Study on the Performance and Mechanism of Separating La from Light Rare Earth Elements Using Single-Column Method with a New Type of Silica-Based Phosphate-Functionalized Resin
Abstract
1. Introduction
2. Results and Discussion
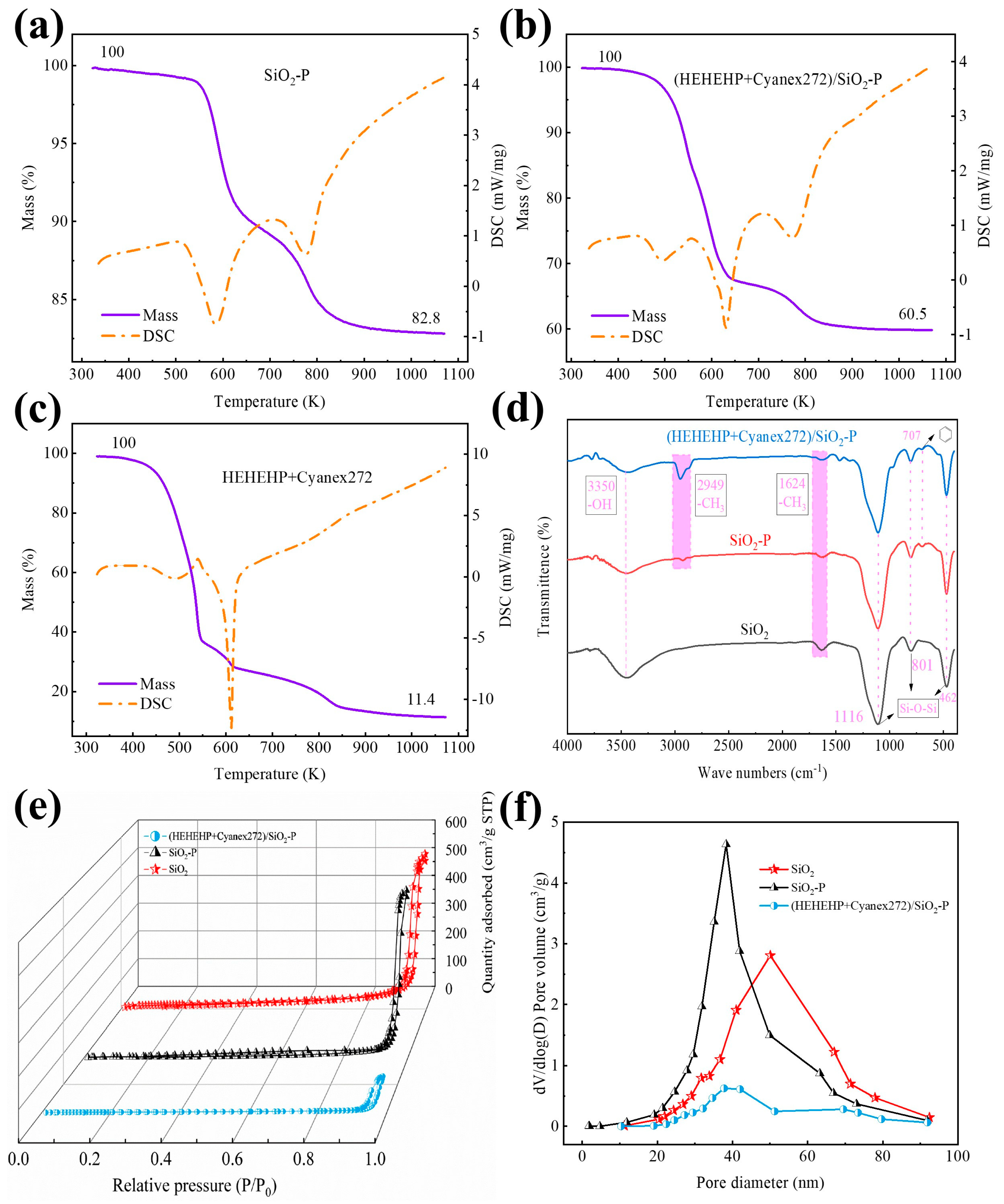
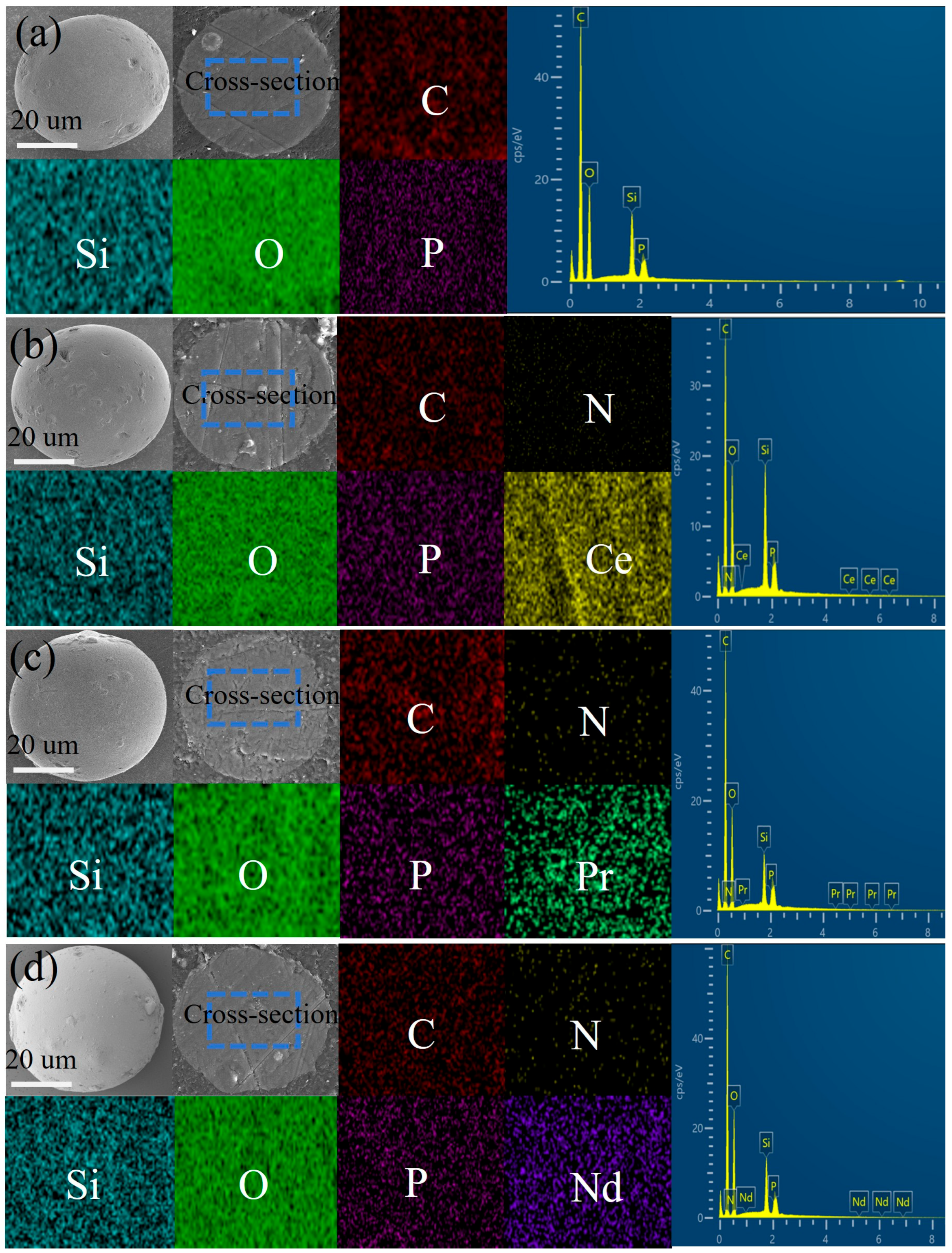
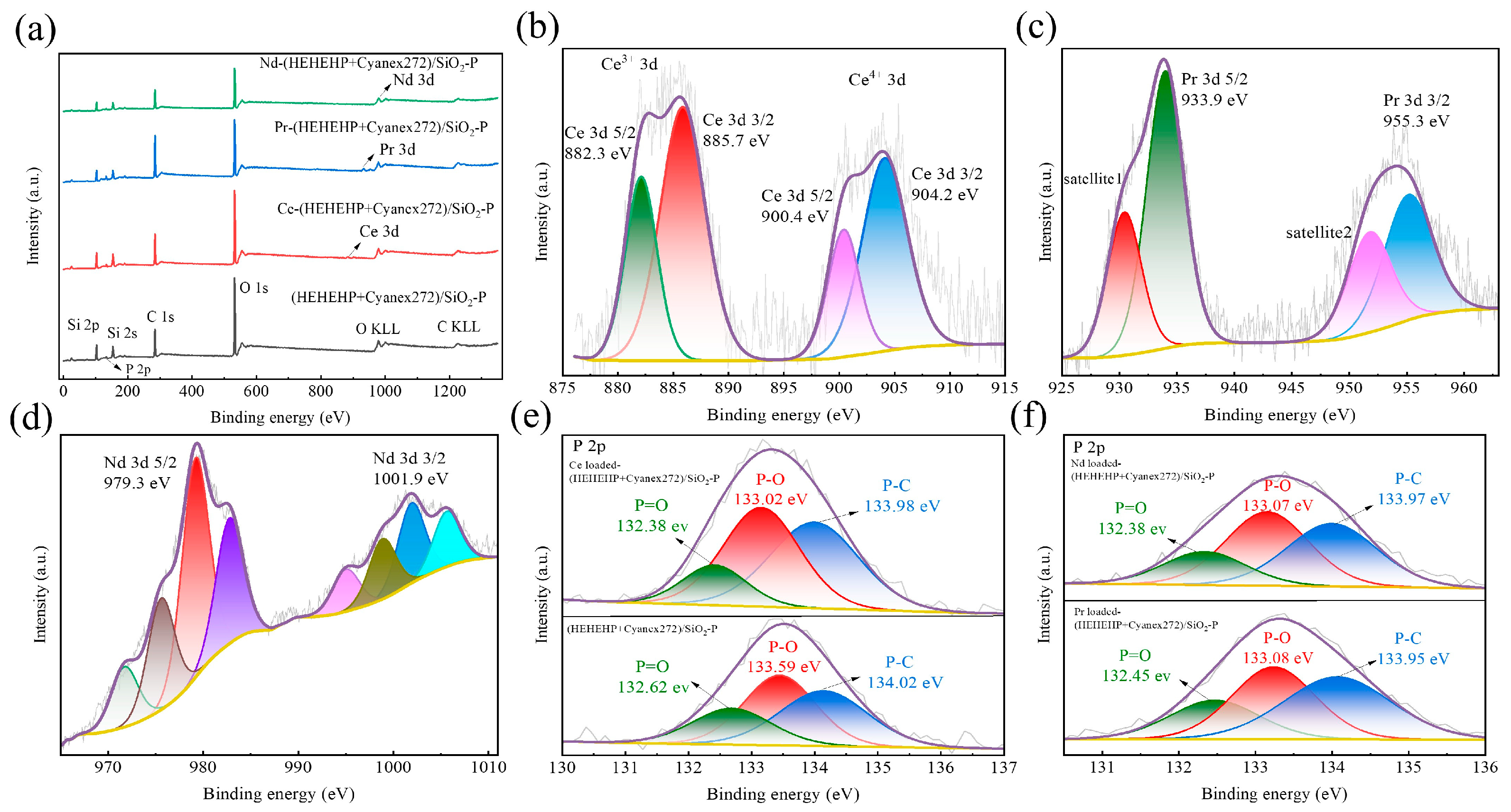
2.1. Characterization
2.2. Batch Experiments
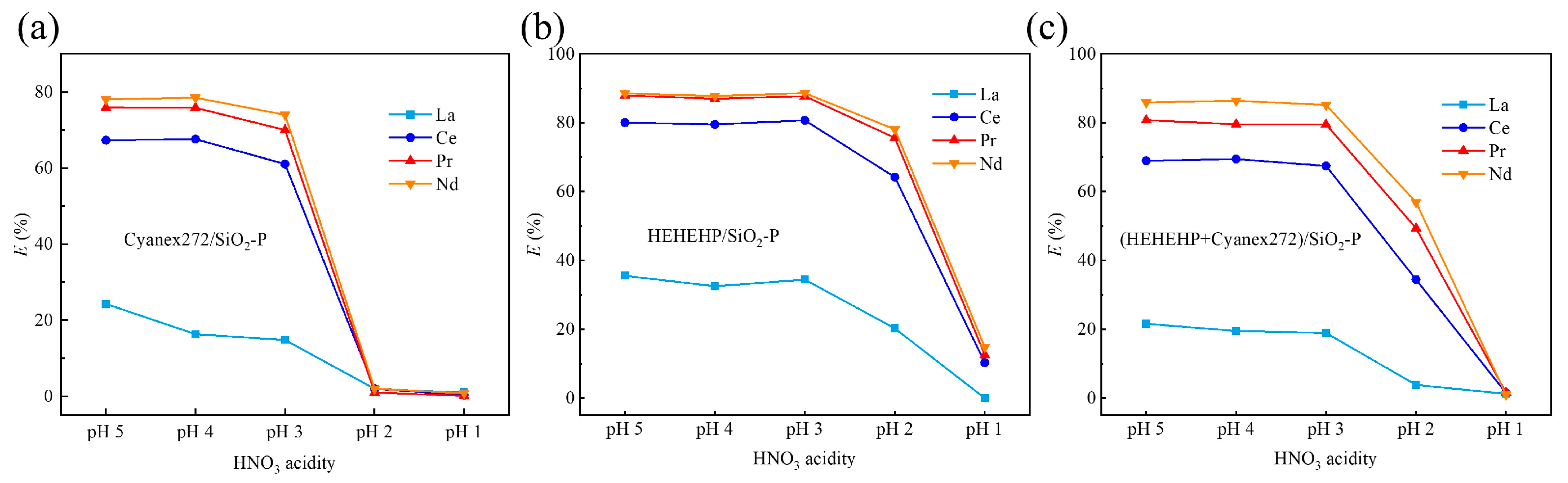
2.2.1. Effect of Acidity
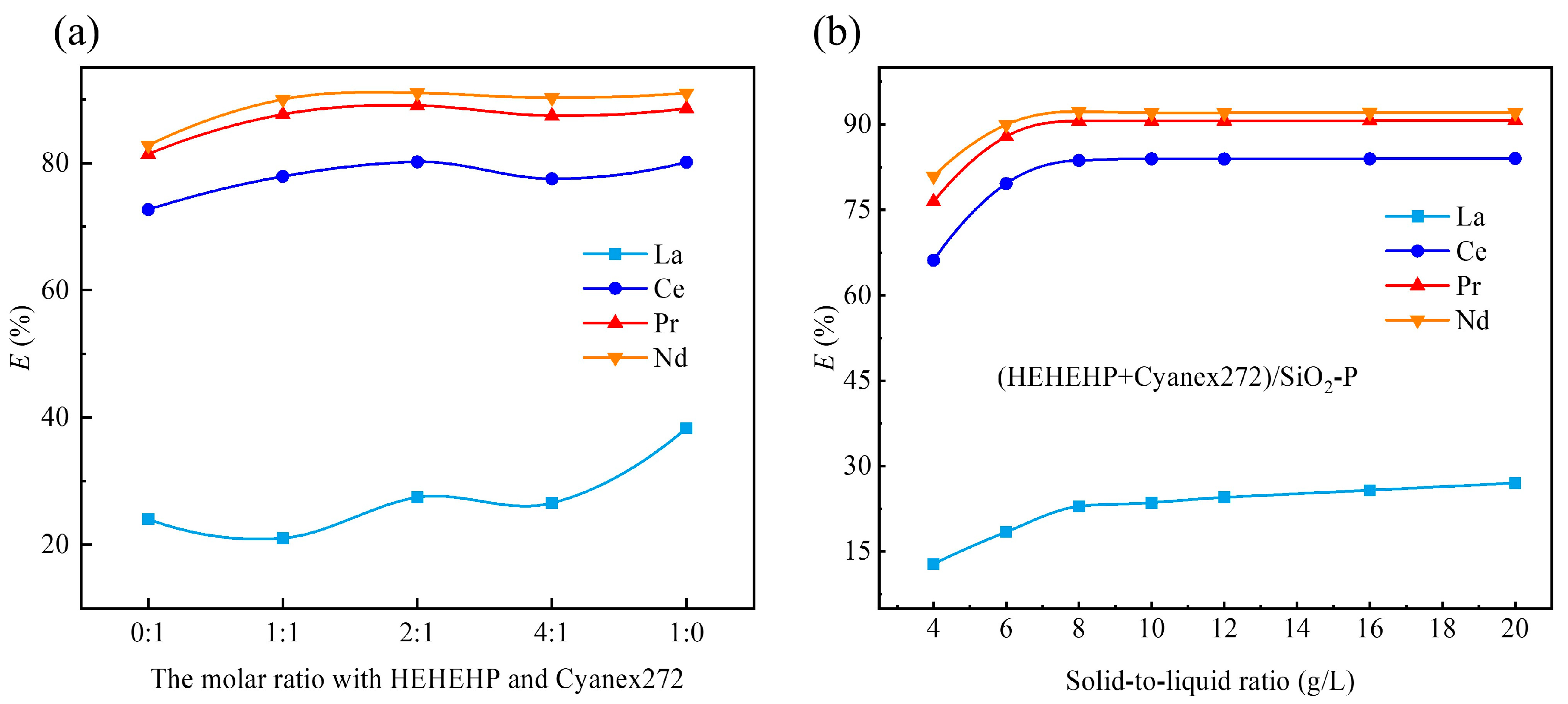
2.2.2. Effect of Mole Ratios of HEHEHP and Cyanex272
2.2.3. Effect of Solid–Liquid Ratio
2.2.4. Kinetics
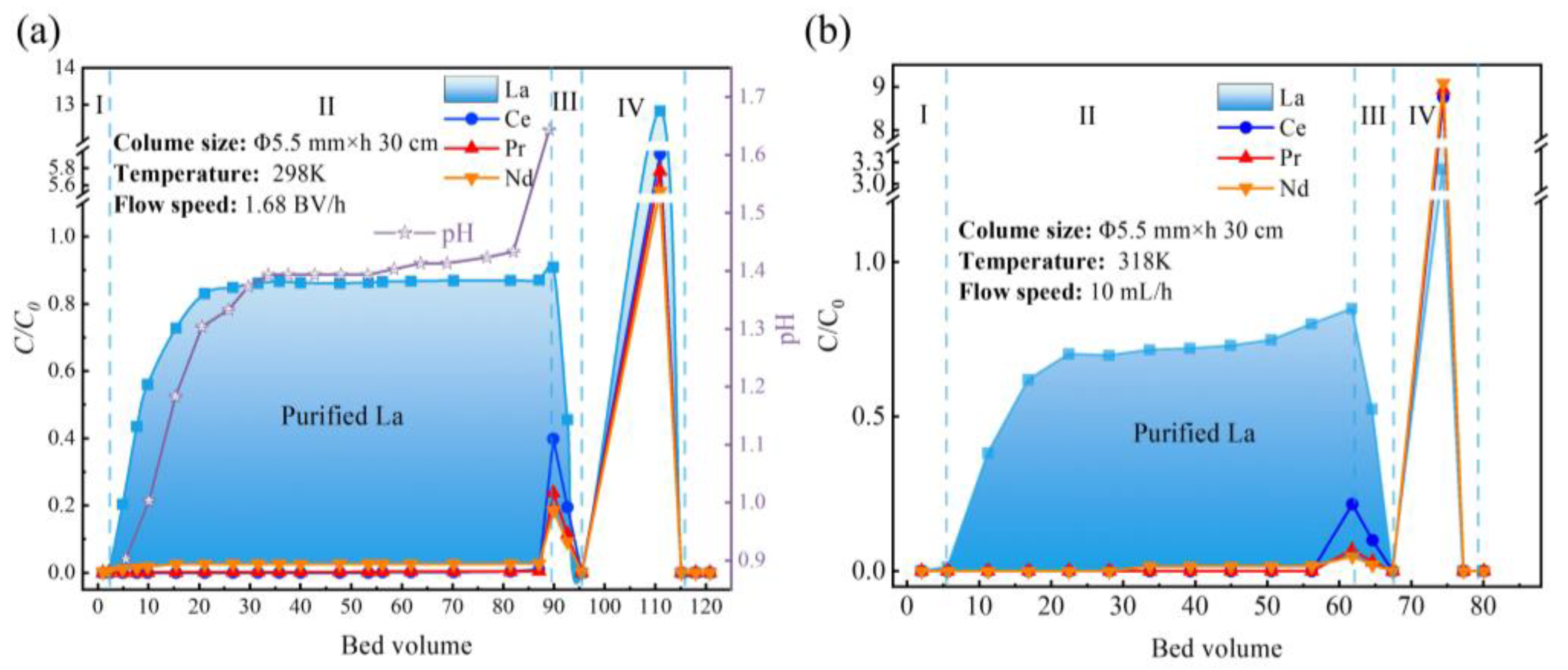
2.3. Column Experiments
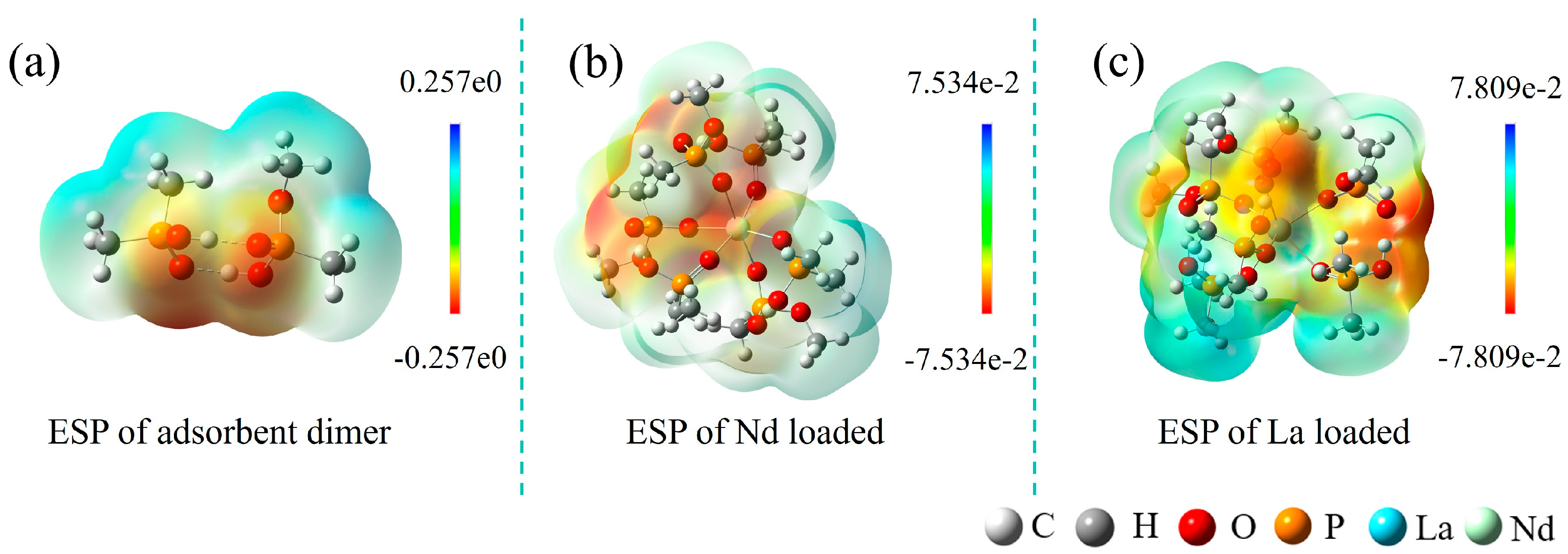
2.4. Study on the Adsorption Mechanism
2.5. Study on Polymer Conformation and Selectivity Mechanism
2.6. Comparison
3. Experimental Section
3.1. Chemicals
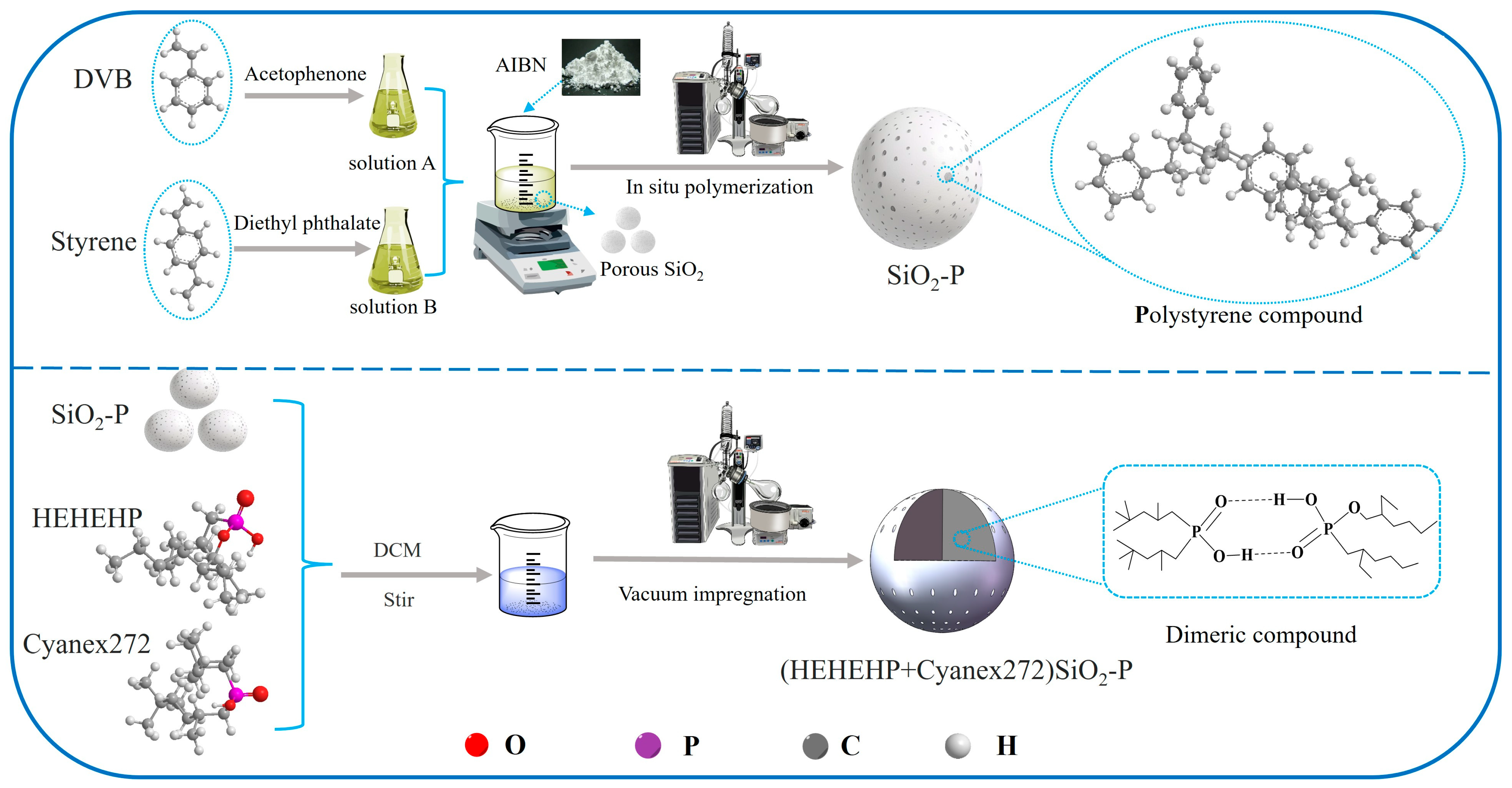
3.2. Synthesis and Characterization of the Resin
3.3. Batch Experiments
3.4. Column Experiments
3.5. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cockell, C.S.; Santomartino, R.; Finster, K.; Waajen, A.C.; Eades, L.J.; Moeller, R.; Rettberg, P.; Fuchs, F.M.; Van Houdt, R.; Leys, N.; et al. Space station biomining experiment demonstrates rare earth element extraction in microgravity and Mars gravity. Nat. Commun. 2020, 11, 5523. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Chen, J.; Qian, X.; Zhong, Y.; Ma, C.; Zhang, H.; Liu, K. The Construction of a Microbial Synthesis System for Rare Earth Enrichment and Material Applications. Adv. Mater. 2023, 35, e2303457. [Google Scholar] [CrossRef]
- Jiang, F.; Yin, S.; Srinivasakannan, C.; Li, S.; Peng, J. Separation of lanthanum and cerium from chloride medium in presence of complexing agent along with EHEHPA (P507) in a serpentine microreactor. Chem. Eng. J. 2018, 334, 2208–2214. [Google Scholar] [CrossRef]
- Dong, Z.; Mattocks, J.A.; Deblonde, G.J.P.; Hu, D.; Jiao, Y.; Cotruvo, J.A., Jr.; Park, D.M. Bridging Hydrometallurgy and Biochemistry: A Protein-Based Process for Recovery and Separation of Rare Earth Elements. ACS Cent. Sci. 2021, 7, 1798–1808. [Google Scholar] [CrossRef]
