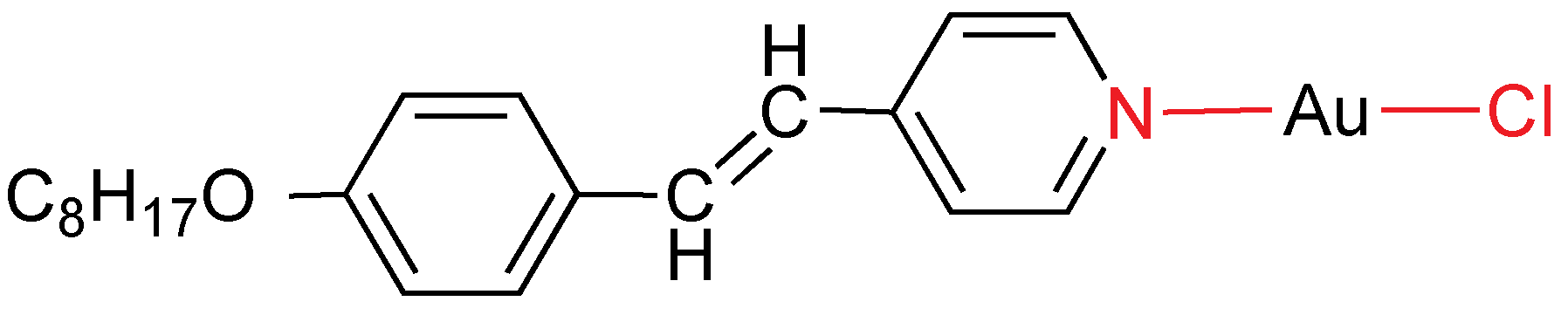
2.6. Stilbazol Ligands
4-alkoxy-4'-stilbazoles are
p-substituted pyridines with liquid crystal behavior. This ligand was used to prepare the first gold metallomesogen in 1986 (
Figure 25) [
41].
Figure 25.
Gold mesogen containing stilbazol.
Figure 25.
Gold mesogen containing stilbazol.
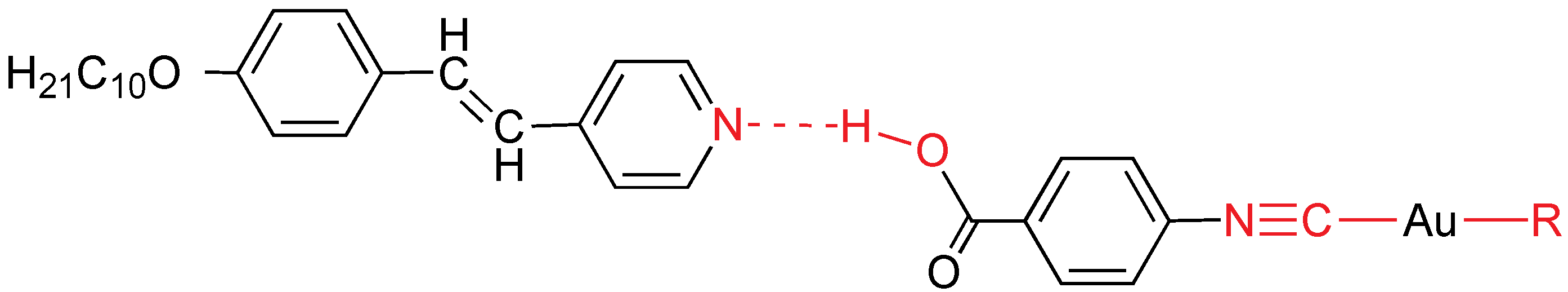
More recently, stilbazol has been selected to prepare metallomesogens by a different way: H-bonds between the pyridine and an acid functional group to give hydrogen bonded liquid crystals. Therefore, mesogens are obtained by using supramolecular chemistry and non-covalent bonds. These bonds are not quite strong, but thermally stable if the temperature is not very high. This is a general strategy that was applied to purely organic liquid crystals and then, to metallomesogens. In fact, it has been known since the early years of the 20th century that H-bond dimerization of 4-alkoxybenzoic acids leads to liquid crystal behavior [
42,
43]. The ligand 4-isocyanobenzoic acid was selected because it possesses an isocyanide coordinating functional group, and can leave a free carboxylic to form supramolecular by means of H-bonds with the nitrogen of the stilbazol molecule. Firstly, 4-isocyanobenzoic acid gold complexes (metallo-organic acids) were prepared, namely [Au
X(CNC
6H
4COOH)] (
X = Cl, fluoroaryl), which can act as proton donors to decyloxystilbazole, giving rise to supramolecular metal complexes displaying liquid crystalline behavior (
Figure 26) [
44,
45]. The chloro fragment exhibits a calamitic SmA mesophase in the short range 170–187 °C, while the fluoroaryl fragments show rod-like SmC mesophase with melting points from 127 to 190 °C, and clearing points in the range 170–190 °C. The fluoroaryl gold(I)-stilbazole aggregates display luminescence in the solid state at room temperature with emission maxima in the range 468–485 nm. The emission has been assigned as an overlapping of the individual emissions coming from the free stilbazole and the gold(I) compound.
