SiO2 Electret Formation via Stamp-Assisted Capacitive Coupling: A Chemophysical Surface Functionalisation
Abstract
1. Introduction
2. Results and Discussion
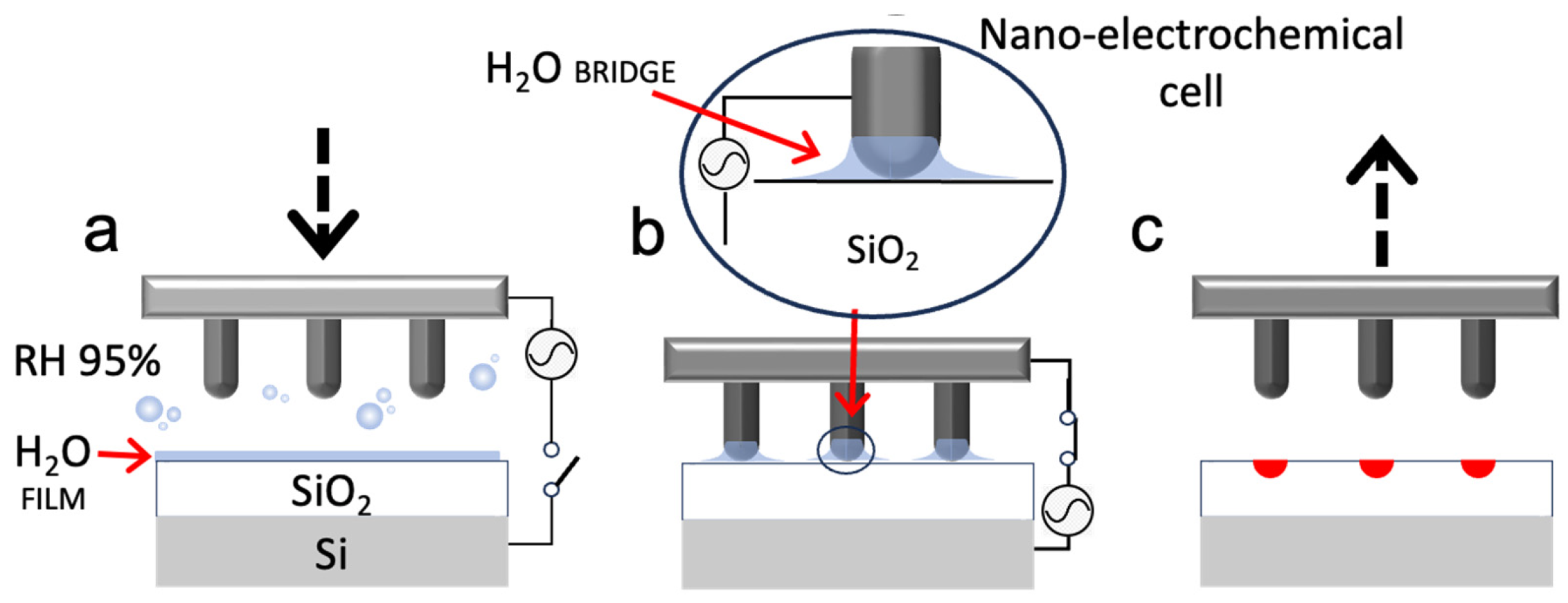
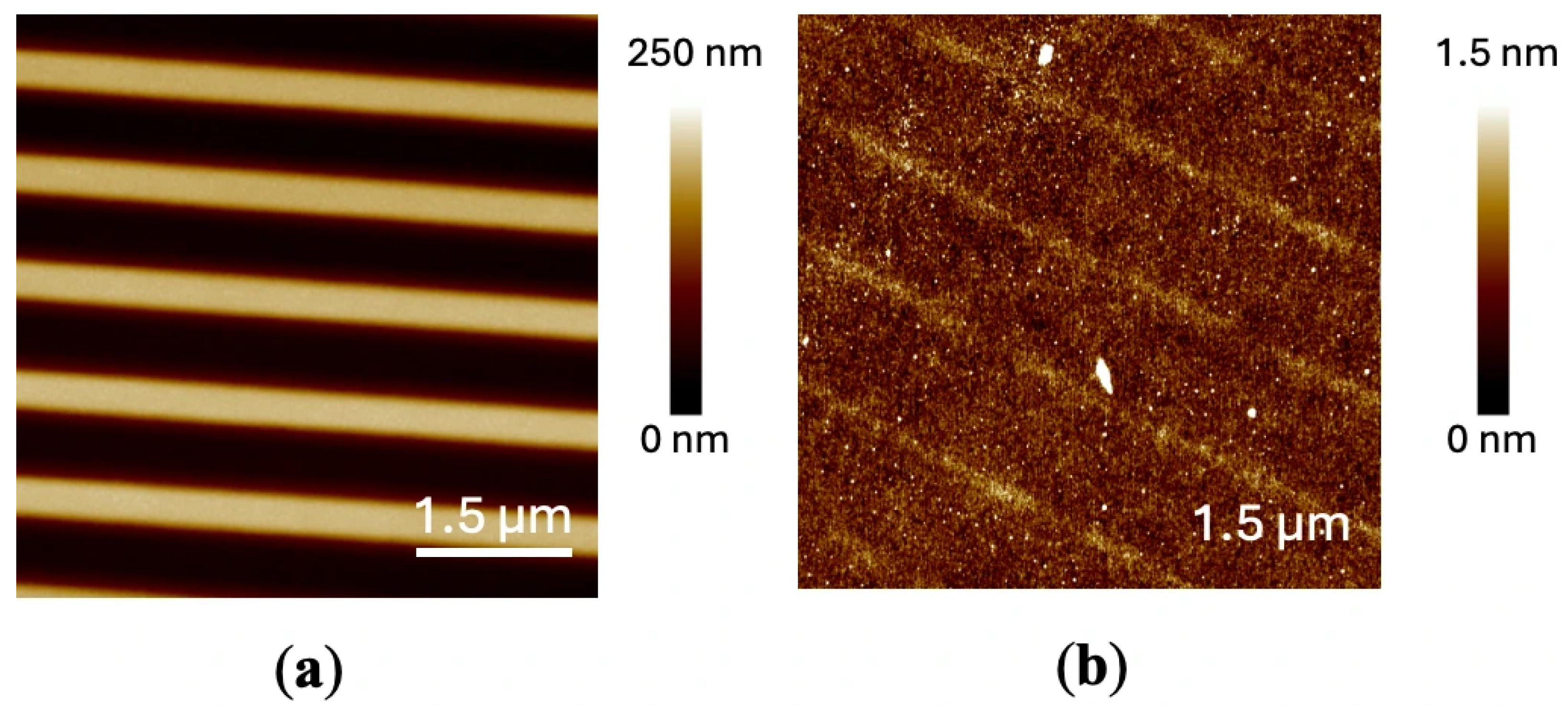
2.1. Process
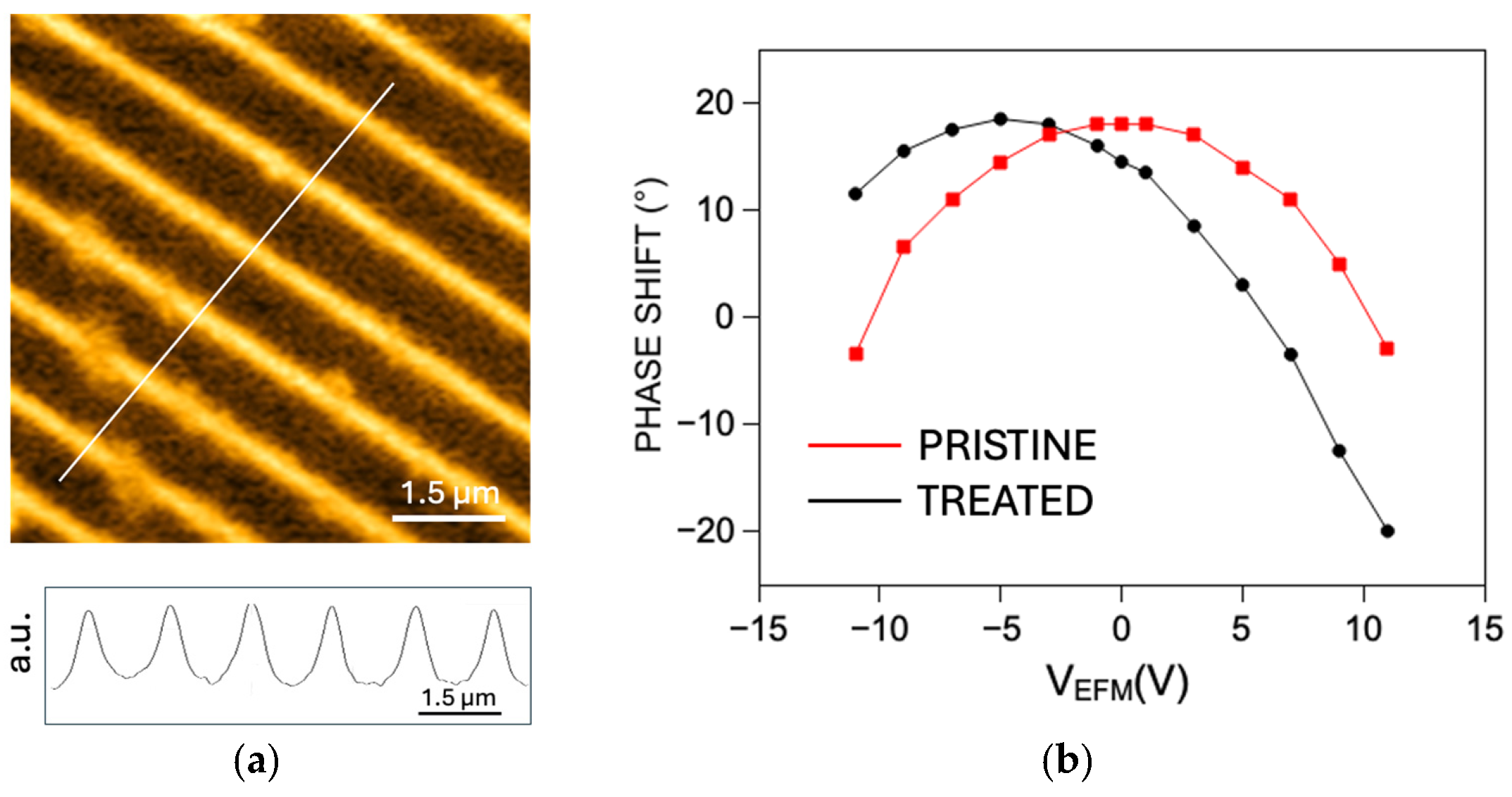
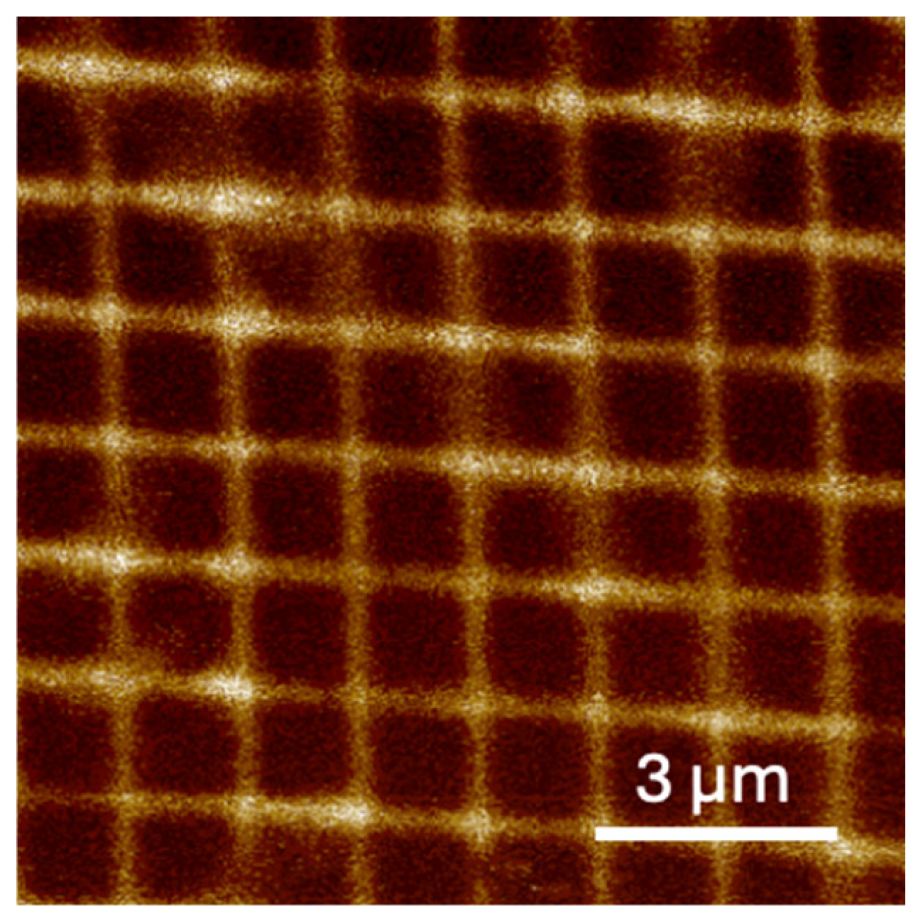
2.2. Electric Force Microscopy
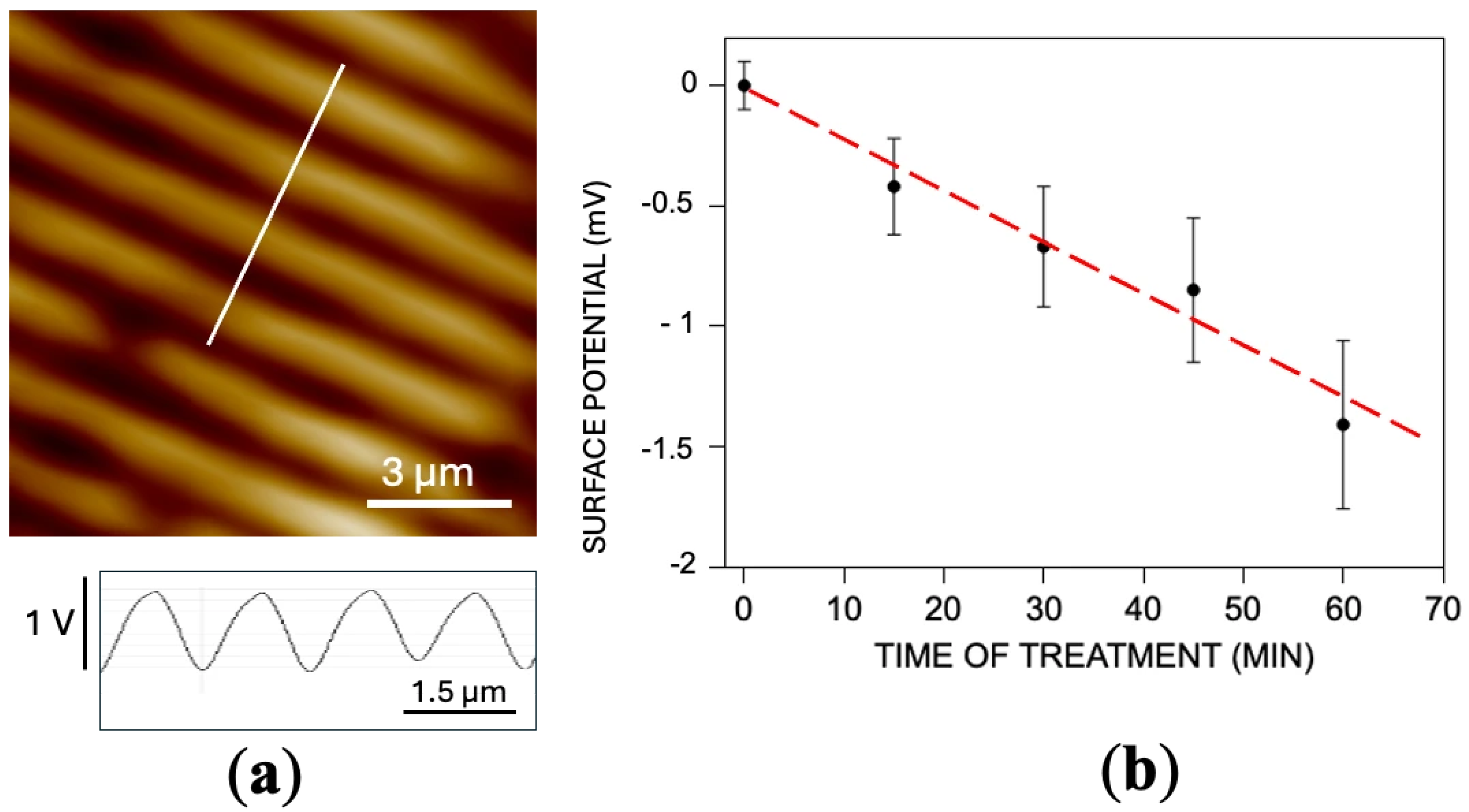
2.3. Kelvin Probe Force Microscopy
3. Materials and Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, F.; Li, H.Y.; Wang, Y.; Kuang, S.Y.; Fan, Y.J.; Wang, Z.L.; Zhu, G. Charge Distribution and Stability of SiO2 Nanoarray Electret. Chemnanomat 2020, 6, 212–217. [Google Scholar] [CrossRef]
- Ma, X.L.; Zhao, D.; Xue, M.Q.; Wang, H.; Cao, T.B. Selective Discharge of Electrostatic Charges on Electrets Using a Patterned Hydrogel Stamp. Angew. Chem. Int. Ed. 2010, 49, 5537–5540. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhao, D.; Tan, X.X.; Cao, T.B.; Zhang, X. AFM Force Mapping for Characterizing Patterns of Electrostatic Charges on SiO2 Electrets. Langmuir 2010, 26, 11958–11962. [Google Scholar] [CrossRef]
- Melucci, M.; Zambianchi, M.; Favaretto, L.; Palermo, V.; Treossi, E.; Montalti, M.; Bonacchi, S.; Cavallini, M. Multicolor, large-area fluorescence sensing through oligothiophene-self-assembled monolayers. Chem. Commun. 2011, 47, 1689–1691. [Google Scholar] [CrossRef] [PubMed]
