Abstract
The present work examines pure and palladium photofixed TiO2 and binary (TiO2/ZnO) photocatalysts for breaking down tartrazine, a food coloring agent, in distilled water. Powders with the following compositions are obtained using the sol-gel process: 100TiO2, 10TiO2/90ZnO, 50TiO2/50ZnO, and 90TiO2/10ZnO. The composite materials are analyzed using SEM-EDS, UV-Vis, DTA-TG, and X-ray diffraction. The synthesized gels are then photo-fixed with UV light to incorporate palladium ions and are also examined for tartrazine (E102) degradation. The photocatalytic tests were carried out in a cylindrical glass reactor illuminated by ultraviolet light. Compared to mixed binary catalysts, the prepared pure TiO2 catalyst demonstrated greater activity in the photodegradation of tartrazine (E102). The further of a specific quantity of zinc oxide reduced the catalytic properties of TiO2. The recombination of photoinduced electron-hole pairs in ZnO may account for this. In comparison to the pure samples, the co-catalytic palladium-modified gels exhibited higher photocatalytic efficiency. Heterojunction and palladium modification of the composites partially captured and transferred the electrons. Consequently, the longer lifetime of the photogenerated charges improved the catalytic activity of the palladium titanium dioxide and binary gels. Additionally, under UV light, pure and palladium photofixed TiO2 and binary sol-gel samples displayed excellent stability for tartrazine photodegradation.
1. Introduction
The food industry is well aware that a variety of synthetic dyes are used to add color to a variety of products, such as baked goods, candies, and sports drinks [1]. In the meantime, the Food and Drug Administration reports that the amount of artificial food dyas consumed per person has grown by 500% in the past 50 years [2]. Despite lacking nutrients, food dyes do contain azo groups and aromatic rings [1,3]. Some food dyes are carcinogenic, genotoxic, and mutagenic [4,5,6]. Tartrazine is the most allergenic azo dye, causing reactions in asthmatics and those who cannot tolerate aspirin [7,8].
The first paper about tartrazine’s potential for adverse reactions appeared in the medical literature in 1959, making it one of the first food additives to cause such concerns [9]. Con-cerns about Tartrazine were first raised in 1958 due to the perceived because both aspirin and this food dye contain benzene rings, they may have structural similarities. Several early studies tried to connect tartrazine to asthma and chronic urticarial [10]. Sensitive individuals have been found to experience allergic reactions to food coloring (E102), and a small percentage of the exposed population may develop intolerances to the color even at dose levels within the corresponding acceptable daily intake of 7.5 mg/kg body weight. Even at low concentrations, this dye is harmful [11,12]. Given these adverse effects, it is critical to clean up food dye-containing wastewater released from facilities in the food industry.
Various methods (e.g., biological, physical, and chemical) have been used to remove harmful dyes from wastewater [13,14]. Enzyme degradation was regarded as an effective biological technique [13], and adsorption on carbonaceous materials (such as carbon nanotubes and activated carbon) is thought to be an effective dye removal method [14,15]. Heterogeneous catalysis, like the technique created with titanium dioxide and zinc oxide catalysts, has been suggested as one of the chemical techniques a more efficient option for treating various organic dyes under ultraviolet light [16,17,18,19,20]. In heterogeneous catalysis, the catalyst absorbs the energy of light, which causes e− to move to the conductive band. This procedure produces e−/h+ carriers, which can create extremely reactive oxygen species like superoxide anions and OH radicals when it reacts with H2O, OH groups, and O2 molecules. After attacking essential organic components (dyes, organic pollutants), these radical species break them down through oxidation reactions [21,22]. In both semiconductors, photocatalytic efficiency is greatly decreased by the recombination of photogenerated electrons and holes within semiconductor particles. The parallels between zinc oxide and titanium dioxide photocatalysts and their respective photocatalytic benefits served as the impetus for this investigation [23,24].
A photocatalyst’s efficiency depends on several important characteristics, including its degree of crystallinity, lattice defects, uncoordinated surface sites, light-exposed surface area, and crystal phase. Using composite materials enables most of the aforementioned properties to be improved, controlled in morphology, and fine-tuned. The creation of suitable mid-band-gap electronic states by composite heterostructures can also change charge migration or result in a red shift in the absorption spectrum. Furthermore, absorption of visible light may result from the materials forming heterojunctions. It is possible to achieve adequate charge separation, a longer enhanced interfacial charge transfer to the adsorbed species, promoting their photooxidation and additional mineralization, and the charge carriers’ lifetime, through the use of heterostructured catalysts with adjustable bandgaps, improved steadiness, and improved catalytic realization [22,25,26]. To achieve more effective photocatalytic degradation, numerous studies have been conducted on nanostructured ZnO/TiO2 composites with various morphologies and configurations [27,28]. Knowing how the variations between TiO2 and ZnO might impact the overall catalytic processes is of interest.
It has been reported that, the noble metals during photocatalysis can successfully stop the deactivation of the photocatalyst at high temperatures and inhibit undesirable redox reactions that consume photogenerated radicals [29]. Among all noble metal co-catalysts palladium is a preferable one due to its lower Fermi levels as well as it expected to reduce the energy band gap by reducing electron-hole recombination [30]. Usually, it has been selected due to its ionic radius as a dopant (~86 pm) bigger than that of Ti+4 ion (~68 pm) which is a challenge due to their large difference [30]. However, a number of studies on the photocatalytic degradation of pollutants on palladium-loaded TiO2 and Pd/ZnO catalysts made using various techniques have been published in the literature [31,32,33,34,35]. To the best of our knowledge, there is no systematic study dealing with the photocatalytic degradation of dyes with the palladium loaded mixed sol-gel derived TiO2/ZnO powders.
This research is an extension of our earlier investigation expanding it toward how the dopant influence on the morphology, thermal and sol-gel derived gels’ catalytic activity [36,37]. The main focus of this work is to verify the effect of palladium—modifying on the catalytic degradation of the azo dye Tartrazine, used as a food colorant E102. It was performed when ultraviolet light is present, utilizing the mixed in different ratio binary gels. A comparison of the prepared gels with those modified with Pd was made, which emphasizes the paper’s originality. In addition, the used food colorant E102 poses a significant threat to human health and requires considerable attention in its use.
2. Results and Discussion
2.1. X-Ray Diffraction and Thermal Stability of the Gels
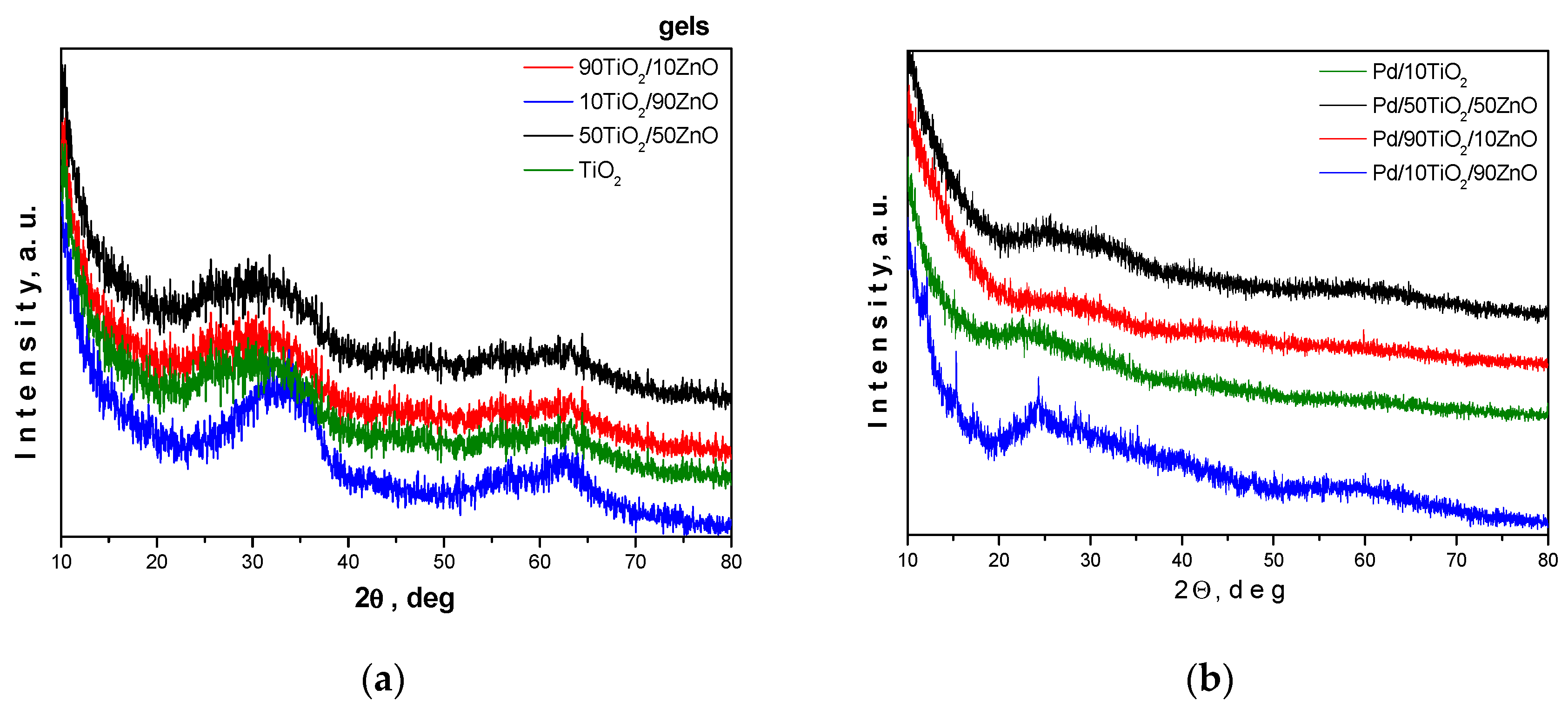
Bright and homogeneous gels were obtained and the gelation occurred immediately. Their visual observations of the gels and Pd modified samples are shown in Figure 1a–g. According to the XRD results all samples are amorphous (Figure 2a). Additionally, we have performed the XRD analysis of the Pd-modified photocatalysts (Figure 2b). As it is seen from the figure, the peak positions were not changed despite the addition of palladium to the TiO2 framework. The absence of peaks corresponding to Pd, PdO, and PdO2 suggests that the palladium ions were incorporated into the titanium anatase structure. In particular, the decreasing of the intensity of the amorphous halo as well as its broadening could be a proof for the substitution of titanium ions. These results well corelate with those obtained by other authors [38,39]. Information for the samples surface was obtained by BET measurements. The prepared gels showed specific surface value about 30 m2/g, while pure TiO2 obtained from Ti(IV) butoxide exhibited about 70 m2/g.

Figure 1.
Pictures of binary gels: (a) 90TiO2/10ZnO, (b) 10TiO2/90ZnO (c) 50TiO2/50ZnO, (d) Pd/TiO2, (e) Pd/90TiO2/10ZnO (f), Pd/50TiO2/50ZnO and (g) Pd/10TiO2/90ZnO.
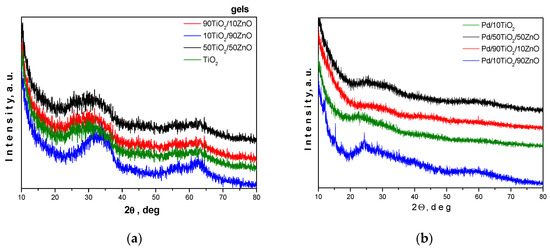
Figure 2.
