Mitigating Lead Toxicity in Halide Perovskite Solar Cells: Strategies for Sustainable Development
Abstract
1. Introduction
2. Environmental Risks of Halide Perovskite Materials
2.1. Lead Toxicity of Halide Perovskites
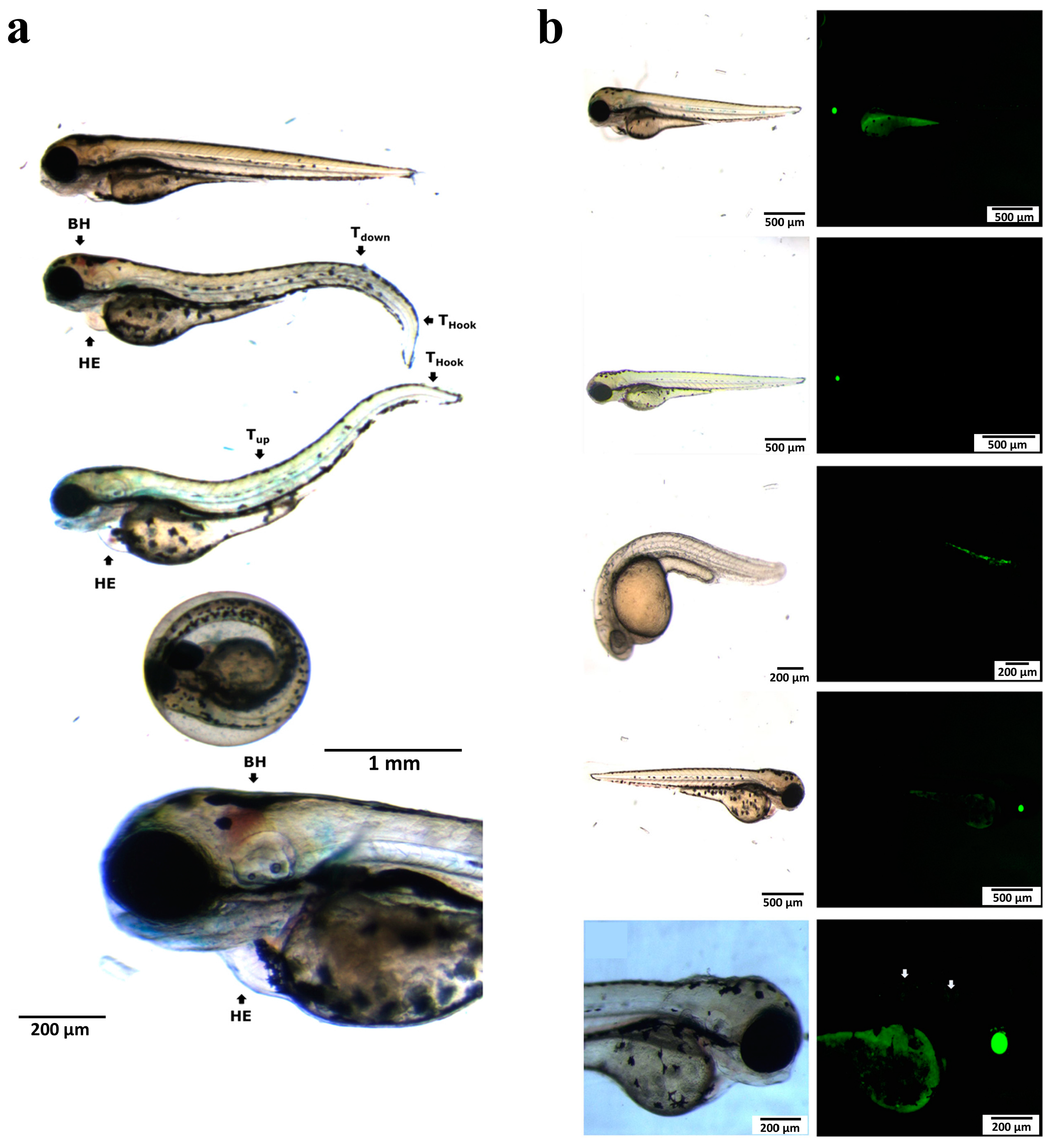
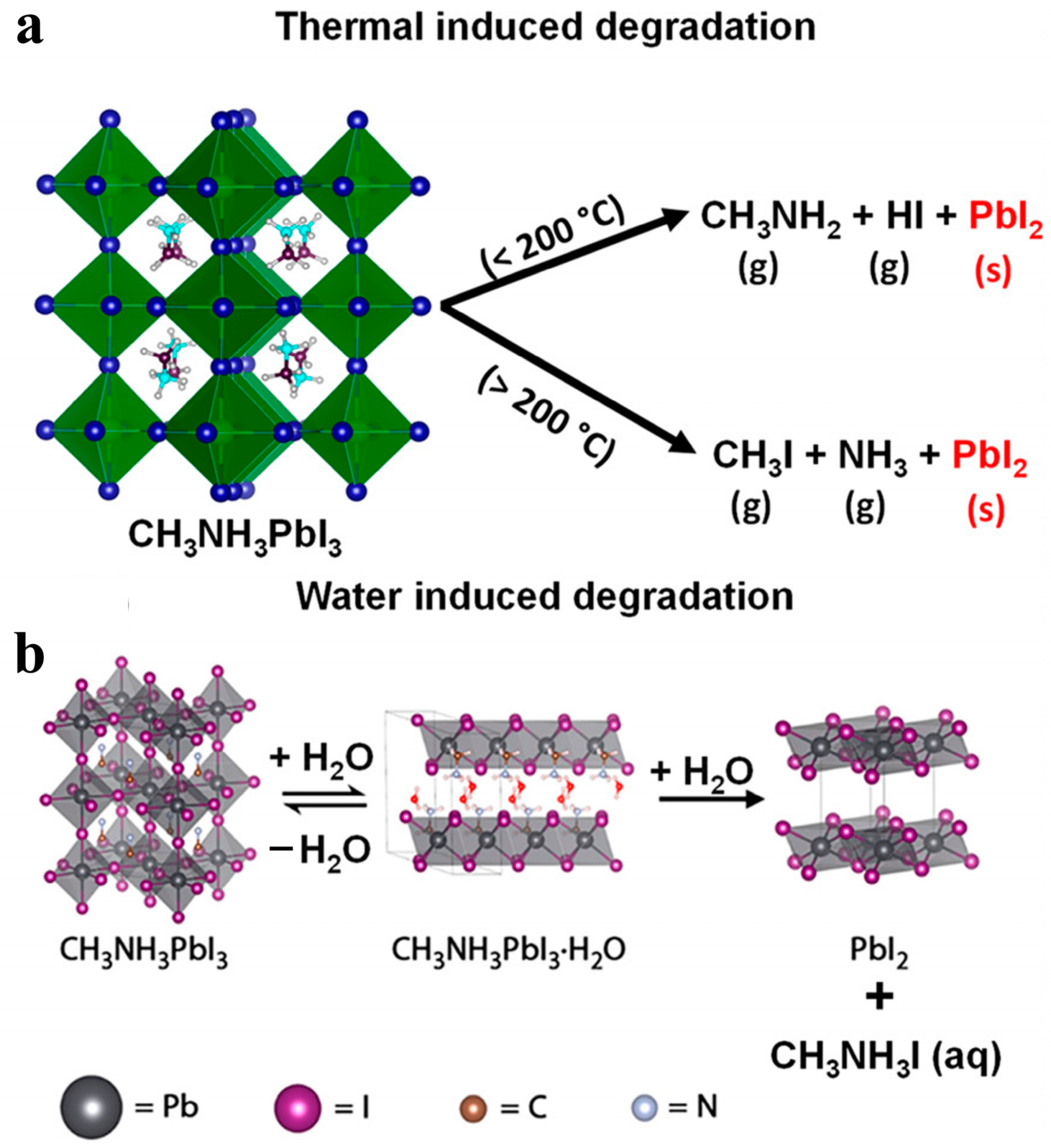
2.2. Mechanism and Effect of Lead Leakage
3. Strategies of Lead Leakage Suppression for Perovskite Solar Cells
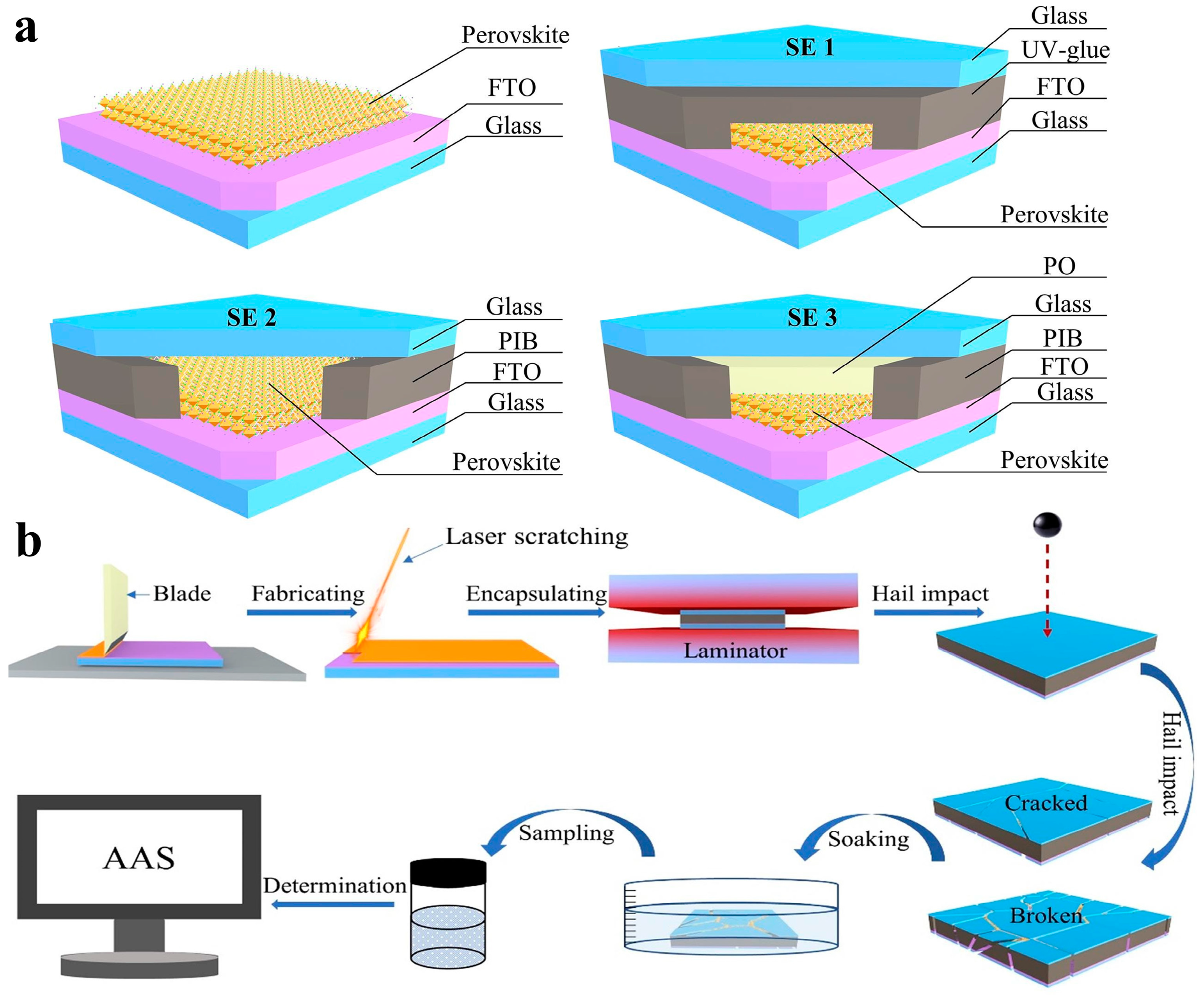
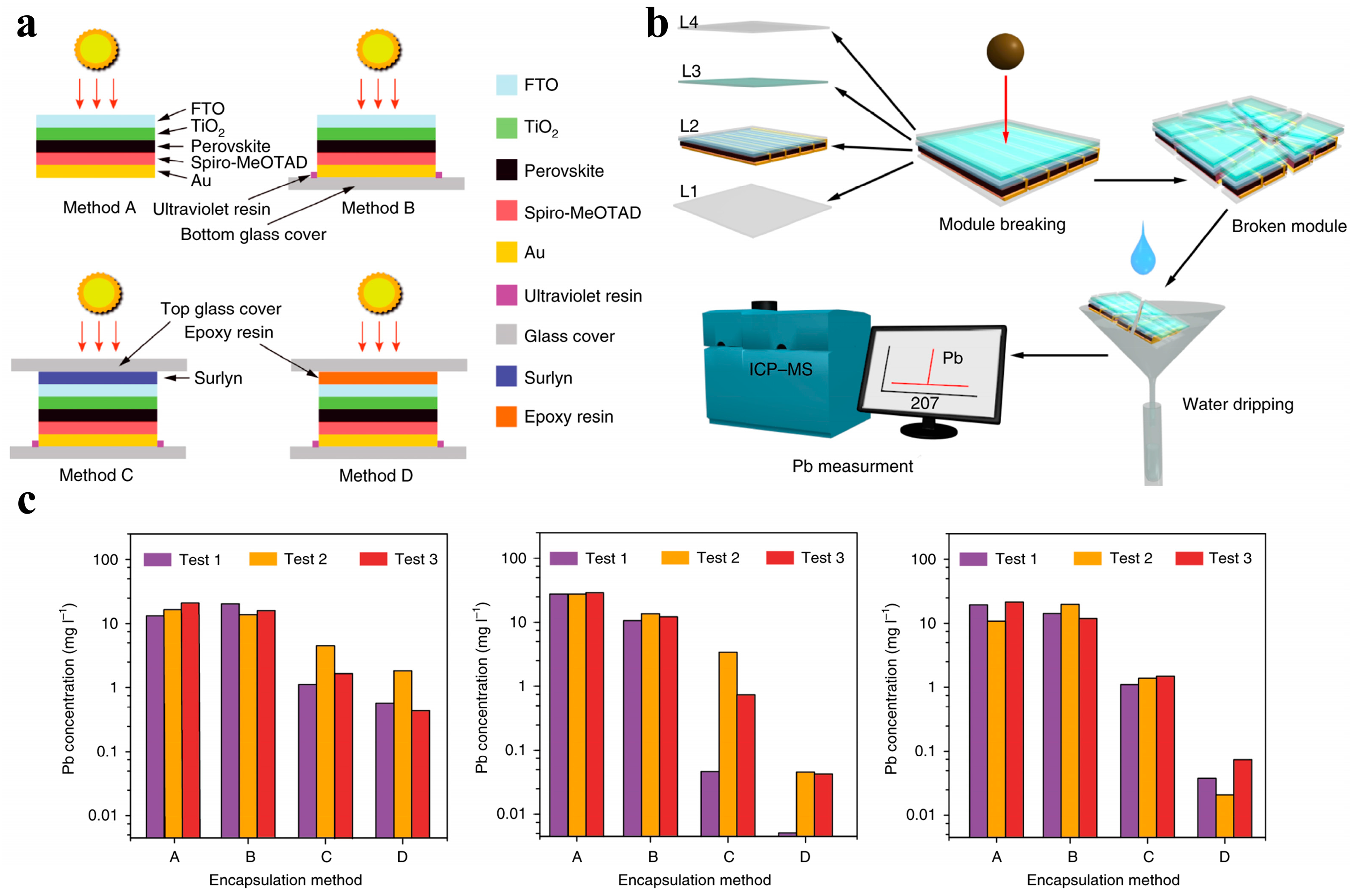
3.1. External Encapsulation
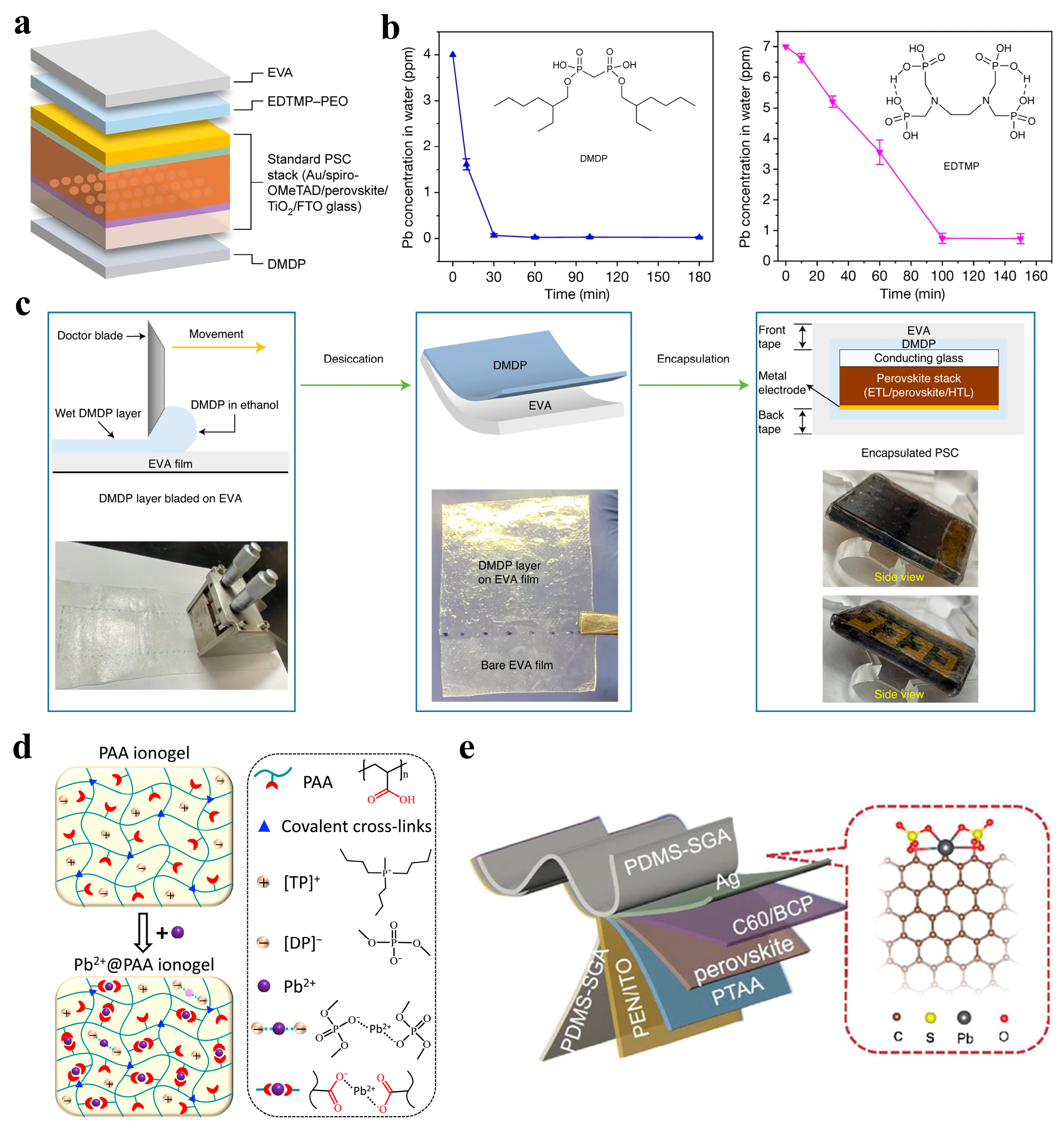
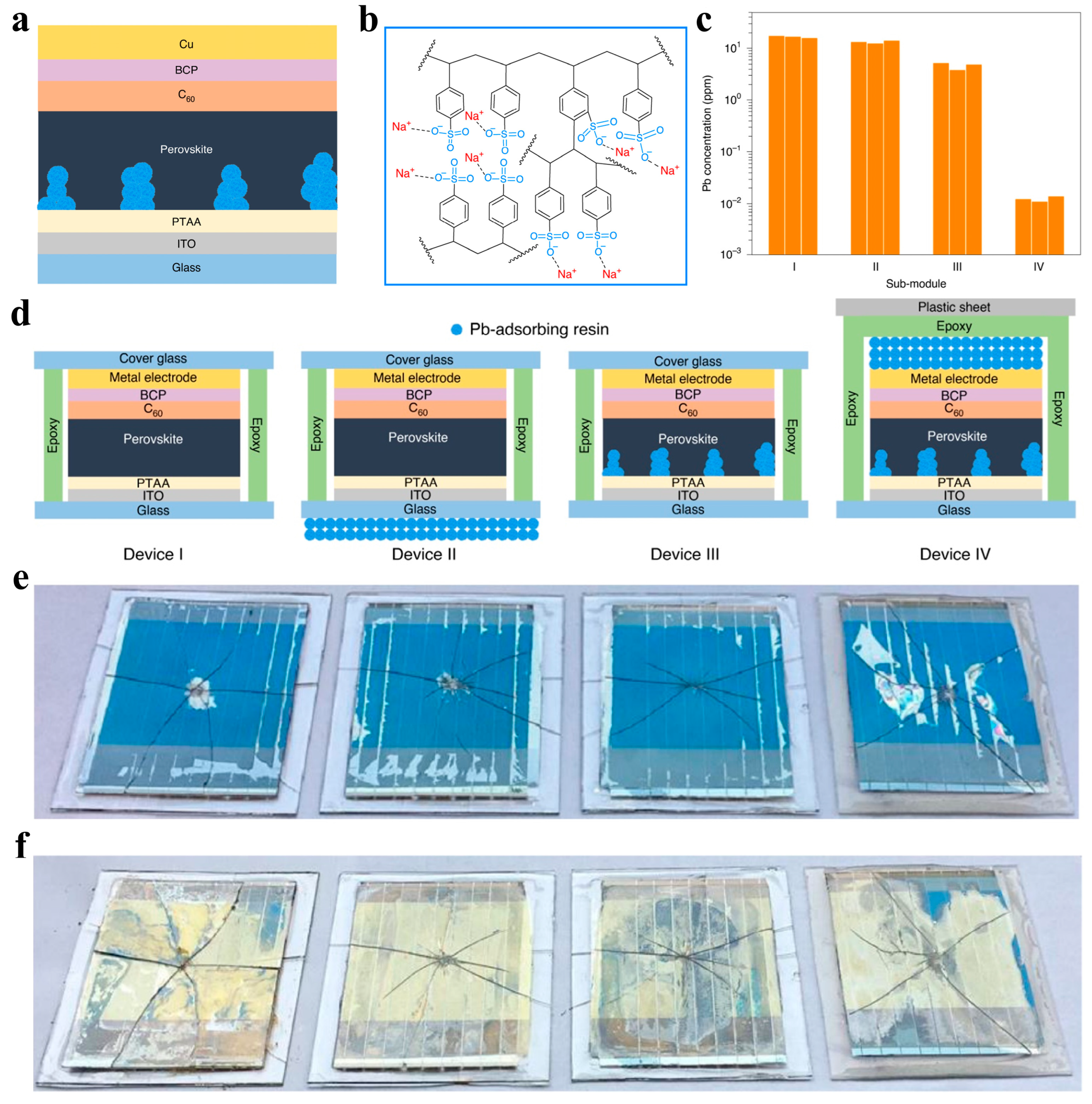
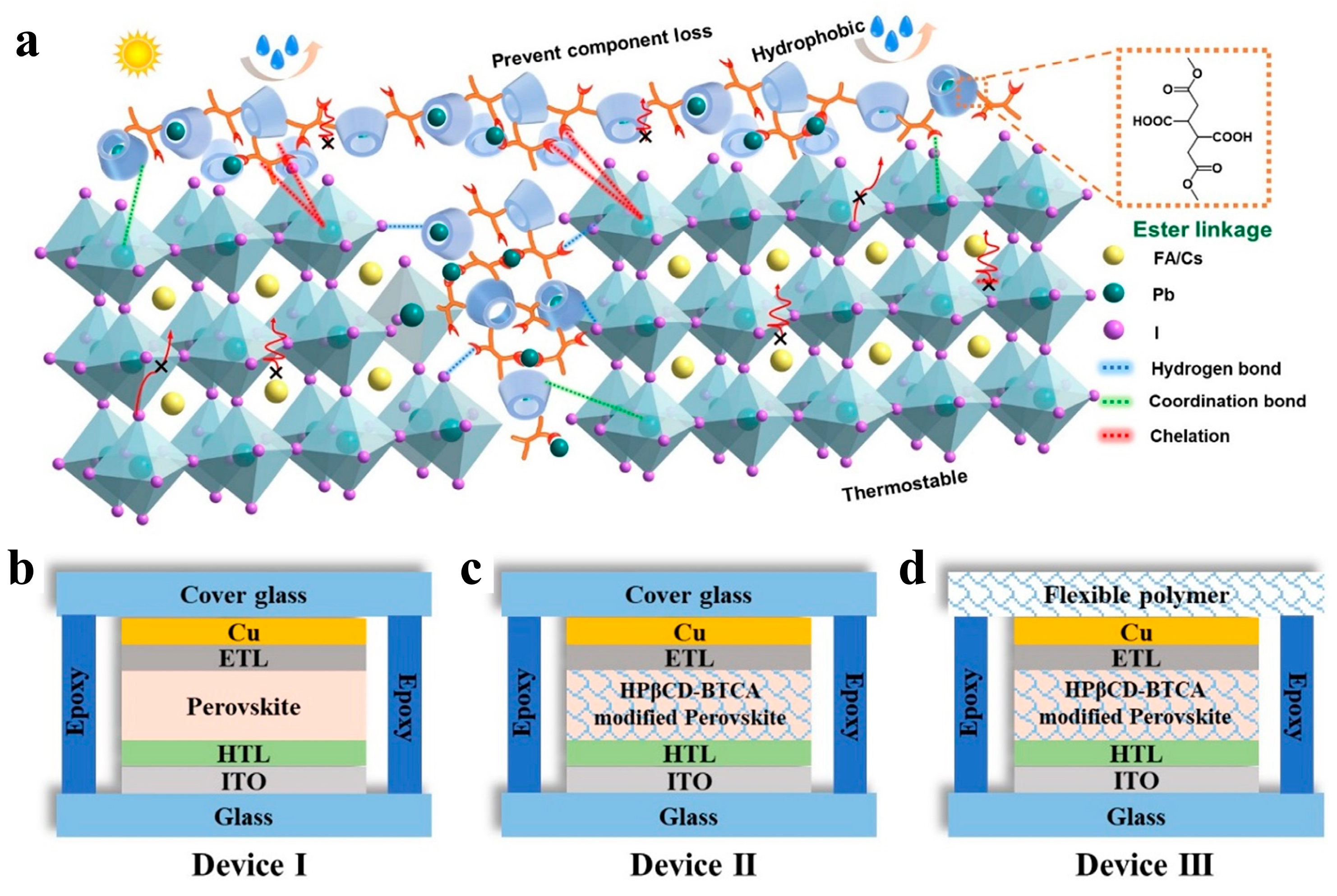
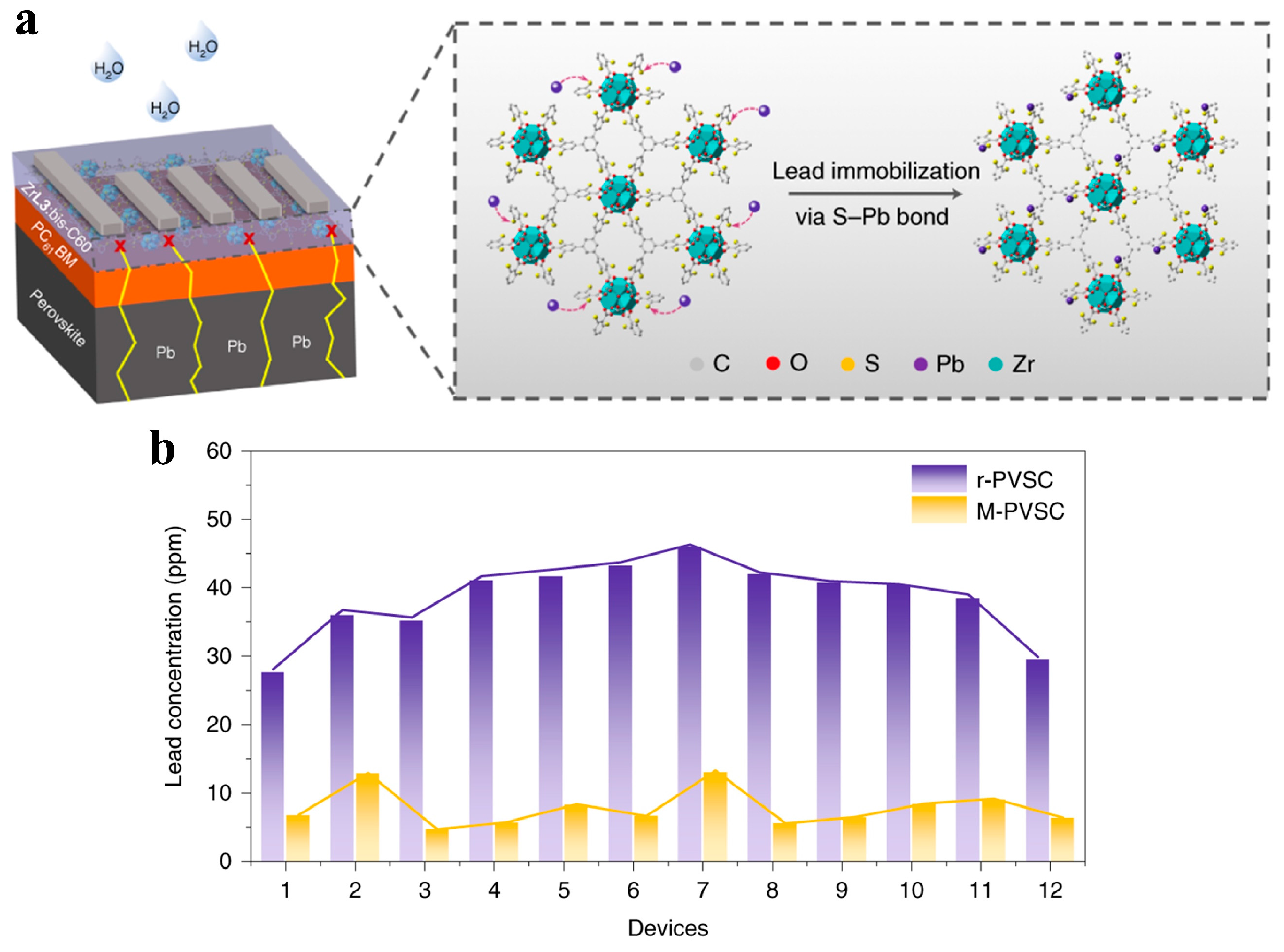
3.2. Lead Immobilization Within PSCs
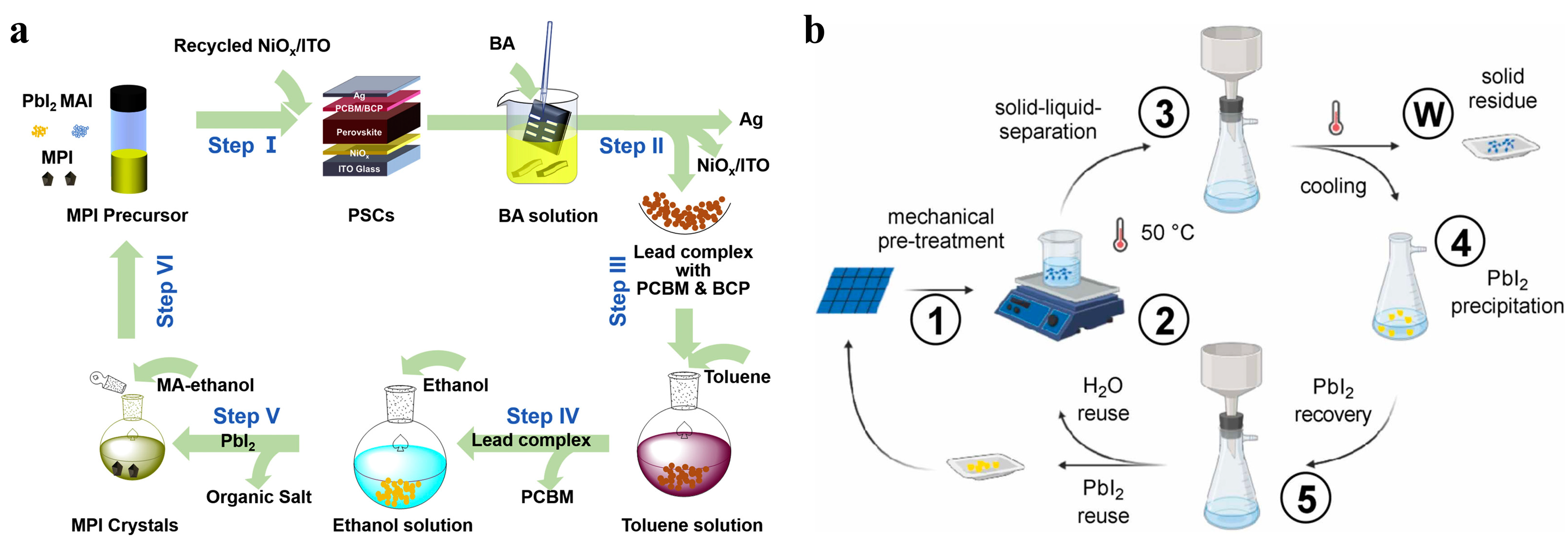
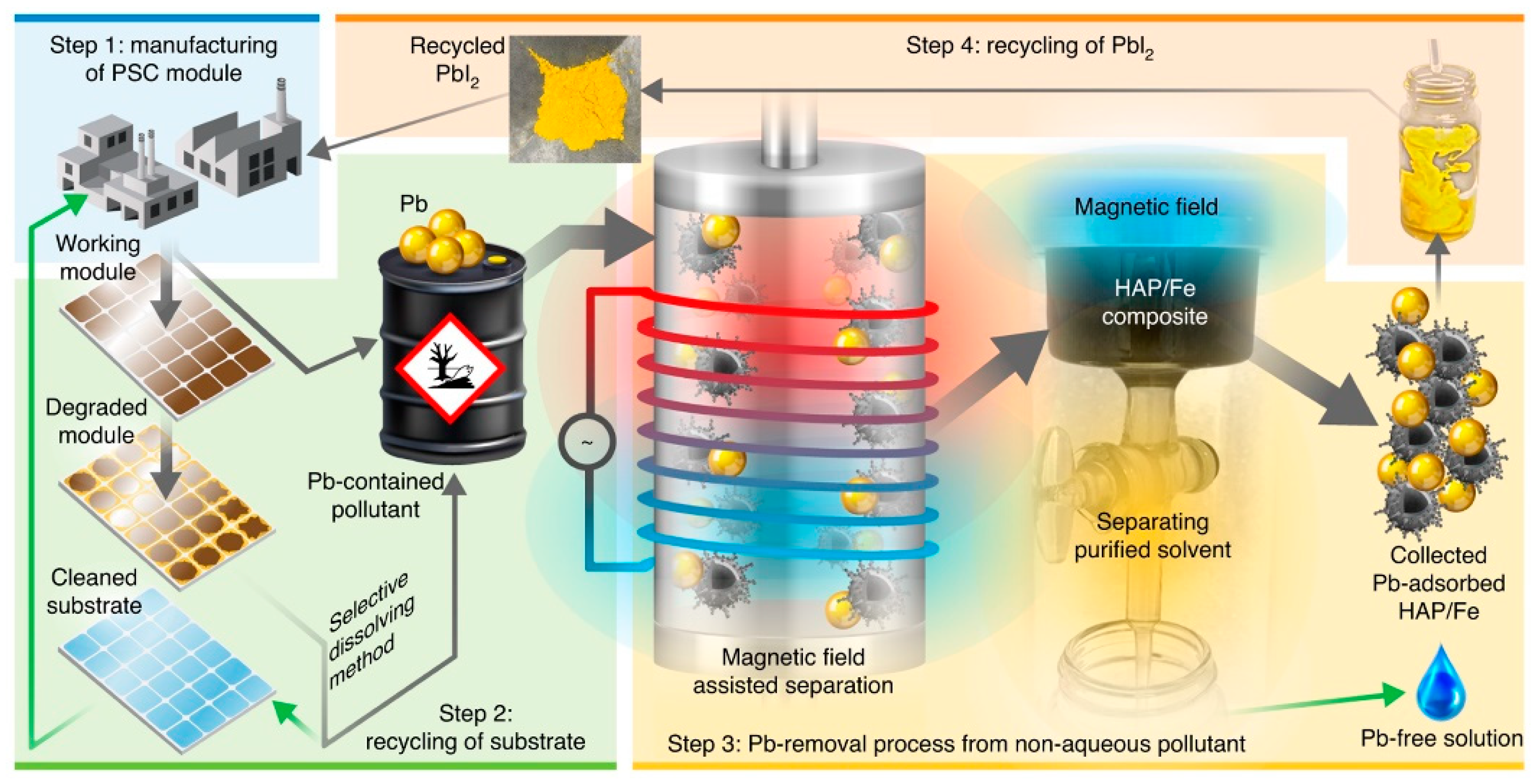
4. Sustainable Lead Recovery for Perovskite Solar Cells
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Yuan, Y.; Shao, Y.; Yan, Y. Understanding the physical properties of hybrid perovskites for photovoltaic applications. Nat. Rev. Mater. 2017, 2, 17042. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Xu, J.; Maxwell, A.; Zhou, W.; Yang, Y.; Zhou, Q.; Bati, A.S.R.; Wan, H.; Wang, Z.; et al. Improved charge extraction in inverted perovskite solar cells with dual-site-binding ligands. Science 2024, 384, 189–193. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Saliba, M.; Buonassisi, T.; Grätzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and challenges of perovskite solar cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef]
- Jiang, Q.; Tong, J.; Scheidt, R.A.; Wang, X.; Louks, A.E.; Xian, Y.; Tirawat, R.; Palmstrom, A.F.; Hautzinger, M.P.; Harvey, S.P.; et al. Compositional texture engineering for highly stable wide-bandgap perovskite solar cells. Science 2022, 378, 1295–1300. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Cao, Y.B.; Zhang, D.; Zhang, Q.; Qiu, X.; Zhou, Y.; Poddar, S.; Fu, Y.; Zhu, Y.; Liao, J.-F.; Shu, L.; et al. High-efficiency, flexible and large-area red/green/blue all-inorganic metal halide perovskite quantum wires-based light-emitting diodes. Nat. Commun. 2023, 14, 4611. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-K.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal halide perovskites for light-emitting diodes. Nat. Mater. 2021, 20, 10–21. [Google Scholar] [CrossRef]