- Mattocks, J.A.; Jung, J.J.; Lin, C.-Y.; Dong, Z.; Yennawar, N.H.; Featherston, E.R.; Kang-Yun, C.S.; Hamilton, T.A.; Park, D.M.; Boal, A.K.; et al. Enhanced rare-earth separation with a metal-sensitive lanmodulin dimer. Nature 2023, 618, 87–93. [Google Scholar] [CrossRef]
- Song, X.; Zhang, J.; Yue, M.; Li, E.; Zeng, H.; Lu, N.; Zhou, M.; Zuo, T. Technique for preparing ultrafine nanocrystalline bulk material of pure rare-earth metals. Adv. Mater. 2006, 18, 1210–1215. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, W.-T.; Sang, F.-N.; Xu, J.-H.; Luo, G.-S.; Wang, Y.-D. Fast extraction and enrichment of rare earth elements from waste water via microfluidic-based hollow droplet. Sep. Purif. Technol. 2017, 174, 352–361. [Google Scholar] [CrossRef]
- Gras, M.; Papaiconomou, N.; Chainet, E.; Tedjar, F.; Billard, I. Separation of cerium(III) from lanthanum(III), neodymium(III) and praseodymium(III) by oxidation and liquid-liquid extraction using ionic liquids. Sep. Purif. Technol. 2017, 178, 169–177. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sudarshan, K.; Kadam, R.M. Optical nanomaterials with focus on rare earth doped oxide: A Review. Mater. Today Commun. 2021, 27, 102277. [Google Scholar] [CrossRef]
- Wilfong, W.C.; Ji, T.; Duan, Y.; Shi, F.; Wang, Q.; Gray, M.L. Critical review of functionalized silica sorbent strategies for selective extraction of rare earth elements from acid mine drainage. J. Hazard. Mater. 2022, 424, 127625. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Q.; Li, F.; Yu, F.; Yu, Z.; Hu, K.; Chen, H.; Li, X.; Wang, C.; Seo, D.H.; et al. Effective separation of neodymium and lanthanum by single-module hollow fiber supported liquid membrane with P507 as extractant. Sep. Purif. Technol. 2024, 340, 126759. [Google Scholar] [CrossRef]
- Han, X.; Xiong, Z.; Wang, S.; Wang, L.; Liang, T. Long-term open-pit mining activities at the world’s largest light rare earth mine significantly affect light rare earth elements in road dust over long distances. J. Hazard. Mater. 2024, 480, 136287. [Google Scholar] [CrossRef]