XRD of the as-prepared gels (a) and Pd-modified samples (b).
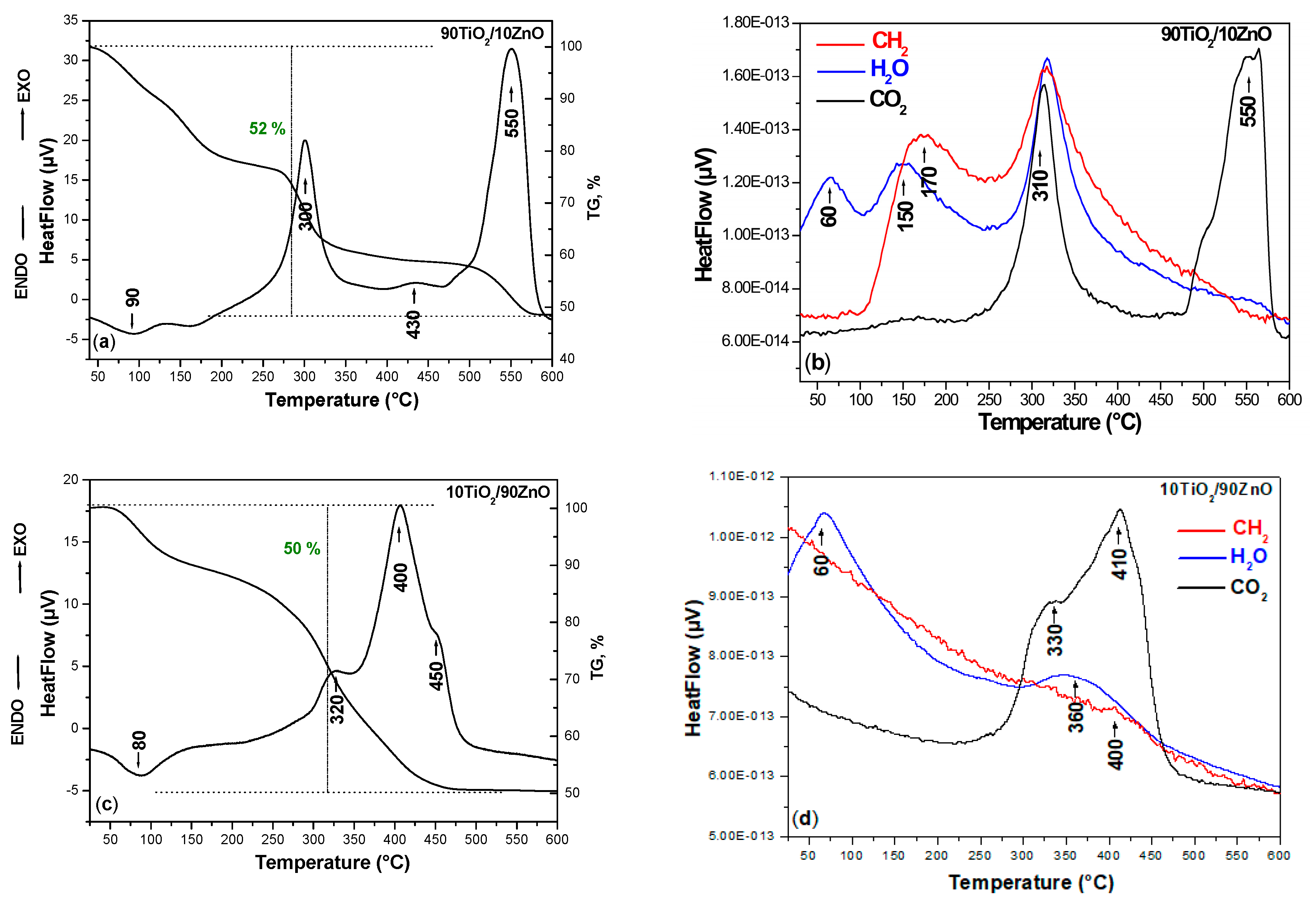
In order to verify the thermal stability of the TiO2/ZnO nonannealed catalysts, the DTA-TG analysis was conducted. The DTA/TG curves of the prepared nonannealed dried gels, are presented in Figure 3a–d. As it can be seen, several peaks are marked on the DTA/TG curves for both samples. The DTA-TG of the pure TiO2 obtained from the Ti(IV) butoxide is not considered in this paper as it has been published elsewhere [40]. Generally, one endothermic peak is observed about 80–90 °C which is to the evaporation of physically adsorbed water (Figure 3a,c). When the temperature is increased the first exothermic peak is observed about 300–320 °C related to the decomposition of the organics. As it is seen from the figure the combustion process occurred more intensively in the sample 90TiO2/10ZnO due to the presence of higher Ti(IV) butoxide amount. In the other 10TiO2/90ZnO sample, this peak is weaker and could be related to the beginning of the thermal decomposition of the ZnO precursor [41,42]. The second exothermic peak is detected at ~400–430 °C and it is sharper in the sample containing higher ZnO content perhaps due to the stronger release of the organics (Figure 3c). According to the TG curves in both samples these peaks are accompanied by a strong weight loss ~50% for both samples and the processes finished at nearly 450 °C. The last exothermic effects at 450 and 500 °C observed in samples 90TiO2/10ZnO and 10TiO2/90ZnO, respectively. Bearing in mind these experimental facts, it could be assumed that these last effects could be related to the oxidation of residual carbon and release of CO2. On the other hand, Siwińska-Stefańska et al. [41] stated that the exothermic effects above 300 °C of the TiO2-ZnO hybrids is due to the thermal decomposition of unreacted zinc acetate. The results have shown that the obtained TiO2/ZnO gels exhibited relatively good thermal stability. Furthermore, titanium dioxide and zinc oxide following thermal degradation have similar thermal stability comparable to that reported in the literature [42,43].
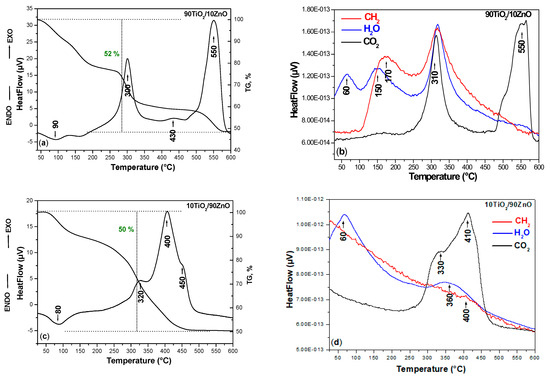
Figure 3.
Differential thermal analysis (DTA)/thermogravimetric (TG) curves (a,c) of the samples and mass spectra recorded for the synthesized gels (b,d).
2.2. SEM Morphology of the Synthesized Gels
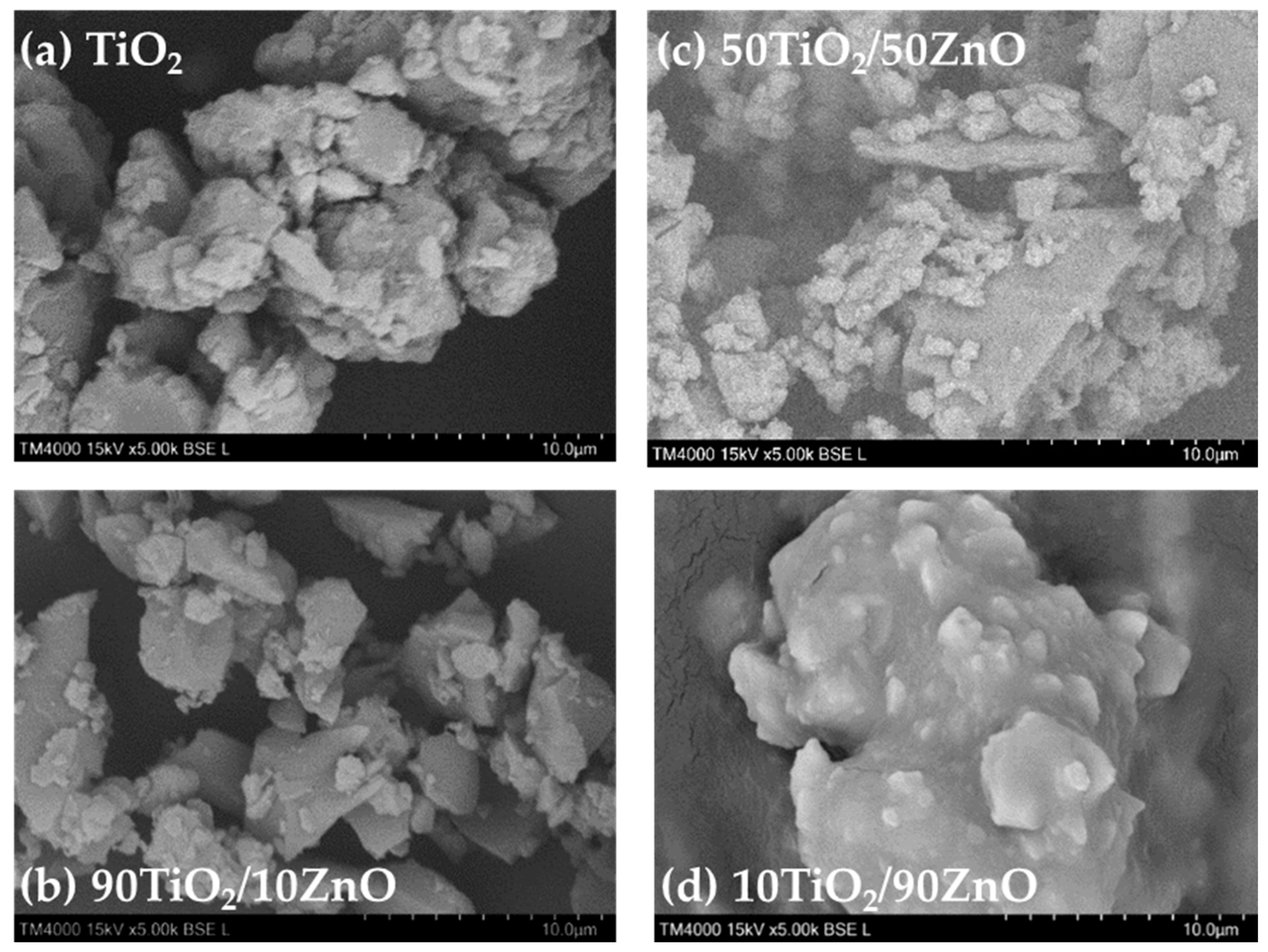
SEM has verified the gels and Pd modified samples morphology and the images are shown in Figure 4a–d and Figure 5a–d, respectively. TiO2 gel, depicted in Figure 4a, ex-hibits a morphology characterized by larger agglomerates ranging from 3 to 13 μm, coated with smaller particles averaging 1.68 ± 0.68 μm in diameter. The SEM micrographs of TiO2/ZnO gels expressed the morphological characteristics of both counterparts as TiO2 and ZnO, depending on the TiO2/ZnO molar ratio. Additionally, all the photographs indicated that the particles obtained by the sol–gel method exhibited strong agglomeration and a less porous structure. The images of as-prepared 90TiO2/10ZnO and 50TiO2/50ZnO showed similarity in their particle shapes (Figure 4b,d). The sample 90TiO2/10ZnO, presented in Figure 4b, displays a distinct morphology. Larger, roughly triangular particles, ranging from 2 to 10 μm in size, are visible. Their surfaces are covered with smaller particulates, exhibiting a narrower size distribution and an average diameter of 1.25 ± 0.27 μm. Similarly, 50TiO2/50ZnO, illustrated in Figure 4d, contains larger, flat particles with a roughly triangular shape, measuring between 5 and 11 μm. These are coated with a significant number of smaller, approximately round secondary particles and a finer granular phase. These granular particles consist of agglomerates with average dimensions of 1.26 ± 0.27 μm. The form of these particles was plate-like or irregular with a particles size above 20 µm. It was clearly observed that the 10TiO2/90ZnO sample exhibited different morphology (Figure 4c) which could predict the difference in the sample’s properties. In sample 10TiO2/90ZnO, shown in Figure 4c, a prominent aggregate approximately 15–20 μm in diameter is observed, featuring a notably smoother surface morphology. However, nodular surface features with an average diameter of 1.18 ± 0.34 μm are also evident. The elemental compositions (mass ratios of Zn/Ti) of the photocatalysts were obtained using EDS (Table 1). No other elements were observed by this method. As shown in the Table 1, the presence of the elemental compositions of Ti and O peaks were endorsed in all samples. Moreover, the measured Zn/Ti mass ratios correspond well to the initial composition.
