Abstract
This study reported covalent organic frameworks (COFs) and their hybrid composites with two-dimensional materials, graphene oxide (GO), graphitic carbon nitride (g-C3N4), and boron nitride (BN), to examine their structural, textural, and gas adsorption properties. Material characterization confirmed the crystallinity of COF-1 and the preservation of framework integrity after integrating the 2D nanomaterials. FT-IR spectra exhibited pronounced vibrational fingerprints of imine linkages and validated the functional groups from the COF and the integrated nanomaterials. TEM images revealed the integration of the two components, porous, layered structures with indications of interfacial interactions between COF and 2D nanosheets. Nitrogen adsorption–desorption isotherms revealed the microporous characteristics of the COFs, with hysteresis loops evident, indicating the development of supplementary mesopores at the interface between COF-1 and the 2D materials. The BET surface area of pristine COF-1 was maximal at 437 m2/g, accompanied by significant micropore and Langmuir surface areas of 348 and 1290 m2/g, respectively, offering enhanced average pore widths and hierarchical porous strcuture. CO2 adsorption tests were investigated showing maximum adsorption capacitiy of 1.47 mmol/g, for COF-1, closely followed by COF@BN at 1.40 mmol/g, underscoring the preserved sorption capabilities of these materials. These findings demonstrate the promise of designed COF-based hybrids for gas capture, separation, and environmental remediation applications.
1. Introduction
The rapid rise of carbon dioxide (CO2) in the atmosphere, caused by human activities such as the combustion of fossil fuels and industrial operations, poses a significant threat to global environmental stability, leading to irreversible climate change [1]. With the expansion of global populations and ongoing industrial development, CO2 emissions are expected to increase, exacerbating climate change [2,3]. Confronting this challenge necessitates immediate and efficient solutions, with carbon capture and sequestration (CCS) technologies emerging as crucial tactics for reducing CO2 emissions, primarily until renewable energy sources are adequately prevalent to satisfy global demand [4,5]. The success of CCS depends on creating new materials that can capture CO2 efficiently, selectively, and cost-effectively [6]. Liquid adsorbents for CO2 capture typically include aqueous amine solutions, ionic liquids, and amino acid salts, each offering high chemical affinities for CO2. These liquids exhibit advantages such as high adsorption capacity and ease of regeneration under controlled conditions via chemical adsorption. However, challenges such as solvent degradation, high energy requirements for regeneration, and corrosiveness, especially in amine-based systems, limit their long-term sustainability and economic viability [7,8]. These limitations have intensified the search for alternative materials that exhibit enhanced performance and durability. Key targets for CCS technology include stationary point sources such as power plants and biogas upgrading systems, where CO2 must be extracted from the gas mixture [9,10]. An optimal CO2 adsorbent must demonstrate substantial adsorption capability, superior selectivity against other gases, durability in humid and high temperatures, and facile regeneration with little energy requirements [11,12,13].
Covalent organic frameworks (COFs) constitute an innovative category of porous materials, entirely composed of light elements such as carbon, hydrogen, boron, nitrogen, and oxygen, interconnected by robust covalent bonds [14,15,16,17]. The frameworks are formed by strategically linking organic moieties through strong covalent bonds, e.g., single bonds (C–C, C–N, C–O, or C–Si) or double bonds (e.g., C=N and N=N) [18,19,20]. They have experienced swift and extensive expansion, leading to the creation of diverse chemical architectures ranging from one-dimensional (1D) chains to intricately two-dimensional (2D) and three-dimensional (3D) networks [21,22,23,24]. The extensive array of reaction routes facilitates the creation of COFs with varied dimensions, topologies, and functional characteristics. COFs encompass low density, high specific surface area, high pore architectures, and stacking configurations that enhance electrical conductivity [24,25]. Moreover, the modular and adjustable characteristics of COF synthesis facilitate the precise design of pore dimensions, functions, and framework structures. The distinctive characteristics of COFs have established them as exceptionally adaptable materials, garnering considerable interest from the scientific community. They have exhibited high potential for various applications, such as environmental control (removal of pollutants from air, water, soil) [17], gas storage and separation [26], photocatalysis [27,28], sensing [29], drug delivery [30], and energy storage [31,32,33]. COFs were intensively investigated as adsorbents for CO2 capture [34,35]. They can capture CO2 from the open air [36]. They can be applied as catalysts for the chemical conversion of CO2 into valuable compounds via several methods, including fixation [37,38], electrocatalytic reduction [39], and photocatalysis [40,41]. COF-based cobalt porphyrins enabled catalytic CO2 reduction in water [42]. However, they have a high aggregation tendency [43]. Combining COF materials with other nanomaterials enables the formation of membranes [44]. Thus, they were conjugated with different materials for MOFs [45], polymers [46], and MXene [47]. COF-based nanocomposites may solve the materials’ challenges and enhance their performance or processing into commercial products, enabling further steps toward commercialization.
Two-dimensional nanomaterials, including carbon-based, e.g., graphene (G), graphene oxide (GO), graphitic carbon nitride (g-C3N4), and boron nitride (BN), received significant attention due to their extraordinary physicochemical properties and wide range of potential applications [48,49]. These materials possess exceptionally high surface areas, facilitating increased interaction with target molecules, making them promising for applications in sensors and biosensors [50], catalysis [51], energy storage [52], and environmental remediation or monitoring [53,54,55]. Their unique anisotropic structures contribute to directional physical and chemical behaviors that can be tailored for specific uses. Furthermore, their ease of integration into functional devices and prototype systems enhances their commercial viability. The structural versatility, high reactivity, and diverse applications make 2D nanomaterials a valuable platform in both academic research and industrial development. COF nanocomposites of 2D nanomaterials were investigated as adsorbents for removing anionic and cationic dyes, offering a 100% adsorption efficiency with superior selectivity relative to the pristine COF [56]. Conjugation of 2D with COF-1 may prevent the aggregation tendency of COFs.
This study involved the synthesis and modification of 2D nanomaterials, such as GO, g-C3N4, and BN, for use in COF-1. The materials were synthesized using a one-pot procedure via the solvothermal method. They were evaluated using X-ray diffraction (XRD), Fourier transform infrared (FT-IR), transmission electron microscopy (TEM), and nitrogen adsorption–desorption isotherms. The materials were investigated as adsorbents for CO2 adsorption.
2. Results and Discussion
2.1. Material Characterization
Figure 1 and Figure 2 show the synthesis procedure for carbon nanomaterials (GO and g-C3N4), BN exfoliation, COF-1, and their composites. Hummer’s method produced graphene oxide (GO) (Figure 1a). Graphite powder and sodium nitrate were gradually incorporated into concentrated sulfuric acid while maintaining constant agitation in an ice bath for 4 h. Subsequently, potassium permanganate was added incrementally while maintaining continuous stirring. The solution was diluted with water, and the temperature was sustained at 37 °C for 2 h. To neutralize unreacted permanganate and finalize oxidation, hydrogen peroxide was introduced while stirring for 1 h, yielding a yellow solution. The resulting suspension was centrifuged, and the GO precipitate was rinsed with double-distilled water to eliminate residual salts and unreacted reagents. GO was dehydrated in a vacuum oven at 60 °C for 2 h.
