Experimental Study on Van der Waals Interactions Between Organic Groups of Quaternary Ammonium Salt Surfactants and Montmorillonite in Aqueous Solutions
Abstract
:1. Introduction
2. Results and Discussion
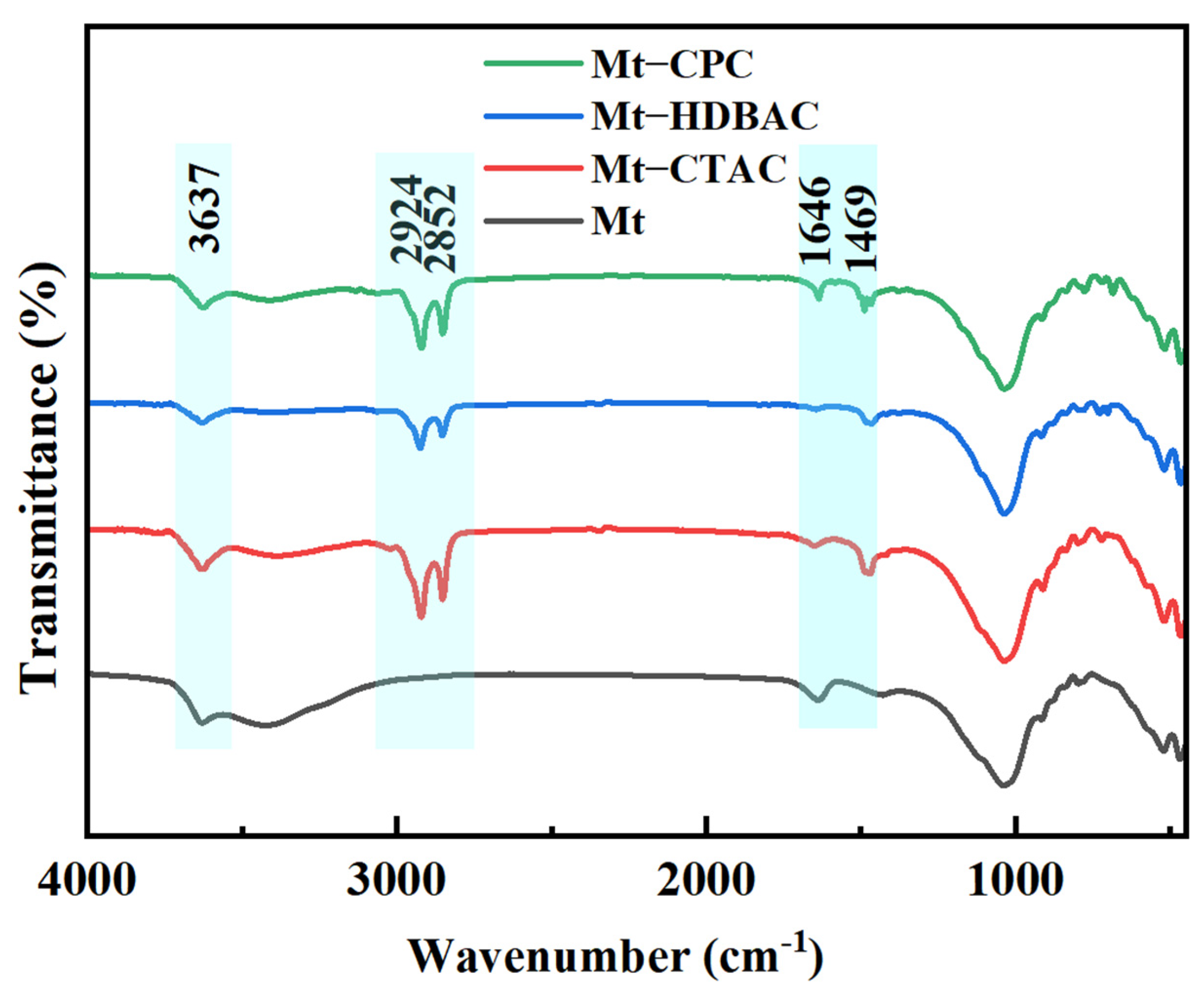
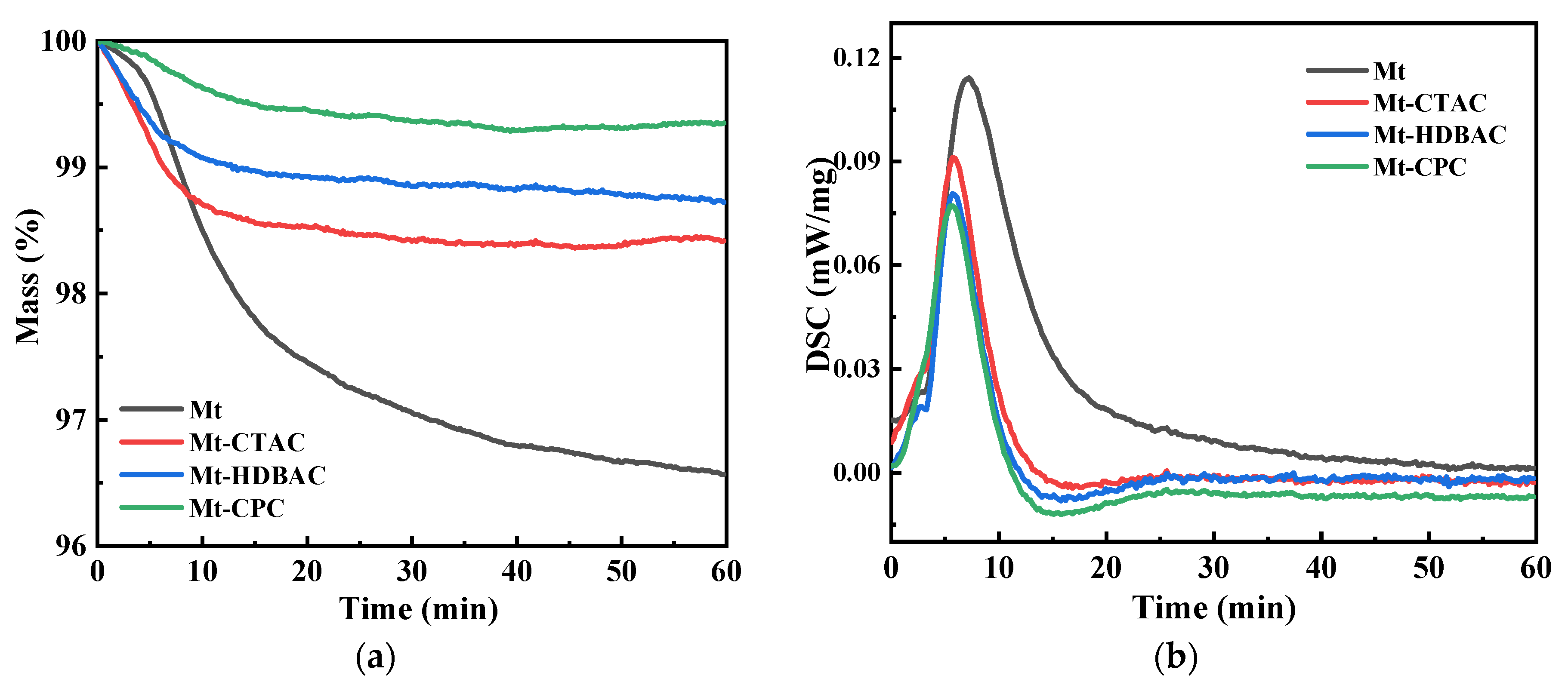
2.1. Physical and Chemical Adsorption Analysis
2.2. Hydrophobic Floc Effect and Dipole Interaction
 -Mnt interaction >
-Mnt interaction >  -Mnt interaction >
-Mnt interaction > 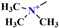 -Mnt interaction.