- Jemli, S.; Pinto, D.; Kanhounnon, W.G.; Ben Amara, F.; Sellaoui, L.; Bonilla-Petriciolet, A.; Dhaouadi, F.; Ameri, R.; Silva, L.F.O.; Bejar, S.; et al. GL-Net: Semantic segmentation for point clouds of shield tunnel via global feature learning and local feature discriminative aggregation. Chem. Eng. J. 2023, 466, 143108. [Google Scholar] [CrossRef]
- Salehi, H.; Maroufi, S.; Nekouei, R.K.; Chinu, K.; Sahajwalla, V. Tailored separation of light rare earth elements using combined oxidative precipitation and multi-stage solvent extraction techniques. Sep. Purif. Technol. 2025, 364, 132566. [Google Scholar] [CrossRef]
- Merroune, A.; Brahim, J.A.; Achiou, B.; Kada, C.; Mazouz, H.; Beniazza, R. Closed-loop purification process of industrial phosphoric acid: Selective recovery of heavy metals and rare earth elements via solvent extraction. Desalination 2024, 580, 117515. [Google Scholar] [CrossRef]
- Belfqueh, S.; Chapron, S.; Giusti, F.; Pellet-Roastaing, S.; Seron, A.; Menad, N.; Arrachart, G. Selective recovery of rare earth elements from acetic leachate of NdFeB magnet by solvent extraction. Sep. Purif. Technol. 2024, 339, 126701. [Google Scholar] [CrossRef]
- Roa, A.; Lopez, J.; Cortina, J.L. Selective separation of light and heavy rare earth elements from acidic mine waters by integration of chelating ion exchange and ligand impregnated resin. Sci. Total Environ. 2024, 954, 176700. [Google Scholar] [CrossRef]
- Tunsu, C.; Menard, Y.; Eriksen, D.O.; Ekberg, C.; Petranikova, M. Recovery of critical materials from mine tailings: A comparative study of the solvent extraction of rare earths using acidic, solvating and mixed extractant systems. J. Clean. Prod. 2019, 218, 425–437. [Google Scholar] [CrossRef]
- Ma, K.-Q.; Han, J.; Yang, C.-T.; Zhang, F.; Yan, H.; Wu, F.-C.; Hu, S.; Shi, L. Advanced solid-phase extraction of tetravalent actinides using a novel hierarchically porous functionalized silica monolith. Sep. Purif. Technol. 2022, 293, 121086. [Google Scholar] [CrossRef]