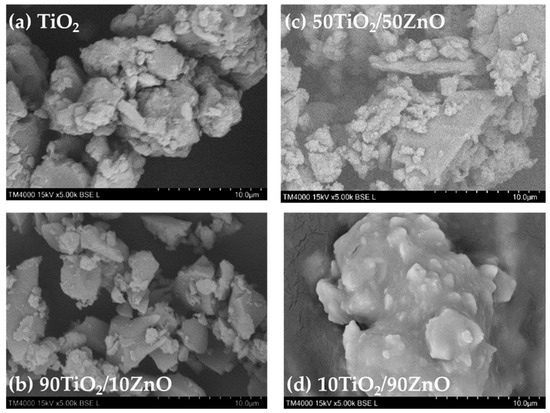
Figure 4.
SEM images of the prepared gels.
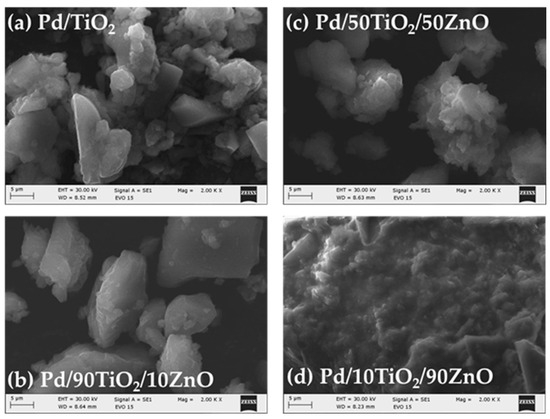
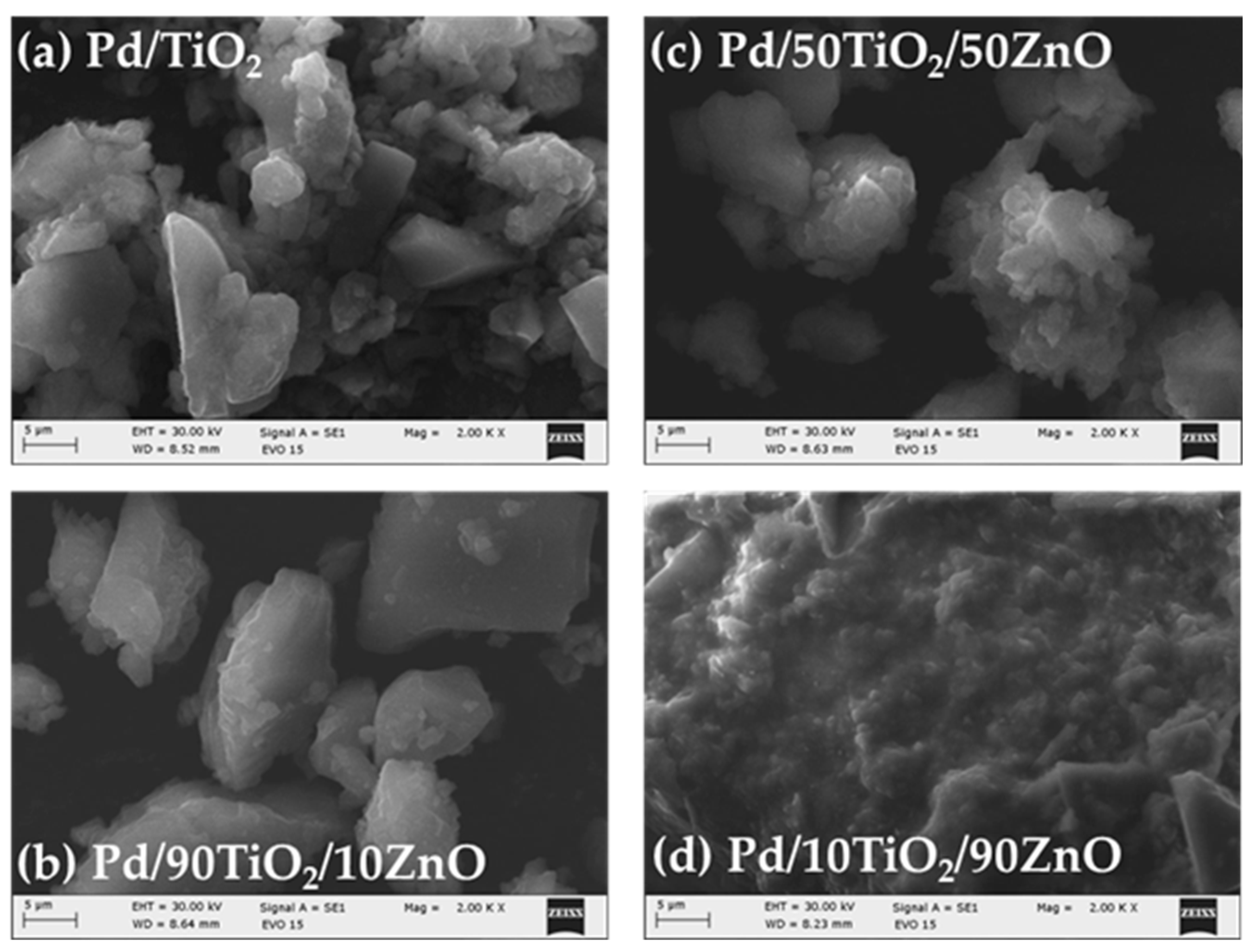
Figure 5.
SEM images of the Pd doped samples.

Table 1.
EDS data of the gels and Pd doped photocatalysts.
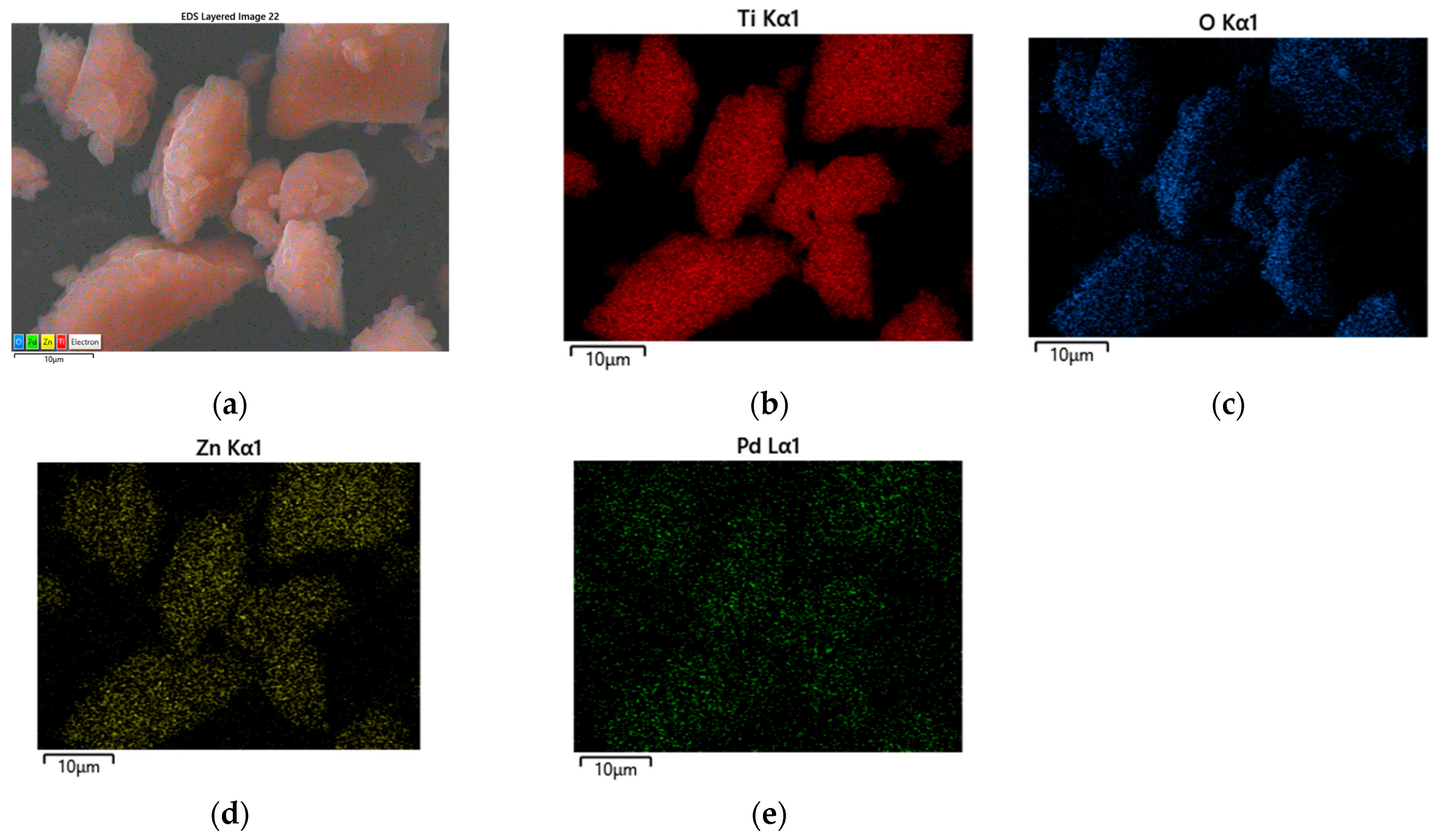
Figure 5a–d represents the surface morphology of Pd modified TiO2/ZnO samples. Generally, there is no change in the morphology of the examined samples after the addition of Pd. It depicts distribution of irregular and well-shaped particles with a certain degree of agglomeration. Perhaps palladium is deposited over the surface of TiO2/ZnO gels which was already stated by several authors [44,45]. The EDS analysis (Table 1) also confirmed its loading on the samples surface which is related to the expected enhancement of the photocatalytic activity. Figure 6a shows the chemical mapping performed under a scanning microscope for sample Pd/90TiO2/10ZnO which eexhibited best photo-catalytic activity. The chemical mapping for the rest of the samples were similar. The mapping shows a homogeneous distribution of Ti, Zn, Pd and O (Figure 6b–e). The presence of all elements observed in the EDS spectrum is summarize in Table 1.
