Gold Thione Complexes
Abstract
:1. Introduction
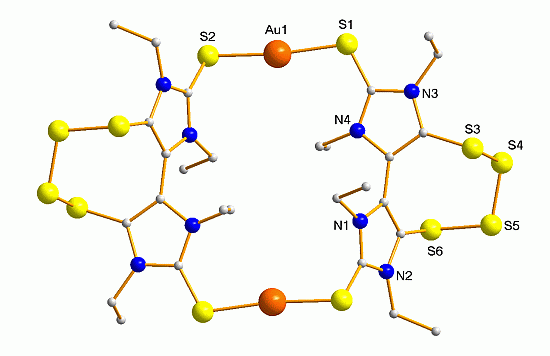
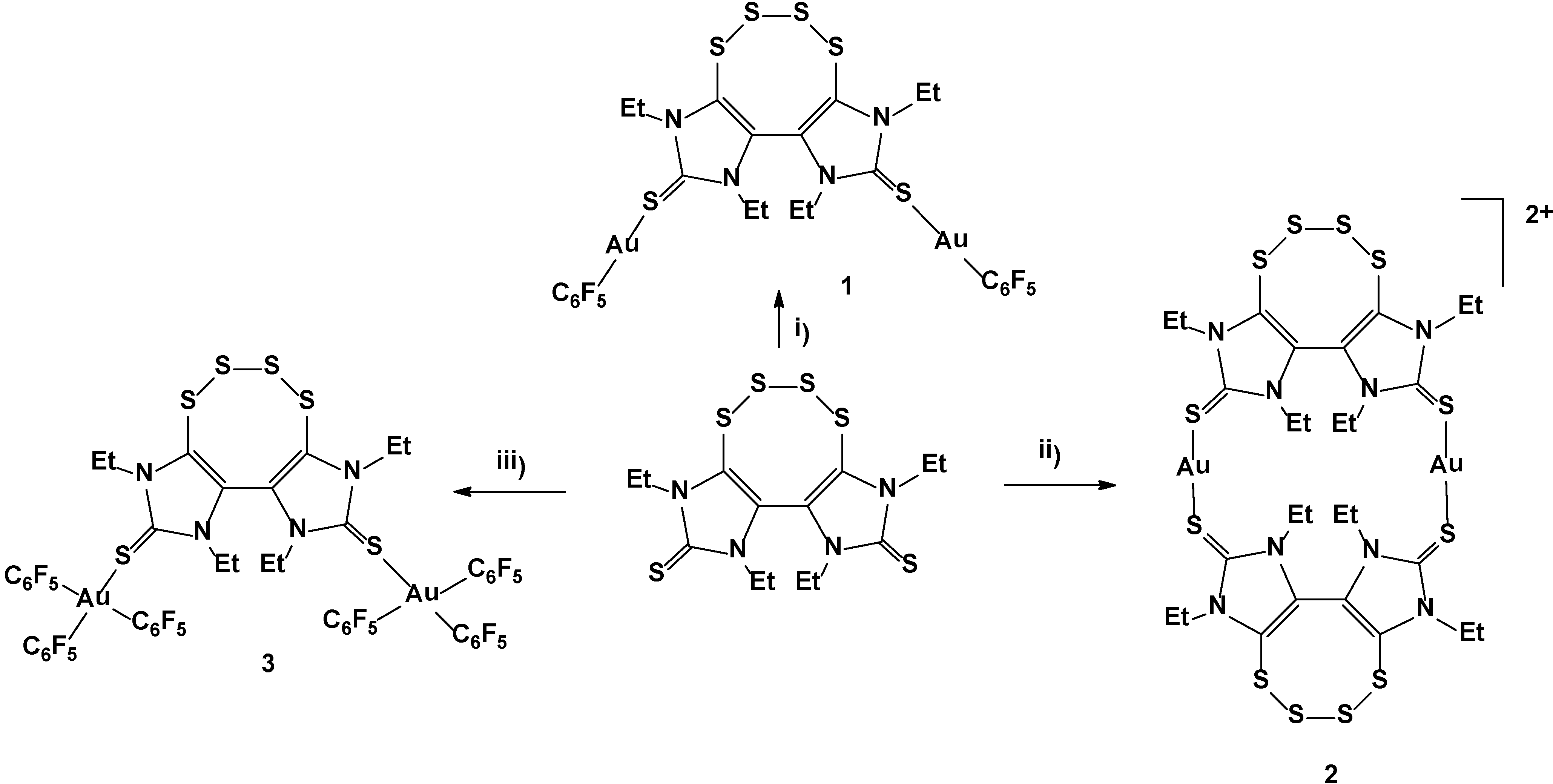
2. Results and Discussion
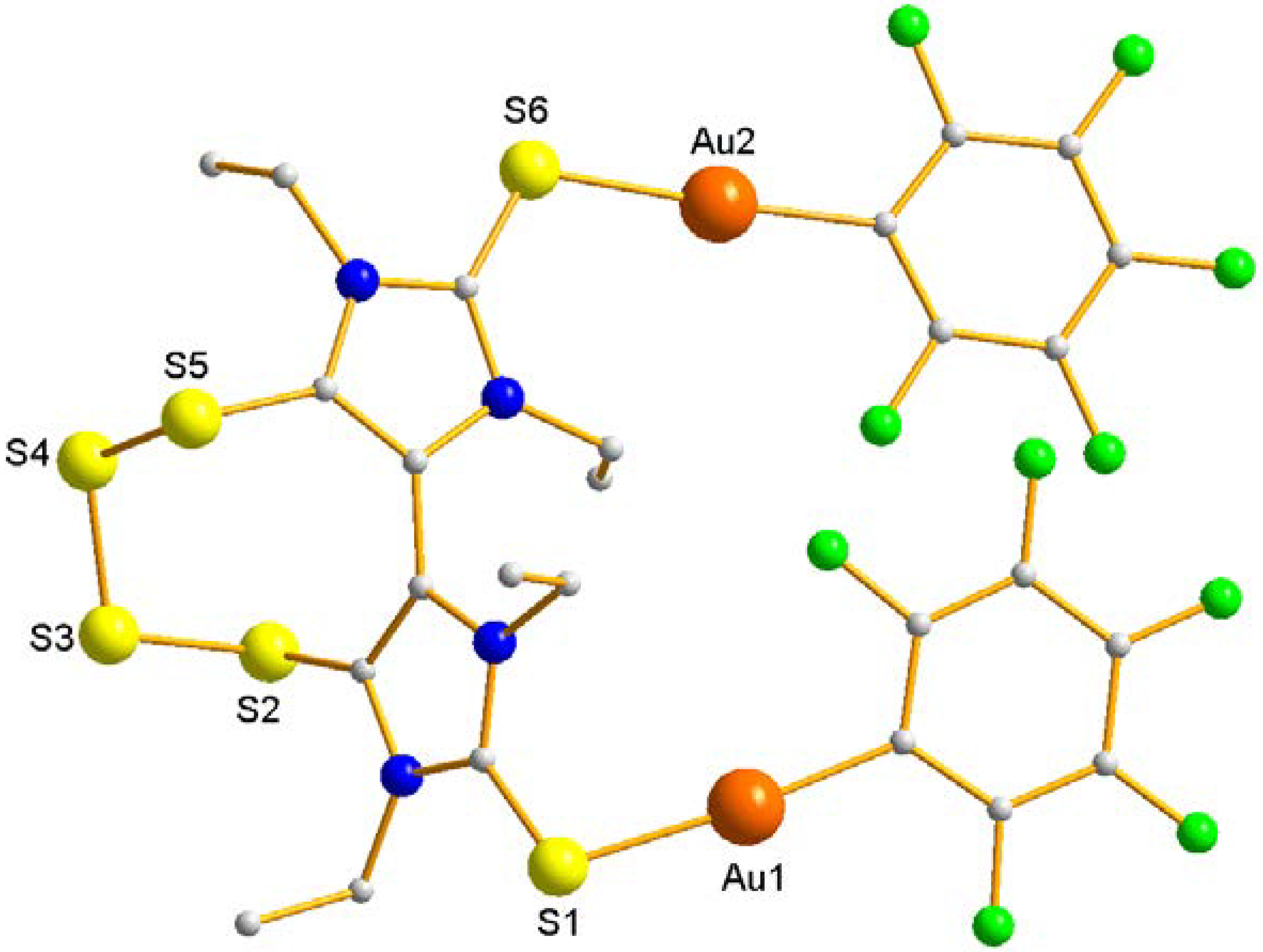
| Distances (Å) | Angles (°) | ||
|---|---|---|---|
| Au(1)–S(1) | 2.333(1) | S(1)–Au(1)–C(1) | 174.8(1) |
| Au(2)–S(6) | 2.309(1) | Au(1)–S(1)–C(7) | 97.8(1) |
| Au(2)–C(21) | 2.028(4) | Au(2)–S(6)–C(15) | 113.7(1) |
| Au(1)–C(1)–C(2) | 121.3(3) | ||
| Au(1)–C(1)–C(6) | 124.1(3) |
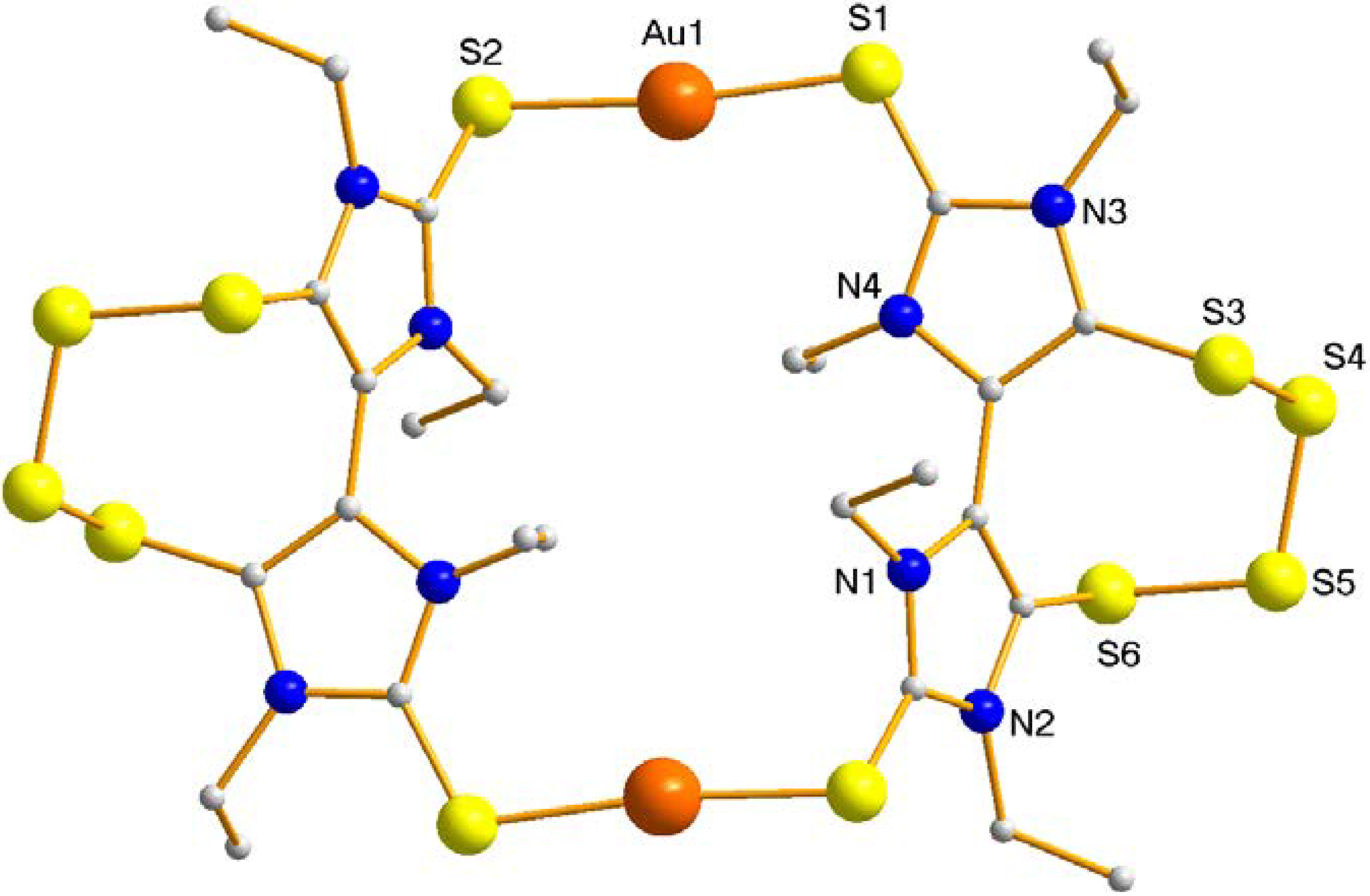
| Distances (Å) | Angles (°) | ||
|---|---|---|---|
| Au(1)–S(1) | 2.289(1) | S(1)–Au(1)–S(2) | 174.8(1) |
| S(2)–C(10) | 1.729(3) | Au(1)–S(1)–C(1) | 111.5(1) |
| S(2)–Au(1) | 2.294(1) | ||
| S(1)–C(1) | 1.709(2) |
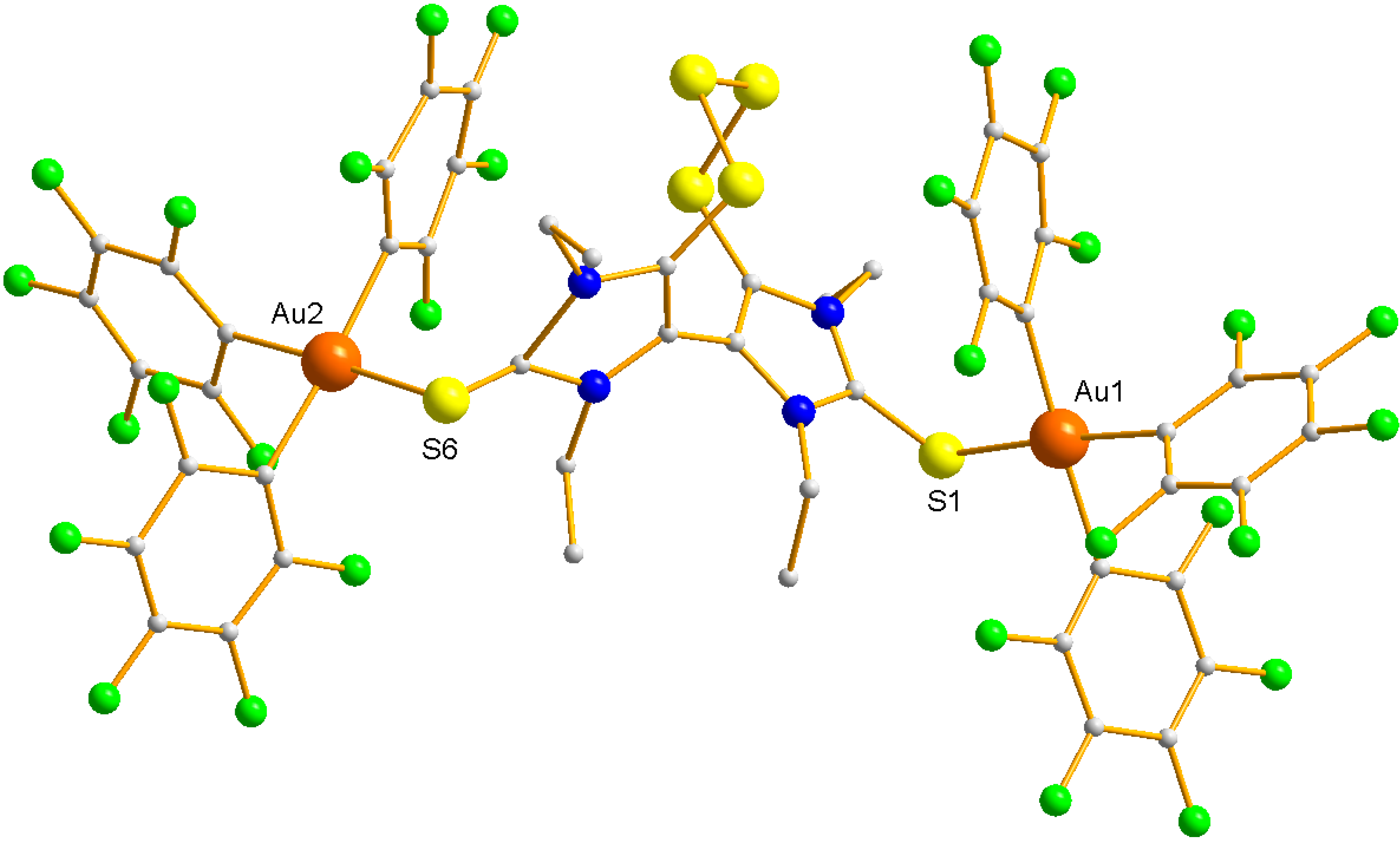
3. Experimental Section
3.1. Instrumentation
3.2. Starting Materials
3.3. General Procedure for the Synthesis of the Complexes 1–3
3.4. Cristallography
| Compound | 1 | 2·2CH2Cl2 |
|---|---|---|
| Chemical Formula | C26H20Au2F10N4S12 | C32H44Au2Cl4F6N8O6S14 |
| Appearance | Colorless plate | Colorless needle |
| Crystal size/mm | 0.42 × 0.23 × 0.06 | 0.20 × 0.08 × 0.06 |
| Crystal system | Triclinic | Triclinic |
