Abstract
Sulfamethoxazole (SMX) is a widely used antibiotic for bacterial infections and is frequently found in surface waters and wastewater treatment plant effluents, where it is commonly co-administered with trimethoprim. Because of its emerging ecological and health risks, the development of effective elimination strategies is urgently required. In this study, a rapid microwave-assisted technique was employed to synthesize a Pd/g-C3N4 photocatalyst for the elimination of SMX in aqueous solution. The structure and optical properties of all samples were characterized using X-ray diffraction (XRD), transmission electron microscopy (TEM), photoluminescence (PL), and UV–visible diffuse reflectance spectroscopy. The photocatalytic performance of Pd/g-C3N4 was systematically evaluated under visible-light irradiation. The results demonstrated that Pd/g-C3N4 achieved a 97% removal efficiency, significantly outperforming pure g-C3N4, which reached only 57% removal. The degradation rate constant for Pd/g-C3N4 was calculated to be 0.0139 min−1, approximately 6.6 times higher than that of bare g-C3N4. This enhanced performance is attributed to the incorporation of Pd nanoparticles, which effectively suppressed the recombination of photogenerated electron–hole pairs and promoted charge separation. The influence of key operational parameters, including pH, SMX concentration, and catalyst dose, were systematically examined. Furthermore, the photocatalytic mechanism of the Pd/g-C3N4 photocatalyst was explored to elucidate its degradation pathways.
1. Introduction
The widespread occurrence of antibiotics in the environment has raised significant concerns regarding their potential impacts on ecosystems and human health [1]. These contaminates can be introduced into the environment through various pathways, such as agricultural runoff, effluents from wastewater treatment plants, aquaculture activities, and pharmaceutical production [2]. Once released, antibiotics can promote the development of antibiotic-resistant bacteria, adversely affect non-target organisms, and disturb ecological balance [2].
Sulfamethoxazole (SMX) is a widely used sulfonamide antibiotic that inhibits bacterial growth by disrupting folic acid synthesis, an essential process for bacterial survival [3]. To enhance its efficacy against a broader spectrum of bacterial infections, SMX is often administered in combination with trimethoprim [4]. However, like other antibiotics, SMX may induce adverse effects, including allergic reactions, gastrointestinal issues, and severe conditions such as Stevens–Johnson syndrome [5].
The effective removal of antibiotics from wastewater is essential to mitigating environmental pollution and preventing the spread of antibiotic-resistant bacteria. Conventional wastewater treatment plants often fail to effectively eliminate these pharmaceutical contaminants, necessitating the implementation of advanced treatment technologies [6]. Advanced Oxidation Processes (AOPs) are a class of water treatment techniques that employ powerful oxidative agents, typically hydroxyl radicals (•OH), to degrade or mineralize organic pollutants—including toxic compounds—into smaller, less harmful substances, or fully convert them into carbon dioxide and water [7]. Photocatalysis, in particular, has garnered significant attention due to its high degradation efficiency and cost-effectiveness in eliminating pharmaceutical pollutants [8].
Graphitic carbon nitride (g-C3N4) has emerged as a promising alternative to conventional semiconductor photocatalysts. Its unique electrical structure, visible-light responsiveness, and exceptional chemical stability have attracted notable attention [9]. Unlike TiO2, g-C3N4 effectively absorbs visible light, which constitutes as a substantial portion of solar radiation, due to its relatively narrow band gap (~2.7 eV) [10]. This enhanced light absorption improves its performance in solar-driven photocatalytic applications, such as the degradation of organic pollutants, water splitting for hydrogen production, and CO2 reduction for renewable fuel synthesis.
In addition to its visible-light responsiveness, g-C3N4 exhibits excellent chemical stability, low toxicity, and ease of synthesis, making it an attractive candidate for practical applications [11]. Ongoing research focuses on optimizing its structure, composition, and surface properties to improve its photocatalytic performance, stability, and selectivity, thereby expanding its applicability in environmental and energy-related fields [11,12]. However, the photocatalytic efficiency of g-C3N4 under visible-light irradiation is significantly hindered by the rapid recombination of photogenerated electron–hole pairs, which limits charge separation and lowers quantum efficiency. These short-lived charge carriers recombine before initiating redox reactions, leading to a reduced photocatalytic activity [13,14]. In addition, its restricted visible-light absorption further reduces light utilization. Therefore, suppressing charge recombination and enhancing charge transfer processes are crucial for improving the photocatalytic performance of g-C3N4.
To overcome these challenges, various strategies have been explored, including morphology control [12], noble metal or metal deposition [15,16], and heterojunction construction with other semiconductors [17,18]. Among these, noble metal deposition, particularly the incorporation of palladium (Pd) nanoparticles, has emerged as an effective approach to mitigating charge recombination and improving charge transport efficiency [19]. Noble metals such as Pd, Au, and Pt possess unique electronic properties that facilitate electron trapping, effectively preventing the rapid recombination of charge carriers [20]. Among them, Pd nanoparticles act as electron sinks by capturing photogenerated electrons from g-C3N4 and promoting their transfer to reaction sites, thereby enhancing redox reactions. Additionally, Pd can serve as a co-catalyst to improve the reaction kinetics of surface redox processes by lowering the activation energy and facilitating interfacial charge migration [19,21]. Previous studies have investigated Pd modification of g-C3N4 using strong reducing agents or photo-deposition methods, which can enhance charge separation but often face limitations such as poor nanoparticle dispersion and aggregation. Compared to these methods, microwave-assisted deposition provides a rapid, efficient, and scalable technique for uniformly integrating Pd nanoparticles onto the g-C3N4 surface.
This study presents a rapid and efficient microwave-assisted polyol method for synthesizing Pd/g-C3N4 photocatalysts in an ethylene glycol (EG) medium, aiming to enhance photocatalytic activity under visible-light irradiation. This method offers a straightforward and scalable approach to fabricating Pd/g-C3N4 with optimized structural and electronic properties for effective degradation of organic contaminants. The incorporated Pd nanoparticles play a crucial role in facilitating electron–hole separation by serving as charge trapping sites, thereby accelerating charge transfer and boosting overall photocatalytic efficiency. The photocatalytic performance of Pd/g-C3N4 was demonstrated through the degradation of sulfamethoxazole (SMX), achieving a 98% degradation rate within 390 min—6.6 times higher than that of pristine g-C3N4. This synthesis strategy highlights strong potential for large-scale environmental remediation as a cost-effective, scalable, and high-performance solution for water pollution and organic pollutant degradation.
2. Results and Discussion
2.1. Characterization
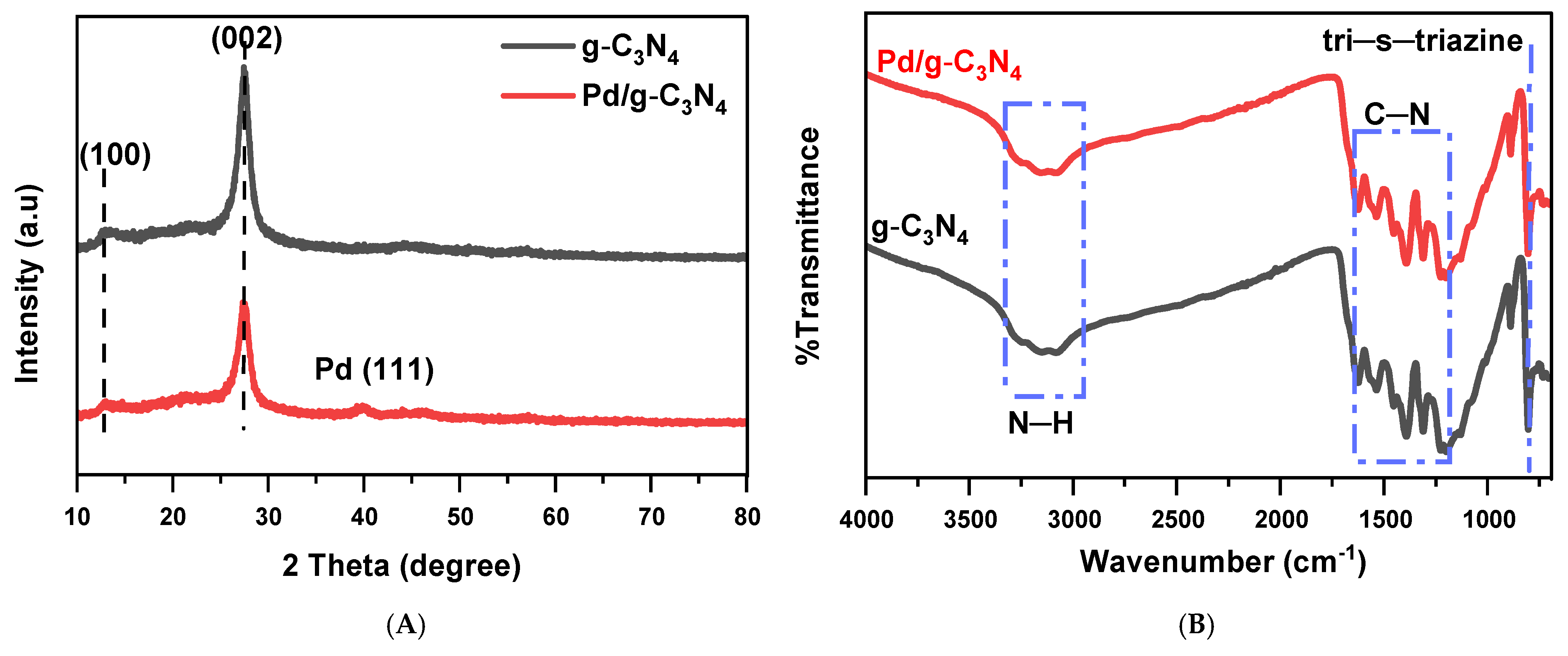
The XRD patterns of g-C3N4 and Pd/g-C3N4 are shown in Figure 1A, where g-C3N4 exhibits two distinct peaks. The peak at 13.10° corresponds to the interlayer structural packing of tri-s-triazine units, while the peak at 27.39° is associated with the interlayer stacking of the conjugated aromatic system (JCPDS 87-1526), reflecting the crystalline structure and arrangement of the material [22]. Upon loading Pd nanoparticles (NPs) onto g-C3N4 nanosheets, a notable change in the XRD pattern is observed. A weak diffraction peak appears at 40.16° (JCPDS 46-1043), corresponding to the (111) plane of Pd, confirming the successful incorporation of Pd NPs [23]. The low intensity of this peak suggests that the Pd NPs are either small in size or present in low abundance relative to the g-C3N4 matrix. Additionally, a reduction in the intensity of the g-C3N4 peaks is observed after Pd NP deposition. Although the Pd nanoparticles are too small to produce a distinct diffraction peak, their interaction with the g-C3N4 matrix can still lead to a reduction in the intensity of the g-C3N4 peak due to induced structural disorder, decreased crystallinity, or X-ray absorption effects.
