Lanthanide Tris-Acetylacetonate Complexes for Luminescent Thermometry: From Isolated Compounds to Hybrid Prussian Blue Core–Silica Shell Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
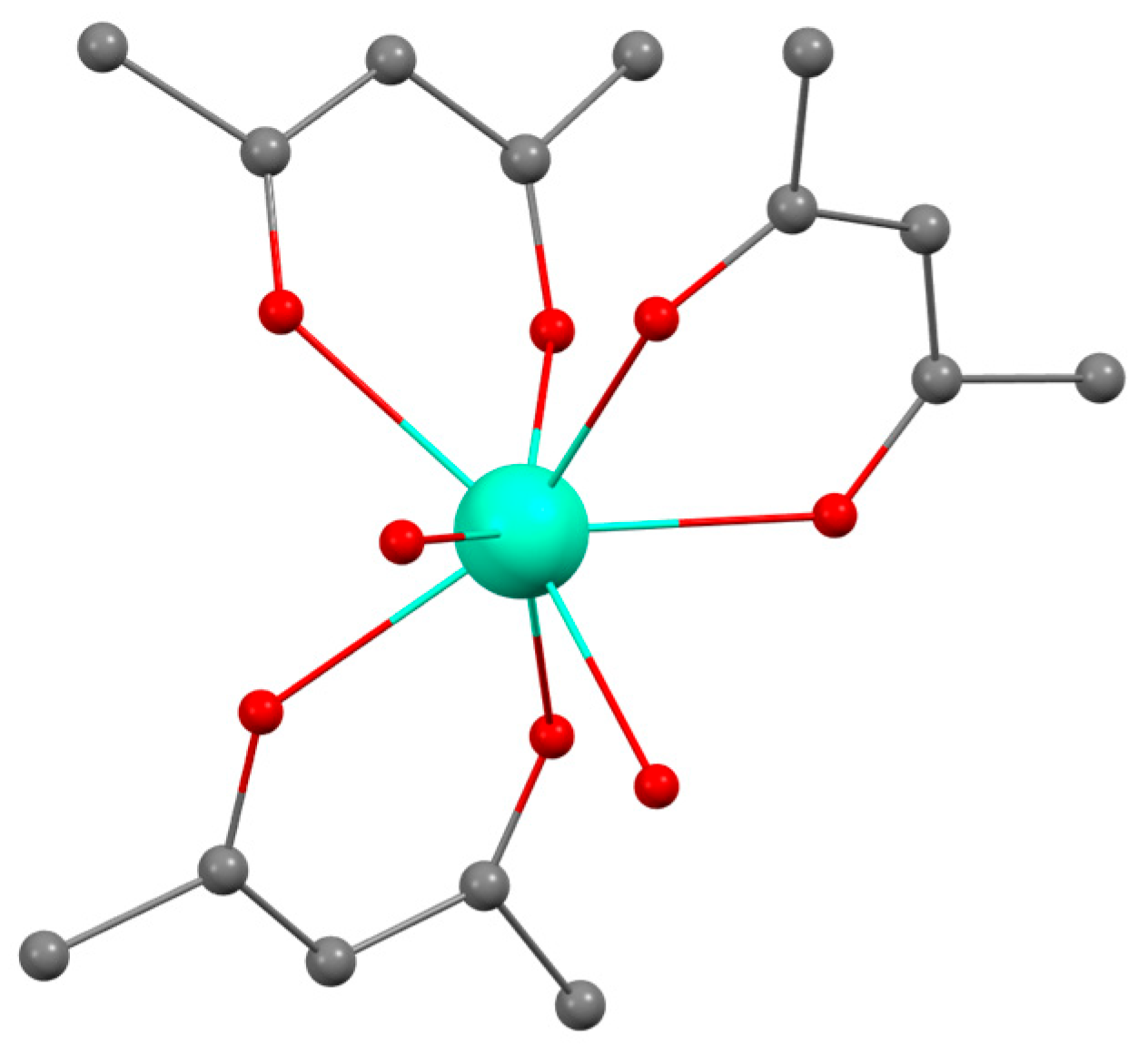
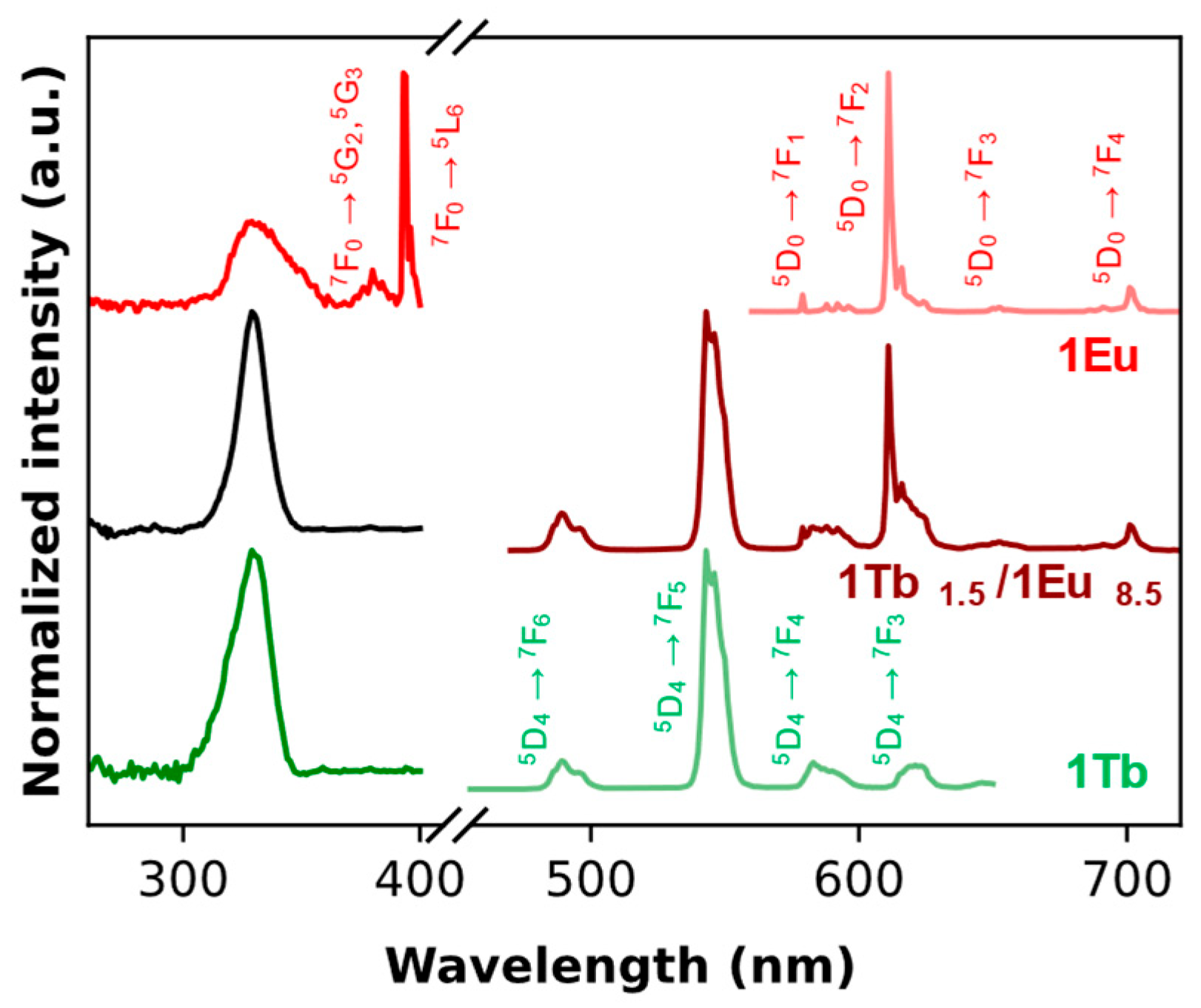
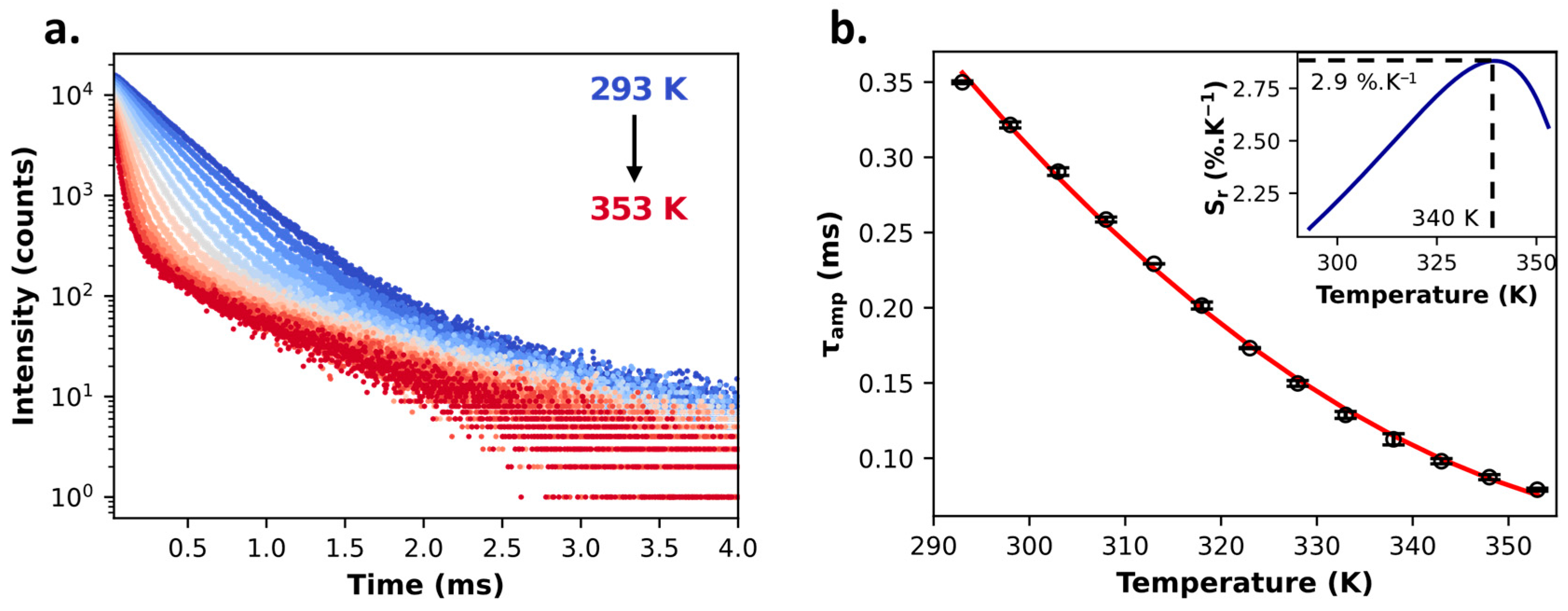
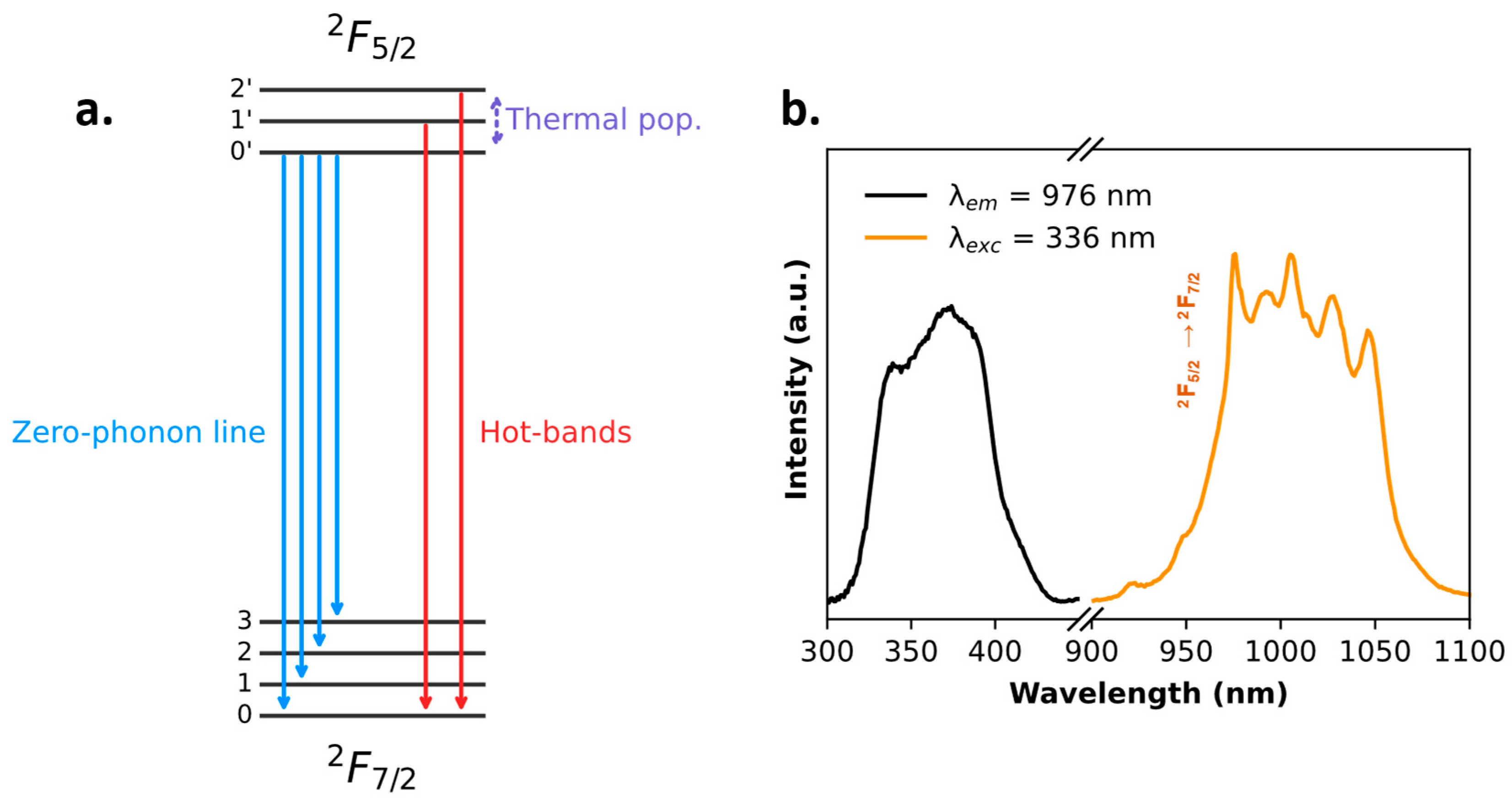
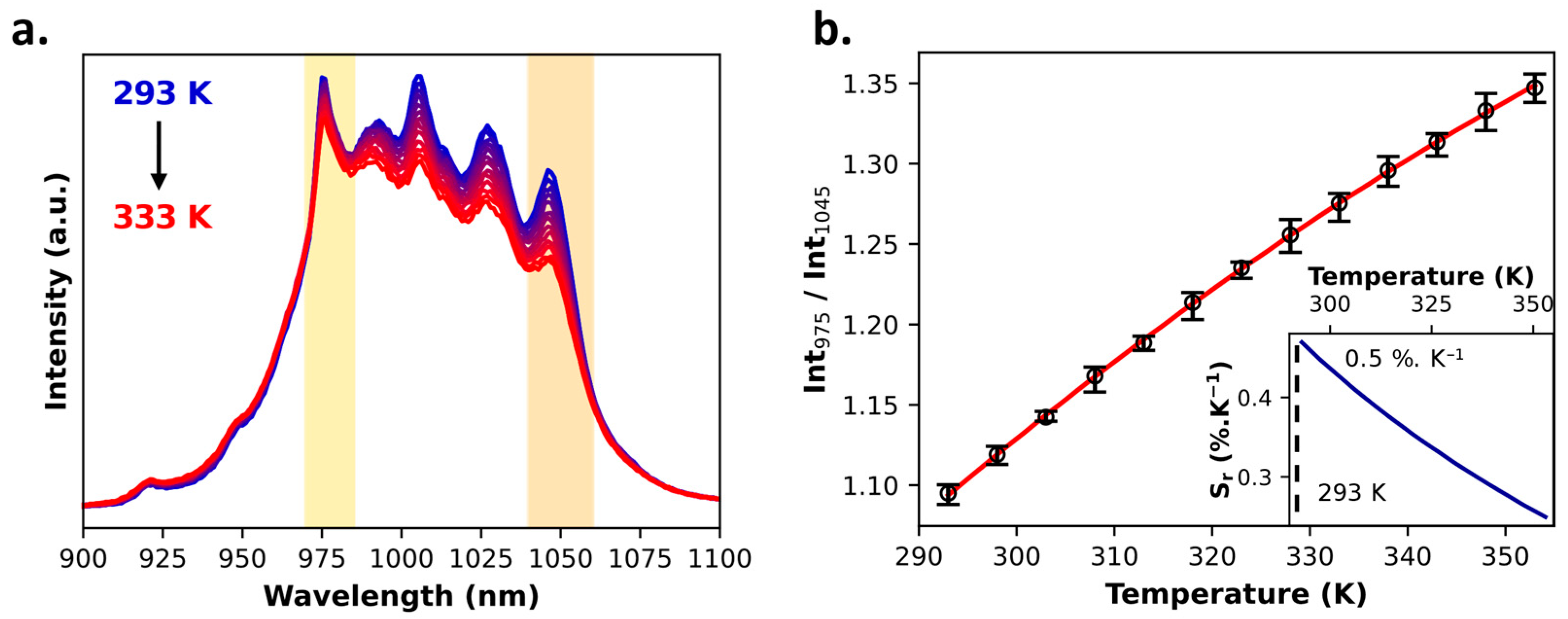
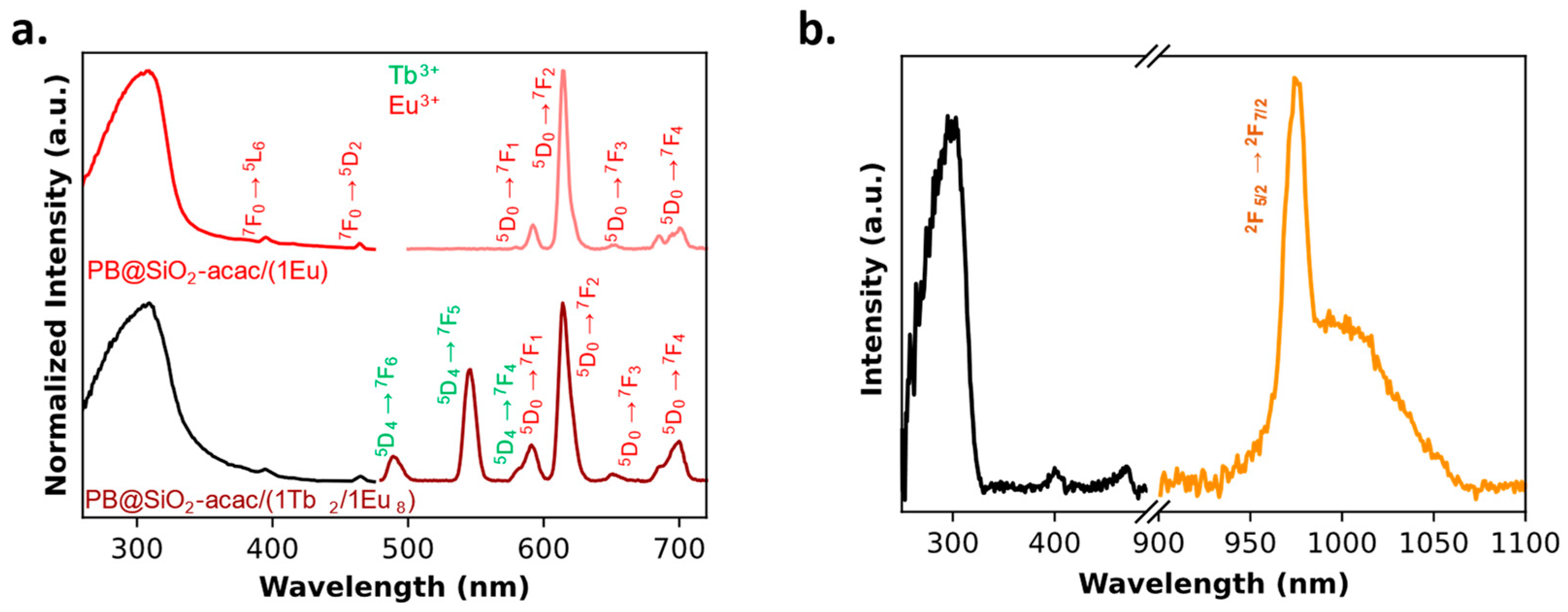
2.1. Tris-Acetylacetonate Ln3+ Compounds as Luminescent Thermometers
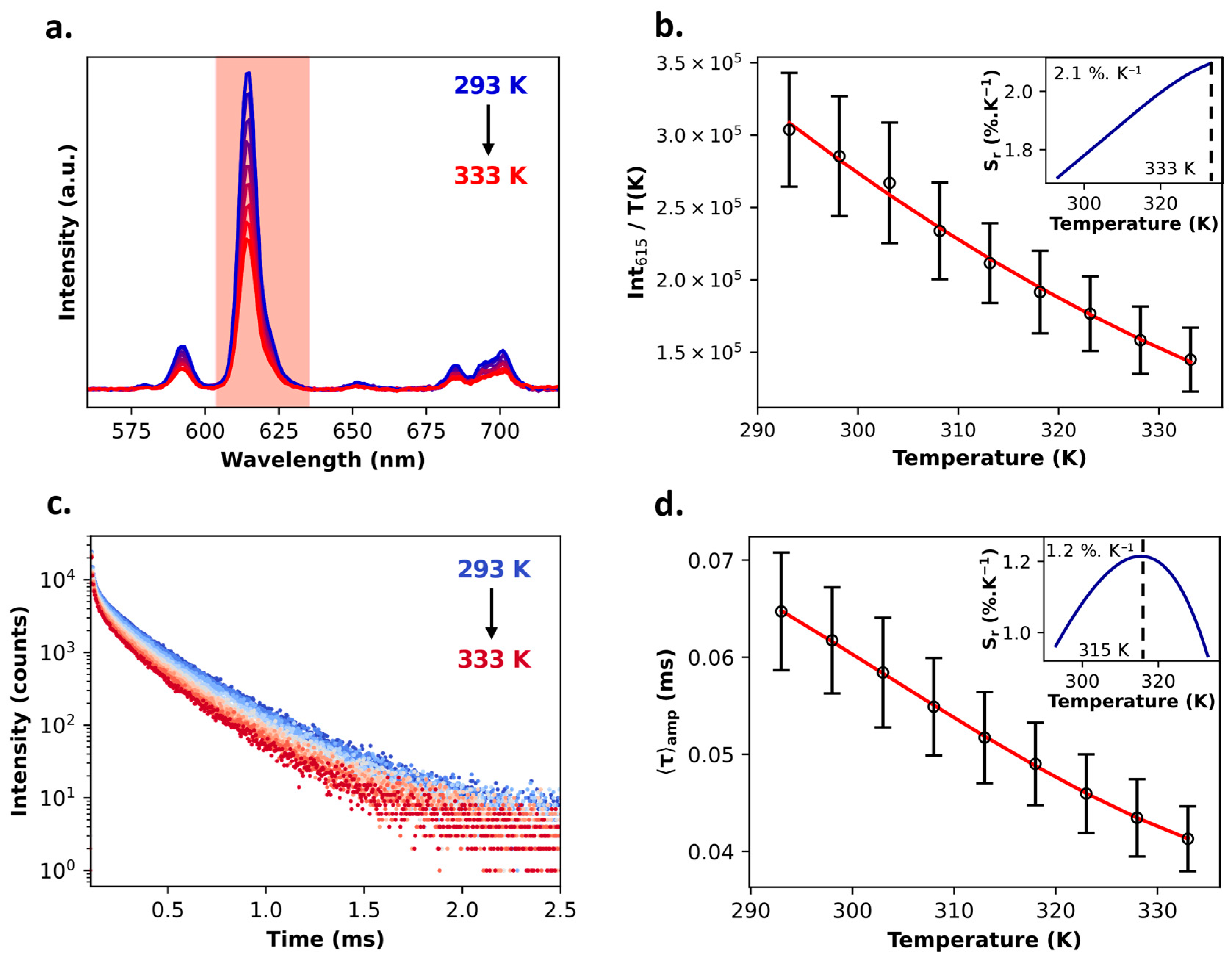
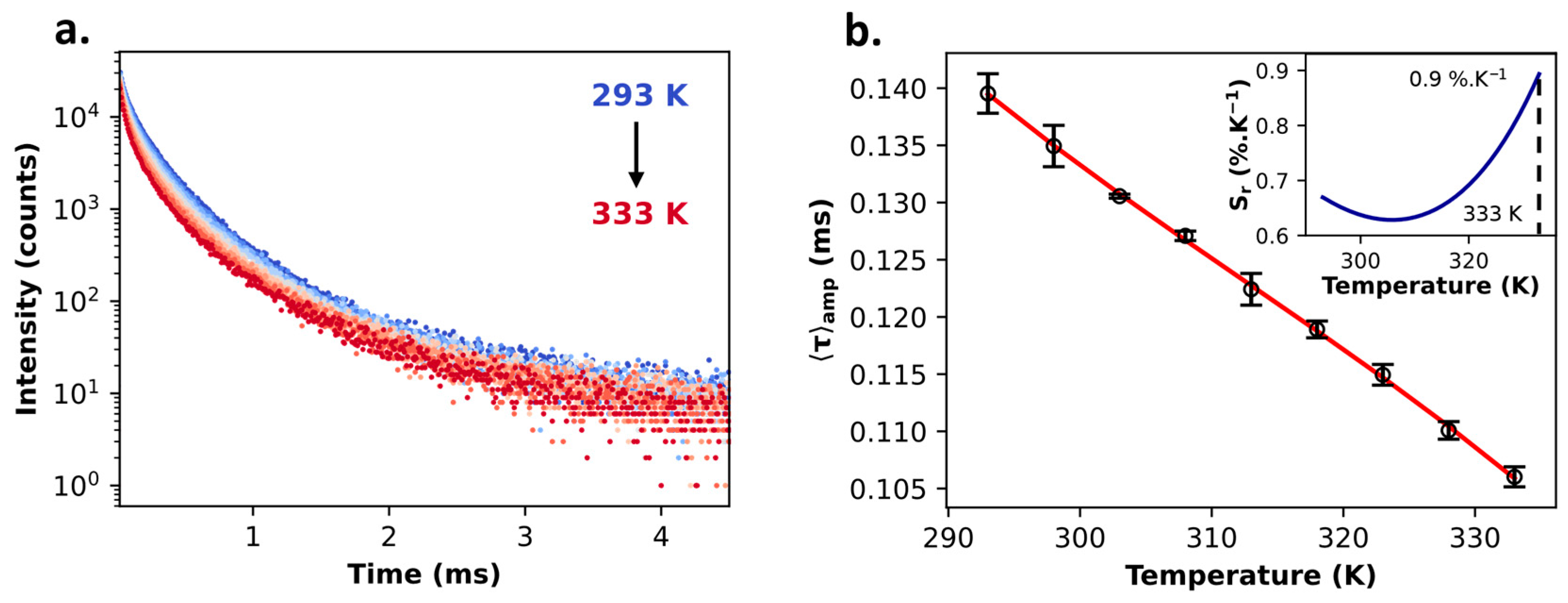
2.2. Multifunctional PB@SiO2 Core@Shell Nanoparticles Loaded by Luminescent Thermometers
3. Materials and Methods
3.1. Syntheses
- Synthesis of 3-[3-(Triethoxysilyl)propyl]pentane-2,4-dione)] (acac-Si) [75]. The synthesis of acac-Si was performed in a two-step procedure. First, 3-iodopropyl)triethoxysilane was synthesized. For this, NaI was dissolved in 500 mL of acetone and (3-chloropropyl)triethoxysilane was added. The mixture was refluxed at 66 °C for 72 h and then the temperature was decreased to room temperature and the mixture was concentrated under reduced pressure. The residue