-Mnt interaction. -Mnt interaction >
-Mnt interaction >  -Mnt interaction >
-Mnt interaction > 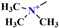 -Mnt interaction.
-Mnt interaction. -Mnt interaction >
-Mnt interaction >  -Mnt interaction >
-Mnt interaction > 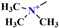 -Mnt interaction.
-Mnt interaction. of CPC, hydrophilic end
of CPC, hydrophilic end  of HDBAC, hydrophilic end
of HDBAC, hydrophilic end 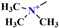 of CTAC, and Mnt are the orientation dipole–induced dipole interactions and the instantaneous dipole–instantaneous dipole interaction under the effect of orientation dipole,
of CTAC, and Mnt are the orientation dipole–induced dipole interactions and the instantaneous dipole–instantaneous dipole interaction under the effect of orientation dipole,  -Mnt interaction >
-Mnt interaction >  -Mnt interaction >
-Mnt interaction > 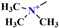 -Mnt interaction.
-Mnt interaction.2.3. Dipole Interaction and Discussion
2.3.1. Adsorption Capacity Characterization of the Effect of Dipoles on the Adsorption of QASs
2.3.2. Characterization of the Effect of Dipole on QASs Adsorption Through Energy Measurement
2.3.3. Average Adsorption Distance, Electrostatic Adsorption Energy, VDW Adsorption Energy, and VDW Constant (C) of QASs
2.3.4. VDW Energy and VDW Constant C of the Organic Groups
 , and its molecular formula is CH3(CH2)11N(CH3)3Cl. Suppose the VDW energy of -CH2 is J/mol, and the value of obtained by combining the three equations according to Equations (5)–(7) is averaged. Then, suppose the VDW energy of -CH3 is J/mol, and the VDW energy of a hydrophilic polar head is J/mol.
, and its molecular formula is CH3(CH2)11N(CH3)3Cl. Suppose the VDW energy of -CH2 is J/mol, and the value of obtained by combining the three equations according to Equations (5)–(7) is averaged. Then, suppose the VDW energy of -CH3 is J/mol, and the VDW energy of a hydrophilic polar head is J/mol.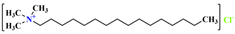 , and its molecular formula is CH3(CH2)15N(CH3)3Cl. Thus, the VDW interaction formula for each organic group is
, and its molecular formula is CH3(CH2)15N(CH3)3Cl. Thus, the VDW interaction formula for each organic group is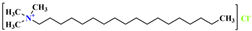 , and its molecular formula is CH3(CH2)17N(CH3)3Cl. The VDW interaction formula for each organic group is
, and its molecular formula is CH3(CH2)17N(CH3)3Cl. The VDW interaction formula for each organic group is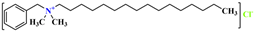 , and its molecular formula is CH3(CH2)15N(CH3)2CH2(C6H5)Cl. C25H46NC6H6Cl. Suppose the VDW interaction energy of the hydrophilic polar head is , and the VDW interaction formula of each organic group is as follows:
, and its molecular formula is CH3(CH2)15N(CH3)2CH2(C6H5)Cl. C25H46NC6H6Cl. Suppose the VDW interaction energy of the hydrophilic polar head is , and the VDW interaction formula of each organic group is as follows: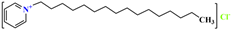 , and its molecular formula is CH3(CH2)17N(CH3)3Cl. C21H38ClN. Suppose the VDW interaction energy of the hydrophilic polar head is , and the VDW interaction formula of each organic group is as follows:
, and its molecular formula is CH3(CH2)17N(CH3)3Cl. C21H38ClN. Suppose the VDW interaction energy of the hydrophilic polar head is , and the VDW interaction formula of each organic group is as follows: -Mnt (−17.533 kJ/mol) >
-Mnt (−17.533 kJ/mol) > 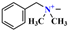 -Mnt (−13.196 kJ/mol) >
-Mnt (−13.196 kJ/mol) > 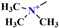 -Mnt (−9.305 kJ/mol), CH3-Mnt (−0.143 kJ/mol) > CH2-Mnt (−0.095 kJ/mol). The dipole moment of pyridine was 5.3. Compared with the pyridine group, the electrostatic center of the benzyl group is located outside the carbon ring, and the carbon ring is more symmetrical; therefore, it has a less oriented dipole. Compared with the benzyl group, the methyl group binds the electron cloud more strongly than the six-carbon ring; thus, it has the least oriented dipole. The order of the dipole is
-Mnt (−9.305 kJ/mol), CH3-Mnt (−0.143 kJ/mol) > CH2-Mnt (−0.095 kJ/mol). The dipole moment of pyridine was 5.3. Compared with the pyridine group, the electrostatic center of the benzyl group is located outside the carbon ring, and the carbon ring is more symmetrical; therefore, it has a less oriented dipole. Compared with the benzyl group, the methyl group binds the electron cloud more strongly than the six-carbon ring; thus, it has the least oriented dipole. The order of the dipole is  >
> 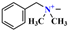 >
> 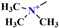 . These results indicate that the larger the hydrophilic dipole of QASs, the greater the interaction between QASs and Mnt, and the greater the VDW energy. CH3 has one more H atom than CH2, and the binding of CH3 on electrons is lower than that of CH2; hence, CH3 has a greater induced dipole and adsorption energy. Additionally, the VDW constants of each functional group can be obtained according to the VDW energy obtained above.
