Kinetics-Controlled Simple Method for the Preparation of Au@Ag Hierarchical Superstructures for SERS Analysis
Abstract
1. Introduction
2. Results and Discussion
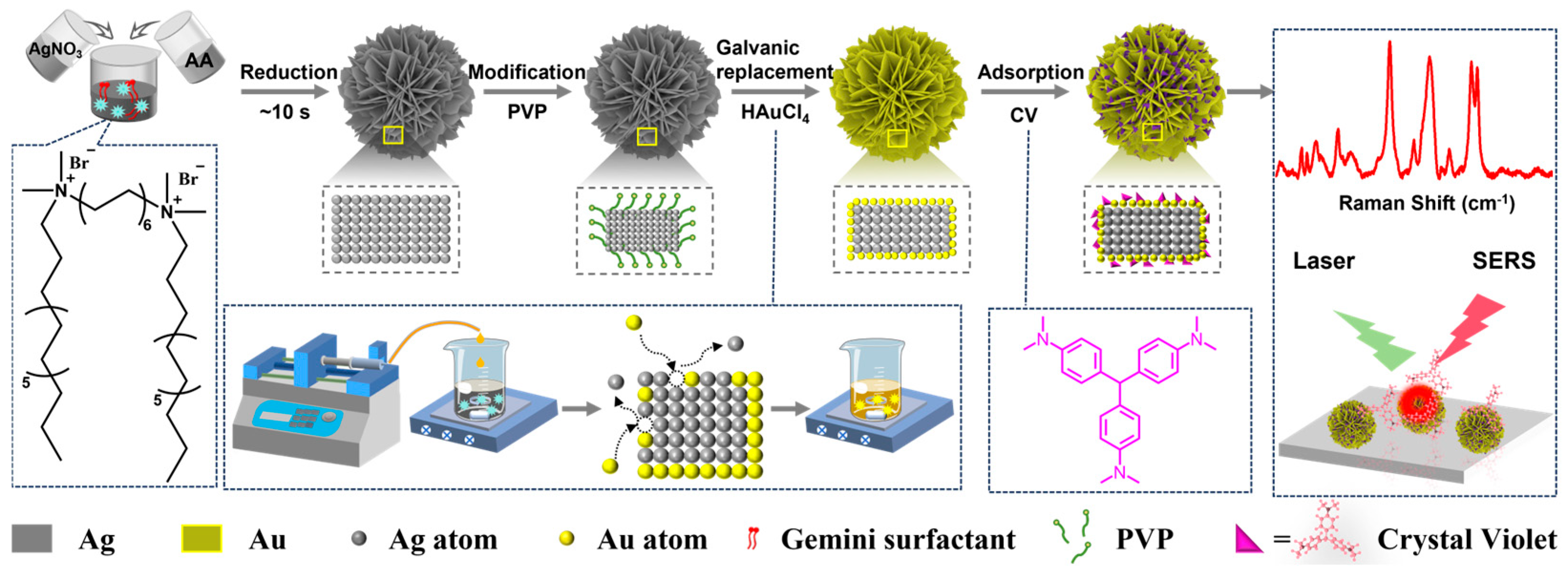
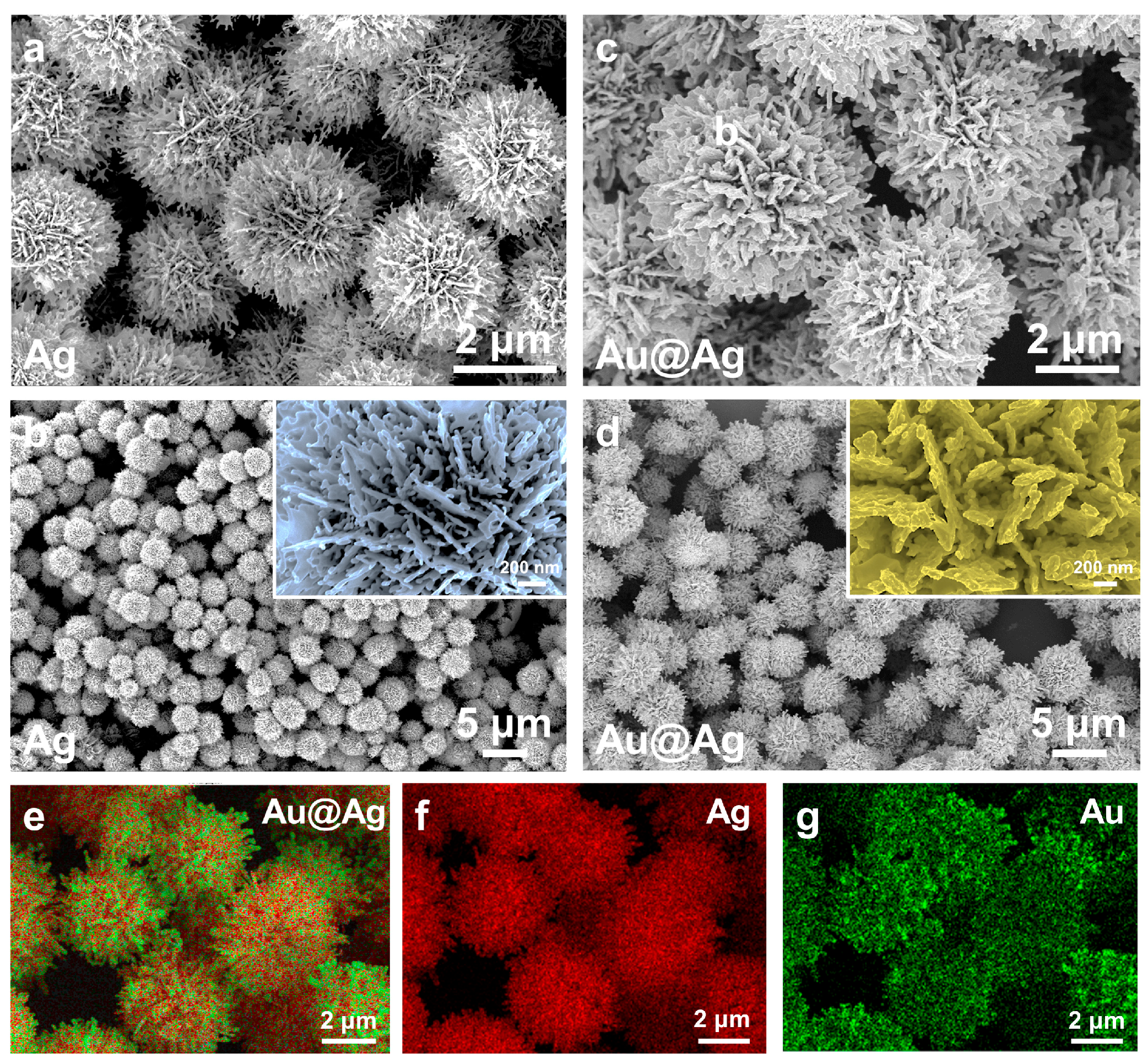
2.1. Synthesis and Characterization of Au@Ag HSs
2.2. Structural Manipulation of Au@Ag HSs
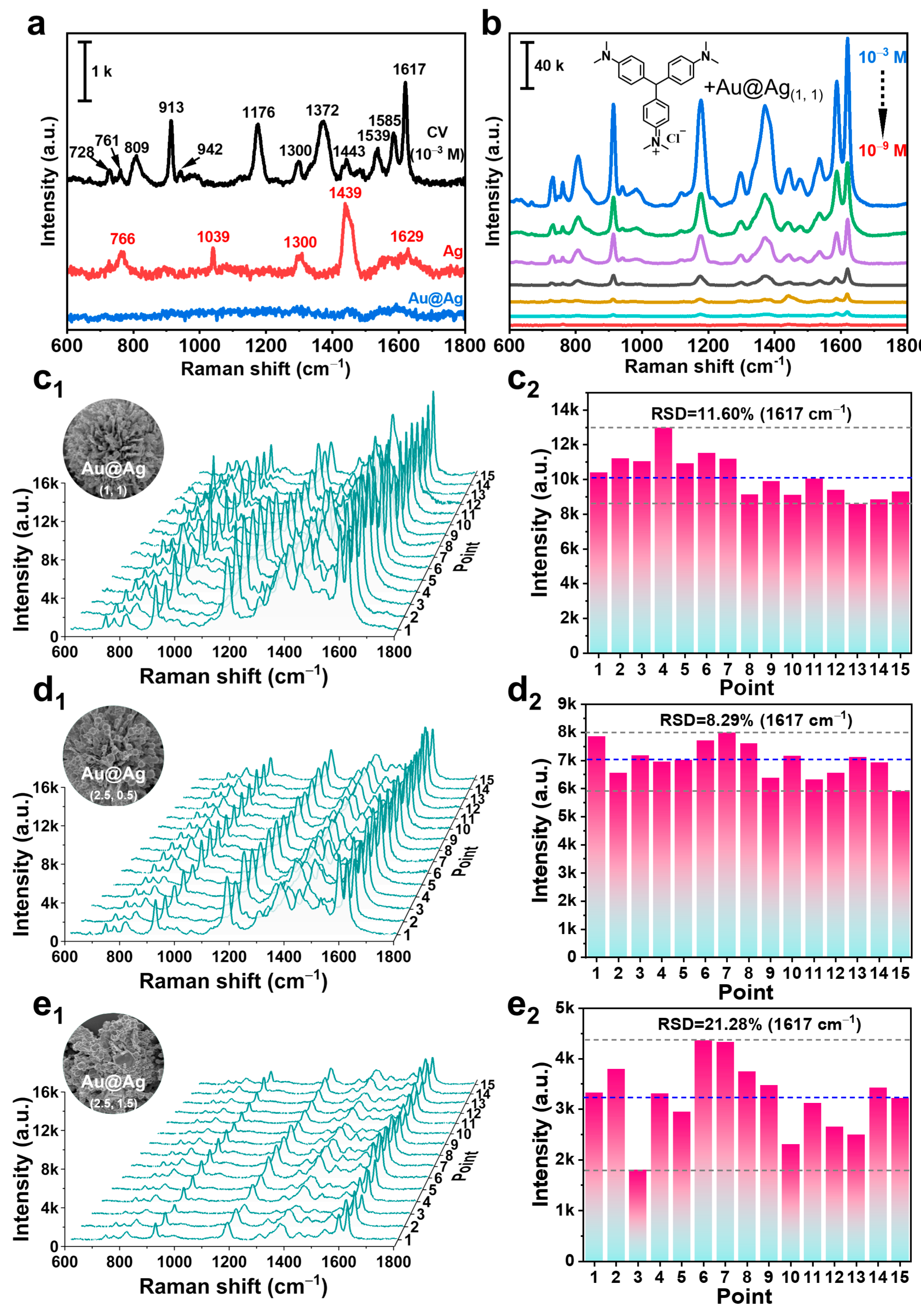
2.3. SERS Properties of Au@Ag HSs
3. Materials and Methods
3.1. Materials
3.2. Instruments and Characterization
3.3. Synthesis of Silver Hierarchical Superstructure (Ag HSs)
3.4. Synthesis of Gold-Coated Silver Hierarchical Superstructure (Au@Ag HSs)
3.5. SERS Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Itoh, T.; Procházka, M.; Dong, Z.; Ji, W.; Yamamoto, Y.S.; Zhang, Y.; Ozaki, Y. Toward a new era of SERS and TERS at the nanometer scale: From fundamentals to innovative applications. Chem. Rev. 2023, 123, 1552–1634. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Hilal, H.; Kim, J.; Park, W.; Haddadnezhad, M.; Lee, J.; Park, W.; Lee, J.; Lee, S.; Jung, I.; et al. All-hot-spot bulk surface-enhanced raman scattering (SERS) substrates: Attomolar detection of adsorbates with designer plasmonic nanoparticles. J. Am. Chem. Soc. 2022, 144, 13285–13293. [Google Scholar] [CrossRef] [PubMed]
- Hilal, H.; Haddadnezhad, M.; Oh, M.J.; Jung, I.; Park, S. Plasmonic dodecahedral-walled elongated nanoframes for surface-enhanced raman spectroscopy. Small 2024, 20, 2304567. [Google Scholar] [CrossRef]
- Tang, X.; Kishimoto, N.; Kitahama, Y.; You, T.T.; Adachi, M.; Shigeta, Y.; Tanaka, S.; Xiao, T.H.; Goda, K. Deciphering the potential of multidimensional carbon materials for surface-enhanced raman spectroscopy through density functional theory. J. Phys. Chem. Lett. 2023, 14, 10208–10218. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, D.; Yu, J.; Pan, T.; Wu, X.; Chen, T.; Gao, C.; Chen, C.; Wang, X.; Wu, A. Amorphous nitrogen-doped carbon nanocages with excellent SERS sensitivity and stability for accurate iden-tification of tumor cells. Anal. Chem. 2023, 95, 4671–4681. [Google Scholar] [CrossRef]