| Space group | P-1 | P-1 |
| a/Å | 9.97740(10) | 9.7616(2) |
| b/Å | 12.1758(2) | 12.9096(3) |
| c/Å | 14.0916(2) | 13.0537(3) |
| α/° | 73.7730(10) | 63.202(2) |
| β/° | 82.3940(10) | 77.979(2) |
| γ/° | 88.3680(10) | 76.346(2) |
| U/Å3 | 1629.16(4) | 1416.97(5) |
| Z | 2 | 1 |
| Dc/g cm−3 | 2.374 | 2.034 |
| M | 1164.75 | 1735.33 |
| F(000) | 1096 | 844 |
| T/°C | −173 | −173 |
| 2θmax/° | 51 | 51 |
| µ(Mo-Kα)/mm−1 | 9.465 | 5.943 |
| Transmission | 0.6005, 0.1092 | 0.7169, 0.3828 |
| No. of reflections measured | 29873 | 27048 |
| No. of unique reflections | 6032 | 5254 |
| Rint | 0.038 | 0.018 |
| Ra (F > 4σ(F)) | 0.024 | 0.045 |
| wR2 (F2, all refl.) | 0.066 | 0.175 |
| No. of reflections used | 6032 | 5254 |
| No. of parameters | 437 | 329 |
| S | 1.059 | 1.041 |
| Max. ∆ρ/eÅ−3 | 1.77 | 0.90 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jia, W.G.; Huang, Y.B.; Lin, Y.J.; Wang, G.L.; Jin, G.-X. Nickel complexes and cobalt coordination polymers with organochalcogen (S, Se) ligands bearing an N-methylimidazole moiety: Syntheses, structures, and properties. Eur. J. Inorg. Chem. 2008, 4063–4073. [Google Scholar]