. These results indicate that the larger the hydrophilic dipole of QASs, the greater the interaction between QASs and Mnt, and the greater the VDW energy. CH3 has one more H atom than CH2, and the binding of CH3 on electrons is lower than that of CH2; hence, CH3 has a greater induced dipole and adsorption energy. Additionally, the VDW constants of each functional group can be obtained according to the VDW energy obtained above.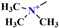 (1.14 × 10−78 J·m6),
(1.14 × 10−78 J·m6), 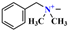 (1.22 × 10)−78 J·m6),
(1.22 × 10)−78 J·m6),  (1.89 × 10−78 J·m6), CH3 (1.75 × 10−80 J·m6), and CH2 (1.17 × 10−80 J·m6). For the hydrophilic end, the VDW constant . is the interaction between the orientation dipole of
(1.89 × 10−78 J·m6), CH3 (1.75 × 10−80 J·m6), and CH2 (1.17 × 10−80 J·m6). For the hydrophilic end, the VDW constant . is the interaction between the orientation dipole of  ,
, 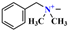 ,
, 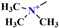 , and the Si-O polar bond of Mnt. is the interaction between the induced dipole between
, and the Si-O polar bond of Mnt. is the interaction between the induced dipole between  ,
, 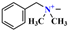 ,
, 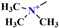 and the Si-O polar bond of Mnt, and is the interaction between the induced dispersion between the instantaneous dipole of
and the Si-O polar bond of Mnt, and is the interaction between the induced dispersion between the instantaneous dipole of  ,
, 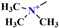 ,
, 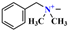 and groups and the instantaneous dipole of Mnt surface. Because C
and groups and the instantaneous dipole of Mnt surface. Because C  = 1.89 × 10−78 J·m6 > C
= 1.89 × 10−78 J·m6 > C 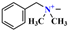 = 1.22 × 10−78 J·m6 > C
= 1.22 × 10−78 J·m6 > C 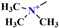 = 1.14 × 10−78 J·m6, the order of the dipole is
= 1.14 × 10−78 J·m6, the order of the dipole is  >
> 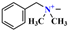 >
> 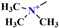 . The difference in attraction between the hydrophilic group and Mnt is caused by the value of the dipole of hydrophilic end; therefore, the VDW energy
. The difference in attraction between the hydrophilic group and Mnt is caused by the value of the dipole of hydrophilic end; therefore, the VDW energy  -Mnt (−17.533 kJ/mol) >
-Mnt (−17.533 kJ/mol) > 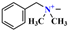 -Mnt (−13.196 kJ/mol) >
-Mnt (−13.196 kJ/mol) > 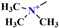 -Mnt (−9.305 kJ/mol). The adsorption difference between QASs and Mnt with the same hydrophobic end is due to the value of the dipole of the hydrophilic end, and the VDW energy decreases in the order CPC > HDBAC > CTAC. Furthermore, the VDW interaction results in the excessive adsorption of QASs on the Mnt surface.
-Mnt (−9.305 kJ/mol). The adsorption difference between QASs and Mnt with the same hydrophobic end is due to the value of the dipole of the hydrophilic end, and the VDW energy decreases in the order CPC > HDBAC > CTAC. Furthermore, the VDW interaction results in the excessive adsorption of QASs on the Mnt surface.3. Materials and Methods
3.1. Samples and Reagents
3.2. Experimental Methods
3.3. Characterization
- (1)
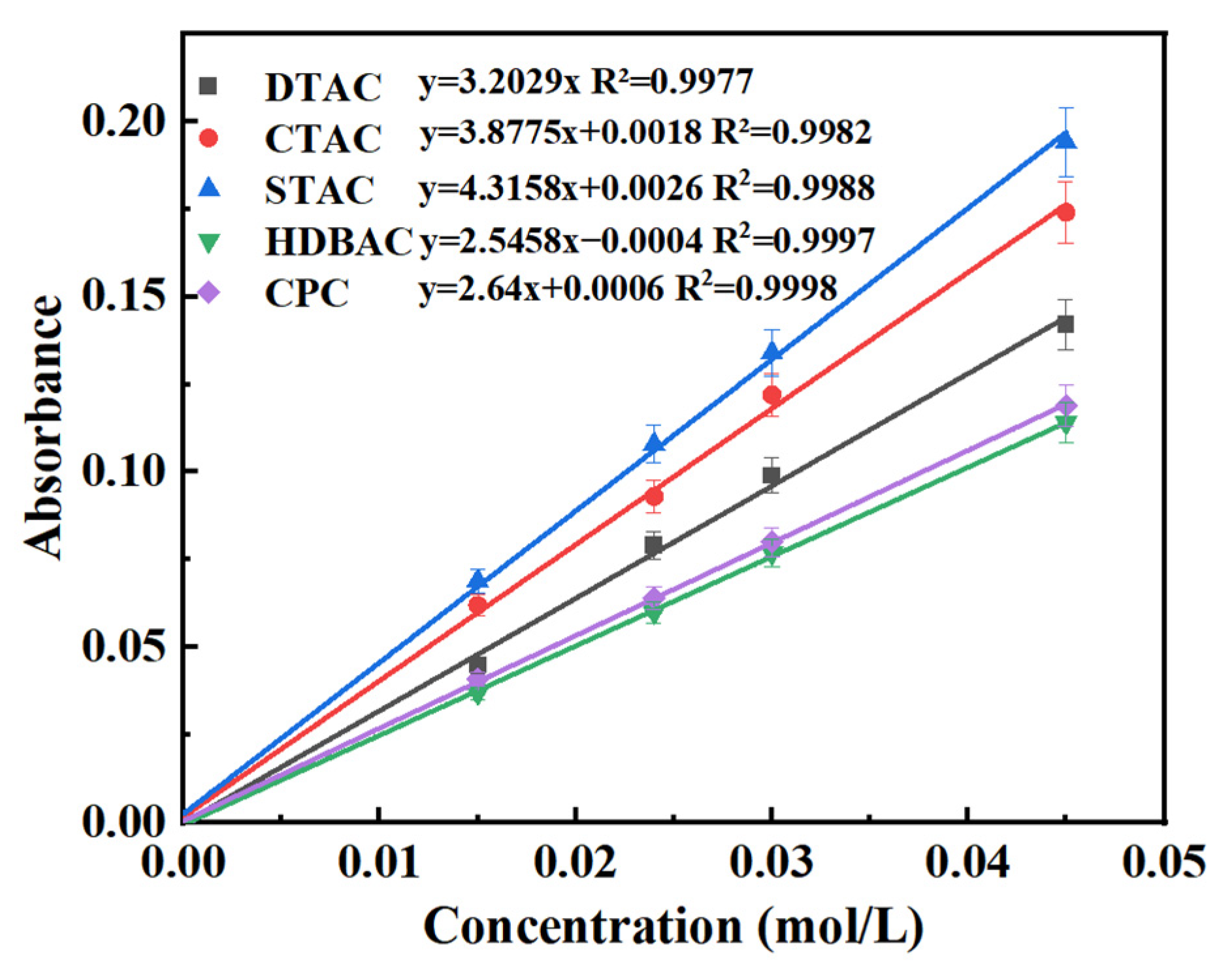
