Photocatalytic Properties of Office-Paper-Waste-Derived Activated Carbon for Efficient Degradation of Organic Pollutants
Abstract
1. Introduction
2. Results and Discussion
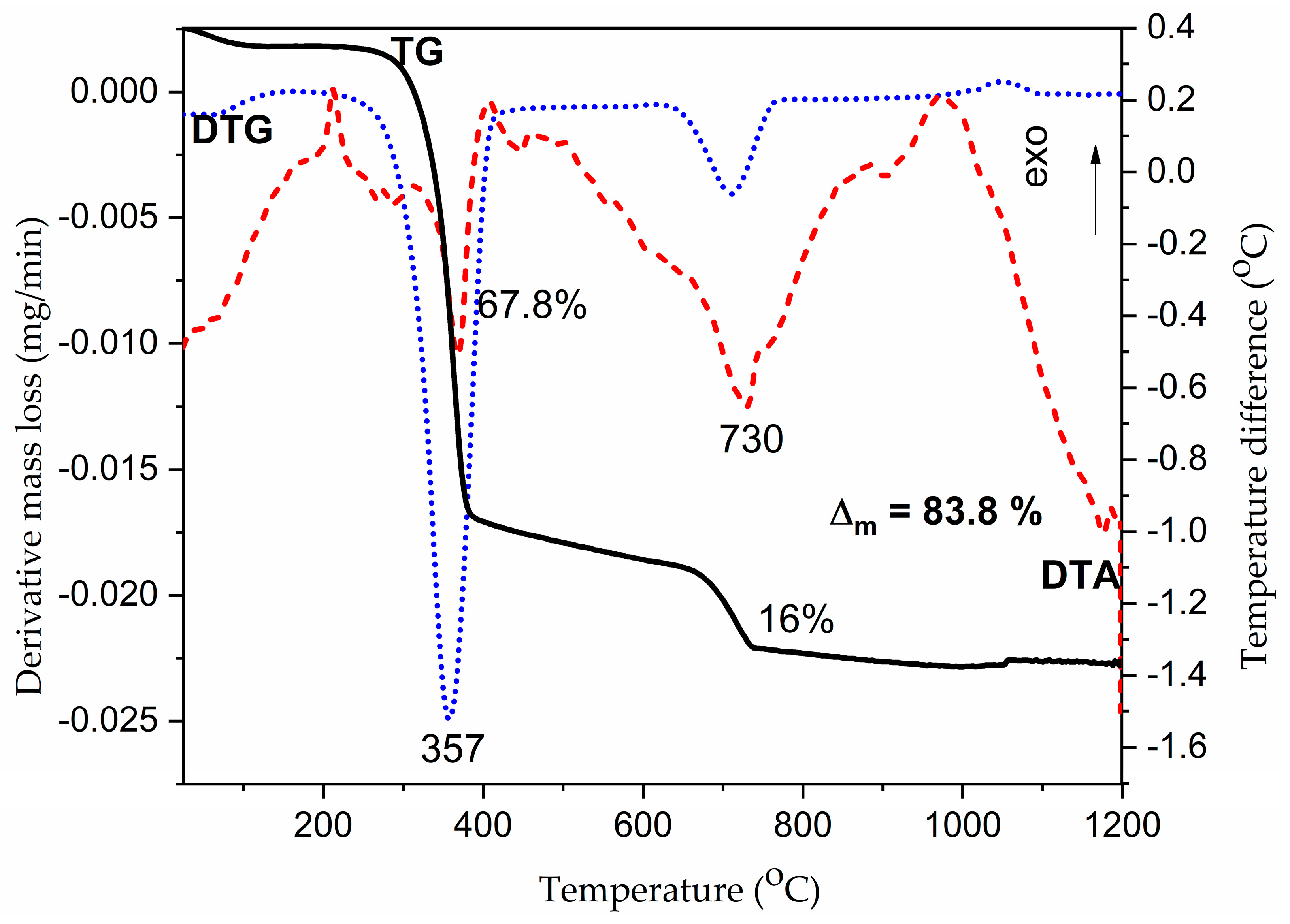
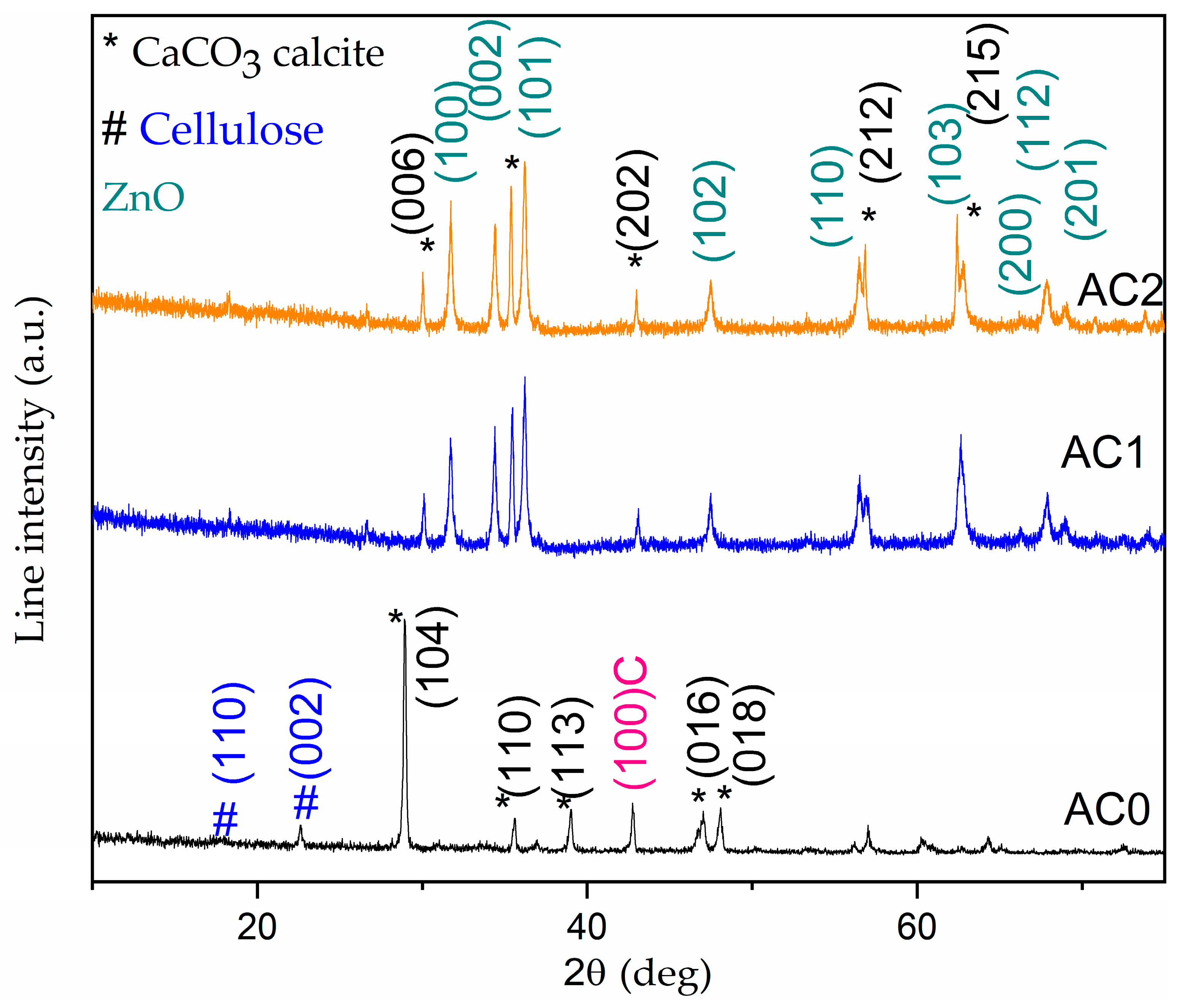
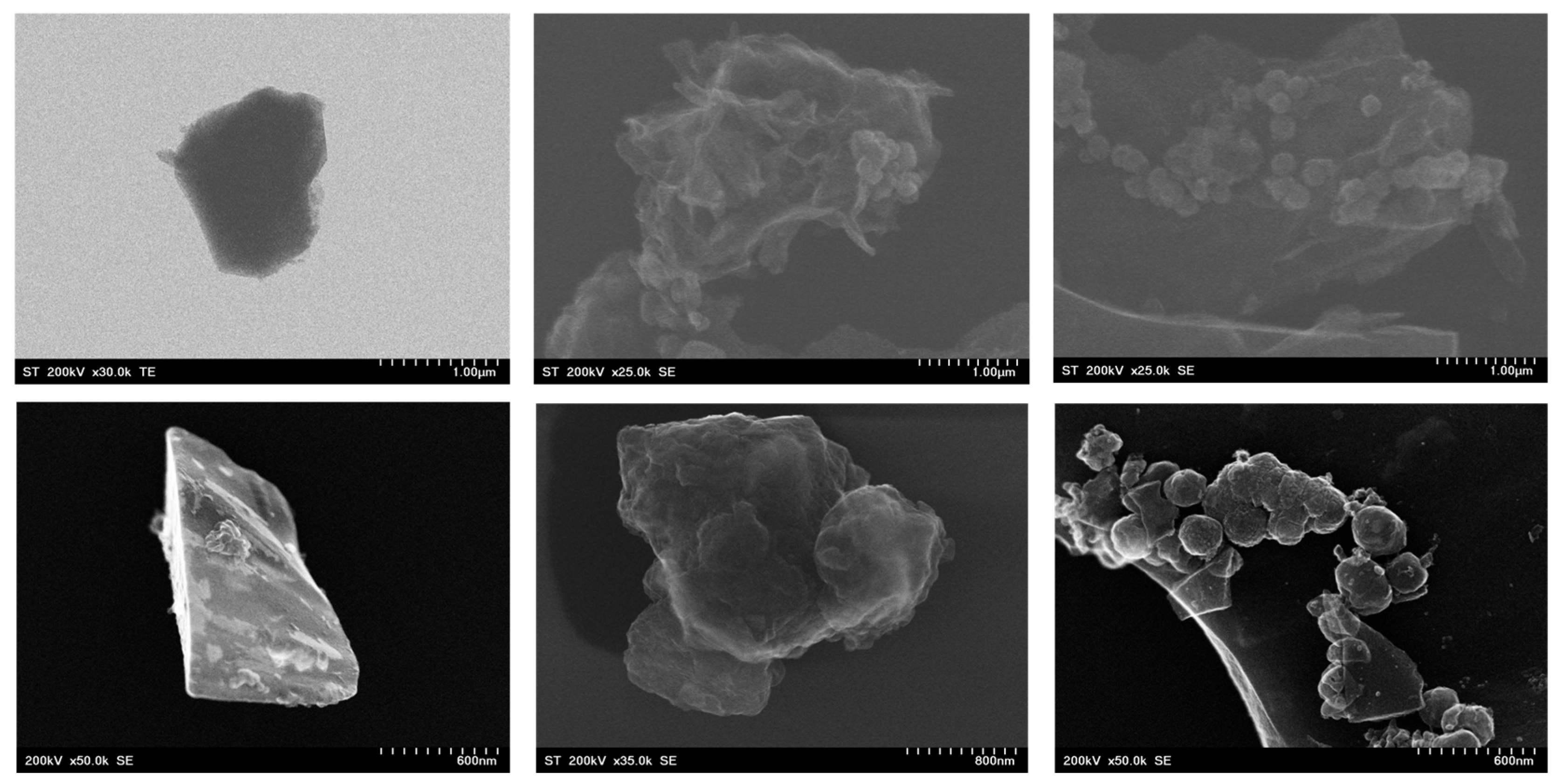
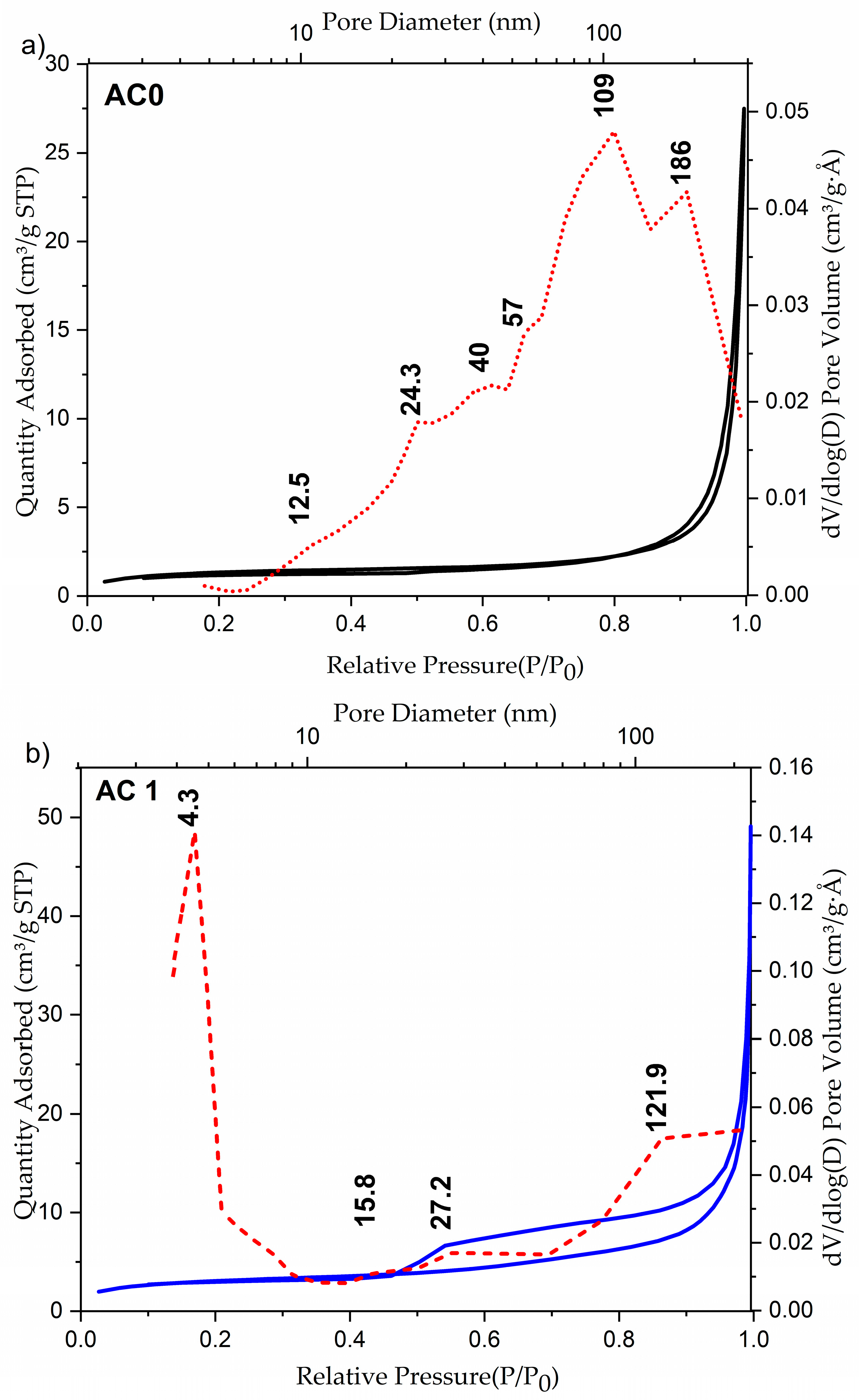
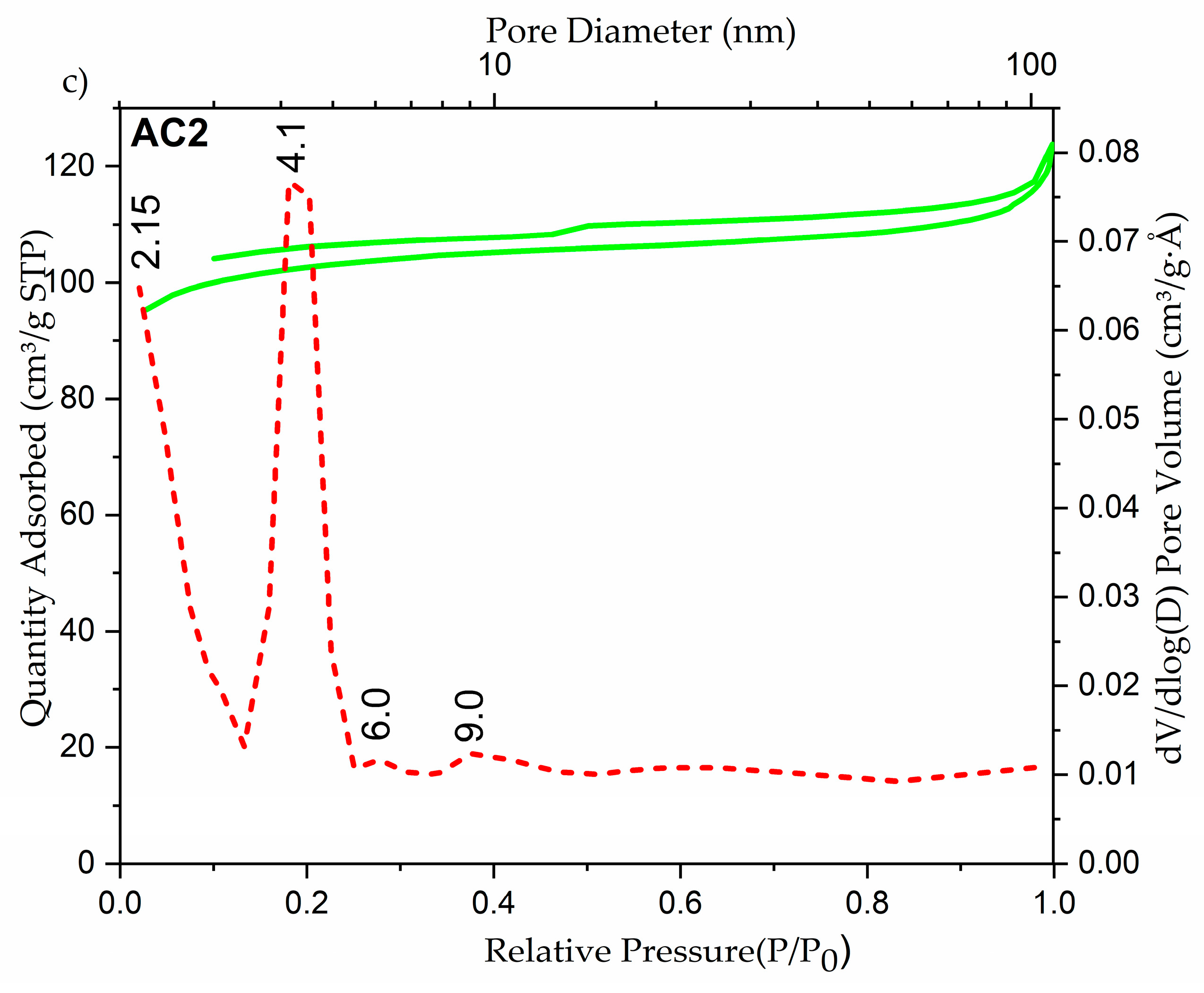
2.1. Structural and Morphological Investigations of AC Samples
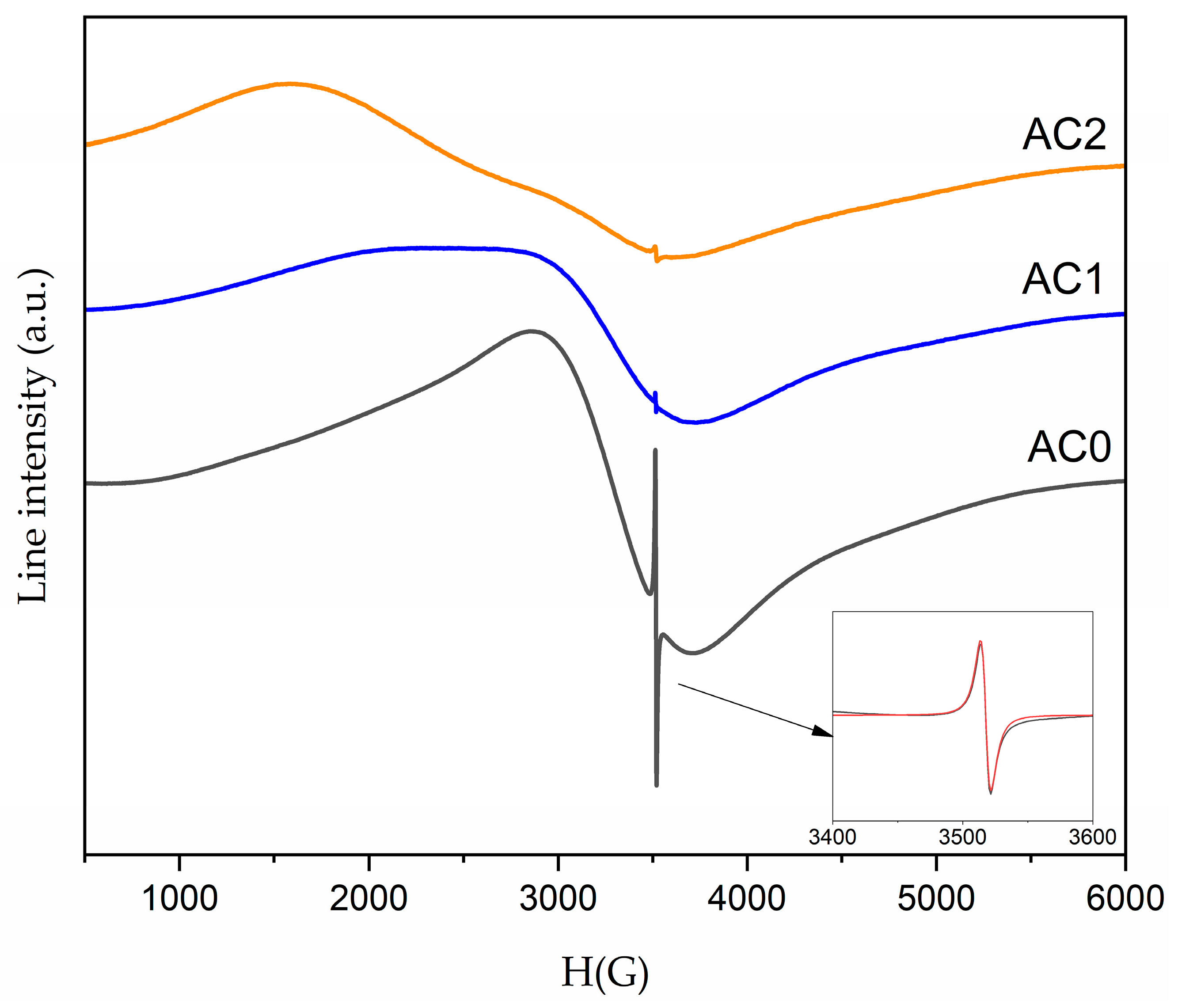
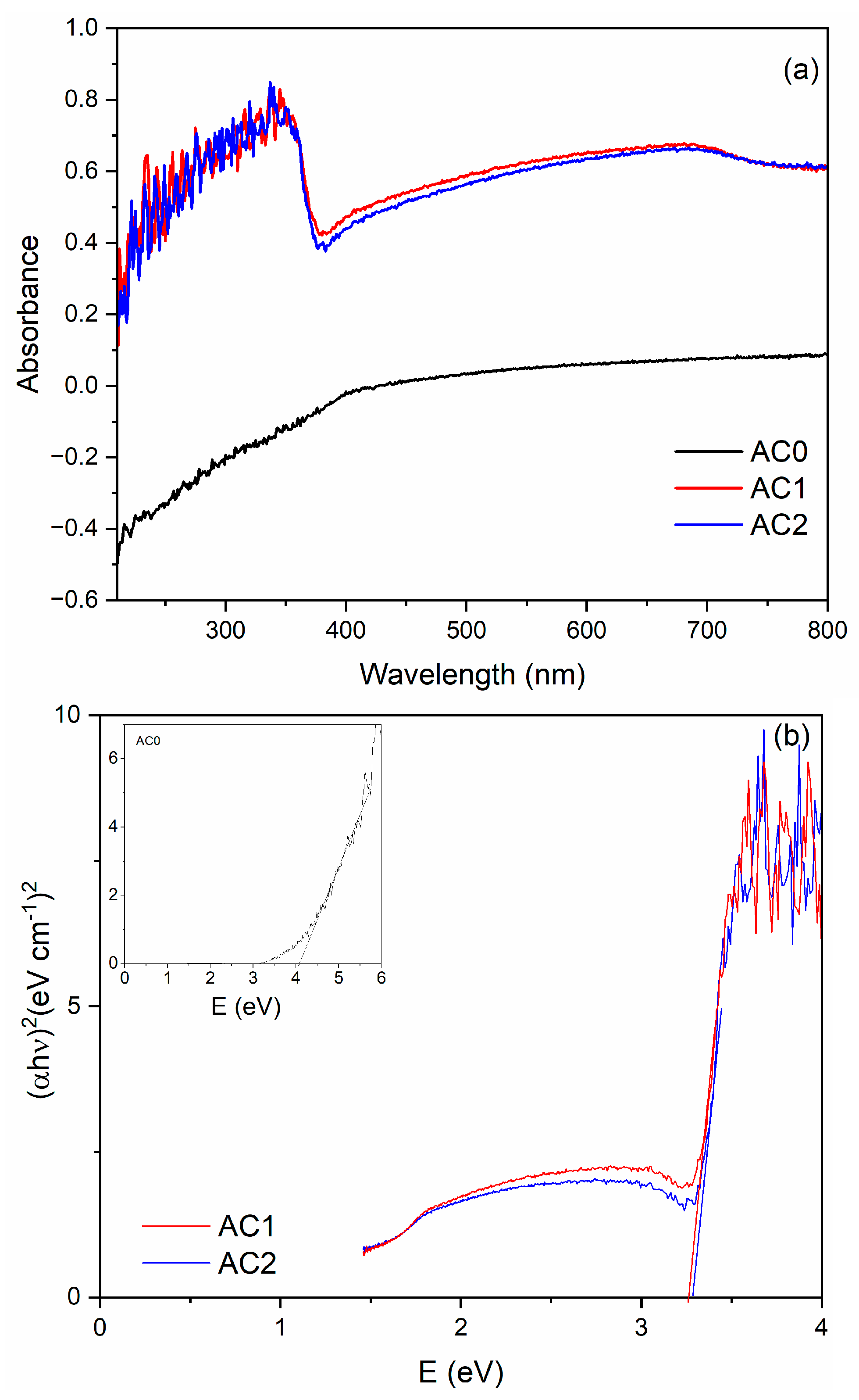
2.2. Optical Properties
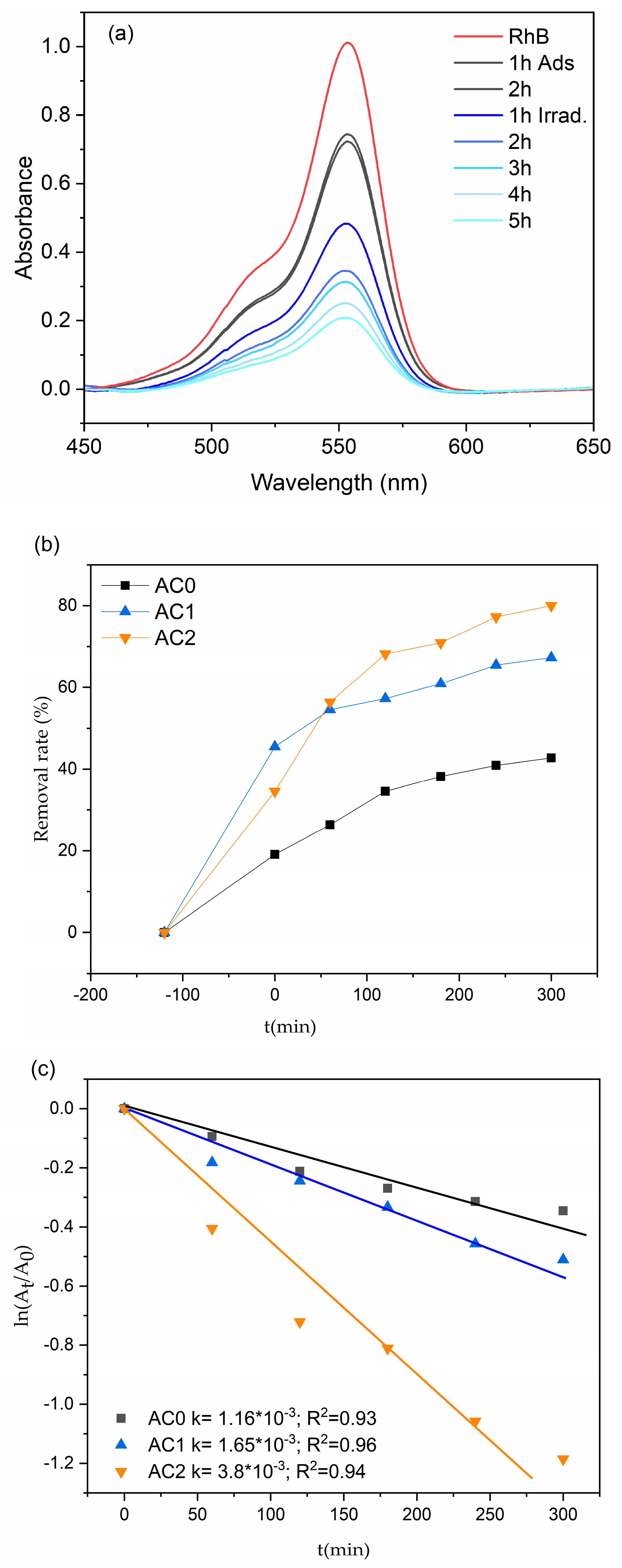
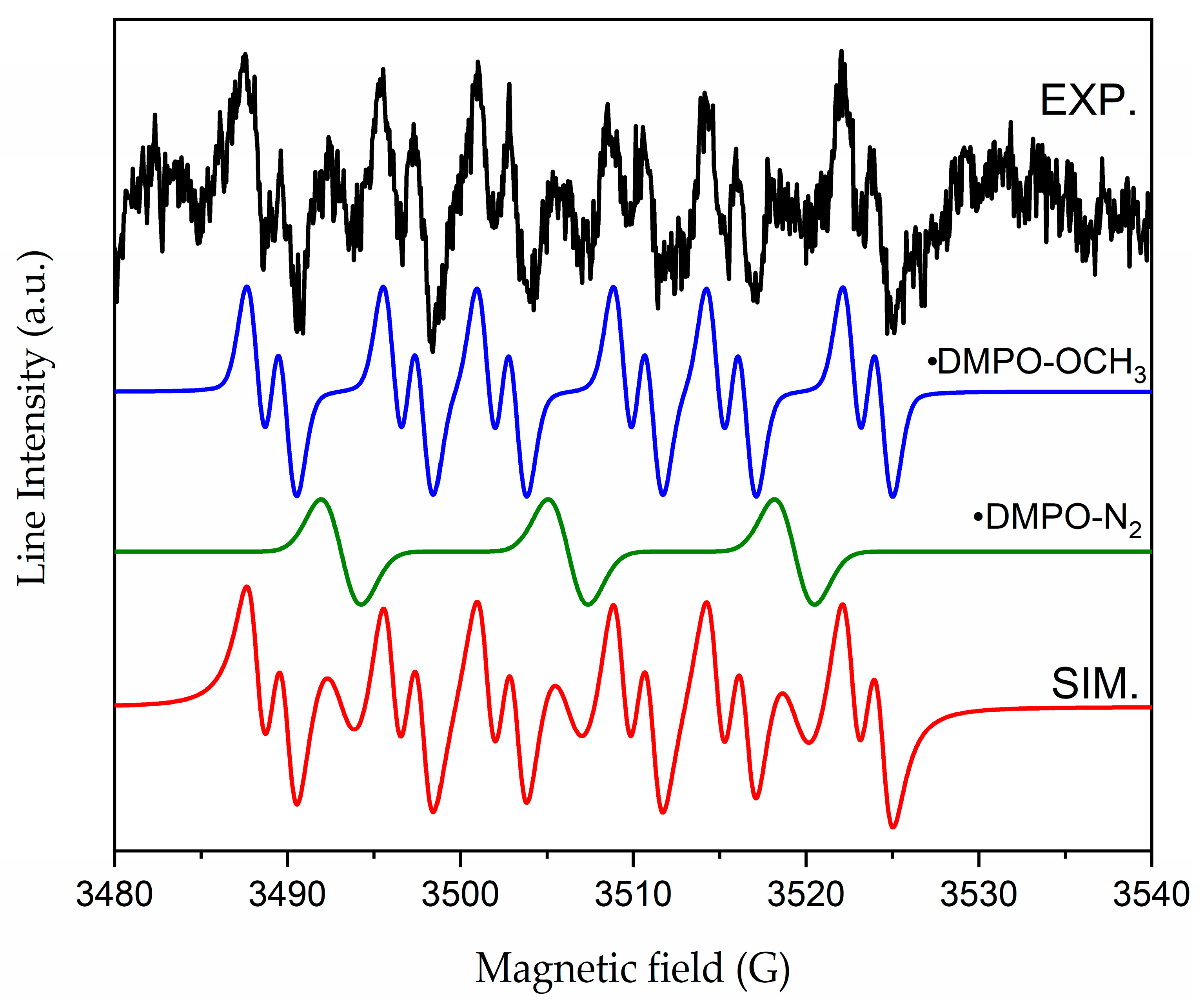
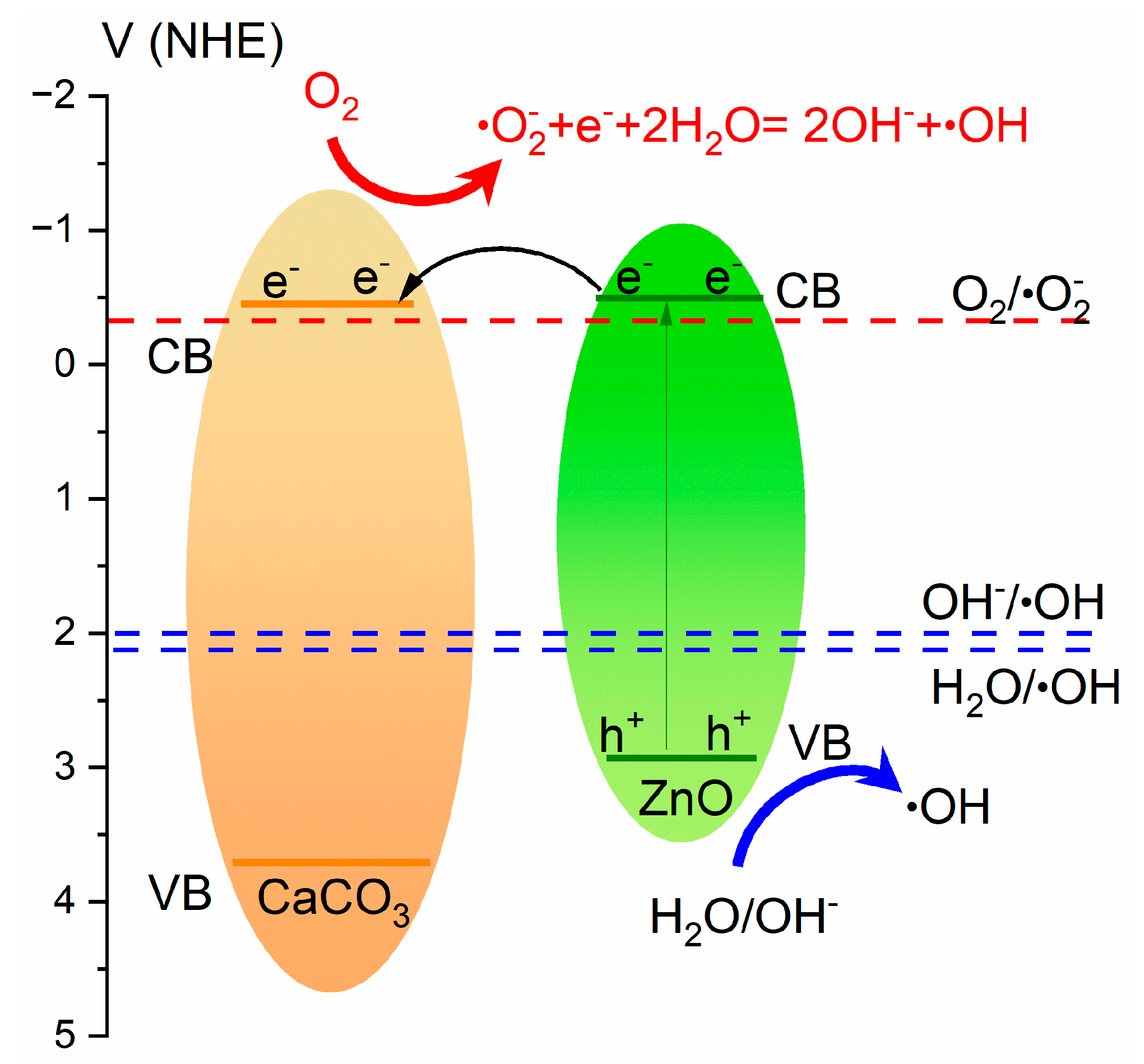
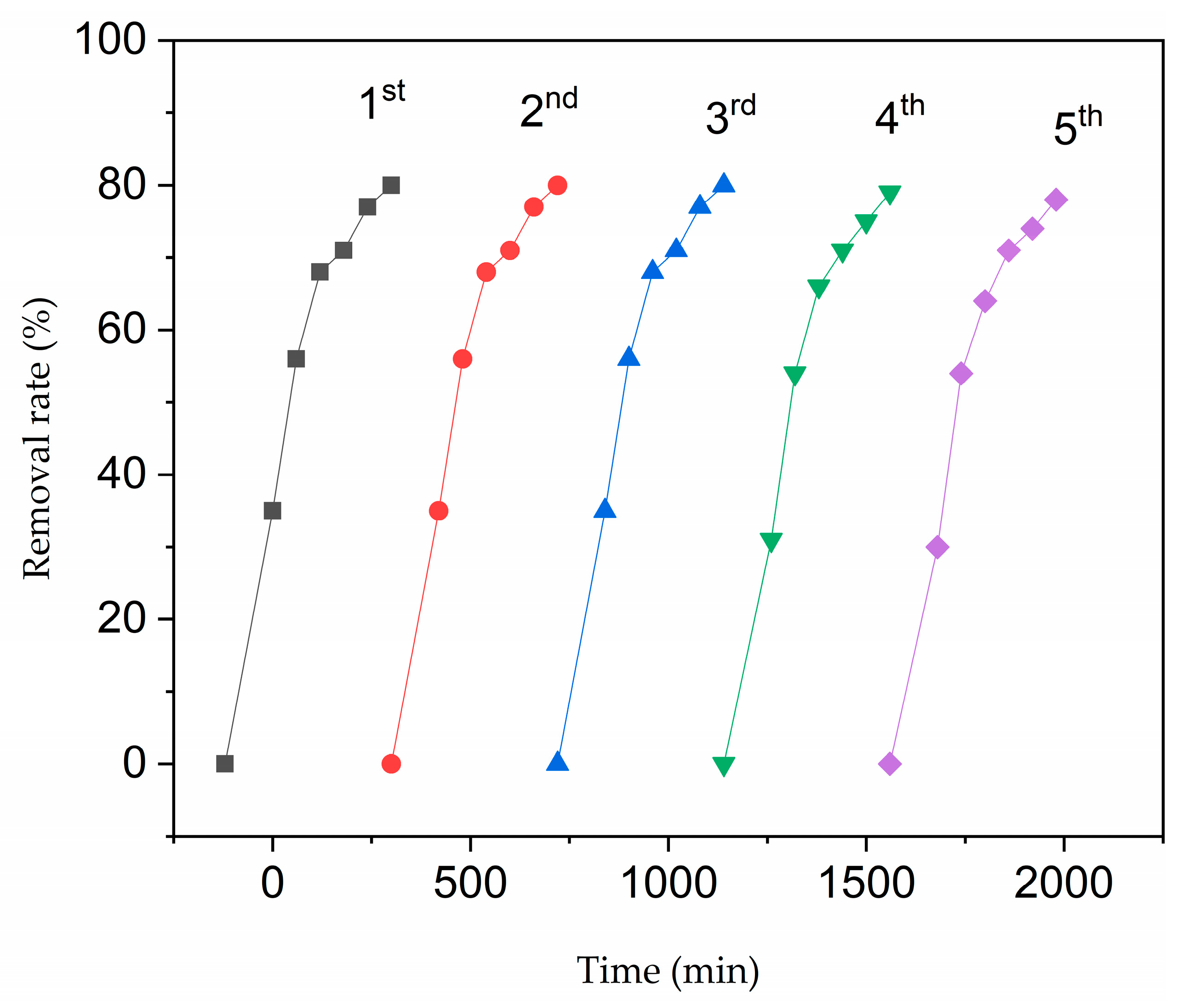
2.3. Photocatalytic Properties
3. Materials and Methods
3.1. Materials
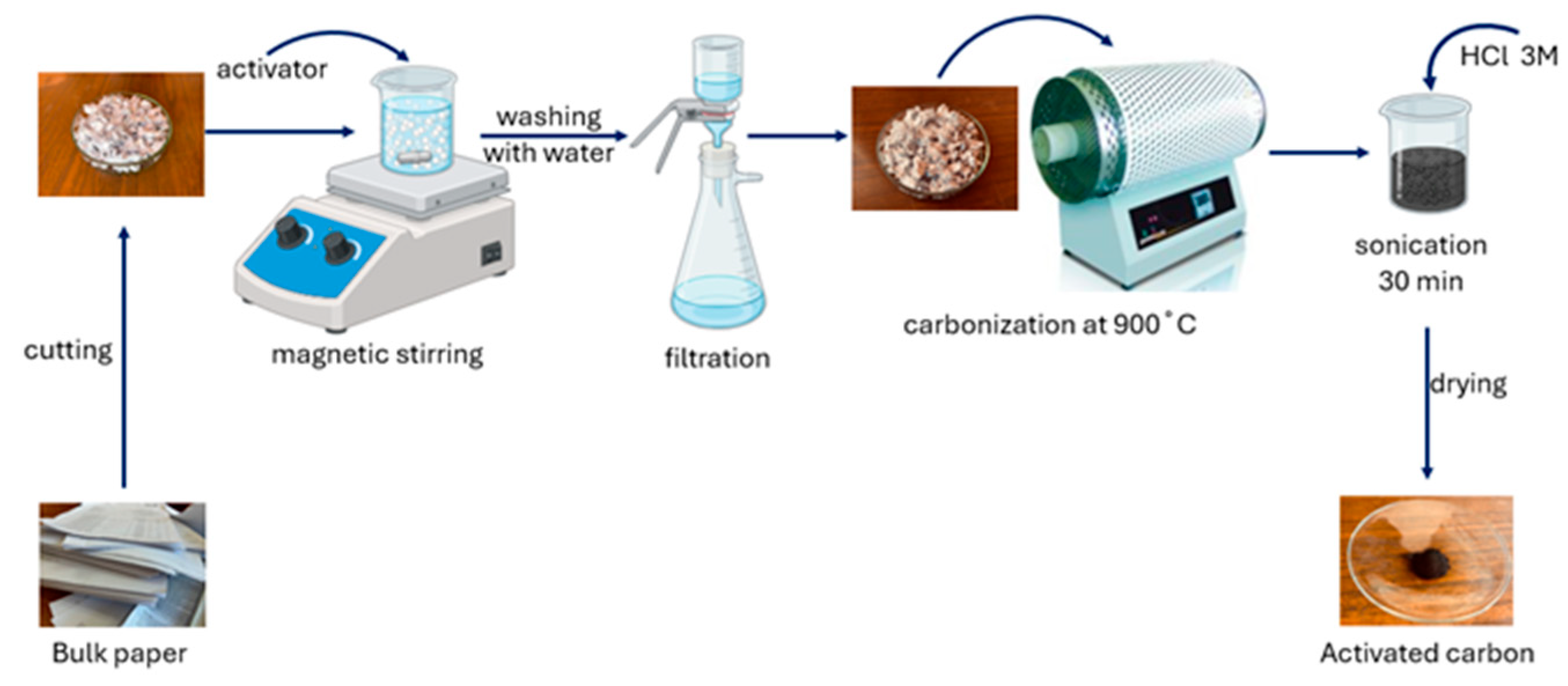
3.2. Sample Preparation
3.3. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, M.; Su, J.; Fang, C.; Cheng, Y.; Li, Y.; Yan, Y.; Lei, W. Synthesis and Properties of Carbon Microspheres from Waste Office Paper. Molecules 2023, 28, 5756. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, R.; Qi, J.; Sui, Y.; Yezeng He, Y.; Meng, Q.; Wei, F.; Ren, Y. Sustainable synthesis of N/S-doped porous carbon sheets derived from waste newspaper for high-performance asymmetric supercapacitor. Mater. Res. Express 2019, 6, 095605. [Google Scholar] [CrossRef]
- Liu, M.C.; Kong, L.B.; Lu, C.; Li, X.M.; Luo, Y.C.; Kang, L. Waste paper based activated carbon monolith as electrode materials for high-performance electric double-layer capacitors. RSC Adv. 2012, 2, 1890–1896. [Google Scholar] [CrossRef]