- Tang, Y.; Jin, P.; Wang, Y.; Li, D.; Chen, Y.; Ran, P.; Fan, W.; Liang, K.; Ren, H.; Xu, X.; et al. Enabling low-drift flexible perovskite photodetectors by electrical modulation for wearable health monitoring and weak light imaging. Nat. Commun. 2023, 14, 4961. [Google Scholar] [CrossRef]
- Li, W.G.; Wang, X.D.; Huang, Y.H.; Kuang, D.B. Ultrasound-assisted crystallization enables large-area perovskite quasi-monocrystalline film for high-sensitive X-ray etection and imaging. Adv. Mater. 2023, 35, 2210878. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yuan, W.; Qian, L.; Chen, S.; Shi, G. High-performance gas sensors based on a thiocyanate ion-doped organometal halide perovskite. Phys. Chem. Chem. Phys. 2017, 19, 12876–12881. [Google Scholar] [CrossRef] [PubMed]
- Senanayak, S.P.; Yang, B.; Thomas, T.H.; Giesbrecht, N.; Huang, W.; Gann, E.; Nair, B.; Goedel, K.; Guha, S.; Moya, X.; et al. Understanding charge transport in lead iodide perovskite thin-film field-effect transistors. Sci. Adv. 2017, 3, e1601935. [Google Scholar] [CrossRef]
- Liu, A.; Zhu, H.; Bai, S.; Reo, Y.; Caironi, M.; Petrozza, A.; Dou, L.; Noh, Y.-Y. High-performance metal halide perovskite transistors. Nat. Electron. 2023, 6, 559–571. [Google Scholar] [CrossRef]
- Deschler, F.; Price, M.; Pathak, S.; Klintberg, L.E.; Jarausch, D.-D.; Higler, R.; Hüttner, S.; Leijtens, T.; Stranks, S.D.; Snaith, H.J.; et al. High photoluminescence efficiency and optically umped Lalsing in solution-rocessed mixed halide perovskite semiconductors. J. Phys. Chem. Lett. 2014, 5, 1421–1426. [Google Scholar] [CrossRef]
- Wu, W.Q.; Rudd, P.N.; Wang, Q.; Yang, Z.; Huang, J. Blading phase-ure formamidinium-alloyed perovskites for high-efficiency solar cells with low photovoltage deficit and improved stability. Adv. Mater. 2020, 32, 2000995. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, L.; Zhu, T.; Chen, H.; Chen, B.; Kubicki, D.J.; Balvanz, A.; Li, C.; Maxwell, A.; Ugur, E.; et al. Suppressed phase segregation for triple-junction perovskite solar cells. Nature 2023, 618, 74–79. [Google Scholar] [CrossRef]