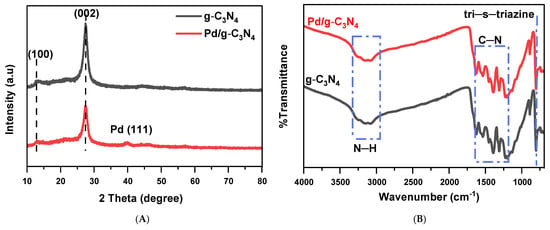
Figure 1.
XRD spectra of two samples (A) and FTIR spectra of two samples (B).
To further investigate the structural characteristics of the photocatalyst, FT-IR spectroscopy was conducted over the wavenumber range of 700–4000 cm−1 (Figure 1B). The FT-IR spectra of g-C3N4 and Pd/g-C3N4 exhibit no significant differences, indicating that Pd deposition does not alter the chemical structure of g-C3N4. The broad absorption peaks observed between 3000 and 3500 cm−1 correspond to N–H stretching vibrations, which may arise from the incomplete polymerization of melem [24] or from hydrogenation of nitrogen atoms during the thermal polymerization process [25]. Peaks in the range of 1200–1600 cm−1 are characteristic of aromatic C–N heterocyclic units [26], while the distinct peak around 806 cm−1 is attributed to the tri-s-triazine units, in agreement with the XRD analysis [13]. Following Pd loading, there are no additional peaks, indicating that the Pd component is present in a low concentration and does not significantly affect the intrinsic chemical and crystalline structure of g-C3N4. These results confirm that Pd deposition preserves the structural integrity of g-C3N4, keeping its chemical framework intact.
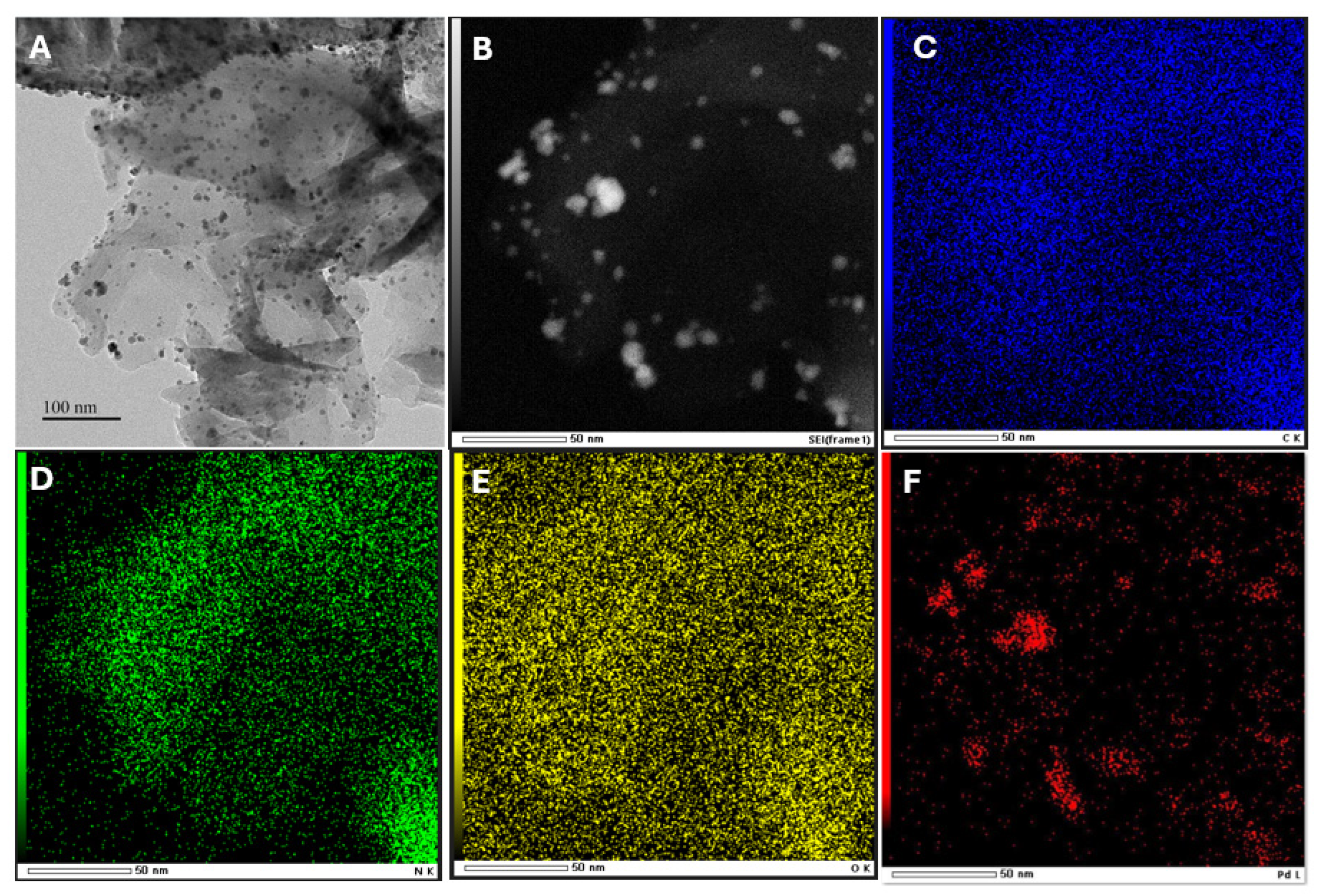
TEM was used to investigate the morphology of Pd/g-C3N4. As shown in Figure 2A, the g-C3N4 nanosheets appear as thin layers, rendering them transparent to electron beams. Additionally, the presence of nanoparticles suggests the successful incorporation of Pd on to the g-C3N4 surface. The elemental composition of the photocatalyst was further confirmed via TEM-EDS mapping (Figure 2C–F), which verified the coexistence of C, N, O, and Pd. These findings confirm that Pd NPs were successfully decorated on the g-C3N4 surface. Based on statistical analysis of TEM images, the median particle size of the Pd nanoparticles was determined to be approximately 5.7 nm (Figure S1). This value was obtained by measuring over 100 nanoparticles across multiple regions of the sample to ensure a representative and reliable size distribution. The small particle size confirms the high dispersion of Pd nanoparticles on the g-C3N4 support and is consistent with the weak Pd nanoparticle diffraction peak observed in the XRD pattern.
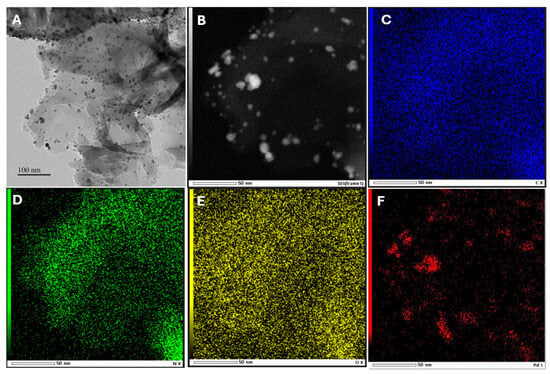
Figure 2.
TEM image of Pd/g-C3N4 (A), STEM of Pd/g-C3N4 (B), and the corresponding EDS element mapping of carbon (C), nitro (D), oxygen (E), palladium (F).