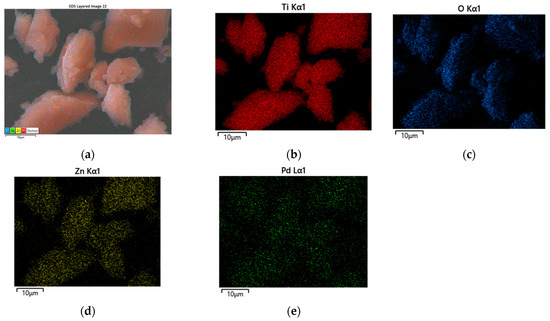
Figure 6.
(a) SEM image elemental mapping of Pd/90TiO2/10ZnO sample; (b) composition map of Ti; (c) composition map of O; (d) composition map of Zn and (e) composition map of Pd.
2.3. Optical Properties of the Gels and Pd Modified Mixed Catalysts
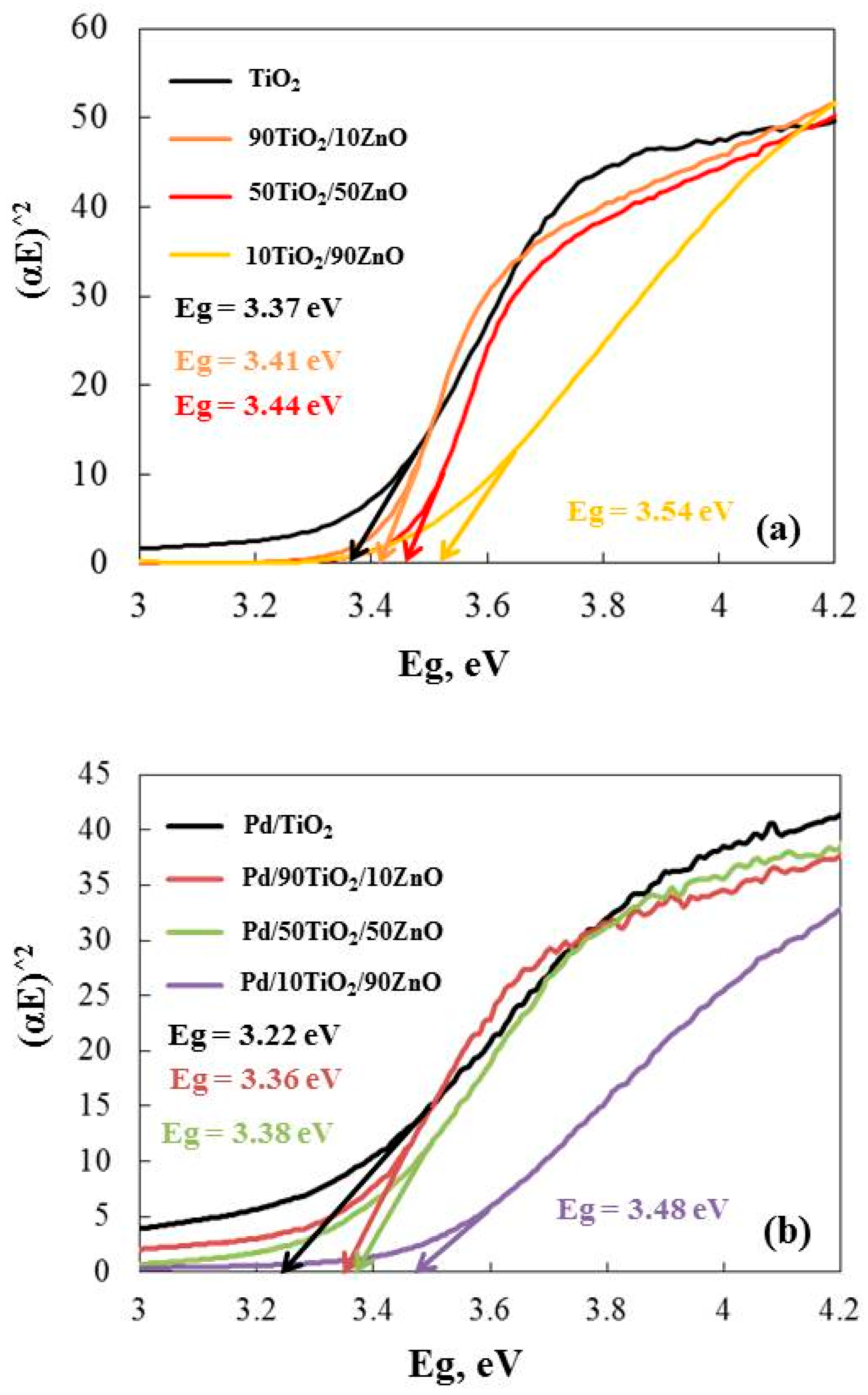
UV-Visible absorption spectroscopy is widely used technique to examine the optical properties of materials. Figure 7 represents the values from the Kubelka–Munk extrapolation to the energy band gap of the gels. The calculated band gaps of the gels are as follows: TiO2 (3.37 eV), 90TiO2/10ZnO (3.41 eV), 50TiO2/50ZnO (3.44 eV) and 10TiO2/90ZnO (3.54 eV). The collected data demonstrates that the band gap (Eg) of the gels narrows with increasing TiO2 content. The lower value of Eg for the sample with higher TiO2 content (90 mo%) may be attributed to the excessively smaller particle size of the TiO2 catalysts, which favor greater surface defects [46]. The calculated Eg values for Pd modified samples are shown in Figure 7b. It has to be noted that the addition of Pd caused the decrease in the band gap values as follow: TiO2 (3.22 eV), 90TiO2/10ZnO (3.36 eV), 50TiO2/50ZnO (3.38 eV) and 10TiO2/90ZnO (3.48 eV). This shift is attributed to the incorporation of palladium ions into the TiO2 and TiO2/ZnO. As a result, induced co-catalytic modification of the semiconductors with Pd, leading to a narrower optical band gap. These results correspond well to those obtained by other authors [44,47,48]. Thus, promising photocatalytic characteristics of the studied materials might be predicted from the observed results.
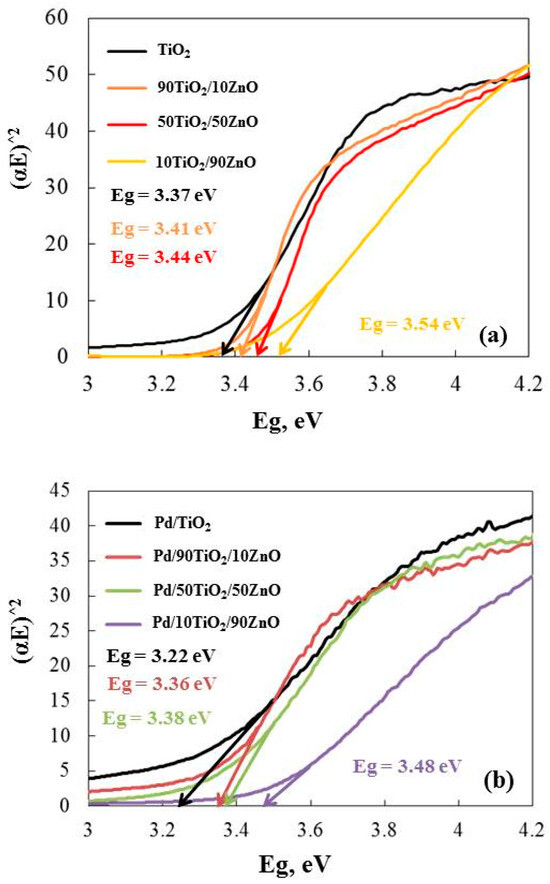
Figure 7.
Energy band gap (Eg) calculated from the Tauc’s equation of as-prepared gels (a) and Pd modified samples (b).
Palladium may cause the semiconductor band gap to decrease because of the band gap’s renormalization effect [47,48]. The exchange interaction in the band’s electrons and the localized electrons of the Pd ions were the cause of the renormalization. The band gap of the palladium samples was altered to pure semiconductors following the potential exchange interaction. The co-catalytic palladium-modified gels’ band gap values dropped to TiO2 (3.22 eV), 90TiO2/10ZnO (3.36 eV), 50TiO2/50ZnO (3.38 eV) and 10TiO2/90ZnO (3.48 eV). In the oxidation and reduction reactions that occurred on the surface, the co-catalytic palladium-modified gels were more effective light-trapping materials because they were able to form more superoxide and hydroxyl radicals. As a result, the palladium ions decreased the photogenerated charge pairs’ (electron-hole) rate of recombination. Consequently, it was anticipated that the Pd photofixed gels would exhibit superior photocatalytic activity.
2.4. Photocatalytic Properties of the Investigated Pure and Pd Modified Samples
Since colored dyes are known to undergo photolysis when exposed to visible light, a control experiment was conducted to ascertain the rates of food colorant removal without of a gel photocatalyst. Tartrazine is known to function as a photosensitizer and is; therefore, more susceptible to photolysis, the results show a loss of up to 5.17% within 75 min. Furthermore, for the pristine gel catalyst (TiO2), a dark phase adsorption experiment using E102 was conducted. The dye concentration has dropped by about 8% from the starting 7.5 ppm. The rate of Tartrazine decolorization was measured spectrophotometrically over time at the wavelength of maximum absorbance, 430 nm.
