The Synthesis and Characterisation of Ru(III)-Substituted Keggin-Type Phosphomolybdates
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
- H3[PMo12O40] HPMo from Sigma Aldrich, St. Louis, MO, USA.
- Molybdenum trioxide from Alfa Aesar, Haverhill, MA, USA.
- Phosphoric acid from Grüssing, Westoverledingen, Germany, as 85 % solution in water (was further diluted with deionized water to a concentration of 36.9 %).
- Ruthenium(III) chloride hydrate was purchased from Sigma Aldrich, St. Louis, MO, USA (38.0 to 42.0 % Ru basis); product number: 84050; batch number: BCCK1103 (40 % Ru according to ICP). The specifications of the supplier correspond to a trihydrate.
- Deionized water was always used as the solvent.
- Lactic acid (LA, 90.3 wt.-% aqueous solution) was purchased from Sigma-Aldrich, St. Louis, MO, USA.
- Propanoic acid (PA, 99.9 %) was purchased from Sigma-Aldrich, St. Louis, MO, USA.
- Propane-1,2-diol (PD, 99.5 %) was purchased from Thermo Fischer Scientific, Waltham, MA, USA.
- Hydrogen (H2, 5.0 grade) was purchased from Linde, Dublin, Ireland.
- Nitrogen (N2, 5.0 grade) was purchased from Linde, Dublin, Ireland.
3.2. General Procedure for Synthesizing the Catalysts
3.3. Characterization of the Catalysts
3.4. Hydrogenation of Lactic Acid (Catalysis)
3.5. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raabe, J.-C.; Hombach, L.; Poller, M.J.; Collauto, A.; Roessler, M.M.; Vorholt, A.; Beine, A.K.; Albert, J. Synthesis and Characterization of Co (II) Substituted Keggin-Type Polyoxometalates as Novel Catalysts for the Hydroformylation of 1-Hexene in a Thermomorphic Solvent System. ChemCatChem 2024, 16, e202400395. [Google Scholar] [CrossRef]
- Raabe, J.-C.; Jameel, F.; Stein, M.; Albert, J.; Poller, M.J. Heteroelements in polyoxometalates: A study on the influence of different group 15 elements on polyoxometalate formation. Dalt. Trans. 2024, 53, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Raabe, J.-C.; Esser, T.; Jameel, F.; Stein, M.; Albert, J.; Poller, M.J. Study on the incorporation of various elements into the Keggin lacunary-type phosphomolybdate [PMo9O34]9− and subsequent purification of the polyoxometalates by nanofiltration. Inorg. Chem. Front. 2023, 10, 4854–4868. [Google Scholar] [CrossRef]
- Raabe, J.; Albert, J.; Poller, M.J. Spectroscopic, Crystallographic, and Electrochemical Study of Different Manganese (II)-Substituted Keggin-Type Phosphomolybdates. Chem. Eur. J. 2022, 28, e202201084. [Google Scholar] [CrossRef]