- Gu, C.; Li, D.; Zeng, S.; Jiang, T.; Shen, X.; Zhang, H. Synthesis and defect engineering of molybdenum oxides and their SERS applications. Nanoscale 2021, 13, 562–5651. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Chu, Y.; He, Q.; Han, Y.; Zhang, Y.; Han, L. A self-assembly hydrophobic oCDs/Ag nanoparticles SERS sensor for ultrasensitive melamine detection in milk. Food Chem. 2023, 402, 134241. [Google Scholar] [CrossRef]
- Hang, Y.; Wang, A.; Wu, N. Plasmonic silver and gold nanoparticles: Shape- and structure-modulated plasmonic functionality for point-of-caring sensing, bio-imaging and medical therapy. Chem. Soc. Rev. 2024, 53, 2932–2971. [Google Scholar] [CrossRef]
- Thakur, A.; Singh, R.; Yadav, V.; Siddhanta, S.; Jayarmaulu, K. Advancing SERS applications of 2D materials through the interplay of rational design and structure-property relationships. Small Methods 2025, e2402056. [Google Scholar] [CrossRef]
- Troncoso-Afonso, L.; Vinnacombe-Willson, G.A.; García-Astrain, C.; Liz-Márzan, L.M. SERS in 3D cell models: A powerful tool in cancer research. Chem. Soc. Rev. 2024, 53, 5118–5148. [Google Scholar] [CrossRef]
- Zhao, Y.; Kumar, A.; Yang, Y. Unveiling practical considerations for reliable and standardized SERS measurements: Lessons from a comprehensive review of oblique angle deposition-fabricated silver nanorod array substrates. Chem. Soc. Rev. 2024, 53, 14–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.; Meng, Y.; Chen, C.; Chen, Q.; Wang, Y.; Dou, S.; Liu, X.; Lu, N. Increasing hotspots density for high-sensitivity SERS detection by assembling array of Ag nanocubes. Talanta 2023, 258, 124408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hu, Z.; Zhang, Y.; Wang, D.; Gong, Z.; Fan, M. Differentiation and identification structural similar chemicals using SERS coupled with different chemometric methods: The example of fluoroquinolones. Microchem. J. 2022, 183, 108023. [Google Scholar] [CrossRef]