- Jia, W.G.; Huang, Y.B.; Lin, Y.J.; Jin, G.-X. Syntheses and structures of half-sandwich iridium(III) and rhodium(III) complexes with organochalcogen (S, Se) ligands bearing N-methylimidazole and their use as catalysts for norbornene polymerization. Dalton Trans. 2008, 5612–5620. [Google Scholar]
- Williams, D.J.; vanDerveer, D; Jones, R.L.; Menaldino, D.S. Main group metal halide complexes with sterically hindered thioureas XI. Complexes of antimony(III) and bismuth(III) chlorides with a new bidentate thiourea 1,1'-methylenebis(3-methyl-2H-imidazole-2-thione). Inorg. Chim. Acta 1989, 165, 173–178. [Google Scholar] [CrossRef]
- Bigoli, F.; Deplano, P.; Devillanova, F.A.; Lippolis, V.; Mercuri, M.L.; Pellinghelli, M.A.; Trogu, E.F. Synthesis, X-ray and spectroscopic characterization of [SnI2(mbit)2](I3)2·2/3I2 obtained throught the one-step reaction of mbit·2I2 with tin metal powder (mbit = 1,1'-bis(3-methyl-4-imidazoline-2-thione)methane). Inorg. Chim. Acta 1998, 267, 115–121. [Google Scholar] [CrossRef]
- Williams, D.J.; Shilatifard, A.; VanDerveer, D.; Lipscomb, L.A.; Jones, R.L. Main group metal halide complexes with sterically hindered thioureas XIII. Crystallographic study of a unique cross-linked polymeric dichlorolead(II) complex with 1,1'-methylenebis(3-methyl-2(3H)-imidazolethione). Inorg. Chim. Acta 1992, 202, 53–57. [Google Scholar] [CrossRef]