- Tetteh, I.K.; Issahaku, I.; Tetteh, A.Y. Recent advances in synthesis, characterization, and environmental applications of activated carbons and other carbon derivatives. Carbon Trends 2024, 14, 100328. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, J.G.; Jun, Y.S.; Park, Y.I. Sustainable utilization of cellulose-rich corn husk for activated carbon in high-performance sodium-ion capacitors. Appl. Surf. Sci. 2025, 695, 162916. [Google Scholar] [CrossRef]
- Alhammadi, Y.; Hai, A.; Taher, H.; Banat, F.; AlMarzooqi, F. Enhancing heavy metals filtration: Synergistic effects of banana peel-derived activated carbon and layered double hydroxides in ultrafiltration membranes. Sep. Purif. Technol. 2025, 362, 131875. [Google Scholar] [CrossRef]
- Liu, P.; He, N.; Li, X.; Li, Y.; Sun, T.; Huhe, T.; Wang, Z.; Lei, T. Anthracite-based activated carbon-supported Ni metal element as a catalyst for carbon dioxide reforming of methane to hydrogen. Renew. Energy 2025, 246, 122867. [Google Scholar] [CrossRef]
- Azam, K.; Shezad, N.; Shafiq, I.; Akhter, P.; Akhtar, F.; Jamil, F.; Shafique, S.; Park, Y.K.; Hussain, M. A review on activated carbon modifications for the treatment of wastewater containing anionic dyes. Chemosphere 2022, 306, 135566. [Google Scholar] [CrossRef]
- Ghaedi, M.; Biyareh, N.; Kokhdan, S.N.; Shamsaldini, S.; Sahraei, R.; Daneshfar, A.; Shahriyar, S. Comparison of the efficiency of palladium and silver nanoparticles loaded on activated carbon and zinc oxide nanorods loaded on activated carbon as new adsorbents for removal of Congo red from aqueous solution: Kinetic and isotherm study. Mat. Sci. Eng. C 2012, 32, 725–734. [Google Scholar] [CrossRef]
- Awad, A.M.; Jalab, R.; Benamor, A.; Nasser, M.S.; Ba-Abbad, M.M.; El-Naas, M.; Mohammad, A.W. Adsorption of organic pollutants by nanomaterial-based adsorbents: An overview. J. Mol. Liq. 2020, 301, 112335. [Google Scholar] [CrossRef]