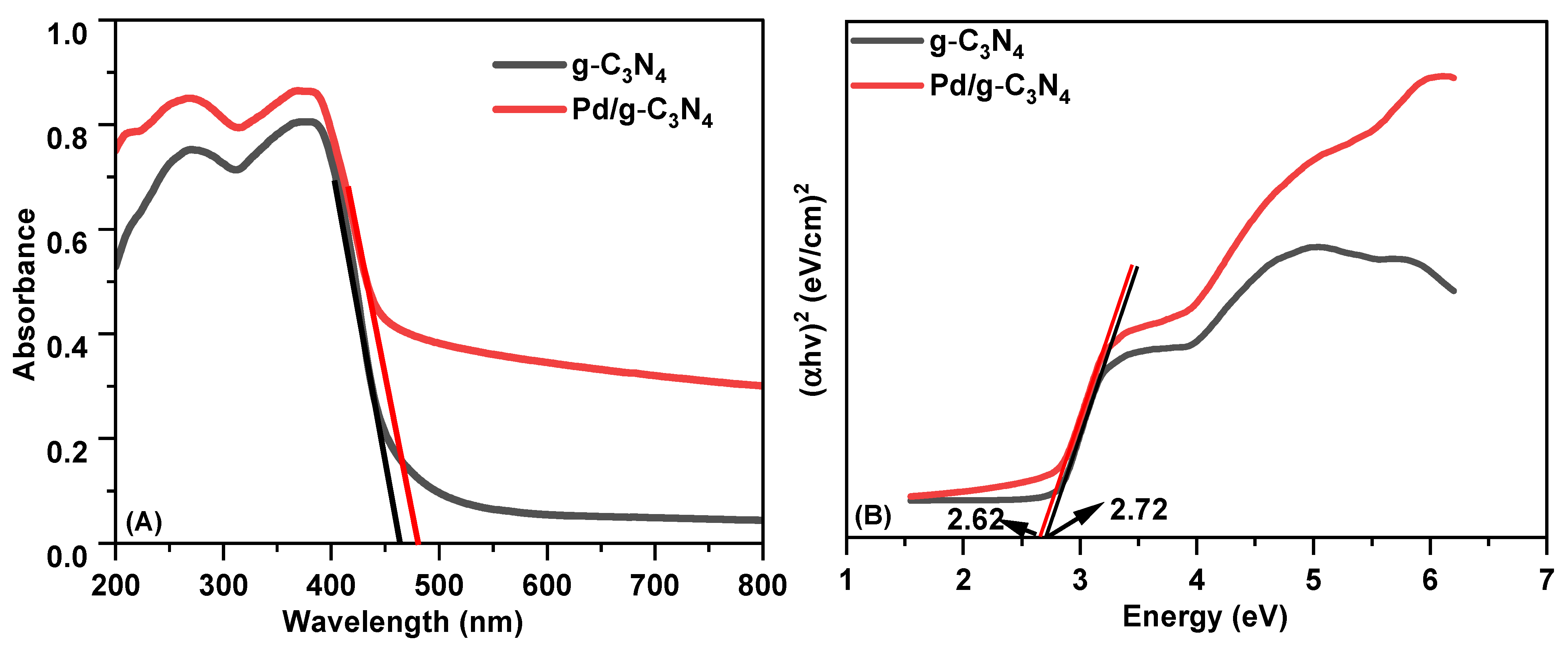
The visible-light absorption properties of the g-C3N4 and Pd/g-C3N4 samples were evaluated by UV-Vis DRS analysis. As shown in Figure 3A, both materials exhibit absorption extending from the UV region into the visible range, with distinct absorption characteristics. The band gap energy (Eg) of the photocatalysts can be determined from their absorption spectra using the Tauc equation:
where A is a proportionality constant, α is the optical absorption coefficient, hν represents the photon energy, and Eg denotes the band gap energy. The exponent n depends on the nature of the optical transition in the semiconductor: for direct band gap transitions, n = 1, while for indirect transitions, n = 4 [14]. Since g-C3N4 is an indirect semiconductor, n is set to 4 in the Tauc equation. The optical band gaps of the synthesized materials were determined from the Tauc plots, as shown in Figure 3B [27]. The Eg values for the g-C3N4 and Pd/g-C3N4 photocatalysts were found to be 2.62 and 2. 72 eV, respectively. The incorporation of Pd nanoparticles did not cause a significant shift in the ban gap compared to pristine g-C3N4.
αhv = A (hν − Eg)n/2
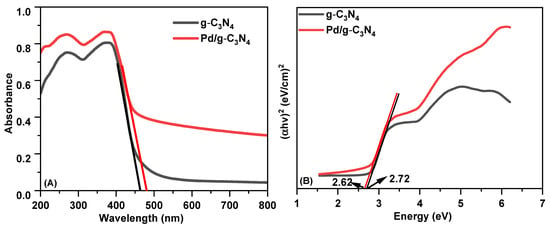
Figure 3.
(A) UV–Vis DRs spectra of two samples; (B) estimated bandgap of two samples.
Figure S2 illustrates the N2 adsorption–desorption isotherms and pore width distribution of the catalysts. The BET surface areas of pure g-C3N4 and Pd/g-C3N4 were measured to be about 77 and 64 m2 g−1, respectively. The introduction of Pd NPs in g-C3N4 did not significantly change the textural properties of the g-C3N4, which can be attributed to the small amount and nanoscale size of the Pd content.
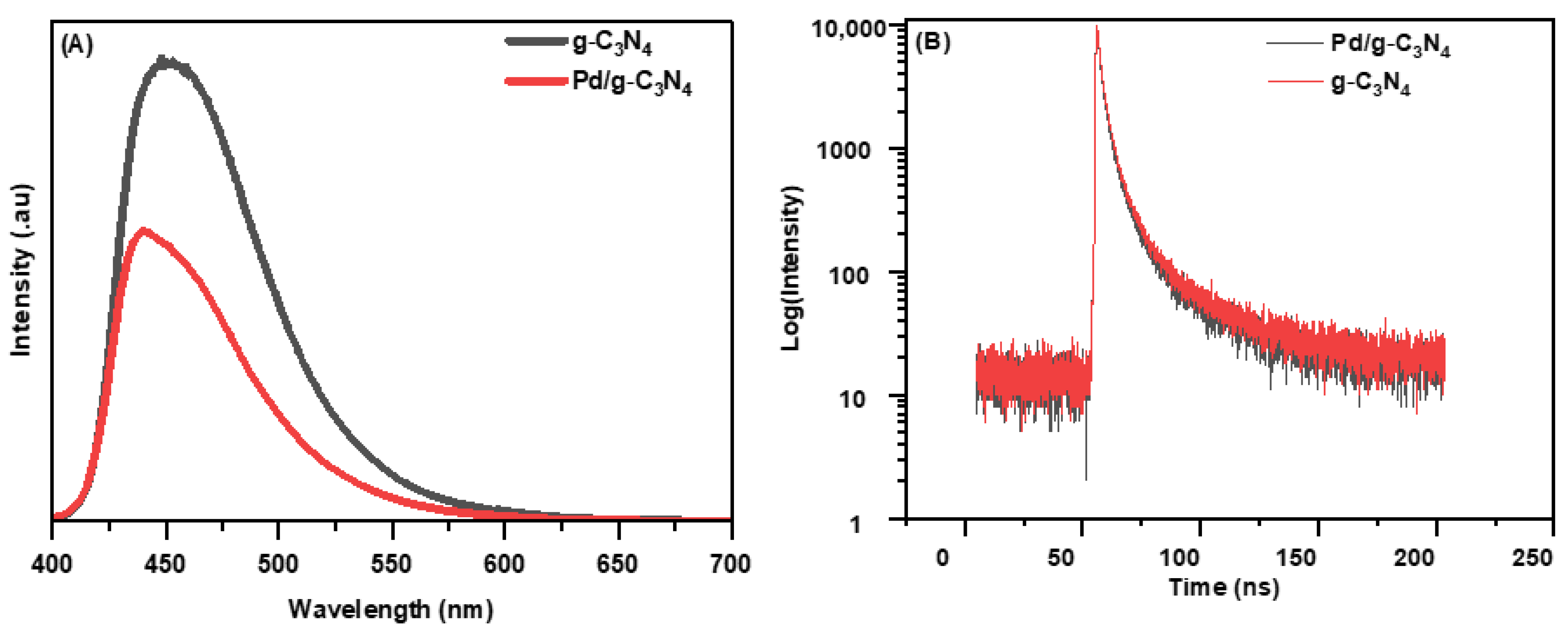
The recombination behavior of photogenerated electron–hole pairs was also examined by analyzing the fluorescence intensities of the semiconductor materials. In general, a higher fluorescence intensity indicates a faster recombination rate of electron–hole pairs [28]. The fluorescence intensity of bare g-C3N4 was higher than that of Pd/g-C3N4, as shown in Figure 4A. With the incorporation of Pd NPs, the fluorescence intensity of the nanocomposite significantly decreases. The lower fluorescence intensity observed in Pd/g-C3N4 suggests an effective charge transfer between g-C3N4 and Pd NPs, thereby successfully suppressing the recombination of photogenerated electron–hole pairs [29].
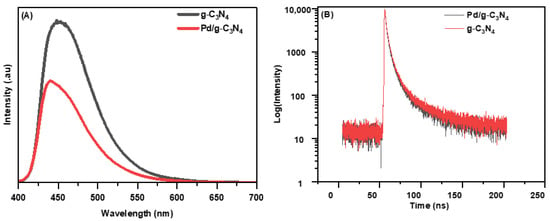
Figure 4.
(A) PL steady spectra and (B) TRPL spectra of two samples.
TRPL spectroscopy was utilized to investigate whether the addition of Pd NPs improves charge transport while decreasing the recombination rate (Figure 4B). Excitation and emission wavelengths of 370 nm and 450 nm, respectively, were used to obtain the TRPL spectra. The charge carrier lifetimes and fitting parameters for each TRPL decay spectrum (Figure S3) were determined using a triexponential function, with the detailed values provided in Table S1. The average lifetime (τav) of charge carriers was calculated using the following equation:
where τ1, τ2, and τ3 represent the time constants, and A1, A2, and A3 are the corresponding pre-exponential factors.
The component τ1 corresponds to rapid radiative recombination at g-C3N4 band edge states, while τ2, and τ3 are associated with charge carrier relaxation in shallow and deep trap states, respectively. The average charge carrier lifetime for Pd/g-C3N4 was determined to be 2.63 ns, compared to 2.97 ns for pristine g-C3N4. Notably, Pd/g-C3N4 exhibited a shorter τav than pure g-C3N4, indicating enhanced charge separation. This reduction in lifetime is attributed to the transfer of photoinduced electrons from the surface of g-C3N4 to the Pd NPs, facilitating a nonradiative decay mechanism. The electron transfer process effectively suppresses the rapid recombination of electron–hole pairs, thereby improving the photocatalytic efficiency of the Pd/g-C3N4 composite [30].
In the Nyquist plot, a smaller semicircle radius indicates a lower charge transfer resistance. As shown in Figure S4, the radius follows the order Pd/g-C3N4 > g-C3N4, suggesting that Pd/g-C3N4 exhibits reduced impedance. This outcome confirms the enhanced charge transfer efficiency at the interface between the Pd° nanoparticles and g-C3N4 matrix, facilitating the more effective charge carrier transport. The efficient electron transport from g-C3N4 to Pd° NPs promotes charge separation and reduces recombination, thereby contributing to improved photocatalytic performance.