- Silva, R.M.; Smith, M.D.; Gardinier, J.R. Anion- and solvent-directed assembly in silver bis(thioimidazolyl)methane chemistry and the silver–sulfur interaction. Inorg. Chem. 2006, 45, 2132–2142. [Google Scholar] [CrossRef]
- Aroz, M.T.; Gimeno, M.C.; Kulcsar, M.; Laguna, A.; Lippolis, V. Group 11 complexes with imidazoline-2-thione or selone derivatives. Eur. J. Inorg. Chem. 2011, 2884–2894. [Google Scholar]
- Aragoni, M.C.; Arca, M.; Devillanova, F.A.; Isaia, F.; Lippolis, V.; Mancini, A.; Pala, L.; Slawin, A.M.Z.; Woollins, J.D. First example of an infinite polybromide 2D-network. Chem. Commun. 2003, 2226–2227. [Google Scholar]
- Mancini, A.; Aragoni, M.C.; Bricklebank, N.; Castellano, C.; Demartin, F.; Isaia, F.; Lippolis, V.; Pintus, A.; Arca, M. Formation of T-shaped versus charge-transfer molecular adducts in the reactions between bis(thiocarbonyl) donors and Br2 and I2. Chem. Asian J. 2013, 8, 639–647. [Google Scholar] [CrossRef]