Figure 1.
Synthesis procedure for (a) GO, (b) g-C3N4, and (c) BN.
Figure 2.
Synthesis procedure for COF-1 and its composites.
g-C3N4 was obtained using direct thermal polymerization of melamine in a semi-closed environment to inhibit sublimation (Figure 1b). In a standard synthesis, melamine was added in a sealed crucible and heated at 550 °C at 10 °C/min in a muffle furnace for 2 h. The resultant yellow solid was rinsed and dried overnight.
BN exfoliation was conducted using a straightforward aqueous reflux technique (Figure 1c). Bulk BN was refluxed in deionized water with stirring at approximately 120 °C for 72 h. The exfoliated BN flakes were obtained by depositing the suspension onto a glass plate, drying, and carefully scraping (Figure 1c).
COF-1 was prepared using a solvothermal method (Figure 2). Melamine and terephthaldehyde were solubilized in DMSO under a nitrogen environment and subjected to rapid stirring. The mixture was conveyed to a closed reaction chamber and heated at 180 °C for 72 h. The product was obtained via filtration, rinsed with THF, acetone, and DMF, and subjected to vacuum drying at 120 °C overnight (Figure 2).
To prepare COF-based nanocomposites (COF-1@GO, COF-1@BN, and COF-1@g-C3N4), each 2D nanomaterial (GO, BN, or g-C3N4) was initially dispersed in DMSO using ultrasonication for 30 min (Figure 2). Subsequently, melamine and terephthaldehyde were included in the dispersion. The mixture was agitated and heated under solvothermal conditions at 180 °C for 72 h. The solid products were filtrated and rinsed with organic solvents to remove unreacted species. The synthesized materials were evaluated by XRD (Figure 3), TEM images (Figure 4 and Figure 5), FT-IR (Figure 6a), DRS (Figure 6b), and nitrogen sorption isotherms (Figure 7; Table 1).
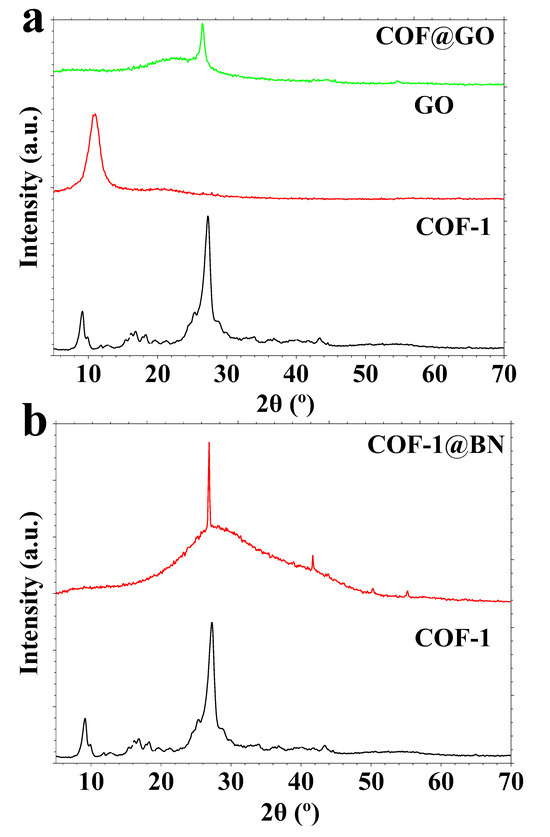
Figure 3.
XRD patterns for (a) GO, COF-1, COF-1@GO, and (b) COF-1@BN.
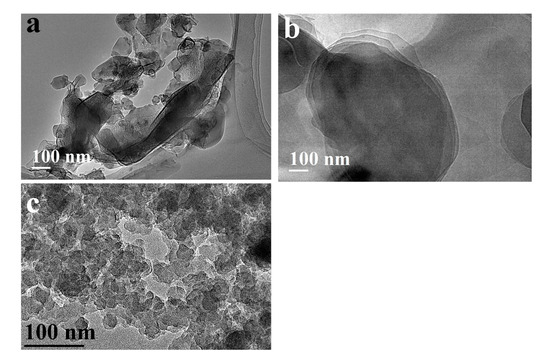
Figure 4.
TEM images for (a) GO, (b) BN, and (c) COF-1.
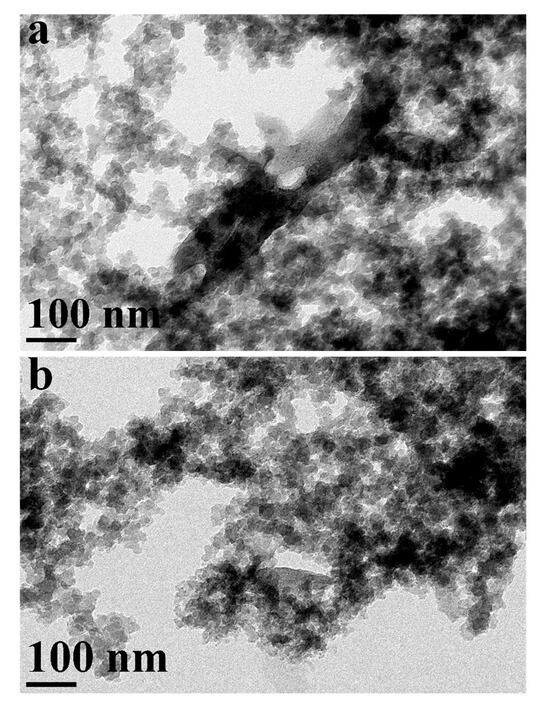
Figure 5.
TEM images for (a) COF-1@GO and (b) COF-1@g-C3N4.
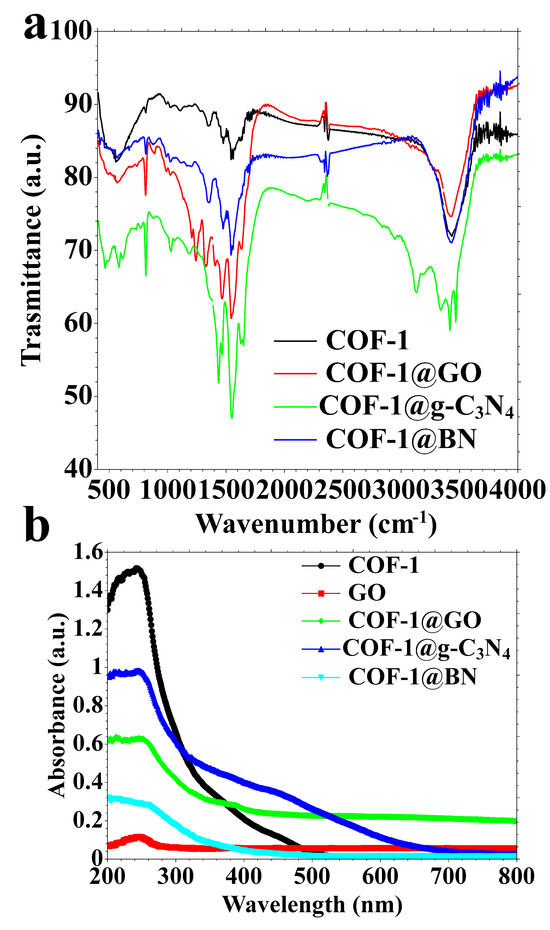
Figure 6.