- Raabe, J.-C.; Aceituno Cruz, J.; Albert, J.; Poller, M.J. Comparative Spectroscopic and Electrochemical Study of V(V)-Substituted Keggin-Type Phosphomolybdates and -Tungstates. Inorganics 2023, 11, 138. [Google Scholar] [CrossRef]
- Lunk, H.J.; Hartl, H. The fascinating polyoxometalates. ChemTexts 2021, 7, 26. [Google Scholar] [CrossRef]
- Pope, M.T.; Müller, A. Polyoxometalate Chemistry from Topology via Self-Assembly to Applications; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; ISBN 0-306-47625-8. [Google Scholar]
- Pope, M.T.; Müller, A. Polyoxometalate Chemistry: An Old Field with New Dimensions in Several Disciplines. Angew. Chem. Int. Ed. Engl. 1991, 30, 34–48. [Google Scholar] [CrossRef]
- Patrut, A.; Bogge, H.; Forizs, E.; Rusu, D.; Lowy, D.A.; Margineanu, D.; Naumescu, A. Spectroscopic and crystal structure investigation of a new bismuth (III) containing polyoxometalate cluster. Rev. Roum. Chim. 2010, 55, 865–870. [Google Scholar]
- Dehghani, R.; Aber, S.; Mahdizadeh, F. Polyoxometalates and Their Composites as Photocatalysts for Organic Pollutants Degradation in Aqueous Media—A Review. CLEAN Soil Air Water 2018, 46, 1800413. [Google Scholar] [CrossRef]
- Avcı Özbek, H. V-subtituted lindqvist-type polyoxometalates: Preparation, structural characterization and antibacterial activity. Chem. Pap. 2023, 77, 5663–5669. [Google Scholar] [CrossRef]
- Lu, F.; Wang, M.; Li, N.; Tang, B. Polyoxometalate-Based Nanomaterials Toward Efficient Cancer Diagnosis and Therapy. Chem. Eur. J. 2021, 27, 6422–6434. [Google Scholar] [CrossRef] [PubMed]
- Gumerova, N.I.; Al-Sayed, E.; Krivosudský, L.; Ĉipĉić-Paljetak, H.; Verbanac, D.; Rompel, A. Antibacterial activity of polyoxometalates against Moraxella catarrhalis. Front. Chem. 2018, 6, 336. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Barsukova-Stuckart, M.; Ibrahim, M.; Ali, S.U.; Khan, A.A.; Kortz, U. Polyoxometalates as potent inhibitors for acetyl and butyrylcholinesterases and as potential drugs for the treatment of Alzheimer’s disease. Med. Chem. Res. 2013, 22, 1224–1228. [Google Scholar] [CrossRef]
- Budych, M.J.W.; Staszak, K.; Bajek, A.; Pniewski, F.; Jastrząb, R.; Staszak, M.; Tylkowski, B.; Wieszczycka, K. The future of polyoxymetalates for biological and chemical apllications. Coord. Chem. Rev. 2023, 493, 215306. [Google Scholar] [CrossRef]
- Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A. Magnetic polyoxometalates: From molecular magnetism to molecular spintronics and quantum computing. Chem. Soc. Rev. 2012, 41, 7464. [Google Scholar] [CrossRef]
- Monakhov, K.Y.; Moors, M.; Vogelsberg, E.; Lorenz, J.; Warneke, J.; Yang, F. Solution-Processable Molecular Oxides for Integrated Memories. In Proceedings of the 2023 IEEE International Interconnect Technology Conference (IITC) and IEEE Materials for Advanced Metallization Conference, Dresden, Germany, 22–25 May 2023; pp. 1–3. [Google Scholar]
- Kikkawa, S.; Fujiki, Y.; Chudatemiya, V.; Nagakari, H.; Shibusawa, K.; Hirayama, J.; Nakatani, N.; Yamazoe, S. Water-Tolerant Superbase Polyoxometalate [H2(Nb6O19)]6− for Homogeneous Catalysis. Angew. Chemie Int. Ed. 2024, 63, 4–9. [Google Scholar] [CrossRef]
- Hill, C.L.; Prosser-McCartha, C.M. Homogeneous catalysis by transition metal oxygen anion clusters. Coord. Chem. Rev. 1995, 143, 407–455. [Google Scholar] [CrossRef]
- Kubas, D.; Beck, J.M.; Kasisari, E.; Schätzler, T.; Becherer, A.; Fischer, A.; Krossing, I. From CO2 to DME: Enhancement through Heteropoly Acids from a Catalyst Screening and Stability Study. ACS Omega 2023, 8, 15203–15216. [Google Scholar] [CrossRef]
- Wesner, A.; Raabe, J.; Poller, M.J.; Meier, S.; Riisager, A.; Albert, J. Conversion of Sugars to Lactic Acid using Homogeneous Niobium-Substituted Polyoxometalate Catalysts. Chem. Eur. J. 2024, 30, e202402649. [Google Scholar] [CrossRef]
- Raabe, J.-C.; Poller, M.J.; Voß, D.; Albert, J. H8[PV5Mo7O40]—A Unique Polyoxometalate for Acid and RedOx Catalysis: Synthesis, Characterization, and Modern Applications in Green Chemical Processes. ChemSusChem 2023, 16, 2013–2015. [Google Scholar] [CrossRef]
- Mürtz, S.D.; Raabe, J.-C.; Poller, M.J.; Palkovits, R.; Albert, J.; Kurig, N. Transition-metal Substituted Polyoxometalates as Soluble RedOx Mediators in Electrocatalytic Biomass Conversion. ChemCatChem 2024, 16, e202301632. [Google Scholar] [CrossRef]
- Huber, M.; Poller, M.J.; Tochtermann, J.; Korth, W.; Jess, A.; Albert, J. Revealing the nitrogen reaction pathway for the catalytic oxidative denitrification of fuels. Chem. Commun. 2023, 16, 2864. [Google Scholar] [CrossRef] [PubMed]