- Mancini, A.; Aragoni, M.C.; Bingham, L.; Castellano, C.; Coles, S.L.; Demartin, F.; Hursthouse, M.B.; Isaia, F.; Lippolis, V.; Maninchedda, G.; et al. Reactivity of fluoro-substituted bis(thiocarbonyl) donors with diiodine: And XRD, FT-Raman, and DFT investigation. Chem. Asian J. 2013, 8, 2071–3078. [Google Scholar]
- Bigoli, F.; Pellinghelli, M.A.; Deplano, P.; Trogu, E.F. Complexes of 4,5,6,7-tetrathiocino[1,2-b:3,4-b']diimidazolyl-1,3,8,10-tetraethyl-2,9-dithione (Et4todit) with group IIb metal halides. Crystal and molecular structure of (Cd(II)Et4toditCl2)n. Inorg. Chim. Acta 1990, 170, 245–249. [Google Scholar] [CrossRef]
- Bigoli, F.; Pellinghelli, M.A.; Deplano, P.; Trogu, E.F. Preparation and characterization of polymeric compounds of copper(I) halogenides with Et4todit = 4,5,6,7-tetrathiocino[1,2-b:3,4-b']diimidazolyl-1,3,8,10-tetraethyl-2,9-dithione. Crystal and molecular structures of [Cu(I)(Et4todit)I]n and [Cu(I)(Et4todit)I]n·n/2Me2CO. Inorg. Chim. Acta 1991, 182, 33–39. [Google Scholar] [CrossRef]
- Bigoli, F.; Pellinghelli, A.; Atzei, D.; Deplano, P.; Trogu, E.F. Synthesis of some 4,5,6,7-tetrathiocino[1,2-b:3,4-b']diimidazolyl-1,3,8,10-tetrasubstituted-2,9-dithiones and crystal structure of the tetraethyl derivative. Phosphorus Sulfur 1988, 37, 189–194. [Google Scholar]