- Cheng, R.; Liang, Z.B.; Zhu, L.; Li, H.; Zhang, Y.; Wang, C.F.; Chen, S. Fibrous nanoreactors frommicrofluidic blow spinning for mass production of highly stable ligand-free perovskite quantum dots. Angew. Chem. Int. Ed. 2022, 61, e202204371. [Google Scholar] [CrossRef]
- Jeong, M.; Choi, I.W.; Go, E.M.; Cho, Y.; Kim, M.; Lee, B.; Jeong, S.; Jo, Y.; Choi, H.W.; Lee, J.; et al. Stable perovskite solar cells with efficiency exceeding 24.8% and 0.3 V voltage loss. Science 2020, 369, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef]
- Wu, T.; Liu, X.; Luo, X.; Lin, X.; Cui, D.; Wang, Y.; Segawa, H.; Zhang, Y.; Han, L. Lead-free tin perovskite solar cells. Joule 2021, 5, 863–886. [Google Scholar] [CrossRef]
- Wang, M.; Wang, W.; Ma, B.; Shen, W.; Liu, L.; Cao, K.; Chen, S.; Huang, W. Lead-free perovskite materials for solar cells. Nano-Micro Lett. 2021, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High-efficiency perovskite solar cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef]
- Awais, M.; Kirsch, R.L.; Yeddu, V.; Saidaminov, M.I. Tin halide perovskites going forward: Frost diagrams offer hints. ACS Mater. Lett. 2021, 3, 299–307. [Google Scholar] [CrossRef]
- Babayigit, A.; Duy Thanh, D.; Ethirajan, A.; Manca, J.; Muller, M.; Boyen, H.-G.; Conings, B. Assessing the toxicity of Pb- and Sn-based perovskite solar cells in model organism Danio rerio. Sci. Rep. 2016, 6, 18721. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, T.; Ding, H.; Yan, C.; Leong, W.L.; Baikie, T.; Zhang, Z.; Sherburne, M.; Li, S.; Asta, M.; Mathews, N.; et al. Lead-free germanium iodide perovskite materials for photovoltaic applications. J. Mater. Chem. A 2015, 3, 23829–23832. [Google Scholar] [CrossRef]
- Li, W.G.; Wang, X.D.; Liao, J.F.; Jiang, Y.; Kuang, D.B. Enhanced on-off ratio photodetectors based on lead-free Cs3Bi2I9 single crystal thin films. Adv. Funct. Mater. 2020, 30, 1909701. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, D.; Wang, X.; Jiang, X.; Liu, B.; Li, B.; Li, Z.; Gao, D.; Zhang, C.; Wang, Y.; et al. Eco-friendly perovskite solar cells: From materials design to device processing and recycling. EcoMat 2023, 5, e12352. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Q.; Lu, Y.; Lu, F.; Mu, X.; Wei, S.-H.; Sui, M. Hydrogenated Cs2AgBiBr6 for significantly improved efficiency of lead-free inorganic double perovskite solar cell. Nat. Commun. 2022, 13, 3397. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, Z.; Xiu, J.; Song, H.; Gatti, T.; He, Z. A critical review on bismuth and antimony halide based perovskites and their derivatives for photovoltaic applications: Recent advances and challenges. J. Mater. Chem. A 2020, 8, 16166–16188. [Google Scholar] [CrossRef]
- Ning, W.; Gao, F. Structural and functional diversity in llead-free halide perovskite materials. Adv. Mater. 2019, 31, 1900326. [Google Scholar] [CrossRef]
- Ke, W.; Kanatzidis, M.G. Prospects for low-toxicity lead-free perovskite solar cells. Nat. Commun. 2019, 10, 965. [Google Scholar] [CrossRef] [PubMed]
- Giustino, F.; Snaith, H.J. Toward lead-ree perovskite solar cells. ACS Energy Lett. 2016, 1, 1233–1240. [Google Scholar] [CrossRef]
- Ravi, V.K.; Mondal, B.; Nawale, V.V.; Nag, A. Don’t let the lead out: New aterial Chemicstry approaches for sustainable lead halide perovskite solar cells. ACS Omega 2020, 5, 29631–29641. [Google Scholar] [CrossRef]
- Babayigit, A.; Ethirajan, A.; Muller, M.; Conings, B. Toxicity of organometal halide perovskite solar cells. Nat. Mater. 2016, 15, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huo, X.; Chen, G.; Luo, X.; Xu, X. Lead (Pb) exposure and heart failure risk. Environ. Sci. Pollut. Res. 2021, 28, 28833–28847. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Boffetta, P.; Apostoli, P. The reproductive toxicity and carcinogenicity of lead: A critical review. Am. J. Ind. Med. 2000, 38, 231–243. [Google Scholar] [CrossRef]
- Wang, Z.-K.; Zhou, X.-L.; Song, X.-B.; Zhuang, D.-M.; Wang, Z.-Y.; Yang, D.-B.; Wang, L. Alleviation of lead-induced apoptosis by puerarin via inhibiting mitochondrial permeability ransitionpore opening in primary cultures of rat proximal tubular cells. Biol. Trace Elem. Res. 2016, 174, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, H.-L.; Jiao, W.-B.; Wang, Q.; Wei, M.; Cantone, I.; Lü, J.; Abate, A. Biological impact of lead from halide perovskites reveals the risk of introducing a safe threshold. Nat. Commun. 2020, 11, 310. [Google Scholar] [CrossRef]