Figure 26.
Supramolecular H-bond mesogens. R = Cl, C6F5, C6F4OC6H13, C6F4C6F4Br.
Figure 26.
Supramolecular H-bond mesogens. R = Cl, C6F5, C6F4OC6H13, C6F4C6F4Br.
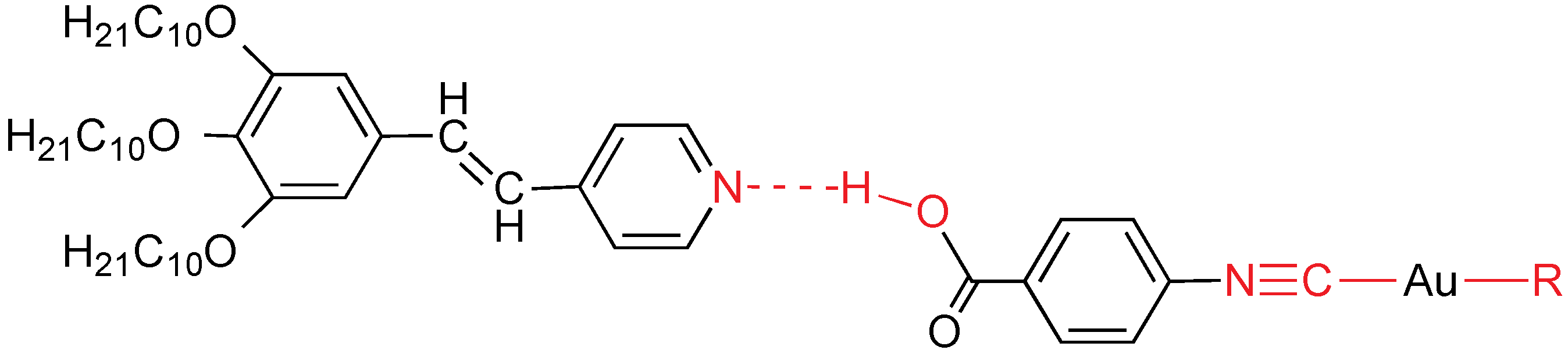
The use of trisalkoxystilbazoles is going to reduce the transition temperatures in order to facilitate more thermally stable metallomesogens. In that way, supramolecular metal complexes formed through hydrogen bonding between tris(3,4,5-decyloxy)stilbazole and several gold-organic acids of the type [Au
R(CNC
6H
4COOH)] have been synthesized (
Figure 27) [
46]. Surprisingly, the presence of three alkoxy chains is not enough to induce columnar mesophases, although it does for similar palladium and platinum compounds containing two isocyanides: [
cis-[
MCl
2(CNC
6H
4COOH)
2] and [
trans-[
MI
2(CNC
6H
4COOH)
2] (
M = Pd, Pt). Remarkably, the hydrogen bond in the gold adduct [Au(C
6F
4OC
10H
21)(CNC
6H
4COOH)] survives on the water surface giving rise to Langmuir films with the molecules arranged parallel to the water surface. The two supramolecular gold(I) adducts emit intensely at room temperature in the solid state, with emission maxima at 497 and 528 nm, respectively. The emission is related to the luminescent stilbazole modified by the H-bonds with the gold fragment.
Figure 27.
Supramolecular H-bond adducts. R = C6F5, C6F4OC10H21.
Figure 27.
Supramolecular H-bond adducts. R = C6F5, C6F4OC10H21.