- Fan, X.; Wu, P.; Qu, C.; Gao, Y.; Yin, P. Functionalized Ag/MOF nanocomposites based on defect engineering for highly sensitive SERS detection of organic dyes. J. Environ. Chem. Eng. 2024, 12, 112240. [Google Scholar] [CrossRef]
- Zhong, L.; Liu, Q.; Wu, P.; Niu, Q.; Zhang, H.; Zheng, Y. Facile on-site aqueous pollutant monitoring using a flexible, ultralight, and robust surface-enhanced raman spectroscopy substrate: Interface self-assembly of au@ag nanocubes on a polyvinyl chloride template. Environ. Sci. Technol. 2018, 52, 5812–5820. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, G.; Chang, Y.; Cheng, Y.; Sun, B.; Wang, L.; Chen, C.; Zhang, H. Electron compensation effect suppressed silver ion release and contributed safety of Au@Ag core-shell nanoparticles. Nano Lett. 2019, 19, 4478–4489. [Google Scholar] [CrossRef]
- Zhang, J.; Winget, S.A.; Wu, Y.; Su, D.; Sun, X.; Xie, Z.; Qin, D. Ag@Au concave cuboctahedra: A unique probe for monitoring au-catalyzed reduction and oxidation reactions by surface-enhanced raman spectroscopy. ACS Nano 2016, 10, 2607–2616. [Google Scholar] [CrossRef]
- Cortie, M.B.; McDonagh, A.M. Synthesis and optical properties of hybrid and alloy plasmonic nanoparticles. Chem. Rev. 2011, 111, 3713–3735. [Google Scholar] [CrossRef]
- He, M.Q.; Ai, Y.; Hu, W.; Guan, L.; Ding, M.; Liang, Q. Recent advances of seed-mediated growth of metal nanoparticles: From growth to applications. Adv. Mater. 2023, 35, e2211915. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Fu, Z.; Qin, D. Galvanic replacement-free deposition of Au on Ag for core-shell nanocubes with enhanced chemical stability and SERS activity. J. Am. Chem. Soc. 2014, 136, 8153–8156. [Google Scholar] [CrossRef]
- Csapo, E.; Oszko, A.; Varga, E.; Juhasz, A.; Buzas, N.; Koroesi, L.; Majzik, A.; Dekany, I. Synthesis and characterization of Ag/Au alloy and core(Ag)-shell(Au) nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 281–287. [Google Scholar] [CrossRef]