- Azam, K.; Raza, R.; Shezad, N.; Shabir, M.; Yang, W.; Ahmad, N.; Shafiq, I.; Akhter, P.; Razzaq, A.; Hussain, M. Development of recoverable magnetic mesoporous carbon adsorbent for removal of methyl blue and methyl orange from wastewater. J. Environ. Chem. Eng. 2020, 8, 104220. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Kittappa, S.; Jais, F.M.; Ramalingam, M.; Mohd, N.S.; Ibrahim, S. Functionalized magnetic mesoporous palm shell activated carbon for enhanced removal of azo dyes. J. Environ. Chem. Eng. 2020, 8, 104081. [Google Scholar] [CrossRef]
- Hasan, M.; Rashid, M.M.; Hossain, M.M.; Al Mesfer, M.K.; Arshad, M.; Danish, M.; Lee, M.; El Jery, A.; Kumar, N. Fabrication of polyaniline/activated carbon composite and its testing for methyl orange removal: Optimization, equilibrium, isotherm and kinetic study. Polym. Test. 2019, 77, 105909. [Google Scholar] [CrossRef]
- Hu, X.; Guo, H.G.; Gholizadeh, M.; Sattari, B.; Liu, Q. Pyrolysis of different wood species: Impacts of C/H ratio in feedstock on distribution of pyrolysis products. Biomass Bioenergy 2019, 120, 28–39. [Google Scholar] [CrossRef]
- Putro, J.N.; Santoso, S.P.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Nanocrystalline cellulose from waste paper: Adsorbent for azo dyes removal. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100260. [Google Scholar] [CrossRef]
- Wu, C.H.; Chang, C.Y.; Tseng, C.H.; Lin, J.P. Pyrolysis product distribution of waste newspaper in MSW. J. Anal. Appl. Pyrolysis 2003, 67, 41–53. [Google Scholar] [CrossRef]
- Hamzeh, Y.; Ashori, A.; Mirzaei, B. Effects of Waste paper sludge on the physico- Mechanical Properties of High-density Polyethylene/Wood Flour Composites. J. Polym. Environ. 2011, 19, 120–124. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Gill, R.A. Fillers for Papermaking: A Review of their Properties, Usage Practices, and their Mechanistic Role. BioResources 2016, 11, 197–207. [Google Scholar] [CrossRef]