- Aragoni, M.C.; Arca, M.; Demartin, F.; Devillanova, F.A.; Garau, A.; Isaia, F.; Lelj, F.; Lippolis, V.; Verani, G. New [M(R,R'timdt)2] Metal-dithiolenes and related compounds (M = Ni, Pd, Pt; R,R'timdt = monoanion of disubstituted imidazolidine-2,4,5-trithiones): An Experimental and theoretical investigation. J. Am. Chem. Soc. 1991, 121, 7098–7107. [Google Scholar]
- Usón, R.; Laguna, A. Polyaryl Derivatives of gold(I), silver(I) and gold(III). In Organometallic Syntheses; King, R.B., Eisch, J.J., Eds.; Elsevier: Amsterdam, Holland, 1986; Volume 3, pp. 322–342. [Google Scholar]
- Usón, R.; Laguna, A.; Navarro, A.; Parish, R.V.; Moore, L.S. Synthesis and reactivity of perchlorate bis(tetrahydrothiophen)gold(I). 197Au Mössbauer spectra of three-coordinate gold(I) complexes. Inorg. Chim. Acta 1986, 112, 295–208. [Google Scholar]
- Usón, R.; Laguna, A.; Laguna, M.; Jiménez, J.; Gómez, M.P.; Sainz, A.; Jones, P.G. Gold complexes with heterocyclic thiones as ligands. X-ray structure determination of [Au(C5H5NS)2]ClO4. J. Chem. Soc. Dalton Trans. 1990, 3457–3463. [Google Scholar]