- Gentili, D.; Sonar, P.; Liscio, F.; Cramer, T.; Ferlauto, L.; Leonardi, F.; Milita, S.; Dodabalapur, A.; Cavallini, M. Logic-gate devices based on printed polymer semiconducting nanostripes. Nano Lett. 2013, 13, 3643–3647. [Google Scholar] [CrossRef]
- Cavallini, M.; Hemmatian, Z.; Riminucci, A.; Prezioso, M.; Morandi, V.; Murgia, M. Regenerable Resistive Switching in Silicon Oxide Based Nanojunctions. Adv. Mater. 2012, 24, 1197–1201. [Google Scholar] [CrossRef]
- Leclère, P.; Surin, M.; Lazzaroni, R.; Kilbinger, A.F.M.; Henze, O.; Jonkheijm, P.; Biscarini, F.; Cavallini, M.; Feast, W.J.; Meijer, E.W.; et al. Surface-controlled self-assembly of chiral sexithiophenes. J. Mater. Chem. 2004, 14, 1959–1963. [Google Scholar] [CrossRef]
- Heaviside, O. Electromagnetic induction and its propagation. Electrician 1885, 15, 230–231. [Google Scholar]
- Gerhard, R. Dielectric materials for electro-active (electret) and/or electro-passive (insulation) applications. In Proceedings of the 2nd International Conference on Electrical Materials and Power Equipment, Guangzhou, China, 7–10 April 2019; pp. 91–96. [Google Scholar]