(a) FT-IR spectra and (b) DRS spectra of COF-1 and its composite.
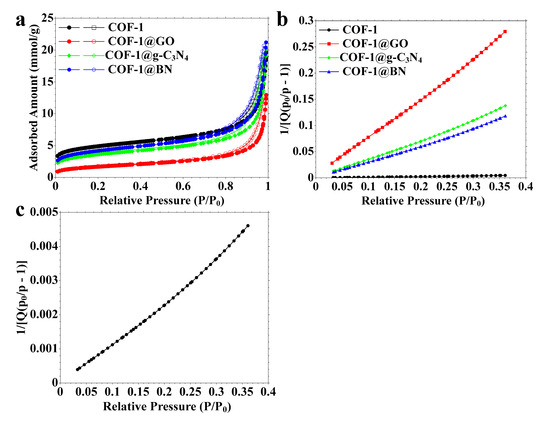
Figure 7.
(a) Nitrogen sorption isotherms for the materials, (b) BET fitting for specific surface area calculations, and (c) the enlarged plot for COF-1 presented in (b).

Table 1.
Surface area and pore volume using different techniques.
The structural characteristics and crystallinity of the produced materials, GO and COF-1, and their nanocomposites (COF-1@GO and COF-1@BN), were investigated via XRD, as illustrated in Figure 3a,b. The XRD pattern of GO has a distinct diffraction peak at a Bragg angle of 2θ = 10.9°, associated with the (001) plane. Employing Bragg’s law (nλ = 2dsinθ) with Cu Kα radiation (λ = 0.15418 nm), this peak corresponds to an interplanar spacing (d-spacing) of 0.81 nm, confirming the successful oxidation of graphite and the formation of GO characterized by interlayer expansion resulting from oxygenated functional groups and water intercalation (Figure 3a).
For COF-1, a unique diffraction pattern is evident, featuring significant peaks at 2θ = 9.0°, 16.8°, 18.3°, and 27.2° (Figure 3a). The associated d-spacings are 0.98 nm, 0.53 nm, 0.48 nm, and 0.33 nm, respectively. The peak at 9.0° is significant due to its occurrence at a low angle, indicating a considerable interlayer distance, characteristic of supramolecules such as COF. This reflection corresponds to the (100) plane in a lattice structure and endorses the establishment of an organized, polymeric COF network. The systematic arrangement of building blocks in two dimensions and the maintenance of long-range order further validate the efficacy of the condensation process between melamine and terephthaldehyde, resulting in a crystalline polymeric structure rather than an amorphous polymer (Figure 3a).
The XRD pattern of the COF-1@GO composite has a prominent diffraction peak at a Bragg angle (2θ) of 26.5° with a d-spacing of 0.34 nm, attributable to the Miller index of (002) reflection of graphitic carbon (Figure 3a). The inclusion of GO shows an insignificant change in the COF structure but notably alters its diffraction profile, indicating the integration of the two components.
The XRD pattern of the COF-1@BN composite displays a distinct peak at 2θ = 26.8° (d = 0.33 nm), aligning with the (002) plane of COF-1 (Figure 3b). It verifies the preservation of COF’s crystalline structure and its stable integration with BN. The high intensity may indicate the highly organized structure of the BN phase inside the hybrid material. Alongside strong diffraction peaks, the large hump within the 20–50° range indicates the existence of amorphous or semi-crystalline domains inside the hybrid composites. Such characteristics generally result from structural disorder, partial exfoliation, or strain effects from the interaction between the COF and the 2D nanomaterials (GO and BN). These disruptions may decrease long-range order while maintaining local crystallinity, which is frequently beneficial for applications such as adsorption or photocatalysis. The XRD data indicate the effective synthesis of crystalline COF-1 with long-range polymeric order, as demonstrated by the low-angle peak at 9.0° and its associated large d-spacing. Incorporating GO and BN into the COF matrix introduces supplementary crystallographic characteristics and alters the diffraction pattern by hybrid material development. These observations confirm the structural integrity and successful production of COF-based composite materials.
TEM images for pristine materials (Figure 4) and their composite (Figure 5) are investigated. Figure 4a displays the TEM image of GO, with a characteristic wrinkled and crumpled sheet-like shape. The distinctive properties of TEM images reflect the thin nature of GO nanosheets, which are prone to developing folds and wrinkles due to structural imperfections and oxygenated functional groups introduced during oxidation. The sheets display pores, presumably due to these imperfections. GO displays wavy particles with gray contrast, indicative of the standard features seen in chemically exfoliated GO (Figure 4a). Figure 4b presents a TEM image of exfoliated BN. The image illustrates a multi-layered structure (Figure 4b). The sheet edges are sharply separated, indicating effective exfoliation of BN following extended reflux in water. The exfoliated edges indicate larger layer spacing, which amplifies BN’s surface area and reactivity, facilitating its accessibility for interaction or composite production. Figure 4c depicts the TEM image of COF-1, illustrating irregular spherical particles with a relatively consistent size of around 20 nm. The small particle size and spherical shape indicate the creation of nanoscale COF particles.
Figure 5 offers more insights into the composite materials. TEM images represent the morphology of the COF-1@GO (Figure 5a) and COF-1@g-C3N4 (Figure 5b). It illustrates that the spherical COF-1 nanoparticles are uniformly dispersed into the crumpled GO sheets (Figure 5a). Figure 5b illustrates the TEM image of the COF-1@g-C3N4 nanocomposite. COF-1 nanoparticles are dispersed throughout the layered structure of g- C3N4, which exhibits particles with flat morphology. The contrast difference verifies the effective deposition of COF-1 onto the g-C3N4 surface. TEM images presented in Figure 4 and Figure 5 validate the synthesis and structural incorporation of COF-1 with diverse two-dimensional nanomaterials. The distinct visualization of morphology, particle size, and hybrid interfaces offers compelling evidence for these composite formations and structural integrity.