- Wesner, A.; Papajewski, M.P.; Schidowski, L.; Ruhmlieb, C.; Poller, M.J.; Albert, J. Supported H8PV5Mo7O40 on activated carbon: Synthesis and Investigation of influencing factors for catalytic performance. Dalt. Trans. 2024, 53, 14065–14076. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Huang, L.; Zhang, Y.; Zhu, Z.; Zhao, J.; Xia, G.-J.; Min, Y. Ionic Liquid/Polyoxometalate Hybrid Catalyst with Tunable Functionalities for Boosting the Selectively Oxidative Transformation of Biobased Carbohydrates. Ind. Eng. Chem. Res. 2023, 62, 18337–18349. [Google Scholar] [CrossRef]
- Tiwari, C.K.; Baranov, M.; Neyman, A.; Neumann, R.; Weinstock, I.A. Selective Oxidation by H5[PV2Mo10O40] in a Highly Acidic Medium. Inorg. Chem. 2020, 59, 11945–11952. [Google Scholar] [CrossRef]
- He, Z.; Hou, Y.; Wei, J.; Ren, S.; Wu, W. Efficient catalytic oxidation of biomass to formic acid coupled with low-energy formaldehyde production from methanol. Green Chem. 2024, 26, 2170–2182. [Google Scholar] [CrossRef]
- Gaspar, A.R.; Evtuguin, D.V.; Neto, C.P. Polyoxometalate-catalyzed oxygen delignification of kraft pulp: A pilot-plant experience. Ind. Eng. Chem. Res. 2004, 43, 7754–7761. [Google Scholar] [CrossRef]
- Gaspar, A.; Evtuguin, D.V.; Neto, C.P. Lignin reactions in oxygen delignification catalysed by Mn(II)-substituted molybdovanadophosphate polyanion. Holzforschung 2004, 58, 640–649. [Google Scholar] [CrossRef]
- Evtuguin, D.V.; Daniel, A.I.D.; Silvestre, A.J.D.; Amado, F.M.L.; Pascoal Neto, C. Lignin aerobic oxidation promoted by molybdovanadophosphate polyanion [PMo7V5O40]8−. Study on the oxidative cleavage of b-O-4 aryl ether structures using model compounds. J. Mol. Catal. A-Chem. 2000, 154, 217–224. [Google Scholar] [CrossRef]
- Efremenko, I.; Neumann, R. Computational Insight into the Initial Steps of the Mars–van Krevelen Mechanism: Electron Transfer and Surface Defects in the Reduction of Polyoxometalates. J. Am. Chem. Soc. 2012, 134, 20669–20680. [Google Scholar] [CrossRef]
- Sloboda-Rozner, D.; Neumann, R. Aqueous biphasic catalysis with polyoxometalates: Oximation of ketones and aldehydes with aqueous ammonia and hydrogen peroxide. Green Chem. 2006, 8, 679–681. [Google Scholar] [CrossRef]
- Benaissa, H.; Davey, P.; Khimyak, Y.; Kozhevnikov, I. Heteropoly compounds as catalysts for hydrogenation of propanoic acid. J. Catal. 2008, 253, 244–252. [Google Scholar] [CrossRef]
- Čolović, M.B.; Lacković, M.; Lalatović, J.; Mougharbel, A.S.; Kortz, U.; Krstić, D.Z. Polyoxometalates in Biomedicine: Update and Overview. Curr. Med. Chem. 2019, 27, 362–379. [Google Scholar] [CrossRef]
- Modvig, A.; Kumpidet, C.; Riisager, A.; Albert, J. Ru-Doped Wells–Dawson Polyoxometalate as Efficient Catalyst for Glycerol Hydrogenolysis to Propanediols. Materials 2019, 12, 2175. [Google Scholar] [CrossRef]