was extracted in pentane, filtered, and concentrated again. Distillation under reduced pressure (vacuum line) gave (3-iodopropyl)triethoxysilane (Tvap = 78–82 °C, Tbath = 100–150 °C). Secondly, tBuOK and tBuOH were placed in a 250 mL two-necked flask with a bubbler, followed by acac. After 10 min, the previously obtained (3-iodopropyl)triethoxysilane was added, and the mixture was heated at 90 °C overnight. The solvent was removed under high vacuum, and the residue was dissolved in pentane, filtered, and concentrated. Distillation under reduced pressure (≈0.01 mbar, heat-on block) afforded the product, collected at Tvap = 87–91 °C (heat-on 125–150 °C). The first fraction distilled at Tvap = 60–65 °C (heat-on 90–120 °C).
- Synthesis of core@shell PB@SiO2-acac nanoparticles. Pristine cubic PB nanoparticles were synthesized by a previously reported co-precipitation method [64]. For this purpose, aqueous solutions of FeCl3·6H2O (10.00 mM, 50 mL) and Na4[Fe(CN)6]·10H2O (11.25 mM, 50 mL) were added to 100 mL of ultrapure water using a peristaltic pump at a constant flow rate of 4 mL·h−1 during 10 h under ambient temperature (25 °C). After complete addition, the