The functional groups and chemical interactions in COF-1 and its composites, COF-1@GO, COF-1@g-C3N4, and COF-1@BN, were examined via FT-IR spectroscopy (Figure 6a). The FT-IR spectrum of COF-1 shows a band at 2920 cm−1 and 3400 cm−1 corresponding to N–H/C–H symmetric stretching and adsorbed O–H groups, presumably from moisture, respectively. The presence of bands at 1540 and 1480 cm−1, indicative of C=N stretching vibrations of the triazine ring, confirms the polymerization and production of the COF (Figure 6a). The characteristic peaks for the aldehyde are absent, indicating the aldehyde’s condensation with the amine. No peaks related to the aldehydes were observed, specifically at 2870 cm−1 (C-H stretching) and 1690 cm−1 (C=O stretching). Notably, when COF-1 is combined with two-dimensional nanomaterials such as GO, g-C3N4, and BN, the FT-IR spectra of COF-1@GO, COF-1@g-C3N4, and COF-1@BN exhibit an insignificant change in the FT-IR spectrum of pure COF-1. These observations suggest that integrating these 2D nanomaterials during COF’s synthesis shows no effect on the polymerization process. The preservation of characteristic COF-1 peaks in all composite spectra verifies the integrity of the COF, suggesting that the interactions between COF-1 and the 2D nanomaterials are predominantly physical interactions with no observation of new bonds (no chemical interactions). The noncovalent bonds are mainly hydrogen bonds and π-π interactions based on the benzene rings present in both COF-1 and 2D nanomaterials.
The DRS spectra illustrated in Figure 6b elucidate the optical absorption properties of the synthesized materials: COF-1, GO, COF-1@GO, COF-1@g-C3N4, and COF-1@BN. COF-1 displays a broad absorption spectrum from 200 to 490 nm, with a peak absorption at 245 nm, indicative of π–π* transitions in its conjugated structure (Figure 6b). GO exhibits an absorption peak at 250 nm, corresponding to π–π* transitions of sp2 hybridized carbon domains, characteristic of GO (Figure 6b). The spectra of the COF-1@GO, COF-1@g-C3N4, and COF-1@BN composites primarily exhibit the absorption profile of COF-1, suggesting that the addition of GO, g-C3N4, or BN does not substantially modify the optical properties of the COF-1 framework in the ultraviolet region. COF-1@g-C3N4 has an extra absorption band at around 450 nm, absent in the other composites (Figure 6b). The absorption at 450 nm is due to the inherent absorption of g-C3N4, a yellow-colored substance recognized for its activity in visible light. The existence of this peak validates the effective incorporation of g-C3N4 into the COF-1 matrix and suggests an expanded light absorption spectrum. The DRS results confirm the structural integrity of COF-1 in all composites and illustrate the functional effect of g-C3N4 in shifting optical absorption toward the visible spectrum.
Figure 7a illustrates the nitrogen adsorption–desorption isotherms for COF-1 and its composites with 2D materials (GO, g-C3N4, and BN). All materials display Type I isotherms characterized by a high uptake at low relative pressures (P/P0 < 0.1), suggesting their microporous characteristics. A hysteresis loop at high relative pressures (P/P0 = 0.8–0.9) is evident in the composites, highlighting the formation of supplementary mesopores or interparticle pores between COF-1 particles and 2D materials (i.e., GO, g-C3N4, and BN). The BET (Brunauer, Emmett and Teller) plots in Figure 7b validate linearity within the suitable relative pressure range, hence endorsing precise surface area calculations, as shown in Table 1.
Table 1 summarizes the specific surface areas (e.g., external surface area, micropore surface area, BET, and Langmuir) and pore sizes (e.g., BET, Barrett-Joyner-Halenda (BJH), and Dollimore-Heal (DH) method). COF-1 has a substantial BET surface area of 348 m2/g and a notable microporosity of 437 m2/g, characterized by small pore diameters ranging from 6.6 to 7.6 Å. Hybridization with GO, g-C3N4, or BN significantly decreases BET surface area (129–305 m2/g), presumably due to partial pore obstruction or structural distortion. These composites exhibit markedly increased average pore sizes (up to approximately 213 Å), as demonstrated by BJH and D-H techniques, highlighting the development of micro and meso-pore structure i.e. hierarchical porous structure. Among the composites, COF/BN has the highest surface area and external surface area (305 and 361 m2/g, respectively), but COF/GO, despite possessing the lowest BET area, features the most prominent pores, indicating a structure characterized by interfacial pores or voids. Data analysis suggests the formation of a hierarchically porous structure suitable for multifunctional applications, including adsorption.
2.2. Carbon Dioxide Adsorption
COF-1 and their composites (e.g., COF-1@GO, COF@g-C3N4, and COF@BN) were investigated for CO2 adsorption (Figure 8a,b). Figure 8a illustrates the CO2 adsorption–desorption isotherms for COF-1 and its composites with GO, g-C3N4, and BN. All materials have characteristic Type I isotherms, characterized by rapid adsorption at low relative pressures (P/P0 < 0.03), indicating strong interactions with CO2 molecules and the existence of microporous regions. The well-aligned adsorption and desorption branches at low pressures indicate reversible physisorption. As pressure increases, the adsorption capacity progressively increases, signifying the high adsorption capacities at high pressure.
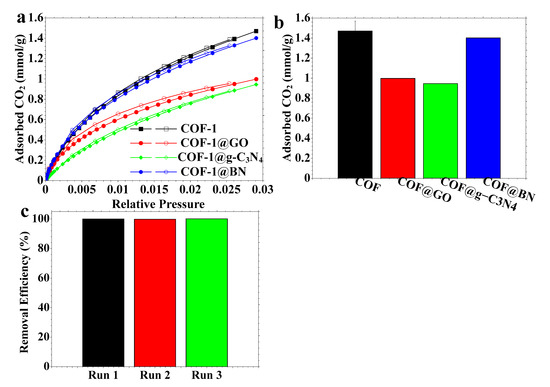
Figure 8.
(a) CO2 adsorption–desorption isotherms, (b) adsorption capacities for different materials, and (c) recyclability of CO2 adsorption using COF-1.
These isotherms’ quantitative CO2 adsorption capabilities further substantiate the material trends (Figure 8b). Pristine COF-1 exhibits the maximum CO2 adsorption at 1.47 mmol/g, owing to its high microporosity and well-defined pore architecture. COF@BN exhibits a near value of 1.40 mmol/g, owing to its hierarchical pore structure that enhances CO2 transport and retention. Conversely, COF@GO and COF@g-C3N4 demonstrate marginally reduced adsorption capabilities of 0.997 and 0.945 mmol/g, respectively (Figure 8b). This decrease may come from partial pore obstruction or a modified framework arrangement resulting from the incorporation of 2D nanosheets. Despite their low capacity, these composites exhibit commendable performance and provide supplementary functions (e.g., structural stability or improved light absorption) relevant to hybrid material applications. The material can be recycled for CO2 adsorption in three successive cycles without a drop in its adsorption capacity (Figure 8c).