- Zhu, Y.; Kim, S.; Ma, X.; Byrley, P.; Yu, N.; Liu, Q.; Sun, X.; Xu, D.; Peng, S.; Hartel, M.C.; et al. Ultrathin-shell epitaxial Ag@Au core-shell nanowires for high-performance and chemically-stable electronic, optical, and mechanical devices. Nano Res. 2021, 14, 4294–4303. [Google Scholar] [CrossRef]
- Shahjamali, M.M.; Salvador, M.; Bosman, M.; Ginger, D.S.; Xue, C. Edge-gold-coated silver nanoprisms: Enhanced stability and applications in organic photovoltaics and chemical sensing. J. Phys. Chem. C 2014, 118, 12459–12468. [Google Scholar] [CrossRef]
- Zaleska-Medynska, A.; Marchelek, M.; Diak, M.; Grabowska, E. Noble metal-based bimetallic nanoparticles: The effect of the structure on the optical, catalytic and photocatalytic properties. Adv. Colloid Interface Sci. 2016, 229, 80–107. [Google Scholar] [CrossRef]
- Lin, S.; Lin, X.; Han, S.; Zhao, H.; Hasi, W.; Wang, L. Highly monodisperse Au@Ag nanospheres: Synthesis by controlled etching route and size-dependent SERS performance of their surperlattices. Nanotechnology 2019, 30, 215601. [Google Scholar] [CrossRef]
- Kim, K.K.; Kim, M.; Pyun, K.; Kim, J.; Min, J.; Koh, S.; Root, S.E.; Kim, J.; Nguyen, B.T.; Nishio, Y.; et al. A substrate-less nanomesh receptor with meta-learning for rapid hand task recognition. Nat. Electron. 2023, 6, 64–75. [Google Scholar] [CrossRef]
- Gholami, M.; Tajabadi, F.; Taghavinia, N.; Moshfegh, A. Chemically-stable flexible transparent electrode: Gold-electrodeposited on embedded silver nanowires. Sci. Rep. 2023, 13, 17511. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Gao, Z.; Liao, X.; Pan, C.; Zhang, Y.; Feng, X. Rapid synthesis of hierarchical, flower-like Ag microstructures with a gemini surfactant as a directing agent for SERS applications. CrystEngComm 2017, 19, 6547–6555. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Ahn, J.; Wang, X.; Qin, D. Defect-assisted deposition of Au on Ag for the fabrication of core-shell nanocubes with outstanding chemical and thermal stability. Chem. Mat. 2019, 31, 1057–1065. [Google Scholar] [CrossRef]