- Çiçekler, M.; Tutuş, A. Recycling Waste Paper into High-Performance Activated Carbon: A Mini Review. In Proceedings of the 2nd International Conference on Modern and Advanced Research (ICMAR 2025), Konya, Turkey, 15–16 January 2025; pp. 158–169. [Google Scholar]
- Li, C.; Sun, Y.; Yi, Z.; Zhang, L.; Zhang, S.; Hu, X. Co-pyrolysis of coke bottle wastes with cellulose, lignin and sawdust: Impacts of the mixed feedstock on char properties. Renew. Energy 2022, 181, 1126–1139. [Google Scholar] [CrossRef]
- Li, C.; Yuan, X.; Sun, Z.; Suvarna, M.; Hu, X.; Wang, X.; Ok, Y.S. Pyrolysis of waste surgical masks into liquid fuel and its life-cycle assessment. Bioresour. Technol. 2022, 346, 126582. [Google Scholar] [CrossRef] [PubMed]
- Abuelnoor, N.; AlHajaj, A.; Khaleel, M.; Vega, L.F.; Mohammad, R.M.; Zahra, A. Activated carbons from biomass-based sources for CO2 capture applications. Chemosphere 2021, 282, 131111. [Google Scholar] [CrossRef]
- Li, M.; Xiao, R. Preparation of a dual Pore Structure Activated Carbon from Rice Husk Char as an Adsorbent for CO2 Capture. Fuel Proc. Technol. 2019, 186, 35–39. [Google Scholar] [CrossRef]
- Zahra, H.; Sawada, D.; Guizani, C.; Ma, Y.; Kumagai, S.; Yoshioka, T.; Sixta, H.; Hummel, M. Close packing of cellulose and chitosan in regenerated cellulose fibers improves carbon yield and structural properties of respective carbon fibers. Biomacromolecules 2020, 21, 4326–4335. [Google Scholar] [CrossRef]
- Hong, D.; Zhou, J.; Hu, C.; Zhou, Q.; Mao, J.; Qin, Q. Mercury removal mechanism of AC prepared by one-step activation with ZnCl2. Fuel 2019, 235, 326–335. [Google Scholar] [CrossRef]