- Luo, T.; Shen, M.; Zhou, J.; Wang, X.; Xia, J.; Fu, Z.; Jin, Y. Chronic exposure to low doses of Pb induces hepatotoxicity at the physiological, biochemical, and transcriptomic levels of mice. Environ. Toxicol. 2019, 34, 521–529. [Google Scholar] [CrossRef]
- Czubowicz, K.; Jęśko, H.; Wencel, P.; Lukiw, W.J.; Strosznajder, R.P. The role of ceramide and sphingosine-1-phosphate in alzheimer’s disease and other neurodegenerative disorders. Mol. Neurobiol. 2019, 56, 5436–5455. [Google Scholar] [CrossRef]
- Mabrouk, A. Thymoquinone attenuates lead-induced nephropathy in rats. J. Biochem. Mol. Toxicol. 2019, 33, e22238. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zeng, Z.; Wang, Q.; Liang, W.; Guo, Y.; Huo, X. Alterations of the gut microbiota and metabolomics in children with e-waste lead exposure. J. Hazard. Mater. 2022, 434, 128842. [Google Scholar] [CrossRef]
- Togao, M.; Nakayama, S.M.M.; Ikenaka, Y.; Mizukawa, H.; Makino, Y.; Kubota, A.; Matsukawa, T.; Yokoyama, K.; Hirata, T.; Ishizuka, M. Bioimaging of Pb and STIM1 in mice liver, kidney and brain using Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) and immunohistochemistry. Chemosphere 2020, 238, 124581. [Google Scholar] [CrossRef] [PubMed]
- Lukačínová, A.; Rácz, O.; Lovásová, E.; Ništiar, F. Effect of lifetime low dose exposure to heavy metals on selected serum proteins of Wistar rats during three subsequent generations. Ecotoxicol. Environ. Saf. 2011, 74, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, F.; He, H.; Berry, J.J.; Zhu, K.; Xu, T. On-device lead sequestration for perovskite solar cells. Nature 2020, 578, 555–558. [Google Scholar] [CrossRef]
- Yang, M.; Tian, T.; Fang, Y.; Li, W.-G.; Liu, G.; Feng, W.; Xu, M.; Wu, W.-Q. Reducing lead toxicity of perovskite solar cells with a built-in supramolecular complex. Nat. Sustain. 2023, 6, 1455–1464. [Google Scholar] [CrossRef]