- The determination wavelength was first identified through spectral analysis. In a 50 mL volumetric flask, 2 mL of a QAS solution (0.045 mol/L) was mixed with 2 mL of 0.15% OP-10 solution, 10 mL of 1.6 × 10−4 mol/L bromothymol blue (BTB) solution, and 5 mL of phosphate buffer (pH = 7.7). The mixture was then diluted to volume with deionized water and shaken thoroughly. Using a JH721 UV–Vis spectrophotometer and a 1 cm pathlength cuvette, the absorption spectra of both the blank BTB solution and the BTB–QAS mixture were recorded over the wavelength range of 400–700 nm, with deionized water as the reference.The maximum absorption wavelength (λ = 576 nm) corresponding to the BTB–QAS complex was selected for subsequent measurements. This wavelength ensured optimal sensitivity, accuracy, and reproducibility in quantifying the adsorption capacity of QASs on montmorillonite. The absorption spectra are presented in Figure 11.
- (2)
- The standard working curve was determined. Each of these QAS aqueous solutions with mass concentrations of 0.015, 0.024, 0.03, and 0.045 mol/L were prepared with deionized water. Additionally, 2 mL of QAS solutions with different mass concentrations were dyed by BTB, and the absorbance was measured using a JH721 UV-Vis spectrophotometer at the determination wavelength using deionized water as the reference solution. The obtained absorbance decrease was used to draw the working curve of the QAS mass concentration, and the standard working curve was obtained by linear fitting of the working curve.
- (3)
- The adsorption capacity was determined. Twenty-five milliliters of the 0.04 mol/L Mnt suspension and 25 mL of the five reagents of 0.045 mol/L were taken, stirred at 400 rad/min for 5 min (magnetic stirrer), allowed to stand for 30 min, and 20 mL of supernatant was centrifuged at 2000 rad/min for 5 min. Then, 2 mL of the supernatant liquid was mixed with the above-prepared solution and shaken well. Deionized water was used as the reference, and the absorbance of each reagent was measured three times at 576 nm, and the final value was averaged. Using the obtained QAS working curve, the adsorption capacity of QASs on the surface of the Mnt particles was calculated using the residual concentration method, and the calculation formula iswhere is the adsorption capacity (mmol/g), is the mass percentage of the reagent aqueous solution (0.5%), is the volume of reagent aqueous solution (mL), is the density of the reagent aqueous solution (1 g/cm3), V is the volume of Mnt suspension (25 mL), m is the mass of Mnt (0.35 g), is the density of Mnt (2 g/cm3), and is the reagent concentration obtained according to the working curve (mol/L).
4. Conclusions
 -Mnt (WVDW = −17.533 kJ/mol, C = 1.89 × 10−78 J·m6) >
-Mnt (WVDW = −17.533 kJ/mol, C = 1.89 × 10−78 J·m6) > 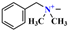 -Mnt (WVDW = −13.196 kJ/mol, C = 1.22 × 10−78 J·m6) >
-Mnt (WVDW = −13.196 kJ/mol, C = 1.22 × 10−78 J·m6) > 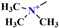 -Mnt (WVDW = −9.305 kJ/mol, C = 1.14 × 10−78 J·m6). The larger the hydrophilic dipole, the stronger the VDW interaction between QASs and Mnt, including orientation dipole–orientation dipole, induced dipole–orientation dipole, and induced dipole–induced dipole. The VDW interaction between QASs and Mnt with different hydrophobic ends decreased in the order STAC > CTAC > DTAC, and the VDW interaction energy between them was STAC-Mnt (WVDW = −11.062 kJ/mol, C = 1.11 × 10−72 J·m6) > CTAC-Mnt (WVDW = −10.863 kJ/mol, C = 1.26 × 10−72 J·m6) > DTAC-Mnt (WVDW = −10.496 kJ/mol, C = 1.62 × 10−72 J·m6), which was due to the superposition of CH2 at the hydrophobic end during adsorption. However, CH3-Mnt (WVDW = −0.143 kJ/mol, C = 1.75 × 10−80 J·m6) > CH2-Mnt (WVDW = −0.095 kJ/mol, C = 1.17 × 10−80 J·m6), and the interaction between these two groups and Mnt mainly induced dipole–induced dipole interactions.