- Yang, P.; Mahmoud, M.E.; Xiang, Y.; Lin, Z.; Ma, X.; Christian, J.H.; Bindra, J.K.; Kinyon, J.S.; Zhao, Y.; Chen, C.; et al. Host–Guest Chemistry in Discrete Polyoxo-12-Palladate (II) Cubes [MO8Pd12L8]n− (M = ScIII, CoII, CuII, L = AsO43−; M = CdII, HgII, L = PhAsO32–): Structure, Magnetism, and Catalytic Hydrogenation. Inorg. Chem. 2022, 61, 18524–18535. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Ma, X.; Mougharbel, A.S.; Haouas, M.; Su, P.; Espenship, M.F.; Taffa, D.H.; Jaensch, H.; Bons, A.-J.; Stuerzer, T.; et al. Discovery of a Neutral 40-Pd II -Oxo Molecular Disk, [Pd40O24(OH)16{(CH3)2AsO2}16]: Synthesis, Structural Characterization, and Catalytic Studies. Inorg. Chem. 2021, 60, 17339–17347. [Google Scholar] [CrossRef]
- Izarova, N.V.; Pope, M.T.; Kortz, U. Noble Metals in Polyoxometalates. Angew. Chemie Int. Ed. 2012, 51, 9492–9510. [Google Scholar] [CrossRef]
- Chen, Y.; Miller, D.J.; Jackson, J.E. Kinetics of Aqueous-Phase Hydrogenation of Organic Acids and Their Mixtures over Carbon Supported Ruthenium Catalyst. Ind. Eng. Chem. Res. 2007, 46, 3334–3340. [Google Scholar] [CrossRef]
- Kluson, P.; Cerveny, L. Selective hydrogenation over ruthenium catalysts. Appl. Catal. A Gen. 1995, 128, 13–31. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Simakova, I.L.; Salmi, T.; Murzin, D.Y. Production of lactic acid/lactates from biomass and their catalytic transformations to commodities. Chem. Rev. 2014, 114, 1909–1971. [Google Scholar] [CrossRef]
- Zhang, Z.; Jackson, J.E.; Miller, D.J. Aqueous-phase hydrogenation of lactic acid to propylene glycol. Appl. Catal. A Gen. 2001, 219, 89–98. [Google Scholar] [CrossRef]
- Popp, L.; Kampe, P.; Fritsch, B.; Hutzler, A.; Poller, M.J.; Albert, J.; Schühle, P. Supported Ruthenium Phosphide as a Promising Catalyst for Selective Hydrogenation of Sugars. Eur. J. Inorg. Chem. 2024, 27, e202400117. [Google Scholar] [CrossRef]
- Odyakov, V.F.; Zhizhina, E.G. New process for preparing aqueous solutions of Mo-V-phosphoric heteropoly acids. Russ. J. Inorg. Chem. 2009, 54, 361–367. [Google Scholar] [CrossRef]
- Odyakov, V.F.; Zhizhina, E.G. A novel method of the synthesis of molybdovanadophosphoric heteropoly acid solutions. React. Kinet. Catal. Lett. 2008, 95, 21–28. [Google Scholar] [CrossRef]