- Suzuki, Y. Recent progress in MEMS electret generator for energy harvesting. IEEJ Trans. Electr. Electron. Eng. 2011, 6, 101–111. [Google Scholar] [CrossRef]
- Kamogashira, T.; Yamasoba, T.; Kikuta, S.; Kondo, K. A Sleep Sensor Made with Electret Condenser Microphones. Clocks Sleep 2025, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, H.O.; Campbell, S.A.; Steward, M.G. Approaching nanoxerography: The use of electrostatic forces to position nanoparticles with 100 nm scale resolution. Adv. Mater. 2002, 14, 1553–1557. [Google Scholar] [CrossRef]
- Zimoch, L.; Schröder, S.; Elzenheimer, E.; Kaps, S.; Strunskus, T.; Faupel, F.; Höft, M.; Adelung, R. Electret integrated magnetic field sensor based on magnetostrictive polymer composite with nT resolution. Sci. Rep. 2025, 15, 1561. [Google Scholar] [CrossRef]
- Yasuda, T.; Komine, R.; Nojiri, R.; Takabe, Y.; Nara, K.; Kaneko, T.; Horigome, S.; Takeda, Y.; Wang, Y.; Kawaguchi, S.; et al. Ultra-Rapidly Responsive Electret-Based Flexible Pressure Sensor via Functional Polymeric Nanoparticle Synthesis. Adv. Funct. Mater. 2024, 34, 2402064. [Google Scholar] [CrossRef]
- Hu, L.; Li, X.; Guo, X.Y.; Xu, M.X.; Shi, Y.Q.; Herve, N.B.; Xiang, R.; Zhang, Q. Electret Modulation Strategy to Enhance the Photosensitivity Performance of Two-Dimensional Molybdenum Sulfide. ACS Appl. Mater. Interfaces 2023, 15, 59704–59713. [Google Scholar] [CrossRef]