-Mnt (WVDW = −9.305 kJ/mol, C = 1.14 × 10−78 J·m6). The larger the hydrophilic dipole, the stronger the VDW interaction between QASs and Mnt, including orientation dipole–orientation dipole, induced dipole–orientation dipole, and induced dipole–induced dipole. The VDW interaction between QASs and Mnt with different hydrophobic ends decreased in the order STAC > CTAC > DTAC, and the VDW interaction energy between them was STAC-Mnt (WVDW = −11.062 kJ/mol, C = 1.11 × 10−72 J·m6) > CTAC-Mnt (WVDW = −10.863 kJ/mol, C = 1.26 × 10−72 J·m6) > DTAC-Mnt (WVDW = −10.496 kJ/mol, C = 1.62 × 10−72 J·m6), which was due to the superposition of CH2 at the hydrophobic end during adsorption. However, CH3-Mnt (WVDW = −0.143 kJ/mol, C = 1.75 × 10−80 J·m6) > CH2-Mnt (WVDW = −0.095 kJ/mol, C = 1.17 × 10−80 J·m6), and the interaction between these two groups and Mnt mainly induced dipole–induced dipole interactions.Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lagaly, G.; Ogawa, M.; Dékány, I. Clay Mineral-Organic Interactions. In Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, ISBN 9780080982588. [Google Scholar] [CrossRef]
- Liu, L.; Kong, C.; Zhao, H.; Lu, F. Elucidating the enhancement of kaolinite flotation by iron content through density functional theory: A study on sodium oleate adsorption efficiency. Int. J. Min. Sci. Technol. 2024, 34, 855–866. [Google Scholar] [CrossRef]
- Bergaya, F.; Detellier, C.; Lambert, J.F.; Lagaly, G. Introduction to Clay-Polymer Nanocomposites (CPN), 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 5. [Google Scholar] [CrossRef]
- Huang, X.; Lewis, S.; Brittain, W.J.; Vaia, R.A. Synthesis of polycarbonate-layered silicate nanocomposites via cyclic oligomers. Macromolecules 2000, 33, 2000–2004. [Google Scholar] [CrossRef]
- Annabi-Bergaya, F. Layered clay minerals. Basic research and innovative composite applications. Microporous Mesoporous Mater. 2008, 107, 141–148. [Google Scholar] [CrossRef]
- Giannelis, E.P.; Krishnamoorti, R.; Manias, E. Polymer-silicate nanocomposites: Model systems for confined polymers and polymer brushes. Adv. Polym. Sci. 1999, 138, 108–147. [Google Scholar] [CrossRef]
- Lebaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: An overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar] [CrossRef]
- Fournaris, K.G.; Karakassides, M.A.; Petridis, D.; Yiannakopoulou, K. Clay—Polyvinylpyridine nanocomposites. Chem. Mater. 1999, 11, 2372–2381. [Google Scholar] [CrossRef]