- Yang, B.; Wu, S.-Z.; Liu, X.-Y.; Yan, Z.-X.; Liu, Y.-X.; Li, Q.-S.; Yu, F.-S.; Wang, J.-L. Solid-phase extraction and separation of heavy rare earths from chloride media using P227-impregnated resins. Rare Met. 2021, 40, 2633–2644. [Google Scholar] [CrossRef]
- Artiushenko, O.; Rojano, W.S.; Nazarkovsky, M.; Azevedo, M.F.M.F.; Saint’Pierre, T.D.; Kai, J.; Zaitsev, V. Recovery of rare earth elements from waste phosphors using phosphonic acid-functionalized silica adsorbent. Sep. Purif. Technol. 2024, 330, 125525. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Deng, B.; Huang, Y.; Liu, X.; Ning, S.; Kuang, S.; Liao, W. Separation and purification of heavy rare earth elements by a silica/ polymer-based β-aminophosphonic acid resin from chloride media. Sep. Purif. Technol. 2025, 354, 129342. [Google Scholar] [CrossRef]
- Wang, J.; Xie, M.; Wang, H.; Xu, S. Solvent extraction and separation of heavy rare earths from chloride media using nonsymmetric (2,3-dimethylbutyl) (2,4,4′-trimethylpentyl)phosphinic acid. Hydrometallurgy 2017, 167, 39–47. [Google Scholar] [CrossRef]
- Yun, S.H.; Jeon, J.H.; Lee, M.S. Recovery of pure metal sulfate solutions from a synthetic sulfuric acid leachate of base-rare earth metals representing spent Ni-MH batteries through selective precipitation, stepwise ionic-liquid/solvent extraction and stripping. Hydrometallurgy 2025, 236, 106510. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, L.; Li, Z.; Zhang, H.; Wei, W.; Huang, X.; Feng, Z. Selective separation of rare earths and aluminum from low-concentration rare earth solution via centrifugal extraction. Sep. Purif. Technol. 2024, 351, 127933. [Google Scholar] [CrossRef]
- Liu, R.; Wei, Y.; Xu, Y.; Usuda, S.; Kim, S.; Yamazaki, H.; Ishii, K. Evaluation study on properties of isohexyl-BTP/SiO2-P resin for direct separation of trivalent minor actinides from HLLW. J. Radioanal. Nucl. Chem. 2012, 292, 537–544. [Google Scholar] [CrossRef]
- Petreanu, I.; Niculescu, V.-C.; Enache, S.; Iacob, C.; Teodorescu, M. Structural Characterization of Silica and Amino-Silica Nanoparticles by Fourier Transform Infrared (FTIR) and Raman Spectroscopy. Anal. Lett. 2023, 56, 390–403. [Google Scholar] [CrossRef]