Carbon dioxide (CO2) can be removed chemically via amine-forming carbamate, which undergoes decomposition over time, decreasing its capturing capacity over time [57]. Furthermore, adsorption is mainly a chemically governed mechanism. Thus, physical adsorption using porous materials like zeolites [58], porous carbons [59], metal–organic frameworks (MOFs) [60], and COFs have attracted considerable interest for their potential in CO2 capture applications (Table 2). Zeolites offer chemical and thermal stability; however, they exhibit performance degradation in humid conditions and require substantial energy for regeneration. Porous carbons facilitate high-pressure CO2 extraction and moisture resistance, although they exhibit selectivity and structural regulation challenges. MOFs characterized by high porosity, structural adaptability, and low energy demands for regeneration exhibit exceptional capabilities for CO2 capture. Nonetheless, their efficacy may decrease in humid environments because water preferentially binds to active metal sites. COFs, as a category of porous crystalline materials, offer a compelling alternative by integrating the advantageous characteristics of MOFs with improved stability and recyclability, even under humid conditions. Two-dimensional materials, such as boron nitride, enable the self-assembly and formation of porous foams with adsorbents like MOFs, resulting in effective CO2 adsorption [61].

Table 2.
Material adsorbents for CO2 adsorption.
A comparison among different materials was tabulated, including COF [62], MOF [61], and carbon materials [63] (Table 2). Assessing materials for CO2 adsorption is complex, as efficacy relies on multiple qualities rather than a singular characteristic. Evaluating materials only based on surface area or porosity may oversimplify performance results and overlook critical factors. There is no absolute correlation or relation between BET surface area and the adsorption capacities of CO2 absorption. Despite the extensive surface areas of activated carbons, their capacity to adsorb CO2 molecules may be constrained if their pore dimensions are inadequately sized or inaccessible. Zeolites and MOFs featuring ultramicropores, possessing moderate surface areas yet tailored micropore structures, can excel in low-pressure applications owing to enhanced van der Waals or electrostatic interactions. CO2 adsorption is significantly influenced by pore size, particularly in ultramicropores (<0.7 nm), where potential overlap enhances binding. Materials with minimal pore volume or surface area can exhibit considerable adsorption owing to the presence of narrowly distributed and accessible micropores. Chemical functionality is essential for CO2 capture, especially at low partial pressures. In diluted CO2 streams, amine-functionalized materials such as COF materials can enhance adsorption via acid–base interactions. Nonetheless, these interactions may influence desorption efficiency or stability. Materials that are purely physisorptive exhibit greater reversibility but lower efficacy under ambient conditions due to their weaker interactions. Composite or hybrid materials, including polymer–inorganic frameworks, can improve CO2 adsorption through synergistic mechanisms. These systems cannot be evaluated against homogeneous materials such as pure zeolites or carbon adsorbents using a singular property metric, as functional domain distribution and interactions influence performance. El-Kaderi et al. reported the synthesis of a two-dimensional mesoporous COF (TDCOF-5) originating from triptycene [62]. The substance was synthesized by condensing 1,4-benzenediboronic acid and hexahydroxytriptycene, resulting in boronate links via a solvothermal process. The synthesis occurred in a mixed solvent system of mesitylene and 1,4-dioxane at 120 °C for five days. The resultant TDCOF-5 exhibited CO2 adsorption capability of 92 mg/g at 273 K and 1 bar pressure [62]. A “self-supporting foam” was reported to create a thermally conductive composite foam consisting of MOFs and boron nitride nanosheets (BNNSs), interconnected by polyethyleneimine (PEI) cross-linking, referred to as MOFs@BNNS-PEI [61]. The adaptable approach facilitates the effective integration of diverse representative MOFs: HKUST-1, MIL-100(Fe), and ZIF-8. Due to these synergistic effects, the MOFs@BNNS-PEI composite demonstrates a markedly enhanced CO2 adsorption capacity, 1.35 to 1.42 times greater than pure MOFs, and a 5.0- to 5.7-fold increase in CO2 desorption rate (Table 2) [61]. HKUST-1@BNNS-PEI, MIL-100@BNNS-PEI, and ZIF-8@BNNS-PEI exhibited CO2 capacities of 4.47, 1.51, and 0.96 mmol/g compared to pristine MOFs (3.15, 1.10, and 0.71 mmol/g), respectively [61]. Activated carbons were synthesized via hydrothermal carbonization of waste biomass [63]. The material was used for CO2 adsorption, offering capacities of 1.45 mmol/g at a low CO2 partial pressure of 10 kPa and a temperature of 0 °C [63]. Our materials exhibit comparable CO2 adsorption compared to reported materials, e.g., COF [62], MOF [61], and carbon materials [63] (Table 2). Our materials exhibit high porosity, enabling high adsorption capacities with high potential for use in the adsorption of other molecules.
3. Experimental
3.1. Materials and Methods
Melamine (Merck, Rahway, NJ, USA), dimethyl sulfoxide (SDFCL, Mumbai, India), and sulfuric acid (SDFCL, Mumbai, India) were used without further purification. Graphite flakes (+100 mesh, ≥75%) and terephthaldehyde were purchased from Fluka (Germany). Boron nitride (BN, powder, size of 1 μm, 98%) was purchased from Sigma-Aldrich (Steinheim, Germany).
3.2. Synthesis of GO
GO was synthesized according to Hummer’s method (Figure 1a) [64,65,66]. Graphite powder (2 g) and sodium nitrate (2 g) were introduced to concentrated sulfuric acid (100 mL). The addition was performed in an ice bath and stirred continuously for 4 h. Thereafter, potassium permanganate (12 g) was gradually introduced while maintaining constant agitation. Afterward, 200 mL of double-distilled water was carefully added, and the temperature was maintained at 37 °C for 2 h. The reaction mixture was further diluted with additional double-distilled water, and the temperature was then elevated to 98 °C and maintained for 2 h. To reduce the excess potassium permanganate, 40 mL of hydrogen peroxide was added while stirring for 1 h, yielding a yellow solution. The suspension was subsequently centrifuged (6000 rpm, 10 min) to separate the GO, which was washed thrice with double-distilled water to eliminate unreacted substances and residual salts.
3.3. Synthesis of g-C3N4
g-C3N4 was synthesized via polymerization of melamine (Figure 1b) [67,68]. A semi-closed system was employed to prevent the sublimation of melamine during heating. Specifically, 5 g of melamine powder was added into a covered alumina crucible and heated in a muffle furnace. The initial heating was conducted at 550 °C for 2 h with a heating rate of 10 °C/min. The material was then washed with water and ethanol, followed by overnight drying.
3.4. Exfoliation of Boron Nitride
The exfoliation of boron nitride was carried out via a water-based method (Figure 1c). Briefly, a bulk boron nitride (2 g) was refluxed (heating at 120 °C) in water for 3 days. The exfoliated material in water was dropped onto a glass plate and then allowed to dry. The flakes were collected and used for COF/BN synthesis.