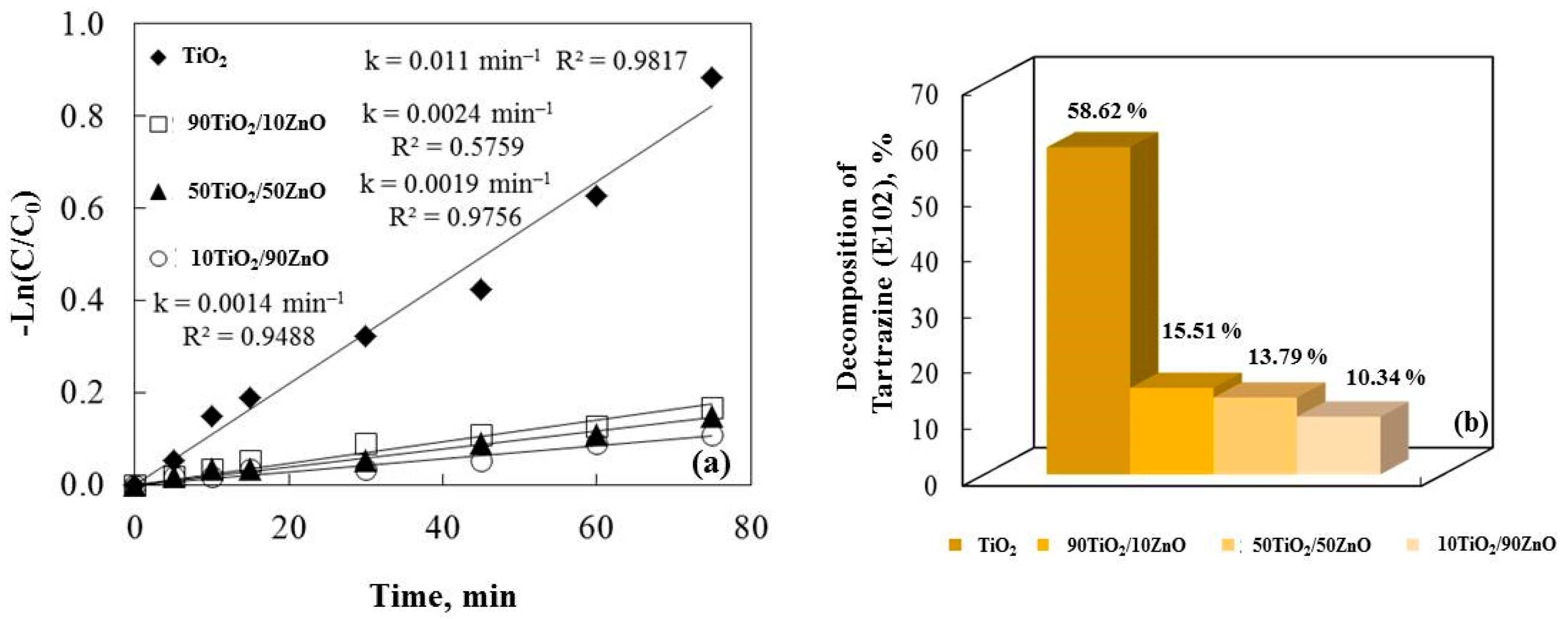
A constant reaction rate for the samples is shown by plotting Ln(C/C0) against irra-diation time in Figure 8a. It is noticeable that increasing the amount of zinc oxide de-creases the photocatalytic efficiency of the no-annealed catalysts. The results demonstrate that TiO2 has a higher efficiency when compared to TiO2/ZnO gels. The causes of these results are unknown, but they most likely result from a complex interaction of various factors. TiO2’s smaller crystallite size and greater surface area cause food coloring to degrade more quickly.
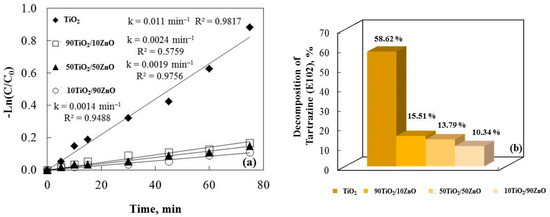
Figure 8.
The rate constants (a) and the degradation percentage of E102 (b) under ultraviolet light illumination for the synthesized gels.
Probably due to the samples’ improved morphology (according to the SEM images, Figure 4), larger specific surface area (BET data), crystalline size, grain growth, and lack of agglomeration, pure gels demonstrated higher photocatalytic efficiency than the TiO2/ZnO catalysts. A higher surface area usually results in more adsorption, which im-proves e−/h+ reactions with the organic molecules. The e−/h+ recombination can be ac-celerated by a greater surface area, which lowers the reaction rate [49]. The specific surface area therefore affects photoactivity; when surface reaction predominates recombination, a photocatalyst with a larger surface area is preferred, and inversely. The degradation percentage of the food colorant confirms the gel catalysts’ rate constant (k).
TiO2 powder tests demonstrate a significantly higher E102 decolorization rate than binary system. The catalytic activity of pure gel is highest, as shown in Figure 8b. The other sample, 90TiO2/10ZnO, has a higher photocatalytic efficiency than 10TiO2/90ZnO. These findings are difficult to fully explain and most likely result from a complex interplay of different factors. The SEM pictures (Figure 4) suggest that the lack of agglomeration and the smaller pore structure are the reasons for the decline in photocatalytic activity as the zinc oxide content rises. This pattern, where a binary photocatalyst performs worse than a pristine one, has also been shown by data from earlier literature [49].
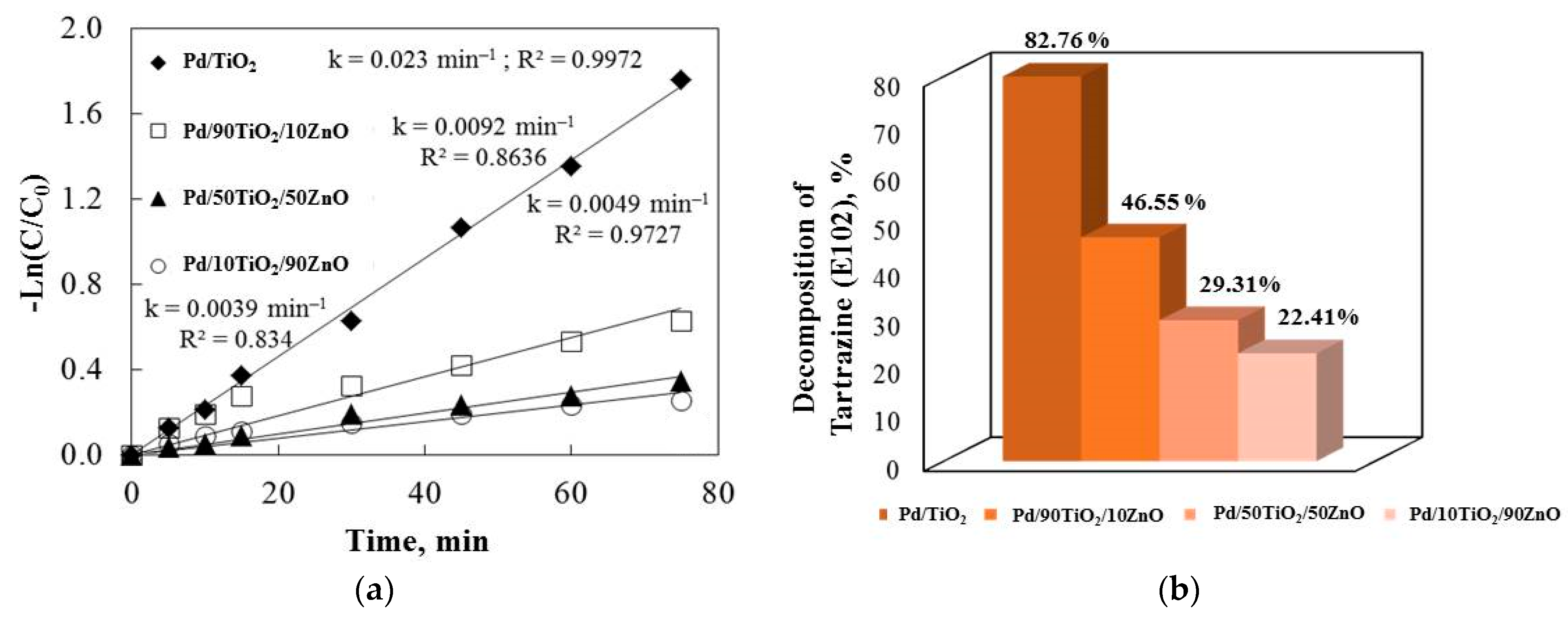
E102 degradation by ultraviolet illumination was used to further examine the catalytic efficiencies of the co-catalytic palladium-modified gels (Figure 9). All of the R2 correlation coefficients were higher than 0.947. The photocatalytic efficiency of co-catalytic modified samples is known to be influenced by a number of factors, such as the synthesis method, band gap, light illumination, crystallite size, surface area, etc. Palladium enhanced the samples’ photocatalytic behavior under ultraviolet illumination, as demonstrated by our photocatalytic experiments.
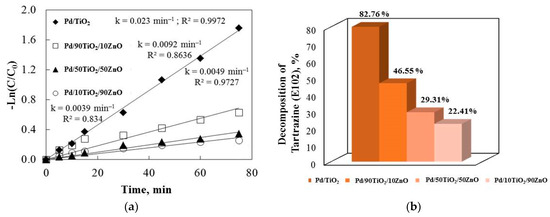
Figure 9.
The rate constants (a) and the degradation percentage of E102 (b) under ultraviolet light illumination for the Pd modified samples.
Figure 9 demonstrates that the palladium-modified catalysts were more effective at decolorizing the E102 than pure TiO2 and TiO2/ZnO gels. When the semiconductor was exposed to UV light, e−/h+ pairs were produced at the surface that was illuminated. These charge carriers’ recombination slowed down the photocatalysis rate. Palladium ions’ capacity to trap electrons may account for the beneficial impact of the co-catalytic modification on the TiO2 and TiO2/ZnO’s photodegradation efficiency. Therefore, ad-sorption onto the surface molecules resulted in a more efficient electron transfer than in the case of pure samples. The electrons were trapped and superoxide anions were created when oxygen was adsorbed onto the photocatalyst surface. Conversely, hydroxyl radicals could be made if the holes at the semiconductor surface oxidize the adsorbed water or hydroxide ions. The production of O2•− is decreased because the electron transfer to palladium ions is significantly faster than oxygen molecules. However, the loading of Pd metal on the TiO2 and TiO2/ZnO surfaces in the co-catalytic palladium-modified samples using the sol-gel method may accelerate the transport of photogenerated electrons to the outer systems. The metal deposits became partially negatively charged as a result of the electrons moving there. By enhancing the electron transfer to the dissolved oxygen molecules, the palladium ion deposits on the surface increased the photoactivity. As a result, trapped electrons from the Pd metal were transferred to the oxygen, reducing the oxygen and producing superoxide anion radicals.