- Xu, S.; Guan, X.; Bian, K.; Zhu, Q.; Dai, N.; Zhao, X.; Qiu, Y.; Zheng, S.; Dong, Y.; Zhong, J.; et al. Electret actuators enabling dual functions of optical ranging and audio feedback to elevate non-contact human-machine interactions. Nano Energy 2024, 125, 109553. [Google Scholar] [CrossRef]
- Mescheder, U.; Müller, B.; Baborie, S.; Urbanovic, P. Properties of SiO2 electret films charged by ion implantation for MEMS-based energy harvesting systems. J. Micromech. Microeng. 2009, 19, 094003. [Google Scholar] [CrossRef]
- Guo, Z.F.; Patil, Y.; Shinohara, A.; Nagura, K.; Yoshida, M.; Nakanishi, T. Organic molecular and polymeric electrets toward soft electronics. Mol. Syst. Des. Eng. 2022, 7, 537–552. [Google Scholar] [CrossRef]
- Bonilla, R.S.; Wilshaw, P.R. Potassium ions in SiO2: Electrets for silicon surface passivation. J. Phys. D Appl. Phys. 2017, 51, 025101. [Google Scholar] [CrossRef]
- Minami, T.; Utsubo, T.; Yamatani, T.; Miyata, T.; Ohbayashi, Y. SiO2 electret thin films prepared by various deposition methods. Thin Solid Films 2003, 426, 47–52. [Google Scholar] [CrossRef]