- Athulya, P.A.; Chandrasekaran, N. Interactions of natural colloids with microplastics in aquatic environment and its impact on FTIR characterization of polyethylene and polystyrene microplastics. J. Mol. Liq. 2023, 369, 120950. [Google Scholar] [CrossRef]
- Akhondi, M.; Jamalizadeh, E. Preparation of cubic and spherical hollow silica structures by polystyrene-poly diallyldimethylammonium chloride and polystyrene-poly ethyleneimine hard templates. Ceram. Int. 2021, 47, 851–857. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, A.; Chai, Z. Synthesis and characterization of novel macroporous silica-polymer-calixcrown hybrid supramolecular recognition materials for effective separation of cesium. J. Hazard. Mater. 2014, 267, 109–118. [Google Scholar] [CrossRef]
- Dehnicke, K.; Greiner, A. Unusual complex chemistry of rare-Earth elements: Large ionic radii-small coordination numbers. Angew. Chem. Int. Ed. 2003, 42, 1340–1354. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, P.; Zitolo, A.; Migliorati, V.; Chillemi, G.; Duvail, M.; Vitorge, P.; Abadie, S.; Spezia, R. Revised Ionic Radii of Lanthanoid(III) Ions in Aqueous Solution. Inorg. Chem. 2011, 50, 4572–4579. [Google Scholar] [CrossRef]
- Bulin, C.; Zheng, R.; Guo, T.; Zhang, B. Incorporating hard-soft acid-base theory in multi-aspect analysis of the adsorption mechanism of aqueous heavy metals by graphene oxide. J. Phys. Chem. Solids 2022, 170, 110934. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.; Kuang, S.; Zhang, Z.; Bian, X.; Liao, W. Synergistic extraction of heavy rare earths by mixture of α-aminophosphonic acid HEHAMP and HEHEHP. J. Rare Earths 2019, 37, 422–428. [Google Scholar] [CrossRef]
- Larochelle, T.; Noble, A.; Strickland, K.; Ahn, A.; Ziemkiewicz, P.; Constant, J.; Hoffman, D.; Glascock, C. Recovery of Rare Earth Element from Acid Mine Drainage Using Organo-Phosphorus Extractants and Ionic Liquids. Minerals 2022, 12, 1337. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Q.; Chen, L.; Zhong, Y.; Hu, F.; Wu, K.; Yin, X.; Wei, Y.; Ning, S. Phosphination of amino-modified mesoporous silica for the selective separation of strontium. J. Hazard. Mater. 2024, 467, 133741. [Google Scholar] [CrossRef]