3.5. Synthesis of COF-1
COF-1 was synthesized via a solvothermal approach (Figure 2) [69]. In a typical procedure, melamine (4 mmol, 0.5 g) and terephthaldehyde (6 mmol, 0.8 g) were dissolved in 25 mL of degassed dimethyl sulfoxide under a nitrogen atmosphere. The reaction mixture was stirred vigorously and heated at 180 °C for 72 h. The resulting precipitate was filtered and thoroughly washed with tetrahydrofuran and N,N-dimethylformamide before being dried under vacuum at 120 °C overnight.
3.6. Synthesis of COF-1-Based Composites
To prepare the nanocomposites, 300 mg of GO, BN, or g-C3N4 was dispersed in 25 mL of DMSO using ultrasonication [56]. Subsequently, melamine (4 mmol) and terephthaldehyde (6 mmol, 0.8 g) were dispersed to the prepared solutions. The mixture was then subjected to solvothermal treatment at 180 °C for 3 days. After cooling, the precipitates were isolated and washed, as mentioned above.
3.7. Characterization
XRD patterns were collected using X’Pert PRO PANAlytical (Bruker, Billerica, MA, USA). TEM (Philips, Amstelplein, the Netherlands) was used to determine the morphology and particle size. Functional groups on the surface were studied using FT-IR (IRTracer-100, SHIMADZU, Kyoto, Japan). Diffuse reflectance spectroscopy (DRS) spectra were recorded using UV-2600i (SHIMADZU, Kyoto, Japan).
3.8. Gases (Nitrogen and CO2) Adsorption
The specific surface areas (Brunauer–Emmett–Teller (BET), Langmuir, micropore surface area, t-plot external surface area, m2/g) were determined using N2 isotherms (at 77 K) using an ASAP 2000 analyzer (Micrometric, Lincoln, UK). The sample was degassed at 110 °C under vacuum for 3 h. Pore size (Å) was evaluated using adsorption average pore width (4 V/A by BET), desorption average pore width (4 V/A by BET), BJH (Barrett, Joyner, and Halenda) adsorption average pore diameter (4 V/A), BJH desorption average pore diameter (4 V/A), D-H (Dollimore–Heal) adsorption average pore diameter (4 V/A), and D-H desorption average pore diameter (4 V/A).
4. Conclusions
This study emphasizes the synthesis and characterization of COF-1 and its composite variants with GO, g-C3N4, and BN. XRD and FT-IR structural analysis validated the integrity of the COF and identified functional groups from both components in the composites. TEM images provided morphological insights, revealing well-organized polycrystalline particles and the efficient incorporation of two-dimensional additives, which facilitated the development of heterogeneous interfaces. Nitrogen sorption analyses showed that the pure COF possessed a high surface area and microporosity, but the integration of 2D materials resulted in the formation of additional mesopores, as demonstrated by hysteresis in the isotherms and enlarged average pore sizes. The results demonstrate the effectiveness of integrating 2D materials in modifying the textural and adsorption properties of COFs, positioning these hybrids as viable options for gas storage and separation technologies.
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Solomon, S.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse gases emissions and global climate change: Examining the influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef] [PubMed]
- Eskander, S.M.S.U.; Fankhauser, S. Reduction in greenhouse gas emissions from national climate legislation. Nat. Clim. Change 2020, 10, 750–756. [Google Scholar] [CrossRef]
- Kabir, M.; Habiba, U.E.; Khan, W.; Shah, A.; Rahim, S.; De los Rios-Escalante, P.R.; Farooqi, Z.-U.-R.; Ali, L.; Shafiq, M. Climate change due to increasing concentration of carbon dioxide and its impacts on environment in 21st century; a mini review. J. King Saud Univ.-Sci. 2023, 35, 102693. [Google Scholar] [CrossRef]
- Montzka, S.A.; Dlugokencky, E.J.; Butler, J.H. Non-CO2 greenhouse gases and climate change. Nature 2011, 476, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Das, R.; Nagaraja, C.M. A review on framework (MOF/COF/POP)-based materials for efficient conversion of CO2 to bio-active oxazolidinones. Inorg. Chem. Front. 2025, 12, 430–478. [Google Scholar] [CrossRef]
- Yoganandan, G.; Saito, R.; Yano, K.; Sakairi, M.; Fushimi, K.; Kitagaki, R.; Elakneswaran, Y.; Senboku, H.; Yoda, Y.; Tsu-jino, M.; et al. Alkaline ethanolamine as dual-functional agent: Effective CO2 capture agent and corrosion inhibitor for structural applications. Chem. Eng. J. 2025, 504, 158810. [Google Scholar] [CrossRef]
- Kopitha, K.; Elakneswaran, Y.; Kitagaki, R.; Saito, R.; Yano, K.; Yoda, Y.; Tsujino, M.; Nishida, A.; Senboku, H.; Fushimi, K.; et al. Enhancing CO2 capture in cement-based materials with alkanolamines: A comprehensive study on efficiency, phase-specific impact, and carbonation mechanisms. Sci. Total Environ. 2024, 957, 177463. [Google Scholar] [CrossRef]
- Kazlou, T.; Cherp, A.; Jewell, J. Feasible deployment of carbon capture and storage and the requirements of climate targets. Nat. Clim. Change 2024, 14, 1047–1055. [Google Scholar] [CrossRef]
- Bukar, A.M.; Asif, M. Technology readiness level assessment of carbon capture and storage technologies. Renew. Sustain. Energy Rev. 2024, 200, 114578. [Google Scholar] [CrossRef]
- He, Y.; Yin, L.; Yuan, N.; Zhang, G. Adsorption and activation, active site and reaction pathway of photocatalytic CO2 reduction: A review. Chem. Eng. J. 2024, 481, 148754. [Google Scholar] [CrossRef]
- Jalilian, M.; Bissessur, R.; Ahmed, M.; Hsiao, A.; He, Q.S.; Hu, Y. A review: Hydrochar as potential adsorbents for wastewater treatment and CO2 adsorption. Sci. Total Environ. 2024, 914, 169823. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Chen, Y.; He, X.; Wang, F.; Cai, Q.; Shen, B. Insights into the adsorption of CO2, SO2 and NOx in flue gas by carbon materials: A critical review. Chem. Eng. J. 2024, 490, 151424. [Google Scholar] [CrossRef]