- Ahn, J.; Wang, D.; Ding, Y.; Zhang, J.; Qin, D. Site-selective carving and co-deposition: Transformation of Ag nanocubes into concave nanocrystals encased by Au-Ag alloy frames. ACS Nano 2018, 12, 298–307. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, Y.; Lyu, M.; Jiang, M.; Hong, Y.; Guo, Z.; Li, J.; Fang, Z. Engineering ZIF-8@Ag core-satellite superstructures through solvent-induced tunable self-assembly for surface-enhanced Raman spectroscopy. Nanoscale 2025, 17, 4687–4694. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, J.; Weng, G.; Liu, Y.; Li, J.; Zhao, J. Study on the roughen process of branches of AuAg nanostars for the improved surface-enhanced Raman scattering (SERS) to detect crystal violet in fish. Sens. Actuators B Chem. 2023, 390, 133936. [Google Scholar] [CrossRef]
- Sakir, M.; Akgul, E.T.; Demir, M. Highly sensitive detection of cationic pollutants on molybdenum carbide (MXene)/Fe2O3/Ag as a SERS substrate. Mater. Today Chem. 2023, 33, 101702. [Google Scholar] [CrossRef]
- Yu, C.; Varghese, L.; Irudayaraj, J. Surface modification of cetyltrimethylammonium bromide-capped gold nanorods to make molecular probes. Langmuir 2007, 23, 9114–9119. [Google Scholar] [CrossRef]
- Kitaw, S.L.; Ahmed, Y.W.; Candra, A.; Wu, T.Y.; Anley, B.E.; Chen, Y.Y.; Cheng, Y.T.; Chen, K.J.; Thammaniphit, C.; Hsu, C.C.; et al. An advanced plasmonic bimetallic nanostar composite for ultra-sensitive SERS detection of crystal violet. Nanoscale 2024, 17, 508–519. [Google Scholar] [CrossRef]
- Sunil, J.; Narayana, C.; Kumari, G.; Jayaramulu, K. Raman spectroscopy, an ideal tool for studying the physical properties and applications of metal-organic frameworks (MOFs). Chem. Soc. Rev. 2023, 52, 3397–3437. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Cheng, Z.; Wei, J.; Yang, L.; Zhong, Z.; Hu, H.; Wang, Y.; Zhou, B.; Li, P. Emerging core–shell nanostructures for surface-enhanced Raman scattering (SERS) detection of pesticide residues. Chem. Eng. J. 2021, 424, 130323. [Google Scholar] [CrossRef]