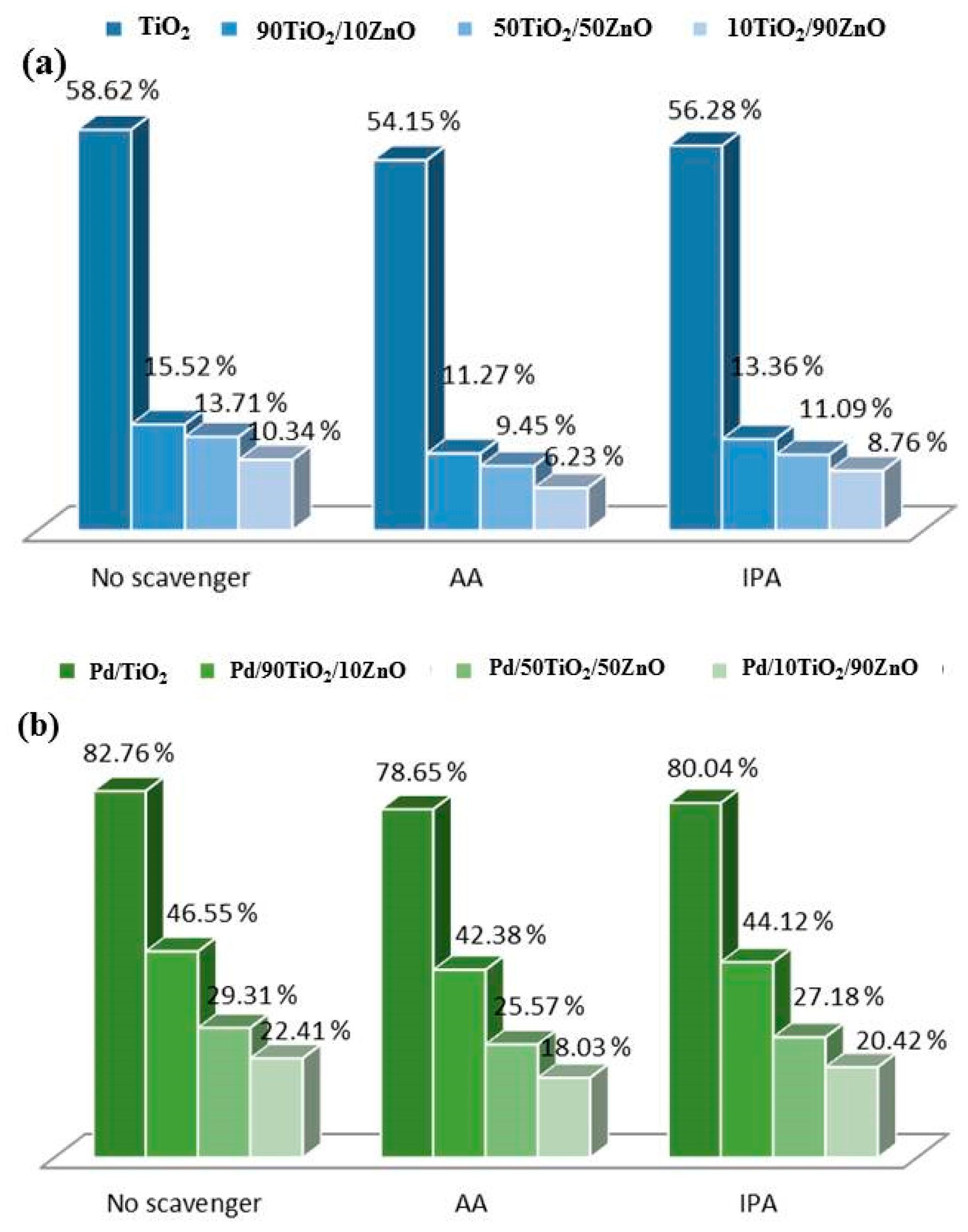
The presence of superoxide and hydroxyl radicals was verified using a radical test (Figure 10). The role of superoxide and OH• in the breakdown of tartrazine was measured using scavengers such as ascorbic acid (AA) and isopropyl alcohol (IPA) [50]. The eight photocatalyst systems all reacted similarly when AA and IPA were included, as seen in Figure 11. AA, on the other hand, showed a more noticeable inhibition, suggesting that the production of superoxide radicals contributes more to the rate of food coloring degradation.
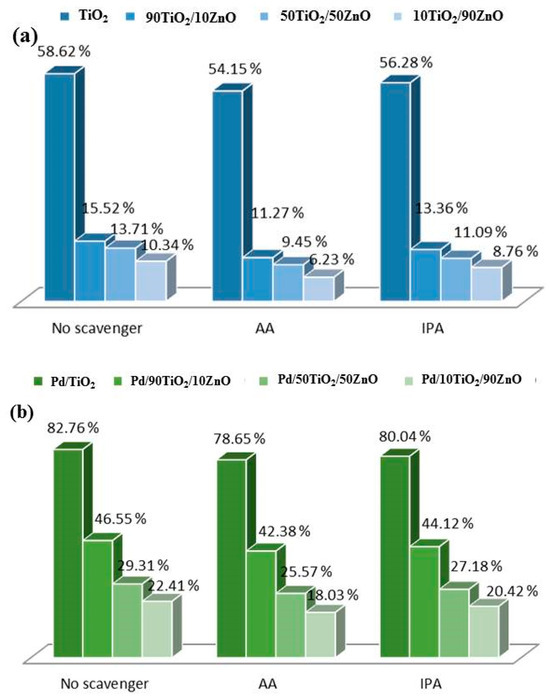
Figure 10.
Scavengers’ radical effects on tartrazine breakdown using (a) pure and (b) Pd-modified TiO2, 90TiO2/10ZnO, 50TiO2/50ZnO and 10TiO2/90ZnO gels.
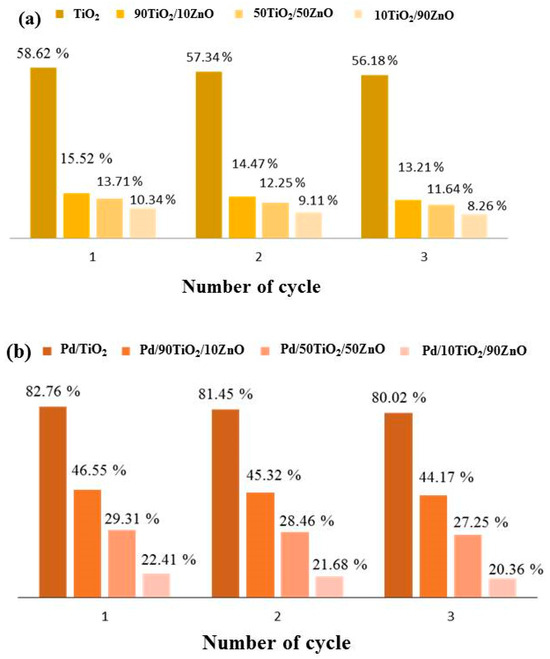
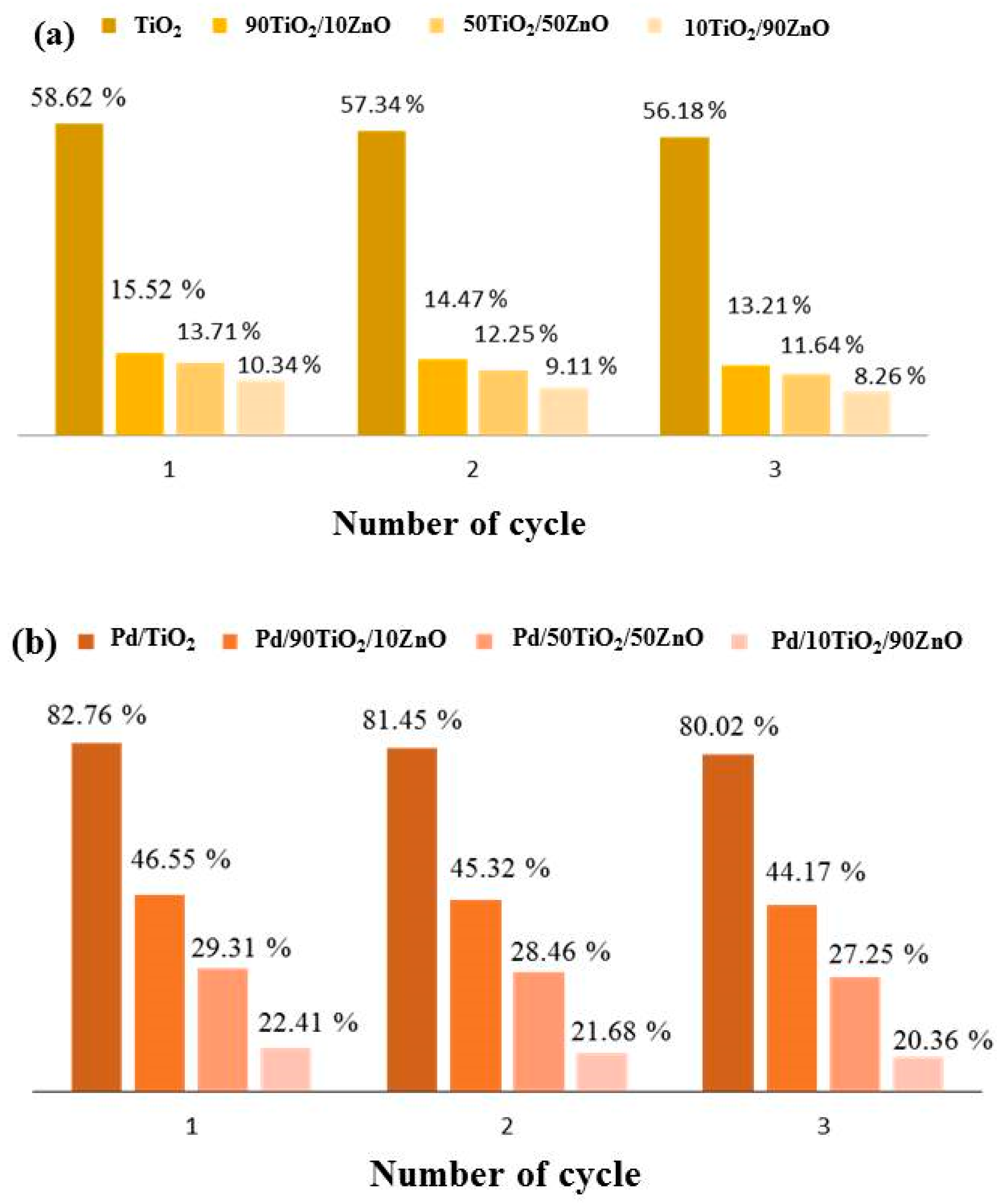
Figure 11.
Decolorization rate of Tatrazine for three successive cycle using (a) pure gels and (b) Pd photo-fixed catalysts.
Figure 11 presents the results of a three-cycle investigation into the recyclability of gels composed of pure and palladium photo-fixed TiO2, 90TiO2/10ZnO, 50TiO2/50ZnO, and 10TiO2/90ZnO. The photocatalytic decomposition of tartrazine decreased by roughly 2% for all catalyst types after three cycles in distilled water, indicating a slight decline in the gels’ catalytic capabilities with each cycle. Despite this decline, it was discovered that the sol-gel gels’ dye decomposition cycle stability was good. These outcomes demonstrate their capacity to be used repeatedly in the breakdown of Tartrazine. Even though the photocatalytic activity somewhat declines with repeated use, TiO2 remains the most stable and efficient catalyst over a number of cycles.
Several factors contribute to the enhanced activity seen in Pd-modified TiO2 and TiO2/ZnO gels. Palladium on the surface of the semiconductor (TiO2 or TiO2/ZnO) probably serves as an electron sink, encouraging interfacial charge transfer between the semiconductor and palladium. This decreases e−/h+ recombination and increases the lifetime of electron-hole pairs. The Schottky barrier formation at the Pd/semiconductor interface amplifies these effects, which are comparable to those seen in other semiconductor photocatalysts modified with noble metals [51,52,53].
The degradation of tartrazine by pure and Pd-modified semiconductors (TiO2 or TiO2/ZnO) is caused by the excitation of an electron (e−) to the conduction band, which results in the creation of a hole (h+) in the valence band when photon energy surpasses the semiconductor bandgap. Following this, reactive species such as hydroxyl radicals and superoxide radicals are produced by the e−/h+ pairs. Organic compounds on or near the photocatalyst surface are broken down by hydroxyl radicals, a strong oxidant.
Equations from (1) through (6) describe the electron–hole reaction in palladium/semiconductor gels that degrade food colorant.
Co-catalytic photofixation of a semiconductor with palladium ions enhances their photocatalytic efficiencies by promoting interfacial charge transfer between the semiconductor (TiO2 or TiO2/ZnO) and palladium and limiting e−/h+ recombination through charge separation.