- Wu, P.; Wang, S.; Li, X.; Zhang, F. Beyond efficiency fever: Preventing lead leakage for perovskite solar cells. Matter 2022, 5, 1137–1161. [Google Scholar] [CrossRef]
- Abate, A. Perovskite solar cells go lead free. Joule 2017, 1, 659–664. [Google Scholar] [CrossRef]
- Chen, S.; Wu, C.; Han, B.; Liu, Z.; Mi, Z.; Hao, W.; Zhao, J.; Wang, X.; Zhang, Q.; Liu, K.; et al. Atomic-scale imaging of CH3NH3PbI3 structure and its decomposition pathway. Nat. Commun. 2021, 12, 5516. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; Zhao, J.; Zhang, Y.; Kong, G.; Li, Q.; Li, N.; Yu, Y.; Xu, N.; Zhang, J.; et al. Atomic scale insights into structure instability and decomposition pathway of methylammonium lead iodide perovskite. Nat. Commun. 2018, 9, 4807. [Google Scholar] [CrossRef]
- Kundu, S.; Kelly, T.L. In situ studies of the degradation mechanisms of perovskite solar cells. EcoMat 2020, 2, e12025. [Google Scholar] [CrossRef]
- Kadro, J.M.; Hagfeldt, A. The end-of-life of perovskite PV. Joule 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Ho, K.; Wei, M.; Sargent, E.H.; Walker, G.C. Grain transformation and degradation mechanism of formamidinium and cesium lead iodide perovskite under humidity and light. ACS Energy Lett. 2021, 6, 934–940. [Google Scholar] [CrossRef]
- Cheacharoen, R.; Boyd, C.C.; Burkhard, G.F.; Leijtens, T.; Raiford, J.A.; Bush, K.A.; Bent, S.F.; McGehee, M.D. Encapsulating perovskite solar cells to withstand damp heat and thermal cycling. Sustain. Energy Fuels 2018, 2, 2398–2406. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, X.; Liu, C.; Meng, X.; Huang, Z.; Yang, J.; Duan, X.; Long, J.; Zhao, Z.; Tan, L.; et al. Water-resistant and flexible perovskite solar cells via a glued interfacial layer. Adv. Funct. Mater. 2019, 29, 1902629. [Google Scholar] [CrossRef]
- Fu, Z.; Xu, M.; Sheng, Y.; Yan, Z.; Meng, J.; Tong, C.; Li, D.; Wan, Z.; Ming, Y.; Mei, A.; et al. Encapsulation of printable mesoscopic perovskite solar cells enables high emperature and long-term outdoor stability. Adv. Funct. Mater. 2019, 29, 1809129. [Google Scholar] [CrossRef]