- Varil, T.; Bergna, D.; Lahti, R.; Romar, H.; Hu, T.; Lassi, U. Activated carbon production from peat using ZnCl2: Characterization and applications. BioResources 2017, 12, 8078–8092. [Google Scholar] [CrossRef]
- Wu, S.; Yan, P.; Yang, W.; Zhou, J.; Wang, H.; Che, L.; Zhu, P. ZnCl2 enabled synthesis of activated carbons from ion-exchange resin for efficient removal of Cu2+ ions from water via capacitive deionization. Chemosphere 2021, 264, 128557. [Google Scholar] [CrossRef]
- Zhao, H.; Zhong, H.; Jiang, Y.; Li, H.; Tang, P.; Li, D.; Feng, Y. Porous ZnCl2-Activated Carbon from Shaddock Peel: Methylene Blue Adsorption Behavior. Materials 2022, 15, 895. [Google Scholar] [CrossRef] [PubMed]
- Nassar, H.; Zyoud, A.; El-Hamouz, A.; Tanbour, R.; Halayqa, N.; Hilal, H.S. Aqueous nitrate ion adsorption/desorption by olive solid waste-based carbon activated using ZnCl2. Sustain. Chem. Pharm. 2020, 18, 100335–100343. [Google Scholar] [CrossRef]
- Son, J.Y.; Choe, S.; Jang, Y.J.; Kim, H. Waste paper-derived porous carbon via microwave-assisted activation for energy storage and water purification. Chemosphere 2024, 355, 141798. [Google Scholar] [CrossRef]
- Puthusseri, D.; Aravindan, V.; Anothumakkool, B.; Kurungot, S.; Madhavi, S.; Ogale, S. From waste paper basket to solid state and Li-HEC ultracapacitor electrodes: A value added journey for shredded office paper. Small 2014, 10, 4395–4402. [Google Scholar] [CrossRef] [PubMed]
- Mrówczyński, D.; Knitter-Piątkowska, A.; Garbowski, T. Non-local sensitivity analysis and numerical homogenization in optimal design of single-wall corrugated board packaging. Materials 2022, 15, 720. [Google Scholar] [CrossRef] [PubMed]