dispersion was centrifuged at 37,565 rcf for 10 min to collect the nanoparticles. The pellet was washed three times with ultrapure water and redispersed in water for storage. The nanoparticles were coated with a mesoporous silica layer following a modified procedure from the literature [76]. A surfactant solution was first prepared by dissolving 700 mg of cetyltrimethylammonium bromide (CTAB) in a mixture of 75 mL ethanol (96%) and 450 mL ultrapure water, stirred overnight at 35 °C (700 rpm). Then, 80 mg of PB nanoparticles was added, followed by 2 mL of tetraethyl orthosilicate (TEOS) and 250 µL of 30% aqueous ammonia. The mixture was stirred for 2 h at 35 °C and then for another 2 h at 80 °C to promote the growth of the silica shell. The resulting PB@SiO2 particles were isolated by centrifugation (37,565 rcf, 10 min). Surfactant removal was performed by two extraction cycles using a 6 g·L−1 NH4NO3 solution in ethanol, with sonication (30 min) followed by centrifugation (37,565 rcf, 10 min). The final purification involved three cycles of dispersion in water and centrifugation at low speed (6000 rcf, 15 min) to eliminate free silica nanoparticles. The purified PB@SiO2 particles were stored in water. The silica-coated nanoparticles were subsequently functionalized with acetylacetonates. For this, 20 mg of PB@SiO2 particles was dispersed in a mixture of 8 mL ethanol and 80 mL toluene. Then, 200 µL of acac–Si (0.62 mmol) was added and the suspension was refluxed at 110 °C under magnetic stirring (700 rpm) overnight. The nanoparticles were collected by centrifugation (37,565 rcf, 10 min) and washed three times with ethanol.
- Characterization of pristine PB nanoparticles: IR (KBr): ν(O–H) = 3635 cm−1 (coordinated water), 3390 cm−1 (crystallized water), δ(O–H) = 1607 cm−1, ν(C≡N) = 2088 cm−1 (Fe3+–C≡N–Fe2+), ν(Fe2+–CN) = 605 cm−1, δ(Fe2+–CN) = 503 cm−1. EDS: Na/Fe atomic ratio of 16/84. Empirical formula: Na0.35Fe3+ [Fe2+ (CN)6]0.84·xH2O. Size (TEM): 95 ± 12 nm.
- Characterization of core@shell PB@SiO2 nanoparticles: IR (KBr): ν(C–H) = 2800–3000 cm−1 (residual CTAB), ν(C≡N) = 2090 cm−1 (Fe3+–C≡N–Fe2+), δ(H–O–H) = 1603 cm−1, δ(CH2, CH3) = 1415 cm−1 (CTAB), ν(Si–O–Si) = 800–1090 cm−1 (SiO2), ν(Fe2+–CN) = 605 cm−1, δ(Fe2+–CN) = 503 cm−1, δ(Si–O–Si) = 475 cm−1. EDS: Si/Fe atomic ratio = 65/35. Size (TEM): 125 ± 15 nm.