- Li, J.; Xia, R.; Qi, W.; Zhou, X.; Cheng, J.; Chen, Y.; Hou, G.; Ding, Y.; Li, Y.; Zhao, Y.; et al. Encapsulation of perovskite solar cells for enhanced stability: Structures, materials and characterization. J. Power Sources 2021, 485, 229313. [Google Scholar] [CrossRef]
- Kim, H.-C.; Jang, T.-W.; Chae, H.-J.; Choi, W.-J.; Ha, M.-N.; Ye, B.-J.; Kim, B.-G.; Jeon, M.-J.; Kim, S.-Y.; Hong, Y.-S. Evaluation and management of lead exposure. Ann. Occup. Environ. Med. 2015, 27, 30. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Li, P.; Ma, J.; Han, L.; Zhang, Y.; Song, Y. Sustainable Pb management in perovskite solar cells toward eco-friendly development. Adv. Energy Mater. 2022, 12, 2201242. [Google Scholar] [CrossRef]
- Yin, J.; Cui, J.; Zhou, H.; Cui, S.; Wang, C.; Guo, J.; Wei, J.; Zhang, X. Encapsulation of UV glue, hydrophobicity of binder and carbon electrode enhance the stability of organic-inorganic hybrid perovskite solar cells up to 5 years. Energy Technol. 2020, 8, 2000513. [Google Scholar] [CrossRef]
- Wan, J.; Yu, X.; Zou, J.; Li, K.; Chen, L.; Peng, Y.; Cheng, Y.-b. Lead contamination analysis of perovskite modules under simulated working conditions. Sol. Energy 2021, 226, 85–91. [Google Scholar] [CrossRef]
- Hailegnaw, B.; Kirmayer, S.; Edri, E.; Hodes, G.; Cahen, D. Rain on methylammonium lead iodide based perovskites: Possible environmental effects of perovskite solar cells. J. Phys. Chem. Lett. 2015, 6, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, J.; Cao, Q.; Pu, X.; Li, Y.; Chen, H.; Zhao, J.; Zhang, Y.; Chen, X.; Li, X. Room temperature nondestructive encapsulation via self-crosslinked fluorosilicone polymer enables damp heat-stable sustainable perovskite solar cells. Nat. Commun. 2023, 14, 1342. [Google Scholar] [CrossRef] [PubMed]
- Mariani, P.; Molina-García, M.Á.; Barichello, J.; Zappia, M.I.; Magliano, E.; Castriotta, L.A.; Gabatel, L.; Thorat, S.B.; Del Rio Castillo, A.E.; Drago, F.; et al. Low-temperature strain-free encapsulation for perovskite solar cells and modules passing multifaceted accelerated ageing tests. Nat. Commun. 2024, 15, 4552. [Google Scholar] [CrossRef]
- IEC 61215-1; Terrestrial Photovoltaic (PV) Modules—Design Qualification and Type Approval—Part 1: Test Requirements. IEC: Geneva, Switzerland, 2021.