- Liu, M.; Xu, Q.; Zeng, G. Ionic Covalent Organic Frameworks in Adsorption and Catalysis. Angew. Chemie 2024, 136, e202404886. [Google Scholar] [CrossRef]
- Waller, P.J.; Gándara, F.; Yaghi, O.M. Chemistry of Covalent Organic Frameworks. Acc. Chem. Res. 2015, 48, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dincă, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The Current Status of MOF and COF Applications. Angew. Chemie Int. Ed. 2021, 60, 23975–24001. [Google Scholar] [CrossRef]
- Ge, S.; Wei, K.; Peng, W.; Huang, R.; Akinlabi, E.; Xia, H.; Shahzad, M.W.; Zhang, X.; Xu, B.B.; Jiang, J. A comprehensive review of covalent organic frameworks (COFs) and their derivatives in environmental pollution control. Chem. Soc. Rev. 2024, 53, 11259–11302. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Z.; Guan, J. Optimization Strategies of Covalent Organic Frameworks and Their Derivatives for Electrocatalytic Applications. Adv. Funct. Mater. 2024, 34, 2310195. [Google Scholar] [CrossRef]
- Lee, W.; Li, H.; Du, Z.; Feng, D. Ion transport mechanisms in covalent organic frameworks: Implications for technology. Chem. Soc. Rev. 2024, 53, 8182–8201. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Su, A.; Gong, C.; Chen, S.; Xia, L.; Zhang, C.; Tao, X.; Li, Y.; Li, Y.; et al. Revolutionizing the structural design and determination of covalent–organic frameworks: Principles, methods, and techniques. Chem. Soc. Rev. 2024, 53, 502–544. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-H.; Xu, C.; Yang, Y.-W. Macrocycle-embedded metal-covalent organic frameworks for catalysis: A bridge between covalent and non-covalent functional frameworks. Coord. Chem. Rev. 2024, 512, 215894. [Google Scholar] [CrossRef]
- Chen, F.; Zheng, H.; Yusran, Y.; Li, H.; Qiu, S.; Fang, Q. Exploring high-connectivity three-dimensional covalent organic frameworks: Topologies, structures, and emerging applications. Chem. Soc. Rev. 2025, 54, 484–514. [Google Scholar] [CrossRef]
- Xue, S.; Ma, X.; Wang, Y.; Duan, G.; Zhang, C.; Liu, K.; Jiang, S. Advanced development of three-dimensional covalent organic frameworks: Valency design, functionalization, and applications. Coord. Chem. Rev. 2024, 504, 215659. [Google Scholar] [CrossRef]
- Daliran, S.; Blanco, M.; Dhakshinamoorthy, A.; Oveisi, A.R.; Alemán, J.; García, H. Defects and Disorder in Covalent Organic Frameworks for Advanced Applications. Adv. Funct. Mater. 2024, 34, 2312912. [Google Scholar] [CrossRef]
- Furukawa, H.; Yaghi, O.M. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef]
- Asayesh-Ardakani, E.; Rahmani, M.; Hosseinian, A.; Ghaffari, S.-B.; Sarrafzadeh, M.-H. Improvement strategies on application of covalent organic frameworks in adsorption, photocatalytic, and membrane processes for organic pollution removal from water. Coord. Chem. Rev. 2024, 518, 216087. [Google Scholar] [CrossRef]
- Qin, L.; Ma, C.; Zhang, J.; Zhou, T. Structural Motifs in Covalent Organic Frameworks for Photocatalysis. Adv. Funct. Mater. 2024, 34, 2401562. [Google Scholar] [CrossRef]
- Xue, R.; Liu, Y.-S.; Huang, S.-L.; Yang, G.-Y. Recent Progress of Covalent Organic Frameworks Applied in Electrochemical Sensors. ACS Sens. 2023, 8, 2124–2148. [Google Scholar] [CrossRef]
- Mehvari, F.; Ramezanzade, V.; Asadi, P.; Singh, N.; Kim, J.; Dinari, M.; Kim, J.S. A panoramic perspective of recent progress in 2D and 3D covalent organic frameworks for drug delivery. Aggregate 2024, 5, e480. [Google Scholar] [CrossRef]
- Dubey, P.; Shrivastav, V.; Boruah, T.; Zoppellaro, G.; Zbořil, R.; Bakandritsos, A.; Sundriyal, S. Unveiling the Potential of Covalent Organic Frameworks for Energy Storage: Developments, Challenges, and Future Prospects. Adv. Energy Mater. 2024, 14, 2400521. [Google Scholar] [CrossRef]
- Shahzad, U.; Marwani, H.M.; Saeed, M.; Asiri, A.M.; Repon, M.R.; Althomali, R.H.; Rahman, M.M. Progress and Perspectives on Promising Covalent-Organic Frameworks (COFs) Materials for Energy Storage Capacity. Chem. Rec. 2024, 24, e202300285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhi, P.; Zhang, J.; Duan, S.; Yao, X.; Liu, S.; Sun, Z.; Jun, S.C.; Zhao, N.; Dai, L.; et al. Engineering Covalent Organic Frameworks Toward Advanced Zinc-Based Batteries. Adv. Mater. 2024, 36, 2313152. [Google Scholar] [CrossRef]
- Zeng, Y.; Zou, R.; Zhao, Y. Covalent Organic Frameworks for CO2 Capture. Adv. Mater. 2016, 28, 2855–2873. [Google Scholar] [CrossRef]
- Olajire, A.A. Recent advances in the synthesis of covalent organic frameworks for CO2 capture. J. CO2 Util. 2017, 17, 137–161. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, T.; Zhang, H.; Chheda, S.; Li, H.; Wang, K.; Ehrling, S.; Giovine, R.; Li, C.; Alawadhi, A.H.; et al. Carbon dioxide capture from open air using covalent organic frameworks. Nature 2024, 635, 96–101. [Google Scholar] [CrossRef]
- Ozdemir, J.; Mosleh, I.; Abolhassani, M.; Greenlee, L.F.; Beitle, R.R.; Beyzavi, M.H. Covalent Organic Frameworks for the Capture, Fixation, or Reduction of CO2. Front. Energy Res. 2019, 7, 77. [Google Scholar] [CrossRef]
- Sani, R.; Dey, T.K.; Sarkar, M.; Basu, P.; Islam, S.M. A study of contemporary progress relating to COF materials for CO2 capture and fixation reactions. Mater. Adv. 2022, 3, 5575–5597. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, M.; Liu, J.; Chen, Y.; Liao, J.; Yang, M.; Cai, Y.; Li, S.; Lan, Y. Covalent Organic Framework Based Functional Materials: Important Catalysts for Efficient CO2 Utilization. Angew. Chemie 2022, 134, e202200003. [Google Scholar] [CrossRef]
- Wu, Z.; Li, W.; Hou, L.; Wei, Q.; Yang, H.; Jiang, Y.; Tang, D. A novel Sunflower-like MOF@COF for improved photocatalytic CO2 reduction. Sep. Purif. Technol. 2023, 311, 123322. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Z.; Zhang, L.; Zhang, Q. Covalent Organic Frameworks for Photocatalytic Reduction of Carbon Dioxide: A Review. ACS Nano 2024, 18, 21804–21835. [Google Scholar] [CrossRef]