- Campano, C.; Miranda, R.; Merayo, N.; Negro, C.; Blanco, A. Direct production of cellulose nanocrystals from old newspapers and recycled newsprint. Carbohydr. Polym. 2017, 173, 489–496. [Google Scholar] [CrossRef]
- Wang, B.; Fitzpatrick, J.R.; Brookfield, A.; Fielding, A.J.; Reynolds, E.; Entwistle, J.; Tong, J.; Spencer, B.F.; Baldock, S.; Hunter, K.; et al. Electron paramagnetic resonance as a tool to determine the sodium charge storage mechanism of hard carbon. Nat. Commun. 2024, 15, 3013. [Google Scholar] [CrossRef]
- Pogacean, F.; Ştefan, M.; Toloman, D.; Popa, A.; Leostean, C.; Turza, A.; Coros, M.; Pana, O.; Pruneanu, S. Photocatalytic and Electrocatalytic Properties of NGr-ZnO Hybrid Materials. Nanomaterials 2020, 10, 1473. [Google Scholar] [CrossRef]
- Râpă, M.; Stefan, M.; Popa, P.A.; Toloman, D.; Leostean, C.; Borodi, G.; Vodnar, D.C.; Wrona, M.; Salafranca, J.; Nerín, C.; et al. Electrospun Nanosystems Based on PHBV and ZnO for Ecological Food Packaging. Polymers 2021, 13, 2123. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Jha, M.K.; Ghimire, J.; Koirala, A.R.; Shrestha, R.M.; Sharma, R.K.; Pant, B.; Park, M.; Pant, H.R. Decoration of Zinc Oxide Nanorods into the Surface of Activated Carbon Obtained from Agricultural Waste for Effective Removal of Methylene Blue Dye. Materials 2020, 13, 5667. [Google Scholar] [CrossRef]
- Naveenkumar, R.; Karthikeyan, B.; Senthilvelan, S. Photocatalytic Degradation of Toxic Rhodamine B Dye by Green-Synthesised Activated Carbon-Supported Cobalt Doped ZnO with Further Assessment of ZnO Nanoparticles in Antimicrobial Applications. Chem. Sel. 2024, 9, e202401448. [Google Scholar] [CrossRef]
- Huang, C.; Xu, F.; Hu, C.; Wang, X.; Wang, D.; Zhong, Y.; Tang, C. Construction of heterostructured CaCO3/NiS with enhanced photocatalytic antibacterial performance under visible light. J. Photochem. Photobiol. A Chem. 2024, 447, 115187. [Google Scholar] [CrossRef]