- Xu, S.; Ning, S.; Wang, Y.; Wang, X.; Dong, H.; Chen, L.; Yin, X.; Fujita, T.; Wei, Y. Precise separation and efficient enrichment of palladium from wastewater by amino-functionalized silica adsorbent. J. Clean. Prod. 2023, 396, 136479. [Google Scholar] [CrossRef]
- Peters, J.A.; Djanashvili, K.; Geraldes, C.F.G.C.; Platas-Iglesias, C. The chemical consequences of the gradual decrease of the ionic radius along the Ln-series. Coord. Chem. Rev. 2020, 406, 213146. [Google Scholar] [CrossRef]
- Wang, X.; Sun, W.; Zhao, J.; Han, H. Organophosphonic acid nitrilotri: A spatially matched depressant of bastnaesite for separation from fluorite. J. Rare Earths 2025, 41, 410–416. [Google Scholar] [CrossRef]
- Hernández-Pérez, D.; Aldana-González, J.; Romero-Romo, M.; Rivera-Hernández, S.; Ezeta-Mejía, A.; Morales-Gil, P.; Sánchez-Piñon, N.; Sampayo-Garrido, A.; Palomar-Pardavé, M. Electrochemical Nucleation and Growth of Neodymium on Glassy Carbon Electrodes using Reline as a Deep Eutectic Solvent. Electrochim. Acta 2025, 535, 146643. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Pan, Z.; Ke, S.; Wang, Y. Allochroic effect of praseodymium doped neodymium molybdate pigment synthesized by solid-state reaction. Ceram. Int. 2022, 48, 20372–20387. [Google Scholar] [CrossRef]
- Song, A.-M.; Yang, M.-J.; Wu, Z.; Yang, Q.; Lin, B.; Liang, R.-P.; Qiu, J.-D. Efficient Separation of Adjacent Rare Earths in One Step by Using Ion-microporous Metal-Organic Frameworks. Adv. Funct. Mater. 2025, 35, 2419093. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, S.; Chen, L.; Li, Z.; Wu, K.; Zhang, Y.; Su, Z.; Yin, X.; Hamza, M.F.; Wei, Y.; et al. Efficient separation of palladium from nitric acid solution by a novel silica-based ion exchanger with ultrahigh adsorption selectivity. Sep. Purif. Technol. 2023, 322, 124326. [Google Scholar] [CrossRef]
- Lin, S.; Bediako, J.K.; Cho, C.-W.; Song, M.-H.; Zhao, Y.; Kim, J.-A.; Choi, J.-W.; Yun, Y.-S. Selective adsorption of Pd(II) over interfering metal ions (Co(II), Ni(II), Pt(IV)) from acidic aqueous phase by metal-organic frameworks. Chem. Eng. J. 2018, 345, 337–344. [Google Scholar] [CrossRef]