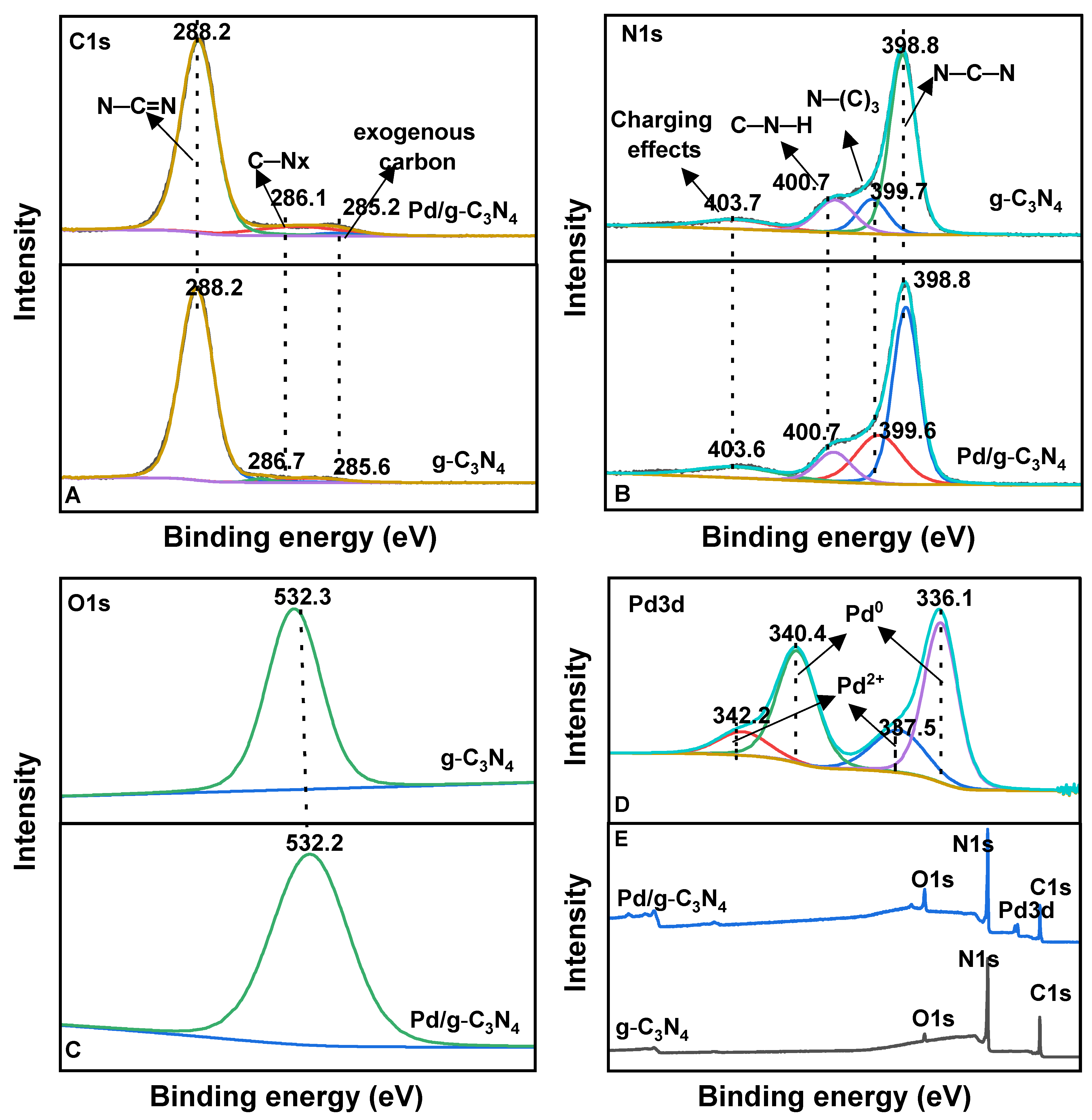
XPS was performed to examine the surface chemical properties and elemental states of the Pd/g-C3N4 and g-C3N4 samples. The survey spectrum (Figure 5E) confirms the presence of C, N, O, and Pd, which is consistent with the XRD findings. The core-level XPS spectra for C 1s, N 1s, O 1s, and Pd 3d are displayed in Figure 5A–D. The C 1s spectrum of g-C3N4 (Figure 5A) is deconvoluted into three peaks corresponding to sp2-hybridized N–C=N, C–NHx, and exogenous carbon [31]. The N 1s spectrum (Figure 5B) exhibits four separate peaks: the pyridinic-like nitrogen (N–C–N) peak at 398 eV, tertiary nitrogen (N–(C)3) at 399.7 eV [32], amino groups (C–N–H) at 400.7 eV, and charging effects at 403.6 eV [33]. The Pd 3d spectrum (Figure 5D) reveals the presence of two Pd species, showing two doublet peaks corresponding to the spin–orbital splitting of Pd 3d5/2 and Pd 3d3/2. The dominant peaks at 336.1 eV and 340.4 eV were attributed to metallic Pd0, while the weaker peaks at 337.5 eV and 342.3 eV were assigned to Pd2⁺, likely resulting from Pd–O or Pd–N bond formation on the catalytic surface [34]. These results show that the amine groups in the g-C3N4 support play a crucial role in stabilizing highly dispersed Pd0 species, thereby partially preventing their oxidation. The atomic concentration (%) was determined by integrating the Pd 3d peaks and normalizing them using the appropriate sensitivity factors (Table S2). The calculated atomic percentage of Pd is approximately 1%, which is consistent with the expected Pd loading.
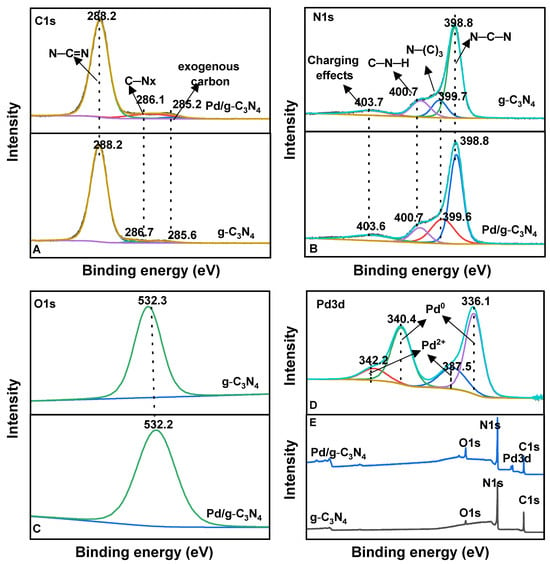
Figure 5.
The core-level spectra of C 1s (A), N 1s (B), O 1s (C), and Pd 3d (D), and XPS survey (E) for two samples.
2.2. Photocatalytic Activity Test
To ensure the adsorption equilibrium was reached during the photocatalytic testing, the catalyst and SMX solution were magnetically stirred in the dark for 60 min before initiating light irradiation. After this dark equilibration period, both samples exhibited a removal efficiency of about 10%. Extending the adsorption period to 390 min did not result in a significant improvement in SMX removal effectiveness, showing that physical adsorption played a minimal role in the overall removal process.
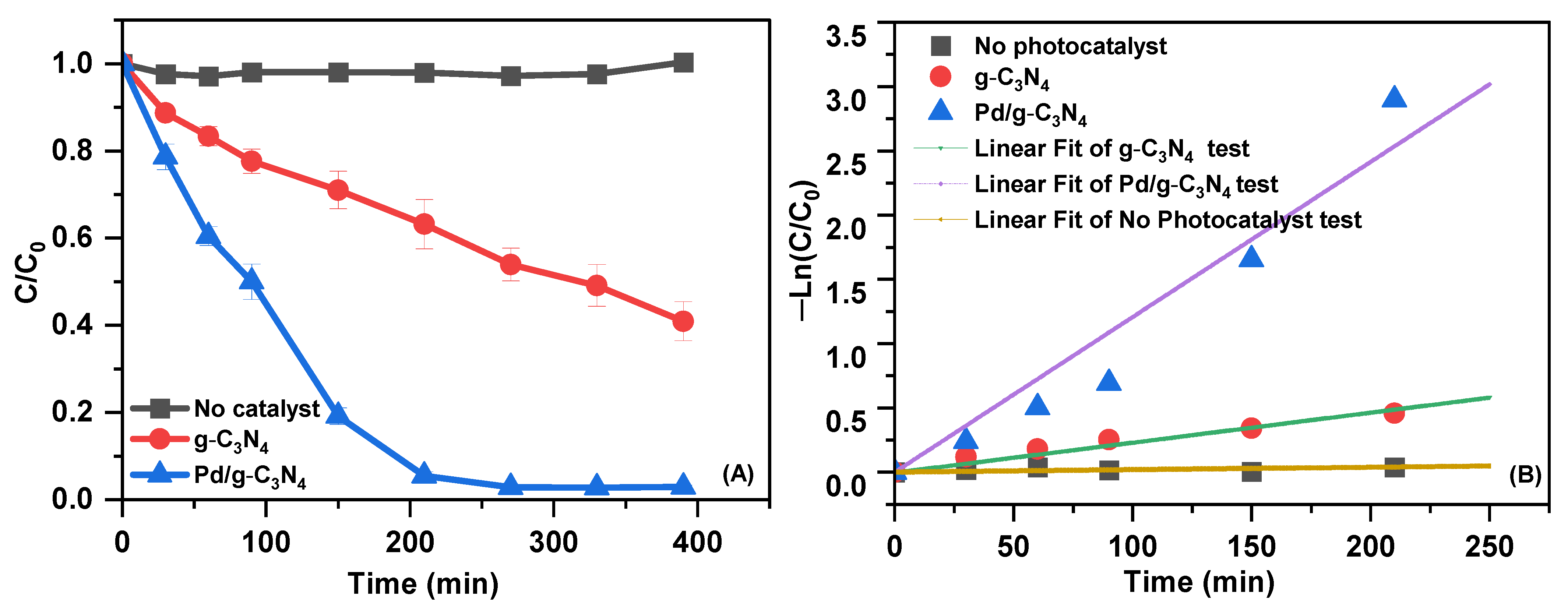
The photocatalytic efficiency of Pd/g-C3N4 was assessed by evaluating the degradation of SMX under visible-light irradiation (Figure 6A). The results reveal that Pd/g-C3N4 demonstrates significantly enhanced photocatalytic activity compared to g-C3N4. SMX degradation in the absence of a photocatalyst was negligible, underscoring the critical role of Pd/g-C3N4 in facilitating the photocatalytic reaction under visible-light irradiation. After 390 min of irradiation, the degradation efficiency reached approximately 59% for g-C3N4 and 97% for Pd/g-C3N4, confirming the superior photocatalytic performance of Pd/g-C3N4. First-order kinetic analysis of SMX degradation (Figure 6B) further supports these findings. The degradation rate constant was determined to be 0.0139 min−1 for Pd/g-C3N4, which is about 6.6 times higher than that of g-C3N4. Table S3 shows a comparison of the photocatalytic degradation of antibiotics over the g-C3N4-based photocatalyst in this study with those reported in the previous literature. The degradation efficiency of Pd/g-C3N4 synthesized via the microwave-assisted method was found to be comparable to or higher than that of previously reported systems, indicating its potential as a promising photocatalyst for the elimination of organic pollutants.