- Lin, S.; Diercks, C.S.; Zhang, Y.B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.; Yaghi, O.M.; et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, L.; Lv, Y.; Tan, T. Facile manufacture of COF-based mixed matrix membranes for efficient CO2 separation. Chem. Eng. J. 2022, 430, 133001. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, H.; Wu, S.; Song, S.; Guo, Z.; Ren, Y.; Zhao, R.; Yang, L.; Wu, Y.; Jiang, Z. Multifunctional covalent organic framework (COF)-Based mixed matrix membranes for enhanced CO2 separation. J. Memb. Sci. 2021, 618, 118693. [Google Scholar] [CrossRef]
- Cheng, Y.; Ying, Y.; Zhai, L.; Liu, G.; Dong, J.; Wang, Y.; Christopher, M.P.; Long, S.; Wang, Y.; Zhao, D. Mixed matrix membranes containing MOF@COF hybrid fillers for efficient CO2/CH4 separation. J. Memb. Sci. 2019, 573, 97–106. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Z.; Ma, T.; Wang, K.; Zhang, H.; Alawadhi, A.H.; Yaghi, O.M. Bonding of Polyethylenimine in Covalent Organic Frameworks for CO2 Capture from Air. J. Am. Chem. Soc. 2024, 146, 35486–35492. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jin, Y.; Li, C.; Fan, Y.; Kawi, S.; Meng, X.; Song, J.; Yang, N. COF/MXene composite membranes compact assembled by electrostatic interactions: A strategy for H2/CO2 separation. J. Memb. Sci. 2024, 700, 122678. [Google Scholar] [CrossRef]
- Yang, F.; Hu, P.; Yang, F.; Hua, X.-J.; Chen, B.; Gao, L.; Wang, K.-S. Photocatalytic applications and modification methods of two-dimensional nanomaterials: A review. Tungsten 2024, 6, 77–113. [Google Scholar] [CrossRef]
- Hu, J.; Dong, M. Recent advances in two-dimensional nanomaterials for sustainable wearable electronic devices. J. Nanobiotechnol. 2024, 22, 63. [Google Scholar] [CrossRef]
- Manoharan, A.K.; Batcha, M.I.K.; Mahalingam, S.; Raj, B.; Kim, J. Recent Advances in Two-Dimensional Nanomaterials for Healthcare Monitoring. ACS Sens. 2024, 9, 1706–1734. [Google Scholar] [CrossRef]
- Wang, D.; Dou, Y.; Zhang, X.; Bi, K.; Panneerselvam, I.R.; Sun, H.; Jiang, X.; Dai, R.; Song, K.; Zhuang, H.; et al. Manufacturing and applications of multi-functional holey two-dimensional nanomaterials—A review. Nano Today 2024, 55, 102162. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abdelhamid, H.N.; Abuelftooh, A.M.; Mohamed, S.G.; Wen, Z.; Sun, X. Covalent organic frameworks (COFs)-derived nitrogen-doped carbon/reduced graphene oxide nanocomposite as electrodes materials for supercapacitors. J. Energy Storage 2022, 55, 105375. [Google Scholar] [CrossRef]
- Ashour, R.M.; Abdelhamid, H.N.; Abdel-Magied, A.F.; Abdel-khalek, A.A.; Ali, M.; Uheida, A.; Muhammed, M.; Zou, X.; Dutta, J. Rare Earth Ions Adsorption onto Graphene Oxide Nanosheets. Solvent Extr. Ion Exch. 2017, 35, 91–103. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Wu, H.-F. Ultrasensitive, Rapid, and Selective Detection of Mercury Using Graphene Assisted Laser Desorption/Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2014, 25, 861–868. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Wu, H.-F. Reduced graphene oxide conjugate thymine as a new probe for ultrasensitive and selective fluorometric determination of mercury(II) ions. Microchim. Acta 2015, 182, 1609–1617. [Google Scholar] [CrossRef]
- Abdellah, A.R.; Abdelhamid, H.N.; El-Adasy, A.-B.A.A.M.; Atalla, A.A.; Aly, K.I. One-pot synthesis of hierarchical porous covalent organic frameworks and two-dimensional nanomaterials for selective removal of anionic dyes. J. Environ. Chem. Eng. 2020, 8, 104054. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Gargiulo, N.; Pepe, F.; Caputo, D. CO2 Adsorption by Functionalized Nanoporous Materials: A Review. J. Nanosci. Nanotechnol. 2014, 14, 1811–1822. [Google Scholar] [CrossRef]
- Kolle, J.M.; Fayaz, M.; Sayari, A. Understanding the Effect of Water on CO2 Adsorption. Chem. Rev. 2021, 121, 7280–7345. [Google Scholar] [CrossRef]
- Lee, G.; Ahmed, I.; Jhung, S.H. CO2 adsorption using functionalized metal–organic frameworks under low pressure: Contribution of functional groups, excluding amines, to adsorption. Chem. Eng. J. 2024, 481, 148440. [Google Scholar] [CrossRef]
- Li, S.; Yu, X.; Liu, L.; Zhou, L.; Jia, Z.; Chen, J.; Zhao, Z.; Zhao, Z. Thermal-Conductive MOFs@BN Self-Supporting Foams for Synchronously Boosting CO2 Adsorption/Desorption. Adv. Funct. Mater. 2024, 34, 2401955. [Google Scholar] [CrossRef]
- Kahveci, Z.; Islamoglu, T.; Shar, G.A.; Ding, R.; El-Kaderi, H.M. Targeted synthesis of a mesoporous triptycene-derived covalent organic framework. CrystEngComm 2013, 15, 1524–1527. [Google Scholar] [CrossRef]
- Hao, W.; Björkman, E.; Lilliestråle, M.; Hedin, N. Activated carbons prepared from hydrothermally carbonized waste biomass used as adsorbents for CO2. Appl. Energy 2013, 112, 526–532. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Hua, P.-Y.; Manikandan, M.; Abdelhamid, H.N.; Wu, H.-F. Graphene nanoflakes as an efficient ionizing matrix for MALDI-MS based lipidomics of cancer cells and cancer stem cells. J. Mater. Chem. B 2014, 2, 7334–7343. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Hussein, K.H. Graphene Oxide as a Carrier for Drug Delivery of Methotrexate. Biointerface Res. Appl. Chem. 2021, 11, 14726–14735. [Google Scholar] [CrossRef]
- Ibrahim, M.; Fayed, M.G.; Mohamed, S.G.; Wen, Z.; Sun, X.; Nasser Abdelhamid, H. High-Performance Lithium-Ion Battery and Supercapacitors Using Covalent Organic Frameworks (COFs)/Graphitic Carbon Nitride (g-C3N4)-Derived Hierarchical N-Doped Carbon. ACS Appl. Energy Mater. 2022, 5, 12828–12836. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation Performance of g-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Schwab, M.G.; Fassbender, B.; Spiess, H.W.; Thomas, A.; Feng, X.; Müllen, K. Catalyst-free Preparation of Melamine-Based Microporous Polymer Networks through Schiff Base Chemistry. J. Am. Chem. Soc. 2009, 131, 7216–7217. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).