- Wang, J.; Sun, S.; Pan, L.; Xu, Z.; Ding, H.; Li, W. Preparation and Properties of CaCO3-Supported Nano-TiO2 Composite with Improved Photocatalytic Performance. Materials 2019, 12, 3369. [Google Scholar] [CrossRef]
- Kuppusamy, M.; Kim, S.W.; Lee, K.P.; Jo, Y.J.; Kim, W.J. Development of TiO2-CaCO3 Based Composites as an Affordable Building Material for the Photocatalytic Abatement of Hazardous NOx from the Environment. Nanomaterials 2024, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Izzati Izhar, N.; Mohd Hir, Z.A.; Rafaie, H.A.; Daud, S. Influence of ZnO content on activated carbon/ZnO for en-hanced acetaminophen removal under very low light intensity: Kinetics, operational variables, and radical trapping response. Kuwait J. Sci. 2025, 52, 100391. [Google Scholar] [CrossRef]
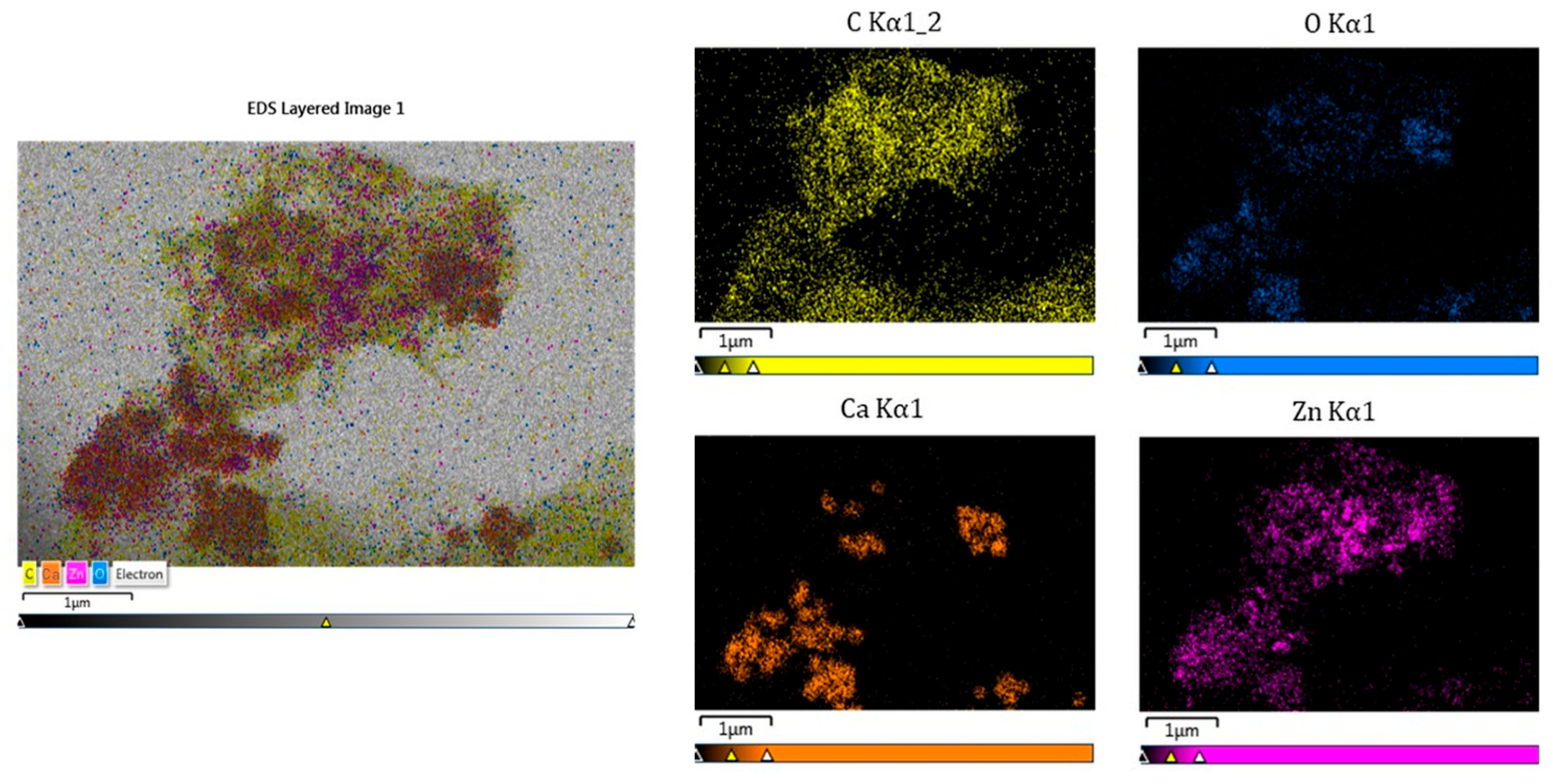
| Samples | Activator | Mass Ratio AC:ZnCl2 |
|---|---|---|
| AC0 | - | - |
| AC1 | ZnCl2 | 1:1 |
| AC2 | ZnCl2 | 1:3 |
| Paper Type | Main Composition | Activated Carbon Characteristics | Recommended Applications | Ref. |
|---|---|---|---|---|
| Office Paper | High-purity cellulose, low lignin | High carbon yield, well-developed microporous structure | Water and air purification | [32] |
| Cardboard | Cellulose, hemicellulose, lignin, additives (adhesives, coatings) | Lower carbon yield, mesoporous structure formation | Adsorption of larger molecules, diverse uses | [33] |
| Newsprint | Mechanical pulp, high lignin content | Lower carbon yield, mixed micro- and mesoporous structure | Large-scale activated carbon production with broad pore size distribution | [34] |