- Characterization of PB@SiO2–acac: IR (KBr): νas(CH2) = 2960 cm−1 and νs(CH2) = 2870–2840 cm−1 (acac, residual CTAB), ν(C≡N) = 2090 cm−1 (Fe3+–C≡N–Fe2+), ν(C=O) = 1710 cm−1 (acac, keto form), δ(H–O–H) = 1603 cm−1, ν(C=C) = 1527 cm−1 (acac), δas(CH3) and δ(CH2)= 1455 cm−1, δs(CH2) = 1415 cm−1, δs(CH3) = 1377cm−1, ν(Si–O–Si) = 800–1090 cm−1, ν(Fe2+–CN) = 605 cm−1, δ(Fe2+–CN) = 503 cm−1, δ(Si–O–Si) = 475 cm−1. EDS: Si/Fe atomic ratio = 65/35. Size (TEM): 138 ± 16 nm. The IR spectra for acac-Si, PB@SiO2 and PB@SiO2-acac are shown in Figure S11, ESI.
- Loading of PB@SiO2-acac nanoparticles with the lanthanide complexes. The encapsulation of the Ln(acac)3(H2O)2 complexes (where Ln = Eu3+, Tb3+/Eu3+, or Yb3+) was achieved by adding 0.27 mmol of the complex (for Eu3+, Yb3+) or the mixture of Tb3+ and Eu3+ complexes with the ratio 1Tb/1Eu = 1.5/8.5 to 15 mg of PB@SiO2-acac nanoparticles dispersed in 80 mL of EtOH. The mixture was stirred under reflux at 80 °C for 2 h. The resulting nanoparticles were then washed twice with EtOH (37,565 rcf, 10 min) and dried at 60 °C for 24 h.
- Characterizations of PB@SiO2–acac/(1Eu): IR (KBr): νas(CH2) = 2960 cm−1 and νs(CH2) = 2870–2840 cm−1 (acac, residual CTAB), ν(C≡N) = 2090 cm−1 (Fe3+–C≡N–Fe2+), ν(C=O) = 1606 cm−1 (acac, enol form), ν(C=C) = 1527 cm−1 (acac), δas(CH3) = 1485 cm−1, δs(CH3) = 1385 cm−1, ν(Si–O–Si) = 800–1090 cm−1, ν(Fe2+–CN) = 605 cm−1, δ(Fe2+–CN) = 503 cm−1, δ(Si–O–Si) = 475 cm−1.
- Characterizations of PB@SiO2–acac/(1Tb2/1Eu8): IR (KBr): νas(CH2) = 2960 cm−1 and νs(CH2) = 2870–2840 cm−1 (acac, residual CTAB), ν(C≡N) = 2090 cm−1 (Fe3+–C≡N–Fe2+), ν(C=O) = 1606 cm−1 (acac, enol form), ν(C=C) = 1527 cm−1 (acac), δas(CH3) = 1485 cm−1, δs(CH3) = 1385 cm−1, ν(Si–O–Si) = 800–1090 cm−1, ν(Fe2+–CN) = 605 cm−1, δ(Fe2+–CN) = 503 cm−1, δ(Si–O–Si) = 475 cm−1. EDS: Si/Fe/Tb/Eu = 43/36/4/17; Tb/Eu = 22/78 Size (TEM): 136 ± 17 nm.
- Characterizations of PB@SiO2–acac/(1Yb): IR (KBr): νas(CH2) = 2960 cm−1 and νs(CH2) = 2870–2840 cm−1 (acac, residual CTAB), ν(C≡N) = 2090 cm−1 (Fe3+–C≡N–Fe2+), ν(C=O) = 1606 cm−1 (acac, enol form), ν(C=C) = 1527 cm−1 (acac), δas(CH3) = 1485cm−1, δs(CH3) = 1360 cm−1, ν(Si–O–Si) = 800–1090 cm−1, ν(Fe2+–CN) = 605 cm−1, δ(Fe2+–CN) = 503 cm−1, δ(Si–O–Si) = 475 cm−1.
3.2. Characterizations
3.3. Photoluminescence Studies and Thermometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dramićanin, M.D. Trends in Luminescence Thermometry. J. Appl. Phys. 2020, 128, 040902. [Google Scholar] [CrossRef]
- Quintanilla, M.; Henriksen-Lacey, M.; Renero-Lecuna, C.; Liz-Marzán, L.M. Challenges for Optical Nanothermometry in Biological Environments. Chem. Soc. Rev. 2022, 51, 4223–4242. [Google Scholar] [CrossRef]
- Jaque, D.; Rosal, B.D.; Rodríguez, E.M.; Maestro, L.M.; Haro-González, P.; Solé, J.G. Fluorescent Nanothermometers for Intracellular Thermal Sensing. Nanomedicine 2014, 9, 1047–1062. [Google Scholar] [CrossRef]
- Ansari, A.A.; Parchur, A.K.; Nazeeruddin, M.K.; Tavakoli, M.M. Luminescent Lanthanide Nanocomposites in Thermometry: Chemistry of Dopant Ions and Host Matrices. Coord. Chem. Rev. 2021, 444, 214040. [Google Scholar] [CrossRef]
- Qiu, X.; Zhou, Q.; Zhu, X.; Wu, Z.; Feng, W.; Li, F. Ratiometric Upconversion Nanothermometry with Dual Emission at the Same Wavelength Decoded via a Time-Resolved Technique. Nat. Commun. 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Savchuk, O.A.; Haro-González, P.; Carvajal, J.J.; Jaque, D.; Massons, J.; Aguiló, M.; Díaz, F. Er:Yb:NaY2F5O up-Converting Nanoparticles for Sub-Tissue Fluorescence Lifetime Thermal Sensing. Nanoscale 2014, 6, 9727–9733. [Google Scholar] [CrossRef]
- Bettinelli, M.; Carlos, L.; Liu, X. Lanthanide-Doped Upconversion Nanoparticles. Phys. Today 2015, 68, 38–44. [Google Scholar] [CrossRef]
- Goderski, S.; Runowski, M.; Woźny, P.; Lavín, V.; Lis, S. Lanthanide Upconverted Luminescence for Simultaneous Contactless Optical Thermometry and Manometry–Sensing under Extreme Conditions of Pressure and Temperature. ACS Appl. Mater. Interfaces 2020, 12, 40475–40485. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, H.; Yue, Y.; Guo, Z.; Yu, J.; Chen, Z.; Gao, J.; Yang, Y.; Qian, G.; Chen, B. A Luminescent Mixed-Lanthanide Metal–Organic Framework Thermometer. J. Am. Chem. Soc. 2012, 134, 3979–3982. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Y.; Xia, T.; Cui, Y.; Yang, Y.; Qian, G. A Highly Sensitive Luminescent Metal–Organic Framework Thermometer for Physiological Temperature Sensing. J. Rare Earths 2018, 36, 561–566. [Google Scholar] [CrossRef]
- Zhao, D.; Rao, X.; Yu, J.; Cui, Y.; Yang, Y.; Qian, G. Design and Synthesis of an MOF Thermometer with High Sensitivity in the Physiological Temperature Range. Inorg. Chem. 2015, 54, 11193–11199. [Google Scholar] [CrossRef] [PubMed]
- Trannoy, V.; Carneiro Neto, A.N.; Brites, C.D.S.; Carlos, L.D.; Serier-Brault, H. Engineering of Mixed Eu3+/Tb3+ Metal-Organic Frameworks Luminescent Thermometers with Tunable Sensitivity. Adv. Opt. Mater. 2021, 9, 2001938. [Google Scholar] [CrossRef]
- Rocha, J.; Brites, C.D.S.; Carlos, L.D. Lanthanide Organic Framework Luminescent Thermometers. Chem. Eur. J. 2016, 22, 14782–14795. [Google Scholar] [CrossRef]
- Cadiau, A.; Brites, C.D.S.; Costa, P.M.F.J.; Ferreira, R.A.S.; Rocha, J.; Carlos, L.D. Ratiometric Nanothermometer Based on an Emissive Ln3+-Organic Framework. ACS Nano 2013, 7, 7213–7218. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Y.; Zhou, X.; Brites, C.D.S.; Ananias, D.; Lin, Z.; Paz, F.A.A.; Rocha, J.; Huang, W.; Carlos, L.D. Visible-Light Excited Luminescent Thermometer Based on Single Lanthanide Organic Frameworks. Adv. Funct. Mater. 2016, 26, 8677–8684. [Google Scholar] [CrossRef]
- Lyubov, D.M.; Neto, A.N.C.; Fayoumi, A.; Lyssenko, K.A.; Korshunov, V.M.; Taydakov, I.V.; Salles, F.; Guari, Y.; Larionova, J.; Carlos, L.D.; et al. Employing Three-Blade Propeller Lanthanide Complexes as Molecular Luminescent Thermometers: Study of Temperature Sensing through a Concerted Experimental/Theory Approach. J. Mater. Chem. C 2022, 10, 7176–7188. [Google Scholar] [CrossRef]