- Jiang, Y.; Qiu, L.; Juarez-Perez, E.J.; Ono, L.K.; Hu, Z.; Liu, Z.; Wu, Z.; Meng, L.; Wang, Q.; Qi, Y. Reduction of lead leakage from damaged lead halide perovskite solar modules using self-healing polymer-based encapsulation. Nat. Energy 2019, 4, 585–593. [Google Scholar] [CrossRef]
- Ottersböck, B.; Oreski, G.; Pinter, G. Comparison of different microclimate effects on the aging behavior of encapsulation materials used in photovoltaic modules. Polym. Degrad. Stab. 2017, 138, 182–191. [Google Scholar] [CrossRef]
- Meng, X.; Hu, X.; Zhang, Y.; Huang, Z.; Xing, Z.; Gong, C.; Rao, L.; Wang, H.; Wang, F.; Hu, T.; et al. A biomimetic self-shield interface for flexible perovskite solar cells with egligible Lead Lleakage. Adv. Funct. Mater. 2021, 31, 2106460. [Google Scholar] [CrossRef]
- Chen, S.; Deng, Y.; Gu, H.; Xu, S.; Wang, S.; Yu, Z.; Blum, V.; Huang, J. Trapping lead in perovskite solar modules with abundant and low-cost cation-exchange resins. Nat. Energy 2020, 5, 1003–1011. [Google Scholar] [CrossRef]
- Chen, S.; Deng, Y.; Xiao, X.; Xu, S.; Rudd, P.N.; Huang, J. Preventing lead leakage with built-in resin layers for sustainable perovskite solar cells. Nat. Sustain. 2021, 4, 636–643. [Google Scholar] [CrossRef]
- Cho, S.-P.; Lee, H.-J.; Seo, Y.-H.; Na, S.-I. Multifunctional passivation agents for improving efficiency and stability of perovskite solar cells: Synergy of methyl and carbonyl groups. Appl. Surf. Sci. 2022, 575, 151740. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; Wang, J.; Tong, J.; Xu, T.; Zhu, K. On-device lead-absorbing tapes for sustainable perovskite solar cells. Nat. Sustain. 2021, 4, 1038–1041. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, M.; Chen, S.; Zhang, Y.; Gu, H.; Deng, Y.; Yang, G.; Fei, C.; Chen, B.; Lin, Y.; et al. Lead-adsorbing ionogel-based encapsulation for impact-resistant, stable, and lead-safe perovskite modules. Sci. Adv. 2021, 7, eabi8249. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, X.; Li, B.; Zhang, S.; Gao, D.; Liu, Y.; Li, X.; Zhang, N.; Hu, X.; Zhi, C.; et al. Sulfonated graphene aerogels enable safe-to-use flexible perovskite olar Mmodules. Adv. Energy Mater. 2021, 12, 2103236. [Google Scholar] [CrossRef]
- Valastro, S.; Smecca, E.; Mannino, G.; Bongiorno, C.; Fisicaro, G.; Goedecker, S.; Arena, V.; Spampinato, C.; Deretzis, I.; Dattilo, S.; et al. Preventing lead leakage in perovskite solar cells with a sustainable titanium dioxide sponge. Nat. Sustain. 2023, 6, 974–983. [Google Scholar] [CrossRef]
- Wu, S.; Li, Z.; Li, M.-Q.; Diao, Y.; Lin, F.; Liu, T.; Zhang, J.; Tieu, P.; Gao, W.; Qi, F.; et al. 2D metal-organic framework for stable perovskite solar cells with minimized lead leakage. Nat. Nanotechnol. 2020, 15, 934–940. [Google Scholar] [CrossRef]
- Zhang, H.; Li, K.; Sun, M.; Wang, F.; Wang, H.; Jen, A.K.Y. Design of superhydrophobic surfaces for table perovskite solar cells with reducing lead leakage. Adv. Energy Mater. 2021, 11, 2102281. [Google Scholar] [CrossRef]
- Binek, A.; Petrus, M.L.; Huber, N.; Bristow, H.; Hu, Y.; Bein, T.; Docampo, P. Recycling perovskite solar cells to void lead waste. ACS Appl. Mater. Interfaces 2016, 8, 12881–12886. [Google Scholar] [CrossRef] [PubMed]
- Poll, C.G.; Nelson, G.W.; Pickup, D.M.; Chadwick, A.V.; Riley, D.J.; Payne, D.J. Electrochemical recycling of lead from hybrid organic–inorganic perovskites using deep eutectic solvents. Green Chem. 2016, 18, 2946–2955. [Google Scholar] [CrossRef]
- Xu, J.; Hu, Z.; Huang, L.; Huang, X.; Jia, X.; Zhang, J.; Zhang, J.; Zhu, Y. In situ recycle of PbI2 as a step towards sustainable perovskite solar cells. Prog. Photovolt. Res. Appl. 2017, 25, 1022–1033. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, L.; Huang, M.; Yu, Y.; Lei, L.; Shao, J.; Zhao, Q.; Wu, Z.; Wang, J.; Yang, S. Cyclic tilization of lead in carbon-based perovskite solar cells. ACS Sustain. Chem. Eng. 2018, 6, 7558–7564. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, J.-S.; Kim, B.J.; Lee, H.; Walsh, A.; Zhu, K.; Kim, D.H.; Jung, H.S. Sustainable lead management in halide perovskite solar cells. Nat. Sustain. 2020, 3, 1044–1051. [Google Scholar] [CrossRef]
- Chen, B.; Fei, C.; Chen, S.; Gu, H.; Xiao, X.; Huang, J. Recycling lead and transparent conductors from perovskite solar modules. Nat. Commun. 2021, 12, 5859. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Guo, Q.; Xiu, J.; Ying, Z.; Ng, K.W.; Huang, L.; Wang, S.; Pan, H.; Tang, Z.; He, Z. Close-loop recycling of perovskite solar cells through dissolution-recrystallization of perovskite by butylamine. Cell Rep. Phys. Sci. 2021, 2, 100341. [Google Scholar] [CrossRef]
- Deng, F.; Song, X.; Li, Y.; Zhang, W.; Tao, X. Facile eco-friendly process for upcycled sustainable perovskite solar cells. Chem. Eng. J. 2024, 489, 151228. [Google Scholar] [CrossRef]
- Schmidt, F.; Amrein, M.; Hedwig, S.; Kober-Czerny, M.; Paracchino, A.; Holappa, V.; Suhonen, R.; Schäffer, A.; Constable, E.C.; Snaith, H.J.; et al. Organic solvent free PbI2 recycling from perovskite solar cells using hot water. J. Hazard. Mater. 2023, 447, 130829. [Google Scholar] [CrossRef]
- Fthenakis, V.M. Life cycle impact analysis of cadmium in CdTe PV production. Renew. Sustain. Energy Rev. 2004, 8, 303–334. [Google Scholar] [CrossRef]
- Vellini, M.; Gambini, M.; Prattella, V. Environmental impacts of PV technology throughout the life cycle: Importance of the end-of-life management for Si-panels and CdTe-panels. Energy 2017, 138, 1099–1111. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Mi, T.; Tian, T.; Yang, M.; Pang, H. Mitigating Lead Toxicity in Halide Perovskite Solar Cells: Strategies for Sustainable Development. Inorganics 2025, 13, 123. https://doi.org/10.3390/inorganics13040123
Li W, Mi T, Tian T, Yang M, Pang H. Mitigating Lead Toxicity in Halide Perovskite Solar Cells: Strategies for Sustainable Development. Inorganics. 2025; 13(4):123. https://doi.org/10.3390/inorganics13040123
Chicago/Turabian StyleLi, Wenguang, Tianci Mi, Tian Tian, Meifang Yang, and Huan Pang. 2025. "Mitigating Lead Toxicity in Halide Perovskite Solar Cells: Strategies for Sustainable Development" Inorganics 13, no. 4: 123. https://doi.org/10.3390/inorganics13040123
APA StyleLi, W., Mi, T., Tian, T., Yang, M., & Pang, H. (2025). Mitigating Lead Toxicity in Halide Perovskite Solar Cells: Strategies for Sustainable Development. Inorganics, 13(4), 123. https://doi.org/10.3390/inorganics13040123