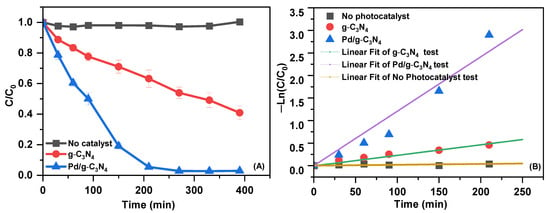
Figure 6.
The degradation rate of SMX (A) and the first-order kinetic curves using as-prepared samples under visible-light irradiation (B).
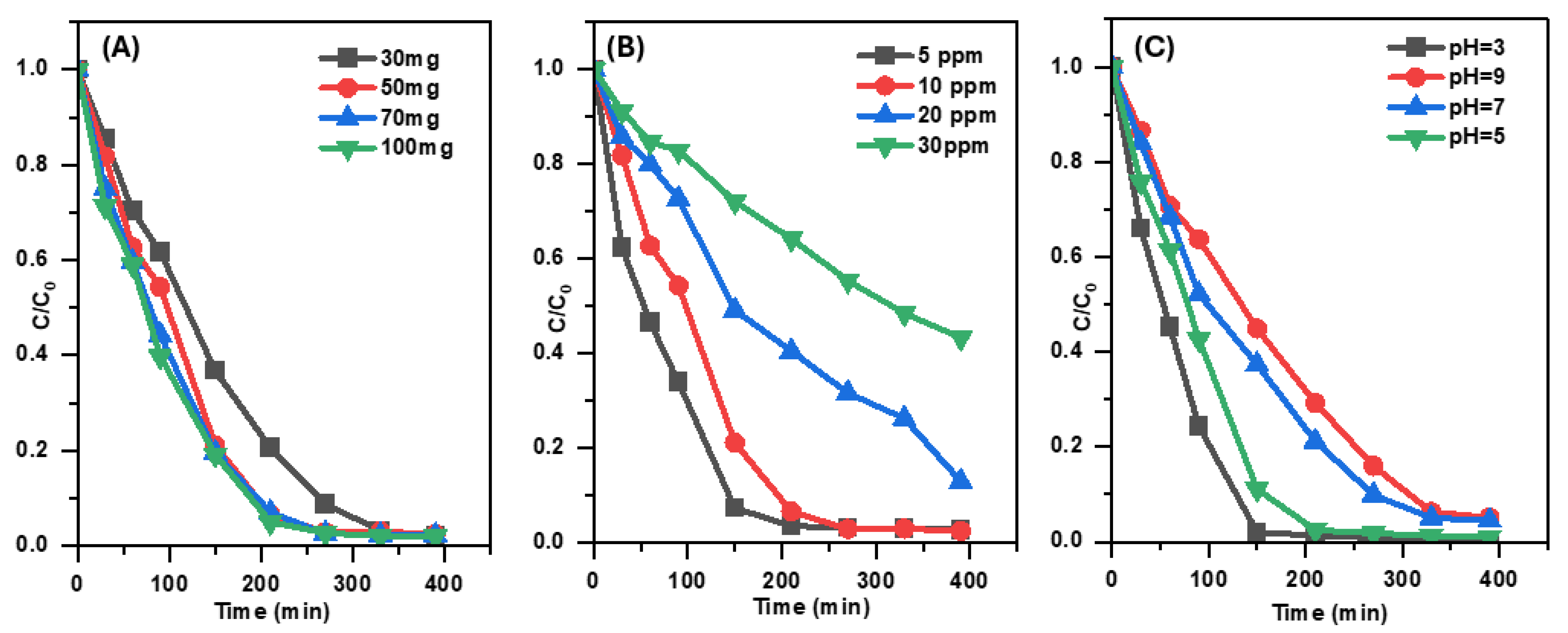
The effect of Pd/g-C3N4 dosage on SMX removal was investigated by varying the catalyst dosage from 0.3 to 1 g L−1 over a 390 min photocatalytic process (Figure 7A). The results indicate that the degradation efficiency of SMX reached approximately 99% when the catalyst dosage was increased to 0.9 g L−1. An increase in catalyst dosage enhanced the number of available photon absorption sites, leading to a corresponding rise in the SMX photodegradation rate. This finding suggests that a higher catalyst dosage facilitates greater photon absorption, thereby improving the overall photocatalytic performance.
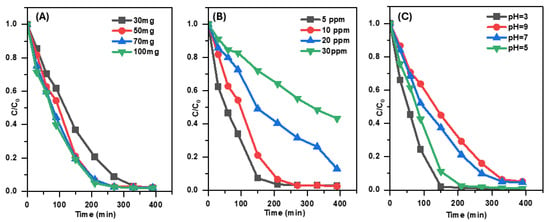
Figure 7.
The impact of catalyst dosage (A), the impact of initial antibiotic concentration (B), and the impact of pH (C) on the removal efficiency of SMX under visible-light irradiation.
The effect of initial SMX concentration on its photocatalytic degradation by Pd/g-C3N4 was investigated at four different concentrations from 5 to 30 mg L−1, while maintaining a constant catalyst dose of 0.5 g L−1 (Figure 7B). The results reveal a clear correlation between the initial SMX concentration and photocatalytic degradation efficiency. After 390 min of visible-light irradiation, the degradation efficiencies for SMX concentrations of 5, 10, 20, and 30 mg L−1 were 97.05%, 96.69%, 87.72%, and 56.62%, respectively. At lower concentrations (5 mg L−1), the catalytic surface effectively absorbed SMX molecules, facilitating the generation of sufficient superoxide radicals (•O2−) to efficiently degrade the antibiotic. However, as the SMX concentration increased, the higher molecular density in the solution partially obstructed light penetration, limiting the photocatalytic reaction. This reduction in light availability led to decreased generation of superoxide radicals and electron–hole pairs, ultimately lowering the overall photocatalytic degradation efficiency. This effect was particularly evident at higher SMX concentrations (20 and 30 mg L−1), where the degradation rate declined. Thus, optimal degradation efficiency is achieved at lower SMX concentrations, as enhanced light absorption and effective radical generation contribute to improved photocatalytic performance.
To assess the effect of pH on SMX degradation, the pH of the antibiotic solution was varied while maintaining a constant catalyst dosage of 0.5 g L−1 and an SMX concentration of 10 mg L−1. The pH was adjusted to 3, 5, 7, and 9, with additional unadjusted control. The degradation efficiencies corresponding to each pH level are presented in Figure 7C. The degradation efficiency followed the order pH 3 > pH 5 > pH 7 > pH 9. These results indicate that an acidic environment (pH 3) promotes the highest photocatalytic degradation efficiency, whereas increasing alkalinity (pH 9) leads to a reduction in SMX removal.
The reusability of a catalyst is a crucial factor in evaluating its effectiveness for practical applications. In our study, the reusability of Pd/g-C3N4 was tested over four consecutive cycles. After each experiment, the catalyst was recovered using a high-speed refrigerated centrifuge and thoroughly washed with ethanol and distilled water before reuse. The results (Figure S5) indicate SMX removal efficiencies of 97%, 94%, 89%, and 88% across the four cycles. These findings suggest that Pd/g-C3N4 retains high photocatalytic activity, with only a slight decrease in performance. This minor decline is likely due to partial catalyst loss during recovery or surface deactivation caused by the accumulation of reaction by-products. Overall, the results demonstrate the good reusability and long-term stability of Pd/g-C3N4, confirming its potential for practical photocatalytic applications.
A leaching test was conducted to investigate the potential leaching of Pd from Pd/g-C3N4 during the photocatalytic degradation of SMX (Figure S6). In this test, 60 min of adsorption and 60 min of photocatalysis, the Pd/g-C3N4 solid was separated from the reaction solution via centrifugation. The remaining SMX solution was subsequently continuously stirred under visible-light irradiation, and its concentration was measured at various time intervals using the same photocatalytic test method. The results revealed that the SMX concentration remained nearly constant after the catalyst was removed, indicating no significant change in the degradation rate. This outcome indicates that any potential leached components from the catalyst did not contribute to SMX removal. Thus, these findings confirm that the photocatalytic degradation of SMX is primarily driven by reactive species generated on the Pd/g-C3N4 catalyst surface.