- Suzuki, M.; Tseeb, V.; Oyama, K.; Ishiwata, S. Microscopic Detection of Thermogenesis in a Single HeLa Cell. Biophys. J. 2007, 92, L46–L48. [Google Scholar] [CrossRef]
- Nexha, A.; Carvajal, J.J.; Pujol, M.C.; Díaz, F.; Aguiló, M. Lanthanide Doped Luminescence Nanothermometers in the Biological Windows: Strategies and Applications. Nanoscale 2021, 13, 7913–7987. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Balabhadra, S.; Carlos, L.D. Lanthanide-Based Thermometers: At the Cutting-Edge of Luminescence Thermometry. Adv. Opt. Mater. 2019, 7, 1801239. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Millán, A.; Carlos, L.D. Chapter 281—Lanthanides in Luminescent Thermometry. In Handbook on the Physics and Chemistry of Rare Earths; Jean-Claude, B., Vitalij, K.P., Eds.; Including Actinides; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 339–427. [Google Scholar]
- Brites, C.D.S.; Lima, P.P.; Silva, N.J.O.; Millán, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Thermometry at the Nanoscale. Nanoscale 2012, 4, 4799–4829. [Google Scholar] [CrossRef]
- Jaque, D.; Vetrone, F. Luminescence Nanothermometry. Nanoscale 2012, 4, 4301–4326. [Google Scholar] [CrossRef]
- Luminescence Thermometry: Methods, Materials and Applications—1st Edition. Available online: https://www.elsevier.com/books/luminescence-thermometry/dramicanin/978-0-08-102029-6 (accessed on 13 June 2022).
- Bünzli, J.-C.; Eliseeva, S. Basics of Lanthanide Photophysics. In Lanthanide Luminescence; Publisher: City, Country, 2010; Volume 7, pp. 1–45. ISBN 978-3-642-21022-8. [Google Scholar]
- Bünzli, J.-C.G.; Piguet, C. Taking Advantage of Luminescent Lanthanide Ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef]
- Tcelykh, L.O.; Kozhevnikova, V.Y.; Goloveshkin, A.S.; Latipov, E.V.; Gordeeva, E.O.; Utochnikova, V.V. Sensing Temperature with Tb-Eu-Based Luminescent Thermometer: A Novel Approach to Increase the Sensitivity. Sens. Actuators Phys. 2022, 345, 113787. [Google Scholar] [CrossRef]
- Sharma, A.R.; Singh, A.R.; Kongasseri, A.A.; Garain, S.; Babu, A.M.; Lonibala, R.; Laishram, R. A Ratiometric Luminescence Thermometer Based on Lanthanide Encapsulated Complexes. Dalton Trans. 2025, 54, 1377–1383. [Google Scholar] [CrossRef]
- Kharcheva, A.V.; Bozhko, A.A.; Sokolovskaya, Y.G.; Borisova, N.E.; Ivanov, A.V.; Patsaeva, S.V. Bimetallic Eu/Tb Complexes for Ratiometric Temperature Sensing with Unusual Enhancement of Eu Luminescence with Temperature. Photonics 2023, 10, 1171. [Google Scholar] [CrossRef]
- Zhao, D.; Yue, D.; Zhang, L.; Jiang, K.; Qian, G. Cryogenic Luminescent Tb/Eu-MOF Thermometer Based on a Fluorine-Modified Tetracarboxylate Ligand. Inorg. Chem. 2018, 57, 12596–12602. [Google Scholar] [CrossRef] [PubMed]
- Popelensky, T.Y.; Utochnikova, V.V. How Does the Ligand Affect the Sensitivity of the Luminescent Thermometers Based on Tb–Eu Complexes. Dalton Trans. 2020, 49, 12156–12160. [Google Scholar] [CrossRef]
- Calado, C.M.S.; Gálico, D.A.; Murugesu, M. Intra-Cluster Energy Transfer Editing in a Dual-Emitting System to Tap into Lifetime Thermometry. Chem. Commun. 2023, 59, 13715–13718. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Zhou, X.; Wu, J.; Li, L.; Wang, Y.; Xiang, G.; Tang, X.; Yang, Z.; Gao, H. Luminescent Properties of Multi-Stimuli-Responsive Ln-BPDC-Phen Lanthanide Complexes. Dyes Pigments 2024, 222, 111871. [Google Scholar] [CrossRef]
- Yang, X.; Zou, H.; Sun, X.; Sun, T.; Guo, C.; Fu, Y.; Wu, C.-M.L.; Qiao, X.; Wang, F. One-Step Synthesis of Mixed Lanthanide Metal-Organic Framework Films for Sensitive Temperature Mapping. Adv. Opt. Mater. 2019, 7, 1900336. [Google Scholar] [CrossRef]
- Tang, H.; Cheng, S.; Zhang, Z.; He, M.; Qian, J.; Li, L. Tailoring Energy Transfer in Mixed Eu/Tb Metal–Organic Frameworks for Ratiometric Temperature Sensing. Molecules 2024, 29, 3914. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, D.; Cui, Y.; Yang, Y.; Qian, G. A Eu/Tb-Mixed MOF for Luminescent High-Temperature Sensing. J. Solid State Chem. 2017, 246, 341–345. [Google Scholar] [CrossRef]
- Outis, M.; Laia, C.A.T.; Oliveira, M.C.; Monteiro, B.; Pereira, C.C.L. A Europium(III) Complex with an Unusual Anion-Cation Interaction: A Luminescent Molecular Thermometer for Ratiometric Temperature Sensing. ChemPlusChem 2020, 85, 580–586. [Google Scholar] [CrossRef]
- Souza, A.S.; Nunes, L.A.O.; Silva, I.G.N.; Oliveira, F.A.M.; da Luz, L.L.; Brito, H.F.; Felinto, M.C.F.C.; Ferreira, R.A.S.; Júnior, S.A.; Carlos, L.D.; et al. Highly-Sensitive Eu3+ Ratiometric Thermometers Based on Excited State Absorption with Predictable Calibration. Nanoscale 2016, 8, 5327–5333. [Google Scholar] [CrossRef]
- Wei, W.; Li, X.; Zhang, Y.-Y.; Zhang, J.-W. Rational Construction of Luminescent Eu-Doped Y-MOF for Ratiometric Temperature Sensing. RSC Adv. 2024, 14, 28340–28344. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Kumagai, M.; Nakanishi, T.; Fushimi, K.; Hasegawa, Y. The Role of π-f Orbital Interactions in Eu(III) Complexes for an Effective Molecular Luminescent Thermometer. Inorg. Chem. 2020, 59, 5865–5871. [Google Scholar] [CrossRef] [PubMed]
- de Souza, K.M.N.; Silva, R.N.; Silva, J.A.B.; Brites, C.D.S.; Francis, B.; Ferreira, R.A.S.; Carlos, L.D.; Longo, R.L. Novel and High-Sensitive Primary and Self-Referencing Thermometers Based on the Excitation Spectra of Lanthanide Ions. Adv. Opt. Mater. 2022, 10, 2200770. [Google Scholar] [CrossRef]
- Kitos, A.A.; Gálico, D.A.; Castañeda, R.; Ovens, J.S.; Murugesu, M.; Brusso, J.L. Stark Sublevel-Based Thermometry with Tb(III) and Dy(III) Complexes Cosensitized via the 2-Amidinopyridine Ligand. Inorg. Chem. 2020, 59, 11061–11070. [Google Scholar] [CrossRef] [PubMed]