- Juberi, A.M.; Saha, P.C.; Faruqe, O.; Park, C. Partial Discharge Characteristics of SiO2/Si3N4 Electret Incorporated AlN Substrates. IEEE Trans. Transp. Electrif. 2024, 10, 7796–7803. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yang, C.H. Electret formation in transition metal oxides by electrochemical amorphization. NPG Asia Mater. 2020, 12, 1. [Google Scholar] [CrossRef]
- Zahmatkeshsaredorahi, A.; Millan-Solsona, R.; Jakob, D.S.; Collins, L.; Xu, X.G. Kelvin probe force microscopy under ambient conditions. Nat. Rev. Methods Prim. 2025, 5, 53. [Google Scholar] [CrossRef]
- Albonetti, C.; Chiodini, S.; Annibale, P.; Stoliar, P.; Martinez, R.V.; Garcia, R.; Biscarini, F. Quantitative phase-mode electrostatic force microscopy on silicon oxide nanostructures. J. Microsc. 2020, 280, 252–269. [Google Scholar] [CrossRef]
- Li, H.Y.; Ying, Z.; Lyu, B.S.; Deng, A.L.; Wang, L.L.; Taniguchi, T.; Watanabe, K.; Shi, Z.W. Electrode-Free Anodic Oxidation Nanolithography of Low-Dimensional Materials. Nano Lett. 2018, 18, 8011–8015. [Google Scholar] [CrossRef] [PubMed]
- Gentili, D.; Calabrese, G.; Lunedei, E.; Borgatti, F.; Mirshokraee, S.A.; Benekou, V.; Tseberlidis, G.; Mezzi, A.; Liscio, F.; Candini, A.; et al. Tuning Electronic and Functional Properties in Defected MoS2 Films by Surface Patterning of Sulphur Atomic Vacancies. Small Methods 2025, 9, 2401486. [Google Scholar] [CrossRef] [PubMed]