To gain a deeper understanding of the SMX photodegradation mechanism over Pd/g-C3N4, trapping tests were conducted using various additives, including IPA, BQ, and AO (Figure S7). The addition of IPA led to a slight decrease in the SMX removal percentage, while the addition of AO and BQ resulted in a more pronounced reduction. These findings suggest that •O2− and h⁺ radicals play a dominant role in the photocatalytic degradation of SMX over Pd/g-C3N4.
Based on the data and analysis provided above, a possible pathway for SMX degradation under visible-light exposure using Pd/g-C3N4 was proposed. During the photocatalytic reaction, visible light excites electrons from the valence band to the conduction band of g-C3N4, generating electron–hole pairs. These charge carriers actively participate in oxidation and reduction reactions, where •O2− and h⁺ interact with SMX molecules, breaking them down into non-toxic byproducts such as H2O and CO2. This proposed degradation mechanism demonstrates the effectiveness of Pd/g-C3N4 as a photocatalyst for SMX elimination under visible-light irradiation.
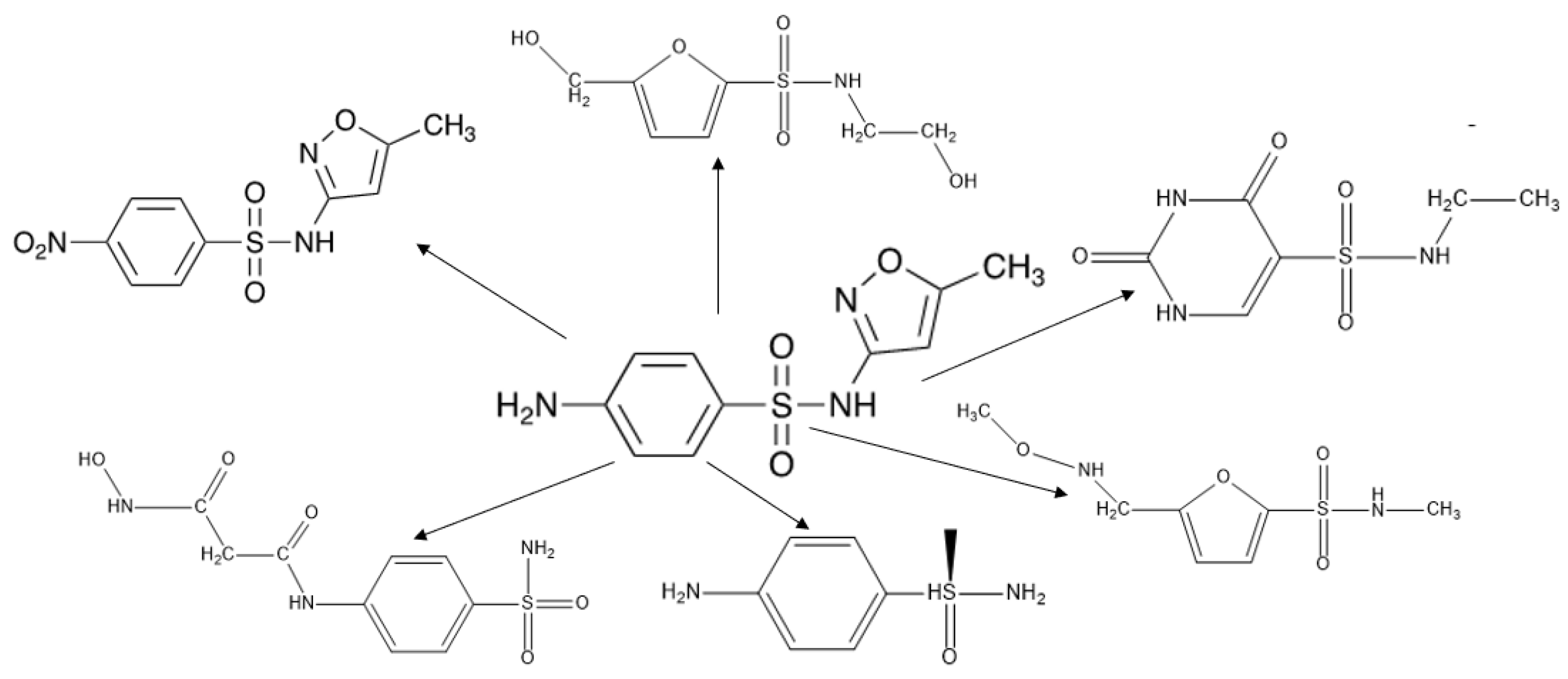
The primary intermediates formed during the SMX degradation process were identified via Q-TOF LC-MS/MS analysis (Figure S8A–C, Table S4). In the positive mode, SMX represented a characteristic peak with a retention time (tR) of 11.4 min and a m/z value of 254. Over the course of 390 min, the SMX (m/z 254) signal gradually decreased and nearly disappeared, confirming the successful degradation of SMX over the photocatalytic reaction (Figure S6). Several primary degradation intermediates were detected during the photocatalytic reaction, including m/z 274 (P1), m/z 284 (P2), m/z 221 (P3), m/z 220 (P4), m/z 219 (P5), and m/z 170 (P6) (Table S5). The detection of these intermediates provides insight into the degradation pathways of SMX, which were further investigated based on their formation and transformation during the reaction (Figure 8).
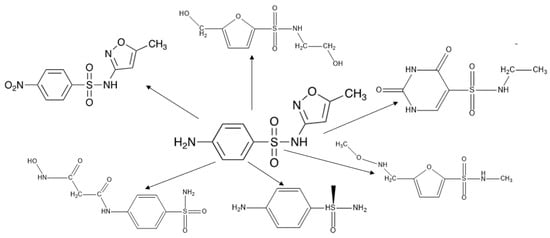
Figure 8.
The proposed degradation pathway of SMX by Pd/g-C3N4 under visible light.
During SMX photocatalytic degradation, the intermediate P1 (m/z = 274) is likely formed through hydroxylation or oxidative alteration of the parent molecule. One possible pathway involves the electrophilic attack of hydroxyl radicals (•OH) on the aromatic ring or other reactive sites of SMX (m/z = 254), leading to the incorporation of an additional hydroxyl (–OH) group. This hydroxylation increases the molecular weight by +16 Da, resulting in an intermediate with m/z = 270 [30]. Subsequent oxidation or hydration reactions, such as the addition of another hydroxyl group or oxidative modification of the amine or sulfonyl moiety, may lead to the formation of m/z = 274.
P2 (m/z = 284, tR = 17.6 min) is generated when the –NH2 group on the benzene ring is converted into a –NO2 group during SMX degradation. After 150 min of photocatalytic degradation, the detection of intermediates with m/z values of 220, 221, and 219 (P3, P4, and P5) suggest key transformation processes, including de-sulfonation, oxidation, and hydroxylation. The cleavage of the S–N bond in SMX (m/z = 254) results in the removal of the sulfonyl group, leading to the formation of an intermediate with m/z = 221. Further oxidation and hydroxylation, facilitated by reactive oxygen species, produce intermediates with m/z = 220 and 219. These results highlight the multi-step nature of SMX degradation, involving a series of chemical transformations that progressively break down the molecule into smaller, less harmful intermediates. The formation of the intermediate with m/z = 170 suggests a significant structural breakdown, likely involving de-sulfonation, oxidation, and cleavage of the aromatic system [35]. A possible pathway begins with the cleavage of the S–N bond in SMX (m/z = 254), leading to the removal of the sulfonyl (-SO2) group and forming intermediates with lower molecular weights. Further oxidation and hydroxylation reactions can modify the remaining structure, resulting in the production of m/z = 190 and 189. Further degradation through ring-opening reactions and decarboxylation processes ultimately generates a compound with m/z = 170. These transformations indicate the progressive breakdown of SMX into smaller organic molecules, which subsequently undergo complete mineralization into CO2, H2O, and inorganic ions.
3. Materials and Methods
3.1. Materials
Melamine (C3H6N6, 99.5%) and ammonium chloride (NH4Cl, 99.5%) were obtained from Junsei Chemical Co, Ltd (Tokyo, Japan). Palladium chloride (PdCl2, 96%) was purchased from Sigma-Adrich(St. Louis, MO, USA). Ethylene glycol (C2H6O2, 99%) and ethyl alcohol (C2H5OH, 70%) were obtained from Dae-Jung Chemicals & Metals Co, Ltd. (Gyeonggi-do, Republic of Korea).
3.2. Synthesis of g-C3N4
Based on our findings, g-C3N4 nanosheets were produced using a one-step thermal treatment [36]. Initially, equal amounts of melamine (5 g) and ammonium chloride (5 g) were thoroughly mixed using a mortar. The mixture was then transferred to a covered aluminum crucible and heated in a furnace at 550 °C for three hours. Once cooled to room temperature, the resulting material was finely ground into a powder, repeatedly washed with distilled water to remove any residual impurities, and then dried in a vacuum oven at 90 °C for 12 h.
3.3. Synthesis of Pd/g-C3N4
Pd/g-C3N4 was synthesized via a microwave-assisted wet chemical reduction method. Initially, 1 g of g-C3N4 nanosheets was dispersed in 50 mL of ethylene glycol (EG) and sonicated for 30 min to ensure uniform dispersion. Subsequently, the Pd precursor (PdCl2) was added to the g-C3N4/EG mixture, followed by continuous stirring for 30 min. The Pd loading was maintained at 1 wt%. The resulting suspension was transferred to a Teflon vessel and subjected to microwave irradiation at 500 W and 145 °C for 10 min. After the reaction, the system was allowed to cool naturally to room temperature. The resulting product was collected by centrifugation, thoroughly washed multiple times with deionized (DI) water to remove any residual precursors, and then dried in a vacuum oven at 90 °C for 12 h to obtain the final Pd/g-C3N4 composite.