- Carrado, K.A. Carrado Synthetic organo- and polymer- clays: Preparation, characterization, and materials applications. Appl. Clay Sci. 2000, 17, 1–23. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Kang, S.; Bai, H.; Gu, W.; Fang, D.; Komarneni, S.; Zhang, Q. Mechanical activation of zero-valent iron (ZVI) in the presence of CaCO3: Improved reactivity of ZVI for enhancing As(III) removal from water. J. Clean. Prod. 2021, 286, 124926. [Google Scholar] [CrossRef]
- Yi, H.; Ai, Z.; Zhao, Y.; Zhang, X.; Song, S. Design of 3D-network montmorillonite nanosheet/stearic acid shape-stabilized phase change materials for solar energy storage. Sol. Energy Mater. Sol. Cells 2020, 204, 110233. [Google Scholar] [CrossRef]
- Lambert, J.F.; Bergaya, F. Smectite-Polymer Nanocomposites, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 5. [Google Scholar] [CrossRef]
- Darder, M.; Colilla, M.; Ruiz-Hitzky, E. Biopolymer-clay nanocomposites based on chitosan intercalated in montmorillonite. Chem. Mater. 2003, 15, 3774–3780. [Google Scholar] [CrossRef]
- Ogawa, M.; Hashizume, T.; Kuroda, K.; Kato, C. Intercalation of 2,2’-Bipyridine and Complex Formation in the Interlayer Space of Montmorillonite by Solid-Solid Reactions. Inorg. Chem. 1991, 30, 584–585. [Google Scholar] [CrossRef]
- Doner, H.E.; Mortland, M.M. Benzene complexes with copper(II)montmorillonite. Science 1969, 166, 1406–1407. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.J.; Mishra, M.K.; Shukla, A.D.; Imae, T.; Shah, D.O. Controlling wettability and hydrophobicity of organoclays modified with quaternary ammonium surfactants. J. Colloid Interface Sci. 2013, 407, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Cambridge, MA, USA, 1981; Volume 53, ISBN 9788578110796. [Google Scholar]
- Butt, H.J.; Kappl, M. Surface and Interfacial Forces; John Wiley & Sons: Hoboken, NJ, USA, 2010; ISBN 9783527408498. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, Y.; Yang, Q.; Khoso, S.A.; Liu, G.; Xu, L.; Hu, Y. Adsorption behaviors and mechanisms of dodecyltrimethyl ammonium chloride and cetyltrimethyl ammonium chloride on illite flotation. Powder Technol. 2018, 331, 218–225. [Google Scholar] [CrossRef]
- Haounati, R.; Ouachtak, H.; El Haouti, R.; Akhouairi, S.; Largo, F.; Akbal, F.; Benlhachemi, A.; Jada, A.; Addi, A.A. Elaboration and properties of a new SDS/CTAB@Montmorillonite organoclay composite as a superb adsorbent for the removal of malachite green from aqueous solutions. Sep. Purif. Technol. 2021, 255, 117335. [Google Scholar] [CrossRef]
- Hong, W.; Meng, J.; Li, C.; Yan, S.; He, X.; Fu, G. Effects of Temperature on Structural Properties of Hydrated Montmorillonite: Experimental Study and Molecular Dynamics Simulation. Adv. Civ. Eng. 2020, 2020, 8885215. [Google Scholar] [CrossRef]