- Gentili, D.; Chini, E.; Cavallini, M. Generation and Tuning of Semiconductor Electronic and Functional Properties through Electrochemical Patterning. Acc. Mater. Res. 2025, 6, 1094–1104. [Google Scholar] [CrossRef]
- Qi, G.C.; Yan, H.; Guan, L.; Yang, Y.L.; Qiu, X.H.; Wang, C.; Li, Y.B.; Jiang, Y.P. Characteristic capacitance in an electric force microscope determined by using sample surface bias effect. J. Appl. Phys. 2008, 103, 114311. [Google Scholar] [CrossRef]
- Lui, C.H.; Liu, L.; Mak, K.F.; Flynn, G.W.; Heinz, T.F. Ultraflat graphene. Nature 2009, 462, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M.; Gentili, D. Atomic Vacancies in Transition Metal Dichalcogenides: Properties, Fabrication, and Limits. Chempluschem 2022, 87, e202100562. [Google Scholar] [CrossRef]
- Du, Y.; Shen, P.H.; Liu, H.F.; Zhang, Z.W.; Yang, F.Y.; Chu, D.P.; Ren, T.L.; Wang, Z.L.; Wei, D. Meta-structured electret heterointerface for resilient and adaptive tele-perception in embodied intelligence. Matter 2025, 8, 102363. [Google Scholar] [CrossRef]
- Gao, H.; Martin, L.S.; Hughes, L.B.; Leitao, N.T.; Put, P.; Zhou, H.; Koyluoglu, N.U.; Meynell, S.A.; Jayich, A.C.B.; Park, H.; et al. Signal amplification in a solid-state sensor through asymmetric many-body echo. Nature 2025, 646, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.T.; Chun, J.K.M.; Bocarsly, A.B. A silicon sensor for SO2. Nature 1996, 382, 214–215. [Google Scholar] [CrossRef]