- Zhizhina, E.G.; Odyakov, V.F.; Simonova, M.V. Catalytic oxidation of organic compounds with oxygen in the presence of Mo-V-phosphoric heteropoly acid solutions. Kinet. Catal. 2008, 49, 773–781. [Google Scholar] [CrossRef]
- Bridgeman, A.J. Computational Study of the Vibrational Spectra ofα- andβ-Keggin Polyoxometalates. Chem. Eur. J. 2004, 10, 2935–2941. [Google Scholar] [CrossRef]
- Lee, J.K.; Melsheimer, J.; Berndt, S.; Mestl, G.; Schlögl, R.; Köhler, K. Transient responses of the local electronic and geometric structures of vanado-molybdo-phoshate catalysts H3+nPVnMo12−nO40 in selective oxidation. Appl. Catal. A Gen. 2001, 214, 125–148. [Google Scholar] [CrossRef]
- Pope, M.T.; Scully, T.F. Geometrical Isomerism Arising from Partial Substitution of Metal Atoms in Isopoly and Heteropoly Complexes. Possibilities for the Keggin Structure. Inorg. Chem. 1975, 14, 953–954. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Poller, M.J.; Bönisch, S.; Bertleff, B.; Raabe, J.-C.; Görling, A.; Albert, J. Elucidating activating and deactivating effects of carboxylic acids on polyoxometalate-catalysed three-phase liquid–liquid-gas reactions. Chem. Eng. Sci. 2022, 264, 118143. [Google Scholar] [CrossRef]
- Xu, M.X.; Lin, S.; Xu, L.-M.; Zhen, S.-L. Crystal structure and properties of H3[PMo12O40]·3C2H6O. Transit. Met. Chem. 2004, 29, 332–335. [Google Scholar] [CrossRef]
- Pyykkö, P.; Atsumi, M. Molecular single-bond covalent radii for elements 1–118. Chem. Eur. J. 2009, 15, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Palatinus, L.; Jacob, D.; Cuvillier, P.; Klementová, M.; Sinkler, W.; Marks, L.D. Structure refinement from precession electron diffraction data. Acta Crystallogr. Sect. A Found. Crystallogr. 2013, 69, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, L.; Andersson, I.; Grate, J.H.; Selling, A. Multicomponent Polyanions. 46. Characterization of the Isomeric Keggin Decamolybdodivanadophosphate Ions in Aqueous Solution by 31P and 51V NMR. Inorg. Chem. 1994, 33, 982–993. [Google Scholar] [CrossRef]
- Selling, A.; Andersson, I.; Grate, J.H.; Pettersson, L. A Potentiometric and (31P,51V) NMR Study of the Aqueous Molybdovanadophosphate System. Eur. J. Inorg. Chem. 2000, 2000, 1509–1521. [Google Scholar] [CrossRef]
- Weinstock, I.A.; Cowan, J.J.; Barbuzzi, E.M.G.; Zeng, H.; Hill, C.L. Equilibria between α and β Isomers of Keggin Heteropolytungstates. J. Am. Chem. Soc. 1999, 121, 4608–4617. [Google Scholar] [CrossRef]