- Errulat, D.; Marin, R.; Gálico, D.A.; Harriman, K.L.M.; Pialat, A.; Gabidullin, B.; Iikawa, F.; Couto, O.D.D.; Moilanen, J.O.; Hemmer, E.; et al. A Luminescent Thermometer Exhibiting Slow Relaxation of the Magnetization: Toward Self-Monitored Building Blocks for Next-Generation Optomagnetic Devices. ACS Cent. Sci. 2019, 5, 1187–1198. [Google Scholar] [CrossRef]
- Kumar, K.; Abe, D.; Komori-Orisaku, K.; Stefańczyk, O.; Nakabayashi, K.; R. Shakirova, J.; P. Tunik, S.; Ohkoshi, S. Neodymium β-Diketonate Showing Slow Magnetic Relaxation and Acting as a Ratiometric Thermometer Based on near-Infrared Emission. RSC Adv. 2019, 9, 23444–23449. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, Y.; Wang, H.; Xiang, L.; Yan, Y.; Li, L.; Xiang, G.; Li, Y.; Jiang, S.; Tang, X.; et al. Nd3+ and Nd3+/Yb3+-Incorporated Complexes as Optical Thermometer Working in the Second Biological Window. Sens. Bio-Sens. Res. 2020, 29, 100345. [Google Scholar] [CrossRef]
- Brunet, G.; Marin, R.; Monk, M.-J.; Resch-Genger, U.; Gálico, D.A.; Sigoli, F.A.; Suturina, E.A.; Hemmer, E.; Murugesu, M. Exploring the Dual Functionality of an Ytterbium Complex for Luminescence Thermometry and Slow Magnetic Relaxation. Chem. Sci. 2019, 10, 6799–6808. [Google Scholar] [CrossRef]
- Karachousos-Spiliotakopoulos, K.; Tangoulis, V.; Panagiotou, N.; Tasiopoulos, A.; Moreno-Pineda, E.; Wernsdorfer, W.; Schulze, M.; Botas, A.M.P.; Carlos, L.D. Luminescence Thermometry and Field Induced Slow Magnetic Relaxation Based on a near Infrared Emissive Heterometallic Complex. Dalton Trans. 2022, 51, 8208–8216. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, D.; Tian, C.; Wang, S.; Li, H. Flexible and Transparent Films Consisting of Lanthanide Complexes for Ratiometric Luminescence Thermometry. J. Colloid Interface Sci. 2018, 519, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Piñol, R.; Antorrena, G.; Brites, C.D.S.; Silva, N.J.O.; Murillo, J.L.; Cases, R.; Díez, I.; Palacio, F.; Torras, N.; et al. Implementing Thermometry on Silicon Surfaces Functionalized by Lanthanide-Doped Self-Assembled Polymer Monolayers. Adv. Funct. Mater. 2016, 26, 200–209. [Google Scholar] [CrossRef]
- Galyametdinov, Y.G.; Krupin, A.S.; Knyazev, A.A. Temperature-Sensitive Chameleon Luminescent Films Based on PMMA Doped with Europium(III) and Terbium(III) Anisometric Complexes. Inorganics 2022, 10, 94. [Google Scholar] [CrossRef]
- Lapaev, D.V.; Nikiforov, V.G.; Lobkov, V.S.; Knyazev, A.A.; Galyametdinov, Y.G. Reusable Temperature-Sensitive Luminescent Material Based on Vitrified Film of Europium(III) β-Diketonate Complex. Opt. Mater. 2018, 75, 787–795. [Google Scholar] [CrossRef]
- de Freitas Silva, G.; da Silva Filho, J.C.; de Castro Andrade, A.A.; Otaguro, H.; Ferri, L.; de Lima Rezende, T.K.; Pasquini, D.; Ferrari, J.L. Synthesis and Luminescent Properties of Cellulose Acetate Butyrate Films Doped with Europium Complex Eu(TTA)3 for Optical Thermometry. Opt. Mater. 2024, 152, 115393. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Lima, P.P.; Silva, N.J.O.; Millán, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Thermometry at the Nanoscale Using Lanthanide-Containing Organic–Inorganic Hybrid Materials. J. Lumin. 2013, 133, 230–232. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Lima, P.P.; Silva, N.J.O.; Millán, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. A Luminescent Molecular Thermometer for Long-Term Absolute Temperature Measurements at the Nanoscale. Adv. Mater. 2010, 22, 4499–4504. [Google Scholar] [CrossRef]
- Mara, D.; Kaczmarek, A.M.; Artizzu, F.; Abalymov, A.; Skirtach, A.G.; Van Hecke, K.; Van Deun, R. Luminescent PMMA Films and PMMA@SiO2 Nanoparticles with Embedded Ln3+ Complexes for Highly Sensitive Optical Thermometers in the Physiological Temperature Range**. Chem. Eur. J. 2021, 27, 6479–6488. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, S.; Gilmanova, D.; Mukhametshina, A.; Nizameev, I.; Kholin, K.; Akhmadeev, B.; Voloshina, A.; Sapunova, A.; Kuznetsova, S.; Daminova, A.; et al. Silica Nanoparticles with Dual Visible–NIR Luminescence Affected by Silica Confinement of Tb(III) and Yb(III) Complexes for Cellular Imaging Application. J. Mater. Sci. 2019, 54, 9140–9154. [Google Scholar] [CrossRef]
- Takei, Y.; Arai, S.; Murata, A.; Takabayashi, M.; Oyama, K.; Ishiwata, S.; Takeoka, S.; Suzuki, M. A Nanoparticle-Based Ratiometric and Self-Calibrated Fluorescent Thermometer for Single Living Cells. ACS Nano 2014, 8, 198–206. [Google Scholar] [CrossRef]
- Pelluau, T.; Sene, S.; Ali, L.M.A.; Félix, G.; Manhes, F.; Neto, A.N.C.; Carlos, L.D.; Albela, B.; Bonneviot, L.; Oliviero, E.; et al. Hybrid Multifunctionalized Mesostructured Stellate Silica Nanoparticles Loaded with β-Diketonate Tb3+/Eu3+ Complexes as Efficient Ratiometric Emissive Thermometers Working in Water. Nanoscale 2023, 15, 14409–14422. [Google Scholar] [CrossRef] [PubMed]
- Harrington, B.; Ye, Z.; Signor, L.; Pickel, A.D. Luminescence Thermometry Beyond the Biological Realm. ACS Nanosci. Au 2024, 4, 30–61. [Google Scholar] [CrossRef]
- Piñol, R.; Brites, C.D.S.; Bustamante, R.; Martínez, A.; Silva, N.J.O.; Murillo, J.L.; Cases, R.; Carrey, J.; Estepa, C.; Sosa, C.; et al. Joining Time-Resolved Thermometry and Magnetic-Induced Heating in a Single Nanoparticle Unveils Intriguing Thermal Properties. ACS Nano 2015, 9, 3134–3142. [Google Scholar] [CrossRef] [PubMed]
- Piñol, R.; Zeler, J.; Brites, C.D.S.; Gu, Y.; Téllez, P.; Carneiro Neto, A.N.; da Silva, T.E.; Moreno-Loshuertos, R.; Fernandez-Silva, P.; Gallego, A.I.; et al. Real-Time Intracellular Temperature Imaging Using Lanthanide-Bearing Polymeric Micelles. Nano Lett. 2020, 20, 6466–6472. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Piñol, R.; Moreno-Loshuertos, R.; Brites, C.D.S.; Zeler, J.; Martínez, A.; Maurin-Pasturel, G.; Fernández-Silva, P.; Marco-Brualla, J.; Téllez, P.; et al. Local Temperature Increments and Induced Cell Death in Intracellular Magnetic Hyperthermia. ACS Nano 2023, 17, 6822–6832. [Google Scholar] [CrossRef]