3. Materials and Methods
3.1. Gels Preparation
Several catalysts with different TiO2 and ZnO content were chosen: pure TiO2, 90TiO2/10ZnO, 50TiO2/50ZnO and 10TiO2/90ZnO (mol%). Ti(IV) n-butoxide Ti(C4H9O)4, (Fluka, Buchs, Switzerland), zinc acetate (Zn(CH3COO)2·2H2O; Merck, Darmstadt, Germany), and citric acid (C6H8O7·H2O; Merck, Darmstadt, Germany) were used as precur-sors, which were then dissolved in C2H6O2. Palladium nitrate (Pd(NO3)2·(H2O)x, 99.8%) was obtained from Thermo Scientific (Waltham, MA, USA). According to literature data [54,55,56,57,58], diols are favorite solvents because they can change metal alkoxides and act as chelating ligands to bridge with other alkoxide groups. Thus, the obtained solutions be-come more stable. The solutions with different precursor concentrations were prepared for sample synthesis using continuous stirring for 5–10 min. Ethylene glycol was used to dissolve each precursor separately in a 1:1 ratio. The zinc acetate solution was then mixed with citric acid and titanium (IV) butoxide at the same time. The prepared solutions’ pH was measured between 4 and 5. After mixing, the gelation process started right away. Although the synthesized gels were uniform, their colors varied because the sample’s color changed from milky white to saturated yellow as the percentage of TiO2 increased. The gelation occurred immediately and aiming to allow full hydrolysis, the gels were aged for about a week at room temperature (23 ± 2 °C). Pure TiO2 gel was obtained from Ti(IV) butoxide and it was used for a comparative analysis to the mixed photocatalysts.
Through photo-deposition, Pd was added to the surface of the as-prepared TiO2 and TiO2/ZnO gels. The sample surface was covered with about 500 μL of an aqueous 5 × 10−3 M Pd2+ solution, which was then allowed to spread into a thin liquid layer. After that, UV light from an LED source (LTPL-C034UVH365, Lite-On Technology Corp., Taiwan, λ = 365 ± 5 nm) was used to illuminate the Pd2+-impregnated catalyst layers.
Using a calibrated research radiometer (Ealing Electro-Optics, Inc., Whetstone, UK), the average illumination intensity of the UV LED source, which was positioned about 35 mm from the TiO2 and TiO2/ZnO surface, was 9.4 ± 0.9 mW/cm2. The estimated illumination time needed to achieve the desired UV dose was based on this pre-established UV irradiance. Before use, the modified photocatalysts were dried at 100 °C for 10 min after being cleaned with distilled water.
3.2. Samples Characterization
The X-ray diffraction method (XRD) was used to identify the crystalline phases of the synthesized materials. A Bruker D8 Advance X-ray diffractometer with Cu Kα (α = 1.5418 Å) was used for the analysis (Billerica, MA, USA). Using the step-scanning mode (Δ2θ = 0.05°), the patterns were acquired over an angular range of 10–80°. The thermal stability of two gels (Ti butoxide and sample A) was determined by differential thermal analysis (LABSYSTM EVO apparatus) with Pt–Pt/Rh thermocouple at a heating rate of 10 K/min in air flow, using Al2O3 as a reference material. The accuracy of the temperature was ±5 °C. The heating of the samples was limited up to 600 °C. Gases evolved (EGA) during the thermal degradations were analyzed by mass spectrometry (MS) with a Pfeiffer OmniStarTM mass spectrometer (Pfeiffer Vacuum Technology AG, Wetzlar, Germany). Mass spectra recorded for the synthesized gels (Figure 3) show m/z of 14, 18 and 44 ascribed to CH2, H2O and CO2, respectively.
The UV-Vis diffused reflectance spectrophotometer “Evolution 300” (Madison, WI, USA) was used to record the optical absorption spectra of the powdered samples in the wavelength range of 200–800 nm, with a magnesium oxide reflectance standard serving as the baseline. The following formula was used to determine the band gap energy of the prepared catalysts:
where α, h, v, Eg, and A stand for the absorption coefficient, Planck’s constant, photon frequency, energy band gap, and a constant, respectively. The Eg values were derived by extrapolating a straight line to the x-axis from plots of (αhv)2 versus hv values obtained from the Kubelka-Munk extrapolation to the gels’ energy band gap.
Using the JSM-5510 scanning electron microscope (SEM) (JEOL, Tokyo, Japan) set to 10 kV of acceleration voltage, the obtained samples were imaged to examine their morphology and microstructure. The JFC-1200 fine coater (JEOL) (Krefeld, Germany) applied a gold coating to the examined samples before observation. The analysis was performed using a Zeiss Evo 15 microscope (Bruker Resolution 126 eV, Berlin, Germany) and energy-dispersive X-ray spectroscopy (EDS, Tokyo, Japan). The specific surface areas (BETs) of the as-prepared were determined by low-temperature (77.4 K) nitrogen adsorption in the NOVA 1200e surface area and pore analyser (Quantachrome, Boynton Beach, FL, USA) at relative pressures p/p0 = 0.1–0.3 using the BET equation.
3.3. Photocatalytic Testing
Tartrazine (C16H9N4Na3O9S2, λmax = 427 nm, ≥85%; ACROS organics (MA, USA)) was used as the model dye pollutant (food coloring, Acid Yellow-23) to assess the photocatalytic decomposition capabilities of the synthesized and examined samples under ultraviolet light illumination. The Evolution 300 UV-VIS spectrophotometer (Thermo Scientific, 50–60 Hz, 150 VA, Madison, WI, USA) with a wavelength range of 300 to 600 nm was used to measure the optical absorption spectra and the pollutant concentration following irradiation. The food coloring’s initial concentration was 7.5 ppm. The 200 mL glass cylindrical reactor used for the photocatalytic test had a UV lamp above it and an electromagnetic stirrer attached. The dark phase lasted for fifteen minutes. Utilizing UV-Vis absorption spectroscopy, aliquot samples (2 mL) were taken at predetermined intervals to examine the pollutant’s degradation during the photocatalytic process. Before spectrophotometer measurement, the aliquot samples of the photo-catalysis with the powders were run through a microfilter to eliminate the suspension. The degree of degradation was determined by
where Ct and Ci are at time t and the initial concentration. The degree of Tartrazine degradation can thus be ascertained. Every photocatalytic test was conducted using distilled water at room temperature (23 ± 2 °C) and a constant stirring speed (200 rpm). Furthermore, no evidence of direct E102 degradation was found through photolysis and adsorption.
A scavenger test was used to look into the reactive species that were causing the tartrazine to degrade. As scavengers, isopropyl alcohol (IPA) and ascorbic acid (AA) were employed to absorb hydroxyl and superoxide radicals, respectively. Six milligrams of each scavenger were used independently in order to pinpoint the precise reactive species that caused the food colorant (50 mL) to degrade due to photocatalysis.
4. Conclusions
Bright and homogeneous TiO2/ZnO gels are obtained using Ti(IV) butoxide as a main precursor and all specimens were characterized using well described methodolo-gies. The as-prepared TiO2/ZnO gels have good thermal stability. According to the UV-Vis results the investigated samples displayed a red shifting of the cut-off in comparison to the pure sol-gel-derived TiO2 gel. The photocatalytic tests under UV light show that TiO2 is more active than binary gels. TiO2’s larger surface area and smaller crystallite size cause food coloring to degrade more quickly. The pure gels showed greater photocatalytic efficiency than the TiO2/ZnO catalysts, most likely because of the samples’ better morphology, greater specific surface area, crystalline size, grain growth, and absence of agglomeration. The enhancement of TiO2 and TiO2/ZnO’s oxidizing power as a result of photogenerated electron-hole pair recombination inhibition and electron transfer from its conduction band to Pd. Tartrazine degradation percentages were highest in the Pd/TiO2 gels (D = 82.76%). Palladium ions’ beneficial effects, superior photostability, and increased photocatalytic efficiency make this system a desirable choice for food dye degradation, helping to develop effective and environmentally friendly wastewater degradation technologies. From the obtained results, it is reasonable to suggest that TiO2/ZnO nonannealed catalysts will continue to be applied in environmental protection as well as other catalytic fields.
Author Contributions
Conceptualization, N.K. and A.B.-N.; methodology, N.K. and A.B.-N.; investigation, N.K. and A.B.-N.; writing—original draft preparation, N.K. and A.B.-N.; writing—N.K. and A.B.-N. All authors have read and agreed to the published version of the manuscript.