- CrysAlisPro, Version 1.171.35.11. Multi-scans absorption correction with SCALE3 ABSPACK scaling algorithm. Agilent Technologies: Waldbronn, Germany, 2011.
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- The Cambridge Crystallographic Data Centre. Available online: http://www.ccdc.cam.ac.uk/data_request/cif (accessed on 2 June 2014).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Caddeo, F.; Fernández-Moreira, V.; Arca, M.; Laguna, A.; Lippolis, V.; Gimeno, M.C. Gold Thione Complexes. Inorganics 2014, 2, 424-432. https://doi.org/10.3390/inorganics2030424
Caddeo F, Fernández-Moreira V, Arca M, Laguna A, Lippolis V, Gimeno MC. Gold Thione Complexes. Inorganics. 2014; 2(3):424-432. https://doi.org/10.3390/inorganics2030424
Chicago/Turabian StyleCaddeo, Francesco, Vanesa Fernández-Moreira, Massimiliano Arca, Antonio Laguna, Vito Lippolis, and M. Concepción Gimeno. 2014. "Gold Thione Complexes" Inorganics 2, no. 3: 424-432. https://doi.org/10.3390/inorganics2030424
APA StyleCaddeo, F., Fernández-Moreira, V., Arca, M., Laguna, A., Lippolis, V., & Gimeno, M. C. (2014). Gold Thione Complexes. Inorganics, 2(3), 424-432. https://doi.org/10.3390/inorganics2030424