- Sundaram, K.M.; Neiwert, W.A.; Hill, C.L.; Weinstock, I.A. Relative Energies of α and β Isomers of Keggin Dodecatungstogallate. Inorg. Chem. 2006, 45, 958–960. [Google Scholar] [CrossRef]
- Neiwert, W.A.; Cowan, J.J.; Hardcastle, K.I.; Hill, C.L.; Weinstock, I.A. Stability and Structure in α- and β-Keggin Heteropolytungstates, [Xn+W12O40](8−n)−, X = p-Block Cation. Inorg. Chem. 2002, 41, 6950–6952. [Google Scholar] [CrossRef]
- Himeno, S.; Takamoto, M.; Ueda, T. Formation of α- and β-Keggin-Type [PW12O40]3− Complexes in Aqueous Media. Bull. Chem. Soc. Jpn. 2005, 78, 1463–1468. [Google Scholar] [CrossRef]
- Evtuguin, D.V.; Pascoal Neto, C.; Rocha, J.; Pedrosa de Jesus, J.D. Oxidative delignification in the presence of molybdovanadophosphate heteropolyanions: Mechanism and kinetic studies. Appl. Catal. A Gen. 1998, 167, 123–139. [Google Scholar] [CrossRef]
- Salavati, H.; Rasouli, N. Synthesis and characterization of supported heteropolymolybdate nanoparticles between silicate layers of Bentonite with enhanced catalytic activity for epoxidation of alkenes. Mater. Res. Bull. 2011, 46, 1853–1859. [Google Scholar] [CrossRef]
- Yamase, T. Photo- and electrochromism of polyoxometalates and related materials. Chem. Rev. 1998, 98, 307–325. [Google Scholar] [CrossRef]
- Raj, N.K.K.; Ramaswamy, A.V.; Manikandan, P. Oxidation of norbornene over vanadium-substituted phosphomolybdic acid catalysts and spectroscopic investigations. J. Mol. Catal. A Chem. 2005, 227, 37–45. [Google Scholar] [CrossRef]
- Barteau, K.P.; Lyons, J.E.; Song, I.K.; Barteau, M.A. UV-visible spectroscopy as a probe of heteropolyacid redox properties: Application to liquid phase oxidations. Top. Catal. 2006, 41, 55–62. [Google Scholar] [CrossRef]
- Weinstock, I.A. Homogeneous-Phase Electron-Transfer Reactions of Polyoxometalates. Chem. Rev. 1998, 98, 113–170. [Google Scholar] [CrossRef]
- Holleman, A.F.; und Nils Wiberg, E.; Fischer, G. Lehrbuch der Anorganischen Chemie; De Gruyter: Berlin, Germany, 2009; ISBN 978-3-11-017770-1. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. C45 (H45), C46 (H46) 3. b Secondary CH2 refined with riding coordinates: C1 (H1A, H1B), C24 (H24A, H24B) 3. c Aromatic/amide H refined with riding coordinates: C2 (H2), C3 (H3), C5 (H5), C6 (H6), C8 (H8), C9 (H9), C11 (H11), C12 (H12), C14 (H14). J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
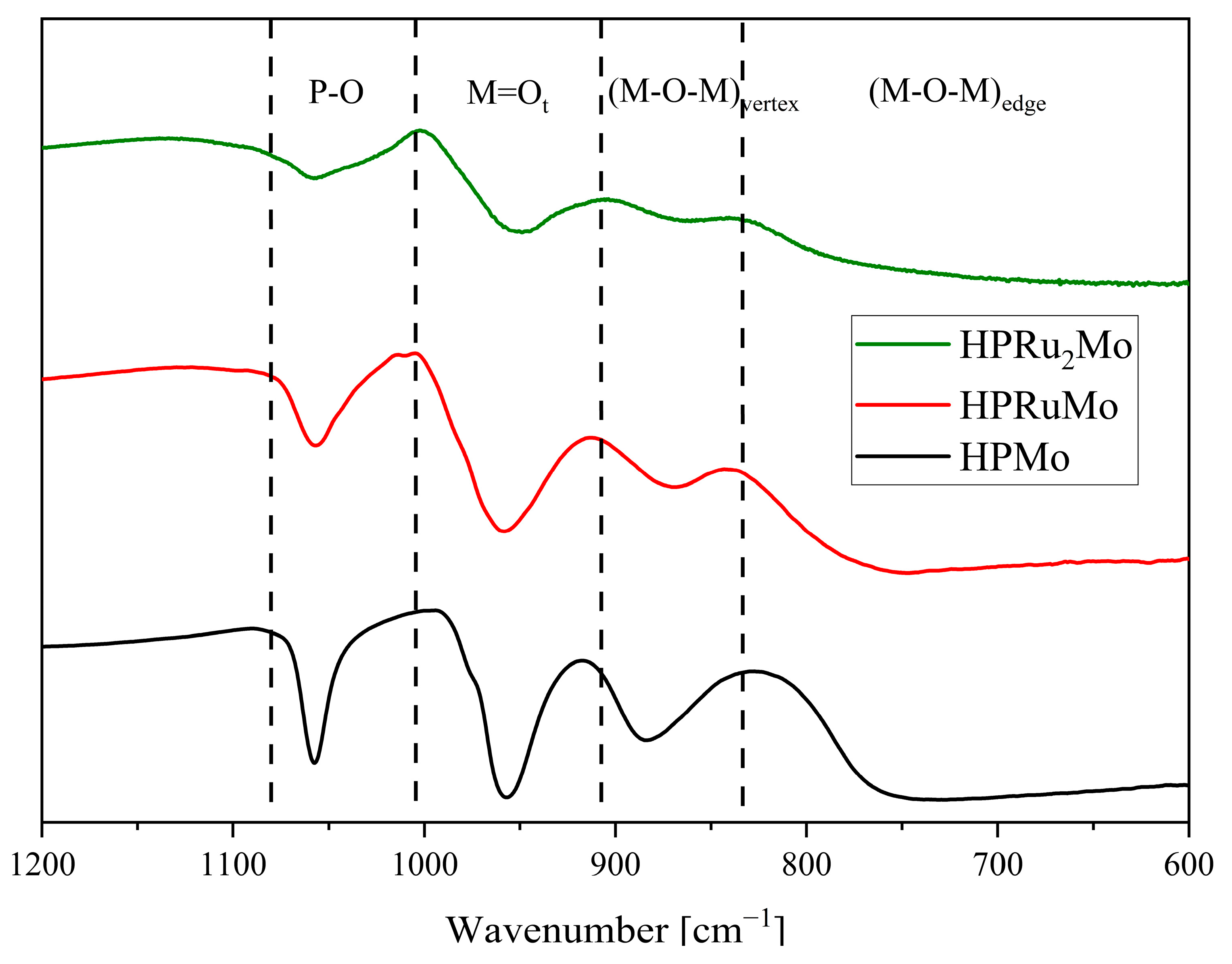
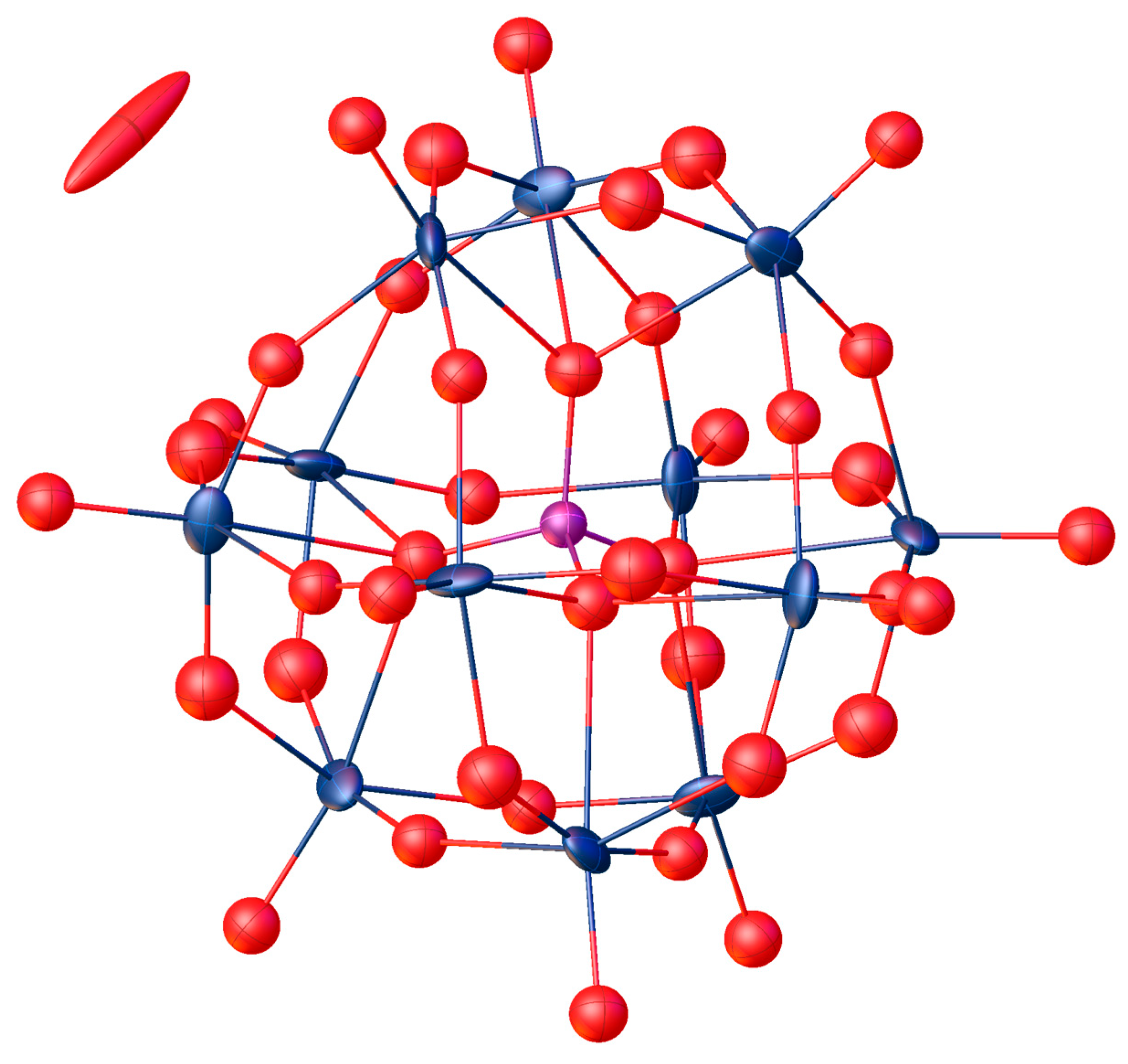

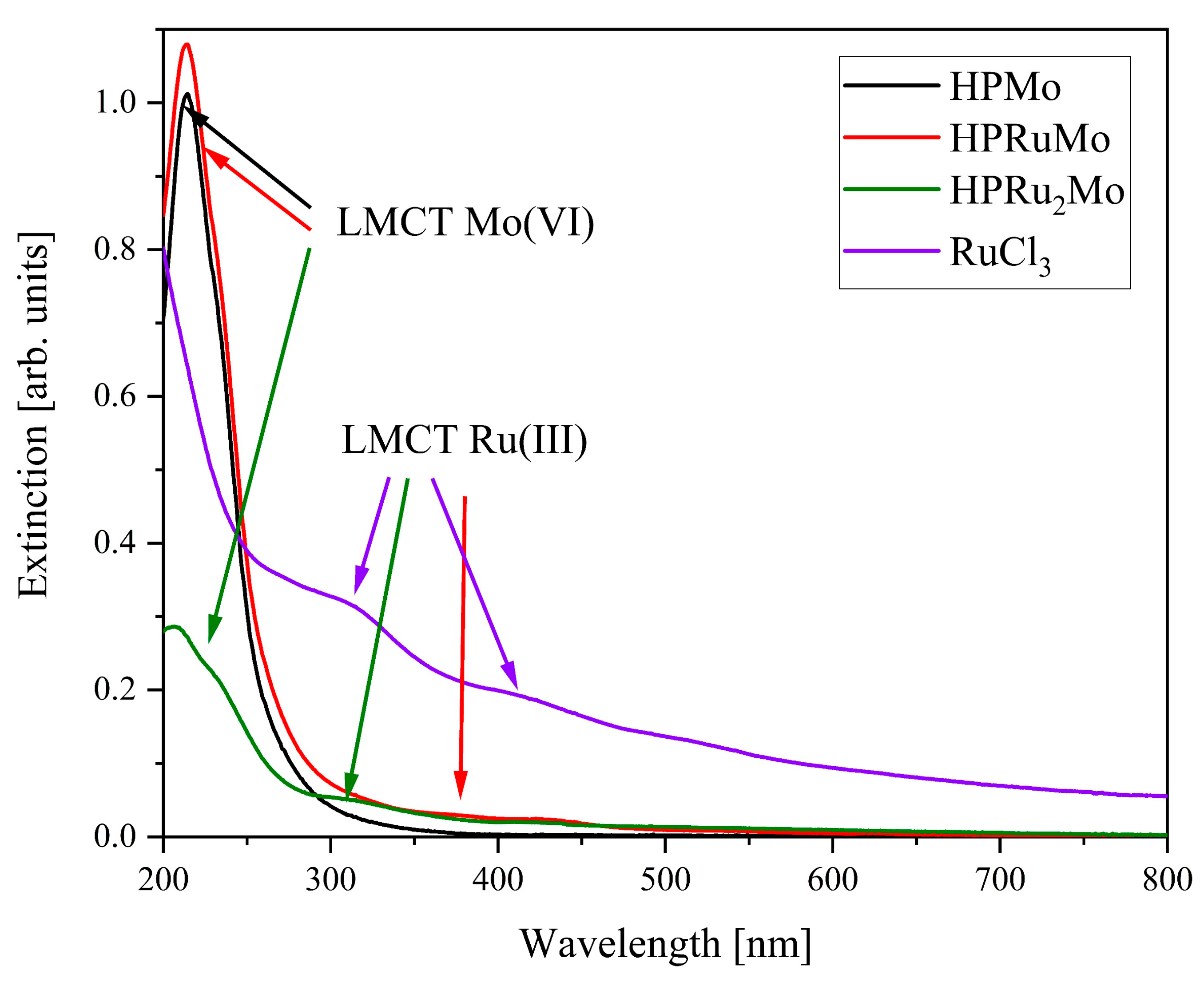
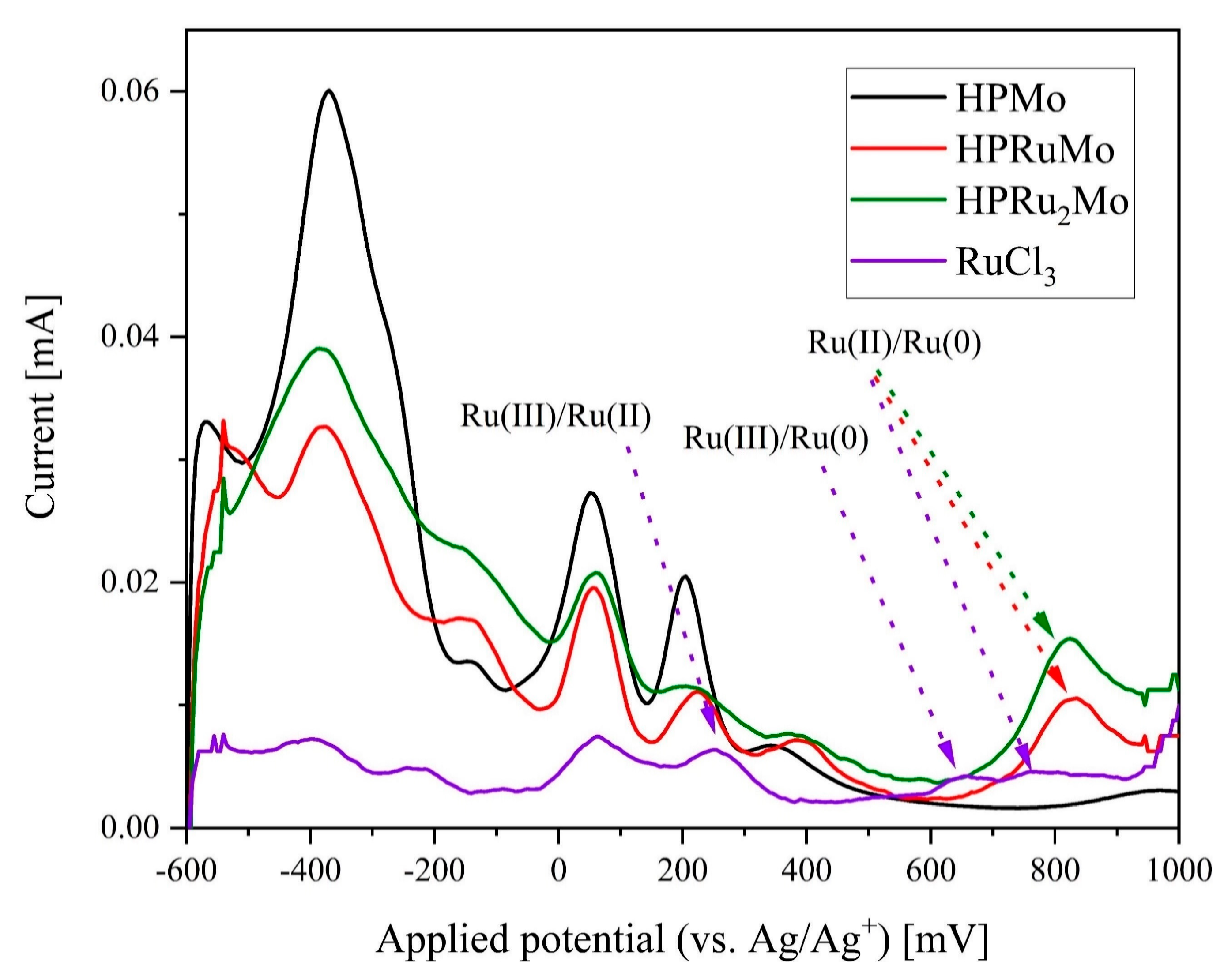