- Shi, M.; Liu, S.; Ning, S.; Liu, J.; Chen, L.; Zeng, D.; Hamza, M.F.; Wei, Y. Exploration and development of carboxyl functionalization ion-exchange resin for efficient separation of trace yttrium from simulated 90Sr decay system. Sep. Purif. Technol. 2025, 376, 133883. [Google Scholar] [CrossRef]
- He, D.; Ning, S.; Liu, J.; Zhang, S.; Chen, L.; Xu, Y.; Feng, Z.; Hamza, M.F.; Wei, Y. Efficiently selective removal of radioactive thorium and uranium from rare earths by trialkyl phosphine oxide modified porous silica-polymer based adsorbent. Sep. Purif. Technol. 2025, 364, 132426. [Google Scholar] [CrossRef]
- Zhang, D.; Macdonald, L.; Raj, P.; Karamalidis, A.K. Thiol-functionalized cellulose adsorbents for highly selective separation of palladium over platinum in acidic aqueous solutions. Chem. Eng. J. 2024, 494, 152948. [Google Scholar] [CrossRef]
- Mustapa, N.R.N.; Malek, N.F.A.; Yusoff, M.M.; Rahman, M.L. Ion imprinted polymers for selective recognition and separation of lanthanum and cerium ions from other lanthanide. Sep. Sci. Technol. 2016, 51, 2762–2771. [Google Scholar] [CrossRef]
- Lee, G.S.; Uchikoshi, M.; Mimura, K.; Isshiki, M. Preparation and Evaluation of High-Purity La2O3. Metal. Mater. Trans. B 2010, 41, 509–519. [Google Scholar] [CrossRef]
- Zheng, X.; Song, Z.; Liu, E.; Zhang, Y.; Li, Z. Preparation of Phosphoric Acid-Functionalized SBA-15 and Its High Efficient Selective Adsorption Separation of Lanthanum Ions. J. Chem. Eng. Data 2020, 65, 746–756. [Google Scholar] [CrossRef]
- Yu, Q.; Ning, S.; Zhang, W.; Wang, X.; Wei, Y. Recovery of scandium from sulfuric acid solution with a macro porous TRPO/SiO2-P adsorbent. Hydrometallurgy 2018, 181, 74–81. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Salehi, H.; Maroufi, S.; Nekouei, R.K.; Sahajwalla, V. Solvent extraction systems for selective isolation of light rare earth elements with high selectivity for Sm and La. Rare Met. 2025, 44, 2071–2084. [Google Scholar] [CrossRef]