- Guselnikova, O.; Lim, H.; Kim, H.J.; Kim, S.H.; Gorbunova, A.; Eguchi, M.; Postnikov, P.; Nakanishi, T.; Asahi, T.; Na, J.; et al. New Trends in Nanoarchitectured SERS Substrates: Nanospaces, 2D Materials, and Organic Heterostructures. Small 2022, 18, e2107182. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.; Lee, Y.; Lee, J.; Park, S.; Park, S.; Nam, J. Synthesis, Assembly, Optical Properties, and Sensing Applications of Plasmonic Gap Nanostructures. Adv. Mater. 2021, 33, e2006966. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, S.; Jiang, S.; Xu, J. Improved SERS Performance on Ag-Coated Amorphous TiO2 Random Nanocavities by the Enhanced Light—Matter Coupling Effect. ACS Sustain. Chem. Eng. 2024, 12, 3234–3242. [Google Scholar] [CrossRef]
- Zana, R.; Benrraou, M.; Rueff, R. Alkanediyl-.alpha.,.omega.-bis(dimethylalkylammonium bromide) surfactants. 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree. Langmuir 1991, 7, 1072–1075. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, M.; Jiang, M.; Yu, H.; Wu, K.; Zhu, P.; Zhu, Y.; Xia, Y.; Li, J. Kinetics-Controlled Simple Method for the Preparation of Au@Ag Hierarchical Superstructures for SERS Analysis. Inorganics 2025, 13, 191. https://doi.org/10.3390/inorganics13060191
Lyu M, Jiang M, Yu H, Wu K, Zhu P, Zhu Y, Xia Y, Li J. Kinetics-Controlled Simple Method for the Preparation of Au@Ag Hierarchical Superstructures for SERS Analysis. Inorganics. 2025; 13(6):191. https://doi.org/10.3390/inorganics13060191
Chicago/Turabian StyleLyu, Mengqi, Ming Jiang, Hanting Yu, Kailiang Wu, Peitao Zhu, Yingke Zhu, Yan Xia, and Juan Li. 2025. "Kinetics-Controlled Simple Method for the Preparation of Au@Ag Hierarchical Superstructures for SERS Analysis" Inorganics 13, no. 6: 191. https://doi.org/10.3390/inorganics13060191
APA StyleLyu, M., Jiang, M., Yu, H., Wu, K., Zhu, P., Zhu, Y., Xia, Y., & Li, J. (2025). Kinetics-Controlled Simple Method for the Preparation of Au@Ag Hierarchical Superstructures for SERS Analysis. Inorganics, 13(6), 191. https://doi.org/10.3390/inorganics13060191