- Du, Y.; Shen, P.; Liu, H.; Zhang, Y.; Jia, L.; Pu, X.; Yang, F.; Ren, T.; Chu, D.; Wang, Z.; et al. Multi-receptor skin with highly sensitive tele-perception somatosensory. Sci. Adv. 2024, 10, eadp8681. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Chini, E.; Gentili, D.; Liscio, A.; Cavallini, M. SiO2 Electret Formation via Stamp-Assisted Capacitive Coupling: A Chemophysical Surface Functionalisation. Inorganics 2026, 14, 21. https://doi.org/10.3390/inorganics14010021
Chini E, Gentili D, Liscio A, Cavallini M. SiO2 Electret Formation via Stamp-Assisted Capacitive Coupling: A Chemophysical Surface Functionalisation. Inorganics. 2026; 14(1):21. https://doi.org/10.3390/inorganics14010021
Chicago/Turabian StyleChini, Edoardo, Denis Gentili, Andrea Liscio, and Massimiliano Cavallini. 2026. "SiO2 Electret Formation via Stamp-Assisted Capacitive Coupling: A Chemophysical Surface Functionalisation" Inorganics 14, no. 1: 21. https://doi.org/10.3390/inorganics14010021
APA StyleChini, E., Gentili, D., Liscio, A., & Cavallini, M. (2026). SiO2 Electret Formation via Stamp-Assisted Capacitive Coupling: A Chemophysical Surface Functionalisation. Inorganics, 14(1), 21. https://doi.org/10.3390/inorganics14010021