- Chen, L.; Jiao, Z.; Yin, X.; Li, W.; Wang, X.; Ning, S.; Wei, Y. Highly efficient removal of strontium from contaminated wastewater by a porous zirconium phosphate material. J. Environ. Manag. 2022, 319, 115718. [Google Scholar] [CrossRef] [PubMed]


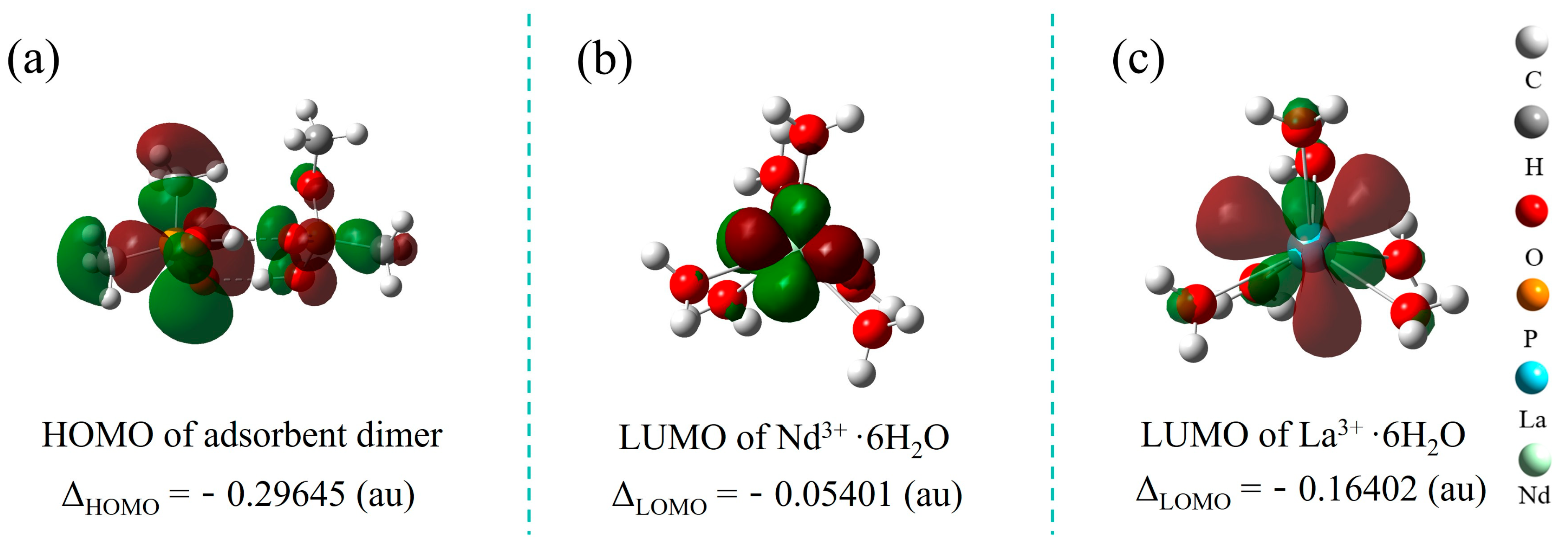
| Reaction | ΔEbinding-energy (kcal/mol) |
|---|---|
| Nd3+·(H2O)6 + 3(HL2)− →Nd (HL2)3 + 6H2O | −108.9 |
| La3+·(H2O)6 + 3(HL2)− →La (HL2)3 + 6H2O | −67.8 |
| Materials | Acidity | Purity | E% | Q (mg/g) | Ref. |
|---|---|---|---|---|---|
| La-IIP-Schiff-base | pH = 6 HNO3 | 298 | 80 | 25.0 | [48] |
| GMZ Bentonite | pH = 6 HCl | 298 | 95 | 42.0 | [49] |
| D2EHPA/DIAION SA 10 A | pH = 3 HCl | 298 | 90 | 67.2 | [49] |
| DEPTS/SBA-15 | pH = 7 HCl | 298 | 95 | 114.8 | [50] |
| (HEHEHP + Cyanex272)/SiO2-P | pH = 4 HNO3 | 298 | 98 | 120.6 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Ning, S.; Liu, J.; Chen, L.; Hamza, M.F.; Wei, Y. Study on the Performance and Mechanism of Separating La from Light Rare Earth Elements Using Single-Column Method with a New Type of Silica-Based Phosphate-Functionalized Resin. Inorganics 2025, 13, 296. https://doi.org/10.3390/inorganics13090296
Huang M, Ning S, Liu J, Chen L, Hamza MF, Wei Y. Study on the Performance and Mechanism of Separating La from Light Rare Earth Elements Using Single-Column Method with a New Type of Silica-Based Phosphate-Functionalized Resin. Inorganics. 2025; 13(9):296. https://doi.org/10.3390/inorganics13090296
Chicago/Turabian StyleHuang, Ming, Shunyan Ning, Juan Liu, Lifeng Chen, Mohammed F. Hamza, and Yuezhou Wei. 2025. "Study on the Performance and Mechanism of Separating La from Light Rare Earth Elements Using Single-Column Method with a New Type of Silica-Based Phosphate-Functionalized Resin" Inorganics 13, no. 9: 296. https://doi.org/10.3390/inorganics13090296
APA StyleHuang, M., Ning, S., Liu, J., Chen, L., Hamza, M. F., & Wei, Y. (2025). Study on the Performance and Mechanism of Separating La from Light Rare Earth Elements Using Single-Column Method with a New Type of Silica-Based Phosphate-Functionalized Resin. Inorganics, 13(9), 296. https://doi.org/10.3390/inorganics13090296