Funding
N. Kaneva is grateful to be financially supported by the Bulgarian NSF project KP-06-N59/11 (КП-06-Н59/11).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
N. Kaneva is grateful to be financially supported by the Bulgarian NSF project KP-06-N59/11 (КП-06-Н59/11). The author A. Bachvarova-Nedelcheva is thankful to the support by European Regional Development Fund under “Research Innovation and Digitization for Smart Transformation” program 2021–2027 under the Project BG16RFPR002-1.014-0006 National Centre of Excellence Mechatronics and Clean Technologies. The research equipment used was from the distributed research infrastructure INFRAMAT, which was supported by the Bulgarian Ministry of Education and Science under contract.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gomes, K.M.S.; de Oliveira, M.V.G.A.; de Sousa Carvalho, F.R.; Menezes, C.C.; Peron, A.P. Citotoxicity of food dyes sunset yellow (E-110), bordeaux red (E-123), and tatrazine yellow (E-102) on Allium cepa L. root meristematic cells. Food Sci. Technol. 2013, 33, 218–223. [Google Scholar] [CrossRef]
- Arnold, L.E.; Lofthouse, N.; Hurt, E. Artificial food colors and attention-deficit/hyperactivity symptoms: Conclusions to dye for. Neurotherapeutics 2012, 9, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15. [Google Scholar] [CrossRef]
- Amchova, P.; Kotolová, H.; Ruda-Kucerova, J. Health safety issues of synthetic food colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Navia-Mendoza, J.; Filho, O.; Zambrano-Intriago, L.; Maddela, N.; Duarte, M.; Quiroz-Fernández, L.; Baquerizo-Crespo, R.; Rodríguez-Díaz, J. Advances in the Application of Nanocatalysts in Photocatalytic Processes for the Treatment of Food Dyes: A Review. Sustainability 2021, 13, 11676. [Google Scholar] [CrossRef]
- Khan, K.; Shah, A.; Nisar, J.; Haleem, A.; Shah, I. Photocatalytic Degradation of Food and Juices Dyes via Photocatalytic Nanomaterials Synthesized through Green Synthetic Route: A Systematic Review. Molecules 2023, 28, 4600. [Google Scholar] [CrossRef]
- König, J. 2—Food colour additives of synthetic origin. In Colour Additives for Foods and Beverages, Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2015; pp. 35–60. [Google Scholar]
- Leek, T. Food additives and reactions: Antioxidants, benzoates, parabens, colorings, flavorings, natural protein-based additives. Encycl. Food Allergy 2024, 862–881. [Google Scholar] [CrossRef]
- Lockey, S. Allergic reactions due to F D and C Yellow No. 5, tartrazine, an aniline dye used as a coloring and identifying agent in various steroids. Ann. Allergy 1959, 17, 719–721. [Google Scholar]
- Stevenson, D.; Simon, R.; Lumry, W. Adverse reactions to tartrazine. J. Allergy Clin. Immunol. 1986, 78, 182–191. [Google Scholar] [CrossRef]
- Choulis, N. Chapter 49—Miscellaneous drugs, materials, medical devices, and techniques. Side Eff. Drugs Annu. 2011, 33, 1009–1029. [Google Scholar]
- Ramesh, M.; Muthuraman, A. Chapter 1—Flavoring and Coloring Agents: Health Risks and Potential Problems. In Natural and Artificial Flavoring Agents and Food Dyes; Handbook of Food Bioengineering; Academic Press: Cambridge, MA, USA, 2018; pp. 1–28. [Google Scholar]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Zare, K.; Gupta, V.K.; Moradi, O.; Makhlouf, A.S.H.; Sillanpää, M.; Nadagouda, M.N.; Sadegh, H.; Shahryari-Ghoshekandi, R.; Pal, A.; Wang, Z. A comparative study on the basis of adsorption capacity between CNTs and activated carbon as adsorbents for removal of noxious synthetic dyes: A review. J. Nanostruct. Chem. 2015, 5, 227–236. [Google Scholar] [CrossRef]
- Vuono, D.; Catizzone, E.; Aloise, A.; Policicchio, A.; Agostino, R.G.; Migliori, M.; Giordano, G. Modelling of adsorption of textile dyes over multi-walled carbon nanotubes: Equilibrium and kinetic. Chin. J. Chem. Eng. 2017, 25, 523–532. [Google Scholar] [CrossRef]
- Prashant, D.; Sarvalkar, P.; Kamble, S.; Powar, P.; Kakade, S.; Jamadar, A.; Thounaojam, P.; Patil, M.; Kalake, S.; Nimbalkar, M.; et al. Synthesized rGO/f-MWCNT-architectured 1-D ZnO nanocomposites for azo dyes adsorption, photocatalytic degradation, and biological applications. Catal. Commun. 2024, 187, 106846. [Google Scholar]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Samarasinghe, L.; Muthukumaran, S.; Baskaran, K. Recent advances in visible light-activated photocatalysts for degradation of dyes: A comprehensive review. Chemosphere 2024, 349, 140818. [Google Scholar] [CrossRef]
- Shen, J.; Chuang, H.; Horng, J. Enhanced photocatalytic mechanism of hydroxyl radical formation in the composite reaction of TiO2/oxidant for azo dye degradation. Desalin. Water Treat. 2017, 96, 153–160. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.; Khataee, A. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Kansal, S.; Singh, M.; Sud, D. Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts. J. Hazard. Mater. 2007, 141, 581–590. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite Titanium Dioxide Nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef] [PubMed]
- Carp, O.; Huisman, C.; Reller, A. Photoinduced Reactivity of Titanium Dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Bachvarova-Nedelcheva, A.; Gegova, R.; Stoyanova, A.; Iordanova, R.; Copcia, V.; Ivanova, N.; Sandu, I. Synthesis, characterization and properties of ZnO/TiO2 powders obtained by combustion gel method. Bulg. Chem.Commun. 2014, 46, 585–593. [Google Scholar]
- Shalaby, A.; Bachvarova-Nedelcheva, A.; Iordanova, R.; Dimitriev, Y.; Stoyanova, A.; Hitkova, H.; Ivanova, N.; Sredkova, M. Sol-gel synthesis and properties of nanocomposites in the Ag/TiO2/ZnO system. J. Optoelectron. Adv. Mater. 2015, 17, 248–256. [Google Scholar]
- Najjar, R.; Shokri, M.; Farsadi, S. Preparation of Pd-doped nano-TiO2 in microemulsion and their application in photodegradation of C.I. Acid Yellow 23. Desalination Water Treat. 2015, 54, 2581–2591. [Google Scholar] [CrossRef]
- Banerjee, A.N.; Nazanin, H.; Joo, S.W. A comparative study of the effect of Pd-doping on the structural, optical, and photocatalytic properties of sol-gel derived anatase TiO2 nanoparticles. Ceram. Int. 2016, 42, 12010–12023. [Google Scholar] [CrossRef]
- Sakthivel, S.; Shankar, M.V.; Palanichamy, M.; Arabindoo, B.; Bahnemann, D.W.; Murugesan, V. Enhancement of photocatalytic activity by metal deposition: Characterisation and photonic efficiency of Pt, Au and Pd deposited on TiO2 catalyst. Water Res. 2004, 38, 3001–3008. [Google Scholar] [CrossRef]
- Babu, N.S.; Lingaiah, N.; Pasha, N.; Kumar, J.V.; Prasad, P.S.S. Influence of particle size and nature of Pd species on the hydrodechlorination of chloroaromatics: Studies on Pd/TiO2 catalysts in chlorobenzene conversion. Catal. Today 2009, 141, 120–124. [Google Scholar] [CrossRef]
- Chan, C.-C.; Chang, C.-C.; Hsu, W.-C.; Wang, S.; Lin, J. Photocatalytic activities of Pd-loaded mesoporous TiO2 thin films. Chem. Eng. J. 2009, 152, 492–497. [Google Scholar] [CrossRef]
- Abdelaal, M.Y.; Mohamed, R.M. Novel Pd/TiO2 nanocomposite prepared by modified sol–gel method for photocatalytic degradation of methylene blue dye under visible light irradiation. J. Alloys Compd. 2013, 576, 201–207. [Google Scholar] [CrossRef]
- Espino-Estévez, M.R.; Fernández-Rodríguez, C.; González-Díaz, O.M.; Araña, J.; Espinós, J.P.; Ortega-Méndez, J.A.; Doña-Rodríguez, J.M. Effect of TiO2–Pd and TiO2–Ag on the photocatalytic oxidation of diclofenac, isoproturon and phenol. Chem. Eng. J. 2016, 298, 82–95. [Google Scholar] [CrossRef]
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Kaneva, N. The Solvent Role for the Decomposition of Paracetamol in Distilled and Drinking Water by Pure and Ag-Modified TiO2 Sol–Gel Powders. Materials 2024, 17, 1791. [Google Scholar] [CrossRef]
- Kaneva, N.; Bachvarova-Nedelcheva, A. The Effect of Heat Treatment on the Sol–Gel Preparation of TiO2/ZnO Catalysts and Their Testing in the Photodegradation of Tartrazine. Appl. Sci. 2024, 14, 9872. [Google Scholar] [CrossRef]
- Kwak, B.S.; Chae, J.; Kim, J.; Kang, M. Enhanced Hydrogen Production from Methanol/Water Photo-Splitting in TiO2 Including Pd Component. Bull. Korean Chem. Soc. 2009, 30, 1047. [Google Scholar]
- Yan, J.; Li, X.; Jin, B.; Zeng, M.; Peng, R. Synthesis of TiO2/Pd and TiO2/PdO Hollow Spheres and Their Visible Light Photocatalytic Activity. Int. J. Photoenergy 2020, 2020, 4539472. [Google Scholar] [CrossRef]
- Yordanov, S.I.; Bachvarova-Nedelcheva, A.D.; Iordanova, R.S.; Stambolova, I.D. Sol-gel synthesis and properties of Sm modified TiO2 nanopowders. Bulg. Chem. Commun. 2018, 50, 42–48. [Google Scholar]
- Wang, L.; Fu, X.; Han, Y.; Chang, E.; Wu, H.; Wang, H.; Li, K.; Qi, X. Preparation, Characterization, and Photocatalytic Activity of TiO2/ZnO Nanocomposites. J. Nanomater. 2013, 2013, 321459. [Google Scholar] [CrossRef]
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Kostov, K.L.; Gegova, R. Sol-gel powder synthesis in the TiO2-TeO2-ZnO system: Structural characterization and properties. Arab. J. Chem. 2020, 13, 7132–7146. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Kubiaka, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO Binary Oxide Systems: Comprehensive Characterization and Tests of Photocatalytic Activity. Materials 2018, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Irine, T.M.; Rathika, A. Improved Photocatalytic Activity of Pd-doped ZnO Nanoparticles Synthesized by Combustion Method. Int. J. Appl. Environ. Sci. 2022, 17, 43–60. [Google Scholar]
- Oliveira, A.F.; Silva, S.A.M.; Rubinger, C.P.; Ider, J.; Rubinger, R.M.; Oliveira, E.T.M.; Doriguetto, A.C.; de Carvalho, H.B. Preparation and characterization of palladium-doped titanium dioxide for solar cell applications. Mater. Sci. Eng. B 2022, 280, 115702. [Google Scholar] [CrossRef]
- Bai, N.; Liu, X.; Li, Z.; Ke, X.; Zhang, K.; Wu, Q. High-efficiency TiO2/ZnO nanocomposites photocatalysts by sol–gel and hydrothermal methods. J. Sol-Gel Sci. Technol. 2021, 99, 92–100. [Google Scholar] [CrossRef]
- Aramendía, M.A.; Borau, V.; Colmenares, J.; Marinas, A.; Marinas, J.; Navío, J.; Urbano, F. Modification of the photocatalytic activity of Pd/TiO2 and Zn/TiO2 systems through different oxidative and reductive calcination treatments. Appl. Catal. Environ. 2008, 80, 88–97. [Google Scholar] [CrossRef]
- Güy, N.; Çakar, S.; Özacar, M. Comparison of palladium/zinc oxide photocatalysts prepared by different palladium doping methods for congo red degradation. J. Colloid Int. Sci. 2016, 466, 128–137. [Google Scholar] [CrossRef]
- Turktena, N.; Bekbolet, M. Photocatalytic performance of titanium dioxide and zinc oxide binary system on degradation of humic matter. J. Photochem. Photobiol. A 2020, 401, 112748. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A New Cd(II)-Based Coordination Polymer for Efficient Photocatalytic Removal of Organic Dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, C.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K.; Zhu, J. Photocatalytic Activity of Ag/ZnO Heterostructure Nanocatalyst: Correlation between Structure and Property. J. Phys. Chem. C 2008, 112, 10773–10777. [Google Scholar] [CrossRef]
- Bera, A.; Basak, D. Pd-nanoparticle-decorated ZnO nanowires: Ultraviolet photosensitivity and photoluminescence proper-ties. Nanotechnology 2011, 22, 265501. [Google Scholar] [CrossRef]
- Li, S.; Cai, J.; Wu, X.; Zheng, F. Sandwich-like TiO2@ZnO-based noble metal (Ag, Au, Pt, or Pd) for better photo-oxidation performance: Synergistic effect between noble metal and metal oxide phases. Appl. Surf. Sci. 2018, 443, 603–612. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yoshikawa, S. Synthesis and thermal analyses of TiO2-derived nanotubes prepared by the hydrothermal method. J. Mater. Res. 2004, 19, 982–985. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Zdarta, J.; Paukszta, D.; Jesionowski, T. The influence of addition of a catalyst and chelating agent on the properties of titanium dioxide synthesized via the sol-gel method. J. Sol-Gel Sci. Technol. 2015, 75, 264–278. [Google Scholar] [CrossRef]
- Lawrence, M.; Dejene, F. Influence of annealing temperature on structural, optical and photocatalytic properties of ZnO–TiO2 composites for application in dye removal in water. Nano-Struct. Nano-Objects 2020, 24, 100594. [Google Scholar]
- Umar, A.; Kumar, R.; Kumar, G.; Algarni, H.; Kim, S. Effect of annealing temperature on the properties and photocatalytic efficiencies of ZnO nanoparticles. J. Alloys Compd. 2015, 648, 46–52. [Google Scholar] [CrossRef]
- Livage, C.; Safari, A.; Klein, L. Glycol-based sol-gel process for the fabrication of ferroelectric PZT thin films: Code: EP28. J. Sol-Gel Sci. Technol. 1994, 2, 605. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).