The photocatalytic performance of the synthesized samples was assessed by degrading SMX (10 mg L−1) under visible-light illumination, utilizing a 300 W Xe-arc lamp equipped with a 400 nm cut-off filter. Prior to irradiation, the catalyst (0.5 g L−1) was dispersed in the SMX solution and stirred in the dark for 1 h with a magnetic stirrer to reach adsorption–desorption equilibrium. Following the initiation of the light irradiation, 1.5 mL of the solution was collected at predefined time intervals (0, 30, 60, 90, 150, 210, 270, 330, and 390 min). The collected samples were then filtered to eliminate solid particles, and the concentration of SMX was determined using a high-performance liquid chromatograph (HPLC, Shimadzu, CDD-10AVP model) equipped with a UV–vis detector (SPD-20A). Separation was performed using a Shim-pack GIS 5 μm-C18 column (4.6 × 250 mm) maintained at 30 °C. SMX detection was conducted at a wavelength of 267 nm. The mobile phase consisted of 90% methanol (v/v) and 10% water (v/v), with a flow rate of 1.0 mL/min. SMX exhibited a retention time of 10 min, with an injection volume of 10 μL.
To study the role of charge carriers in the photodegradation of SMX, ammonium oxalate monohydrate (AO, 10−2 mol L−1, Junsei, 99%), p-benzoquinone (BQ, 3 × 10−2 mol L−1, Sigma-Aldrich, reagent grade, ≥98%), and isopropyl alcohol (IPA, 2 × 10−1 mol L−1, Sigma-Aldrich, anhydrous, ≥99.5%) were employed as scavengers for holes (h+), (•O2−), and hydroxyl radicals (•OH), respectively. The experimental conditions and processes were conducted following the aforementioned method, with the exception that the scavengers were added to the catalyst–antibiotic suspension prior to irradiation.
Photocatalytic reaction intermediates were analyzed using an Ultra High-Resolution Q-TOF LC-MS/MS system (Bruker, maXis-HD). Separation was performed using a WATERS BEH C18 column (2.1 mm × 100 mm, 1.7 µm) at a flow rate of 0.2 mL min−1, with an injection volume of 20 µL. The mobile phase consisted of water (A) and 10 mM ammonium acetate in methanol (B), both containing 0.1% formic acid. Gradient elution conditions are detailed in Table S2. The mass spectrometry (MS) system was operated in positive electrospray ionization (ESI) mode, scanning a mass range from m/z 50 to m/z 1000 to capture full-scan mass spectra.
4. Conclusions
This study demonstrates the successful development of Pd-decorated g-C3N4 via microwave-assisted synthesis, providing a rapid and efficient approach to fabricating high-performance photocatalysts for SMX degradation. The incorporation of Pd NPs facilitates charge separation and suppresses electron–hole recombination, thereby significantly improving photocatalytic activity. Compared to bare g-C3N4, Pd/g-C3N4 achieved a 97% SMX removal efficiency, with a degradation rate constant 6.6 times higher. This study also confirmed that increasing the catalyst dosage improves degradation efficiency, while acidic conditions (pH 3) provide optimal performance. These findings emphasize the strong potential of Pd/g-C3N4 for real-world water treatment scenarios, particularly in the removal of pharmaceutical contaminants like SMX. After five consecutive cycles, only a slight decrease in degradation efficiency was observed, likely due to the physical loss of a small fraction of catalyst during each run. To further improve the reusability of the catalyst, future work may focus on enhancing the structural integrity of the catalyst–such as improving Pd particle anchoring or exploring protective coatings on a suitable substrate–to minimize catalyst loss and maintain high performance during extended use in practical water treatment systems.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/inorganics13040118/s1. Figure S1: Particle size from TEM image and fit distribution curve, Figure S2: N2 adsorption–desorption isotherms of g-C3N4 (A), Pd/g-C3N4 (B), and the pore-size distribution curves of g-C3N4 (C), Pd/g-C3N4 (D). Figure S3: TRPL emission decay curves recorded for Pd/g-C3N4 and g-C3N4. τ is the lifetime calculated by fitting to a bi-exponential decay function. Figure S4: EIS Nyquist plots of g-C3N4 and Pd/g-C3N4. Figure S5: Photocatalytic SMX removal rates over Pd/g-C3N4 composites after four reaction cycles. Figure S6: Leaching test using Pd/g-C3N4. Figure S7: Effects of different scavengers on the photocatalytic degradation of sulfamethoxazole over Pd/g-C3N4 catalysts under visible light irradiation. Figure S8A: Total ion chromatogram of SMX and extracted ion chromatograms for intermediates during photocatalytic degradation reaction at different time intervals (0 min). Figure S8B: Total ion chromatogram of SMX and extracted ion chromatograms for intermediates during photocatalytic degradation reaction at different time intervals (150 min). Figure S8C: Total ion chromatogram of SMX and extracted ion chromatograms for intermediates during photocatalytic degradation reaction at different time intervals (390 min). Figure S9: Area evolution of SMZ and intermediates (in positive mode). Table S1: Results of TRPL data of Pd/g-C3N4 and g-C3N4. Table S2: Atomic Percentage (at.%) from XPS. Table S3: Comparison of photocatalytic activity of g-C3N4 modified with metals for the degradation of antibiotics. Table S4: The gradient elution. Table S5: Mass spectrometry pieces information and proposed structure for SMX. References [36,37,38,39,40,41,42] are cited in the supplementary materials.
Author Contributions
Conceptualization, L.-A.T.H.; methodology, L.-A.T.H., T.D.N. and T.L.; validation, L.-A.T.H.; formal analysis, T.D.N. and T.L.; data curation, L.-A.T.H.; writing—original draft, L.-A.T.H.; writing—review and editing, T.D.N. and T.L.; supervision, T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a research grant of Pukyong National University (2021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Georgia Ștefan, M.; Loghin, F. Antibiotics in the Environment: Causes and Consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Al Masud, M.A.; Shin, W.S.; Septian, A.; Samaraweera, H.; Khan, I.J.; Mohamed, M.M.; Billah, M.M.; López-Maldonado, E.A.; Rahman, M.M.; Islam, A.R.M.T.; et al. Exploring the Environmental Pathways and Challenges of Fluoroquinolone Antibiotics: A State-of-the-Art Review. Sci. Total Environ. 2024, 926, 171944. [Google Scholar] [CrossRef] [PubMed]
- Lade, H.; Kim, J.S. Bacterial Targets of Antibiotics in Methicillin-Resistant Staphylococcus aureus. Antibiotics 2021, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Minato, Y.; Dawadi, S.; Kordus, S.L.; Sivanandam, A.; Aldrich, C.C.; Baughn, A.D. Mutual Potentiation Drives Synergy between Trimethoprim and Sulfamethoxazole. Nat. Commun. 2018, 9, 1003. [Google Scholar] [CrossRef]
- Kocak, Z.; Hatipoglu, C.A.; Ertem, G.; Kinikli, S.; Tufan, A.; Irmak, H.; Demiroz, A.P. Trimethoprim-Sulfamethoxazole Induced Rash and Fatal Hematologic Disorders. J. Infect. 2006, 52, e49–e52. [Google Scholar] [CrossRef]
- Ganthavee, V.; Trzcinski, A.P. Removal of Pharmaceutically Active Compounds from Wastewater Using Adsorption Coupled with Electrochemical Oxidation Technology: A Critical Review. J. Ind. Eng. Chem. 2023, 126, 20–35. [Google Scholar] [CrossRef]
- Jamil, T. Role of Advance Oxidation Processes (AOPs) in Textile Wastewater Treatment: A Critical Review. Desalination Water Treat. 2024, 318, 100387. [Google Scholar] [CrossRef]
- Le-Duy, N.; Hoang, L.A.T.; Nguyen, T.D.; Lee, T. Pd Nanoparticles Decorated BiVO4 Pine Architectures for Photocatalytic Degradation of Sulfamethoxazole. Chemosphere 2023, 321, 138118. [Google Scholar] [CrossRef]
- Bhanderi, D.; Lakhani, P.; Modi, C.K. Graphitic Carbon Nitride (g-C3N4) as an Emerging Photocatalyst for Sustainable Environmental Applications: A Comprehensive Review. RSC Sustain. 2023, 2, 265–287. [Google Scholar] [CrossRef]
- Ding, F.; Yang, D.; Tong, Z.; Nan, Y.; Wang, Y.; Zou, X.; Jiang, Z. Graphitic Carbon Nitride-Based Nanocomposites as Visible-Light Driven Photocatalysts for Environmental Purification. Environ. Sci. Nano 2017, 4, 1455–1469. [Google Scholar] [CrossRef]