- Montes-H, G.; Geraud, Y.; Duplay, J.; Reuschlé, T. ESEM observations of compacted bentonite submitted to hydration/dehydration conditions. Colloids Surf. Physicochem. Eng. Asp. 2005, 262, 14–22. [Google Scholar] [CrossRef]
- Ezquerro, C.S.; Ric, G.I.; Miñana, C.C.; Bermejo, J.S. Characterization of montmorillonites modified with organic divalent phosphonium cations. Appl. Clay Sci. 2015, 111, 1–9. [Google Scholar] [CrossRef]
- Song, S.; Lopez-Valdivieso, A.; Reyes-Bahena, J.L.; Bermejo-Perez, H.I.; Trass, O. Hydrophobic flocculation of galena fines in aqueous suspensions. J. Colloid Interface Sci. 2000, 227, 272–281. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, L.; Min, F.; Zhang, M.; Song, S. Hydrophobic agglomeration of colloidal kaolinite in aqueous suspensions with dodecylamine. Colloids Surf. Physicochem. Eng. Asp. 2013, 434, 281–286. [Google Scholar] [CrossRef]
- Peng, C.; Zhong, Y.; Min, F. Adsorption of alkylamine cations on montmorillonite (001) surface: A density functional theory study. Appl. Clay Sci. 2018, 152, 249–258. [Google Scholar] [CrossRef]
- Rotenberg, B.; Morel, J.-P.; Marry, V.; Turq, P.; Morel-Desrosiers, N. On the driving force of cation exchange in clays: Insights from combined microcalorimetry experiments and molecular simulation. Geochim. Cosmochim. Acta 2009, 73, 4034–4044. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Guo, Z.; Liu, Y.; Guo, J.; Zhang, S. Wettability modification and restraint of moisture re-adsorption of lignite using cationic gemini surfactant. Colloids Surf. Physicochem. Eng. Asp. 2016, 508, 286–293. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, F. Analysis of surface wettability of synthetic magnetite. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2014, 29, 679–683. [Google Scholar] [CrossRef]
- Matusik, J.; Kłapyta, Z. Characterization of kaolinite intercalation compounds with benzylalkylammonium chlorides using XRD, TGA/DTA and CHNS elemental analysis. Appl. Clay Sci. 2013, 83–84, 433–440. [Google Scholar] [CrossRef]



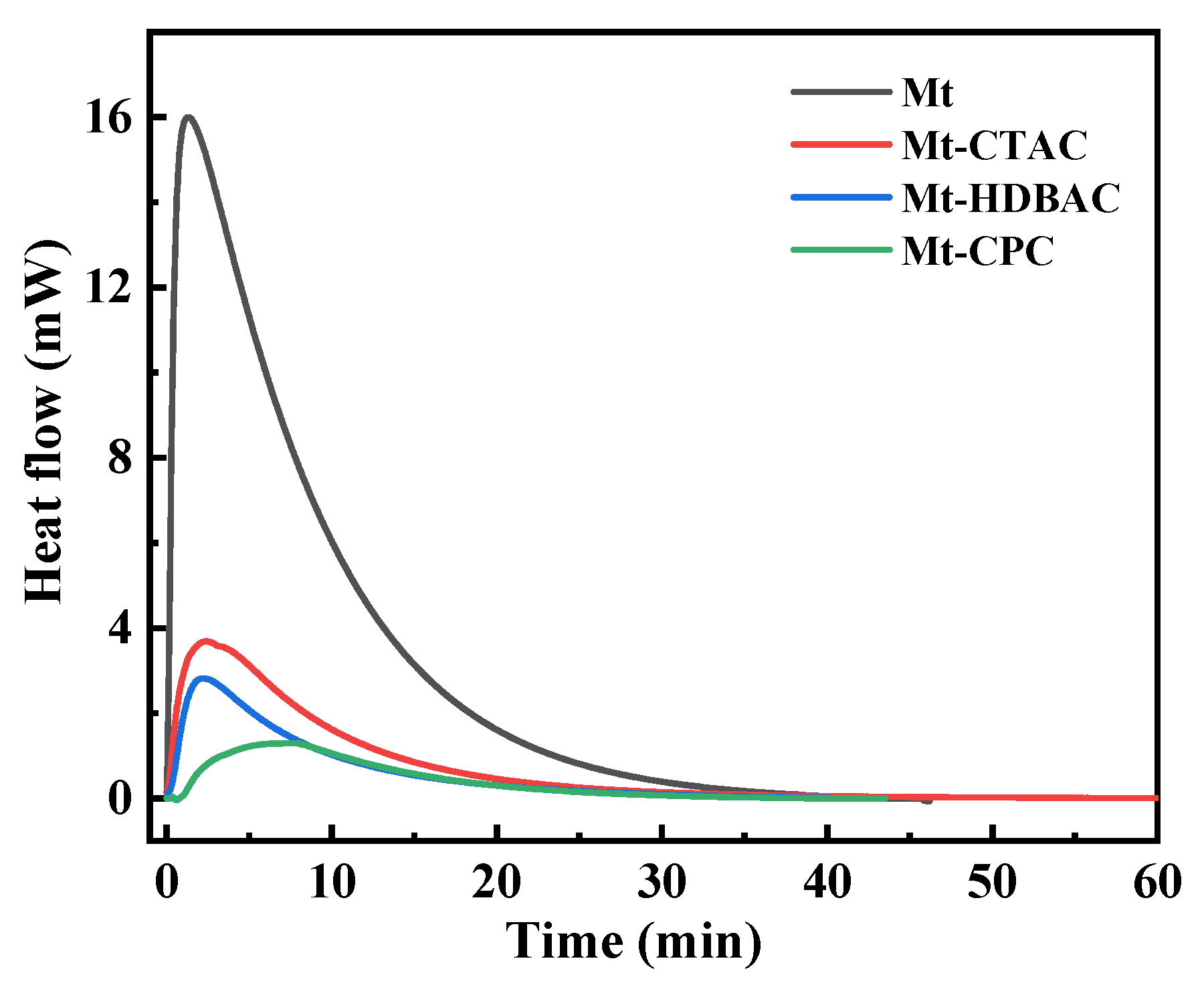
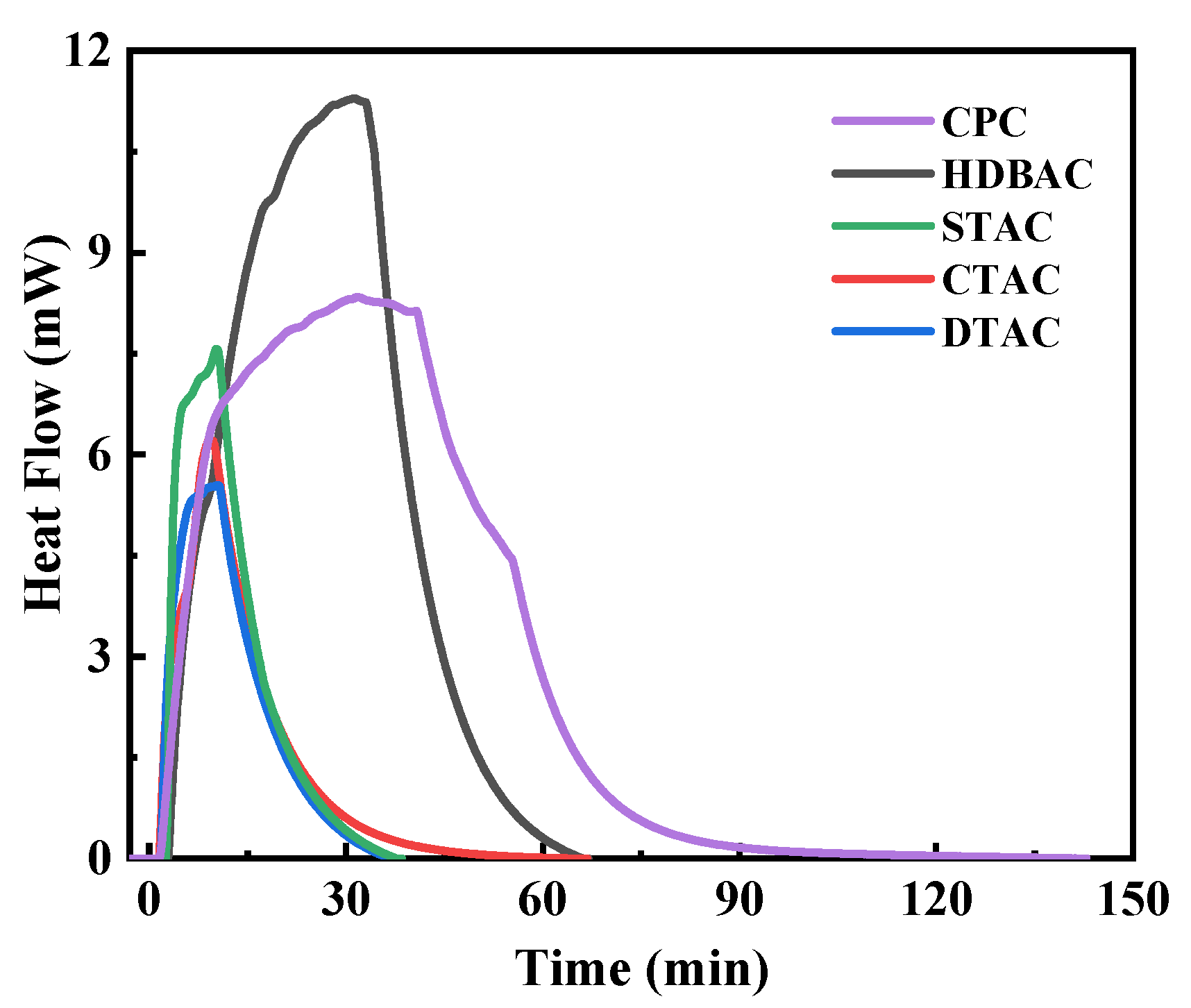


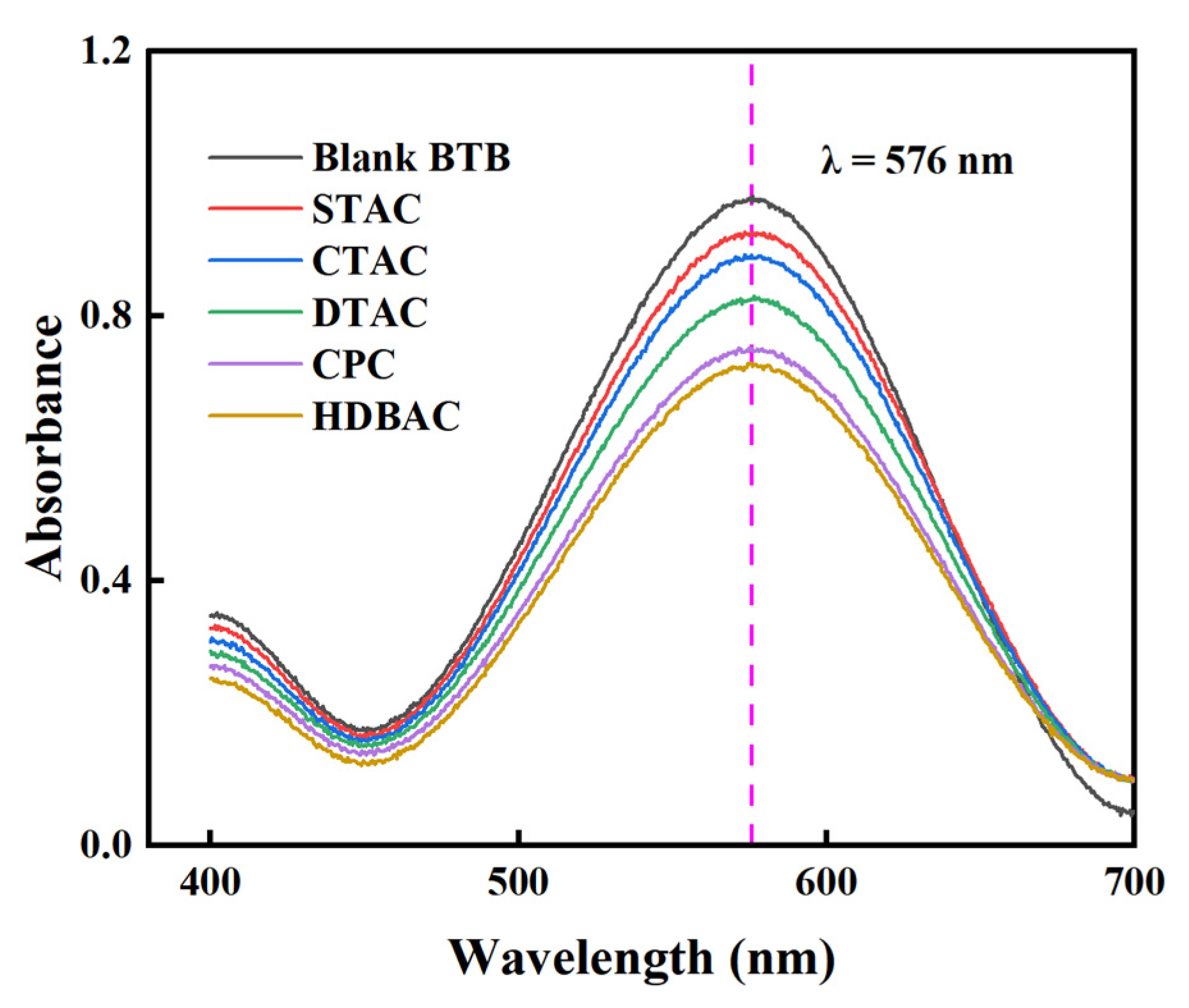
| Sample | Mnt | Mnt-CTAC | Mnt-HDBAC | Mnt-CPC |
|---|---|---|---|---|
| Mass change (%) | 3.43 ± 0.08 | 1.57 ± 0.04 | 1.28 ± 0.05 | 0.65 ± 0.02 |
| Heat value change (kJ/mol) | 10.84 ± 0.26 | 6.7 ± 0.19 | 5.43 ± 0.15 | 3.93 ± 0.12 |
| Sample | DTAC | CTAC | STAC | HDBAC | CPC |
|---|---|---|---|---|---|
| Reagent adsorption capacity (mmol/g) | 2.496 ± 0.063 | 2.524 ± 0.048 | 2.533 ± 0.035 | 2.541 ± 0.041 | 2.596 ± 0.057 |
| Electrostatic adsorption capacity (mmol/g) | 0.688 ± 0.012 | 0.672 ± 0.014 | 0.663 ± 0.008 | 0.658 ± 0.013 | 0.685 ± 0.011 |
| VDW adsorption capacity (mmol/g) | 1.808 ± 0.021 | 1.852 ± 0.018 | 1.87 ± 0.017 | 1.883 ± 0.016 | 1.911 ± 0.018 |