- Nigoghossian, K.; Bouvet, B.; Félix, G.; Sene, S.; Costa, L.; Milhet, P.-E.; Carneiro Neto, A.N.; Carlos, L.D.; Oliviero, E.; Guari, Y.; et al. Magneto-Induced Hyperthermia and Temperature Detection in Single Iron Oxide Core-Silica/Tb3+/Eu3+(Acac) Shell Nano-Objects. Nanomaterials 2022, 12, 3109. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Fuertes, M.C.; Angelomé, P.C.; Martínez, E.D.; Lima, P.P.; Soler-Illia, G.J.A.A.; Carlos, L.D. Tethering Luminescent Thermometry and Plasmonics: Light Manipulation to Assess Real-Time Thermal Flow in Nanoarchitectures. Nano Lett. 2017, 17, 4746–4752. [Google Scholar] [CrossRef]
- Larquey, A.; Bellahsene, H.; Félix, G.; Beaudhuin, M.; Pelluau, T.; Bouvet, B.; Guari, Y.; Sene, S.; Larionova, J. New Heater@luminescent Thermometer Nano-Objects: Prussian Blue Core@silica Shell Loaded with a β-Diketonate Tb3+/Eu3+ Complex. Mater. Chem. Front. 2024, 9, 131–146. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of Europium(III) Spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Anderson, B.R.; Gunawidjaja, R.; Eilers, H. Soluble Sm-Based Ternary Complexes for Non-Contact Molecular Thermometry. J. Lumin. 2018, 204, 341–348. [Google Scholar] [CrossRef]
- Anderson, B.R.; Gunawidjaja, R.; Eilers, H. Two-Color Thermosensors Based on [Y1−xDyx(Acetylacetonate)3(1,10-Phenanthroline)] Molecular Crystals. Appl. Phys. B 2017, 123, 62. [Google Scholar] [CrossRef]
- Katagiri, S.; Hasegawa, Y.; Wada, Y.; Yanagida, S. Thermo-Sensitive Luminescence Based on the Back Energy Transfer in Terbium(III) Complexes. Chem. Lett. 2004, 33, 1438–1439. [Google Scholar] [CrossRef]
- Vanden Bussche, F.; Kaczmarek, A.M.; Veerapandian, S.K.P.; Everaert, J.; Debruyne, M.; Abednatanzi, S.; Morent, R.; De Geyter, N.; Van Speybroeck, V.; Van Der Voort, P.; et al. N-Rich Porous Polymer with Isolated Tb3+-Ions Displays Unique Temperature Dependent Behavior through the Absence of Thermal Quenching. Chem. Eur. J. 2020, 26, 15596–15604. [Google Scholar] [CrossRef]
- Shen, C.; Fan, Y.; Liu, G.; Wang, Y.; Lu, P. The crystal and molecular structures of gadolinium, terbium, erbium And thulium acetylacetonate trihydrate. J. Chin. Univ. 1983, 4, 769. [Google Scholar]
- Bednarkiewicz, A.; Marciniak, L.; Carlos, L.D.; Jaque, D. Standardizing Luminescence Nanothermometry for Biomedical Applications. Nanoscale 2020, 12, 14405–14421. [Google Scholar] [CrossRef]
- Zatryb, G.; Klak, M.M. On the Choice of Proper Average Lifetime Formula for an Ensemble of Emitters Showing Non-Single Exponential Photoluminescence Decay. J. Phys. Condens. Matter 2020, 32, 415902. [Google Scholar] [CrossRef] [PubMed]
- Sillen, A.; Engelborghs, Y. The Correct Use of “Average” Fluorescence Parameters. Photochem. Photobiol. 1998, 67, 475–486. [Google Scholar] [CrossRef]
- Puccini, A.; Liu, N.; Hemmer, E. Lanthanide-Based Nanomaterials for Temperature Sensing in the near-Infrared Spectral Region: Illuminating Progress and Challenges. Nanoscale 2024, 16, 10975–10993. [Google Scholar] [CrossRef]
- Yuan, B.; He, X.; Chen, Y.; Wang, K. Preparation of Nanosilica/Polynorbornene Nanocomposite by Covalently Immobilized Silica-Supported Acetylacetonate Palladium(II) Dichloride Catalyst. Macromol. Chem. Phys. 2011, 212, 2378–2388. [Google Scholar] [CrossRef]
- Hu, J.-J.; Chen, Y.; Li, Z.-H.; Peng, S.-Y.; Sun, Y.; Zhang, X.-Z. Augment of Oxidative Damage with Enhanced Photodynamic Process and MTH1 Inhibition for Tumor Therapy. Nano Lett. 2019, 19, 5568–5576. [Google Scholar] [CrossRef] [PubMed]


| Thermometer | Signal Type | Readout Mode | Window | Srmax | δT | |
|---|---|---|---|---|---|---|
| 1Tb | Intensity | Non-ratiometric | Visible | 1.0%·K−1 (at 353 K) | 1.7 K (at 353 K) | 5.7 K |
| 1Eu | Intensity | Non-ratiometric | Visible | 6.5%·K−1 (at 338 K) | 0.1 K (at 303 K) | 0.2 K |
| Lifetime | Self-referenced | 2.9%·K−1 (at 340 K) | 0.02 K (at 313 K) | 0.5 K | ||
| 1Tb1.5/1Eu8.5 | Intensity | Ratiometric (double-band approach) | Visible | 3.2%·K−1 (at 320 K) | 1.1 K (at 333 K) | 1.5 K |
| 1Yb | Intensity | Ratiometric (single-band approach) | NIR | 0.5%·K−1 (at 293 K) | 0.5 K (at 303 K) | 1.6 K |
| Samples | Loading | Signal Type | Readout Mode | Window | Srmax | δT | |
|---|---|---|---|---|---|---|---|
| PB@SiO2-acac/(1Eu) | 0.28 | Intensity | Non-ratiometric | Visible | 2.1%·K−1 (at 333 K) | 6.7 K (at 313 K) | 7.6 K |
| Lifetime | Self-referenced | 1.2%·K−1 (at 315 K) | 7.2 K (at 318 K) | 8.1 K | |||
| PB@SiO2-acac/(1Tb2/1Eu8) | 0.48 | Intensity | Ratiometric | Visible | 0.6%·K−1 (at 293 K) | 21 K (at 323 K) | 21.6 K |
| Lifetime | Self-referenced | 0.9%·K−1 (at 333 K) | 0.21 K (at 303 K) | 1.1 K | |||
| PB@SiO2-acac/(1Yb) | 0.31 | Intensity | Ratiometric (Single band approach) | NIR | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larquey, A.; Félix, G.; Sene, S.; Larionova, J.; Guari, Y. Lanthanide Tris-Acetylacetonate Complexes for Luminescent Thermometry: From Isolated Compounds to Hybrid Prussian Blue Core–Silica Shell Nanoparticles. Inorganics 2025, 13, 304. https://doi.org/10.3390/inorganics13090304
Larquey A, Félix G, Sene S, Larionova J, Guari Y. Lanthanide Tris-Acetylacetonate Complexes for Luminescent Thermometry: From Isolated Compounds to Hybrid Prussian Blue Core–Silica Shell Nanoparticles. Inorganics. 2025; 13(9):304. https://doi.org/10.3390/inorganics13090304
Chicago/Turabian StyleLarquey, Aurore, Gautier Félix, Saad Sene, Joulia Larionova, and Yannick Guari. 2025. "Lanthanide Tris-Acetylacetonate Complexes for Luminescent Thermometry: From Isolated Compounds to Hybrid Prussian Blue Core–Silica Shell Nanoparticles" Inorganics 13, no. 9: 304. https://doi.org/10.3390/inorganics13090304
APA StyleLarquey, A., Félix, G., Sene, S., Larionova, J., & Guari, Y. (2025). Lanthanide Tris-Acetylacetonate Complexes for Luminescent Thermometry: From Isolated Compounds to Hybrid Prussian Blue Core–Silica Shell Nanoparticles. Inorganics, 13(9), 304. https://doi.org/10.3390/inorganics13090304