| Compound | Target Stoichiometry | P/Ru/Mo Ratio a | Hydration Water Content [mol/mol-POM] b |
|---|---|---|---|
| HPRu1Mo c | H6[PRuMo11O40] | 1.22/1.12/11 | 11 |
| HPRu2Mo d | H9[PRu2Mo10O40] | 1.22/2.08/10 | 12 |
| Precipitate | Ru2(MoO4)3 | -/2.20/3.00 | 6 |
| POM | P-Oa [Å] | Oa-M [Å] | M-Ob-M [Å] | M=Ot [Å] |
|---|---|---|---|---|
| HPMo | 1.534 | 2.439 | 1.916 | 1.674 |
| HPRu1Mo | 1.554 | 2.452 | 1.767 | 1.699 |
| Catalyst | Conversion [mol%] | Yield [mol%] | Selectivity [%] | ||
|---|---|---|---|---|---|
| PD | PA | PD | PA | ||
| Control | 0 | 0 | 0 | 0 | 0 |
| H3[PMo12O40] b | 7 | 0 | 9 | 0 | 100 |
| RuCl3 a | 20 | 9 | 6 | 47 | 31 |
| HPRu1Mo a,b | 33 | 4 | 24 | 12 | 73 |
| HPRu2Mo b | 36 | 12 | 15 | 35 | 42 |
| Compound | Molybdenum Trioxide | 36.9 % Phosphoric Acid in Water | Volume of Water [mL] | Ruthenium(III) Chloride x Hydrate | Volume of Water [mL] | Volume of Ethanol [mL] |
|---|---|---|---|---|---|---|
| HPRuMo | 2.590 g 17.99 mmol 11 equiv. | 0.435 g 1.636 mmol 1 equiv. | 80 | 0.414 g 1.638 mmol Ru 1 equiv. | 15 | 15 |
| HPRu2Mo | 2.353 g 16.35 mmol 10 equiv. | 0.469 g 1.766 mmol 1.08 equiv. | 80 | 0.825 g 3.265 mmol Ru 1.99 equiv. | 15 | 15 |
| Product | ||||||
| m [g] | M [g/mol] * | Lattice water | n [mmol] | n H3PO4 [mmol] ** | Yield [%] | |
| HPRuMo | 3.075 | 2031.5755 | 11 | 1.514 | 1.636 | 92.52 |
| HPRu2Mo | 3.363 | 2057.7446 | 12 | 1.634 | 1.766 | 92.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papajewski, M.; Raabe, J.-C.; Anwari, H.; Voß, D.; Albert, J.; Poller, M.J. The Synthesis and Characterisation of Ru(III)-Substituted Keggin-Type Phosphomolybdates. Inorganics 2025, 13, 176. https://doi.org/10.3390/inorganics13060176
Papajewski M, Raabe J-C, Anwari H, Voß D, Albert J, Poller MJ. The Synthesis and Characterisation of Ru(III)-Substituted Keggin-Type Phosphomolybdates. Inorganics. 2025; 13(6):176. https://doi.org/10.3390/inorganics13060176
Chicago/Turabian StylePapajewski, Max, Jan-Christian Raabe, Hamid Anwari, Dorothea Voß, Jakob Albert, and Maximilian J. Poller. 2025. "The Synthesis and Characterisation of Ru(III)-Substituted Keggin-Type Phosphomolybdates" Inorganics 13, no. 6: 176. https://doi.org/10.3390/inorganics13060176
APA StylePapajewski, M., Raabe, J.-C., Anwari, H., Voß, D., Albert, J., & Poller, M. J. (2025). The Synthesis and Characterisation of Ru(III)-Substituted Keggin-Type Phosphomolybdates. Inorganics, 13(6), 176. https://doi.org/10.3390/inorganics13060176