| Sample | Mnt | Mnt-CTAC | Mnt-HDBAC | Mnt-CPC |
|---|---|---|---|---|
| Hydration heat (kJ/mol) | −16.569 ± 0.245 | −4.350 ± 0.128 | −2.858 ± 0.102 | −1.900 ± 0.097 |
| Sample | DTAC | CTAC | STAC | HDBAC | CPC |
|---|---|---|---|---|---|
| Reagent adsorption heat (kJ/mol) | −8.045 ± 0.112 | −8.219 ± 0.103 | −8.322 ± 0.098 | −11.969 ± 0.126 | −16.412 ± 0.147 |
| Sample | Formulas | Mnt | Mnt-DTAC | Mnt-CTAC | Mnt-STAC | Mnt-HDBAC | Mnt-CPC |
|---|---|---|---|---|---|---|---|
| Hydrophilic group | - | - | 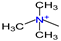 |  |  | 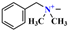 |  |
| Zeta potential (mV) | - | −15.6 ± 0.8 | 41 ± 1.2 | 43 ± 1.0 | 44 ± 0.9 | 44.6 ± 1.1 | 43.5 ± 0.7 |
| Electrostatic adsorption energy (kJ/mol) | - | 2.451 | 2.644 | 2.740 | 2.798 | 2.692 | |
| Average adsorption distance (nm) | - | 2.13 | 2.03 | 1.98 | 1.95 | 2.00 | |
| VDW energy (kJ/mol) | - | −10.496 | −10.863 | −11.062 | −14.767 | −19.104 | |
| VDW constant C (J·m6) | - | 1.62 × 10−72 | 1.26 × 10−72 | 1.11 × 10−72 | 1.37 × 10−72 | 2.06 × 10−72 |
| Functional Group | 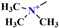 | 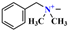 |  | -CH3 | -CH2- |
|---|---|---|---|---|---|
| VDW energy (kJ/mol) | −9.305 | −13.196 | −17.533 | −0.143 | −0.095 |
| VDW constant (J·m6) | 1.14 × 10−78 | 1.22 × 10−78 | 1.89 × 10−78 | 1.75 × 10−80 | 1.17 × 10−80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Chang, M.; Pan, Y.; Xu, W.; Li, R.; Zhu, W.; Li, H. Experimental Study on Van der Waals Interactions Between Organic Groups of Quaternary Ammonium Salt Surfactants and Montmorillonite in Aqueous Solutions. Inorganics 2025, 13, 119. https://doi.org/10.3390/inorganics13040119
Fu Y, Chang M, Pan Y, Xu W, Li R, Zhu W, Li H. Experimental Study on Van der Waals Interactions Between Organic Groups of Quaternary Ammonium Salt Surfactants and Montmorillonite in Aqueous Solutions. Inorganics. 2025; 13(4):119. https://doi.org/10.3390/inorganics13040119
Chicago/Turabian StyleFu, Yongzheng, Ming Chang, Yuhao Pan, Wennan Xu, Rui Li, Wenzhao Zhu, and Hongliang Li. 2025. "Experimental Study on Van der Waals Interactions Between Organic Groups of Quaternary Ammonium Salt Surfactants and Montmorillonite in Aqueous Solutions" Inorganics 13, no. 4: 119. https://doi.org/10.3390/inorganics13040119
APA StyleFu, Y., Chang, M., Pan, Y., Xu, W., Li, R., Zhu, W., & Li, H. (2025). Experimental Study on Van der Waals Interactions Between Organic Groups of Quaternary Ammonium Salt Surfactants and Montmorillonite in Aqueous Solutions. Inorganics, 13(4), 119. https://doi.org/10.3390/inorganics13040119