| Mixed Paper Waste | Cellulose, lignin | Variable carbon yield and pore structure | Cost-effective activated carbon for various applications | [20] |
| Office paper | Cellulose, CaCO3 | Lower carbon yield, mixed micro- and mesoporous structure | Water purification | This study |
| Sample | SBET (m2/g) | Pores Volume (cm3/g) | Dpores (nm) |
|---|---|---|---|
| AC0 | 4.49 | 0.043 | 54.27 |
| AC1 | 71.2 | 0.0612 | 33.93 |
| AC2 | 318.45 | 0.043 | 5.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varadi, A.; Popa, A.; Toloman, D.; Leostean, C.; Perhaiţă, I.; Dan, M.; Rostas, A.M.; Tripon, S.; Macavei, S.; Stefan, M. Photocatalytic Properties of Office-Paper-Waste-Derived Activated Carbon for Efficient Degradation of Organic Pollutants. Inorganics 2025, 13, 196. https://doi.org/10.3390/inorganics13060196
Varadi A, Popa A, Toloman D, Leostean C, Perhaiţă I, Dan M, Rostas AM, Tripon S, Macavei S, Stefan M. Photocatalytic Properties of Office-Paper-Waste-Derived Activated Carbon for Efficient Degradation of Organic Pollutants. Inorganics. 2025; 13(6):196. https://doi.org/10.3390/inorganics13060196
Chicago/Turabian StyleVaradi, Ana, Adriana Popa, Dana Toloman, Cristian Leostean, Ioana Perhaiţă, Monica Dan, Arpad Mihai Rostas, Septimiu Tripon, Sergiu Macavei, and Maria Stefan. 2025. "Photocatalytic Properties of Office-Paper-Waste-Derived Activated Carbon for Efficient Degradation of Organic Pollutants" Inorganics 13, no. 6: 196. https://doi.org/10.3390/inorganics13060196
APA StyleVaradi, A., Popa, A., Toloman, D., Leostean, C., Perhaiţă, I., Dan, M., Rostas, A. M., Tripon, S., Macavei, S., & Stefan, M. (2025). Photocatalytic Properties of Office-Paper-Waste-Derived Activated Carbon for Efficient Degradation of Organic Pollutants. Inorganics, 13(6), 196. https://doi.org/10.3390/inorganics13060196