- Qi, K.; Liu, S.-Y.; Zada, A. Graphitic Carbon Nitride, a Polymer Photocatalyst. J. Taiwan Inst. Chem. Eng. 2020, 109, 111–123. [Google Scholar] [CrossRef]
- Hoang, L.A.T.; Le, N.D.; Nguyen, T.D.; Lee, T. One-Step Synthesis of g-C3N4 Nanosheets with Enhanced Photocatalytic Performance for Organic Pollutants Degradation Under Visible Light Irradiation. Top. Catal. 2023, 66, 194–204. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Lai, J.; Pan, R.; Fan, Y.; Wu, X.; Ou, M.; Zhu, Y.; Fu, L.; Shi, F.; et al. Two-Dimensional Graphitic Carbon Nitride/N-Doped Carbon with a Direct Z-Scheme Heterojunction for Photocatalytic Generation of Hydrogen. Nanoscale Adv. 2021, 3, 6580–6586. [Google Scholar] [CrossRef]
- Rashidizadeh, A.; Ghafuri, H.; Rezazadeh, Z. Improved Visible-Light Photocatalytic Activity of g-C3N4/CuWO4 Nanocomposite for Degradation of Methylene Blue. Proceedings 2019, 41, 43. [Google Scholar]
- Zhang, M.; Duan, Y.; Jia, H.; Wang, F.; Wang, L.; Su, Z.; Wang, C. Defective Graphitic Carbon Nitride Synthesized by Controllable Co-Polymerization with Enhanced Visible Light Photocatalytic Hydrogen Evolution. Catal. Sci. Technol. 2017, 7, 452–458. [Google Scholar] [CrossRef]
- Huang, M.; Yang, Z.; Lu, L.; Xu, J.; Wang, W.; Yang, C. The Preparation of G-C3N4/CoAl-LDH Nanocomposites and Their Depollution Performances in Cement Mortars under UV-Visible Light. Catalysts 2022, 12, 443. [Google Scholar] [CrossRef]
- Meng, A.; Yang, R.; Li, W.; Li, Z.; Zhang, J. Enhanced Photocatalytic Hydrogen Production through Tuning Charge Transfer in TiO2/CdS Se1–-DETA Nanocomposites with S-Scheme Heterojunction Structure. J. Mater. 2024, 11, 100919. [Google Scholar] [CrossRef]
- Li, W.; Meng, A.Y.; Li, Z.; Zhang, J.F.; Fu, J.W. S-Scheme CeO2/Cd7.23Zn2.77S10-DETA Heterojunctions for Superior Cocatalyst-Free Visible-Light Photocatalytic Hydrogen Evolution. J. Cent. South. Univ. 2024, 31, 4572–4585. [Google Scholar] [CrossRef]
- Godin, R.; Wang, Y.; Zwijnenburg, M.A.; Tang, J.; Durrant, J.R. Time-Resolved Spectroscopic Investigation of Charge Trapping in Carbon Nitrides Photocatalysts for Hydrogen Generation. J. Am. Chem. Soc. 2017, 139, 5216–5224. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Zhang, M.; Luo, D.; Ye, S.; Weng, B. Surface Plasmon Resonance-Mediated Photocatalytic H2 Generation. ChemSusChem 2024, 17, e202400513. [Google Scholar] [CrossRef]
- Palanisamy, T.; Mitra, S.; Batra, N.; Smajic, J.; Emwas, A.-H.; Roqan, I.; Costa, P. Carbon Nitride Thin Film-Sensitized Graphene Field-Effect Transistor: A Visible-Blind Ultraviolet Photodetector. Adv. Mater. Interfaces 2022, 9, 2200313. [Google Scholar] [CrossRef]
- Xu, H.; Yan, J.; She, X.; Xu, L.; Xia, J.; Xu, Y.; Song, Y.; Huang, L.; Li, H. Graphene-Analogue Carbon Nitride: Novel Exfoliation Synthesis and Its Application in Photocatalysis and Photoelectrochemical Selective Detection of Trace Amount of Cu2+. Nanoscale 2014, 6, 1406–1415. [Google Scholar] [CrossRef]
- Liu, Y.N.; Zhou, X.; Wang, X.; Liang, K.; Yang, Z.K.; Shen, C.C.; Imran, M.; Sahar, S.; Xu, A.W. Hydrogenation/Oxidation Induced Efficient Reversible Color Switching between Methylene Blue and Leuco-Methylene Blue. RSC Adv. 2017, 7, 30080–30085. [Google Scholar] [CrossRef]
- Chang, X.; Fan, H.; Lei, L.; Wu, X.; Wang, W.; Ma, L. Generation Mechanism of the Defects in G-C3N4 Synthesized in N2 Atmosphere and the Method for Improving Photocatalysis Activity. Catalysts 2023, 13, 269. [Google Scholar] [CrossRef]
- Tiong, P.; Lintang, H.O.; Endud, S.; Yuliati, L. Improved Interfacial Charge Transfer and Visible Light Activity of Reduced Graphene Oxide-Graphitic Carbon Nitride Photocatalysts. RSC Adv. 2015, 5, 94029–94039. [Google Scholar] [CrossRef]
- Zhong, X.; Meng, F.; Dong, Y.; Zhao, J.; Zhang, H.; Du, Y. S-Scheme Heterojunction between Donor-Acceptor Linear Polymer and g-C3N4 via Strengthened Internal Electric Field for Enhanced Photocatalytic Activity. Mater. Today Energy 2025, 48, 101773. [Google Scholar] [CrossRef]
- Liang, B.; Rao, Y.; Duan, X. The Electrical Properties and Modulation of G-C3N4/β-As and g-C3N4/β-Sb Heterostructures: A First Principles Study. RSC Adv. 2019, 9, 38724–38729. [Google Scholar] [CrossRef]
- Zhang, L.; Mohamed, H.H.; Dillert, R.; Bahnemann, D. Kinetics and Mechanisms of Charge Transfer Processes in Photocatalytic Systems: A Review. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 263–276. [Google Scholar]
- Paul, D.R.; Gautam, S.; Panchal, P.; Nehra, S.P.; Choudhary, P.; Sharma, A. ZnO-Modified g-C3N4: A Potential Photocatalyst for Environmental Application. ACS Omega 2020, 5, 3828–3838. [Google Scholar] [CrossRef]
- Gong, H.; Chu, W.; Xu, K.; Xia, X.; Gong, H.; Tan, Y.; Pu, S. Efficient Degradation, Mineralization and Toxicity Reduction of Sulfamethoxazole under Photo-Activation of Peroxymonosulfate by Ferrate (VI). Chem. Eng. J. 2020, 389, 124084. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, T.; Gu, Y.; Yan, X.; Lu, N.; Liu, H.; Xu, Z.; Xing, Y.; Song, Y.; Zhang, Z.; et al. An Electrochemical Thrombin Aptasensor Based on the Use of Graphite-like C3N4 Modified with Silver Nanoparticles. Microchim. Acta 2020, 187, 163. [Google Scholar] [CrossRef]
- Peng, H.; Mo, Z.; Liao, S.; Liang, H.; Yang, L.; Luo, F.; Song, H.; Zhong, Y.; Zhang, B. High Performance Fe- and N- Doped Carbon Catalyst with Graphene Structure for Oxygen Reduction. Sci. Rep. 2013, 3, 1765. [Google Scholar] [CrossRef]
- Vezzù, K.; Bach Delpeuch, A.; Negro, E.; Polizzi, S.; Nawn, G.; Bertasi, F.; Pagot, G.; Artyushkova, K.; Atanassov, P.; Di Noto, V. Fe-Carbon Nitride “Core-Shell” Electrocatalysts for the Oxygen Reduction Reaction. Electrochim. Acta 2016, 222, 1778–1791. [Google Scholar] [CrossRef]
- Thøgersen, A.; Mayandi, J.; Vines, L.; Sunding, M.F.; Olsen, A.; Diplas, S.; Mitome, M.; Bando, Y. Composition and Structure of Pd Nanoclusters in SiOx Thin Film. J. Appl. Phys. 2011, 109, 084329. [Google Scholar] [CrossRef]
- Sági, G.; Csay, T.; Szabó, L.; Pátzay, G.; Csonka, E.; Takács, E.; Wojnárovits, L. Analytical Approaches to the OH Radical Induced Degradation of Sulfonamide Antibiotics in Dilute Aqueous Solutions. J. Pharm. Biomed. Anal. 2015, 106, 52–60. [Google Scholar] [CrossRef]
- Song, Y.; Qi, J.; Tian, J.; Gao, S.; Cui, F. Construction of Ag/g-C3N4 photocatalysts with visible-light photocatalytic activity for sulfamethoxazole degradation. Chem. Eng. J. 2018, 341, 547–555. [Google Scholar] [CrossRef]
- Senasu, T.; Lorwanishpaisarn, N.; Hemavibool, K.; Nijpanich, S.; Chanlek, N.; Nanan, S. Construction of g-C3N4/BiOCl/CdS heterostructure photocatalyst for complete removal of oxytetracycline antibiotic in wastewater. Sep. Purif. Technol. 2022, 306, 122735. [Google Scholar] [CrossRef]
- Guo, X.; He, S.; Meng, Z.; Wang, Y.; Peng, Y. Ag@ZIF-8/g-C3N4 Z-scheme photocatalyst for the enhanced removal of multiple classes of antibiotics by integrated adsorption and photocatalytic degradation under visible light irradiation. RSC Adv. 2022, 12, 17919–17931. [Google Scholar] [CrossRef]
- Ning, P.; Chen, H.; Pan, J.; Liang, J.; Qin, L.; Chen, D.; Huang, Y. Surface defect-rich g-C3N4/TiO2 Z-scheme heterojunction for efficient photocatalytic antibiotic removal: Rational regulation of free radicals and photocatalytic mechanism. Catal. Sci. Technol. 2020, 10, 8295–8304. [Google Scholar] [CrossRef]
- Lu, G.; Li, X.; Lu, P.; Guo, H.; Wang, Z.; Zhang, Q.; Li, Y.; Sun, W.; An, J.; Zhang, Z. Z-Type Heterojunction MnO2@g-C3N4 Photocatalyst-Activated Peroxymonosulfate for the Removal of Tetracycline Hydrochloride in Water. Toxics 2024, 12, 70. [Google Scholar] [CrossRef]
- Xue, J.; Ma, S.; Zhou, Y.; Zhang, Z.; He, M. Facile Photochemical Synthesis of Au/Pt/g-C3N4with Plasmon-Enhanced Photocatalytic Activity for Antibiotic Degradation. ACS Appl. Mater. Interfaces 2015, 7, 9630–9637. [Google Scholar] [CrossRef] [PubMed]
- Van Pham, V.; Truong, T.K.; Hai, L.V.; La, H.P.P.; Nguyen, H.T.; Lam, V.Q.; Tong, H.D.; Nguyen, T.Q.; Sabbah, A.; Chen, K.-H.; et al. S-Scheme α-Fe2O3/g-C3N4 Nanocomposites as Heterojunction Photocatalysts for Antibiotic Degradation. ACS Appl. Nano Mater. 2022, 5, 4506–4514. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).