2.7. Triazine Ligand
2,4,6-Triarylamino-1,3,5-triazines equipped by 6 or 9 peripheral alkoxy chains are discotic liquid crystals at room temperature. These compounds are combined with carboxylic acids to form H-bonded adducts, and self-organize into liquid crystalline columnar mesophases [
47,
48,
49]. It is important to note that only one acid enters, because of the steric requirements. The same strategy has been applied by integrating metal complexes containing carboxylic acid functions, to get reinforced H-bonds and more stable metallomesogens. Room temperature columnar mesophases are obtained in supramolecular aggregates from 9-alkoxy chains triazine and
para-isocyanobenzoic metal complexes, namely, [
M(CNC
6H
4CO
2H)(CO)
x] (
M = Fe,
x = 4;
M = Cr, Mo, W,
x = 5) [
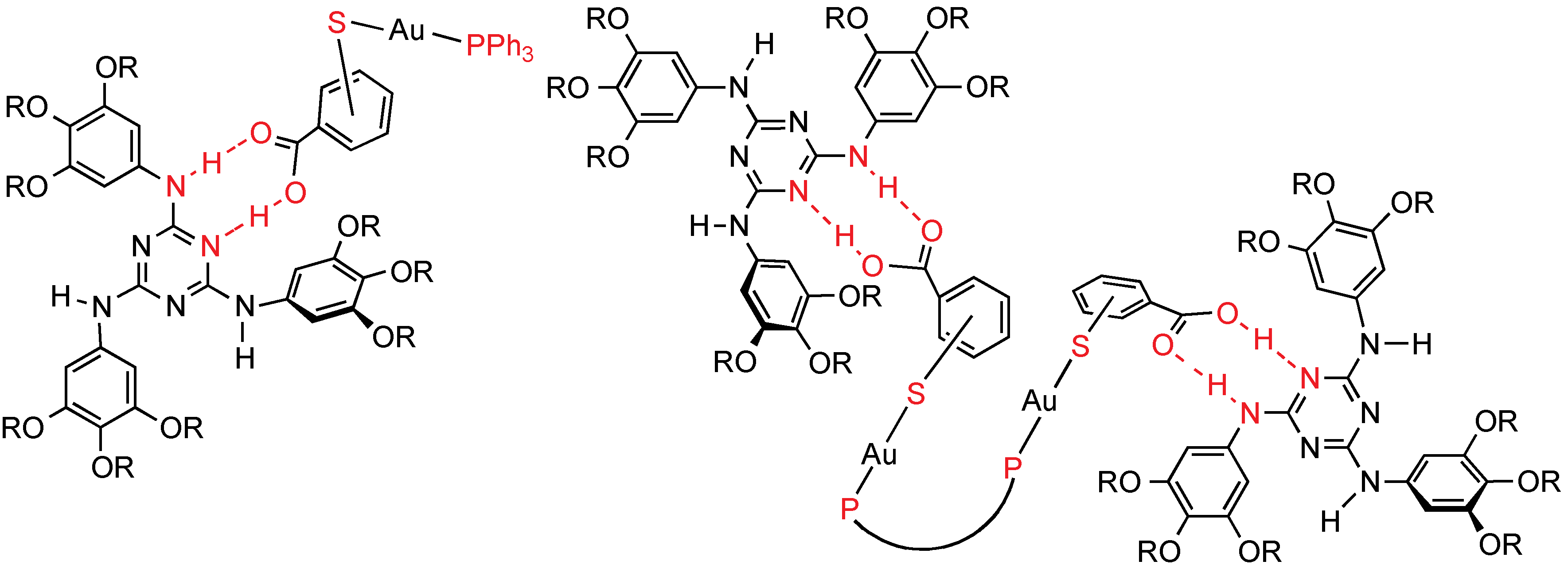
50]. The corresponding gold(I) isocyanobenzoic acid complexes does not produce such adducts, likely because the weakness of the H-bonding interactions with the triazine and the high insolubility of the starting gold complexes. Very recently, mono- and dinuclear thiolatobenzoic gold(I) adducts with the same triazine mesogen have been reported (
Figure 28) [
51]. The second ligand attached to the gold center is a large mono- or diphosphine (triphenylphosphine or BINAP: 2,2'-Bis(diphenylphosphino)-1,1'-binaphthalene). However, the supramolecular adducts also lead to a stable Col
hex mesophase at room temperature. The isotropic transition temperature is close or lower (0 to −14 °C) than for the free triazine (57 °C), while for the previous metallo-acids (Fe, Cr, Mo, and W) is higher. The diffractograms in the mesophase show a new broad peak, which can be related to scattering gold-gold distances from closest neighbor. It is remarkable that the hexagonal packing into columnar mesophases found for the free triazine was systematically preserved in the metal-containing supramolecular adducts. The triazine structure is able to swallow metal-organic fragments, even containing large non-mesomorphic ligands as phosphines.
Figure 28.
Supramolecular H-bond mesogens (ortho, para substitution). R: C10H21; P–P: R-BINAP, S-BINAP.
Figure 28.
Supramolecular H-bond mesogens (ortho, para substitution). R: C10H21; P–P: R-BINAP, S-BINAP.